Abstract
Parkinson disease is a chronic progressive syndrome with a broad array of clinical features. Different investigators have suggested the heterogeneous motor manifestations of early Parkinson disease can be conceptualized through a taxonomy of clinical subtypes including tremor‐predominant and postural instability and gait difficulty‐predominant subtypes. Although it is theoretically valuable to distinguish subtypes of Parkinson disease, the reality is that few patients fit these discrete categories well and many transition from exhibiting elements of one subtype to elements of another. In the time since the initial description of the postural instability and gait difficulty‐predominant subtype, Parkinson disease clinical research has blossomed in many ways – including an increased emphasis on the role of medical comorbidities and extranigral pathologies in Parkinson disease as markers of prognostic significance. By conceptualizing the pathogenesis of an expansive disease process in the limited terms of categorical motor subtypes, we run the risk of overlooking or misclassifying clinically significant pathogenic risk factors that lead to the development of motor milestones such as falls and related axial motor disability. Given its critical influence on quality of life and overall prognosis, we are in need of a model of postural instability and gait difficulty–predominant features in Parkinson disease that emphasizes the overlooked pathological influence of aging and medical comorbidities on the development of axial motor burden and postural instability and gait difficulty‐predominant features. This Point of View proposes thinking of postural instability and gait difficulties in Parkinson disease not as a discrete subtype, but rather as multidimensional continuum influenced by several overlapping age‐related pathologies.
Introduction
No two individuals with Parkinson disease (PD) are alike. Many endogenous and exogenous factors influence the severity and breadth of the clinical manifestations of PD. This is true of motor manifestations of PD, where clinical diagnostic criteria1, 2, 3 offer multiple routes (e.g., the presence of rest tremor or asymmetry or rigidity) leading to a diagnosis of probable PD. The heterogeneous nature of PD's clinical manifestations does not represent a failure of our diagnostic approach. It is an expected feature of an insidiously progressive chronic neurologic condition that affects nervous systems of differing age, underlying integrity, and capacity for repair.
Many movement disorders clinicians and investigators attempt to make sense of motor feature variability by conceiving PD as disorder with discrete underlying motor subtypes. In the contemporary UPDRS‐era of PD clinical research, this motor subtype approach was conceptualized by Jankovic et al.4 in their retrospective review of the DATATOP trial, which remains one of largest natural history studies of PD. Based on empiric, investigator‐determined UPDRS characteristics, the 800 subjects with early PD were classified as exhibiting either postural instability and gait difficulty‐predominant disease (PIGD; n = 441), tremor‐predominant disease (n = 233), or an indeterminate subtype (n = 126). These groups differed in their ability to perform activities of daily living (ADL) and also in key nonmotor features, leading investigators to conclude that these data supported the existence of discrete clinical PD subtypes. This same classification system has now been updated for the MDS‐UPDRS motor scale.5
More recently, there have been attempts to further refine the categorization of PD motor subtypes using both empirically derived categorizations and factor analysis‐based data‐driven approaches.6 If subtypes represent a biologically valid categorization of PD, they should have unique pathogenic substrates giving rise to distinct symptom/feature clusters. This scenario would allow for targeted, subtype‐specific observational and interventional studies aimed at altering disease progression in the subtypes of PD. The opposite is also true – if PIGD is a state marker rather than an early trait marker, we may be overlooking the contributing role of salient risk factors to the development of the PIGD state. The validity, then, of the motor subtype classification is an important issue for clinical research progress.
Limitations of Motor Subtypes
Many overlapping subtypes in the literature lead to confusing terminology
The terminology used to describe gait and axial motor features is ambiguous, overlapping, and at times confusing. Jankovic et al. referred to the PIGD subtype as one where axial symptoms on history and features observed during the motor exam were over‐represented relative to tremor items. Other investigators use the term akinetic‐rigid to describe a similar group of tremor‐free subjects with significant axial and appendicular rigidity.7 Others still have used the term “bradykinesia/rigidity and PIGD‐dominant” disease with what appears to be a primary goal of excluding tremor‐predominant subjects.8 What is most likely is that each investigator subdivides PD motor subtypes based on the underlying hypothesis in question. Although this allows for reasonable intergroup comparisons within a single study, it leads to an imprecise body of literature characterizing the dominant factors associated with the so‐called PIGD subtype.
