Abstract
Accurate genetic information transfer is essential for life. As a key enzyme involved in the first step of gene expression, RNA polymerase II (Pol II) must maintain high transcriptional fidelity while it reads along DNA template and synthesizes RNA transcript in a stepwise manner during transcription elongation. DNA lesions or modifications may lead to significant changes in transcriptional fidelity or transcription elongation dynamics. In this review, we will summarize recent progress towards understanding the molecular basis of RNA Pol II transcriptional fidelity control and impacts of DNA lesions and modifications on Pol II transcription elongation.
Keywords: Transcription fidelity, RNA polymerase II, DNA damage, Epigenetic DNA modifications, Structural biology, Chemical biology, Computational biology
1. Introduction
Transcription is the first key step for gene expression. RNA Polymerase II (Pol II) is the central enzyme responsible for pre-mRNA and noncoding RNA synthesis in eukaryotic cells (Kornberg, 2007). Transcriptional mutagenesis induced by damaged DNA templates may generate erroneous nascent mRNAs and as a result, lead to malfunction noncoding RNA and miscoded/misfolded proteins (Saxowsky and Doetsch, 2006). These transcription errors can have transient or heritable phenotypic changes for the cell, which may also be involved in tumour development (Bregeon and Doetsch, 2011; Gordon et al., 2009; Gordon et al., 2015; Morreall, Petrova, and Doetsch, 2013; Saxowsky and Doetsch, 2006; Viswanathan, You, and Doetsch, 1999). Another threat to the fidelity of RNA synthesis is from the nucleotide pool, in which the damaged ribonucleotides can be directly incorporated, inducing altered secondary or 3D structures of non-coding RNA transcripts or specificities of codon–anticodon recognition for incorporation of amino acids during translation (Bregeon and Doetsch, 2011). Therefore, maintaining an accurate genetic information transfer (high transcriptional fidelity) is essential for the process of life (Libby and Gallant, 1991).
RNA Pol II can be viewed as a highly specific “DNA reader” as well as an “RNA writer”. During transcription, Pol II moves along and recognizes the DNA template strand, and selects the matched nucleotide triphosphate (NTP) for synthesizing complementary RNA transcripts with a very low error rate (less than 0.001%) (Imashimizu et al., 2014; Kaplan, 2013; Liu, Bushnell, and Kornberg, 2013; Martinez-Rucobo and Cramer, 2013; Svejstrup, 2013; Svetlov and Nudler, 2013; Xu et al., 2014; Zhang and Wang, 2013). Pol II active site is highly evolved to select for the cognate substrate that matches the DNA template through a major conformational change of trigger loop to seal the active site and form an extensive interaction network with cognate substrate poised for nucleotide addition and to exclude non-cognate substrates (Kaplan, Larsson, and Kornberg, 2008; Kellinger et al., 2012; Kireeva et al., 2008; Wang et al., 2006; Yuzenkova et al., 2010). A combination of genetic, biochemical, and structural studies have provided extensive details on the molecular mechanisms of RNA polymerase transcription and its fidelity control in the last decade (Bregeon et al., 2003; Erie, Yager, and Vonhippel, 1992; Gnatt et al., 2001; Huang et al., 2010; Jeon and Agarwal, 1996; Kaplan, Larsson, and Kornberg, 2008; Kashkina et al., 2006; Kettenberger, Armache, and Cramer, 2003; Kettenberger, Armache, and Cramer, 2004; Kireeva et al., 2008; Koyama et al., 2007; Nesser, Peterson, and Hawley, 2006; Reines, Conaway, and Conaway, 1999; Thomas, Platas, and Hawley, 1998; Trinh et al., 2006; Walmacq et al., 2012; Wang et al., 2009; Wang et al., 2006; Westover, Bushnell, and Kornberg, 2004; Westover, Bushnell, and Kornberg, 2004; Yuzenkova et al., 2010). At least three fidelity checkpoints are utilized to ensure high transcriptional accuracy (Kellinger et al., 2012; Xu et al., 2014; Zhang and Wang, 2013). These fidelity checkpoints include insertion step (specific nucleotide selection and incorporation), extension step (from a matched vs a mismatched 3′-RNA terminus), and proofreading step (removal of misincorporated nucleotides from the 3′-RNA terminus) (Figure 1) (Kellinger et al., 2012; Xu et al., 2014; Yuzenkova et al., 2010; Zhang and Wang, 2013).
Figure 1.
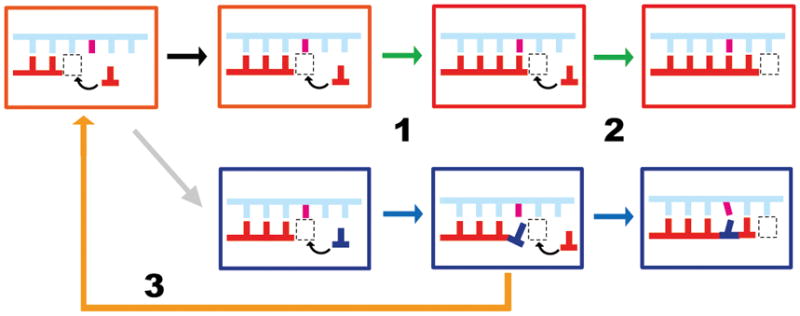
Scheme of three fidelity checkpoint steps during Pol II transcription elongation. During the insertion step (step 1), the matched nucleotide (red) is selected over incorrect substrate (blue). During the extension step (step 2), the RNA transcript can be preferentially extended from a matched pair. During the proofreading step (step 3), cleavage of the RNA transcript containing a mismatched end is significantly more efficient than that for the fully matched end. The template DNA is shown in cyan and non-template DNA is omitted. The RNA primer is shown in red. The active site of Pol II is shown in the boxed area.
In addition to its critical roles in gene expression, RNA Pol II has also been proposed to be a highly selective sensor to detect abnormities in DNA, such as DNA lesions, non-canonical DNA structures, and specific sequence motifs, and plays important roles in DNA damage tolerance and DNA repair (Belotserkovskii, Mirkin, and Hanawalt, 2013; Hanawalt and Spivak, 2008; Lindsey-Boltz and Sancar, 2007; Ljungman and Lane, 2004; Saxowsky and Doetsch, 2006; Tornaletti and Hanawalt, 1999). In fact, all cells and organisms constantly face dreadful threats of genomic DNA lesions caused by endogenous factors and environmental agents (Hoeijmakers, 2009; Jackson and Bartek, 2009). Every day, there are more than one million DNA lesions generated in a single cell (Lindahl and Barnes, 2000), and some of them cause significant DNA structural and chemical alterations. These alterations within highly transcribed genomic regions would significantly interfere with Pol II transcription process (Hanawalt and Spivak, 2008; Laine and Egly, 2006; Saxowsky and Doetsch, 2006; Svejstrup, 2007). Biochemical and genetic studies have shown that DNA damage processing by Pol II is lesion-specific (Hanawalt and Spivak, 2008; Lagerwerf et al., 2011; Lindsey-Boltz and Sancar, 2007; Saxowsky and Doetsch, 2006; Svejstrup, 2007; Tornaletti and Hanawalt, 1999). Pol II can bypass, backtrack, or stall at DNA lesions, leading to distinct downstream consequences. Pol II transcriptional lesion bypass is sometimes associated with misincorporation within the RNA transcript, termed transcriptional mutagenesis (Doetsch, 2002; Saxowsky and Doetsch, 2006). On the other hand, Pol II stalling at bulky DNA lesions initiates a specialized DNA repair pathway, termed transcription-coupled repair (TCR) (Hanawalt and Spivak, 2008; Lagerwerf et al., 2011; Saxowsky and Doetsch, 2006; Tornaletti and Hanawalt, 1999), which preferentially removes DNA lesions in the transcribed strand (Bohr et al., 1985; Fousteri and Mullenders, 2008; Hanawalt and Spivak, 2008; Lagerwerf et al., 2011; Mellon et al., 1986; Mellon, Spivak, and Hanawalt, 1987; Sarker et al., 2005). Arrested Pol II by DNA lesion is proposed as the initial signal for the recruitment of repair proteins involved in TCR though the detailed recruiting mechanism remains to be elucidated (Malik et al., 2010; Sarker et al., 2005; Troelstra et al., 1993; Troelstra et al., 1992; van den Boom et al., 2004; van Gool et al., 1997). Defects in genes involved in TCR cause premature aging and human diseases, such as Cockayne Syndrome, Xeroderma Pigmentosum, Trichothiodystrophy, UV-sensitive syndrome, and Cerebro-Oculo-Facio-Skeletal Syndrome (Hanawalt, 2008; Hanawalt and Spivak, 2008; Jaakkola et al., 2010; Laugel et al., 2010). Finally, persistently arrested Pol II undergoes ubiquitylation and subsequent degradation (Lindsey-Boltz and Sancar, 2007; Svejstrup, 2007; Wilson, Harreman, and Svejstrup, 2013).
In this review, we will focus on discussing recent progresses that provide novel insights into the molecular basis and chemical perspectives of Pol II transcriptional fidelity control during transcription elongation through structural, computational and chemical biology approaches. In addition, we will discuss the current views of the functional interplay between Pol II transcriptional machinery and DNA modifications and lesions.
2. A combined approach to understand the molecular basis of Pol II transcriptional fidelity
A combination of genetic, biochemical, and structural studies have shed new light on the Pol II transcription mechanism at an atomic level over the last decade (Bregeon et al., 2003; Erie, Yager, and Vonhippel, 1992; Gnatt et al., 2001; Huang et al., 2010; Jeon and Agarwal, 1996; Kaplan, Larsson, and Kornberg, 2008; Kashkina et al., 2006; Kettenberger, Armache, and Cramer, 2003; Kettenberger, Armache, and Cramer, 2004; Kireeva et al., 2008; Koyama et al., 2007; Nesser, Peterson, and Hawley, 2006; Reines, Conaway, and Conaway, 1999; Thomas, Platas, and Hawley, 1998; Trinh et al., 2006; Walmacq et al., 2012; Wang et al., 2009; Westover, Bushnell, and Kornberg, 2004; Westover, Bushnell, and Kornberg, 2004; Yuzenkova et al., 2010; Yuzenkova and Zenkin, 2010; Zhang et al., 2006). Several excellent reviews focusing on Pol II enzymatic catalysis and transcriptional regulation were published elsewhere (Kaplan, 2013; Liu, Bushnell, and Kornberg, 2013; Martinez-Rucobo and Cramer, 2013; Svejstrup, 2013; Svetlov and Nudler, 2013; Zhang and Wang, 2013). In this section, we will focus on recent progress towards understanding of mechanisms of three key fidelity checkpoint steps in controlling Pol II transcriptional fidelity at the elongation phase.
2.1 Structural biology studies
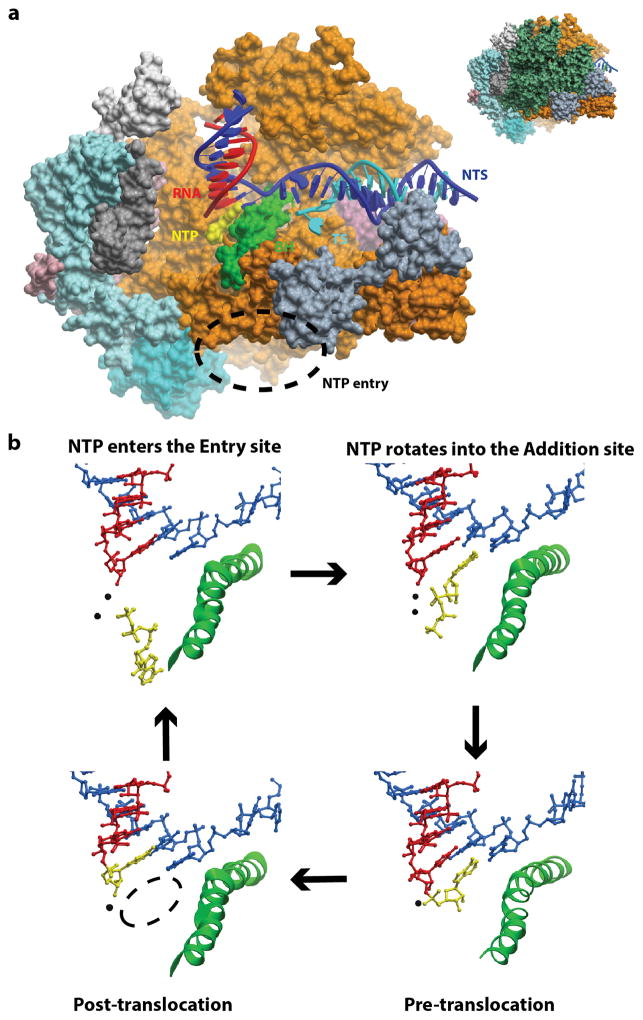
The first crystal structure of Pol II transcribing elongation complex was captured in a ‘pre-translocation’ state in which the Pol II active site is still occupied by the newly incorporated nucleotide (Gnatt et al., 2001). This crystal structure revealed several important structural features: RNA and DNA are held by the Pol II clamp in a closed conformation; the template DNA bends and crosses over the bridge helix to be delivered into the Pol II active site; the RNA forms a short upstream RNA-DNA hybrid as an intermediate between A and B form with the template DNA strand extending from the Pol II active site. The “pre-translocation” state is the state immediately after nucleotide addition. In order to make the active site available for the next round of nucleotide addition, Pol II must translocate one base pair downstream along the DNA template into a new state, termed the “post-translocation” state (Figure 2a). The Pol II complex in a post-translocation state was solved using a synthetic scaffold (Westover, Bushnell, and Kornberg, 2004; Westover, Bushnell, and Kornberg, 2004). Intriguingly, Pol II protein in the post-translocation state has an almost identical conformation with that in the pre-translocation state.
Figure 2.
Structures of Pol II elongation complexes (EC) reveals key steps of the nucleotide addition cycle. (a) Overall architecture of RNA Pol II EC (PDB ID: 2E2H). Rbp2 is omitted here to reveal the position of RNA/DNA scaffold and active site. The incoming NTP enters Pol II active site through the secondary channel of Pol II at bottom (dashed circle). Bridge helix (BH) is shown in green. RNA, template DNA (TS), and non-template DNA (NTS) are shown in red, blue, and cyan, respectively (b) Nucleotide addition cycle during Pol II transcription elongation. The NTP can first bind to the “entry site”, followed by rotating into the “addition site” to form the matched base pair with the DNA template. After the catalysis, Pol II translocate one base-step forward from a pre-translocation state to form a new post-translocation state, creating a new active site for the next round of nucleotide addition cycle.
