Abstract
Various stem cell sources are being explored to treat diabetes since the proof-of-concept for cell therapy was laid down by transplanting cadaveric islets as a part of Edmonton protocol in 2000. Human embryonic stem (hES) cells derived pancreatic progenitors have got US-FDA approval to be used in clinical trials to treat type 1 diabetes mellitus (T1DM). However, these progenitors more closely resemble their foetal counterparts and thus whether they will provide long-term regeneration of adult human pancreas remains to be demonstrated. In addition to lifestyle changes and administration of insulin sensitizers, regeneration of islets from endogenous pancreatic stem cells may benefit T2DM patients. The true identity of pancreatic stem cells, whether these exist or not, whether regeneration involves reduplication of existing islets or ductal epithelial cells transdifferentiate, remains a highly controversial area. We have recently demonstrated that a novel population of very small embryonic-like stem cells (VSELs) is involved during regeneration of adult mouse pancreas after partial-pancreatectomy. VSELs (pluripotent stem cells in adult organs) should be appreciated as an alternative for regenerative medicine as these are autologous (thus immune rejection issues do not exist) with no associated risk of teratoma formation. T2DM is a result of VSELs dysfunction with age and uncontrolled proliferation of VSELs possibly results in pancreatic cancer. Extensive brainstorming and financial support are required to exploit the potential of endogenous VSELs to regenerate the pancreas in a patient with diabetes.
Keywords: Diabetes, ES cells, islets, pancreas, stem cells, VSELs
Diabetes is one of the major non-communicable diseases in the world with majority of patients belonging to India, China and USA. Along with associated complications like heart disease and stroke, diabetes results in increased morbidity and mortality and it is expected that by the year 2025, India alone will have more than 70 million diabetics1,2. Diabetes is a metabolic disorder associated with progressive loss or dysfunction of β-cells of pancreas. Onset of type 1 diabetes mellitus (T1DM) occurs when the β-cell mass is reduced to less than 20 per cent due to autoimmune effect, whereas the declining β-cell mass is unable to meet the age-related increased insulin demands of the body in type 2 (T2DM) as a result of insulin resistance and in due course the β-cells are lost by apoptosis. Thus, in both T1 and T2DM, restoration of a functional β-cell mass constitutes the central goal of diabetes therapy. Besides a change in lifestyle and administration of insulin sensitizers to T2DM patients, an unmet need exists to regenerate the pancreas (implying regenerating healthy β-islets). In case of T1DM, transplantation of islets (derived from cadavers, foetal or from non-human xeno-source or from the stem cells) may help whereas in case of T2DM attempts are being made to facilitate endogenous regeneration of islets in the pancreas. There was a rush in the clinics to transplant autologous bone marrow stem cells and mesenchymal stem cells (MSCs) and handful of pilot studies showed marginal benefit. Basic scientists are still divided as to whether pancreatic regeneration occurs by reduplication of existing islets, trans-differentiation of ductal epithelial cells or whether neogenesis of islets involves stem cells. Elaborate studies conducted over more than a decade, from Harvard University3 have demonstrated a role for PDX-1 positive progenitors during regeneration of mouse pancreas and lineage tracing studies have shown that these cells possibly arise from the ductal epithelium by de-differentiation involving regression of cells to an earlier stage of a progenitor which can differentiate into islets and acinar cells during regeneration3. A study reported in 20134 using tamoxifen independent INSCremTmG compound mice could not generate any evidence by flow cytometry in support of neogenesis of β-cells. We have recently demonstrated how very small embryonic-like stem cells (VSELs) are involved during pancreas regeneration5 and also how VSELs have eluded the scientific community for so long6. This article provides an overview of the various approaches used to regenerate pancreas in patients with diabetes, recent advances including our contributions and also a novel approach that may be explored in future.
