Abstract
In pathogenic Neisseria species the type IV pili (Tfp) are of primary importance in host–pathogen interactions. Tfp mediate initial bacterial attachment to cell surfaces and formation of microcolonies via pilus–pilus interactions. Based on genome analysis, many non-pathogenic Neisseria species are predicted to express Tfp, but aside from studies on Neisseria elongata, relatively little is known about the formation and function of pili in these organisms. Here, we have analysed pilin expression and the role of Tfp in Neisseria cinerea. This non-pathogenic species shares a close taxonomic relationship to the pathogen Neisseria meningitidis and also colonizes the human oropharyngeal cavity. Through analysis of non-pathogenic Neisseria genomes we identified two genes with homology to pilE, which encodes the major pilin of N. meningitidis. We show which of the two genes is required for Tfp expression in N. cinerea and that Tfp in this species are required for DNA competence, similar to other Neisseria. However, in contrast to the meningococcus, deletion of the pilin gene did not impact the association of N. cinerea to human epithelial cells, demonstrating that N. cinerea isolates can adhere to human epithelial cells by Tfp-independent mechanisms.
Introduction
The bacterial genus Neisseria includes two pathogens, Neisseria meningitidis and Neisseria gonorrhoeae, but also several commensal species. These commensal species can be isolated from humans and various animals (Berger, 1962) although they rarely cause disease. Several of the commensal Neisseria species can be found in the human oral cavity or nasopharynx (Gold et al., 1978; Knapp & Hook, 1988; Kraal et al., 2014; Kristiansen et al., 2012; Sheikhi et al., 2015) and must therefore be able to associate with cells of the human airways. This has been shown in vitro for some species, such as Neisseria lactamica (Green et al., 2011); however, relatively little is known about the molecular mechanisms that mediate interaction of the non-pathogenic Neisseria with the human host.
In N. meningitidis, type IV pili (Tfp) are of primary importance in epithelial cell interactions and, in the presence of capsule, are the main adhesins for initial binding to cells (Nassif et al., 1993, 1994; Virji et al., 1992, 1993). Meningococcal Tfp have been proposed to bind to CD46, a membrane-bound complement regulator (Johansson et al., 2003; Källström et al., 1997), and the platelet activating factor receptor (pAFR) at the epithelial surface (Jen et al., 2013). In addition, in the absence of capsule, outer membrane opacity proteins Opa and Opc mediate cell attachment by binding to carcinoembryonic antigen cell adhesion molecules (CEACAMs), heparin sulphate proteoglycans or integrins (Bos et al., 1997; Gómez-Duarte et al., 1997; van Putten & Paul, 1995). Several autotransporters have also been implicated in adhesion to epithelial cells, including App (Serruto et al., 2003), NhhA (Msf) (Scarselli et al., 2006) and MspA (Turner et al., 2006), as well as Neisseria adhesin A (NadA), which binds to β1-integrins (Nägele et al., 2011) and contributes to attachment and invasion (Capecchi et al., 2005). Additional adhesins have been described, including ACP (Hung et al., 2013) and TspA (Oldfield et al., 2007), although the host receptors they target are as yet undetermined. Likewise, experiments have shown that blocking tetraspanins can reduce adhesion of N. meningitidis (and N. lactamica) to epithelial cells, but the bacterial ligands remain unknown (Green et al., 2011).
Commensal Neisseria possess several recognized virulence genes that have been characterized in N. meningitidis (Bennett et al., 2009; Snyder & Saunders, 2006; Stabler et al., 2005) including important adhesins such as the opacity proteins (Toleman et al., 2001; Wolff & Stern, 1995) and NadA (Muzzi et al., 2013). They also express, or have the potential to express, Tfp (Aho et al., 1987, 1997, 2000; Higashi et al., 2011). Tfp are polymers of PilE (pilin), the major structural component of the pilus fibre (Parge et al., 1995). Typical pilin features include an N-terminal class III signal peptide (Paetzel et al., 2002), N-methylphenylalanine as the first and a glutamate as the fifth amino acid of the mature protein, an N-terminal hydrophobic domain (Dalrymple & Mattick, 1987) and a surface-exposed C-terminal region (D-region) flanked by two cysteine residues (Parge et al., 1995). Strains of N. meningitidis express one of two distinct pilins, termed class I and class II, and both class I- and class II-expressing N. meningitidis can cause disease in humans. Class I pilin is closely related to gonococcal pilin, and both bind the monoclonal antibody SM1 (Virji & Heckels, 1983; Virji et al., 1989). Meningococcal class II pilins are generally smaller in size compared with class I pilins and phylogenetically related to pilins from commensal Neisseria (Aho et al., 1997; Cehovin et al., 2010; Wörmann et al., 2014). Pilins from commensal species also fail to bind SM1 (Virji & Heckels, 1983; Wörmann et al., 2014), and it has been proposed that pilin from commensal Neisseria and class II pilins from N. meningitidis share a common genetic ancestry (Saunders et al., 1993).
Tfp have been extensively studied in the pathogenic Neisseria (Carbonnelle et al., 2009; Trivedi et al., 2011) but, aside from studies on the role of Tfp in competence of Neisseria elongata (Higashi et al., 2011), far less is known about Tfp in commensal species. Interestingly, pathogenic Neisseria appear to have only one pilE gene whereas several commensal Neisseria species harbour two putative pilE genes (Aho et al., 2000; Marri et al., 2010; Wörmann et al., 2014). Two, tandemly located pilin genes have also been reported in the Tfp-producing genera Eikenella, Kingella and Xanthomonas (Ojanen-Reuhs et al., 1997; Tønjum et al., 1993; Weir et al., 1996). In the plant pathogen Xanthomonas campestris, genes encoding the two pilin homologues fimA and fimB are expressed differentially, with fimA being transcribed at much higher levels compared with fimB. Deletion of fimA dramatically reduces the formation of bacterial aggregates but has no effect on the initial colonization of the mutant to tomato leaves, suggesting that components other than FimA mediate the initial interaction between the bacterium and the tomato leaf surface (Ojanen-Reuhs et al., 1997).
In this work we have investigated the involvement of the two putative pilE genes in Tfp formation and function in Neisseria cinerea. N. cinerea shares a close taxonomic relationship with the pathogenic Neisseria (Bennett et al., 2012; Marri et al., 2010) and colonizes the human oropharyngeal cavities in healthy adults (Knapp & Hook, 1988) and children (Sheikhi et al., 2015). Genome sequencing analysis has reported the presence of genes required for Tfp biogenesis in this species, suggesting that N. cinerea has the capacity to produce Tfp (Marri et al., 2010). Here we show that N. cinerea does indeed produce Tfp and that they are required for DNA competence in this bacterium. Of the two putative PilE expression loci in N. cinerea, pilE2 appears to be dispensable for Tfp biogenesis and DNA competence. In addition, we have identified Tfp-independent adherence to human epithelial cells, suggesting that for certain N. cinerea isolates Tfp are not the primary determinant of host colonization.
Methods
Bacterial strains and growth conditions
All strains used in this study are listed in Table 1. N. cinerea and N. meningitidis strains were grown at 37 °C and 5 % CO2 in brain heart infusion (BHI) (Oxoid) medium or on BHI agar supplemented with 5 % (v/v) horse serum (Oxoid). Escherichia coli strains were grown at 37 °C on Luria Broth (LB) agar medium or in liquid LB at 37 °C with 180 r.p.m. shaking. Where appropriate, the medium was supplemented with kanamycin (N. cinerea 75 μg ml− 1, E. coli 50 μg ml− 1), spectinomycin (65 μg ml− 1) or carbenicillin (100 μg ml− 1).
Table 1. Bacterial strains used in this study.
Antibiotics were used at the following concentrations: for E. coli, carbenicillin (Carb) at 100 μg ml− 1 and kanamycin (Kan) at 50 μg ml− 1; for N. cinerea, kanamycin at 75 μg ml− 1 and spectinomycin (Spec) at 65 μg ml− 1.
| Strain | Relevant features | Reference |
|---|---|---|
| Neisseria cinerea | ||
| CCUG 346T (346T) | N. cinerea WT | Bennett et al. (2012) |
| CCUG 27178A (27178A) | N. cinerea WT | Bennett et al. (2012) |
| 346TΔpilE1 | Deletion mutagenesis, pilE1 deficient; KanR | This study |
| 346TΔpilE2 | Deletion mutagenesis, pilE2 deficient; KanR | This study |
| 346TΔpilE1/2 | Deletion mutagenesis, pilE1 and pilE2 deficient; KanR | This study |
| 27178AΔpilE1/2 | Deletion mutagenesis, pilE1 and pilE2 deficient; KanR | This study |
| 346TΔpilE1 : : pilE2 | pilE1 replaced with pilE2; KanR | This study |
| 346TΔpilD | Deletion mutagenesis, pilD deficient; SpecR | This study |
| 346TΔpilE1 : : pilE1-his | pilE1 replaced with pilE1 fused to a 3′ his-tag; KanR | This study |
| 346TΔpilE1 : : pilE2-his | pilE1 replaced with pilE2 fused to a 3′ his-tag; KanR | This study |
| 346T_pNCC1sfGFP(WTsfGFP) | sfGFP-expressing 346T | This study |
| 346TDpilE1/2_pNCC1sfGFP(ΔpilE1/2sfGFP) | sfGFP-expressing 346TΔpilE1/2 | This study |
| Neisseria meningitidis | ||
| 8013 | N. meningitidis WT | Rusniok et al. (2009) |
| 8013ΔpilE | Deletion mutagenesis, pilE deficient; KanR | Wörmann et al. (2014) |
| Escherichia coli | ||
| XL1Blue | Cloning strain | Agilent Technology |
| XL1Blue pUC19Kan | pUC19 containing kanamycin resistance marker | This study |
| XL1Blue pUC19ΔpilE1 | pilE1 deletion construct; CarbR KanR | This study |
| XL1Blue pUC19ΔpilE2 | pilE2 deletion construct; CarbR KanR | This study |
| XL1Blue pUC19ΔpilE1/2 | Construct for pilE1 and pilE2 deletion; CarbR KanR | This study |
| XL1Blue pUC19ΔpilE1 : : pilE2 | pilE2 placed under pilE1 promoter control; CarbR KanR | This study |
| XL1Blue pET21bpilE1 | pilE1 with 3′ his-tag under IPTG-inducible promoter control; CarbR | This study |
| XL1Blue pET21bpilE2 | pilE2 with 3′ his-tag under IPTG-inducible promoter control; CarbR | This study |
| XL1Blue pUC19ΔpilE1 : : pilE1-hisXL1Blue pUC19ΔpilE1 : : pilE2-his | pilE1 replaced with pilE1 fused to 3′ his-tag; CarbR KanR pilE1 replaced with pilE2 fused to 3′ his-tag; CarbR KanR | This study |
Sequence annotation and analysis
The genome sequences of the Neisseria strains analysed in this work are publicly available online in the PubMLST Neisseria BIGSdb (http://pubmlst.org/neisseria/). This database was developed by Keith Jolley and is sited at the University of Oxford (Jolley & Maiden, 2010). Homologues of pilE were identified in selected non-pathogenic Neisseria genomes using the database as described previously (Wörmann et al., 2014). Coding sequences flanking the putative pilE genes were identified based on homology to previously annotated sequences in the Integrated Microbial Genomes (IMG) database (https://img.jgi.doe.gov/cgi-bin/w/main.cgi). Putative bacterial promoter sequences were identified by manually inspecting pilE loci for highly similar sequences to the bacterial RpoD σ70 promoter consensus sequence ( − 35 box TTGACA and − 10 box TATAAT; Hawley & McClure, 1983) and to the RpoN (σ54)-dependent promoter consensus sequence ( − 24 TGGCA and − 12 TTGC) (Schaefer et al., 2015). Putative Rho-independent terminator sequences were identified using the online bioinformatic software ARNold (Naville et al., 2011). Amino acid alignments were carried out using the online analysis tool clustal w (http://www.ebi.ac.uk/Tools/msa/clustalw2). Homologues of other Tfp components were identified by blast analysis of the genome sequences in PubMLST using nucleotide sequences of pilus components described in N. meningitidis strain 8013 (Rusniok et al., 2009). Sequences were extracted and analysed manually to identify full-length coding sequence based on 8013 homologues. The amino acid sequences were derived using the online Emboss Transeq tool (http://www.ebi.ac.uk/Tools/st/emboss_transeq/). Alignment and identity matrices were obtained using clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/).
