Aging is associated with attenuated exercise hyperemia and vasodilation. Chronic endurance exercise training augments exercise hyperemia and vasodilation during steady-state exercise in older adults. In the current study, we found that chronic exercise improves contraction-induced rapid vasodilation in both the arm and leg of older adults. Furthermore, our current results suggest that exercise capacity is associated with peak dilator responses in both the arm and leg of older adults.
Keywords: aging, vasodilation, hyperemia, exercise training
Abstract
Aging is associated with attenuated contraction-induced rapid onset vasodilation (ROV). We sought to examine whether chronic exercise training would improve ROV in older adults. Additionally, we examined whether a relationship between cardiorespiratory fitness and ROV exists in young and older adults. Chronically exercise-trained older adults (n = 16; 66 ± 2 yr, mean ± SE) performed single muscle contractions in the forearm and leg at various intensities. Brachial and femoral artery diameter and blood velocity were measured using Doppler ultrasound. Vascular conductance (VC) was calculated as the quotient of blood flow (ml/min) and mean arterial pressure (mmHg). These data were compared with our previously published work from an identical protocol in 16 older untrained (66 ± 1 yr, mean ± SE) and 14 young (23 ± 1 yr) adults. Peak (ΔVCpeak) and total vasodilator (VCtotal) responses were greater in trained compared with untrained older adults across leg exercise intensities (P < 0.05). There were no differences in responses between trained older and young adults in the arm or leg at any exercise intensity (P > 0.05). Comparison of ΔVCpeak in a subset of subjects at an absolute workload in the leg revealed that trained older adults exhibited augmented responses relative to untrained older adults. Exercise capacity (V̇o2 peak) was associated with ΔVCpeak and VCtotal across arm (r = 0.59–0.64) and leg exercise intensities (r = 0.55–0.68, P < 0.05) in older adults. Our data demonstrate that 1) chronic exercise training improves ROV in the arm and leg of trained older adults, such that age-related differences in ROV are abolished, and 2) VO2peak is associated with ΔVCpeak responses in both limbs of older adults.
NEW & NOTEWORTHY
Aging is associated with attenuated exercise hyperemia and vasodilation. Chronic endurance exercise training augments exercise hyperemia and vasodilation during steady-state exercise in older adults. In the current study, we found that chronic exercise improves contraction-induced rapid vasodilation in both the arm and leg of older adults. Furthermore, our current results suggest that exercise capacity is associated with peak dilator responses in both the arm and leg of older adults.
aging is associated with impairments in vascular function and the regulation of skeletal muscle blood flow, as well as reductions in aerobic exercise capacity (1, 17, 24, 50). These age-related impairments in the regulation of skeletal muscle blood flow, particularly during exercise, are apparent after a single skeletal muscle contraction (6, 7, 9, 19, 21) and persist under steady-state conditions (8, 23, 33, 34, 36). Blunted skeletal muscle blood flow responses in older adults have been attributed to alterations in local vasodilatory mechanisms, elevations in sympathetic vasoconstrictive activity, or an impaired ability to modulate sympathetic vasoconstriction during exercise (13, 17, 22, 26, 42). Collectively, these adverse changes in the vasculature and decreased limb blood flow can potentially lead to an inadequate matching of oxygen delivery to the metabolic demand of the contracting muscle (1, 23, 40).
Rapid onset vasodilation (ROV) describes the immediate increase in blood flow and vasodilation elicited with a single muscle contraction (10, 41, 48, 49) and is thought to serve a critical role in modulating the blood flow response during steady-state exercise conditions (41, 44). The influence of age on ROV is well characterized in the human forearm (6, 7, 9, 21) and, more recently, in the leg (19). Collectively, these studies indicate that older adults exhibit attenuated peak and total hyperemic and vasodilator responses following a single muscle contraction (6, 7, 9, 21), which appears to be independent of limb (19). At least in the forearm, these age-related decrements in contraction-induced rapid vasodilation are thought to be due in part to diminished nitric oxide (NO) bioavailability or signaling and enhanced α-adrenergic vasoconstrictor tone (7, 9). However, to date, all studies examining ROV in both young and older adults have exclusively examined the hyperemic and vasodilator responses in sedentary or recreationally active individuals.
Chronic exercise training has been shown to ameliorate many age-associated changes in vascular function (26, 28, 43), while physical inactivity is a risk factor for the development of chronic diseases (5, 32). Indeed, there is a strong inverse relationship between cardiorespiratory fitness (e.g., maximal oxygen consumption) and overall mortality (4, 15), highlighting the benefits of lifelong exercise and high levels of physical activity. However, it is unclear whether the attenuated ROV in contracting muscle of older adults is due to aging per se or the reduction of physical activity and functional fitness that often accompanies the aging process (14, 24). Moreover, it is unknown whether long-term exercise training can prevent the blunting of ROV that occurs in untrained older adults. With this information as background, we tested the hypothesis that chronic endurance exercise training in older adults would ameliorate the age-related impairments in contraction-induced rapid vasodilation. Additionally, we sought to examine whether a relationship between cardiorespiratory fitness and ROV exists in young and older adults.