Islets obtained from the cadavers, foetal tissue or from xeno-source: Success of Edmonton protocol7 provided the proof of concept for cellular therapy to treat T1DM and current status was reviewed recently8,9. Islets isolated from cadavers infused in immuno-suppressed patients with diabetes through the portal vein resulted in quick and sustained insulin production7. However, need for alternative source of regulated insulin release is acutely felt due to scarcity of cadaveric islets. Use of foetal islets or those obtained from pigs have associated ethical and immunological concerns. In addition, use of pigs as a source of islets has associated issues like zoonoses.
Stem cells as a source of islets: Use of stem cells including pluripotent stem cells (PSCs), multipotent adult stem cells and progenitors holds lot of promise to treat a variety of diseases. Use of medicines and antibiotics can cure a disease whereas stem cells may be able to replace diseased cells with healthy cells and as a result the patient becomes free of the disease. Both pluripotent and adult stem cells have been used as a source for pancreatic islets. Approval given by US-FDA to study the efficacy and safety of embryonic stem cells derived pancreatic progenitors in T1DM patients is a major step in the field10.
Pluripotent stem cells: Pluripotent stem cells (PSCs) have the ability to self-renew and differentiate into three germ layers including ectoderm, endoderm and mesoderm, and hence can play an important role in regenerative medicine and cell therapy. PSCs are obtained from the inner cell mass of blastocyst (embryonic stem cells, ES) or from the foetal genital ridge (embryonic germ cells, EG). Human ES cell lines were first reported in 1998 by Prof. Thomson and his group11 whereas human EG cell lines were reported by Prof. Shamblott in the same year12. Technology also exists to derive PSCs from adult somatic cells by reprogramming them to embryonic state using a cocktail of factors (induced pluripotent stem cells, iPS) or by allowing factors present in the oocyte cytoplasm to reprogramme somatic cells (therapeutic cloning). Prof. Yamanaka shared the Nobel Prize for Medicine in 2012 for iPS technology13 whereas Prof. Mitalipov's group in 201314 was the first to derive human ES cell line by somatic cell nuclear transfer (SCNT).
The concept of ES cell therapy is simple and involves differentiation of ES cells (which can be expanded in large numbers in vitro due to their immortal status) into pancreatic progenitors for transplantation. The ES cells derived pancreatic progenitors can be packed in immuno-isolatory capsules prior to subcutaneous transplantation under the skin (thus avoid life-long immuno-suppressive therapy) and even if a teratoma forms - it would remain contained in the device and could be safely removed. These encapsulated cells (expected to mature into islets on transplantation) will have the ability to secrete appropriate amount of insulin in a glucose-responsive manner over a period of time. This will be a more physiological approach compared to daily insulin injections and are expected to remain functional over a longer time.
Jiang et al15 observed that 30 per cent of transplanted mice showed reduction in hyperglycaemia on transplanting insulin positive cells, obtained by differentiating ES cells, for over a period of six months. Thus proof of concept for use of human ES cells for diabetes was established, however, the process remains highly inefficient. Schulz et al16 developed a scalable system for producing functional progenitors and Bruin et al17 improved the differentiation protocol further which resulted in grafts containing >80 per cent endocrine cells and resulted in single hormonal cells expressing either insulin or glucagon or somatostatin in contrast to earlier polyhormonal cells. Kirk et al18 have demonstrated that human insulin is secreted by seven weeks after transplantation of encapsulated pancreatic progenitors and by 20 wk enough human insulin is produced to ameliorate alloxan-induced diabetic symptoms. Endocrine cells that differentiated were monohormonal and insulin was produced in response to a glucose challenge. Thus, impressive progress has been made and the recent approval from US-FDA for a clinical trial using encapsulated cell replacement therapy termed VC-01 from ViaCyte in T1DM patients appears to be very promising9. Tabar and Studer19 have discussed the existing challenges in translating ES based cell therapies to the clinic. These include issues related to huge costs involved, scalability, clinical grade of stem cell products, genetic, epigenetic and safety concerns, etc. Although ES/iPS theoretically have the ability to differentiate into functional beta cells but the field has not advanced as expected20.