Strain construction
Primers used in this study are listed in Table 2. Plasmid pUC19ΔpilE1 was constructed for the deletion of pilE1 in N. cinerea. Approximately 500 bp of sequence 5′ and 3′ of pilE1 was amplified from CCUG 346T (346T) genomic DNA with primer pairs MW55/MW57 and MW59/MW56, respectively, and the ORF of a kanamycin resistance gene was amplified from pUC19Kan using primers MW60/MW58. The resultant products were fused by splice overlap extension PCR (SOE PCR) (Horton et al., 1989) using primers MW55/MW56. The final PCR product was digested with EcoRI/KpnI, ligated into pUC19, and recombinant plasmids transformed into cells of E. coli strain XL1 Blue (Agilent). Following digestion from pUC19 to remove plasmid-encoded beta-lactam resistance, the deletion construct was gel extracted and subsequently transformed into cells of N. cinerea 346T as follows: N. cinerea was harvested from overnight growth on solid media into PBS and a 10 μl aliquot was spotted onto BHI agar and allowed to dry. The purified pilE1 deletion construct (approx. 500 ng) was added to the bacteria and allowed to dry. Bacteria were incubated at 37 °C, 5 % CO2 for 5 h. Transformants were selected by streaking bacterial colonies onto BHI agar containing kanamycin. Genomic DNA was extracted and verified by PCR and sequencing, resulting in strain 346TΔpilE1.
Table 2. Primers used in this study.
| Number | Sequence |
|---|---|
| MW55 | GGTGAATTCACACCTGCCTGCCGAACACGGCGGC |
| MW56 | CCGGTACCCATTTTGCCGCAGGAGGCAGACCG |
| MW57 | AATATGGCTCATGGGATAACTCCTAAGTTTAAAGTTTAAGTG |
| MW58 | AGGAGTTATCCCATGAGCCATATTCAACGGGAAACGTCTTGC |
| MW59 | GAGTTTTTCTAAATAATAGGATTGGCGATTGGATAAGGCAGG |
| MW60 | CAATCCTATTATTTAGAAAAACTCATCGAGCATCAAATGAAAC |
| MW61 | GGTGAATTCCTGCCGCTTCTTTGGGTGGTTTG |
| MW62 | CCGGTACCGCAGCCTGCAACCCGGATTCACTC |
| MW63 | AAGAACAAACCTCAACCATCATCGATGAATTGTGTCTCAAAATC |
| MW64 | CGATGATGGTTGAGGTTTGTTCTTCATATTTATATTTTTTATGG |
| MW65 | TGGCATGCATCGGTCTGCCGCCGTTAAGCCCGTCCGTAATCCACG |
| MW66 | ACGGCGGCAGACCGATGCATGCCAACAGATAAAACGAAAGGCCCAG |
| MW67 | AAAGCAATCCAACAACCATCATCGATGAATTGTGTCTCAAAATC |
| MW69 | CGATGATGGTTGTTGGATTGCTTTCATGGGATAACTCCTAAG |
| MW207 | GCTAGCCGTTGCAGCAGTTG |
| MW208 | GTCCTCTACAAAGCGTTCTAC |
| MW229 | TGCCGCCGTTAAATAATAGGATTGGCGATTGGATAAGGCAGG |
| MW230 | AGGAGTTATCCCATGAAGAACAAACCTTACGGGTTTACGCTG |
| MW231 | CAATCCTATTATTTAACGGCGGCAGACGGCAGGCAGCGACCTC |
| MW232 | GGTGAATTCTCATACCAATCCATACCAACTCC |
| MW234 | TTTGTTCTTCATGGGATAACTCCTAAGTTTAAAGTTTAAGTG |
| CH11 | TAGCGGTTATACTTTCCCTGCAAA |
| CH12 | TAGGGTTTAGGCTCAGGTCG |
| CH13 | CCTCTATTTACAACCATCTGCCC |
| CH14 | GACCTCGGCTCTATCGTCC |
| CH15 | CGCCATATGAAAGCAATCCAAAAAGG |
| CH16 | ACGCCTCGAGGGGTTTAGGCTCAGGTCGGC |
| CH17 | CGCCATATGAAGAACAAACCTTACGG |
| CH18 | ACGCCTCGAGACGGCGGCAGACGGCAGGCAG |
| CH19 | AGGAGTTATCCCATGAAAGCAATCCAAAAAGGTTTCACCCTG |
| CH20 | GATTGCTTTCATGGGATAACTCCTAAGTTTAAAGTTTAAGTG |
| CH21 | CAATCCTATTATTTAGCAGCCGGATCTCAGTGGTGGTGGTGG |
| CH22 | TCCGGCTGCTAAATAATAGGATTGGCGATTGGATAAGGCAGG |
| GL146 | AACATGATGAGTGATCGTTAAATTTAACCGGAAAAGGCTTC |
| GL91 | CCAACCTGCCATCACGAGATTTCGATTCCACCGCCGCCTTTTACAGTACTTTTACGATGCTTTC |
| GL147 | CGGAATTGCGGCCGCGGAATTCTCATGTTTGACAGCTTATAGTCCCAATCCTGTAGCCCA |
| GL143 | GCCGGCCCTAGTGCTAGCGGATCCCTCAGACGGCATTTTATTTTGCTGTG |
| GL92 | AAGGCGGCGGTGGAATCG |
| GL140 | ATAAGCTGTCAAACATGAGAATTCCGC |
| GL145 | ATGCCGTCTGAAGCCTTTTCCGGTTAAATTTAACGATCACTCATCATGTTC- |
| GL153 | CTGGAAAGCGGGCAGTGAGGTACACGAAAAACAAGTTAAGG |
| GL144 | CACAGCAAAATAAAATGCCGTCTGAGGGATCCGCTAGCACTAGGGCCGGC |
| GL152 | TAACTTGTTTTTCGTGTACCTCACTGCCCGCTTTCCAGTC |
| GL95 | GTAAAACGACGGCCAGTGTTTAAACGAATTCGAGCTCGGTACCCGGGGATCCTCTAGAGTCGACCTGCAGGCATG-CAAGCTTTTAATTAA |
| GL96 | TTAATTAAAAGCTTGCATGCCTGCAGGTCGACTCTAGAGGATCCCCGGGTACCGAGCTCGAATTCGTTTAAACAC-TGGCCGTCGTTTTAC |
| GL23 | GGGGGCATGCATGAGCAAAGGAGAAGAACT |
| GL24 | GGGGCATATGTCATTTGTAGAGCTCatCCATGC |
| GL155 | TTGACAGCTAGCTCAGTCCTAGGTATAATGCTAGCCCAACATGTTACACAATAATGGAGTAATGAACATATGAGCA-AAGGAGAAGAAC |
| GL168 | GGGTTAATTAATTGACAGCTAGCTCAGTCC |
| GL169 | GGGTCTAGATTTCATTTGTAGAGCTCATCC |
Relevant restriction sites in primer sequences are underlined.
The same strategy was used to construct all N. cinerea mutants used in this study (Table 1), using specific primers. For the deletion of pilE2, approximately 500 bp of sequence corresponding to regions flanking pilE2 were amplified from genomic N. cinerea 346T DNA using MW64/MW61 and MW62/MW65, respectively, and a kanamycin resistance gene was amplified using primers MW66/MW63. PCR products were fused using primers MW61/MW62. To delete both pilE genes in N. cinerea ∼500 bp 5′ of pilE1 and ∼500 bp 3′ of pilE2 were amplified from 346T genomic DNA with primer pairs MW69/MW55 and MW62/MW65 and the kanamycin resistance gene was amplified using primers MW66/MW67. Primers MW55/MW62 were used in SOE PCR.
Strain 346TΔpilE1 : : pilE2 was constructed to study expression of pilE2 under the control of the pilE1 promoter. Approximately 1 kb upstream of pilE1 and the pilE2 coding sequence were amplified from 346T genomic DNA using primer pairs MW232/MW234 and MW230/MW231, respectively. A kanamycin resistance gene and a sequence region corresponding to ∼500 bp downstream of pilE2 were amplified from pUC19ΔpilE2 with primers MW229/MW62. The resultant PCR products were fused by PCR with primers MW232/MW62.
In order to detect PilE1 and PilE2 protein expression in N. cinerea we replaced the endogenous pilE1 ORF with a sequence encoding a C-terminal His-tagged pilin. First, plasmids pET21bpilE1 and pET21bpilE2 were constructed by amplifying pilE1 and pilE2 from 346T genomic DNA with primer pairs CH15/CH16 and CH17/CH18, respectively. The resultant PCR products were digested with NdeI/XhoI and ligated into pET21b (Millipore). Plasmids were transformed into cells of XL1Blue, yielding strains XL1Blue pET21bpilE1 and XL1Blue pET21bpilE2. Next, pilE1 and pilE2 fused to the 3′ sequence coding for a C-terminal His-tag were amplified from pET21bpilE1 and pET21bpilE2 using primer pairs CH19/CH21 and MW230/CH21. Approximately 1 kb 5′ of pilE1 was amplified from 346T genomic DNA using primer pair MW232/CH20 or MW230/CH21. A kanamycin resistance gene and a sequence region corresponding to ∼500 bp downstream of pilE2 were amplified from pUC19ΔpilE2 with primers CH22/MW62. These DNA fragments were fused by PCR using primers MW232/MW62. All strains were confirmed by PCR and sequencing (Source Bioscience).
Strain 346T_pNCC1sfGFP was constructed for the visualization of bacteria by fluorescence microscopy. Plasmid pNCC1 was designed based on plasmid pGCC4 previously used for complementation in N. gonorrhoeae (Mehr & Seifert, 1998). First, two regions of the N. cinerea 346T genome were amplified by PCR, using primers GL146/GL91 and GL147/GL143, respectively. The resulting fragments of 2137 and 1328 nt comprised ORFs NEIS0479–0481 and NEIS0482, respectively, to allow integration into the genome of N. cinerea through homologous recombination and insertion of alleles for complementation into the intergenic region between NEIS0481 and NEIS0482. Next, three fragments containing aph(3′)-II/neoR for kanamycin selection in E. coli, ermC for erythromycin selection in N. cinerea, and an IPTG-inducible lac promoter were amplified from plasmid pGCC4 (Addgene, plasmid no. 37058), with primers GL92/140, GL145/153 and GL144/152, respectively. All amplifications were carried out using Q5 DNA polymerase (NEB). The five PCR products were purified and assembled using Gibson Assembly (NEB and Gibson et al., 2009). This resulted in plasmid pNCC0, which was then digested with PmeI and PacI (NEB). Primers GL95 and GL96, which form a multiple cloning site based on sequences from pUC19, were annealed and digested with PmeI and PacI, prior to ligation into pNCC0, giving rise to pNCC1 (Fig. S1, available in the online Supplementary Material). To generate pNCC1-sfGFP, the ORF encoding superfolder GFP was amplified from plasmid pXG10-SF (Corcoran et al., 2012; a kind gift from Dr Yanjie Chao and Professor Dr Jörg Vogel, University of Würzburg) and cloned into pGEM-T Easy (Promega). To achieve sufficient expression of chromosomally encoded sfGFP for visualization of bacteria by fluorescence microscopy, first, sfGFP was amplified from pGEMTsfGFP using primers GL155/GL169 to add a constitutive promoter BBa_J23119 (Anderson collection, iGEM; http://parts.igem.org/Promoters/Catalog/Anderson) and a strong 5′ UTR LII-11 (Berg et al., 2009). This product was then amplified using primers GL168/GL169 to introduce restriction sites for PacI and XbaI. Following restriction digest, the fragment was ligated into pNCC1 digested with the same enzymes. All plasmids were verified by sequencing. Strain 346T_pNCC1sfGFP was made by transformation of 346T with BglII-linearized, purified pNCC1sfGFP plasmid. Chromosomal DNA isolated from strain 346TΔpilE1/2 was used to move the ΔpilE1/2 deletion into 346T_pNCC1sfGFP, resulting in strain 346TΔpilE1/2_pNCC1sfGFP. All strains were confirmed by antibiotic resistance, PCR and sequencing.