METHODS
Subjects
Sixteen older endurance exercise-trained (9 men, 7 women, age 59–85 yr) subjects volunteered to participate in this study. To address our primary aim, data from our previous study, which included 16 older and 14 young sedentary to recreationally active adults (19), were used for comparison with the present group of older exercise-trained adults (see Table 1 for complete demographics). All subjects completed a general health history screening and written informed consent and were generally healthy, free of any diagnosed cardiovascular or metabolic complications, nonobese (body mass index ≤ 30 kg/m2), nonsmokers, and not taking any vasoactive medications. Exercise-trained older adults self-reported chronic endurance exercise training ≥4 days per week, ≥1 h per session per day, and for at least the past year. On average, subjects reported meeting these training requirements for the past 19 ± 4 yr, and several of them were still actively competing in races (both running and cycling). Trained status was confirmed with a graded, maximal exercise stress test. Studies were performed after an overnight fast, and subjects refrained from exercise, alcohol, and caffeine for 24 h before reporting to the laboratory. Young female subjects were studied during the early follicular phase of their menstrual cycle or the placebo phase of oral contraceptives to control for the potential influence of sex hormones on primary outcome variables (25). All older female subjects were postmenopausal and were not taking any form of hormone replacement therapy. All study protocols were approved by the Institutional Review Board at the University of Iowa.
Table 1.
Subject characteristics
| Variable | Young Adults (n = 14) | Untrained Older Adults (n = 16) | Trained Older Adults (n = 16) |
|---|---|---|---|
| Age, yr | 23 ± 1 | 66 ± 1* | 66 ± 2* |
| Men/women | 9/5 | 10/6 | 9/7 |
| Height, cm | 174 ± 2 | 172 ± 2 | 170 ± 2 |
| Weight, kg | 75 ± 3 | 78 ± 3 | 72 ± 2 |
| Body mass index, kg/m2 | 24.7 ± 0.6 | 26.5 ± 0.7 | 24.7 ± 0.7 |
| Percent body fat | 27.1 ± 1.9 | 31.9 ± 1.7 | 28.0 ± 2.0 |
| Forearm muscle mass, kg | 0.94 ± 0.08 | 0.90 ± 0.06 | 0.85 ± 0.07 |
| Thigh muscle mass, kg | 7.5 ± 0.5 | 6.9 ± 0.3 | 7.0 ± 0.3 |
| V̇o2peak, ml·kg−1·min−1 | 44.3 ± 2.6 | 30.6 ± 1.5* | 37.5 ± 1.9† |
| MVC, kg | 41 ± 3 | 41 ± 3 | 40 ± 3 |
| WRmax, W | 41 ± 4 | 29 ± 2* | 37 ± 3 |
| Systolic pressure, mmHg | 117 ± 2 | 123 ± 3 | 123 ± 3 |
| Diastolic pressure, mmHg | 72 ± 2 | 75 ± 2 | 77 ± 2 |
| Mean arterial pressure, mmHg | 87 ± 2 | 91 ± 2 | 92 ± 2 |
Values are means ± SE. V̇o2peak, peak exercise oxygen consumption; MVC, maximal voluntary contraction; WRmax, work rate maximum.
P < 0.05 vs. young adults.
P < 0.05 vs. untrained older adults.
Pre-Study Day Measurements
Body composition, forearm, and leg tissue mass.
Body composition was determined by dual-energy X-ray absorptiometry (DEXA; Hologic software version APEX 4.0). Total mass and fat-free mass of the left forearm and right leg were determined from regional analysis from the whole-body DEXA scan using bony landmarks for normalization of blood flow and vascular conductance responses. Body mass index was calculated as body weight (kg) divided by height (m) squared.
Measurement of exercise capacity.
Peak exercise oxygen consumption (V̇o2 peak; ml·kg−1·min−1) was determined in all subjects using respiratory gas analysis (Parvo Medics TrueOne 2400, Sandy, UT) during incremental treadmill exercise using a Bruce protocol performed to exhaustion as previously described (16).
Determination of work rate maximum.
Work rate maximum (WRmax) was determined from a single leg knee extensor incremental maximal exercise test completed during a familiarization session prior to the study day as previously described (19). Briefly, subjects were seated in a semirecumbent position on a modified adjustable bucket seat that accommodated variable body and leg lengths allowing each subject's lower leg to move through a 90–180° range of motion during the knee extension exercise. Resistance was developed by a custom-made computer-controlled leg ergometer. Briefly, resistance (torque) was developed by an alternating current motor turning at a constant rpm that was transferred to the leg shaft, against which subjects contracted. The computer monitored the elapsed time and the angle of the leg and controlled the actual torque presented to the leg during contraction. In this way, the subject was required to develop enough power to extend the leg through a full range of motion. WRmax testing consisted of an initial workload of 5 W that incrementally increased every minute by 3 W and 5 W in female and male subjects, respectively. Subjects kicked dynamically through a full range of motion at a cadence of 40 kicks per minute. The single leg knee extensor incremental maximal test continued until the subject could not maintain a full range of motion or a cadence of 40 contractions per minute. The final workload completed was recorded as maximal kicking load, from which relative workloads were calculated.
Study Day Measurements
Heart rate and systemic blood pressure.
Heart rate was recorded via continuous three-lead electrocardiogram, and systemic blood pressure was assessed (beat-to-beat) via finger plethysmography (Nexfin; Edwards Lifesciences, Irvine, CA) on the nonexercising hand. Brachial artery pressure was measured in duplicate using an automated cuff (Cardiocap/5, Datex-Ohmeda, Louisville, CO) prior to beginning exercise trials while the subjects were in a supine position following 15 min of rest.
Single muscle contractions.