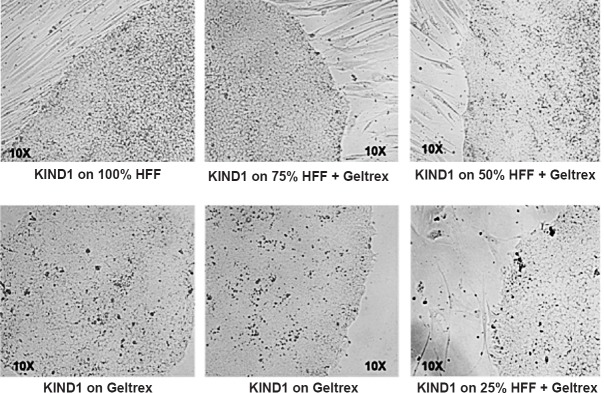
Our group after having derived two well-characterized human ES cell lines (KIND1 and KIND2)21 and studying their propensity22, carried out studies to differentiate KIND1 ES cells into pancreatic progenitors23. Adapting our cell lines to feeder-free state was a big achievement wherein the ES cells initially grown on human foetal fibroblast feeder support were gradually transitioned to feeder-free state and yet the cells maintained their pluripotent characteristics. KIND1 cells have been gradually transitioned into feeder-free state (Fig. 1), expanded in feeder-free state for almost 160 passages and these still maintained a normal karyotype and pluripotent characteristics. Feeder-free KIND1 hES cells were then gradually differentiated into pancreatic progenitors using a modified version of protocol24. For differentiation of KIND1 cells into pancreatic progenitors, briefly the KIND1 feeder-free cells at 80 per cent confluence are used for a 16 days differentiation protocol wherein the cells gradually transition from undifferentiated state- definitive endoderm- primitive gut tube- to finally pancreatic progenitor stage23.
Fig. 1.
Establishment of feeder-free culture of human embryonic stem cell. KIND1 cells growing on human feeder fibroblasts (HFF) were transitioned to feeder-free culture system by gradually reducing feeder density while maintaining the concentration of Geltrex (synthetic extracellular matrix).
All cells in human body have the same basic genetic information, yet different cells have different morphology and function. Strict epigenetic control by several protein complexes such as polycomb group complexes, trithorax group proteins, histone acetylases, histone deacteylases, DNA methylation enables this feat. Amongst these, polycomb group proteins have received greater attention since these are required during differentiation at embryonic stage and their aberrant expression leads to tumour formation25,26. Several polycomb group proteins associate with each other and form large protein complexes called Polycomb Repressive complexes (PRC), two of the well studied PRCs are PRC1 and PRC2. On comparing the epigenetic profile of the pancreatic progenitors obtained after 16 days in culture to adult human pancreas the PRC1 and PRC2 transcripts showed minimal expression in the adult human pancreas compared to high expression in the pancreatic progenitors23 (Fig. 2). Could this difference in the epigenetic profiles of adult pancreatic cells and the pancreatic progenitors derived from the ES cells prevent long-term benefits after stem cell therapy, remains to be answered. Also these differences could suggest that the differentiated progeny more closely resembles their foetal counterparts rather than the adult (we did not have access to foetal pancreatic tissue sample to do a comparison) as recently concluded by Tabar and Studer19. Dimmeler et al27 also conclude that although now we know a lot about stem cells biology, a huge gap needs to be bridged before these cells can be successfully utilized in the clinic. Further studies are required to evaluate whether this epigenetic status of ES-derived progenitors gets ameliorated after transplantation in diabetic mice following further maturation in vivo.
Fig. 2.
