Abstract
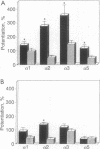
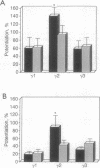
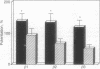
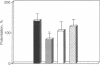
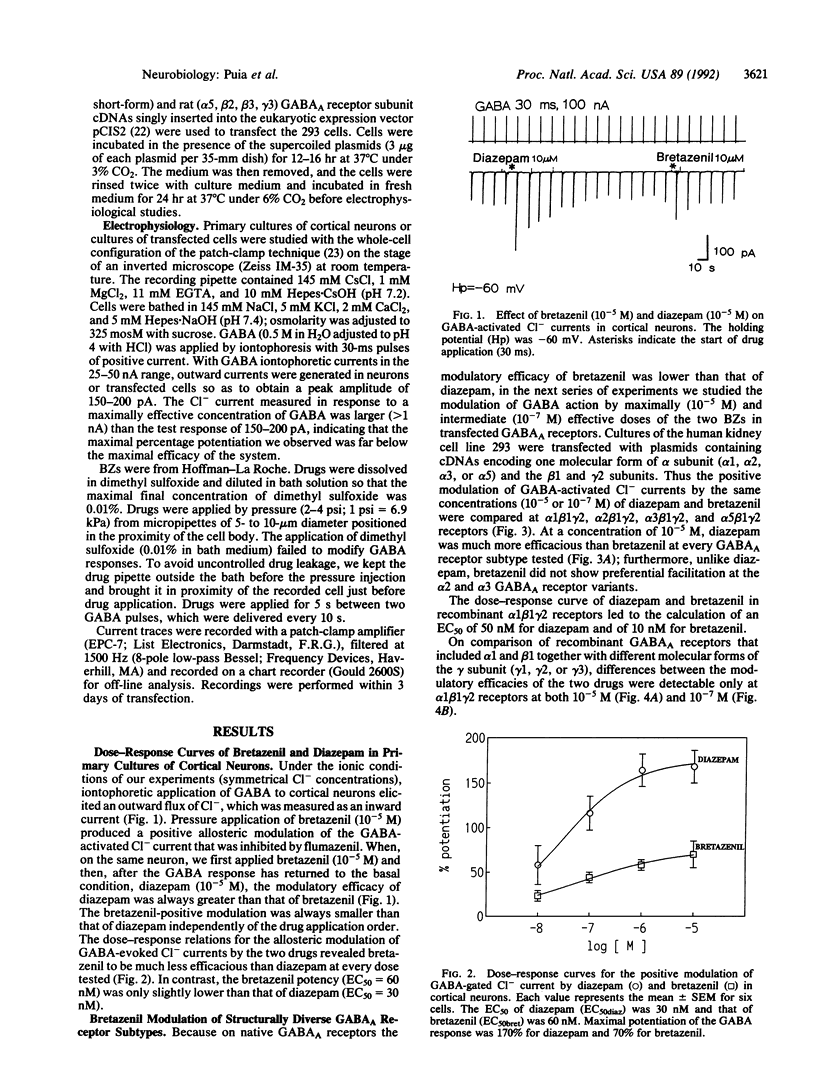
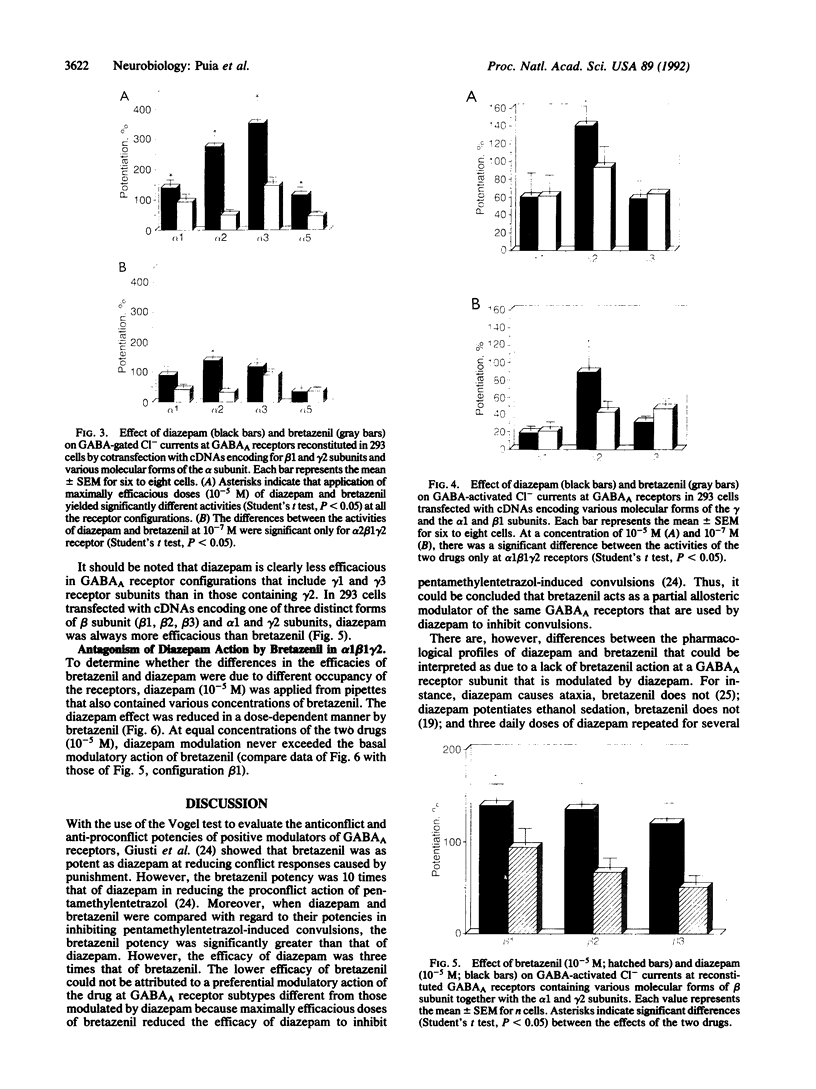
In central nervous system gamma-aminobutyric acid (GABA) inhibits neuronal activity by acting on GABA type A (GABAA) receptors. These heterooligomeric integral membrane proteins include a GABA-gated Cl- channel and various allosteric modulatory sites where endogenous modulators and anxiolytic drugs act to regulate GABA action. In vivo, various anxiolytic drugs exhibit a wide range of variability in their modulatory efficacy and potency of GABA action. For instance, bretazenil modulatory efficacy is much lower than that of diazepam. Such low efficacy could be due either to a preferential modulation of specific GABAA receptor subtypes or to a low modulatory efficacy at every GABAA receptor subtype. To address these questions we studied drug-induced modifications of GABA-activated Cl- currents in native GABAA receptors of cortical neurons in primary cultures and in recombinant GABAA receptors transiently expressed in transformed human embryonic kidney cells (293) after transfection with cDNAs encoding different molecular forms of alpha, beta, and gamma subunits of GABAA receptors. In cortical neurons the efficacy of bretazenil was lower than that of diazepam, whereas the potency of the two drugs was similar. In cells transfected with gamma 2 subunits and various molecular forms of alpha and beta subunits bretazenil efficacy was always lower than that of diazepam. However, in cells transfected with gamma 1 or gamma 3 subunits and various forms of alpha and beta subunits the efficacy of both diazepam and bretazenil was lower and always of similar magnitude. When bretazenil and diazepam were applied together to GABAA receptors including a gamma 2 subunit, the action of diazepam was curtailed in a manner related to the dose of bretazenil.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alho H., Ferrarese C., Vicini S., Vaccarino F. Subsets of GABAergic neurons in dissociated cell cultures of neonatal rat cerebral cortex show co-localization with specific modulator peptides. Brain Res. 1988 Apr 1;467(2):193–204. doi: 10.1016/0165-3806(88)90023-5. [DOI] [PubMed] [Google Scholar]
- Belzung C., Misslin R., Vogel E. Behavioural effects of the benzodiazepine receptor partial agonist RO 16-6028 in mice. Psychopharmacology (Berl) 1989;97(3):388–391. doi: 10.1007/BF00439456. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E., Guidotti A., Toffano G. Molecular mechanisms mediating the action of diazepam on GABA receptors. Br J Psychiatry. 1978 Sep;133:239–248. doi: 10.1192/bjp.133.3.239. [DOI] [PubMed] [Google Scholar]