Subjects performed dynamic single contractions in both the arm and leg as previously described (19). Briefly, single forearm contractions were performed with a handgrip device at 10%, 20%, and 40% of the subject's maximal voluntary contraction (MVC), determined (using a handgrip dynamometer) as the average of three maximal squeezes performed on the pre-study measurement day. Single knee extension contractions were performed at 20%, 40%, and 60% WRmax. Subjects were instructed to contract and relax on a verbal command from laboratory personnel. WRmax and MVC intensities were randomized prior to the experimental protocol, and each contraction intensity was performed in duplicate to calculate the average response for each subject for a given condition. Each contraction (leg and arm) was visually observed by the laboratory personnel to ensure proper timing of contraction. Two minutes of relaxation were given between each contraction to allow continuous measures of limb hemodynamics postcontraction. All single muscle contractions (knee extensions and forearm contractions) were performed on the same day.
Measurement of blood flow.
Brachial and common femoral (∼2–3 cm proximal to bifurcation) artery diameter and blood velocity were determined with a 12-MHz linear array Doppler probe (model M12L; Vivid 7, General Electric, Milwaukee, WI). Blood velocity was measured with a probe insonation angle previously calibrated to 60°. Measured velocity waveforms were synchronized to a data acquisition system (WinDaq; DATAQ Instruments, Akron, OH) via a Doppler audio transformer (18). Artery diameter measurements were obtained at end diastole at rest (before contraction) and 1-min postcontraction. Limb blood flow (BF) was calculated as the product of mean blood velocity (cm/s) and artery cross-sectional area (cm2) and expressed as milliliters per minute (ml/min).
Data analysis and statistics.
Data were collected at 250 Hz and analyzed offline with signal-processing software (WinDaq; DATAQ Instruments). Beat-to-beat mean arterial pressure (MAP) was derived from the Nexfin pressure waveform and was recorded simultaneously with beat-to-beat blood velocity measurements. Heart rate was determined from the electrocardiogram. Baseline BF and MAP represent an average of the last 30 s of the resting time period before each muscle contraction and were used to quantify the hyperemic response. Vascular conductance (VC) was calculated as BF/MAP × 100 (and expressed as ml·min−1·100 mmHg−1). Rapid hyperemic and vasodilator responses are expressed as the change in (Δ) BF and VC from baseline, respectively. Of particular interest are the peak (ΔVCpeak) and total (VCtotal) dilator responses postcontraction. Total BF (ml) and VC (ml/100 mmHg) were defined as the area under the curve over 30 postcontraction cardiac cycles after respective baseline values were subtracted for a given flow or conductance curve. To account for the possible influence of muscle mass, BF and VC responses were also examined after normalizing for muscle mass (kg).
All values are expressed as mean ± SE. Analysis of variance (ANOVA) was used to analyze demographic variables between groups. To address the primary question of whether chronic exercise training offsets the age-related impairment in contraction-induced ROV, comparisons were made between the older trained adults and our previously published data on young and older untrained adults (19). Independent, one-way ANOVAs were used to compare groups across exercise intensities in both the arm (10%, 20%, and 40%) and leg (20%, 40%, and 60%). When significance was detected, Tukey's post hoc analysis was used to identify differences between groups. Pearson's correlation coefficients were calculated to assess the relationship between exercise capacity (V̇o2 peak) and ROV (peak and total VC). All statistical analyses were completed using SigmaPlot software version 11.0 (Systat Software, San Jose, CA). Statistical difference was set a priori at P < 0.05.