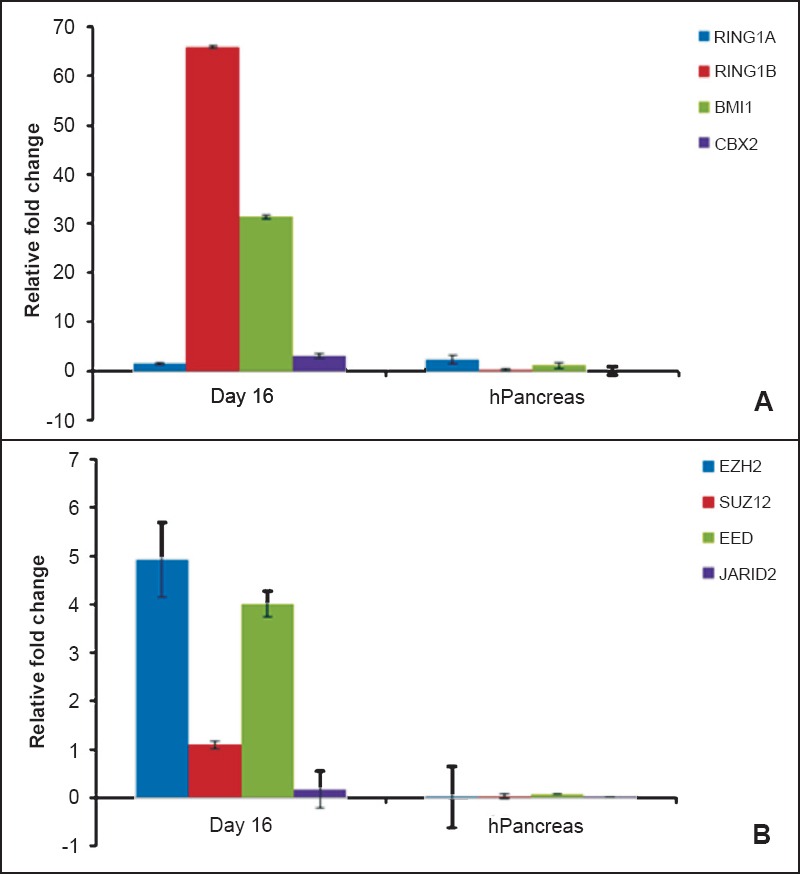
Comparison of polycomb repressive complexes (PRC) expression in pancreatic progenitors derived from human embryonic stem (hES) cells on day 16 and adult human pancreas. Expression of (A) PRC1 (RING1A, RING1B, BMI1 and CBX2) and (B) PRC2 (SUZ12, EZH2, EED and JARID2) complexes was compared by qRT-PCR. Results revealed distinct epigenetic differences of pancreatic progenitors derived from hES cells and adult human pancreas when compared to that of undifferentiated human ES cells used as reference sample; analyzed by CtΔΔ method. Expression of polycomb group (PcG) proteins in adult human pancreas (normalized to 18S, Ct40) was shown by our group earlier (Ref. 23). These differences may ameliorate after transplantation or possibly are the underlying cause to explain that hES cells differentiate into their fetal counterparts and may thus not be very useful to regenerate adult pancreas. Results are representative of five biological replicates.
[Source: Adapted from Ref. 23, Figures 6 & 9, reproduced with permission].
Adult stem cells: Besides the pluripotent stem cells as a source of islets, various groups have used adult stem cells like bone marrow and mesenchymal cells to treat diabetes. Two broad directions were followed including (i) clinicians directly tested efficacy of autologous stem cells in treating the pancreas in the clinic, and (ii) basic scientists studied ‘trans-differentiation potential’ of mesenchymal stem cells (from various sources) into islets.