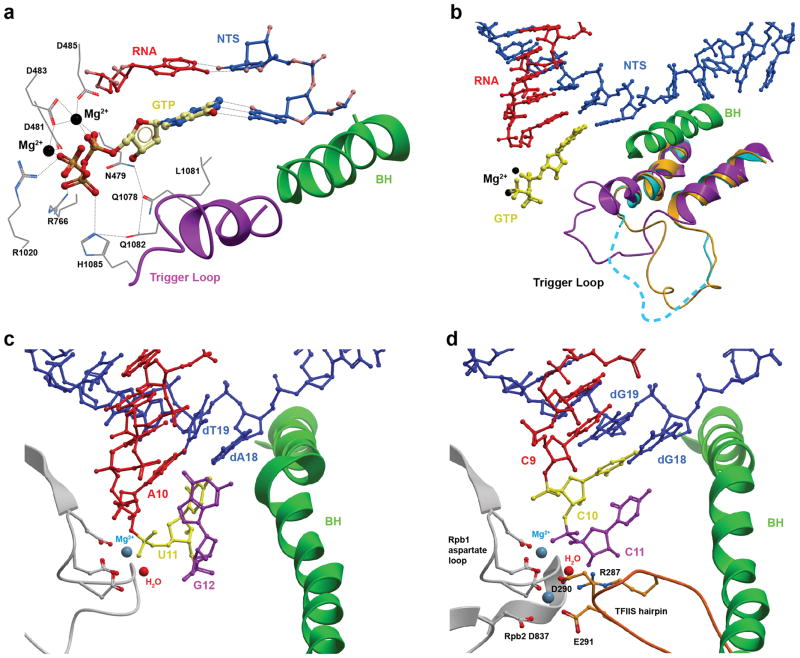
The availability of Pol II elongation complex structure in a post-translocation state also promotes us to address the essential question related to transcriptional fidelity control: how is the nucleotide substrate selected and bound to the Pol II active site? A series of crystal structures of Pol II elongation complexes with matched or mismatched substrates bound in the active site were determined (Kettenberger, Armache, and Cramer, 2004; Westover, Bushnell, and Kornberg, 2004; Westover, Bushnell, and Kornberg, 2004; Zhang et al., 2006). These structural studies provide the molecular basis of nucleotide selection and addition by Pol II, the first checkpoint step of transcriptional fidelity (Figure 2b). Intriguingly, only the matched NTP binds to the canonical “addition site” (or A site), in which the nucleobase of substrate forms a Watson-Crick base pair with the DNA template base (Figure 3a), whereas the mismatched NTP binds to an inverted conformation away from the DNA template, referred to as the “entry site” or E site (Hanawalt and Spivak, 2008; Kettenberger, Armache, and Cramer, 2003; Wang et al., 2006; Westover, Bushnell, and Kornberg, 2004). Importantly, upon the binding of the matched NTP, a conserved motif of Pol II, termed the trigger loop, switches (and folds) from an inactive open state to an active closed state (Figure 3a and 3b)–(Wang et al., 2006). Like many other nucleic acid enzymes, the closure of the active site is a common strategy to facilitate its enzymatic efficiency and selectivity (Beard and Wilson, 2006; Cramer, 2002; Doublie, Sawaya, and Ellenberger, 1999). Indeed, mutations in the trigger loop and nearby active site, or blockage of trigger loop movement by alpha-amanitin significantly affect Pol II transcription fidelity and catalytic efficiency (Braberg et al., 2013; Kaplan, Larsson, and Kornberg, 2008; Kireeva et al., 2008; Larson et al., 2012; Nedialkov et al., 2013; Strathern et al., 2012; Tan et al., 2008; Toulokhonov et al., 2007; Walmacq et al., 2012; Walmacq et al., 2009; Wang et al., 2006). Pol II also shares a universal two-metal ion catalytic mechanism with other nucleic acid enzymes (Figure 3a and 3b) (De Vivo, Dal Peraro, and Klein, 2008; Feng, Dong, and Cao, 2006; Schmidt et al., 2010; Sosunov et al., 2003; Steitz and Steitz, 1993). All three moieties of the substrate (nucleobase, sugar, and triphosphate) are recognized by Pol II through an extensive substrate recognition network (Wang et al., 2006). The nucleobase of the substrate is base paired with the DNA template and sandwiched by the 3′-RNA primer and Pol II trigger loop. These interactions ensure the correct nucleobase positioning in both lateral and vertical directions (Huang et al., 2010; Wang et al., 2006). In sharp contrast, the mismatched nucleotide is bound to the “E site”, in which the trigger loop is in the inactive open conformation. Therefore, the rate of mismatched nucleotide incorporation (trigger loop independent nucleotide addition) is several orders of magnitude slower than the rate of correct substrate incorporation (trigger loop dependent nucleotide addition) (Kellinger et al., 2012; Yuzenkova et al., 2010). This key mechanistic difference contributes to the high transcriptional fidelity of insertion step.
Figure 3.
NTP recognition by Pol II EC. (a) Interaction networks between the binding NTP and Pol II EC in the active site (PDB ID 2E22H). Part of bridge helix (BH, in green), part of trigger loop (TL, in violet), RNA/DNA hybrid chain (in red/blue), NTP (in yellow) and the Pol II residues interacting with the NTP are shown. (b) The closing motion of the TL can stabilize the matched NTP in the active site and further facilitates the catalytic reaction. The various TL forms captured by X-ray studies (PDB ID: 2E2H, 1R9T, and 2YU9) are shown in magenta, cyan, and orange, respectively. (c) Misincorporations can lead to a backtracked state of the Pol EC (PDB ID: 3GTG). The misincorporated RNA is shown in magenta. (d) The crystal structure of Pol II active site during TFIIS-mediated cleavage of RNA transcript (PDB ID: 3PO3). The Mg2+ ions and water are modelled in (c) and (d).
Structural studies also provide insights into the molecular basis of the second fidelity checkpoint step. In this step, RNA transcript extension from a mismatched 3′-RNA terminus is extremely slower than the extension from a fully matched end. Indeed, the fully matched upstream RNA:DNA hybrid and nearby active site residues are essential for the proper alignment of the 3′-RNA terminus with active site metal ions and incoming NTP. In the Pol II structures containing a mismatched RNA terminus, the mismatched 3′-RNA terminus either adopts frayed states or causes Pol II to move in a reverse direction into backtracked states, extruded toward the “secondary channel” of Pol II, which is the same channel for NTP diffusion (Figure 3c) (Cheung and Cramer, 2011; Sydow et al., 2009; Wang et al., 2009). Consequently, the mismatched 3′-RNA terminus is in the inactive conformation and partially overlaps with the canonical substrate binding site. The next round of nucleotide binding and addition is greatly compromised.
Pol II proofreading activity is important for the removal of the mismatched nucleotide in the 3′-RNA terminus. This proofreading mechanism enables Pol II to cleave the dinucleotide containing a mismatched 3′-RNA terminus through backtracking-dependent proofreading mechanism to regenerate a new post-translocation state, allowing Pol II to have a “second chance” to reselect the correct nucleotide for incorporation and elongation. In fact, slow forward extension from the mismatched 3′-RNA terminus also provides a time window that allows for Pol II backtracking and proofreading (Cheung and Cramer, 2011; Jeon and Agarwal, 1996; Kalogeraki et al., 2005; Koyama et al., 2007; Thomas, Platas, and Hawley, 1998; Wang et al., 2009). Structural studies of backtracked Pol II provide a detailed understanding of these backtracked states and proofreading mechanisms. The single stranded 3′-RNA terminus containing the misincorporated nucleotide extrudes toward the secondary channel (Figure 3c), or the same channel for NTP diffusion and binding. Dinucleotides or short oligomers containing the misincorporated nucleotide are preferentially removed (Cheung and Cramer, 2011; Kettenberger, Armache, and Cramer, 2003; Walmacq et al., 2012; Wang et al., 2009). Two independent proofreading mechanisms are found: intrinsic cleavage and TFIIS-mediated cleavage (Figure 3c and 3d) (Awrey et al., 1997; Cheung and Cramer, 2011; Erie et al., 1993; Izban and Luse, 1993; Jeon and Agarwal, 1996; Johnson and Chamberlin, 1994; Kalogeraki et al., 2005; Kettenberger, Armache, and Cramer, 2004; Koyama et al., 2003; Koyama et al., 2007; Reines, Chamberlin, and Kane, 1989; Rudd, Izban, and Luse, 1994; Sigurdsson, Dirac-Svejstrup, and Svejstrup, 2010; Thomas, Platas, and Hawley, 1998; von Hippel, 1998; Wang et al., 2009; Weilbaecher et al., 2003; Yuzenkova et al., 2010; Zenkin, Yuzenkova, and Severinov, 2006). In the intrinsic cleavage mode (Figure 3c), Pol II removes one or two nucleotides from the 3′-RNA terminus without the recruitment of external transcription factors. A metal bound water molecule from free solution (Figure 3c) is expected to attack the phosphodiester backbone of RNA transcript for intrinsic cleavage. On the other hand, for TFIIS-stimulated cleavage mode, transcription factor TFIIS is recruited to the backtracked complex to stimulate the transcript cleavage in the case of extensive backtracking (Cheung and Cramer, 2011; Kettenberger, Armache, and Cramer, 2003; Kettenberger, Armache, and Cramer, 2004; Powell, Bartholomew, and Reines, 1996; Wang et al., 2009) (Figure 3d). The domain III of TFIIS is inserted into the Pol II active site and provides residues for chelating magnesium ions that is important for cleavage (Figure 3d). The inserted domain III of TFIIS also pushes Pol II trigger loop into an open (inactive) conformation. Therefore, to re-enter the productive Pol II elongation mode, the inserted domain III of TFIIS needs to be displaced from the secondary channel, allowing the Pol II trigger loop to access its closed conformation during nucleotide addition cycles (Cheung and Cramer, 2011; Kettenberger, Armache, and Cramer, 2003; Kettenberger, Armache, and Cramer, 2004; Powell, Bartholomew, and Reines, 1996; Wang et al., 2009; Wang et al., 2006). Multiple rounds of backtracking, cleavage, and reactivation could happen when Pol II encounters DNA lesions or translocation barriers (Awrey et al., 1998; Awrey et al., 1997; Erie et al., 1993; Izban and Luse, 1993; Jeon and Agarwal, 1996; Johnson and Chamberlin, 1994; Kalogeraki et al., 2005; Kettenberger, Armache, and Cramer, 2004; Koyama et al., 2003; Koyama et al., 2007; Reines, Chamberlin, and Kane, 1989; Rudd, Izban, and Luse, 1994; Sigurdsson, Dirac-Svejstrup, and Svejstrup, 2010; Thomas, Platas, and Hawley, 1998; von Hippel, 1998; Wang et al., 2009; Weilbaecher et al., 2003; Yuzenkova et al., 2010; Zenkin, Yuzenkova, and Severinov, 2006). This provides a mechanism for increasing overall transcriptional bypass by either suppressing DNA-lesion induced transcriptional mutagenesis or providing enough time for the recruitment of DNA repair machinery.
2.2 Revealing Pol II transcriptional dynamics through computational biology approaches
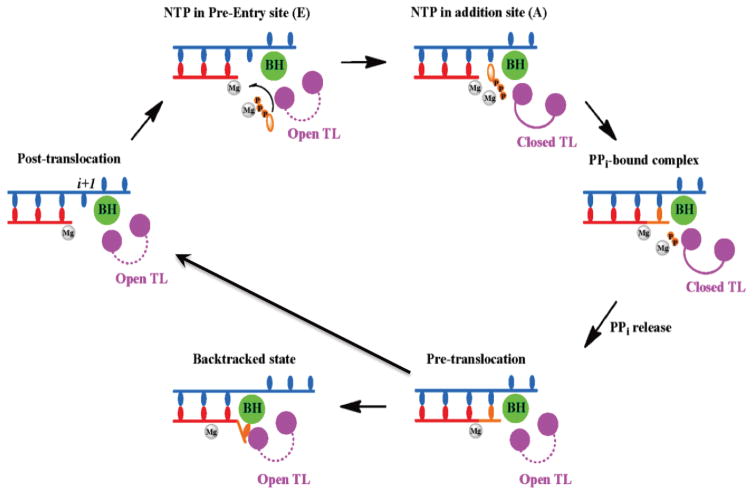
While the crystal structures of Pol II elongation complexes have provided atomic structural snapshots of Pol II transcription machinery at different states (Cheung, Sainsbury, and Cramer, 2011; Cramer, Bushnell, and Kornberg, 2001; Gnatt et al., 2001; Kornberg, 2007; Wang et al., 2009; Wang et al., 2006), little is known about the transcriptional dynamics or the detailed conformational transition from one state to another (Brueckner et al., 2009; Svetlov and Nudler, 2009). Molecular dynamics (MD) simulations provide us a powerful complementary approach to gain novel understandings of the dynamic transcription process (Batada et al., 2004; Da, Wang, and Huang, 2012; Feig and Burton, 2010; Feig and Burton, 2010; Huang et al., 2010; Kireeva et al., 2012). Indeed, the great advances in computational power, algorithms, and force field in the last decade allow us to apply molecular dynamics simulation to study the Pol II elongation complex in a biologically relevant time scale. Here we review some of the recent computational studies, mainly focusing on NTP loading and recognition, as well as pyrophosphate ion (PPi) release and translocation.
Crystal structures revealed multiple nucleotide binding sites (entry site, addition site, and pre-insertion site as intermediates). However, it is not clear how and through which routes nucleotides diffuse from free solution to the Pol II active site or how pyrophosphate releases from active site to solution. Batada et al addressed the NTP diffusion question using a computational biology approach (Batada et al., 2004) and revealed a functional role for the “entry site” in facilitating NTP diffusion into the “addition site” (Figure 4). Once the NTP reaches the addition site, it is further recognized and stabilized by the closed trigger loop via several specific interactions between the NTP and trigger loop residues (Wang et al., 2006). The functional roles of trigger loop residues (such as Leu1081 and His1085 (Rpb1)) in positioning and stabilizing the NTP were systematically evaluated by all-atom MD simulations (Huang et al., 2010). The MD simulations revealed that the protonated His1085 is the most favorable form when interacting with the β-phosphate atom of NTP. Both H1085F and H1085Y mutants can compromise the interaction between NTP and the trigger loop. These computational results are consistent with the experimental observations that H1085Y can induce cell growth defects and H1085F (or H1085A) is lethal to the cell (Braberg et al., 2013; Irvin et al., 2014; Larson et al., 2012; Toulokhonov et al., 2007). In addition, the authors suggested that hydrophobic interactions between Leu1081 of the side chain and the nucleotide base of the incoming NTP is important for stabilizing the nucleotide base in the correct position.
Figure 4.
A complete nucleotide addition cycle depicted from both structural and computational evidence during the transcription elongation process. This process mainly consists of the following steps: (1) The NTP (in orange) binds to the post-translocation state of Pol EC, accompanied by the TL closing motion. (2) Catalytic reaction takes place and one pyrophosphate ion (PPi) is formed. (3) PPi releases from the active site, followed by the TL opening motion. (4) The pre-translocation state of Pol II EC translocates to the post-translocation state to reinitiate another NAC. (5) When misincorporation occurs, the Pol II EC can move in reverse direction to form a backtracked state. The NTP- and modeled PPi-bound Pol II EC complexes are shown in the inset where TL residues Leu1081 and His1085 are highlighted in cyan.