RESULTS
Subject characteristics are shown in Table 1. Young, untrained older, and trained older subjects were of similar height, weight, body mass, and percent body fat (P > 0.05). Brachial blood pressures, forearm and leg muscle masses, and forearm MVC were also similar between groups (P > 0.05 for all). There were no differences in WRmax between trained and untrained older adults. Compared with young adults, untrained older adults had a lower WRmax (P < 0.05), but there were no differences between trained older adults and young adults. Lastly, and important to the current study, exercise-trained older adults had higher V̇o2 peak compared with their untrained counterparts (P < 0.05).
Rapid Hyperemic and Vasodilator Responses to Single Muscle Contractions in Trained Older, Untrained Older, and Young Adults.
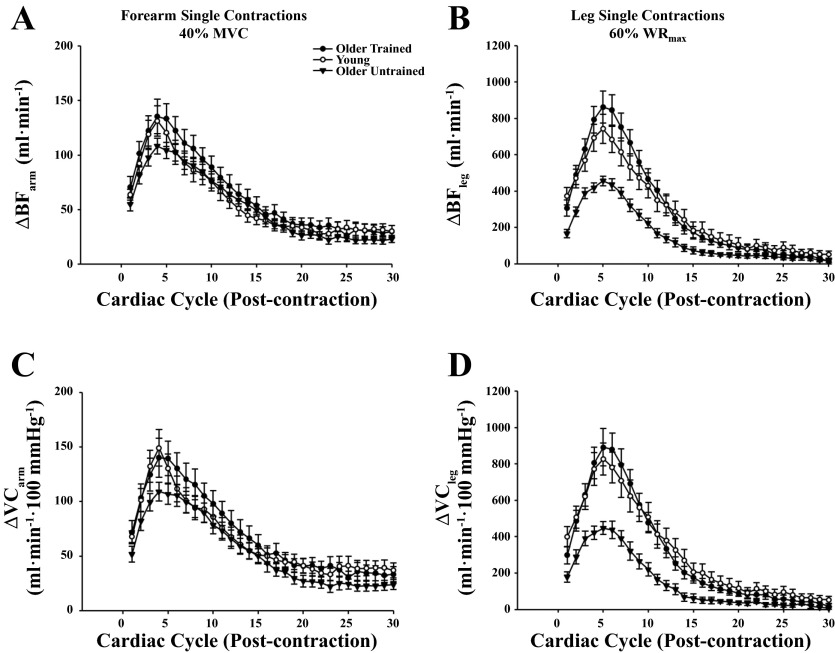
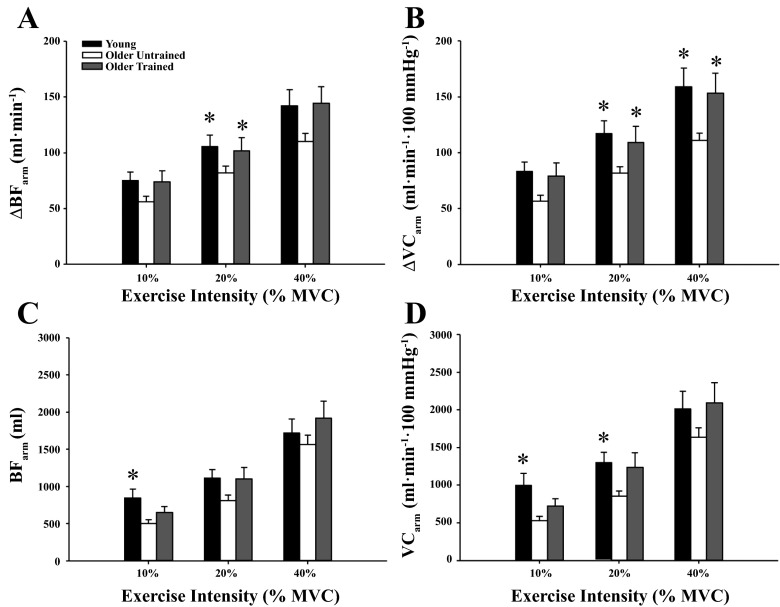
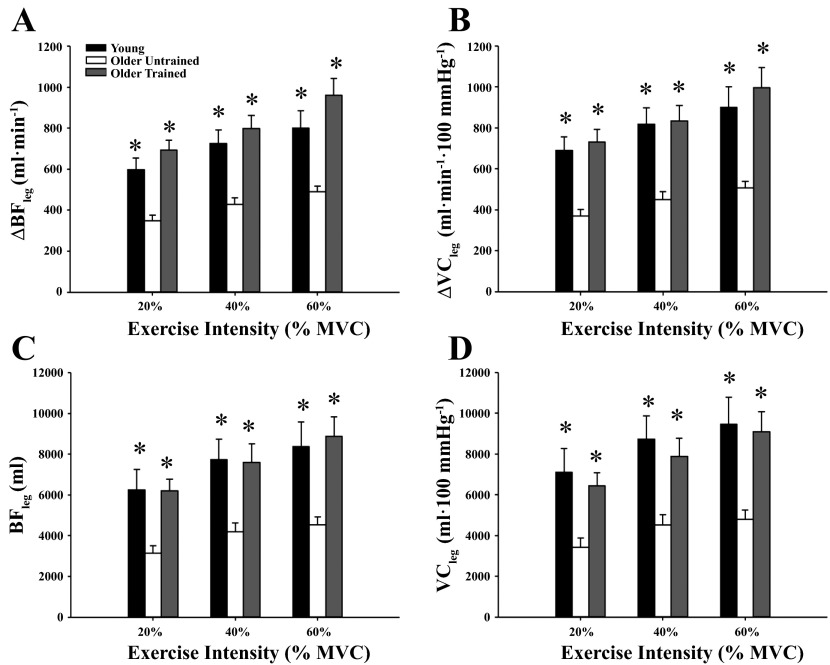
Figure 1 illustrates the temporal rapid hyperemic and vasodilator responses at the highest arm (Fig. 1, A and C; 40% MVC) and leg (Fig. 1, B and D; 60% WRmax) exercise intensities in the three groups of subjects (exercise-trained older adults, untrained older adults, and young adults). The responses in the young and untrained older adults have been previously published (19) and are included to highlight the effect of chronic exercise training on the temporal vasodilator response following a single muscle contraction in the arm and leg. The peak responses for the arm and leg tended to occur in the first 3–5 cardiac cycles, and this timing of peak responses was similar between groups. The peak hyperemic and vasodilator responses in the arm were greater in trained older adults at moderate exercise intensities (20–40% MVC) compared with their older untrained counterparts (P < 0.05; Fig. 2, A and B). There were no differences between trained and untrained older adults for total BF at any exercise intensity (Fig. 2, C and D). Additionally, there were no differences across contraction intensities for the peak and total hyperemic and vasodilator responses in the arm between trained older adults and young adults (P > 0.05). Peak and total hyperemic (Fig. 3, A and C) and vasodilator (Fig. 3, B and D) responses were greater in the older trained adults across leg exercise intensities compared with older untrained adults (P < 0.05) while there were no differences between trained older and young adults (P > 0.05). Normalizing responses to muscle mass elicited similar results, in that peak arm hyperemic and vasodilator responses were greater in trained older adults at moderate exercise intensities compared with untrained counterparts (data not shown P < 0.05). Additionally, peak and total hyperemic and vasodilator responses were greater in trained older adults across leg exercise intensities compared with untrained older adults, with no differences between trained older and young adults (data not shown, P > 0.05).
Fig. 1.
Hyperemic [change (Δ) in blood flow (BF); A and B] and vasodilator [Δ vascular conductance (VC); C and D] responses over 30 cardiac cycles following single muscle contractions at the highest arm (A and C; 40% MVC) and leg (B and D; 60% WRmax) exercise intensities.
Fig. 2.
Peak (A and B) and total (C and D) hyperemic and vasodilator responses in the arm for young, untrained older, and trained older adults. Peak hyperemic and vasodilator responses (20% and 40% MVC) were greater in trained compared with untrained older adults. There were no differences in hyperemic and vasodilator responses between trained older adults and young adults. *P < 0.05 vs. untrained older adults.