- Cutting G. R., Lu L., O'Hara B. F., Kasch L. M., Montrose-Rafizadeh C., Donovan D. M., Shimada S., Antonarakis S. E., Guggino W. B., Uhl G. R. Cloning of the gamma-aminobutyric acid (GABA) rho 1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draguhn A., Verdorn T. A., Ewert M., Seeburg P. H., Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+. Neuron. 1990 Dec;5(6):781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- Eaton D. L., Wood W. I., Eaton D., Hass P. E., Hollingshead P., Wion K., Mather J., Lawn R. M., Vehar G. A., Gorman C. Construction and characterization of an active factor VIII variant lacking the central one-third of the molecule. Biochemistry. 1986 Dec 30;25(26):8343–8347. doi: 10.1021/bi00374a001. [DOI] [PubMed] [Google Scholar]
- Giusti P., Guidetti G., Costa E., Guidotti A. The preferential antagonism of pentylenetetrazole proconflict responses differentiates a class of anxiolytic benzodiazepines with potential antipanic action. J Pharmacol Exp Ther. 1991 Jun;257(3):1062–1068. [PubMed] [Google Scholar]
- Haefely W., Martin J. R., Schoch P. Novel anxiolytics that act as partial agonists at benzodiazepine receptors. Trends Pharmacol Sci. 1990 Nov;11(11):452–456. doi: 10.1016/0165-6147(90)90126-s. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Khrestchatisky M., MacLennan A. J., Chiang M. Y., Xu W. T., Jackson M. B., Brecha N., Sternini C., Olsen R. W., Tobin A. J. A novel alpha subunit in rat brain GABAA receptors. Neuron. 1989 Dec;3(6):745–753. doi: 10.1016/0896-6273(89)90243-2. [DOI] [PubMed] [Google Scholar]
- Krnjević K. Glutamate and gamma-aminobutyric acid in brain. Nature. 1970 Oct 10;228(5267):119–124. doi: 10.1038/228119a0. [DOI] [PubMed] [Google Scholar]
- Levitan E. S., Schofield P. R., Burt D. R., Rhee L. M., Wisden W., Köhler M., Fujita N., Rodriguez H. F., Stephenson A., Darlison M. G. Structural and functional basis for GABAA receptor heterogeneity. Nature. 1988 Sep 1;335(6185):76–79. doi: 10.1038/335076a0. [DOI] [PubMed] [Google Scholar]
- Lüddens H., Pritchett D. B., Köhler M., Killisch I., Keinänen K., Monyer H., Sprengel R., Seeburg P. H. Cerebellar GABAA receptor selective for a behavioural alcohol antagonist. Nature. 1990 Aug 16;346(6285):648–651. doi: 10.1038/346648a0. [DOI] [PubMed] [Google Scholar]
- Miller L. G., Galpern W. R., Greenblatt D. J., Lumpkin M., Shader R. I. Chronic benzodiazepine administration. VI. A partial agonist produces behavioral effects without tolerance or receptor alterations. J Pharmacol Exp Ther. 1990 Jul;254(1):33–38. [PubMed] [Google Scholar]