The PPi release after nucleotide incorporation is the least understood step in nucleotide addition cycle. Several important questions remain to be addressed. How does the PPi release along the secondary channel? What is the relationship between the PPi release and the opening motion of the trigger loop (TL)? The kinetics of PPi release and its possible release paths were recently investigated using MD simulations (Da, Wang, and Huang, 2012). Intriguingly, the MD simulations results suggest that PPi adopts a hopping model through several conserved positively charged residues of Pol II to release from the active site to the secondary channel. Within each hopping site, (Mg-PPi)2− can form favorable interactions with Pol II and greatly increase its residence time. Single mutant simulations further suggest that H1085 and K752 (Rbp1) aid PPi exit from the active site after catalysis, whereas K619 facilitates its passage through the secondary channel. In addition, the authors reported that PPi release was tightly coupled with the trigger loop tip motion, but the dynamics of PPi release is much faster than the full opening of trigger loop and translocation (Figure 4). Therefore, these results argued that PPi release cannot be directly coupled with translocation (so-called power stroke model), which in general occurs in longer timescales than PPi release (Malinen et al., 2012). This study provides novel mechanistic insights into the dynamics of PPi release.
How RNA Pol II translocates along the DNA template is a long-standing question in the transcription field. Very limited mechanistic insights can be obtained from structural studies, as the structures of Pol II elongation complexes in pre- and post- translocation states are virtually identical except the RNA at the 3′-terminus. Even for computational biology approach, direct simulation of Pol II translocation at the biologically relevant timescale (milliseconds) for such a huge system (nearly half a million atoms) is not yet possible even with specialized simulation hardware. Recently, Huang, Wang, and colleagues used Markov State Models to overcome the “timescale gap” of conventional simulations (Silva et al., 2014). Intriguingly, their simulations revealed that RNA Pol II translocation is driven by thermal energy and does not require input of any additional chemical energy. Furthermore, the authors identified two novel metastable translocation intermediate states that are too transient to be captured by structural studies. The authors further suggested an important role for the bridge helix in facilitating translocation by lowering the free energy barriers. This dynamic view of translocation, though built on the mini-scaffold lacking a full transcriptional bubble and in the absence of NTP, represents a substantial advance over the current understanding based on static snapshots provided by X-ray structures. It would be of great interest to simulate Pol II transcription with a full transcriptional bubble and in the presence of NTP to fully recapitulate the Pol II translocation process in the near future.
2.3 Understanding chemical interactions and intrinsic structural features in controlling Pol II transcriptional fidelity by synthetic nucleic acid analogues
Previous structural studies revealed a substrate recognition network with multiple types of chemical interactions including base stacking, hydrogen bonding, hydrophobic interactions, and salt bridge interactions (Huang et al., 2010; Wang et al., 2006). However, it is unclear how each of these interactions contributes to the transcriptional fidelity of Pol II. Dissecting individual contributions of these chemical interactions will provide insight into the molecular mechanism by which Pol II reads the DNA template and how DNA modifications and lesions will affect transcriptional fidelity by altering the chemical functional groups and structural features of nucleic acids.
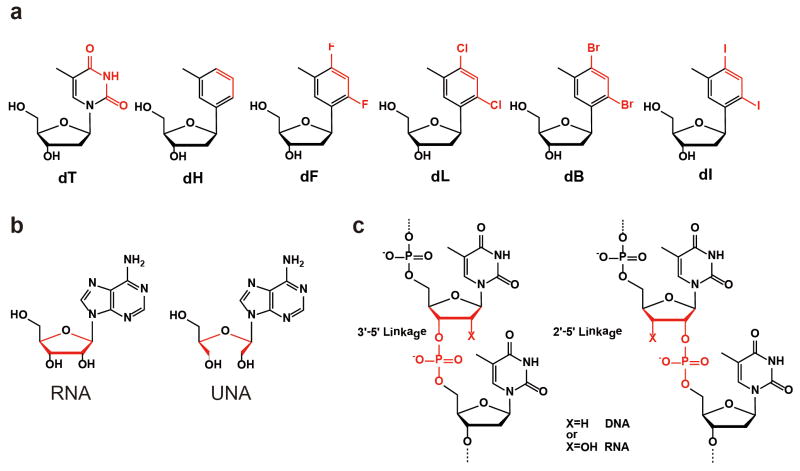
We recently employed a chemical biology approach to systematically examine the individual contributions of chemical interactions (such as hydrogen bonds and base stacking) and nucleic acid structural motifs in Pol II transcriptional fidelity control. In collaboration with the Kool group, a series of synthetic ‘hydrogen-bond deficient’ nucleoside analogues were used as chemical probes to dissect hydrogen-bonding interactions governing RNA Pol II transcriptional fidelity (Kellinger et al., 2012). Nonpolar thymidine analogues were synthesized to mimic the shape of thymine nucleoside with overall 1.0 Å increment in size from the smallest dH to the largest dI (Figure 5a) (Kim et al., 2005; Kim and Kool, 2005). This system allows us to measure the effects of the loss of hydrogen bonding at the individual “checkpoint” steps (Khakshoor et al., 2012). In addition, the size effect of the template nucleobase can be evaluated by comparing among the five analogues.
Figure 5.
Utilization of nucleotide analogues to dissect the individual contributions of chemical interactions and structural motifs to the transcriptional fidelity. (a) Chemical structures of several ‘hydrogen-bonding deficient’ thymidine (dT) analogues (dH, dF, dL, dB, dI) with sub-angstrom increments in size to probe the effect of hydrogen-bonding base pairing. (b) The unlocked nucleoside was designed to evaluate the roles of the sugar backbone in the transcriptional fidelity. (c) Phosphodiester linkage was mutated to elucidate the contribution of phosphodiester linkage orientation.
This study reveals distinct contributions of hydrogen bonds at each step of the fidelity checkpoints. In the insertion step, Pol II loses the discrimination power between ATP and UTP for the dF, dL, dB, and dI templates, indicating the significant role of hydrogen bonding in ensuring correct substrate incorporation in the first fidelity checkpoint step. This is in sharp contrast to some of the high fidelity DNA polymerases where dNTP incorporation is relatively less affected by hydrogen bond deficiency. Unexpectedly, we revealed a different role of hydrogen bonding during the extension step. The loss of hydrogen bonds between the matched 3′-terminus base pair does not drastically affect CTP incorporation specificity. More strikingly, we observed significant increases (~100–1000 fold) of extension from a mismatched pair upon the removal of the wobble hydrogen bonding. These results suggest the unexpected contribution of wobble pair hydrogen bonding in preventing mismatched extension. These two different aspects indicate that while the canonical hydrogen bonding assures the correct nucleotide insertion in the first fidelity checkpoint step, the wobble hydrogen bonding is important in preventing mismatch extension in the second fidelity checkpoint step. In the third checkpoint step of Pol II transcription, we found that loss of Watson-Crick hydrogen bonds leads to a significant increase in backtracking and cleavage activities. Interestingly, nonpolar analogues with larger sizes (dB and dI) can compensate for the loss of hydrogen bonds at the 3′-end of RNA, presumably by increased base stacking that could strengthen the RNA:DNA duplex stability. Taken together, this study systematically evaluated the effects of hydrogen bonding as well as contributions of nucleobase size and stacking at individual transcriptional fidelity checkpoint steps.
Wang, Wengel, and co-workers further investigated the contribution of the sugar backbone to transcriptional fidelity using sugar analogues (Xu et al., 2013). The sugar backbone is the central structural moiety connecting all of the peripheral nucleic acid functional groups: the nucleobase, 2′- and 3′-OH, and phosphate groups. However, the contribution of this core moiety in maintaining accurate genetic information transfer is unclear. To reveal the contribution of the sugar backbone to Pol II transcriptional fidelity, a “sugar backbone mutant” ribonucleotide analogue, termed unlocked nucleoside (UNA), was selected for comparative studies (Figure 5b) (Langkjaer, Pasternak, and Wengel, 2009). The UNA analogue contains all of the same peripheral functional groups of the “wild type” ribonucleotide except that it misses a bond that connects the C2′ and C3′ atoms of the ribose ring. Importantly, UNA residues maintain the capability to form Watson-Crick base pairing within DNA strands and form a similar duplex structure in comparison with a RNA/DNA hybrid (Pasternak and Wengel, 2010). We found that the sugar backbone is an underappreciated, dominant factor in controlling all three checkpoint steps of Pol II transcriptional fidelity (Xu et al., 2013). Nucleobase discrimination is completely abolished following the replacement with the unlocked sugar in every single fidelity checkpoint step. In fact, the sugar backbone integrity is a prerequisite for correct nucleotide selection in Pol II transcription. Pol II has an unexpectedly strong discrimination power for interrogating substrate ribose integrity, which is even ~100-fold stronger than its ability to discriminate against a mismatched base and 103–104 stronger than its ability to discriminate against dNTPs (Xu et al., 2013). Therefore, the correct peripheral functional groups of the nucleotide per se are insufficient for efficient Pol II transcription. Rather, the correct spatial arrangement of peripheral functional groups (restrained by the sugar backbone) is essential in maintaining high Pol II catalytic activity and fidelity. Indeed, the Pol II active site is not fully pre-assembled and the binding of the nucleotide substrate with the correct spatial arrangement of its functional groups is important for closure of the trigger loop and full assembly of Pol II active site poised for catalysis (Kaplan, Larsson, and Kornberg, 2008; Malinen et al., 2012; Yuzenkova et al., 2010; Zhang et al., 2006).
Phosphodiester linkage is also critical for maintaining RNA Pol II transcriptional fidelity. The non-enzymatic RNA polymerization introduces backbone heterogeneity with a mixture of 2′–5′ and 3′–5′ linkages (Figure 5c) (Bowler et al., 2013; Ekland and Bartel, 1996; Ertem and Ferris, 1996; Ferris and Ertem, 1992; Inoue and Orgel, 1982; Usher and McHale, 1976; Usher and McHale, 1976). Pol II is a key modern enzyme responsible for synthesizing 3′–5′-linked RNA with high fidelity. It is not clear how Pol II selectively recognizes the 3′–5′ linkage over 2′–5′ linkage. Recently, Wang and collaborators systematically investigated how phosphodiester linkages of nucleic acids govern Pol II transcriptional efficiency and fidelity (Xu et al., 2014). Intriguingly, it was revealed that Pol II recognizes phosphodiester linkages in an asymmetric (strand-specific) manner. These results reveal essential contributions of the template phosphodiester linkages to Pol II transcription and provide new understanding on nucleic acid recognition and genetic information transfer during molecular evolution. Intriguingly, Pol II stalls at a 2′–5′ linkage in a strikingly similar manner as a CPD lesion or Cyclo-dA lesion, suggesting a likely common recognition mode for these three modifications (Walmacq et al., 2012; Walmacq et al., 2015).
The nucleic acid analogues are useful to probe specific interactions between Pol II functional domains and nucleic acid moieties. Wang and collaborators recently utilized a series of synthetic nucleotide analogues and alpha-amanitin to reveal the chemical interactions and substrate structural signatures governing Pol II trigger loop closure (Xu et al., 2014). Investigations of hydrogen bonding deficient nucleotide analogues suggested that the formation of Watson-Crick hydrogen bonds is dispensable in promoting trigger loop closure, and the loss of hydrogen bonds can be fully rescued by increasing the size (base stacking) of a nucleobase. In addition, we also found the sugar pucker ring conformation plays a much more important role in facilitating nucleotide incorporation and trigger loop closure than that of hydrogen bonds via 2′-OH. Employing synthetic nucleotide analogues, this study highlighted the important contributions of π-π stacking (base stacking) and van der Waals interactions (sugar pucker) in governing the Pol II substrate recognition and full trigger loop closure.
Collectively, these studies using synthetic nucleic acid analogues highlight the importance of chemical interactions and the intrinsic structural features (backbones) of nucleic acids in controlling Pol II transcriptional fidelity that could not be achieved by conventional biological observations and analyses (Benner, 2004; Benner and Sismour, 2005; Kellinger et al., 2012; Khakshoor and Kool, 2011; Xu et al., 2014; Xu et al., 2013; Zhang and Wang, 2013). These studies also provide a framework for understanding the effect of disrupted chemical interactions and structural features by DNA lesions on Pol II transcriptional fidelity. This synthetic chemical biology approach may be extended to understand the mechanisms of other RNA polymerases as well as other nucleic acid enzymes in future studies.
3. DNA lesion-induced transcriptional mutagenesis
Pol II can bypass certain types of DNA lesions in either an error-free or error-prone manner (Doetsch, 2002; Saxowsky and Doetsch, 2006; Scicchitano, 2005; Scicchitano, Olesnicky, and Dimitri, 2004; Tornaletti, 2005; Wang et al., 2010; Waters et al., 2009; Xu et al., 2015). Error-prone transcription bypass may lead to miscoded mRNA or malfunctioning non-coding RNAs (Bregeon and Doetsch, 2011; Doetsch, 2002; Liu, Zhou, and Doetsch, 1995). Impacts of several common DNA lesions on Pol II transcription are thoroughly summarized and reviewed elsewhere (Hanawalt and Spivak, 2008; Saxowsky and Doetsch, 2006; Scicchitano, 2005; Scicchitano, Olesnicky, and Dimitri, 2004; Tornaletti, 2005). The effects of DNA lesions on transcriptional fidelity appear to be lesion-specific and position-specific. Using alkylation damage as an example, different alkylated nucleotides or different alkylation positions in the same nucleotide may cause distinct consequences during transcriptional lesion bypass (Cline et al., 2004; Dimitri et al., 2008; Dimitri et al., 2008; You et al., 2015; You et al., 2014).
In recent years, there has been substantial progress towards understanding how pol II recognizes and processes DNA lesions, which also provides structural mechanistic insights into the impact of DNA lesions on transcriptional fidelity (error-prone vs error-free modes). Here we summarize some recent progresses of structural studies in this area, focusing on the following categories of DNA lesions and modifications: oxidative DNA lesions, 1,2-intrastrand DNA lesions, monofunctional bulky DNA lesions, and epigenetic DNA modifications.