Fig. 3.
Peak (A and B) and total (C and D) hyperemic and vasodilator responses in the leg for young and untrained and trained older adults. All parameters of the ROV response (peak and total) were greater in trained older adults compared with untrained older adults across leg exercise intensities. There were no differences between trained older adult responses and young adults. *P < 0.05 vs. untrained older adults.
Relationship Between Exercise Capacity and ROV Within the Arm and Leg
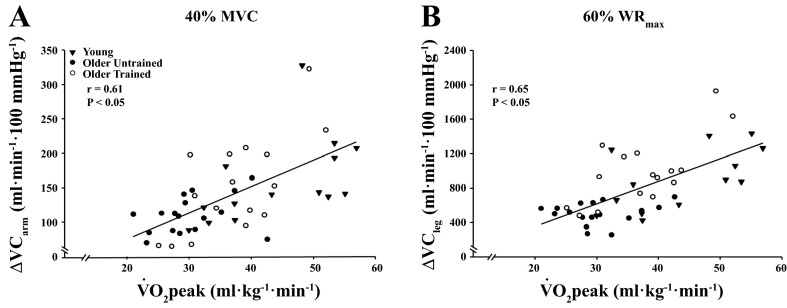
Figure 4 illustrates the relationship between V̇o2 peak and peak vasodilator responses at the highest exercise intensities in the arm (Fig. 4A) and leg (Fig. 4B). V̇o2 peak was moderately associated with arm peak and total VC across exercise intensities (r = 0.31–0.61, P < 0.05 for all) in the group as a whole. When separated by age, V̇o2 peak was associated with peak (r = 0.59–0.64, P < 0.05) and total (r = 0.39–0.52, P < 0.05) arm VC across exercise intensities in older adults, but only at 40% in young adults (r = 0.53, P < 0.05). Within the leg, V̇o2 peak was associated with peak (r = 0.55–0.65, P < 0.05) and total (r = 0.39–0.50, P < 0.05) VC across exercise intensities in the entire group. When separated by age, V̇o2 peak was moderately associated with peak leg vasodilator responses across exercise intensities in older adults (r = 0.55–0.68, P < 0.01 for all), and at 40–60% WRmax in young adults (r = 0.60 and 0.65, respectively, P < 0.05 for both). When separated by training status (trained older vs. untrained adults) there were no associations between V̇o2 peak or total VC in the arm or leg of untrained older adults (P > 0.05). Conversely, trained older adults exhibited associations between V̇o2 peak and peak ΔVC across arm exercise intensities (r = 0.68–0.70, P < 0.05 for all) and at 40% and 60% WRmax in the leg (r = 0.62–0.70, P < 0.05).
Fig. 4.
Relationship between exercise capacity (V̇o2 peak) and peak vasodilator responses (ΔVCpeak) in the arm (A) and leg (B) of young and older adults.
ROV, Age, and Physical Fitness: Influence of Workload
As untrained older adults had a lower leg WRmax it could be argued that because of the tight relationship between blood flow and metabolic rate, any age- or physical fitness-related differences may be due to differences in the absolute work performed between groups. To address this issue, we compared the peak and total responses normalized for work rate (e.g., flow and/or conductance per watt) between groups. Normalizing the data in this way revealed results similar to those presented above for the absolute hyperemic and vasodilator responses. Trained older adults still demonstrated higher peak hyperemic (98 ± 8 vs. 68 ± 8, 56 ± 4 vs. 41 ± 4, and 44 ± 3 vs. 32 ± 3 ml·min−1·W−1) and vasodilator responses (105 ± 10 vs. 73 ± 9, 59 ± 5 vs. 43 ± 5, and 46 ± 4 vs. 33 ± 3 ml·min−1·100 mmHg−1·W−1) across exercise intensities (20–60% WRmax) compared with untrained older adults (P < 0.05 for all). Additionally, the total hyperemic response normalized for work rate was higher at 20% (908 ± 102 vs. 640 ± 110) and 60% (416 ± 47 vs. 296 ± 34) WRmax in trained vs. untrained older adults (P < 0.05 for both).