- Olsen R. W., Tobin A. J. Molecular biology of GABAA receptors. FASEB J. 1990 Mar;4(5):1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Sontheimer H., Shivers B. D., Ymer S., Kettenmann H., Schofield P. R., Seeburg P. H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989 Apr 13;338(6216):582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Puia G., Vicini S., Seeburg P. H., Costa E. Influence of recombinant gamma-aminobutyric acid-A receptor subunit composition on the action of allosteric modulators of gamma-aminobutyric acid-gated Cl- currents. Mol Pharmacol. 1991 Jun;39(6):691–696. [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Shivers B. D., Killisch I., Sprengel R., Sontheimer H., Köhler M., Schofield P. R., Seeburg P. H. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989 Sep;3(3):327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Shivers B. D., Killisch I., Sprengel R., Sontheimer H., Köhler M., Schofield P. R., Seeburg P. H. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989 Sep;3(3):327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Draguhn A., Ymer S., Seeburg P. H., Sakmann B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron. 1990 Jun;4(6):919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- Vicini S., Mienville J. M., Costa E. Actions of benzodiazepine and beta-carboline derivatives on gamma-aminobutyric acid-activated Cl- channels recorded from membrane patches of neonatal rat cortical neurons in culture. J Pharmacol Exp Ther. 1987 Dec;243(3):1195–1201. [PubMed] [Google Scholar]
- Vicini S. Pharmacologic significance of the structural heterogeneity of the GABAA receptor-chloride ion channel complex. Neuropsychopharmacology. 1991 Jan;4(1):9–15. [PubMed] [Google Scholar]
- Wilson-Shaw D., Robinson M., Gambarana C., Siegel R. E., Sikela J. M. A novel gamma subunit of the GABAA receptor identified using the polymerase chain reaction. FEBS Lett. 1991 Jun 24;284(2):211–215. doi: 10.1016/0014-5793(91)80687-x. [DOI] [PubMed] [Google Scholar]
- Yakushiji T., Fukuda T., Oyama Y., Akaike N. Effects of benzodiazepines and non-benzodiazepine compounds on the GABA-induced response in frog isolated sensory neurones. Br J Pharmacol. 1989 Nov;98(3):735–740. doi: 10.1111/j.1476-5381.1989.tb14600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ymer S., Draguhn A., Köhler M., Schofield P. R., Seeburg P. H. Sequence and expression of a novel GABAA receptor alpha subunit. FEBS Lett. 1989 Nov 20;258(1):119–122. doi: 10.1016/0014-5793(89)81630-8. [DOI] [PubMed] [Google Scholar]
- Ymer S., Draguhn A., Wisden W., Werner P., Keinänen K., Schofield P. R., Sprengel R., Pritchett D. B., Seeburg P. H. Structural and functional characterization of the gamma 1 subunit of GABAA/benzodiazepine receptors. EMBO J. 1990 Oct;9(10):3261–3267. doi: 10.1002/j.1460-2075.1990.tb07525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Sayed H. Y. The effect of ultrasonic scaling on the integrity of the apical seal of roots obturated with gutta percha cones. (Dye penetration study). Egypt Dent J. 1990 Jul;36(3):235–242. [PubMed] [Google Scholar]