3.1 Oxidative DNA lesion-induced transcriptional mutagenesis
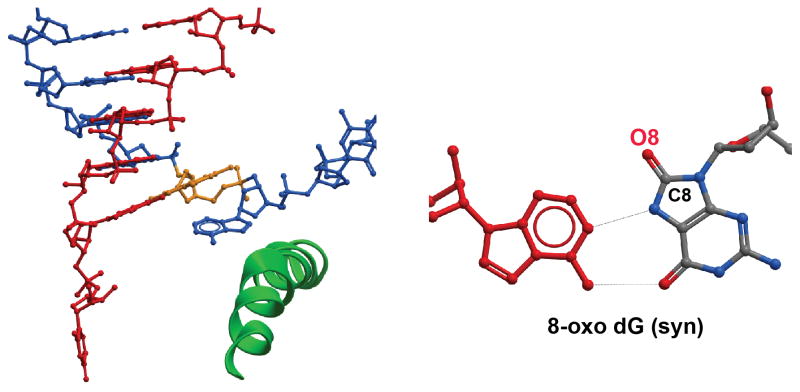
Oxidative DNA damage is one of the most abundant genomic DNA lesions induced by reactive oxygen species (ROS). Among these oxidative lesions, 8-oxo dG is well documented as a mutagenic DNA lesion (Saxowsky et al., 2008). Pol II can bypass this lesion and incorporate either a matched cytosine or a mismatched adenine opposite 8-oxo dG (Kuraoka et al., 2007; Tornaletti et al., 2004). Structural studies of Pol II with a template containing a site-specific 8-oxo dG confirmed that 8-oxo dG adopts a canonical anti conformation when paired with a cytosine as a Watson Crick base pair, and a syn conformation to form a Hoogsteen base pair with adenine (Figure 6a) (Damsma and Cramer, 2009). Therefore, the presence of an 8-oxo dG-lesion leads to great increases of efficiencies of ATP mis-insertion and extension step from an 8-oxo dG:rA base pair. Meanwhile, the presence of 8-carbonyl group reduces the stability of an 8-oxo dG at anti conformation and thus reduces the efficiencies of cognate CTP insertion and extension. Taken together, the combined effects cause an error-prone transcriptional bypass. Notably, Pol II essentially shares the same mechanism of adenine misincorporation at 8-oxo dG with some high fidelity DNA polymerases (Brieba et al., 2004; Eoff et al., 2007). These results suggest that, at least in the case of 8-oxo dG, the DNA lesion-induced changes in the structural features of nucleic acids play a very important role in controlling the potential functional outputs for RNA polymerases and high fidelity DNA polymerases.
Figure 6.
RNA Pol II recognition of 8-oxo dG lesion. 8-oxo dG adopts an a syn- form when pairing with adenine in the Pol II active site (PDB ID: 3I4N).
3.2. 1,2-intrastrand DNA lesions cause strong Pol II blockage and transcriptional mutagenesis
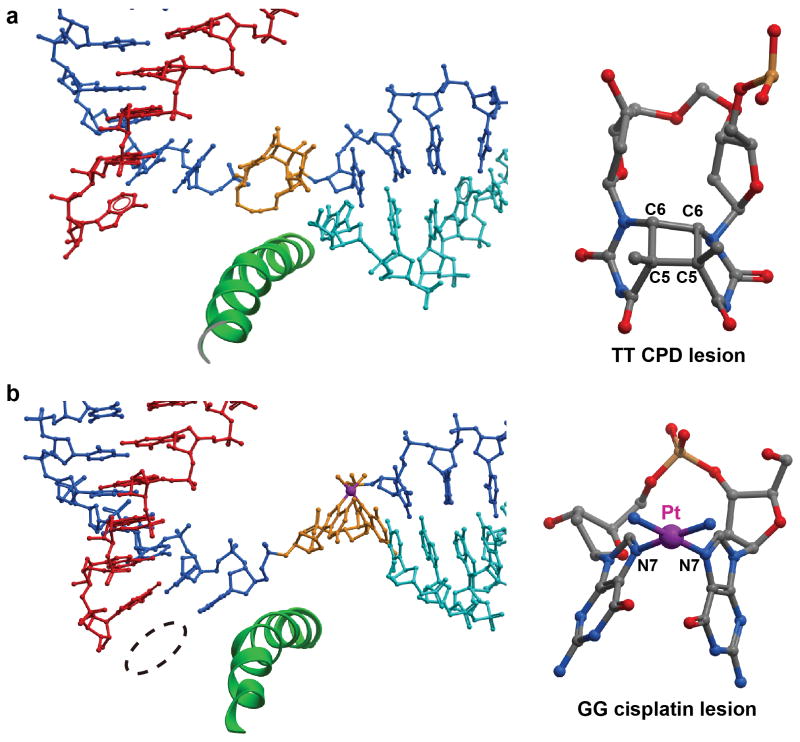
UV-induced Cyclobutane pyrimidine dimer (CPD) lesion and cisplatin-induced 1,2-dGpG intrastrand cross-links are two well-studied 1,2-intrastrand DNA lesions that greatly distort the DNA duplex structure (Figure 7) (Park et al., 2002; Todd and Lippard, 2009). These two DNA lesions strongly block Pol II transcription and initiate transcription-coupled repair (Corda et al., 1993; Cullinane et al., 1999; Donahue et al., 1994; Jung and Lippard, 2006; Kalogeraki et al., 2005; Kalogeraki, Tornaletti, and Hanawalt, 2003; Lee et al., 2002; Mei Kwei et al., 2004; Mello, Lippard, and Essigmann, 1995; Mellon, Spivak, and Hanawalt, 1987; Tornaletti et al., 1997; Tornaletti et al., 2003; Tornaletti, Reines, and Hanawalt, 1999; Wang and Lippard, 2005). Interestingly, structural studies of Pol II complexes stalled at the intrastrand cross-links caused by CPD lesions or cisplatin-induced DNA lesions suggested somewhat different mechanisms of Pol II recognition and processing of these two 1,2-intrastrand DNA lesions (Figure 7a and 7b) (Brueckner et al., 2007; Damsma et al., 2007; Walmacq et al., 2012; Wang et al., 2006). CPD lesions are less bulky than cisplatin-induced DNA lesions. Therefore, CPD lesions can be delivered into the Pol II active site. Indeed, a structure captures a CPD-stalled intermediate state in which the CPD lesion is accommodated right above the bridge helix and partially reaches into the +1 site (PDB ID 4A93). The presence of a CPD DNA lesion greatly distorts the DNA template and therefore, a CPD lesion is a poor template to support template-dependent AMP incorporation opposite to 3′-thymine. Further translocation of the CPD lesion from +1 to −1 position is strongly disfavored. UMP misincorporation opposite the 5′-thymine is also reported (Figure 7a) (Brueckner et al., 2007; Walmacq et al., 2012). In contrast, the structure of the Pol II complex stalled at a 1,2-dGpG cisplatin-induced DNA lesion revealed that the lesion is accommodated at the +2/+3 position, and no stable structure of Pol II complex with a cisplatin-induced DNA lesion at position +1/+2 is captured. Indeed, the designed scaffold with a cisplatin-induced DNA lesion at position +1/+2 strongly favors backtracking and moves the cisplatin-induced DNA lesion at +2/+3 position. These results indicate the cisplatin-induced DNA lesion might be too bulky to cross over the bridge helix and be delivered into the active site (Figure 7b). Slow non-template dependent AMP misincorporation is observed opposite the cisplatin-induced DNA lesion.
Figure 7.
Structural basis of the 1,2-crosslinked DNA lesion-containing Pol II ECs. (a) The crystal structure of Pol II EC stalled at CPD lesion (PDB ID: 4A93). (b) The crystal structure of Pol II EC stalled by the 1,2-dGpG cisplatin DNA lesion (PDB ID: 2R7Z).
3.3. Pol II blockage and bypass at monofunctional bulky DNA lesions
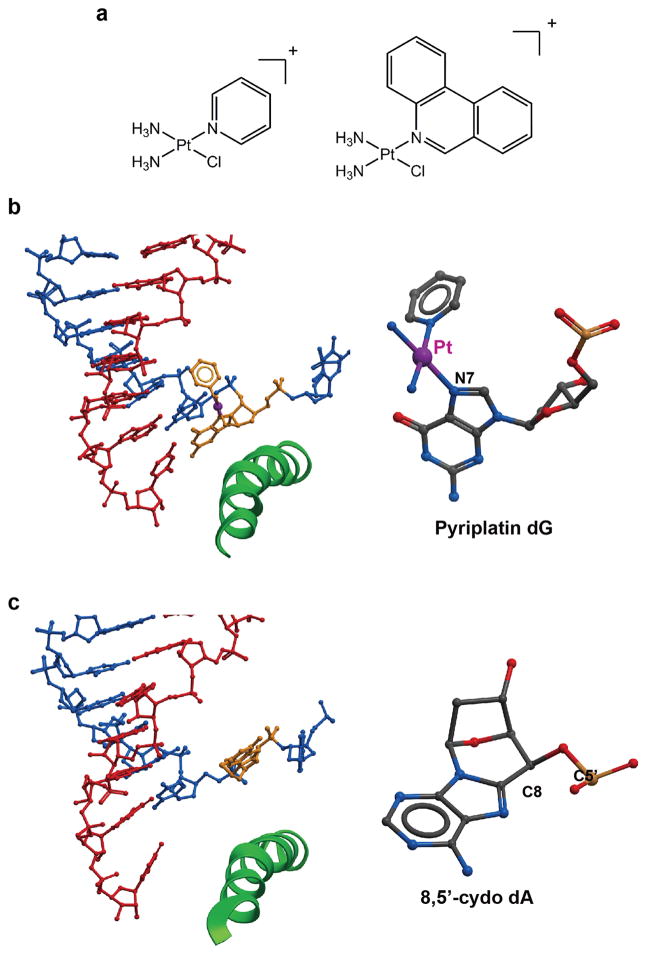
Unlike bifunctional cisplatin that forms 1,2-dGpG DNA lesions, pyriplatin (cis-diamminepyridinechloroplatinum(II)) and phenanthriplatin (cis-diamminephenanthridinechloroplatinum(II)) (Figure 8a) form monofunctional bulky platinum-induced DNA lesions at dG and dA sites on the DNA template without distorting the DNA duplex structure. These monofunctional platinum compounds may escape detection from DNA damage recognition proteins XPC/HR23B and subsequent global nucleotide excision repair. These compounds have also demonstrated unique anticancer activities against cisplatin-resistant cancer cell lines (Park et al., 2012). It is therefore particularly interesting to understand how these monofunctional platinum compounds accommodate within the Pol II active site and affect Pol II transcription.
Figure 8.
Recognition of the monofunctional bulky DNA lesion by Pol II EC. (a) Chemical structures of mono-functional platinum complexes, pyriplatin and phenanthriplatin. (b) The crystal structure of the Pol II EC stalled at a pyriplatin- DNA monofunctional adduct (PDB ID: 3M3Y). (c) RNA Pol II recognition of 8,5′-cydo dA (PDB ID: 4X6A). This bulky monofunctional oxidative damage fails to move over the bridge helix but stays in the “above-bridge-helix” conformation.
The structural studies of Pol II complex stalled at a pyriplatin-DNA monofunctional adduct revealed novel insights into how Pol II recognizes and processes this bulky monofunctional platinum-induced DNA lesion (Wang et al., 2010). Intriguingly, in sharp contrast to 1,2-dGpG cisplatin-induced DNA lesions, the pyriplatin-DNA adduct can cross over the bridge helix and accommodate into the Pol II active site to support efficient CMP incorporation. Indeed, the correct CMP nucleotide can form a canonical Watson-Crick base pair with pyriplatin-modified guanosine (Figure 8b). The bulky pyridine ligand extrudes toward the major grove without disrupting the overall structure of the RNA/DNA duplex. Intriguingly, the platinum DNA adduct interacts with conserved bridge helix residues to stabilize the pre-translocation state and the presence of the bulky ligand also introduces a strong steric barrier for allowing the next template nucleobase to cross over the bridge helix to reach +1 template position during translocation. Therefore, the mechanism of transcriptional inhibition by pyriplatin differs significantly from that of cisplatin or UV damage (Wang et al., 2010).
The impacts of the phenanthriplatin-dG DNA lesion on transcription bypass and transcriptional fidelity were also systematically investigated recently (Kellinger et al., 2013). Strikingly, the presence of a phenanthriplatin-dG DNA lesion at +1 position does not affect the insertion step. The specificity for CMP incorporation is almost the same as for undamaged dG on the template. Pol II maintains a very high discrimination power at the first checkpoint step even in the presence of the bulky phenanthriplatin-induced DNA lesion. In sharp contrast, the presence of the phenanthriplatin-induced DNA lesion causes Pol II to significantly slow down at the extension step and Pol II switches to an error-prone mode during lesion bypass. The presence of the phenanthriplatin-induced DNA lesion does not change proofreading activity in the third checkpoint step. The fact that Pol II can switch from an efficient error-free mode to a slow error-prone mode during lesion bypass somewhat resembles how DNA polymerases switch in translesion DNA synthesis (Friedberg, Lehmann, and Fuchs, 2005; Goodman, 2002). The difference is that translesion DNA synthesis requires enzyme switching between multiple DNA polymerases, whereas translesion RNA bypass requires only a single polymerase (Pol II) but at distinct modes (trigger loop dependent vs trigger loop independent mode).
Cyclopurine, such as 8,5′-cyclo-2′-deoxyadenosine (CydA), is a monofunctional bulky oxidative DNA lesion caused by ROS (Wang, 2008) that also strongly blocks RNA polymerase II and interferes with gene transcription in mammalian cells (Jaruga and Dizdaroglu, 2008). Pol II can also undergo slow bypass and generate both error-free and error-prone transcripts with AMP misincorporated immediately downstream from the lesion (Brooks et al., 2000; Marietta and Brooks, 2007; You et al., 2012). Recently, Kashlev and Wang lab presented biochemical and crystallographic evidences for the molecular mechanism of CydA recognition and lesion bypass by RNA Pol II (Figure 8c) (Walmacq et al., 2015). Interestingly, unlike pyriplatin or phenanthriplatin induced lesions, CydA-DNA lesions fail to support efficient UMP incorporation. In fact, the mode of CydA-DNA lesion bypass by Pol II is somewhat similar to that of CPD-lesions. The crystal structure of the Pol II complex containing a CydA lesion at the +1 register reveals a strikingly similar location of the DNA lesion in comparison with the CPD DNA lesion (Brueckner et al., 2007). Both lesions accommodate above the bridge helix. As a result, CydA lesion cannot support template-dependent UMP incorporation, rather preferential non-template dependent AMP incorporation is observed (A-rule). Similar to CPD lesions, Pol II also gets stalled due to the difficult loading of the template base (5′) next to CydA into the active site.
3.4. Functional interplay between epigenetic DNA modifications and Pol II transcription
In addition to DNA lesions caused by endogenous or environment chemical agents, the DNA template also undergoes enzyme-catalyzed epigenetic DNA modification such as dynamic DNA methylation and demethylation, which are important in stem cell renewal and cell differentiation. During TET-mediated active DNA demethylation, 5-methylcytosine (5mC) can be oxidized to 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC), and 5-carboxylcytosine (5caC) by TET enzyme in a step-wise manner (Branco, Ficz, and Reik, 2011; He et al., 2011; Ito et al., 2011; Munzel, Globisch, and Carell, 2011; Wu and Zhang, 2011). 5fC and 5caC can be further removed by thymine DNA glycosylase (TDG) in conjunction with base excision repair to regenerate unmodified cytosine. Emerging evidence suggests that these oxidized 5-methylcytosines (Oxi-mCs, such as 5hmC, 5fC and 5caC) may have critical impacts on transcription. Indeed, genome-wide mapping of these Oxi-mCs revealed specific enrichment at enhancers, promoters, and gene bodies (Shen et al., 2013; Song et al., 2013). A number of protein complexes involved in transcription, chromatin remodelling, splicing, and DNA repair have been identified to selectively bind synthetic DNA containing Oxi-mCs (Hashimoto et al., 2014; Iurlaro et al., 2013; Spruijt et al., 2013).