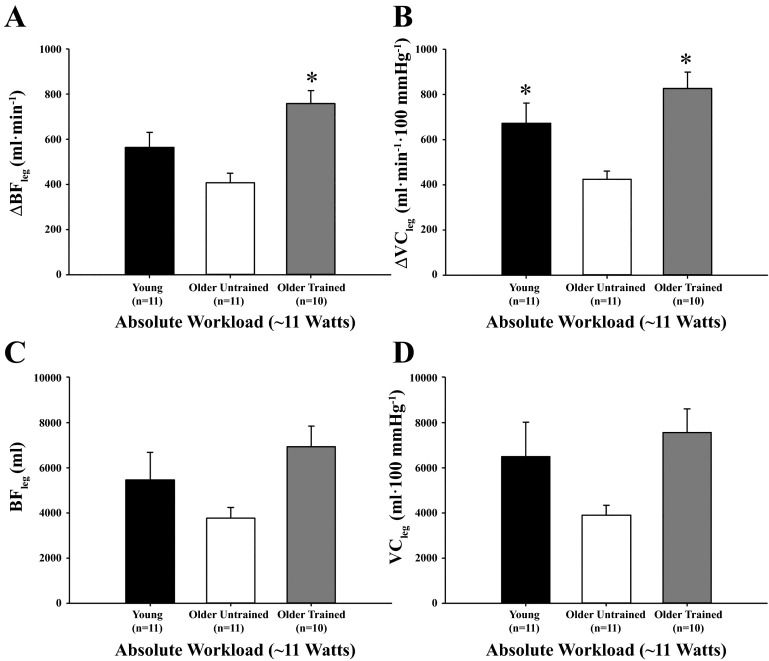
To further address the potential confound of work rate between groups, we compared the peak and total hyperemic and vasodilator responses in a subset of subjects from each of the three groups that performed similar absolute workloads (mean ∼10–11 W for each group). The mean ± SE workloads for the subset of trained older (n = 10), untrained older (n = 11), and young (n = 11) adults were 10.6 ± 0.4, 10.5 ± 0.3, and 10.3 ± 0.3 W, respectively. As illustrated in Fig. 5, peak hyperemic (Fig. 5A) and vasodilator responses (Fig. 5B) were greater in trained older adults compared with untrained older adults (P < 0.05 for both) when matched for workload, while peak ΔVC was lower in untrained older adults compared with young adults (P < 0.05). Total hyperemic (Fig. 5C) and vasodilator (Fig. 5D) responses were not different between groups (P = 0.07 for both). When the responses were normalized for muscle mass in the subset of subjects matched for workload, there were no differences between groups (P = 0.09–0.17) for peak and total ΔBF and ΔVC. In the same subset of subjects, V̇o2 peak was associated with ΔVCpeak in the group as a whole (r = 0.48, P < 0.01), but not when separated by training status (r = −0.08–0.44, P > 0.05 for all). Total VC and V̇o2 peak were not associated in any group, regardless of how responses were expressed (r = −0.54-0.39, P > 0.05 for all). There were no significant associations between V̇o2 peak and ΔVCpeak (normalized for muscle mass), in the group as a whole (r = 0.15, P > 0.05) or when separated by training status (trained, untrained older adults, and young; r = −0.43–0.48, P > 0.05 for all).
Fig. 5.
Peak (A and B) and total (C and D) hyperemic and vasodilator responses in the leg at an absolute workload of ∼11 W for young adults and untrained and trained older adults. Both parameters of peak ROV responses were greater in trained older adults compared with untrained older adults at an absolute workload. *P < 0.05 vs. untrained older adults.
DISCUSSION
We and others have previously shown that contraction-induced rapid hyperemic and vasodilator responses are attenuated in untrained or sedentary older adults (6, 7, 9, 19, 21) and that this attenuation appears to be similar between the arm and leg (19). In the present cross-sectional study, we demonstrate for the first time that 1) older adults with a history of chronic endurance exercise training demonstrate greater contraction-induced rapid hyperemic and vasodilator responses within both the arm and leg compared with their age-matched counterparts and 2) vasodilator responsiveness to single muscle contractions is moderately associated with exercise capacity (V̇o2 peak). These conclusions are supported by the fact that chronically trained older adults demonstrated a substantially greater peak ROV in the arm (Fig. 2) as well as an approximately twofold greater peak and total ROV response in the leg (Fig. 3) compared with untrained older adults across a range of relative (percent) workloads. Moreover, the group differences between the older trained and untrained adults still persist when 1) the ROV responses are normalized to the absolute work rate performed and 2) the groups are matched for a similar absolute workload (Fig. 5). Finally, our findings also demonstrate that exercise capacity is positively related to the peak ROV response, regardless of whether the peak ROV responses are expressed at the same relative exercise intensity or matched for workload. Taken together, these results suggest that chronic endurance exercise offsets the age-related attenuation in ROV in both the arm and leg of older adults.
Benefits of Exercise Training on Blood Flow in Older Adults
During submaximal dynamic exercise there is a general decline in steady-state blood flow and vasodilation with age (23, 33–39), which may be offset with exercise training (1, 31). The decline in exercise blood flow with aging is presumably due to an imbalance between local vasodilator and vasoconstrictor signaling, elevated sympathetic vasoconstrictive tone, as well as an impaired ability to blunt sympathetic vasoconstriction within the contracting muscle (12, 13, 20–22, 42). Conversely, lifelong physical activity (27) as well as exercise interventions (26) appear to preserve the ability to blunt sympathetic vasoconstrictive tone (functional sympatholysis) in the leg of older adults and maintain sufficient oxygen delivery in older adults (38, 50). However, it should be noted that the documented benefits of lifelong physical activity or chronic exercise training on the regulation of muscle blood flow during exercise may be sex specific. That is, exercise-trained older women do not appear to exhibit the same preservation of exercise hyperemia and vasodilation that trained older men do (31, 34, 35). In the present study, chronic exercise training appears to offset the age-related attenuation of peak and total hyperemic and vasodilator responses following a single muscle contraction in the leg of both older men and women. However, this study was not adequately powered to detect sex by exercise-training interactions, and therefore we cannot make any conclusions on whether chronic exercise training is less effective in improving ROV in older women compared with men.
Training Benefits Beyond “Active” Tissue
Exercise-training adaptations to vascular structure and function are not solely restricted to the active limb (e.g., metabolically active limb) and may be conferred to the vasculature perfusing nonexercising tissue (e.g., inactive skeletal muscle) (2, 30). In the context of the present study, the majority of exercise-trained older adults reported participation primarily in activities involving the lower limbs, and as illustrated in Figs. 2 and 3, this chronic exercise training offsets age-related impairment of ROV responses in both limbs. Along these lines, lower-body exercise training not only results in an increase in femoral artery diameter but also preserves endothelial function in the brachial artery (e.g., “inactive” limb), such that age-related differences in both conduit and resistance arteries are abolished (11, 52). These favorable vascular adaptations in conduit arteries to exercise training in “inactive” skeletal muscle are hypothesized to be mainly due to repeated bouts of elevated shear stress, with endothelial adaptations evident even after a single bout of acute exercise (47). Taken together, these data confirm and highlight the systemic benefits of chronic exercise training on the vasculature in older adults.