DNA competence assay
Transformation reactions were performed on BHI agar. Bacteria were harvested from overnight growth on solid media and resuspended in PBS. An aliquot of bacteria (107 c.f.u. in 10 μl) was spotted on BHI agar and 5 μg of chromosomal DNA extracted from a spectinomycin-resistant N. cinerea pilD mutant (unpublished) was added to the bacteria. Plates were incubated at 37 °C, 5 % CO2 for 5 h. Bacteria were recovered by scraping from the plate and resuspending in PBS to a final concentration of 5 × 108 c.f.u. ml− 1. Dilutions were plated to BHI agar and spectinomycin-containing BHI agar. The transformation frequency was determined by comparing the number of transformants with the total number of plated bacteria and expressed as a percentage. Experiments were repeated on three independent occasions.
RNA isolation and Northern blotting
Liquid cultures of N. cinerea grown overnight were diluted 1 : 100 in 20 ml BHI, and grown at 37 °C with shaking until reaching an OD600 of ∼0.5. Bacteria were pelleted by centrifugation for 10 min at 4369 g and 4 °C. The supernatant was discarded and total RNA was isolated using the Fast RNA Blue kit (MP Biomedicals) followed by DNase treatment (Roche). Total RNA (20 μg) was separated in 1.5 % formaldehyde gels in HEPES buffer, transferred to a Hybond-N membrane and cross-linked by UV light. Probes for detecting pilE1 and pilE2 transcripts were generated by PCR with primers CH11/CH12 and CH13/CH14. Control probes to N. cinerea tmRNA were generated by PCR with primers MW207/MW208. Probes were end-labelled with γ-ATP (Perkin Elmer). Membranes were prehybridized in hybridization buffer (GE Healthcare) for 1 h at 60 °C and subsequently incubated with labelled probes overnight at 60 °C in hybridization buffer. The following day, membranes were washed once with 2 × SSC (1 × SSC: 150 mM NaCl, 15 mM Na3C6H5O7, pH 7)/0.1 % Tween 20 and twice with 0.5 × SSC/0.1 % Tween 20, and signals were detected using a fluorescence image analyser (Fuji, FLA-5000). RNA isolation and Northern blotting was performed on three independent occasions.
Western blotting
To detect His-tagged pilins in N. cinerea, the OD600 of an overnight culture was taken prior to collecting 1 ml of culture by centrifugation at 21 100 g for 5 min. Proteins in the supernatant were precipitated with a final concentration of 10 % TCA (supernatant fraction). The remaining bacterial pellet was resuspended in SDS-PAGE sample buffer and samples were normalized to OD600 readings (1 ml of a culture with an OD600 reading of 1 was resuspended in 100 μl SDS-PAGE sample buffer) (cell fraction). TCA-precipitated and acetone-washed protein pellets from the supernatant fraction were resuspended in 2 × SDS-PAGE sample buffer and normalized to OD600 as described above. All samples were boiled for 30 min, centrifuged at 21 100 g for 5 min and 10 μl aliquots were separated in 12 % SDS-PAGE gels. Proteins were transferred to a PVDF membrane (Millipore). Membranes were blocked with 5 % milk in 0.1 % Tween in PBS (PBST). Antibody incubation was performed in PBST. For the detection of His-tagged pilin proteins, a horseradish peroxidase (HRP)-conjugated anti-His antibody (Sigma) was used at a 1 : 10 000 dilution. For the detection of RecA, anti-RecA antibody (Abcam; 1 : 5000) followed by goat anti-rabbit HRP-conjugated IgG (Santa Cruz Technology; 1 : 10 000) was used. Cross-reactivity was visualized using an ECL detection kit (GE Healthcare). The experiment was performed three times.
Cell culture and adhesion assays
Human bronchial epithelial A549 cells were cultured in DMEM (Sigma) containing 10 % FCS (Sigma) and Detroit 562 human pharyngeal carcinoma epithelial cells were cultured in EMEM (Sigma) supplemented with 1 % non-essential amino acids (Sigma), 2 mM l-glutamine (Sigma), 1 mM sodium pyruvate (Sigma), 0.1 % lactalbumin hydrolysate (Sigma) and 10 % FCS. Cells (5 × 105) were seeded in 24-well plates (Corning Life Sciences, ref. 3524) and incubated at 37 °C with 5 % CO2. Prior to infection, cells were washed three times with culture medium. N. cinerea or N. meningitidis strains were harvested from overnight growth on BHI agar, resuspended in PBS and diluted in culture medium to give 1.5 × 107 c.f.u. ml− 1. The concentration of all bacterial suspensions was verified by plating serial dilutions to solid media. Bacteria suspensions (1 ml) were added to cells at an m.o.i. of 30, and incubated at 37 °C and 5 % CO2 for 30 min, 1.5 h or 3 h. Infected cells were washed three times with PBS to remove non-adherent bacteria. Cells were lysed with 1 ml 1 % saponin-PBS at 37 °C and 5 % CO2 for 10 min and the number of cell-associated bacteria was determined by plating serial dilutions onto solid media. To compare adherence of N. cinerea to different surfaces, glass coverslips (VWR, ref. 631-0146) and plastic coverslips (Sarstedt, ref. 83.1840.002) were placed in a 24-well plate. A549 or Detroit 562 cells (5 × 105) were seeded onto glass coverslips in a 24-well plate and incubated at 37 °C with 5 % CO2. Prior to infection, cells were washed three times with culture medium. Bacterial suspensions were prepared as described above and added to cells or glass or plastic coverslips and incubated at 37 °C and 5 % CO2 for 1.5 h. Coverslips were washed once with PBS, transferred to a new well and washed a further three times with PBS. Cells were incubated with 1 ml 1 % saponin-PBS at 37 °C and 5 % CO2 for 10 min and appropriate dilutions were plated to solid media. Adherence was expressed as percentage of recovered bacteria compared with input. Experiments were repeated on three independent occasions.
Electron microscopy
For visualization of surface fibres by negative staining, N. cinerea strains were grown on BHI agar overnight at 37 °C in the presence of 5 % CO2 and Formvar coated grids were touched onto single colonies. Grids were washed three times for 2 min on 10 μl drops of water, before staining for 30 s with 1 % uranyl acetate. Grids were air-dried and then viewed using an FEI Tecnai 12 transmission electron microscope operated at 120 kV. Images were captured with a 4 megapixel Gatan Ultrascan 1000 CCD camera. ImageJ was used to estimate the diameter of fibres by calculating the mean of ten independent measurements across the fibre, using a minimum of three fibres per image.
For scanning electron microscopy (SEM), A549 cells were cultured on glass coverslips as described above and infected for 1.5 h at an m.o.i. of 30. Coverslips with infected cells were washed three times with PBS and fixed for 20 min with 1 ml of PBS containing 0.5 % glutaraldehyde (AppliChem) and 2 % paraformaldehyde (Sigma). The coverslips were washed three times with PBS, then stained with 1 % osmium tetroxide (TAAB Laboratories) in PBS for 1 h, washed three times with deionized water and taken through an ethanol dehydration series (30, 50, 70, 80, 90, 95 % ethanol for 5 min each, then 100 % ethanol three times for 20 min). Coverslips were dried for 3 min with hexamethyldisilazane (Sigma), then mounted on carbon tape onto an SEM stub and coated with ∼7.5 nm gold using a Q150R ES sputter coater (Quorum Technologies). Images were taken using a Zeiss Merlin Compact Field Emission Gun SEM operated at 3.5 kV.
Fluorescence microscopy
For visualization of bacterial interaction with cells by fluorescence microscopy, A549 cells were seeded onto glass coverslips as described above at a density of 105 cells per well. Cells were inoculated with bacteria at an m.o.i. of 30 and incubated at 37 °C in 5 % CO2 for 1.5 h. Cells were then washed three times with PBS and fixed for 20 min with 1 ml of PBS containing 3 % paraformaldehyde. Coverslips were washed in PBS/0.05 % Tween 20 and incubated for 1 h in PBST containing 2 % BSA as a blocking agent. Cells were visualized using DAPI (Sigma; 1 : 500 dilution) to stain nuclei and Alexa Fluor 647-conjugated phalloidin (Life Technologies; 1 : 40 final dilution) to stain actin. Images were taken using a CCD3 inverted Zeiss microscope. For quantitative analysis of microcolony formation, at least 300 cells were analysed and adherent microcolonies (defined as a bacterial clump containing either ≤ 5 bacteria or >5 bacteria) were quantified. Experiments were repeated on three independent occasions.
Statistical analysis
Error bars on all graphs show the standard deviation. Statistical significance was calculated using Prism6 unpaired Student's t-test. A P value < 0.05 was considered statistically significant.
Results
Organization and analysis of the pilE locus in N. cinerea
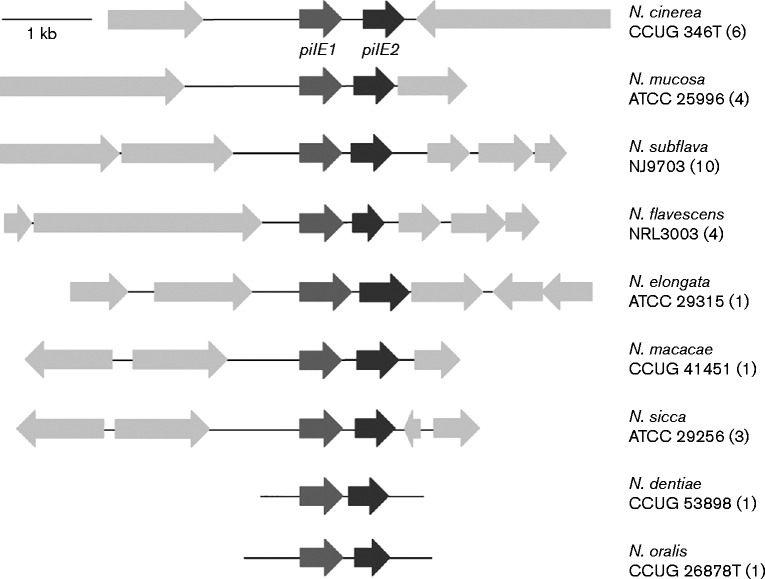
In a previous study we analysed sequences related to pilE in genomes of pathogenic and non-pathogenic Neisseria belonging to 14 different species. We showed that a single pilE gene was present in N. lactamica and Neisseria polysaccharea (a feature shared with N. meningitidis and N. gonorrhoeae), but that nine other species of Neisseria have two putative pilE genes (Wörmann et al., 2014). Analysis of the genomic context of the putative pilE genes in these species revealed that they are located adjacent to each other (Fig. 1). Furthermore, analysis of the adjacent ORFs showed that the flanking sequences are not conserved between the different non-pathogenic Neisseria species and do not resemble those adjacent to either the class I or class II pilE in N. meningitidis.
Fig. 1.
Schematic representation of the pilE locus in nine commensal Neisseria species. Putative pilE genes are highlighted, and predicted ORFs in the regions flanking pilE are shown. A representative isolate is shown for each species and the isolate name is given. The number of isolates analysed within each species is shown in parentheses. Bar, 1 kb.