PIGD subtypes are not reproducible using cluster/factor analyses
If clinically derived motor subtypes have biological validity, one would expect that they could be derived both through clinical observations/empirical approaches and through data‐driven approaches such as cluster and/or factor analysis. A systematic review of published PD subtype cluster analyses,8 however, found significant heterogeneity between studies, with the most consistent subgroups being “old‐age‐at‐onset and rapid disease progression” versus “young‐age‐at‐onset and slow disease progression”. Motor subgroupings were inconsistently assessed in these studies, although two studies reported clustering of “tremor‐predominant” disease versus a “nontremor‐predominant” subtype.9, 10 Although these analyses lend support to the designation of a tremor‐predominant subtype, they fail to distinguish an alternative motor cluster approximating a PIGD subtype. The utility of a motor subtype that combines all nontremor features of PD together is of questionable value.
PIGD‐subtype propensity is influenced by age and medical comorbidities
Natural history studies in PD inform our understanding of real‐world prognostic factors. In the CamPaIGN study, an incident PD cohort study (n = 142) based in the UK, the level of medical comorbidities was an independent predictor of the likelihood of progressing to Hoehn and Yahr stage 3. Other observational PD studies have shown comorbidity burden to be a clear risk factor for gait and mobility symptoms.11, 12 Compared to middle‐age subjects, older individuals diagnosed with PD tend to have disproportionately more axial motor impairments and a greater burden of comorbidities.13 Axial motor features are preferentially associated with impaired quality of life in PD.14 PIGD severity proximate to the time of PD diagnosis is also associated with an increased mortality risk.15 A recent prognostic modeling study in newly diagnosed PD showed that the development of postural instability at approximately 5 years is, as might be expected, predicted by older age and subthreshold‐level axial motor burden at baseline.16 The common thread here is that older age + more medical comorbidities give rise to greater axial motor burden which leads to disability in PD and poor prognosis. There is no reason to think that this combination of broad risk factors is only relevant in the newly diagnosed individual with PIGD‐subtype PD. More likely, these salient risk factors (age and medical comorbidities) are continually accruing in all PD‐affected individuals and influencing important prognostic concerns. While there certainly may be situations in which postural abnormalities are an early clinical feature based solely or largely on the underlying PD pathogenic cascade, it is far more common that comorbid conditions synergize with PD pathology to produce these debilitating symptoms throughout the natural history of the disease. Breaking down the PIGD‐subtype silo might allow for studies that place greater emphasis on how to modify the effects of these common pathogenic risk factors in all individuals with PD.
Discrete motor subtypes do not account for transitions between subtypes
Clinical subtypes are prognostically most significant if they (1) each predict a unique clinical course and (2) are mutually exclusive. The PD motor subtype classification taxonomy lacks these features. In the absence of either of these characteristics, what we think of as discrete subtypes may be staging markers for PD progression, rather than trait markers. There is evidence to support this staging concept as it relates to the purported PIGD subtype. Clinically defined postural instability is relatively uncommon early in the disease course2 and is a key staging marker on the modified Hoehn and Yahr scale17 marking the transition from early, mild disease to late, severe disease. In a longitudinal cohort study of 171 nondemented PD subjects in Norway, Alves et al. demonstrated an essentially unidirectional stage transition pattern from tremor‐predominant & indeterminate subtypes toward the PIGD subtype with advancing disease duration.18 This stage transition was associated with markedly increased risk of incident dementia. Similarly, Vu et al. tracked motor changes in 795 subjects from the DATATOP study. Across the cohort, the rate of progression of tremor features was twice as slow as other motor elements.19 PIGD‐related motor features were identified as being not only the most levodopa resistant relative to other varieties of parkinsonian motor burden but also were unique in their continued gradual worsening in severity even at the end of the 8 years follow‐up.19
PIGD trait versus state concepts in early PD
The initial DATATOP‐derived PIGD subtype was applied to a cohort of clinical trial subjects relatively proximate to their initial diagnosis of PD.4 Many investigators continue to use the PIGD subtype to distinguish a more disabling subtype of early PD. The formula used to categorize PD patients as having the PIGD subtype involves calculating the ratio of tremor‐related items on the MDS‐UPDRS to PIGD‐related items.5 If the ratio is ≤0.90, then an individual is classified as having the PIGD subtype. Individuals with scores between 0.90 and 1.15 are categorized as “indeterminate” and those with score ≥1.15 are categorized as having tremor‐dominant disease. If we agree that not all forms of recently diagnosed PD are equivalent in their clinical manifestations, disability, neuropathological burden, or rate of progression, then it may be worth considering whether our PIGD motor subtype is correctly identifying disease heterogeneity in “early PD” or instead, erroneously lumping together individuals without significant tremor, who have underlying pathology ranging from mild to advanced. Although recent reviews have argued in favor of the trait‐like properties of the PIGD subtype,20, 21 this bivariate categorization system provides much less nuanced information about disease burden than, for example, a combined ordinal score comprised simply of the denominator in the aforementioned tremor/PIGD ratio equation.