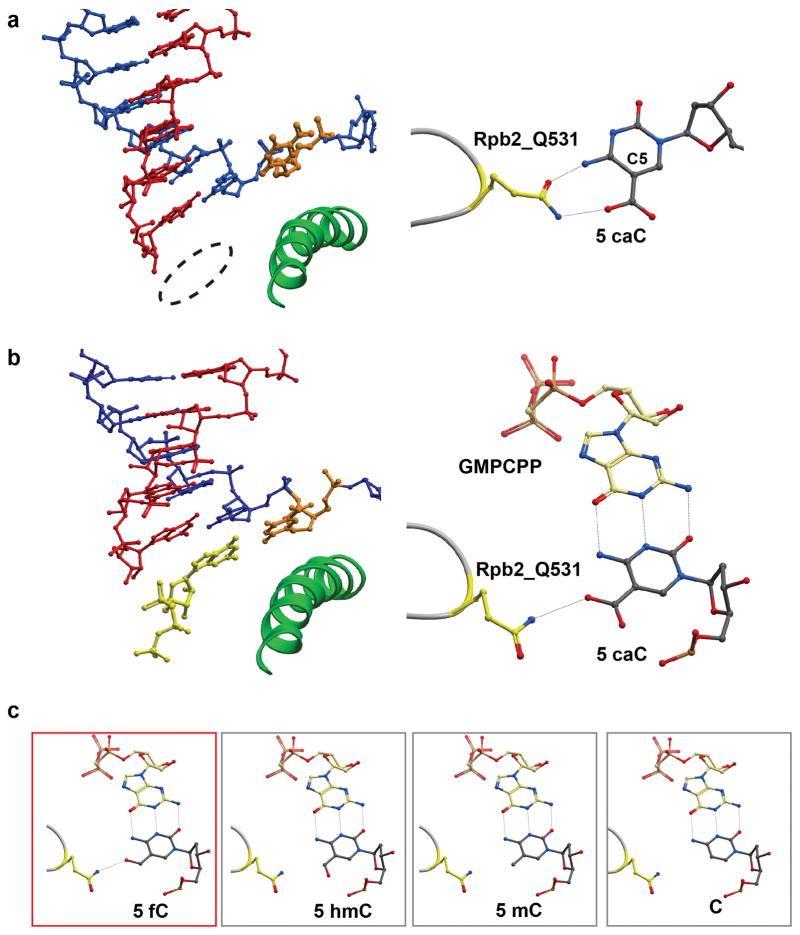
It is interesting to note that the modifications of these Oxi-mCs are located at the 5-positions of cytosine facing toward major grooves, which do not interfere with Watson-Crick base pairing (Raiber et al., 2015). However, given the distinct chemical nature of the functional groups in these oxidized methylcytosines and potential to interact differently with protein residues, whether and how these modifications affect RNA Pol II transcription deserve exploration. To this end, the transcriptional effects of these oxidized intermediates at biochemical, structural and cellular levels were investigated recently (Kellinger et al., 2012; Wang et al., 2015; You et al., 2014). The in vitro transcription results using purified mammalian and yeast Pol II reveals transient pausing of Pol II at 5fC or 5caC site in comparison with other forms of cytosine (Kellinger et al., 2012). Further follow-up studies elucidated the molecular basis of Pol II retardation at 5fC/5caC sites. RNA Pol II can recognize 5caC through a specific interaction between its conserved epi-DNA recognition loop in the polymerase and the carboxyl group of 5caC (Figure 9a and 9b) (Wang et al., 2015). This hydrogen bonding interaction causes a positional shift for incoming GTP, thus compromising nucleotide addition. The global effect of increased 5fC/5caC levels on transcription was also determined, revealing that such DNA modifications indeed retarded Pol II elongation on gene bodies in vivo, which is consistent with previous in vitro observation. Modeling studies indicated that this epi-loop can also recognize 5fC but not 5hmC, 5mC or C during nucleotide incorporation (Figure 9c). These results demonstrate the functional impact between cytosine modifications and transcription activities and suggest a novel role for Pol II to function as a specific and direct epigenetic sensor during transcription elongation.
Figure 9.
Recognition of DNA demethylation intermediates by Pol II EC. (a) The crystal structure of the Pol II EC with 5cac, in which the key residue Q531 of Rpb2 subunit interacts with 5cac (PDB ID: 4Y52). About half of 5caC adopts the “above-bridge-helix” conformation. (b) Q531 can form hydrogen bond with 5caC results in a positional shift of the template away from the canonical position (PDB ID: 4Y7N). (c) Models of similar interaction between Q531 and 5fC template, but not for 5hmC, 5mC and C templates.
Note: The color version of figures are available online.
4. Conclusions
Recent studies combining structural biology, computational biology and synthetic chemical biology approaches provide different perspectives in understanding the molecular basis of transcriptional fidelity and functional interplay between DNA modifications and Pol II transcription. Structural biology reveals the snapshots of static atomic structures and detailed interaction network during Pol II transcription. Computational biology provides detailed dynamic information and conformational transitions of the Pol II transcription process. Synthetic chemical biology provides us with novel chemical tools to further dissect individual contributions of chemical interactions and nucleic acid structural motifs in controlling Pol II transcriptional fidelity. Taking all the evidence together, we now obtain a much more comprehensive understanding of the molecular mechanisms of Pol II transcriptional fidelity maintenance, which provides a framework to understand how DNA lesions and modifications affect Pol II transcriptional fidelity.
Recent mechanistic studies of the functional interplay between DNA lesions and modifications and Pol II transcription machinery greatly enhance our understanding of how Pol II deals with a variety of DNA modifications as it moves along the DNA template during transcription. It has now become clearer that in addition to the ‘routine job’ for RNA Pol II to synthesize a RNA transcript complementary to DNA template, the ‘secret identity’ of RNA Pol II starts to appear. Indeed, it has been proposed that Pol II is a sensor for a variety of DNA lesions. Arrested Pol II at bulky DNA lesions triggers transcription-coupled repair, a specific DNA repair pathway, or ubiquitination (Bregeon et al., 2003; Burns et al., 2010; Cline et al., 2004; Dimitri et al., 2008; Dimitri et al., 2008; Dimitri et al., 2008; Donahue et al., 1996; Jung and Lippard, 2006; Kalogeraki, Tornaletti, and Hanawalt, 2003; Kuraoka et al., 2007; Lee et al., 2002; Mei Kwei et al., 2004; Monti et al., 2011; Neil, Belotserkovskii, and Hanawalt, 2012; Perlow and Broyde, 2003; Perlow et al., 2002; Schinecker et al., 2003; Tornaletti et al., 1997; Tornaletti, Maeda, and Hanawalt, 2006; Tornaletti et al., 2004; Tornaletti et al., 2003; Tornaletti, Reines, and Hanawalt, 1999; Wang et al., 2006). Recent research efforts also uncovered that Pol II can pause at natural unconventional DNA structures and epigenetic DNA modifications (Belotserkovskii et al., 2010; Belotserkovskii et al., 2013; Ditlevson et al., 2008; Krasilnikova et al., 2007; Salinas-Rios, Belotserkovskii, and Hanawalt, 2011; Tornaletti, 2009; Tornaletti, Park-Snyder, and Hanawalt, 2008). Taken together, Pol II may work as a scanner or integrator to scan the genome and sense a variety of DNA modifications and lesions, or unnatural DNA sequences or structures and triggers distinct downstream processes such as alternative splicing, chromatin remodelling, termination, and DNA repair. Further mechanistic studies using multidisciplinary approaches are needed to gain a comprehensive understanding of the functional interplay between DNA lesions/modifications and transcriptional machinery. This knowledge would also pave the way for developing novel therapies for transcription-related diseases in the future.
Footnotes
Declarations of Interest
D.W. acknowledges the NIH (GM102362), Kimmel Scholars award from the Sidney Kimmel Foundation for Cancer Research, and start-up funds from the Skaggs School of Pharmacy and Pharmaceutical Sciences, UCSD.
References
- Awrey DE, Weilbaecher RG, Hemming SA, Orlicky SM, Kane CM, Edwards AM. Transcription elongation through DNA arrest sites. A multistep process involving both RNA polymerase II subunit RPB9 and TFIIS. J Biol Chem. 1997;272:14747–54. doi: 10.1074/jbc.272.23.14747. [DOI] [PubMed] [Google Scholar]
- Awrey DE, Shimasaki N, Koth C, Weilbaecher R, Olmsted V, Kazanis S, Shan X, Arellano J, Arrowsmith CH, Kane CM, Edwards AM. Yeast transcript elongation factor (TFIIS), structure and function. II: RNA polymerase binding, transcript cleavage, and read-through. J Biol Chem. 1998;273:22595–605. doi: 10.1074/jbc.273.35.22595. [DOI] [PubMed] [Google Scholar]
- Batada NN, Westover KD, Bushnell DA, Levitt M, Kornberg RD. Diffusion of nucleoside triphosphates and role of the entry site to the RNA polymerase II active center. Proc Natl Acad Sci U S A. 2004;101:17361–4. doi: 10.1073/pnas.0408168101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard WA, Wilson SH. Structure and mechanism of DNA polymerase Beta. Chem Rev. 2006;106:361–82. doi: 10.1021/cr0404904. [DOI] [PubMed] [Google Scholar]
- Belotserkovskii BP, Liu R, Tornaletti S, Krasilnikova MM, Mirkin SM, Hanawalt PC. Mechanisms and implications of transcription blockage by guanine-rich DNA sequences. Proc Natl Acad Sci U S A. 2010;107:12816–21. doi: 10.1073/pnas.1007580107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belotserkovskii BP, Mirkin SM, Hanawalt PC. DNA sequences that interfere with transcription: implications for genome function and stability. Chem Rev. 2013;113:8620–37. doi: 10.1021/cr400078y. [DOI] [PubMed] [Google Scholar]
- Belotserkovskii BP, Neil AJ, Saleh SS, Shin JH, Mirkin SM, Hanawalt PC. Transcription blockage by homopurine DNA sequences: role of sequence composition and single-strand breaks. Nucleic Acids Res. 2013;41:1817–28. doi: 10.1093/nar/gks1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner SA. Understanding Nucleic Acids Using Synthetic Chemistry. Acc Chem Res. 2004;37:784–97. doi: 10.1021/ar040004z. [DOI] [PubMed] [Google Scholar]
- Benner SA, Sismour AM. Synthetic biology. Nat Rev Genet. 2005;6:533–43. doi: 10.1038/nrg1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr VA, Smith CA, Okumoto DS, Hanawalt PC. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985;40:359–69. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Bowler FR, Chan CK, Duffy CD, Gerland B, Islam S, Powner MW, Sutherland JD, Xu J. Prebiotically plausible oligoribonucleotide ligation facilitated by chemoselective acetylation. Nat Chem. 2013;5:383–9. doi: 10.1038/nchem.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braberg H, Jin H, Moehle EA, Chan YA, Wang S, Shales M, Benschop JJ, Morris JH, Qiu C, Hu F, Tang LK, Fraser JS, Holstege FC, Hieter P, Guthrie C, Kaplan CD, Krogan NJ. From structure to systems: high-resolution, quantitative genetic analysis of RNA polymerase II. Cell. 2013;154:775–88. doi: 10.1016/j.cell.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nat Rev Genet. 2011;13:7–13. doi: 10.1038/nrg3080. [DOI] [PubMed] [Google Scholar]
- Bregeon D, Doddridge ZA, You HJ, Weiss B, Doetsch PW. Transcriptional mutagenesis induced by uracil and 8-oxoguanine in Escherichia coli. Mol Cell. 2003;12:959–70. doi: 10.1016/s1097-2765(03)00360-5. [DOI] [PubMed] [Google Scholar]
- Bregeon D, Doetsch PW. Transcriptional mutagenesis: causes and involvement in tumour development. Nat Rev Cancer. 2011;11:218–27. doi: 10.1038/nrc3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieba LG, Eichman BF, Kokoska RJ, Doublie S, Kunkel TA, Ellenberger T. Structural basis for the dual coding potential of 8-oxoguanosine by a high-fidelity DNA polymerase. EMBO J. 2004;23:3452–61. doi: 10.1038/sj.emboj.7600354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PJ, Wise DS, Berry DA, Kosmoski JV, Smerdon MJ, Somers RL, Mackie H, Spoonde AY, Ackerman EJ, Coleman K, Tarone RE, Robbins JH. The oxidative DNA lesion 8,5′-(S)-cyclo-2′-deoxyadenosine is repaired by the nucleotide excision repair pathway and blocks gene expression in mammalian cells. J Biol Chem. 2000;275:22355–62. doi: 10.1074/jbc.M002259200. [DOI] [PubMed] [Google Scholar]
- Brueckner F, Hennecke U, Carell T, Cramer P. CPD damage recognition by transcribing RNA polymerase II. Science. 2007;315:859–62. doi: 10.1126/science.1135400. [DOI] [PubMed] [Google Scholar]
- Brueckner F, Armache KJ, Cheung A, Damsma GE, Kettenberger H, Lehmann E, Sydow J, Cramer P. Structure-function studies of the RNA polymerase II elongation complex. Acta Crystallogr D Biol Crystallogr. 2009;65:112–20. doi: 10.1107/S0907444908039875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JA, Dreij K, Cartularo L, Scicchitano DA. O6-methylguanine induces altered proteins at the level of transcription in human cells. Nucleic Acids Res. 2010;38:8178–87. doi: 10.1093/nar/gkq706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AC, Cramer P. Structural basis of RNA polymerase II backtracking, arrest and reactivation. Nature. 2011;471:249–53. doi: 10.1038/nature09785. [DOI] [PubMed] [Google Scholar]
- Cheung AC, Sainsbury S, Cramer P. Structural basis of initial RNA polymerase II transcription. EMBO J. 2011;30:4755–63. doi: 10.1038/emboj.2011.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline SD, Riggins JN, Tornaletti S, Marnett LJ, Hanawalt PC. Malondialdehyde adducts in DNA arrest transcription by T7 RNA polymerase and mammalian RNA polymerase II. Proc Natl Acad Sci U S A. 2004;101:7275–80. doi: 10.1073/pnas.0402252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda Y, Job C, Anin MF, Leng M, Job D. Spectrum of DNA--platinum adduct recognition by prokaryotic and eukaryotic DNA-dependent RNA polymerases. Biochemistry. 1993;32:8582–8. doi: 10.1021/bi00084a027. [DOI] [PubMed] [Google Scholar]
- Cramer P, Bushnell DA, Kornberg RD. Structural basis of transcription: RNA polymerase II at 2.8 angstrom resolution. Science. 2001;292:1863–76. doi: 10.1126/science.1059493. [DOI] [PubMed] [Google Scholar]
- Cramer P. Multisubunit RNA polymerases. Curr Opin Struct Biol. 2002;12:89–97. doi: 10.1016/s0959-440x(02)00294-4. [DOI] [PubMed] [Google Scholar]
- Cullinane C, Mazur SJ, Essigmann JM, Phillips DR, Bohr VA. Inhibition of RNA polymerase II transcription in human cell extracts by cisplatin DNA damage. Biochemistry. 1999;38:6204–12. doi: 10.1021/bi982685+. [DOI] [PubMed] [Google Scholar]
- Da LT, Wang D, Huang X. Dynamics of pyrophosphate ion release and its coupled trigger loop motion from closed to open state in RNA polymerase II. J Am Chem Soc. 2012;134:2399–406. doi: 10.1021/ja210656k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsma GE, Alt A, Brueckner F, Carell T, Cramer P. Mechanism of transcriptional stalling at cisplatin-damaged DNA. Nat Struct Mol Biol. 2007;14:1127–33. doi: 10.1038/nsmb1314. [DOI] [PubMed] [Google Scholar]