We therefore sought to determine the contribution of the two putative pilE genes to Tfp formation and function. We selected N. cinerea for further study, as this species is found in the human nasopharynx, similar to N. meningitidis (Knapp & Hook, 1988). We analysed six genome sequences of N. cinerea isolates available in PubMLST (http://pubmlst.org/neisseria/). Of these, one isolate (isolate CCUG 5746) had a single pilE gene which clustered phylogenetically with pilE from N. lactamica, N. polysaccharea and class II meningococci (Wörmann et al., 2014). The remaining five N. cinerea isolates possessed two putative pilE genes located in tandem. Both ORFs encode a predicted protein of 147–158 aa with characteristic features of type IV pilins (Craig et al., 2004) (i.e. a class III signal peptide, a hydrophobic N-terminal domain, a phenylalanine at position one and a glutamate residue at position five of the mature protein, as well as a D-region in the C terminus) (Fig. S2). The predicted signal peptide sequence of the first protein (PilE1) from the five N. cinerea isolates was identical to the signal peptide from N. meningitidis class II pilins (MKAIQKG) (Wörmann et al., 2014); the second protein (PilE2) possessed a slightly different signal peptide (MKNKPYG). The length of the predicted D-region was between 14 and 16 aa in both proteins and both lacked the motif recognized by mAb SM1 (EYYLN) at the end of the conserved N terminus (Virji et al., 1989). The size of the D-region and the absence of an SM1 motif are also features shared with class II pilins from meningococci. Comparison of the amino acid sequence of the putative pilins from the five N. cinerea isolates revealed that PilE1 displays some sequence diversity within the C-terminal region (Fig. S2a). In contrast, PilE2 is highly conserved over the full-length of the protein (Fig. S2b).
Finally we analysed the regions 5′ and 3′ of the putative pilE genes in the five N. cinerea isolates (Fig. S3). Predicted Rho-independent terminator sequences were found on both strands downstream of pilE1 and pilE2. A sequence closely resembling the consensus RpoN (σ54)-dependent promoter was identified upstream of pilE1 in all isolates, consistent with previous data (Rendón et al., 2013), but this was not found adjacent to pilE2. We also identified elements resembling RpoD (σ70)-dependent − 10 and − 35 bacterial promoter sequences immediately upstream of both pilE1 and pilE2, although the sequence 5′ of pilE2 was less conserved between isolates, with one (CCUG 28662) lacking a consensus − 35 sequence upstream of pilE2. A putative ribosome-binding site (RBS) was identified eight nucleotides upstream of the putative start codon (ATG) of the pilE1 ORF, but no obvious RBS was identified upstream of the putative pilE2 ORF.
Identification of Tfp genes in N. cinerea genomes
Previous analysis of Tfp in N. meningitidis has identified 15 proteins essential for pilus biogenesis (PilC1/2, D, E, F, G, H, I, J, K, M, N, O, P, Q and W) and seven additional proteins that contribute to Tfp functions (PilT, T2, U, V, X, Z and ComP) (Brown et al., 2010; Carbonnelle et al., 2005). We therefore performed blast analysis of the genomes of the five N. cinerea isolates to identify homologues of these genes. As shown in Table S1, all the genes were found in each strain, except that only one pilC gene was identified. The coding sequences were extracted and translated, and putative proteins were aligned using Clustal Omega (Fig. S4). The putative PilC in N. cinerea isolates was shorter than the meningococcal PilC1 protein and had less than 50 % amino acid sequence identity. The remaining N. cinerea pilus components had at least 70 % amino acid sequence identity to their counterparts in N. meningitidis 8013. Homologues of all the proteins that have motifs of type IV pilins (i.e PilH, PilI, PilJ, PilK as well as PilX, PilV and ComP; Berry & Pelicic, 2015) were identified in the genomes of all five strains and in each case these were distinct from the two pilE homologues found in each strain.
Pilin transcription and translation in N. cinerea CCUG 346T
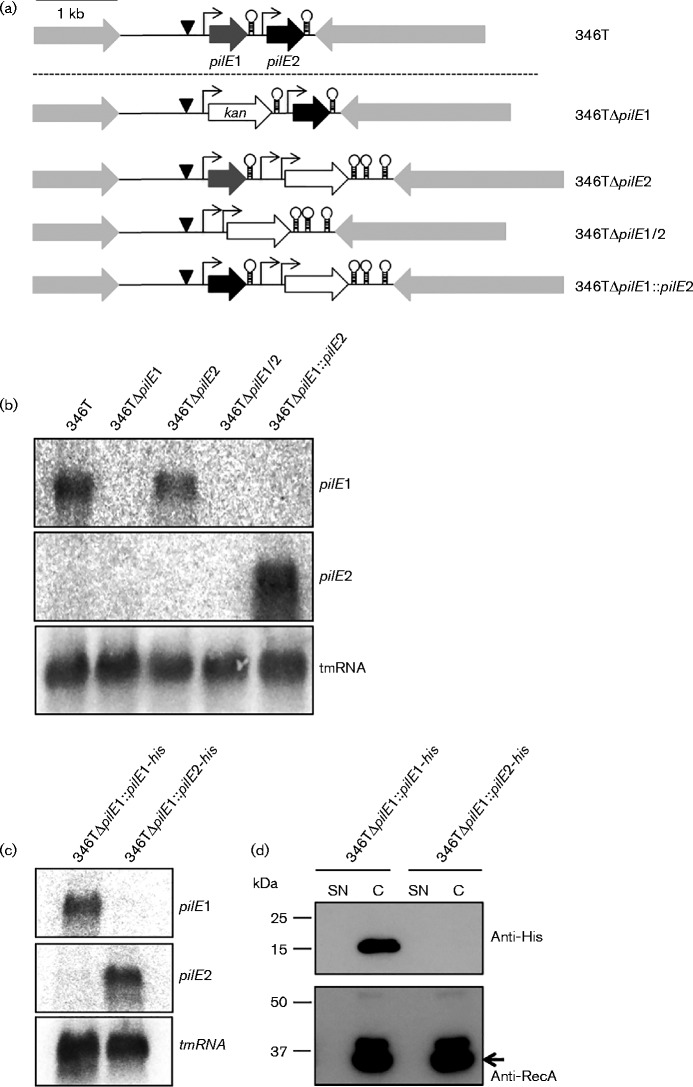
To analyse the transcription and translation of each putative pilE gene, we first constructed strains lacking each or both gene(s). For this, we chose isolate CCUG 346T (Fig. 2a) and examined the expression of pilE1 and pilE2 transcripts in the WT and mutants by Northern blotting (Fig. 2b). We designed probes which hybridized to unique regions within each sequence and which were therefore specific for each transcript. We observed a single transcript for pilE1 in the WT strain and in 346TΔpilE2, which was absent from 346TΔpilE1 and 346TΔpilE1/2, indicating that the pilE1 gene is expressed and that the absence of pilE2 does not impact the transcription of pilE1. In contrast, we were unable to detect a transcript for pilE2, even in the WT strain, suggesting that the two pilin genes are not co-transcribed, and that pilE2 is not transcribed under the conditions tested. To rule out inefficacy of the pilE2 probe, we constructed a strain in which we replaced the pilE1 coding sequence with the coding sequence from pilE2, thus placing pilE2 under control of the pilE1 promoter (Fig. 2a). In this strain, we were able to detect a pilE2 transcript (Fig. 2b), suggesting that in WT N. cinerea 346T, pilE2 may only be transcribed under specific conditions, or that RNA transcripts expressed from the pilE2 promoter are unstable.
Fig. 2.
Analysis of pilE1 and pilE2 transcript and protein expression in N. cinerea CCUG 346T. (a) Schematic representation of the WT pilE locus in N. cinerea 346T and isogenic mutants used to analyse transcript expression. Putative promoters are indicated by arrows (σ70) and triangles (σ54), and Rho-independent terminators by stem–loops. A kanamycin resistance cassette with promoter and bidirectional rrnB terminators was used for the construction of mutants, except for 346TΔpilE1, in which only the ORF was used. Bar, 1 kb. (b) Northern blotting of total RNA from N. cinerea 346T WT and mutants. pilE1 transcript was detected in both the WT and 346TΔpilE2. pilE2 transcript was undetectable in the WT under the conditions tested but was detected when expressed from the pilE1 promoter, in 346TΔpilE1 : : pilE2. tmRNA was used as loading control. (c) Northern blot analysis of total RNA from 346TΔpilE1 : : pilE1-his and 346ΔpilE1 : : pilE2-his. pilE1 and pilE2 transcripts were detected, respectively. tmRNA was used as loading control. (d) Detection of His-tagged PilE1 and PilE2 in supernatant (SN) and cell extracts (C) from strains 346TΔpilE1 : : pilE1-his and 346TΔpilE1 : : pilE2-his. RecA detection was used as loading control. No signal was obtained for PilE2.
The predicted proteins encoded by pilE1 and pilE2 have a molecular mass of 15.2 and 15.9 kDa respectively, and share 37 % sequence identity. We were unable to detect any proteins of this size in whole-cell extracts from WT N. cinerea using a panel of antibodies which recognize meningococcal class I or class II pilins including SM1, anti-PilE peptide antisera and murine sera against meningococcal pilin proteins (Wörmann et al., 2014; data not shown). Therefore, to examine pilin protein expression in 346T, we constructed strains in which pilE1 was replaced with either pilE1 or pilE2 fused to a 3′ sequence encoding a poly-histidine tag. Transcription of pilE1-his or pilE2-his was confirmed by Northern blotting (Fig. 2c) and whole-cell extracts were analysed by Western blotting using anti-His-tag antibody (Fig. 2d). A protein of the expected size for His-tagged PilE1 protein was detected in extracts of 346TΔpilE1 : : pilE1-his. However, no protein corresponding to the predicted size of His-tagged PilE2 was visible in the cell fraction of 346TΔpilE1 : : pilE2-his. To investigate whether PilE2 is secreted, we also analysed the culture supernatants. No signal could be detected in the culture supernatants of 346TΔpilE1 : : pilE2-his (Fig. 2d).
pilE2 is not required for Tfp expression in N. cinerea CCUG 346T
We next tested whether we could visualize Tfp fibres on the surface of N. cinerea cells. Using electron microscopy we were able to detect cell surface fibres in WT N. cinerea 346T and in a strain lacking pilE2 (Fig. 3a). ImageJ analysis estimated a mean diameter of these fibres of 5.4 ± 1 nm, which is consistent with the diameter of Tfp (Berry & Pelicic, 2015). No fibres were visible on the strain lacking pilE1 or the double mutant (not shown). Taken together, these data indicate that N. cinerea 346T produces Tfp and that, of the two putative pilins, PilE1 is sufficient for fibre formation.
Fig. 3.
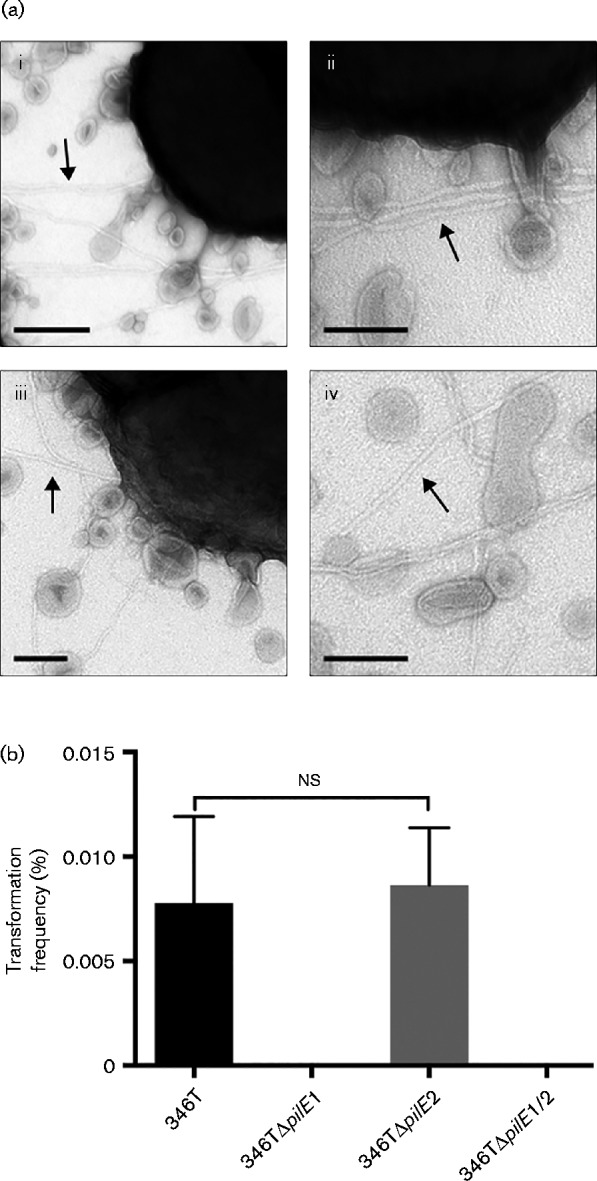
WT N. cinerea strain 346T and 346TΔpilE2 express Tfp. (a) Negative-staining transmission electron microscopy of Tfp in WT (i, ii) and 346TΔpilE2 mutant (iii, iv). Filaments consistent with Tfp were evident on the surface of both strains. A large number of membranous bleb-like structures were also visible. Bars, 200 nm (i) and 100 nm (ii–iv). (b) N. cinerea strains were transformed using chromosomal DNA isolated from a spectinomycin-resistant 346T ΔpilD mutant. The number of transformants compared with the number of recipient cells is shown as a percentage. Results are the mean+sd of three independent experiments. No transformants were obtained for 346TΔpilE1 and 346TΔpilE1/2. ns, Not significant (unpaired Student's t-test, P = 0.7822).