Several times a month, I tell newly diagnosed PD patients that I believe they have “early Parkinson disease.” What I tend to mean is that they have newly diagnosed clinically probable PD and have not yet attained any irreversible disease‐specific milestones such as disabling motor fluctuations, recurrent falls, or dementia. How patients and their families interpret my words may be something different entirely. They might imagine themselves as setting foot in a fast moving river that will carry them in a direction opposite their wishes, ultimately culminating in a poor outcome in the unseen distant future. By lumping all subjects without substantial tremor and with variable degrees of axial motor impairments and into one category, the current tremor‐predominant subtype versus PIGD‐predominant subtype model fails to appropriately describe the topography of this river and may harmfully contribute toward the invisible gap between medical providers and patients in these prognostic discussions.
As clinicians, we choose to initiate a subtype‐specific conversation not because we believe it to be perfectly accurate, but rather because we hope it will be useful to the patient and family sitting in front of us. Since we lack detailed prognostic models, our ability to advise patients more specifically about ways to understand the natural history of their specific condition and modify their disease progression are inherently poor. Shifting our nomenclature away from trait‐like subtype concepts and toward a state‐like concept of progressively worsening PIGD burden might (1) encourage researchers to investigate shared pathogenic risk elements in PD that contribute to progressive PIGD burden and (2) improve the precision, accuracy, and overall quality of our meaningful prognostic conversations in clinic.
New Schema
PIGD features are a meaningful measure of disease burden in all individuals affected by PD. Rather than subtyping individuals as PIGD (+) or PIGD (−), we may derive greater benefit from construing PIGD burden as continuous marker of disease progression in PD. This shift in approach would encourage mechanistic and natural history studies to track the progression of PIGD features from inception to low‐level burden to the development of clinically meaningful milestones (Fig. 1). To date, differences seen between motor subtypes with in vivo biomarker or imaging studies may have been interpreted as lending further support the to the PIGD‐as‐an‐early trait marker concept. These same biomarkers, however, might be better characterized not as markers of early subtypes per se, but rather as markers of ordinal disease progression in PD. Validation studies of these biomarkers and of the same clinical elements used as a denominator in MDS‐UPDRS discrete subtype classification algorithm could be pursued as a measure of continuous PIGD disease burden.5
Figure 1.
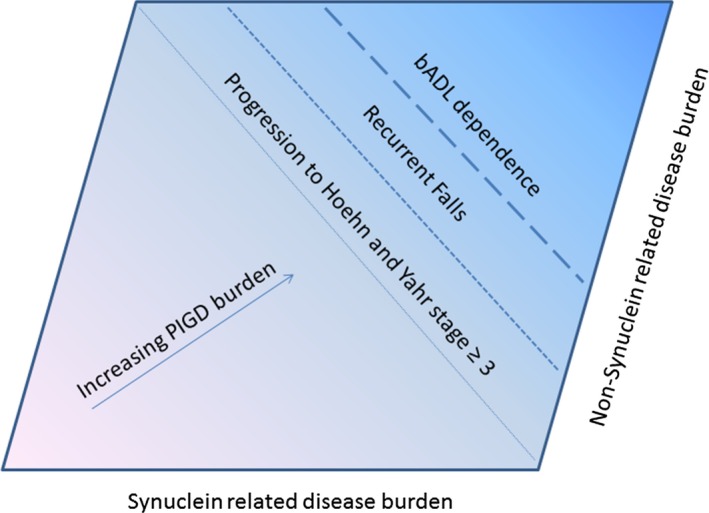
A PIGD continuum model for all individuals with Parkinson disease. A figure depicting the breath of postural instability and gait difficulty (PIGD) features in all individuals affected by Parkinson disease. PIGD features become progressively worse as synuclein‐related factors and other age‐related factors shift disease burden from the less‐affected lower left hand corner to the more affected upper right hand corner. PIGD, postural instability and gait difficulty‐predominant; bADL, basic activities of daily living.
This approach might also help improve our understanding of the pathological role of modifiable conditions that are not currently a focus of clinical care or ongoing PD trials. These include but are not limited to targeted interventions toward improving or modifying vision, osteopenia and osteoarthritis, polyneuropathy, sarcopenia, microvascular risk factors, amyloidopathy, and other neuronal and non‐neuronal comorbidities that influence the development of PIGD features. These conditions influence gait and balance morbidity in PD and in many cases, already have approved treatment approaches in other disease states that carry important potential for improving clinical outcomes in PD.