Possible Mechanisms
This study provides insight into how chronic endurance exercise training offsets age-related attenuations in contraction-induced ROV; however, it is important to emphasize that discerning the underlying mechanisms modulating this response was not the purpose of the present investigation. Instead, this study was designed to highlight and characterize the relationship between chronic exercise training, exercise capacity, and age-related attenuations in contraction-induced ROV. Therefore we will only briefly highlight plausible mechanisms by which exercise training may offset age-related attenuation of the ROV response to single muscle contractions. Previous evidence from our laboratory demonstrates that age-related impairments in contraction-induced ROV in the arm are in part due to a reduction in NO bioavailability or signaling, as well as an increase in sympathetic vasoconstriction (7, 9). In this context, evidence from habitually exercising older adults indicates that lifelong exercise training preserves both NO signaling (28, 46) as well as functional sympatholysis within the leg vasculature in older individuals (27). Therefore it is plausible that the mechanisms by which chronic exercise training offsets age-related attenuation of ROV in older adults are similar to those observed under steady-state conditions during submaximal dynamic exercise.
Experimental Considerations
It should be noted that our current study design does not allow us to examine whether endurance exercise-trained older adults exhibit similar ROV responses relative to trained young adults. Evidence suggests that young, highly trained adults exhibit augmented exercise blood flow responses at submaximal and maximal workloads relative to young sedentary counterparts in both the arm and leg (51). Therefore it can be assumed that young trained adults might exhibit augmented ROV responses; however, this has yet to be confirmed. Given that the aim of the study was to examine how chronic exercise training influences characteristics of ROV in a cross-sectional manner, we could not control which specific type of chronic exercise subjects participated in. Furthermore, as we present a large range of chronic exercise-trained older adults, we were unable to control the number of years the group as a whole participated in. Along these lines, improvements to vascular function are apparent even with short-term exercising training interventions, particularly in populations with established vascular dysfunction (11, 26, 29, 45, 52). Additionally, while the older trained and untrained groups were well matched for many characteristics besides exercise training and V̇o2, there was a trend (P = 0.11) for a higher percent body fat in the untrained compared with exercise-trained older adults. Since contraction-induced rapid onset vasodilatation has been shown to be blunted with obesity (3), differences in body composition could in part contribute to the augmented ROV responses observed in the exercise-trained group. However, we believe these limitations should not detract from the present study; instead, it should be viewed in a larger picture, in that older adults with a history of performing habitual endurance exercise demonstrate an augmented contraction-induced rapid vasodilation compared with their sedentary older peers.
Conclusion
To our knowledge, this is the first study to demonstrate that chronic exercise training or physical activity offsets the age-related attenuation in contraction-induced rapid vasodilation in both the arm and leg. Additionally, exercise capacity is associated with contraction-induced rapid hyperemic and vasodilator responses in older adults. These findings extend upon previous studies demonstrating the ability of chronic aerobic exercise to offset age-related impairments of endothelial function as well as steady-state exercise hyperemia and vasodilation, further highlighting the benefits of lifelong exercise training.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Research Grant HL-105467 (to D. P. Casey) and NIH CTSA U54TR001356.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
W.E.H., K.U., and D.P.C. performed experiments; W.E.H. and D.P.C. analyzed data; W.E.H. and D.P.C. interpreted results of experiments; W.E.H. prepared figures; W.E.H. drafted manuscript; W.E.H., K.U., and D.P.C. edited and revised manuscript; W.E.H., K.U., and D.P.C. approved final version of manuscript; D.P.C. conception and design of research.
ACKNOWLEDGMENTS
The authors are grateful to the study volunteers for their participation. We thank David Treichler, Charles Ganger IV, Aaron Schneider, Samuel Norton, and Cynthia Pauley for their technical assistance.