N. cinerea CCUG 346T is naturally transformable and pilE2 is dispensable for DNA competence
Tfp mediate DNA uptake and transformation in both pathogenic and commensal Neisseria (Chen & Dubnau, 2004; Higashi et al., 2011; Seifert et al., 1990). To test whether Tfp are necessary for DNA competence in N. cinerea and which putative pilE gene(s) are required for this event, we transformed 346T WT, 346TΔpilE1, 346TΔpilE2 and 346TΔpilE1/2 with genomic DNA isolated from a spectinomycin-resistant N. cinerea strain (Fig. 3b). Transformants that were resistant to spectinomycin could be recovered from the WT and from the 346TΔpilE2 strain with similar frequencies (WT 0.0078 ± 0.004134 %; 346TΔpilE2 0.0086 ± 0.002743 %), consistent with both the lack of detectable expression of PilE2 in the WT and the ability of the 346TΔpilE2 mutant to express Tfp. No transformants were obtained from 346TΔpilE1 or 346TΔpilE1/2 in agreement with the lack of detectable Tfp on the surface of these mutants. In summary, these data suggest that Tfp play an important role in N. cinerea DNA transformation and that, of the two putative pilins, pilE1 is required for DNA competence in 346T.
N. cinerea CCUG 346T binds to human epithelial cells in a pilus-independent manner
Tfp are important adhesive organelles in many organisms. In pathogenic Neisseria, they are required for the initial interaction with host cells, and pilus-deficient mutants of N. meningitidis and N. gonorrhoeae are markedly impaired in adherence (Carbonnelle et al., 2009; Virji et al., 1991). To analyse the adhesive properties of N. cinerea we examined the association of N. cinerea 346T and 346TΔpilE1/2 to human respiratory epithelial cells. In addition, we tested whether N. cinerea adheres to abiotic surfaces. We infected human bronchial epithelial A549 cells, human pharyngeal epithelial Detroit 562 cells, and glass or plastic coverslips for 1.5 h and quantified bacteria after washing. Low levels of the WT and pilus mutant could be recovered from either glass or plastic coverslips. Strikingly, both the N. cinerea WT and the pilus-deficient mutant adhered in equal levels to both A549 and Detroit 562 cells (Fig. 4a). To investigate this further, we analysed binding of N. cinerea to Detroit 562 cells infected for different lengths of time. In addition, as a control for Tfp-dependent adhesion, we included the N. meningitidis WT strain 8013 and the corresponding pilus-deficient mutant 8013ΔpilE. Infected cells were incubated for 30 min, 1.5 h or 3 h, and cell-associated bacteria were quantified by plating. As shown in Fig. 4(b), after 0.5 h of infection, the level of cell association of WT N. cinerea was slightly higher than that of WT N. meningitidis. After 1.5 h infection, the adhesion of N. cinerea was similar to WT N. meningitidis, while following a longer incubation (3 h), the number of N. meningitidis associated with cells increased but there was no further increase in the association of N. cinerea. As expected, the pilus-deficient strain of N. meningitidis was strongly impaired in adherence compared with the WT at each time. In contrast, the pilus-deficient mutant 346TΔpilE1/2 consistently showed slightly higher levels of adherence to Detroit 562 cells compared with the WT, confirming our finding that adhesion of this strain is Tfp-independent. To rule out that different growth rates or saponin sensitivity affected the recovery of bacteria, we followed the growth of N. cinerea WT and the pilE1/2 mutant in culture medium over a time period of 3 h and tested survival in 1 % saponin. There was no significant growth difference between 346T WT and the mutant and 1 % saponin did not affect the viability of 346T or 346TΔpilE1/2 (data not shown).
Fig. 4.
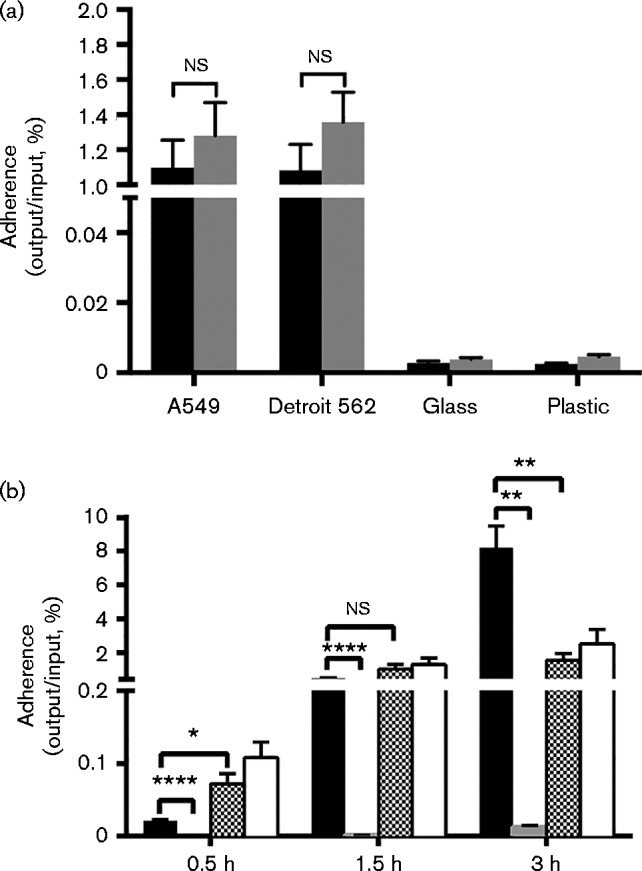
Adherence of N. cinerea CCUG 346T WT and pilus-deficient mutant to biotic and abiotic surfaces. (a) A549 cells, Detroit 562 cells and glass or plastic coverslips were infected for 1.5 h with N. cinerea 346T WT (black bars) or 346TΔpilE1/2 (grey bars). Results are the mean+sd of three experiments. ns, Not significant. (b) Detroit 562 cells were infected for 0.5, 1.5 or 3 h with 346T (chequered bars) or 346TΔpilE1/2 (white bars). Cell-associated bacteria were quantified by plating. N. meningitidis 8013 (black bars) and the corresponding pilE mutant (grey bars) were used as a control for Tfp-dependent adherence. Results are the mean+sd of three experiments. ns, Not significant; asterisks indicate significance (unpaired Student's t-test; *P = 0.01–0.05, **P = 0.001–0.01, ****P < 0.0001).
Microscopy analysis of adhesion of N. cinerea CCUG 346T and CCUG346TΔpilE1/2 to epithelial cells
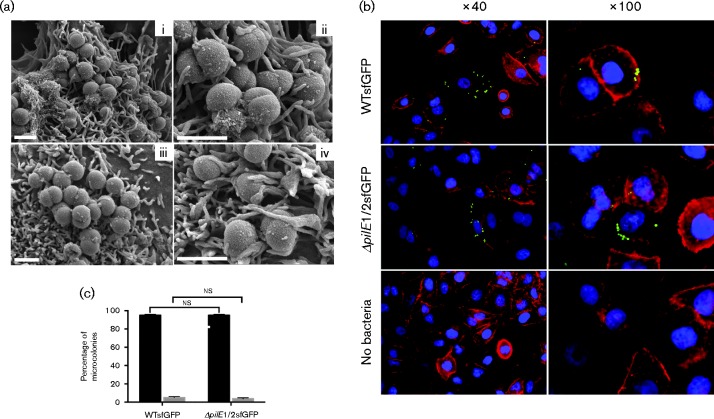
In light of our finding that N. cinerea 346T binds to epithelial cells in a Tfp-independent manner, we also examined the adhesion of the WT and mutant by microscopy. The use of SEM demonstrated that both strains were present on the surface of the cells. In each case, single bacteria, diplococci or bacteria in microcolonies could be seen in close contact with cellular protrusions that were similar in size and aspect to microvilli (Fig. 5a). We also constructed strains of 346T and the pilE1/2 mutant expressing GFP for analysis by fluorescence microscopy. A549 cells were infected with either WT or pilE1/2 mutant and at 1.5 h post-infection cells were fixed and stained with DAPI and phalloidin to visualize nuclei and actin, respectively. Again, for each strain, diplococci or small microcolonies were clearly visible (Fig. 5b). To quantify this we determined the number of adhesion events (i.e. the number of individual or groups of bacteria) visible in 10 fields (300 cells in total per strain) and ascertained the size of the microcolonies (Fig. 5c). Approximately equal numbers of adhesion events were observed in total for each strain (171 for 346T and 190 for 346TΔpilE1/2), and in each case >90 % of these were small microcolonies composed of fewer than five bacteria. Thus, microscopy analyses also indicated there are no differences in adhesion of the WT N. cinerea and the corresponding strain lacking Tfp.
Fig. 5.
Microscopy analysis of N. cinerea binding to human epithelial cells. (a) SEM analysis of A549 cells infected with WT 346T (i, ii) or 346TΔpilE1/2 (iii, iv) for 1.5 h. Bacterial microcolonies in close association with host cells could be observed for both the WT strain and pilus-deficient mutant. Both strains appear to form intimate contact with cellular protrusions. Bars, 1 μm. (b) A549 cells were infected with WT 346T or 346TΔpilE1/2 expressing GFP at an m.o.i. of 30. At 1.5 h post-infection, cells were fixed and stained for nuclei (blue) and actin (red). Both WT and mutant could be clearly visualized on cells. Images were taken using a CCD3 inverted Zeiss microscope. (c) Quantitative analysis of microcolony formation. At least 300 cells were analysed per experiment and the number of bacteria per microcolony was expressed as a percentage. Black bars correspond to microcolonies consisting of ≤ 5 bacteria; grey bars correspond to microcolonies of >5 bacteria. WT and pilE1/2 mutant formed microcolonies of similar size. ns, Not significant.
Tfp-independent adhesion is also a property of other N. cinerea strains
Finally, we determined whether Tfp-independent binding to epithelial cells is a property shared by other N. cinerea isolates. Isolate CCUG 27178A (27178A) was also shown to express fibres consistent with Tfp by electron microscopy (Fig. S5) and the pilE region of this isolate has 92 % nucleotide identity with the region from 346T (Fig. S3). PilE1 and PilE2 share 76 and 98 % identity with the corresponding proteins from 346T, respectively. A549 cells were infected with either N. cinerea 27178A WT or mutant 27178AΔpilE1/2. N. meningitidis strains 8013 and 8013ΔpilE, as well as 346T and 346TΔpilE1/2 were included as controls. Infected cells were incubated for 1.5 h and cell-associated bacteria were quantified by plating. The N. cinerea 27178A isolate displayed a similar pilus-independent adhesion to A549 epithelial cells, as loss of pilE1/2 did not impact cell association (Fig. 6).
Fig. 6.