PIGD is important to all people affected by PD. By deemphasizing the PIGD subtype as a trait maker and increasing our focus toward the PIGD continuum, we can keep this key topic in the foreground of our approach toward therapeutic design and other ongoing concepts of what it means to modify disease progression in PD.
Conflict of Interests
Dr. Kotagal reports grants from National Institute of Aging and from Veterans Affairs Health System during the conduct of the study.
Acknowledgements
Thanks to Roger Albin, Bill Dauer, and the reviewers for their helpful feedback.
References
- 1. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;56:33–39. [DOI] [PubMed] [Google Scholar]
- 3. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30:1591–1601. [DOI] [PubMed] [Google Scholar]
- 4. Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson's disease: a base‐line analysis of the DATATOP cohort.The Parkinson Study Group. Neurology 1990;40:1529–1534. [DOI] [PubMed] [Google Scholar]
- 5. Stebbins GT, Goetz CG, Burn DJ, et al. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson's disease rating scale: comparison with the unified Parkinson's disease rating scale. Mov Disord 2013;28:668–670. [DOI] [PubMed] [Google Scholar]
- 6. Marras C. Subtypes of Parkinson's disease: state of the field and future directions. Curr Opin Neurol 2015;28:382–386. [DOI] [PubMed] [Google Scholar]
- 7. Rajput AH, Voll A, Rajput ML, et al. Course in Parkinson disease subtypes: a 39‐year clinicopathologic study. Neurology 2009;73:206–212. [DOI] [PubMed] [Google Scholar]
- 8. van Rooden SM, Heiser WJ, Kok JN, et al. The identification of Parkinson's disease subtypes using cluster analysis: a systematic review. Mov Disord 2010;25:969–978. [DOI] [PubMed] [Google Scholar]
- 9. Lewis SJ, Foltynie T, Blackwell AD, et al. Heterogeneity of Parkinson's disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry 2005;76:343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reijnders JS, Ehrt U, Lousberg R, et al. The association between motor subtypes and psychopathology in Parkinson's disease. Parkinsonism Relat Disord 2009;15:379–382. [DOI] [PubMed] [Google Scholar]
- 11. King LA, Priest KC, Nutt J, et al. Comorbidity and functional mobility in persons with Parkinson disease. Arch Phys Med Rehabil 2014;95:2152–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parashos SA, Wielinski CL, Giladi N, Gurevich T. Falls in Parkinson disease: analysis of a large cross‐sectional cohort. J Parkinson's Dis 2013;3:515–522. [DOI] [PubMed] [Google Scholar]
- 13. Diederich NJ, Moore CG, Leurgans SE, et al. Parkinson disease with old‐age onset: a comparative study with subjects with middle‐age onset. Arch Neurol 2003;60:529–533. [DOI] [PubMed] [Google Scholar]
- 14. Post B, Muslimovic D, van Geloven N, et al. Progression and prognostic factors of motor impairment, disability and quality of life in newly diagnosed Parkinson's disease. Mov Disord 2011;26:449–456. [DOI] [PubMed] [Google Scholar]
- 15. Lo RY, Tanner CM, Albers KB, et al. Clinical features in early Parkinson disease and survival. Arch Neurol 2009;66:1353–1358. [DOI] [PubMed] [Google Scholar]
- 16. Velseboer DC, de Bie RM, Wieske L, et al. Development and external validation of a prognostic model in newly diagnosed Parkinson disease. Neurology 2016. Mar 15;86(11):986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goetz CG, Poewe W, Rascol O, et al. Movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 2004;19:1020–1028. [DOI] [PubMed] [Google Scholar]
- 18. Alves G, Larsen JP, Emre M, et al. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov Disord 2006;21:1123–1130. [DOI] [PubMed] [Google Scholar]
- 19. Vu TC, Nutt JG, Holford NH. Progression of motor and nonmotor features of Parkinson's disease and their response to treatment. Br J Clin Pharmacol 2012;74:267–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marras C, Lang A. Parkinson's disease subtypes: lost in translation? J Neurol Neurosurg Psychiatry 2013;84:409–415. [DOI] [PubMed] [Google Scholar]
- 21. Thenganatt MA, Jankovic J. Parkinson disease subtypes. JAMA Neurol 2014;71:499–504. [DOI] [PubMed] [Google Scholar]