- Damsma GE, Cramer P. Molecular basis of transcriptional mutagenesis at 8-oxoguanine. J Biol Chem. 2009;284:31658–63. doi: 10.1074/jbc.M109.022764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vivo M, Dal Peraro M, Klein ML. Phosphodiester cleavage in ribonuclease H occurs via an associative two-metal-aided catalytic mechanism. J Am Chem Soc. 2008;130:10955–62. doi: 10.1021/ja8005786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitri A, Burns JA, Broyde S, Scicchitano DA. Transcription elongation past O6-methylguanine by human RNA polymerase II and bacteriophage T7 RNA polymerase. Nucleic Acids Res. 2008;36:6459–71. doi: 10.1093/nar/gkn657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitri A, Goodenough AK, Guengerich FP, Broyde S, Scicchitano DA. Transcription processing at 1,N2-ethenoguanine by human RNA polymerase II and bacteriophage T7 RNA polymerase. J Mol Biol. 2008;375:353–66. doi: 10.1016/j.jmb.2007.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitri A, Jia L, Shafirovich V, Geacintov NE, Broyde S, Scicchitano DA. Transcription of DNA containing the 5-guanidino-4-nitroimidazole lesion by human RNA polymerase II and bacteriophage T7 RNA polymerase. DNA Repair (Amst) 2008;7:1276–88. doi: 10.1016/j.dnarep.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditlevson JV, Tornaletti S, Belotserkovskii BP, Teijeiro V, Wang G, Vasquez KM, Hanawalt PC. Inhibitory effect of a short Z-DNA forming sequence on transcription elongation by T7 RNA polymerase. Nucleic Acids Res. 2008;36:3163–70. doi: 10.1093/nar/gkn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch PW. Translesion synthesis by RNA polymerases: occurrence and biological implications for transcriptional mutagenesis. Mutat Res. 2002;510:131–40. doi: 10.1016/s0027-5107(02)00258-0. [DOI] [PubMed] [Google Scholar]
- Donahue BA, Yin S, Taylor JS, Reines D, Hanawalt PC. Transcript cleavage by RNA polymerase II arrested by a cyclobutane pyrimidine dimer in the DNA template. Proc Natl Acad Sci U S A. 1994;91:8502–6. doi: 10.1073/pnas.91.18.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue BA, Fuchs RP, Reines D, Hanawalt PC. Effects of aminofluorene and acetylaminofluorene DNA adducts on transcriptional elongation by RNA polymerase II. J Biol Chem. 1996;271:10588–94. doi: 10.1074/jbc.271.18.10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doublie S, Sawaya MR, Ellenberger T. An open and closed case for all polymerases. Structure. 1999;7:R31–5. doi: 10.1016/S0969-2126(99)80017-3. [DOI] [PubMed] [Google Scholar]
- Ekland EH, Bartel DP. RNA-catalysed RNA polymerization using nucleoside triphosphates. Nature. 1996;382:373–76. doi: 10.1038/382373a0. [DOI] [PubMed] [Google Scholar]
- Eoff RL, Irimia A, Angel KC, Egli M, Guengerich FP. Hydrogen bonding of 7,8-dihydro-8-oxodeoxyguanosine with a charged residue in the little finger domain determines miscoding events in Sulfolobus solfataricus DNA polymerase Dpo4. J Biol Chem. 2007;282:19831–43. doi: 10.1074/jbc.M702290200. [DOI] [PubMed] [Google Scholar]
- Erie DA, Yager TD, Vonhippel PH. The single-nucleotide addition cycle in transcription - a biophysical and biochemical perspective. Annual Review of Biophysics and Biomolecular Structure. 1992;21:379–415. doi: 10.1146/annurev.bb.21.060192.002115. [DOI] [PubMed] [Google Scholar]
- Erie DA, Hajiseyedjavadi O, Young MC, von Hippel PH. Multiple RNA polymerase conformations and GreA: control of the fidelity of transcription. Science. 1993;262:867–73. doi: 10.1126/science.8235608. [DOI] [PubMed] [Google Scholar]
- Ertem G, Ferris JP. Synthesis of RNA oligomers on heterogeneous templates. Nature. 1996;379:238–40. doi: 10.1038/379238a0. [DOI] [PubMed] [Google Scholar]
- Feig M, Burton ZF. RNA polymerase II flexibility during translocation from normal mode analysis. Proteins. 2010;78:434–46. doi: 10.1002/prot.22560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig M, Burton ZF. RNA polymerase II with open and closed trigger loops: active site dynamics and nucleic acid translocation. Biophys J. 2010;99:2577–86. doi: 10.1016/j.bpj.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Dong L, Cao W. Catalytic mechanism of endonuclease v: a catalytic and regulatory two-metal model. Biochemistry. 2006;45:10251–9. doi: 10.1021/bi060512b. [DOI] [PubMed] [Google Scholar]
- Ferris JP, Ertem G. Oligomerization of ribonucleotides on montmorillonite: reaction of the 5′-phosphorimidazolide of adenosine. Science. 1992;257:1387–9. doi: 10.1126/science.1529338. [DOI] [PubMed] [Google Scholar]
- Fousteri M, Mullenders LH. Transcription-coupled nucleotide excision repair in mammalian cells: molecular mechanisms and biological effects. Cell Res. 2008;18:73–84. doi: 10.1038/cr.2008.6. [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Lehmann AR, Fuchs RPP. Trading places: how do DNA polymerases switch during translesion DNA synthesis? Mol Cell. 2005;18:499–505. doi: 10.1016/j.molcel.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Gnatt AL, Cramer P, Fu J, Bushnell DA, Kornberg RD. Structural basis of transcription: an RNA polymerase II elongation complex at 3.3 A resolution. Science. 2001;292:1876–82. doi: 10.1126/science.1059495. [DOI] [PubMed] [Google Scholar]
- Goodman MF. Error-prone repair DNA polymerases in prokaryotes and eukaryotes. Annu Rev Biochem. 2002;71:17–50. doi: 10.1146/annurev.biochem.71.083101.124707. [DOI] [PubMed] [Google Scholar]
- Gordon AJ, Halliday JA, Blankschien MD, Burns PA, Yatagai F, Herman C. Transcriptional infidelity promotes heritable phenotypic change in a bistable gene network. PLoS Biol. 2009;7:e44. doi: 10.1371/journal.pbio.1000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AJ, Satory D, Halliday JA, Herman C. Lost in transcription: transient errors in information transfer. Curr Opin Microbiol. 2015;24:80–7. doi: 10.1016/j.mib.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawalt PC. Emerging links between premature ageing and defective DNA repair. Mech Ageing Dev. 2008;129:503–5. doi: 10.1016/j.mad.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawalt PC, Spivak G. Transcription–coupled DNA repair: two decades of progress and surprises. Nat Rev Mol Cell Biol. 2008;9:958–70. doi: 10.1038/nrm2549. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Olanrewaju YO, Zheng Y, Wilson GG, Zhang X, Cheng X. Wilms tumor protein recognizes 5-carboxylcytosine within a specific DNA sequence. Genes Dev. 2014;28:2304–13. doi: 10.1101/gad.250746.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L, Sun Y, Li X, Dai Q, Song CX, Zhang K, He C, Xu GL. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333:1303–7. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–85. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- Huang X, Wang D, Weiss DR, Bushnell DA, Kornberg RD, Levitt M. RNA polymerase II trigger loop residues stabilize and position the incoming nucleotide triphosphate in transcription. Proc Natl Acad Sci U S A. 2010;107:15745–50. doi: 10.1073/pnas.1009898107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imashimizu M, Shimamoto N, Oshima T, Kashlev M. Transcription elongation. Heterogeneous tracking of RNA polymerase and its biological implications. Transcription. 2014;5:e28285. doi: 10.4161/trns.28285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Orgel LE. Oligomerization of (guanosine 5′-phosphor)-2-methylimidazolide on poly(C). An RNA polymerase model. J Mol Biol. 1982;162:201–17. doi: 10.1016/0022-2836(82)90169-3. [DOI] [PubMed] [Google Scholar]
- Irvin JD, Kireeva ML, Gotte DR, Shafer BK, Huang I, Kashlev M, Strathern JN. A genetic assay for transcription errors reveals multilayer control of RNA polymerase II fidelity. PLoS Genet. 2014;10:e1004532. doi: 10.1371/journal.pgen.1004532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–3. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iurlaro M, Ficz G, Oxley D, Raiber EA, Bachman M, Booth MJ, Andrews S, Balasubramanian S, Reik W. A screen for hydroxymethylcytosine and formylcytosine binding proteins suggests functions in transcription and chromatin regulation. Genome Biol. 2013;14:R119. doi: 10.1186/gb-2013-14-10-r119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izban MG, Luse DS. SII-facilitated transcript cleavage in RNA polymerase II complexes stalled early after initiation occurs in primarily dinucleotide increments. J Biol Chem. 1993;268:12864–73. [PubMed] [Google Scholar]
- Jaakkola E, Mustonen A, Olsen P, Miettinen S, Savuoja T, Raams A, Jaspers NG, Shao H, Wu BL, Ignatius J. ERCC6 founder mutation identified in Finnish patients with COFS syndrome. Clin Genet. 2010;78:541–7. doi: 10.1111/j.1399-0004.2010.01424.x. [DOI] [PubMed] [Google Scholar]
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–8. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaruga P, Dizdaroglu M. 8,5′-Cyclopurine-2′-deoxynucleosides in DNA: mechanisms of formation, measurement, repair and biological effects. DNA Repair (Amst) 2008;7:1413–25. doi: 10.1016/j.dnarep.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Jeon C, Agarwal K. Fidelity of RNA polymerase II transcription controlled by elongation factor TFIIS. Proc Natl Acad Sci U S A. 1996;93:13677–82. doi: 10.1073/pnas.93.24.13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TL, Chamberlin MJ. Complexes of yeast RNA polymerase II and RNA are substrates for TFIIS-induced RNA cleavage. Cell. 1994;77:217–24. doi: 10.1016/0092-8674(94)90314-x. [DOI] [PubMed] [Google Scholar]
- Jung Y, Lippard SJ. RNA polymerase II blockage by cisplatin-damaged DNA. Stability and polyubiquitylation of stalled polymerase. J Biol Chem. 2006;281:1361–70. doi: 10.1074/jbc.M509688200. [DOI] [PubMed] [Google Scholar]
- Kalogeraki VS, Tornaletti S, Hanawalt PC. Transcription arrest at a lesion in the transcribed DNA strand in vitro is not affected by a nearby lesion in the opposite strand. J Biol Chem. 2003;278:19558–64. doi: 10.1074/jbc.M301060200. [DOI] [PubMed] [Google Scholar]
- Kalogeraki VS, Tornaletti S, Cooper PK, Hanawalt PC. Comparative TFIIS-mediated transcript cleavage by mammalian RNA polymerase II arrested at a lesion in different transcription systems. DNA Repair (Amst) 2005;4:1075–87. doi: 10.1016/j.dnarep.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Kaplan CD, Larsson K-M, Kornberg RD. The RNA polymerase II trigger loop functions in substrate selection and is directly targeted by alpha-amanitin. Mol Cell. 2008;30:547–56. doi: 10.1016/j.molcel.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan CD. Basic mechanisms of RNA polymerase II activity and alteration of gene expression in Saccharomyces cerevisiae. Biochim Biophys Acta. 2013;1829:39–54. doi: 10.1016/j.bbagrm.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashkina E, Anikin M, Brueckner F, Pomerantz RT, McAllister WT, Cramer P, Temiakov D. Template misalignment in multisubunit RNA polymerases and transcription fidelity. Mol Cell. 2006;24:257–66. doi: 10.1016/j.molcel.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Kashkina E, Anikin M, Tahirov TH, Kochetkov SN, Vassylyev DG, Temiakov D. Elongation complexes of Thermus thermophilus RNA polymerase that possess distinct translocation conformations. Nucleic Acids Res. 2006;34:4036–45. doi: 10.1093/nar/gkl559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellinger MW, Song CX, Chong J, Lu XY, He C, Wang D. 5-formylcytosine and 5-carboxylcytosine reduce the rate and substrate specificity of RNA polymerase II transcription. Nat Struct Mol Biol. 2012;19:831–3. doi: 10.1038/nsmb.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellinger MW, Ulrich S, Chong J, Kool ET, Wang D. Dissecting chemical interactions governing RNA polymerase II transcriptional fidelity. J Am Chem Soc. 2012;134:8231–40. doi: 10.1021/ja302077d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellinger MW, Park GY, Chong J, Lippard SJ, Wang D. Effect of a monofunctional phenanthriplatin-DNA adduct on RNA polymerase II transcriptional fidelity and translesion synthesis. J Am Chem Soc. 2013;135:13054–61. doi: 10.1021/ja405475y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenberger H, Armache K-J, Cramer P. Architecture of the RNA polymerase II-TFIIS complex and implications for mRNA cleavage. Cell. 2003;114:347–57. doi: 10.1016/s0092-8674(03)00598-1. [DOI] [PubMed] [Google Scholar]
- Kettenberger H, Armache K-J, Cramer P. Complete RNA polymerase II elongation complex structure and its interactions with NTP and TFIIS. Mol Cell. 2004;16:955–65. doi: 10.1016/j.molcel.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Khakshoor O, Kool ET. Chemistry of nucleic acids: impacts in multiple fields. Chem Commun (Camb) 2011;47:7018–24. doi: 10.1039/c1cc11021g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakshoor O, Wheeler SE, Houk KN, Kool ET. Measurement and theory of hydrogen bonding contribution to isosteric DNA base pairs. J Am Chem Soc. 2012;134:3154–63. doi: 10.1021/ja210475a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Delaney JC, Essigmann JM, Kool ET. Probing the active site tightness of DNA polymerase in subangstrom increments. Proc Natl Acad Sci U S A. 2005;102:15803–8. doi: 10.1073/pnas.0505113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Kool ET. A series of nonpolar thymidine analogues of increasing size: DNA base pairing and stacking properties. J Org Chem. 2005;70:2048–53. doi: 10.1021/jo048061t. [DOI] [PubMed] [Google Scholar]
- Kireeva ML, Nedialkov YA, Cremona GH, Purtov YA, Lubkowska L, Malagon F, Burton ZF, Strathern JN, Kashlev M. Transient reversal of RNA polymerase II active site closing controls fidelity of transcription elongation. Mol Cell. 2008;30:557–66. doi: 10.1016/j.molcel.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kireeva ML, Opron K, Seibold SA, Domecq C, Cukier RI, Coulombe B, Kashlev M, Burton ZF. Molecular dynamics and mutational analysis of the catalytic and translocation cycle of RNA polymerase. BMC Biophys. 2012;5:11. doi: 10.1186/2046-1682-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg RD. The molecular basis of eucaryotic transcription. Cell Death Differ. 2007;14:1989–97. doi: 10.1038/sj.cdd.4402251. [DOI] [PubMed] [Google Scholar]
- Koyama H, Ito T, Nakanishi T, Kawamura N, Sekimizu K. Transcription elongation factor S-II maintains transcriptional fidelity and confers oxidative stress resistance. Genes Cells. 2003;8:779–88. doi: 10.1046/j.1365-2443.2003.00677.x. [DOI] [PubMed] [Google Scholar]
- Koyama H, Ito T, Nakanishi T, Sekimizu K. Stimulation of RNA polymerase II transcript cleavage activity contributes to maintain transcriptional fidelity in yeast. Genes Cells. 2007;12:547–59. doi: 10.1111/j.1365-2443.2007.01072.x. [DOI] [PubMed] [Google Scholar]