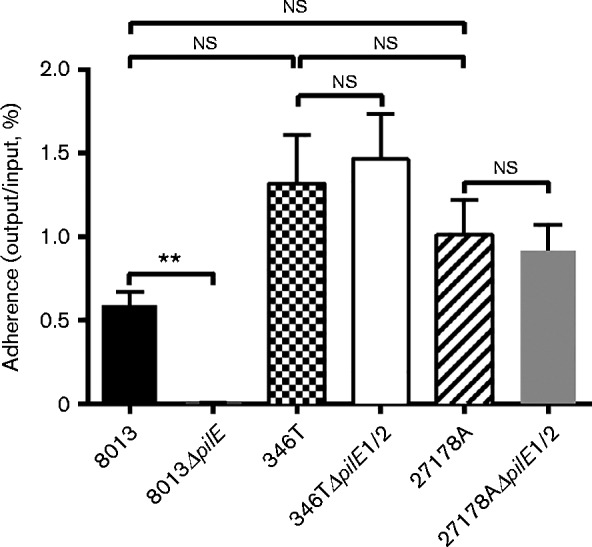
N. cinerea CCUG 27178A can also bind to A549 cells independently of pilE. The N. cinerea WT isolate 27178A (black/white striped bar) and a mutant lacking both putative pilE genes (27178ΔpilE1/2, grey bar) were used to investigate the adhesion of a second N. cinerea strain to A549 cells. N. meningitidis 8013 (black bar) and isogenic pilus-deficient mutant (8013ΔpilE) were used as a control for Tfp-dependent adherence and 346T (black/white chequered bar) and 346TΔpilE1/2 (white bar) were included for comparison. Results show the mean+sd of data from five independent experiments. Statistical significance was calculated using an unpaired Student's t-test. 8013ΔpilE was impaired for adhesion (**P = 0.001–0.01); ns, not significant.
Discussion
Tfp biogenesis genes have been identified in both pathogenic and commensal Neisseria species (Marri et al., 2010). PilE is the major constituent of Tfp and some commensal Neisseria have been reported to contain two putative pilE genes (Aho et al., 2005; Marri et al., 2010). Here, we present evidence that, of the two putative pilE genes in N. cinerea 346T, pilE1 is transcribed and does not appear to form a co-transcriptional unit with pilE2. Transcription of pilE2 was not detected in the WT strain in the conditions tested, suggesting that pilE1 and pilE2 are differentially regulated and pilE2 may only be expressed under certain conditions. pilE transcription is regulated by integration host factor, RpoN and Neisseria pil activator, Npa, in N. elongata (Rendón et al., 2013). RpoN recognition sequences were identified upstream of pilE1 in N. cinerea; however, these sequence elements were absent from the region 5′ to pilE2, consistent with our hypothesis that if pilE2 is expressed, it may be subject to a distinct regulatory mechanism. Interestingly, analysis of the sequence upstream of pilE2 did not reveal an obvious RBS, which questions whether PilE2 protein expression occurs at all in WT N. cinerea. However, pilE2 is highly conserved in different N. cinerea isolates and a number of different commensal Neisseria species contain a second pilE gene, suggesting it may have a function. Replacing the coding sequence of pilE1 with that of pilE2, and hence placing pilE2 under pilE1 promoter control, resulted in a detectable pilE2 transcript by Northern blotting, but we were still unable to detect any His-tagged PilE2 protein in this strain. This suggests that post-transcriptional mechanisms affect pilE2 mRNA or protein stability, and supports the hypothesis that this gene and/or protein may only be stably expressed in specific conditions.
Despite the absence of detectable pilE2 expression, we observed fibres on the surface of WT N. cinerea by electron microscopy, demonstrating that this gene is dispensable for Tfp formation. Moreover, WT N. cinerea 346T and 346TΔpilE2, but not the pilE1 mutant, were transformable. As pilE2 appears to be dispensable for fibre formation and genetic transformation, the role of this gene product remains to be determined. One possibility is that, rather than encoding a major subunit (PilE), it may be an accessory pilin that is not required for fibre synthesis. In pathogenic Neisseria, seven proteins have sequences characteristic of pilin subunits. PilH, PilI, PilJ and PilK are essential for pilus biogenesis, and the minor pilins PilV, PilX and ComP are incorporated into the Tfp fibre and contribute to specific functions (Brown et al., 2010; Carbonnelle et al., 2005, 2006). For example, PilX is required for the formation of bacterial aggregates, ComP is essential for DNA competence and PilV triggers reorganization of the host cell surface (Aas et al., 2002; Hélaine et al., 2005; Mikaty et al., 2009; Wolfgang et al., 1999). Mutants of the minor pilins PilX, PilV and ComP display Tfp on the bacterial surface but show defects in specific Tfp-linked properties (Brown et al., 2010). Genome sequence analysis has demonstrated that N. cinerea harbours genes encoding homologues of all the type IV pilins (Marri et al., 2010) and we confirmed this using blast analysis of the genomes of N. cinerea strains used in this work. Genes encoding proteins with at least 71 % identity to each of these 8013 pilin-like proteins (PilH, I, J, K and PilX, PilV and ComP) were identified in all genomes. Thus, based on the presence of motifs that define type IV pilins, PilE2 may be an additional pilin in N. cinerea, which confers specific functions rather than being required for Tfp formation. Another possibility is that pilE2 is a pseudogene or an ancestral version of pilS, the silent pilin sequences which serve as donors for recombination and generation of pilin antigenic variants in some strains of N. meningitidis or N. gonorrhoeae (Haas & Meyer, 1986; Hagblom et al., 1985). Our inability to detect expression of this gene in WT N. cinerea provides some support for this.
A comparison of adhesion of N. cinerea to abiotic surfaces and to human epithelial cells revealed that the bacteria adhere most effectively to the cells. Interestingly, the pilE mutant of N. cinerea 346T reproducibly showed no defect in association to two different human epithelial cell lines; both the WT and the pilus-deficient mutant associated with cells at similar levels to the WT meningococcal strain 8013. These findings are distinct from those with pathogenic Neisseria, in which mutants lacking Tfp are significantly impaired in their ability to interact with epithelial cell lines (Nassif et al., 1994, 1999; Virji et al., 1992, 1993) and show reduced binding to explanted organ cultures (Exley et al., 2009; Rayner et al., 1995). Piliated N. meningitidis attaches to the microvillous surface of non-ciliated cells (Stephens et al., 1983); following attachment the microvilli alter in structure to surround the bacteria, eventually forming a mesh-like structure around the microcolony (Rayner et al., 1995). We observed similar structures and processes by SEM, which showed microcolonies of both the WT and the non-piliated N. cinerea strains in close association with the cell surface, and bacteria frequently in contact with, or surrounded by, cellular protrusions consistent with microvilli. These data suggest that 346T might express adhesins other than Tfp that allow the bacterium to attach to the human epithelium. Tfp-independent binding of N. gonorrhoeae to immobilized glycolipids has been reported previously, and is suggested to be due to the presence of a lectin-like adhesin on the gonococcal surface, which recognizes a carbohydrate receptor (Deal et al., 1987). This proposed adhesin was identified as a 36 kDa gonococcal surface protein that is not associated with pili (Paruchuri et al., 1990). However, the gene number or sequence was not defined, making it difficult to search for homologues in N. cinerea genomes.
Tfp are particularly important for initial adhesion of pathogenic Neisseria which expresses a polysaccharide capsule. They induce a series of signalling events in host cells that leads to recruitment of receptors and rearrangement of the cytoskeleton, resulting in cortical plaque formation at the site of the microcolony (Merz & So, 1997; Mikaty et al., 2009). However, in non-encapsulated strains and/or at later times post-infection when piliation is reduced (Pujol et al., 1997, 1999), diffuse adherence occurs, through intimate adhesion mediated by outer-membrane proteins such as Opa and Opc (Virji et al., 1992, 1996; Weir et al., 1996). N. cinerea CCUG 346T lacks a polysaccharide capsule and thus surface expressed adhesins may make a significant contribution at early stages of attachment to cells. Strains of N. lactamica, N. subflava and N. flavescens have been shown to express Opa-like proteins that interact with human CEACAMs (Toleman et al., 2001), and genome analysis has also identified opa gene homologues in N. polysaccharea, but not in N. cinerea strain 14685 (Marri et al., 2010). Using blast analysis of the N. cinerea genome contigs available in PubMLST we were unable to identify opa homologues in the isolates used in this study. In contrast, homologues of other adhesins, including the autotransporters MspA and TspA, and NadA are present in the genome of 346T (http://pubmlst.org/neisseria/; Muzzi et al., 2013). In addition, the PilC protein that is associated with Tfp in pathogenic Neisseria has been shown to impact adhesion (Kirchner & Meyer, 2005; Rudel et al., 1995). Meningococci have two pilC genes (pilC1 and pilC2) but our analysis indicated that N. cinerea 346T has only one. Although the draft nature of the genome sequence means that we cannot exclude the presence of a second pilC, previous work has also reported a single PilC orthologue in N. cinerea (Marri et al., 2010). PilC orthologues in different species have conserved C-terminal domains but vary in the N-terminal domain, which is implicated in adhesion (Morand et al., 2001). The lack of sequence similarity in the N-terminal region of N. cinerea and meningococcal PilC (Fig. S4) suggests that these proteins could also have different adhesion properties. Notably, while the number of cell-associated meningococci increased over time, we found no increase in cell-associated N. cinerea from 1.5 h after infection, onwards. Increased recovery of adherent meningococci can result from increasing size of microcolonies and/or new adhesion events. Microcolony formation requires pilus–pilus interactions in N. meningitidis and N. gonorrhoeae (Hélaine et al., 2005); thus this observation is in agreement with the lack of involvement of Tfp in the adhesion of 346T. Furthermore, this may reflect saturation of available receptors for N. cinerea and suggests that N. cinerea 346T employs different mechanisms for colonization of human cells, compared with the pathogenic Neisseria.
Importantly the pilus-independent adhesion of N. cinerea was also observed for a second isolate, 27178A. Deletion of both pilE1 and pilE2 in this strain also had no impact on association with epithelial cells. Thus our work highlights that N. cinerea isolates harbour different pilE loci compared with pathogenic Neisseria, and can adhere to epithelial cells independently of Tfp. Given that pathogenic and commensal Neisseria species reside in the same host niche, this may have important consequences for co-colonization or competition. Recent human challenge studies have demonstrated that commensals can impact nasopharyngeal carriage of pathogens (Deasy et al., 2015) and raise the possibility of using non-pathogenic Neisseria strains as ‘bacterial medicine’ (Deasy et al., 2015). An increased understanding of the molecular mechanisms underlying the association of specific isolates of commensal Neisseria with the human epithelium will not only improve our understanding of host–microbe interactions, but may also be informative for such approaches.
Acknowledgements
We thank Felicia Tan for help with RNA techniques. We are grateful to Dr Julia Bennett and Professor Martin Maiden for providing N. cinerea strains and helpful discussion. Electron microscopy was carried out in the Dunn School EM facility and we thank Dr Anna Pielach for preparation of SEM samples. This publication made use of the Neisseria Multi Locus Sequence Typing website (http://pubmlst.org/neisseria/). The development of this site has been funded by the Wellcome Trust and European Union. M. W. is funded by the Wellcome Trust. G. L. is supported by an EP Abraham Departmental studentship.