REFERENCES
- 1.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation 100: 1085–1094, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Birk GK, Dawson EA, Atkinson C, Haynes A, Cable NT, Thijssen DH, Green DJ. Brachial artery adaptation to lower limb exercise training: role of shear stress. J Appl Physiol 112: 1653–1658, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Blain GM, Limberg JK, Mortensen GF, Schrage WG. Rapid onset vasodilatation is blunted in obese humans. Acta Physiol (Oxf) 205: 103–112, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair SN, Kohl HW III, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA 262: 2395–2401, 1989. [DOI] [PubMed] [Google Scholar]
- 5.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol 2: 1143–1211, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson RE, Kirby BS, Voyles WF, Dinenno FA. Evidence for impaired skeletal muscle contraction-induced rapid vasodilation in aging humans. Am J Physiol Heart Circ Physiol 294: H1963–H1970, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Casey DP, Joyner MJ. Influence of alpha-adrenergic vasoconstriction on the blunted skeletal muscle contraction-induced rapid vasodilation with aging. J Appl Physiol 113: 1201–1212, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casey DP, Ranadive SM, Joyner MJ. Aging is associated with altered vasodilator kinetics in dynamically contracting muscle: role of nitric oxide. J Appl Physiol 119: 232–241, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casey DP, Walker BG, Ranadive SM, Taylor JL, Joyner MJ. Contribution of nitric oxide in the contraction-induced rapid vasodilation in young and older adults. J Appl Physiol 115: 446–455, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clifford PS. Skeletal muscle vasodilatation at the onset of exercise. J Physiol 583: 825–833, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 100: 164–170, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic alpha-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 567: 311–321, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiPietro L. Physical activity in aging: changes in patterns and their relationship to health and function. J Gerontol A Biol Sci Med Sci 56, Spec No 2: 13–22, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Farrell SW, Kampert JB, Kohl HW III, Barlow CE, Macera CA, Paffenbarger RS Jr, Gibbons LW, and Blair SN. Influences of cardiorespiratory fitness levels and other predictors on cardiovascular disease mortality in men. Med Sci Sports Exerc 30: 899–905, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Fielding RA, Frontera WR, Hughes VA, Fisher EC, Evans WJ. The reproducibility of the Bruce protocol exercise test for the determination of aerobic capacity in older women. Med Sci Sports Exerc 29: 1109–1113, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Hearon CM Jr, Dinenno FA. Regulation of skeletal muscle blood flow during exercise in ageing humans. J Physiol 594: 2261–2273, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herr MD, Hogeman CS, Koch DW, Krishnan A, Momen A, Leuenberger UA. A real-time device for converting Doppler ultrasound audio signals into fluid flow velocity. Am J Physiol Heart Circ Physiol 298: H1626–H1632, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes WE, Ueda K, Treichler DP, Casey DP. Rapid onset vasodilation with single muscle contractions in the leg: influence of age. Physiol Rep 3: e12516, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Modulation of postjunctional alpha-adrenergic vasoconstriction during exercise and exogenous ATP infusions in ageing humans. J Physiol 589: 2641–2653, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch DW, Leuenberger UA, Proctor DN. Augmented leg vasoconstriction in dynamically exercising older men during acute sympathetic stimulation. J Physiol 551: 337–344, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Milanovic Z, Pantelic S, Trajkovic N, Sporis G, Kostic R, James N. Age-related decrease in physical activity and functional fitness among elderly men and women. Clin Interv Aging 8: 549–556, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Mortensen SP, Nyberg M, Gliemann L, Thaning P, Saltin B, Hellsten Y. Exercise training modulates functional sympatholysis and alpha-adrenergic vasoconstrictor responsiveness in hypertensive and normotensive individuals. J Physiol 592: 3063–3073, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mortensen SP, Nyberg M, Winding K, Saltin B. Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the ageing human leg. J Physiol 590: 6227–6236, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyberg M, Blackwell JR, Damsgaard R, Jones AM, Hellsten Y, Mortensen SP. Lifelong physical activity prevents an age-related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J Physiol 590: 5361–5370, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyberg M, Seidelin K, Andersen TR, Overby NN, Hellsten Y, Bangsbo J. Biomarkers of vascular function in premenopausal and recent postmenopausal women of similar age: effect of exercise training. Am J Physiol Regul Integr Comp Physiol 306: R510–R517, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Padilla J, Simmons GH, Bender SB, Arce-Esquivel AA, Whyte JJ, Laughlin MH. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology (Bethesda) 26: 132–145, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Proctor DN. Sex-specific influence of aging on exercising leg blood flow. J Appl Physiol 104: 655–664, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 16, Suppl 1: 3–63, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol 95: 1963–1970, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Proctor DN, Koch DW, Newcomer SC, Le KU, Smithmyer SL, Leuenberger UA. Leg blood flow and V̇o2 during peak cycle exercise in younger and older women. Med Sci Sports Exerc 36: 623–631, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Proctor DN, Le KU, Ridout SJ. Age and regional specificity of peak limb vascular conductance in men. J Appl Physiol 98: 193–202, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation 13: 315–327, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol 85: 68–75, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Ridout SJ, Parker BA, Proctor DN. Age and regional specificity of peak limb vascular conductance in women. J Appl Physiol 99: 2067–2074, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Saltin B, Mortensen SP. Inefficient functional sympatholysis is an overlooked cause of malperfusion in contracting skeletal muscle. J Physiol 590: 6269–6275, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunders NR, Tschakovsky ME. Evidence for a rapid vasodilatory contribution to immediate hyperemia in rest-to-mild and mild-to-moderate forearm exercise transitions in humans. J Appl Physiol 97: 1143–1151, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 579: 227–236, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol 587: 5541–5549, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shoemaker JK, Tschakovsky ME, Hughson RL. Vasodilation contributes to the rapid hyperemia with rhythmic contractions in humans. Can J Physiol Pharmacol 76: 418–427, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Spence AL, Carter HH, Naylor LH, Green DJ. A prospective randomized longitudinal study involving 6 months of endurance or resistance exercise: conduit artery adaptation in humans. J Physiol 591: 1265–1275, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation 101: 2896–2901, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 55: 312–318, 2010. [DOI] [PubMed] [Google Scholar]
- 48.Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol 96: 639–644, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Tschakovsky ME, Shoemaker JK, Hughson RL. Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol Heart Circ Physiol 271: H1697–H1701, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Wahren J, Saltin B, Jorfeldt L, Pernow B. Influence of age on the local circulatory adaptation to leg exercise. Scand J Clin Lab Invest 33: 79–86, 1974. [DOI] [PubMed] [Google Scholar]
- 51.Walther G, Nottin S, Karpoff L, Perez-Martin A, Dauzat M, Obert P. Flow-mediated dilation and exercise-induced hyperaemia in highly trained athletes: comparison of the upper and lower limb vasculature. Acta Physiol (Oxf) 193: 139–150, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. Am J Physiol Heart Circ Physiol 290: H1271–H1277, 2006. [DOI] [PubMed] [Google Scholar]