- Krasilnikova MM, Kireeva ML, Petrovic V, Knijnikova N, Kashlev M, Mirkin SM. Effects of Friedreich’s ataxia (GAA)n*(TTC)n repeats on RNA synthesis and stability. Nucleic Acids Res. 2007;35:1075–84. doi: 10.1093/nar/gkl1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraoka I, Suzuki K, Ito S, Hayashida M, Kwei JS, Ikegami T, Handa H, Nakabeppu Y, Tanaka K. RNA polymerase II bypasses 8-oxoguanine in the presence of transcription elongation factor TFIIS. DNA Repair (Amst) 2007;6:841–51. doi: 10.1016/j.dnarep.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Lagerwerf S, Vrouwe MG, Overmeer RM, Fousteri MI, Mullenders LH. DNA damage response and transcription. DNA Repair (Amst) 2011;10:743–50. doi: 10.1016/j.dnarep.2011.04.024. [DOI] [PubMed] [Google Scholar]
- Laine JP, Egly JM. When transcription and repair meet: a complex system. Trends Genet. 2006;22:430–6. doi: 10.1016/j.tig.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Langkjaer N, Pasternak A, Wengel J. UNA (unlocked nucleic acid): a flexible RNA mimic that allows engineering of nucleic acid duplex stability. Bioorg Med Chem. 2009;17:5420–5. doi: 10.1016/j.bmc.2009.06.045. [DOI] [PubMed] [Google Scholar]
- Larson MH, Zhou J, Kaplan CD, Palangat M, Kornberg RD, Landick R, Block SM. Trigger loop dynamics mediate the balance between the transcriptional fidelity and speed of RNA polymerase II. Proc Natl Acad Sci U S A. 2012;109:6555–60. doi: 10.1073/pnas.1200939109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugel V, Dalloz C, Durand M, Sauvanaud F, Kristensen U, Vincent MC, Pasquier L, Odent S, Cormier-Daire V, Gener B, Tobias ES, Tolmie JL, Martin-Coignard D, Drouin-Garraud V, Heron D, Journel H, Raffo E, Vigneron J, Lyonnet S, Murday V, Gubser-Mercati D, Funalot B, Brueton L, Sanchez Del Pozo J, Munoz E, Gennery AR, Salih M, Noruzinia M, Prescott K, Ramos L, Stark Z, Fieggen K, Chabrol B, Sarda P, Edery P, Bloch-Zupan A, Fawcett H, Pham D, Egly JM, Lehmann AR, Sarasin A, Dollfus H. Mutation update for the CSB/ERCC6 and CSA/ERCC8 genes involved in Cockayne syndrome. Hum Mutat. 2010;31:113–26. doi: 10.1002/humu.21154. [DOI] [PubMed] [Google Scholar]
- Lee K-B, Wang D, Lippard SJ, Sharp PA. Transcription-coupled and DNA damage-dependent ubiquitination of RNA polymerase II in vitro. Proc Natl Acad Sci USA. 2002;99:4239–44. doi: 10.1073/pnas.072068399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby R, Gallant J. The role of RNA polymerase in transcriptional fidelity. Mol Microbiol. 1991 doi: 10.1111/j.1365-2958.1991.tb01872.x. [DOI] [PubMed] [Google Scholar]
- Lindahl T, Barnes DE. Repair of endogenous DNA damage. Cold Spring Harb Symp Quant Biol. 2000;65:127–33. doi: 10.1101/sqb.2000.65.127. [DOI] [PubMed] [Google Scholar]
- Lindsey-Boltz LA, Sancar A. RNA polymerase: the most specific damage recognition protein in cellular responses to DNA damage? Proc Natl Acad Sci U S A. 2007;104:13213–4. doi: 10.1073/pnas.0706316104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhou W, Doetsch PW. RNA polymerase bypass at sites of dihydrouracil: implications for transcriptional mutagenesis. Mol Cell Biol. 1995;15:6729–35. doi: 10.1128/mcb.15.12.6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bushnell DA, Kornberg RD. RNA polymerase II transcription: structure and mechanism. Biochim Biophys Acta. 2013;1829:2–8. doi: 10.1016/j.bbagrm.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman M, Lane DP. Transcription - guarding the genome by sensing DNA damage. Nat Rev Cancer. 2004;4:727–37. doi: 10.1038/nrc1435. [DOI] [PubMed] [Google Scholar]
- Malik S, Chaurasia P, Lahudkar S, Durairaj G, Shukla A, Bhaumik SR. Rad26p, a transcription-coupled repair factor, is recruited to the site of DNA lesion in an elongating RNA polymerase II-dependent manner in vivo. Nucleic Acids Res. 2010;38:1461–77. doi: 10.1093/nar/gkp1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinen AM, Turtola M, Parthiban M, Vainonen L, Johnson MS, Belogurov GA. Active site opening and closure control translocation of multisubunit RNA polymerase. Nucleic Acids Res. 2012;40:7442–51. doi: 10.1093/nar/gks383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marietta C, Brooks PJ. Transcriptional bypass of bulky DNA lesions causes new mutant RNA transcripts in human cells. EMBO Rep. 2007;8:388–93. doi: 10.1038/sj.embor.7400932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Rucobo FW, Cramer P. Structural basis of transcription elongation. Biochim Biophys Acta. 2013;1829:9–19. doi: 10.1016/j.bbagrm.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Mei Kwei JS, Kuraoka I, Horibata K, Ubukata M, Kobatake E, Iwai S, Handa H, Tanaka K. Blockage of RNA polymerase II at a cyclobutane pyrimidine dimer and 6-4 photoproduct. Biochem Biophys Res Commun. 2004;320:1133–8. doi: 10.1016/j.bbrc.2004.06.066. [DOI] [PubMed] [Google Scholar]
- Mello JA, Lippard SJ, Essigmann JM. DNA adducts of cis-diamminedichloroplatinum(II) and its trans isomer inhibit RNA polymerase II differentially in vivo. Biochemistry. 1995;34:14783–91. doi: 10.1021/bi00045a020. [DOI] [PubMed] [Google Scholar]
- Mellon I, Bohr VA, Smith CA, Hanawalt PC. Preferential DNA repair of an active gene in human cells. Proc Natl Acad Sci U S A. 1986;83:8878–82. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I, Spivak G, Hanawalt PC. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987;51:241–9. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Monti P, Broxson C, Inga A, Wang RW, Menichini P, Tornaletti S, Gold B, Fronza G. 3-Methyl-3-deazaadenine, a stable isostere of N3-methyl-adenine, is efficiently bypassed by replication in vivo and by transcription in vitro. DNA Repair (Amst) 2011;10:861–8. doi: 10.1016/j.dnarep.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morreall JF, Petrova L, Doetsch PW. Transcriptional mutagenesis and its potential roles in the etiology of cancer and bacterial antibiotic resistance. J Cell Physiol. 2013;228:2257–61. doi: 10.1002/jcp.24400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel M, Globisch D, Carell T. 5-Hydroxymethylcytosine, the sixth base of the genome. Angew Chem Int Ed Engl. 2011;50:6460–8. doi: 10.1002/anie.201101547. [DOI] [PubMed] [Google Scholar]
- Nedialkov YA, Opron K, Assaf F, Artsimovitch I, Kireeva ML, Kashlev M, Cukier RI, Nudler E, Burton ZF. The RNA polymerase bridge helix YFI motif in catalysis, fidelity and translocation. Biochim Biophys Acta. 2013;1829:187–98. doi: 10.1016/j.bbagrm.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil AJ, Belotserkovskii BP, Hanawalt PC. Transcription blockage by bulky end termini at single-strand breaks in the DNA template: differential effects of 5′ and 3′ adducts. Biochemistry. 2012;51:8964–70. doi: 10.1021/bi301240y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesser NK, Peterson DO, Hawley DK. RNA polymerase II subunit Rpb9 is important for transcriptional fidelity in vivo. Proc Natl Acad Sci USA. 2006;103:3268–73. doi: 10.1073/pnas.0511330103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park GY, Wilson JJ, Song Y, Lippard SJ. Phenanthriplatin, a monofunctional DNA-binding platinum anticancer drug candidate with unusual potency and cellular activity profile. Proc Natl Acad Sci U S A. 2012;109:11987–92. doi: 10.1073/pnas.1207670109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Zhang K, Ren Y, Nadji S, Sinha N, Taylor JS, Kang C. Crystal structure of a DNA decamer containing a cis-syn thymine dimer. Proc Natl Acad Sci U S A. 2002;99:15965–70. doi: 10.1073/pnas.242422699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak A, Wengel J. Thermodynamics of RNA duplexes modified with unlocked nucleic acid nucleotides. Nucleic Acids Res. 2010;38:6697–706. doi: 10.1093/nar/gkq561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlow RA, Kolbanovskii A, Hingerty BE, Geacintov NE, Broyde S, Scicchitano DA. DNA adducts from a tumorigenic metabolite of benzo[a]pyrene block human RNA polymerase II elongation in a sequence- and stereochemistry-dependent manner. J Mol Biol. 2002;321:29–47. doi: 10.1016/s0022-2836(02)00593-4. [DOI] [PubMed] [Google Scholar]
- Perlow RA, Broyde S. Extending the understanding of mutagenicity: structural insights into primer-extension past a benzo[a]pyrene diol epoxide-DNA adduct. J Mol Biol. 2003;327:797–818. doi: 10.1016/s0022-2836(03)00187-6. [DOI] [PubMed] [Google Scholar]
- Powell W, Bartholomew B, Reines D. Elongation factor SII contacts the 3′-end of RNA in the RNA polymerase II elongation complex. J Biol Chem. 1996;271:22301–4. doi: 10.1074/jbc.271.37.22301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiber EA, Murat P, Chirgadze DY, Beraldi D, Luisi BF, Balasubramanian S. 5-Formylcytosine alters the structure of the DNA double helix. Nat Struct Mol Biol. 2015;22:44–9. doi: 10.1038/nsmb.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reines D, Chamberlin MJ, Kane CM. Transcription elongation factor SII (TFIIS) enables RNA polymerase II to elongate through a block to transcription in a human gene in vitro. J Biol Chem. 1989;264:10799–809. [PubMed] [Google Scholar]
- Reines D, Conaway RC, Conaway JW. Mechanism and regulation of transcriptional elongation by RNA polymerase II. Curr Opin Cell Biol. 1999;11:342–6. doi: 10.1016/S0955-0674(99)80047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd MD, Izban MG, Luse DS. The active site of RNA polymerase II participates in transcript cleavage within arrested ternary complexes. Proc Natl Acad Sci USA. 1994;91:8057–61. doi: 10.1073/pnas.91.17.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas-Rios V, Belotserkovskii BP, Hanawalt PC. DNA slip-outs cause RNA polymerase II arrest in vitro: potential implications for genetic instability. Nucleic Acids Res. 2011;39:7444–54. doi: 10.1093/nar/gkr429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker AH, Tsutakawa SE, Kostek S, Ng C, Shin DS, Peris M, Campeau E, Tainer JA, Nogales E, Cooper PK. Recognition of RNA polymerase II and transcription bubbles by XPG, CSB, and TFIIH: insights for transcription-coupled repair and Cockayne Syndrome. Mol Cell. 2005;20:187–98. doi: 10.1016/j.molcel.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Saxowsky TT, Doetsch PW. RNA polymerase encounters with DNA damage: transcription-coupled repair or transcriptional mutagenesis? Chem Rev. 2006;106:474–88. doi: 10.1021/cr040466q. [DOI] [PubMed] [Google Scholar]
- Saxowsky TT, Meadows KL, Klungland A, Doetsch PW. 8-Oxoguanine-mediated transcriptional mutagenesis causes Ras activation in mammalian cells. Proc Natl Acad Sci U S A. 2008;105:18877–82. doi: 10.1073/pnas.0806464105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinecker TM, Perlow RA, Broyde S, Geacintov NE, Scicchitano DA. Human RNA polymerase II is partially blocked by DNA adducts derived from tumorigenic benzo[c]phenanthrene diol epoxides: relating biological consequences to conformational preferences. Nucleic Acids Res. 2003;31:6004–15. doi: 10.1093/nar/gkg771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BH, Burgin AB, Deweese JE, Osheroff N, Berger JM. A novel and unified two-metal mechanism for DNA cleavage by type II and IA topoisomerases. Nature. 2010;465:641–4. doi: 10.1038/nature08974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scicchitano DA, Olesnicky EC, Dimitri A. Transcription and DNA adducts: what happens when the message gets cut off? DNA Repair (Amst) 2004;3:1537–48. doi: 10.1016/j.dnarep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Scicchitano DA. Transcription past DNA adducts derived from polycyclic aromatic hydrocarbons. Mutat Res. 2005;577:146–54. doi: 10.1016/j.mrfmmm.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Shen L, Wu H, Diep D, Yamaguchi S, D’Alessio AC, Fung HL, Zhang K, Zhang Y. Genome-wide Analysis Reveals TET- and TDG-Dependent 5-Methylcytosine Oxidation Dynamics. Cell. 2013;153:692–706. doi: 10.1016/j.cell.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson S, Dirac-Svejstrup AB, Svejstrup JQ. Evidence that transcript cleavage is essential for RNA polymerase II transcription and cell viability. Mol Cell. 2010;38:202–10. doi: 10.1016/j.molcel.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva DA, Weiss DR, Pardo Avila F, Da LT, Levitt M, Wang D, Huang X. Millisecond dynamics of RNA polymerase II translocation at atomic resolution. Proc Natl Acad Sci U S A. 2014;111:7665–70. doi: 10.1073/pnas.1315751111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CX, Szulwach KE, Dai Q, Fu Y, Mao SQ, Lin L, Street C, Li Y, Poidevin M, Wu H, Gao J, Liu P, Li L, Xu GL, Jin P, He C. Genome-wide Profiling of 5-Formylcytosine Reveals Its Roles in Epigenetic Priming. Cell. 2013;153:678–91. doi: 10.1016/j.cell.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosunov V, Sosunova E, Mustaev A, Bass I, Nikiforov V, Goldfarb A. Unified two-metal mechanism of RNA synthesis and degradation by RNA polymerase. EMBO J. 2003;22:2234–44. doi: 10.1093/emboj/cdg193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruijt CG, Gnerlich F, Smits AH, Pfaffeneder T, Jansen PW, Bauer C, Munzel M, Wagner M, Muller M, Khan F, Eberl HC, Mensinga A, Brinkman AB, Lephikov K, Muller U, Walter J, Boelens R, van Ingen H, Leonhardt H, Carell T, Vermeulen M. Dynamic readers for 5-(hydroxy)methylcytosine and its oxidized derivatives. Cell. 2013;152:1146–59. doi: 10.1016/j.cell.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Steitz TA, Steitz JA. A general two-metal-ion mechanism for catalytic RNA. Proc Natl Acad Sci U S A. 1993;90:6498–502. doi: 10.1073/pnas.90.14.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathern JN, Jin DJ, Court DL, Kashlev M. Isolation and characterization of transcription fidelity mutants. Biochim Biophys Acta. 2012;1819:694–9. doi: 10.1016/j.bbagrm.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svejstrup JQ. Contending with transcriptional arrest during RNAPII transcript elongation. Trends Biochem Sci. 2007;32:165–71. doi: 10.1016/j.tibs.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Svejstrup JQ. RNA polymerase II transcript elongation. Biochim Biophys Acta. 2013;1829:1. doi: 10.1016/j.bbagrm.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Svetlov V, Nudler E. Macromolecular micromovements: how RNA polymerase translocates. Curr Opin Struct Biol. 2009;19:701–7. doi: 10.1016/j.sbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetlov V, Nudler E. Basic mechanism of transcription by RNA polymerase II. Biochim Biophys Acta. 2013;1829:20–8. doi: 10.1016/j.bbagrm.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydow JF, Brueckner F, Cheung AC, Damsma GE, Dengl S, Lehmann E, Vassylyev D, Cramer P. Structural basis of transcription: mismatch-specific fidelity mechanisms and paused RNA polymerase II with frayed RNA. Mol Cell. 2009;34:710–21. doi: 10.1016/j.molcel.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Tan L, Wiesler S, Trzaska D, Carney HC, Weinzierl ROJ. Bridge helix and trigger loop perturbations generate superactive RNA polymerases. J Biol. 2008;7:40. doi: 10.1186/jbiol98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Platas AA, Hawley DK. Transcriptional fidelity and proofreading by RNA polymerase II. Cell. 1998;93:627–37. doi: 10.1016/s0092-8674(00)81191-5. [DOI] [PubMed] [Google Scholar]