Supplementary Data
Supplementary Data
Abbreviations:
- CEACAM
carcinoembryonic antigen cell adhesion molecule
- PBST
0.1 % Tween in PBS
- RBS
ribosome-binding site
- SEM
scanning electron microscopy
- SOE
splice overlap extension
- Tfp
type IV pili
References
- Aas F. E., Wolfgang M., Frye S., Dunham S., Løvold C., Koomey M. (2002). Competence for natural transformation in Neisseria gonorrhoeae: components of DNA binding and uptake linked to type IV pilus expression Mol Microbiol 46 749–760 10.1046/j.1365-2958.2002.03193.x . [DOI] [PubMed] [Google Scholar]
- Aho E. L., Murphy G. L., Cannon J. G. (1987). Distribution of specific DNA sequences among pathogenic and commensal Neisseria species Infect Immun 55 1009–1013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aho E. L., Botten J. W., Hall R. J., Larson M. K., Ness J. K. (1997). Characterization of a class II pilin expression locus from Neisseria meningitidis: evidence for increased diversity among pilin genes in pathogenic Neisseria species Infect Immun 65 2613–2620 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aho E. L., Keating A. M., McGillivray S. M. (2000). A comparative analysis of pilin genes from pathogenic and nonpathogenic Neisseria species Microb Pathog 28 81–88 10.1006/mpat.1999.0325 . [DOI] [PubMed] [Google Scholar]
- Aho E. L., Urwin R., Batcheller A. E., Holmgren A. M., Havig K., Kulakoski A. M., Vomhof E. E., Longfors N. S., Erickson C. B., other authors (2005). Neisserial pilin genes display extensive interspecies diversity FEMS Microbiol Lett 249 327–334 10.1016/j.femsle.2005.06.035 . [DOI] [PubMed] [Google Scholar]
- Bennett J. S., Thompson E. A., Kriz P., Jolley K. A., Maiden M. C. (2009). A common gene pool for the Neisseria FetA antigen Int J Med Microbiol 299 133–139 10.1016/j.ijmm.2008.06.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. S., Jolley K. A., Earle S. G., Corton C., Bentley S. D., Parkhill J., Maiden M. C. (2012). A genomic approach to bacterial taxonomy: an examination and proposed reclassification of species within the genus Neisseria Microbiology 158 1570–1580 10.1099/mic.0.056077-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg L., Lale R., Bakke I., Burroughs N., Valla S. (2009). The expression of recombinant genes in Escherichia coli can be strongly stimulated at the transcript production level by mutating the DNA-region corresponding to the 5′-untranslated part of mRNA Microb Biotechnol 2 379–389 10.1111/j.1751-7915.2009.00107.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger U. (1962). [Studies on acid formation by saccharolytic, saprophytic Neisseria] Arch Hyg Bakteriol 146 55–60 (in German). [PubMed] [Google Scholar]
- Berry J. L., Pelicic V. (2015). Exceptionally widespread nanomachines composed of type IV pilins: the prokaryotic Swiss Army knives FEMS Microbiol Rev 39 134–154 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos M. P., Grunert F., Belland R. J. (1997). Differential recognition of members of the carcinoembryonic antigen family by Opa variants of Neisseria gonorrhoeae Infect Immun 65 2353–2361 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. R., Helaine S., Carbonnelle E., Pelicic V. (2010). Systematic functional analysis reveals that a set of seven genes is involved in fine-tuning of the multiple functions mediated by type IV pili in Neisseria meningitidis Infect Immun 78 3053–3063 10.1128/IAI.00099-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi B., Adu-Bobie J., Di Marcello F., Ciucchi L., Masignani V., Taddei A., Rappuoli R., Pizza M., Aricò B. (2005). Neisseria meningitidis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells Mol Microbiol 55 687–698 10.1111/j.1365-2958.2004.04423.x . [DOI] [PubMed] [Google Scholar]
- Carbonnelle E., Hélaine S., Prouvensier L., Nassif X., Pelicic V. (2005). Type IV pilus biogenesis in Neisseria meningitidis: PilW is involved in a step occurring after pilus assembly, essential for fibre stability and function Mol Microbiol 55 54–64 10.1111/j.1365-2958.2004.04364.x . [DOI] [PubMed] [Google Scholar]
- Carbonnelle E., Helaine S., Nassif X., Pelicic V. (2006). A systematic genetic analysis in Neisseria meningitidis defines the Pil proteins required for assembly, functionality, stabilization and export of type IV pili Mol Microbiol 61 1510–1522 10.1111/j.1365-2958.2006.05341.x . [DOI] [PubMed] [Google Scholar]
- Carbonnelle E., Hill D. J., Morand P., Griffiths N. J., Bourdoulous S., Murillo I., Nassif X., Virji M. (2009). Meningococcal interactions with the host Vaccine 27 (Suppl. 2), B78–B89 10.1016/j.vaccine.2009.04.069 . [DOI] [PubMed] [Google Scholar]
- Cehovin A., Winterbotham M., Lucidarme J., Borrow R., Tang C. M., Exley R. M., Pelicic V. (2010). Sequence conservation of pilus subunits in Neisseria meningitidis Vaccine 28 4817–4826 10.1016/j.vaccine.2010.04.065 . [DOI] [PubMed] [Google Scholar]
- Chen I., Dubnau D. (2004). DNA uptake during bacterial transformation Nat Rev Microbiol 2 241–249 10.1038/nrmicro844 . [DOI] [PubMed] [Google Scholar]
- Corcoran C. P., Podkaminski D., Papenfort K., Urban J. H., Hinton J. C., Vogel J. (2012). Superfolder GFP reporters validate diverse new mRNA targets of the classic porin regulator, MicF RNA Mol Microbiol 84 428–445 10.1111/j.1365-2958.2012.08031.x . [DOI] [PubMed] [Google Scholar]
- Craig L., Pique M. E., Tainer J. A. (2004). Type IV pilus structure and bacterial pathogenicity Nat Rev Microbiol 2 363–378 10.1038/nrmicro885 . [DOI] [PubMed] [Google Scholar]
- Dalrymple B., Mattick J. S. (1987). An analysis of the organization and evolution of type 4 fimbrial (MePhe) subunit proteins J Mol Evol 25 261–269 10.1007/BF02100020 . [DOI] [PubMed] [Google Scholar]
- Deal C. D., Stromberg N., Nyberg G., Normark S., Karlsson K. A., So M. (1987). Pilin independent binding of Neisseria gonorrhoeae to immobilized glycolipids Antonie van Leeuwenhoek 53 425–430 10.1007/BF00415497 . [DOI] [PubMed] [Google Scholar]
- Deasy A. M., Guccione E., Dale A. P., Andrews N., Evans C. M., Bennett J. S., Bratcher H. B., Maiden M. C., Gorringe A. R., Read R. C. (2015). Nasal inoculation of the commensal Neisseria lactamica inhibits carriage of Neisseria meningitidis by young adults: a controlled human infection study Clin Infect Dis 60 1512–1520 . [DOI] [PubMed] [Google Scholar]
- Exley R. M., Sim R., Goodwin L., Winterbotham M., Schneider M. C., Read R. C., Tang C. M. (2009). Identification of meningococcal genes necessary for colonization of human upper airway tissue Infect Immun 77 45–51 10.1128/IAI.00968-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. G., Young L., Chuang R. Y., Venter J. C., Hutchison C. A., III, Smith H. O. (2009). Enzymatic assembly of DNA molecules up to several hundred kilobases Nat Methods 6 343–345 10.1038/nmeth.1318 . [DOI] [PubMed] [Google Scholar]
- Gold R., Goldschneider I., Lepow M. L., Draper T. F., Randolph M. (1978). Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children J Infect Dis 137 112–121 10.1093/infdis/137.2.112 . [DOI] [PubMed] [Google Scholar]
- Gómez-Duarte O. G., Dehio M., Guzmán C. A., Chhatwal G. S., Dehio C., Meyer T. F. (1997). Binding of vitronectin to Opa-expressing Neisseria gonorrhoeae mediates invasion of HeLa cells Infect Immun 65 3857–3866 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. R., Monk P. N., Partridge L. J., Morris P., Gorringe A. R., Read R. C. (2011). Cooperative role for tetraspanins in adhesin-mediated attachment of bacterial species to human epithelial cells Infect Immun 79 2241–2249 10.1128/IAI.01354-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas R., Meyer T. F. (1986). The repertoire of silent pilus genes in Neisseria gonorrhoeae: evidence for gene conversion Cell 44 107–115 10.1016/0092-8674(86)90489-7 . [DOI] [PubMed] [Google Scholar]
- Hagblom P., Segal E., Billyard E., So M. (1985). Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae Nature 315 156–158 10.1038/315156a0 . [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. (1983). Compilation and analysis of Escherichia coli promoter DNA sequences Nucleic Acids Res 11 2237–2255 10.1093/nar/11.8.2237 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélaine S., Carbonnelle E., Prouvensier L., Beretti J. L., Nassif X., Pelicic V. (2005). PilX, a pilus-associated protein essential for bacterial aggregation, is a key to pilus-facilitated attachment of Neisseria meningitidis to human cells Mol Microbiol 55 65–77 10.1111/j.1365-2958.2004.04372.x . [DOI] [PubMed] [Google Scholar]
- Higashi D. L., Biais N., Weyand N. J., Agellon A., Sisko J. L., Brown L. M., So M. (2011). N. elongata produces type IV pili that mediate interspecies gene transfer with N. gonorrhoeae PLoS One 6 e21373 10.1371/journal.pone.0021373 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. (1989). Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension Gene 77 61–68 10.1016/0378-1119(89)90359-4 . [DOI] [PubMed] [Google Scholar]
- Hung M. C., Heckels J. E., Christodoulides M. (2013). The adhesin complex protein (ACP) of Neisseria meningitidis is a new adhesin with vaccine potential MBio 4 e00041-13 10.1128/mBio.00041-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen F. E., Warren M. J., Schulz B. L., Power P. M., Swords W. E., Weiser J. N., Apicella M. A., Edwards J. L., Jennings M. P. (2013). Dual pili post-translational modifications synergize to mediate meningococcal adherence to platelet activating factor receptor on human airway cells PLoS Pathog 9 e1003377 10.1371/journal.ppat.1003377 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson L., Rytkonen A., Bergman P., Albiger B., Källström H., Hökfelt T., Agerberth B., Cattaneo R., Jonsson A. B. (2003). CD46 in meningococcal disease Science 301 373–375 10.1126/science.1086476 . [DOI] [PubMed] [Google Scholar]
- Jolley K. A., Maiden M. C. (2010). BIGSdb: scalable analysis of bacterial genome variation at the population level BMC Bioinformatics 11 595 10.1186/1471-2105-11-595 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källström H., Liszewski M. K., Atkinson J. P., Jonsson A. B. (1997). Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria Mol Microbiol 25 639–647 10.1046/j.1365-2958.1997.4841857.x . [DOI] [PubMed] [Google Scholar]
- Kirchner M., Meyer T. F. (2005). The PilC adhesin of the Neisseria type IV pilus-binding specificities and new insights into the nature of the host cell receptor Mol Microbiol 56 945–957 10.1111/j.1365-2958.2005.04600.x . [DOI] [PubMed] [Google Scholar]
- Knapp J. S., Hook E. W., III (1988). Prevalence and persistence of Neisseria cinerea and other Neisseria spp. in adults J Clin Microbiol 26 896–900 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal L., Abubucker S., Kota K., Fischbach M. A., Mitreva M. (2014). The prevalence of species and strains in the human microbiome: a resource for experimental efforts PLoS One 9 e97279 10.1371/journal.pone.0097279 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen P. A., Diomandé F., Ouédraogo R., Sanou I., Sangaré L., Ouédraogo A. S., Ba A. K., Kandolo D., Dolan Thomas J., other authors (2012). Carriage of Neisseria lactamica in 1- to 29-year-old people in Burkina Faso: epidemiology and molecular characterization J Clin Microbiol 50 4020–4027 10.1128/JCM.01717-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marri P. R., Paniscus M., Weyand N. J., Rendón M. A., Calton C. M., Hernández D. R., Higashi D. L., Sodergren E., Weinstock G. M., other authors (2010). Genome sequencing reveals widespread virulence gene exchange among human Neisseria species PLoS One 5 e11835 10.1371/journal.pone.0011835 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehr I. J., Seifert H. S. (1998). Differential roles of homologous recombination pathways in Neisseria gonorrhoeae pilin antigenic variation, DNA transformation and DNA repair Mol Microbiol 30 697–710 10.1046/j.1365-2958.1998.01089.x . [DOI] [PubMed] [Google Scholar]