- Todd RC, Lippard SJ. Inhibition of transcription by platinum antitumor compounds. Metallomics. 2009;1:280–91. doi: 10.1039/b907567d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornaletti S, Donahue BA, Reines D, Hanawalt PC. Nucleotide sequence context effect of a cyclobutane pyrimidine dimer upon RNA polymerase II transcription. J Biol Chem. 1997;272:31719–24. doi: 10.1074/jbc.272.50.31719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornaletti S, Hanawalt PC. Effect of DNA lesions on transcription elongation. Biochimie. 1999;81:139–46. doi: 10.1016/s0300-9084(99)80046-7. [DOI] [PubMed] [Google Scholar]
- Tornaletti S, Reines D, Hanawalt PC. Structural characterization of RNA polymerase II complexes arrested by a cyclobutane pyrimidine dimer in the transcribed strand of template DNA. J Biol Chem. 1999;274:24124–30. doi: 10.1074/jbc.274.34.24124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornaletti S, Patrick SM, Turchi JJ, Hanawalt PC. Behavior of T7 RNA polymerase and mammalian RNA polymerase II at site-specific cisplatin adducts in the template DNA. J Biol Chem. 2003;278:35791–7. doi: 10.1074/jbc.M305394200. [DOI] [PubMed] [Google Scholar]
- Tornaletti S, Maeda LS, Kolodner RD, Hanawalt PC. Effect of 8-oxoguanine on transcription elongation by T7 RNA polymerase and mammalian RNA polymerase II. DNA Repair (Amst) 2004;3:483–94. doi: 10.1016/j.dnarep.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Tornaletti S. Transcription arrest at DNA damage sites. Mutat Res. 2005;577:131–45. doi: 10.1016/j.mrfmmm.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Tornaletti S, Maeda LS, Hanawalt PC. Transcription arrest at an abasic site in the transcribed strand of template DNA. Chem Res Toxicol. 2006;19:1215–20. doi: 10.1021/tx060103g. [DOI] [PubMed] [Google Scholar]
- Tornaletti S, Park-Snyder S, Hanawalt PC. G4-forming sequences in the non-transcribed DNA strand pose blocks to T7 RNA polymerase and mammalian RNA polymerase II. J Biol Chem. 2008;283:12756–62. doi: 10.1074/jbc.M705003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornaletti S. Transcriptional processing of G4 DNA. Mol Carcinog. 2009;48:326–35. doi: 10.1002/mc.20513. [DOI] [PubMed] [Google Scholar]
- Toulokhonov I, Zhang J, Palangat M, Landick R. A central role of the RNA polymerase trigger loop in active-site rearrangement during transcriptional pausing. Mol Cell. 2007;27:406–19. doi: 10.1016/j.molcel.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Trinh V, Langelier M-F, Archambault J, Coulombe B. Structural perspective on mutations affecting the function of multisubunit RNA polymerases. Microbiol Mol Biol Rev. 2006;70:12–36. doi: 10.1128/MMBR.70.1.12-36.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh V, Langelier MF, Archambault J, Coulombe B. Structural perspective on mutations affecting the function of multisubunit RNA polymerases. Microbiol Mol Biol Rev. 2006;70:12–36. doi: 10.1128/MMBR.70.1.12-36.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troelstra C, van Gool A, de Wit J, Vermeulen W, Bootsma D, Hoeijmakers JH. ERCC6, a member of a subfamily of putative helicases, is involved in Cockayne’s syndrome and preferential repair of active genes. Cell. 1992;71:939–53. doi: 10.1016/0092-8674(92)90390-x. [DOI] [PubMed] [Google Scholar]
- Troelstra C, Hesen W, Bootsma D, Hoeijmakers JH. Structure and expression of the excision repair gene ERCC6, involved in the human disorder Cockayne’s syndrome group B. Nucleic Acids Res. 1993;21:419–26. doi: 10.1093/nar/21.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher DA, McHale AH. Nonenzymic joining of oligoadenylates on a polyuridylic acid template. Science. 1976;192:53–4. doi: 10.1126/science.1257755. [DOI] [PubMed] [Google Scholar]
- Usher DA, McHale AH. Hydrolytic stability of helical RNA: a selective advantage for the natural 3′,5′-bond. Proc Natl Acad Sci U S A. 1976;73:1149–53. doi: 10.1073/pnas.73.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boom V, Citterio E, Hoogstraten D, Zotter A, Egly JM, van Cappellen WA, Hoeijmakers JH, Houtsmuller AB, Vermeulen W. DNA damage stabilizes interaction of CSB with the transcription elongation machinery. J Cell Biol. 2004;166:27–36. doi: 10.1083/jcb.200401056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool AJ, Citterio E, Rademakers S, van Os R, Vermeulen W, Constantinou A, Egly JM, Bootsma D, Hoeijmakers JH. The Cockayne syndrome B protein, involved in transcription-coupled DNA repair, resides in an RNA polymerase II-containing complex. EMBO J. 1997;16:5955–65. doi: 10.1093/emboj/16.19.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan A, You HJ, Doetsch PW. Phenotypic change caused by transcriptional bypass of uracil in nondividing cells. Science. 1999;284:159–62. doi: 10.1126/science.284.5411.159. [DOI] [PubMed] [Google Scholar]
- von Hippel PH. An integrated model of the transcription complex in elongation, termination, and editing. Science. 1998;281:660–5. doi: 10.1126/science.281.5377.660. [DOI] [PubMed] [Google Scholar]
- Walmacq C, Kireeva ML, Irvin J, Nedialkov Y, Lubkowska L, Malagon F, Strathern JN, Kashlev M. Rpb9 subunit controls transcription fidelity by delaying NTP sequestration in RNA polymerase II. J Biol Chem. 2009;284:19601–12. doi: 10.1074/jbc.M109.006908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmacq C, Cheung AC, Kireeva ML, Lubkowska L, Ye C, Gotte D, Strathern JN, Carell T, Cramer P, Kashlev M. Mechanism of translesion transcription by RNA polymerase II and its role in cellular resistance to DNA damage. Mol Cell. 2012;46:18–29. doi: 10.1016/j.molcel.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmacq C, Wang L, Chong J, Scibelli K, Lubkowska L, Gnatt A, Brooks PJ, Wang D, Kashlev M. Mechanism of RNA polymerase II bypass of oxidative cyclopurine DNA lesions. Proc Natl Acad Sci U S A. 2015;112:E410–9. doi: 10.1073/pnas.1415186112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–20. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- Wang D, Bushnell DA, Westover KD, Kaplan CD, Kornberg RD. Structural basis of transcription: role of the trigger loop in substrate specificity and catalysis. Cell. 2006;127:941–54. doi: 10.1016/j.cell.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Bushnell DA, Huang X, Westover KD, Levitt M, Kornberg RD. Structural basis of transcription: backtracked RNA polymerase II at 3.4 angstrom resolution. Science. 2009;324:1203–6. doi: 10.1126/science.1168729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Zhu G, Huang X, Lippard SJ. X-ray structure and mechanism of RNA polymerase II stalled at an antineoplastic monofunctional platinum-DNA adduct. Proc Natl Acad Sci U S A. 2010;107:9584–9. doi: 10.1073/pnas.1002565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhou Y, Xu L, Xiao R, Lu X, Chen L, Chong J, Li H, He C, Fu XD, Wang D. Molecular basis for 5-carboxycytosine recognition by RNA polymerase II elongation complex. Nature. 2015;523:621–5. doi: 10.1038/nature14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sheppard TL, Tornaletti S, Maeda LS, Hanawalt PC. Transcriptional inhibition by an oxidized abasic site in DNA. Chem Res Toxicol. 2006;19:234–41. doi: 10.1021/tx050292n. [DOI] [PubMed] [Google Scholar]
- Wang Y. Bulky DNA lesions induced by reactive oxygen species. Chem Res Toxicol. 2008;21:276–81. doi: 10.1021/tx700411g. [DOI] [PubMed] [Google Scholar]
- Waters LS, Minesinger BK, Wiltrout ME, D’Souza S, Woodruff RV, Walker GC. Eukaryotic translesion polymerases and their roles and regulation in DNA damage tolerance. Microbiol Mol Biol Rev. 2009;73:134–54. doi: 10.1128/MMBR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilbaecher RG, Awrey DE, Edwards AM, Kane CM. Intrinsic transcript cleavage in yeast RNA polymerase II elongation complexes. J Biol Chem. 2003;278:24189–99. doi: 10.1074/jbc.M211197200. [DOI] [PubMed] [Google Scholar]
- Westover KD, Bushnell DA, Kornberg RD. Structural basis of transcription: separation of RNA from DNA by RNA polymerase II. Science. 2004;303:1014–6. doi: 10.1126/science.1090839. [DOI] [PubMed] [Google Scholar]
- Westover KD, Bushnell DA, Kornberg RD. Structural basis of transcription: nucleotide selection by rotation in the RNA polymerase II active center. Cell. 2004;119:481–9. doi: 10.1016/j.cell.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Wilson MD, Harreman M, Svejstrup JQ. Ubiquitylation and degradation of elongating RNA polymerase II: the last resort. Biochim Biophys Acta. 2013;1829:151–7. doi: 10.1016/j.bbagrm.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Wu H, Zhang Y. Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation. Genes Dev. 2011;25:2436–52. doi: 10.1101/gad.179184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Plouffe SW, Chong J, Wengel J, Wang D. A chemical perspective on transcriptional fidelity: dominant contributions of sugar integrity revealed by unlocked nucleic acids. Angew Chem Int Ed Engl. 2013;52:12341–5. doi: 10.1002/anie.201307661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Butler KV, Chong J, Wengel J, Kool ET, Wang D. Dissecting the chemical interactions and substrate structural signatures governing RNA polymerase II trigger loop closure by synthetic nucleic acid analogues. Nucleic Acids Res. 2014;42:5863–70. doi: 10.1093/nar/gku238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Da L, Plouffe SW, Chong J, Kool E, Wang D. Molecular basis of transcriptional fidelity and DNA lesion-induced transcriptional mutagenesis. DNA Repair (Amst) 2014;19:71–83. doi: 10.1016/j.dnarep.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Zhang L, Chong J, Xu J, Huang X, Wang D. Strand-specific (asymmetric) contribution of phosphodiester linkages on RNA polymerase II transcriptional efficiency and fidelity. Proc Natl Acad Sci U S A. 2014;111:E3269–76. doi: 10.1073/pnas.1406234111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Wang W, Zhang L, Chong J, Huang X, Wang D. Impact of template backbone heterogeneity on RNA polymerase II transcription. Nucleic Acids Res. 2015;43:2232–41. doi: 10.1093/nar/gkv059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You C, Dai X, Yuan B, Wang J, Wang J, Brooks PJ, Niedernhofer LJ, Wang Y. A quantitative assay for assessing the effects of DNA lesions on transcription. Nat Chem Biol. 2012;8:817–22. doi: 10.1038/nchembio.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You C, Ji D, Dai X, Wang Y. Effects of Tet-mediated oxidation products of 5-methylcytosine on DNA transcription in vitro and in mammalian cells. Sci Rep. 2014;4:7052. doi: 10.1038/srep07052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You C, Wang P, Dai X, Wang Y. Transcriptional bypass of regioisomeric ethylated thymidine lesions by T7 RNA polymerase and human RNA polymerase II. Nucleic Acids Res. 2014;42:13706–13. doi: 10.1093/nar/gku1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You C, Wang J, Dai X, Wang Y. Transcriptional inhibition and mutagenesis induced by N-nitroso compound-derived carboxymethylated thymidine adducts in DNA. Nucleic Acids Res. 2015;43:1012–8. doi: 10.1093/nar/gku1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzenkova Y, Bochkareva A, Tadigotla VR, Roghanian M, Zorov S, Severinov K, Zenkin N. Stepwise mechanism for transcription fidelity. BMC Biol. 2010;8:54. doi: 10.1186/1741-7007-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzenkova Y, Zenkin N. Central role of the RNA polymerase trigger loop in intrinsic RNA hydrolysis. Proc Natl Acad Sci U S A. 2010;107:10878–83. doi: 10.1073/pnas.0914424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenkin N, Yuzenkova Y, Severinov K. Transcript-assisted transcriptional proofreading. Science. 2006;313:518–20. doi: 10.1126/science.1127422. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang D. Understanding the Molecular Basis of RNA Polymerase II Transcription. Isr J Chem. 2013;53:442–49. doi: 10.1002/ijch.201300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang X, Chen L, Wu J, Su D, Lu WJ, Hwang M-T, Yang G, Li S, Wei M, Davis L, Breyer MD, Guan Y. Liver X receptor agonist TO-901317 upregulates SCD1 expression in renal proximal straight tubule. Am J Physiol Renal Physiol. 2006;290:F1065–73. doi: 10.1152/ajprenal.00131.2005. [DOI] [PubMed] [Google Scholar]