- Merz A. J., So M. (1997). Attachment of piliated, Opa- and Opc- gonococci and meningococci to epithelial cells elicits cortical actin rearrangements and clustering of tyrosine-phosphorylated proteins Infect Immun 65 4341–4349 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikaty G., Soyer M., Mairey E., Henry N., Dyer D., Forest K. T., Morand P., Guadagnini S., Prévost M. C., other authors (2009). Extracellular bacterial pathogen induces host cell surface reorganization to resist shear stress PLoS Pathog 5 e1000314 10.1371/journal.ppat.1000314 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand P. C., Tattevin P., Eugene E., Beretti J. L., Nassif X. (2001). The adhesive property of the type IV pilus-associated component PilC1 of pathogenic Neisseria is supported by the conformational structure of the N-terminal part of the molecule Mol Microbiol 40 846–856 10.1046/j.1365-2958.2001.02452.x . [DOI] [PubMed] [Google Scholar]
- Muzzi A., Mora M., Pizza M., Rappuoli R., Donati C. (2013). Conservation of meningococcal antigens in the genus Neisseria MBio 4 e00163-13 10.1128/mBio.00163-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nägele V., Heesemann J., Schielke S., Jiménez-Soto L. F., Kurzai O., Ackermann N. (2011). Neisseria meningitidis adhesin NadA targets β1 integrins: functional similarity to Yersinia invasin J Biol Chem 286 20536–20546 10.1074/jbc.M110.188326 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif X. (1999). Interactions between encapsulated Neisseria meningitidis and host cells Int Microbiol 2 133–136 . [PubMed] [Google Scholar]
- Nassif X., Lowy J., Stenberg P., O'Gaora P., Ganji A., So M. (1993). Antigenic variation of pilin regulates adhesion of Neisseria meningitidis to human epithelial cells Mol Microbiol 8 719–725 10.1111/j.1365-2958.1993.tb01615.x . [DOI] [PubMed] [Google Scholar]
- Nassif X., Beretti J. L., Lowy J., Stenberg P., O'Gaora P., Pfeifer J., Normark S., So M. (1994). Roles of pilin and PilC in adhesion of Neisseria meningitidis to human epithelial and endothelial cells Proc Natl Acad Sci U S A 91 3769–3773 10.1073/pnas.91.9.3769 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojanen-Reuhs T., Kalkkinen N., Westerlund-Wikström B., van Doorn J., Haahtela K., Nurmiaho-Lassila E. L., Wengelnik K., Bonas U., Korhonen T. K. (1997). Characterization of the fimA gene encoding bundle-forming fimbriae of the plant pathogen Xanthomonas campestris pv. vesicatoria J Bacteriol 179 1280–1290 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield N. J., Bland S. J., Taraktsoglou M., Dos Ramos F. J., Robinson K., Wooldridge K. G., Ala'Aldeen D. A. (2007). T-cell stimulating protein A (TspA) of Neisseria meningitidis is required for optimal adhesion to human cells Cell Microbiol 9 463–478 10.1111/j.1462-5822.2006.00803.x . [DOI] [PubMed] [Google Scholar]
- Paetzel M., Karla A., Strynadka N. C., Dalbey R. E. (2002). Signal peptidases Chem Rev 102 4549–4580 10.1021/cr010166y . [DOI] [PubMed] [Google Scholar]
- Parge H. E., Forest K. T., Hickey M. J., Christensen D. A., Getzoff E. D., Tainer J. A. (1995). Structure of the fibre-forming protein pilin at 2.6 Å resolution Nature 378 32–38 10.1038/378032a0 . [DOI] [PubMed] [Google Scholar]
- Paruchuri D. K., Seifert H. S., Ajioka R. S., Karlsson K. A., So M. (1990). Identification and characterization of a Neisseria gonorrhoeae gene encoding a glycolipid-binding adhesin Proc Natl Acad Sci U S A 87 333–337 10.1073/pnas.87.1.333 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol C., Eugène E., de Saint Martin L., Nassif X. (1997). Interaction of Neisseria meningitidis with a polarized monolayer of epithelial cells Infect Immun 65 4836–4842 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol C., Eugène E., Marceau M., Nassif X. (1999). The meningococcal PilT protein is required for induction of intimate attachment to epithelial cells following pilus-mediated adhesion Proc Natl Acad Sci U S A 96 4017–4022 10.1073/pnas.96.7.4017 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner C. F., Dewar A., Moxon E. R., Virji M., Wilson R. (1995). The effect of variations in the expression of pili on the interaction of Neisseria meningitidis with human nasopharyngeal epithelium J Infect Dis 171 113–121 10.1093/infdis/171.1.113 . [DOI] [PubMed] [Google Scholar]
- Rendón M. A., Hockenberry A. M., McManus S. A., So M. (2013). Sigma factor RpoN (σ54) regulates pilE transcription in commensal Neisseria elongata Mol Microbiol 90 103–113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel T., Scheuerpflug I., Meyer T. F. (1995). Neisseria PilC protein identified as type-4 pilus tip-located adhesin Nature 373 357–359 10.1038/373357a0 . [DOI] [PubMed] [Google Scholar]
- Rusniok C., Vallenet D., Floquet S., Ewles H., Mouzé-Soulama C., Brown D., Lajus A., Buchrieser C., Médigue C., other authors (2009). NeMeSys: a biological resource for narrowing the gap between sequence and function in the human pathogen Neisseria meningitidis Genome Biol 10 R110 10.1186/gb-2009-10-10-r110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. R., Wakeman J., Sims G., O'Sullivan H., Hart C. A., Virji M. (1993). Piliation in Neisseria meningitidis and its consequences J Med Microbiol 39 7–9. [Google Scholar]
- Scarselli M., Serruto D., Montanari P., Capecchi B., Adu-Bobie J., Veggi D., Rappuoli R., Pizza M., Aricò B. (2006). Neisseria meningitidis NhhA is a multifunctional trimeric autotransporter adhesin Mol Microbiol 61 631–644 10.1111/j.1365-2958.2006.05261.x . [DOI] [PubMed] [Google Scholar]
- Schaefer J., Engl C., Zhang N., Lawton E., Buck M. (2015). Genome wide interactions of wild-type and activator bypass forms of σ54 Nucleic Acids Res 43 7280–7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert H. S., Ajioka R. S., Paruchuri D., Heffron F., So M. (1990). Shuttle mutagenesis of Neisseria gonorrhoeae: pilin null mutations lower DNA transformation competence J Bacteriol 172 40–46 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serruto D., Adu-Bobie J., Scarselli M., Veggi D., Pizza M., Rappuoli R., Aricò B. (2003). Neisseria meningitidis App, a new adhesin with autocatalytic serine protease activity Mol Microbiol 48 323–334 10.1046/j.1365-2958.2003.03420.x . [DOI] [PubMed] [Google Scholar]
- Sheikhi R., Amin M., Rostami S., Shoja S., Ebrahimi N. (2015). Oropharyngeal colonization with Neisseria lactamica, other nonpathogenic Neisseria species and Moraxella catarrhalis among young healthy children in Ahvaz, Iran Jundishapur J Microbiol 8 e14813 10.5812/jjm.14813 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder L. A., Saunders N. J. (2006). The majority of genes in the pathogenic Neisseria species are present in non-pathogenic Neisseria lactamica, including those designated as ‘virulence genes’ BMC Genomics 7 128 10.1186/1471-2164-7-128 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabler R. A., Marsden G. L., Witney A. A., Li Y., Bentley S. D., Tang C. M., Hinds J. (2005). Identification of pathogen-specific genes through microarray analysis of pathogenic and commensal Neisseria species Microbiology 151 2907–2922 10.1099/mic.0.28099-0 . [DOI] [PubMed] [Google Scholar]
- Stephens D. S., Hoffman L. H., McGee Z. A. (1983). Interaction of Neisseria meningitidis with human nasopharyngeal mucosa: attachment and entry into columnar epithelial cells J Infect Dis 148 369–376 10.1093/infdis/148.3.369 . [DOI] [PubMed] [Google Scholar]
- Toleman M., Aho E., Virji M. (2001). Expression of pathogen-like Opa adhesins in commensal Neisseria: genetic and functional analysis Cell Microbiol 3 33–44 10.1046/j.1462-5822.2001.00089.x . [DOI] [PubMed] [Google Scholar]
- Tønjum T., Weir S., Bøvre K., Progulske-Fox A., Marrs C. F. (1993). Sequence divergence in two tandemly located pilin genes of Eikenella corrodens Infect Immun 61 1909–1916 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi K., Tang C. M., Exley R. M. (2011). Mechanisms of meningococcal colonisation Trends Microbiol 19 456–463 10.1016/j.tim.2011.06.006 . [DOI] [PubMed] [Google Scholar]
- Turner D. P., Marietou A. G., Johnston L., Ho K. K., Rogers A. J., Wooldridge K. G., Ala'Aldeen D. A. (2006). Characterization of MspA, an immunogenic autotransporter protein that mediates adhesion to epithelial and endothelial cells in Neisseria meningitidis Infect Immun 74 2957–2964 10.1128/IAI.74.5.2957-2964.2006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Putten J. P., Paul S. M. (1995). Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrhoeae entry into human mucosal cells EMBO J 14 2144–2154 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M., Heckels J. E. (1983). Antigenic cross-reactivity of Neisseria pili: investigations with type- and species-specific monoclonal antibodies J Gen Microbiol 129 2761–2768 . [DOI] [PubMed] [Google Scholar]
- Virji M., Heckels J. E., Potts W. J., Hart C. A., Saunders J. R. (1989). Identification of epitopes recognized by monoclonal antibodies SM1 and SM2 which react with all pili of Neisseria gonorrhoeae but which differentiate between two structural classes of pili expressed by Neisseria meningitidis and the distribution of their encoding sequences in the genomes of Neisseria spp J Gen Microbiol 135 3239–3251 . [DOI] [PubMed] [Google Scholar]
- Virji M., Kayhty H., Ferguson D. J., Alexandrescu C., Heckels J. E., Moxon E. R. (1991). The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells Mol Microbiol 5 1831–1841 10.1111/j.1365-2958.1991.tb00807.x . [DOI] [PubMed] [Google Scholar]
- Virji M., Alexandrescu C., Ferguson D. J., Saunders J. R., Moxon E. R. (1992). Variations in the expression of pili: the effect on adherence of Neisseria meningitidis to human epithelial and endothelial cells Mol Microbiol 6 1271–1279 10.1111/j.1365-2958.1992.tb00848.x . [DOI] [PubMed] [Google Scholar]
- Virji M., Saunders J. R., Sims G., Makepeace K., Maskell D., Ferguson D. J. (1993). Pilus-facilitated adherence of Neisseria meningitidis to human epithelial and endothelial cells: modulation of adherence phenotype occurs concurrently with changes in primary amino acid sequence and the glycosylation status of pilin Mol Microbiol 10 1013–1028 10.1111/j.1365-2958.1993.tb00972.x . [DOI] [PubMed] [Google Scholar]
- Virji M., Watt S. M., Barker S., Makepeace K., Doyonnas R. (1996). The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae Mol Microbiol 22 929–939 10.1046/j.1365-2958.1996.01548.x . [DOI] [PubMed] [Google Scholar]
- Weir S., Lee L. W., Marrs C. F. (1996). Identification of four complete type 4 pilin genes in a single Kingella denitrificans genome Infect Immun 64 4993–4999 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff K., Stern A. (1995). Identification and characterization of specific sequences encoding pathogenicity associated proteins in the genome of commensal Neisseria species FEMS Microbiol Lett 125 255–263 10.1111/j.1574-6968.1995.tb07366.x . [DOI] [PubMed] [Google Scholar]
- Wolfgang M., van Putten J. P., Hayes S. F., Koomey M. (1999). The comP locus of Neisseria gonorrhoeae encodes a type IV prepilin that is dispensable for pilus biogenesis but essential for natural transformation Mol Microbiol 31 1345–1357 10.1046/j.1365-2958.1999.01269.x . [DOI] [PubMed] [Google Scholar]
- Wörmann M. E., Horien C. L., Bennett J. S., Jolley K. A., Maiden M. C., Tang C. M., Aho E. L., Exley R. M. (2014). Sequence, distribution and chromosomal context of class I and class II pilin genes of Neisseria meningitidis identified in whole genome sequences BMC Genomics 15 253 10.1186/1471-2164-15-253 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data