Cell therapy using adult stem cells: Bhansali et al28,29 injected autologous bone marrow stem cells directly into the pancreas via the gastrodudenal artery in T2DM and observed certain degree of success. Dave et al30 transplanted autologous adipose tissue transdifferentiated insulin-making cells in two patients with T1DM which resulted in reduced insulin requirement over long term and in a separate study reported that these stem cells were better than bone marrow derived haematopoietic stem cells transplantation31. Another group32 showed that autologous bone marrow stem cells when transplanted through intrahepatic route in two T1DM patients resulted in increased c-peptide and HbA1c levels and reversed the production of anti-pancreatic islet antibody during 12 months follow up. Liu et al33 have transplanted Wharton's Jelly derived MSCs (through intravenous and intrapancreatic endovascular injection) in 22 T2DM patients. The signs of inflammation, glucose and HbA1c levels were reduced and an improvement of C-peptide levels was observed. They concluded that therapeutic benefit may be due to improved systemic inflammation and/or immunological regulation. However, these are pilot studies showing marginal benefit and are similar to several pilot studies undertaken using autologous bone marrow stem cells to improve cardiac function. But data are now accumulating that on conducting double blind trials the beneficial effect of stem cell therapy to treat heart diseases may not exist. A meta-analysis of six clinical trials on use of bone marrow stem cell therapy to treat heart disease showed that the effect of therapy on the left ventricular ejection fraction was zero34.
Basic research using adult stem cells: Several groups have attempted to culture and differentiate MSCs into islets in vitro 35-39. Gopurappilly et al40 used MSCs isolated from the pancreas to differentiate into islets. The foetal islets can be expanded in culture to obtain MSCs41. Parekh et al42 used 270 cord blood samples to evaluate the ability of cord blood mononuclear cells to differentiate into islets and concluded that a sub-set of ‘pancreas committed cells’ existed whose numbers increased after the mice underwent partial pancreatectomy. To conclude, these attempts by various investigators have remained inefficient and the concept remains controversial as it involves de-differentiation followed by re-differentiation into a different lineage since MSCs are mesodermal in origin whereas beta cells are endodermal. Although phenotypic changes are reported of MSCs changing into islets, a robust functional ability of the differentiated islet-like structures has not been demonstrated.
MSCs have also been injected directly in the pancreas and being niche providing cells, these have helped alleviate diabetes symptoms through several mechanisms, such as by improving metabolic control in animal models, counteracting autoimmunity, enhancing islet engraftment and survival or as a source of growth factors and cytokines43. It is of interest to note that injecting MSCs not only helps improve pancreatic functions, but also heal associated symptoms like diabetic foot, nephropathy, neuropathy, etc. Thus the effect of MSCs appears to be more generalized and most probably is a niche effect rather than true regeneration.
Regenerating the islets in pancreas: Major issue in T2DM is insulin resistance and patients are advised lifestyle changes (diet and exercise) along with insulin sensitizers to improve the sensitivity of muscle, fat and liver for insulin and thus resulting in reduced blood sugar. The sensitivity of various body organs to insulin is reduced and hence insulin requirement is greatly increased but beta cells are unable to secrete. As a result T2DM patients lose their ability to produce insulin and may also benefit if their pancreas could be made functional again by regenerating insulin producing islets from endogenous stem cells. This has led to an interest in understanding endogenous pancreatic stem cells/ progenitors and whether one can target them as a cure for type 2 diabetes. It may thus be possible to replenish the damaged islets in T2DM by stimulating the endogenous stem cells and thus reverse symptoms of diabetes. However, the true identity of pancreatic stem cells remains unresolved20.
Pancreas is one of the organs besides lung and liver that shows huge potential to regenerate. Regeneration of pancreas occurs successfully even after almost 80-90 per cent of pancreatectomy in mice44. The pancreas of mice with streptozotocin induced diabetes can also regenerate after pancreatectomy and reverse diabetes symptoms45,46. However, the underlying mechanism how this regeneration occurs, is still controversial. Three schools of thoughts exist including (i) reduplication of existing islets, (ii) involvement of ductal epithelium, and (iii) neogenesis of new islets from stem/progenitor cells. However, the existence and identity of such stem cells/progenitors remain obscure till date as direct proof of their existence is still lacking.
Bonner-Weir's group47 from the Harvard Stem Cell Institute, USA, has made seminal contributions and developed the concept of ductal epithelium (DE) as a source of pancreatic progenitors that can regenerate adult pancreas after partial pancreatectomy. Pancreatic regeneration is understood to recapitulate embryonic development with a burst of epithelium in the ductal epithelium. They suggest that DE cells undergo de-differentiation to an earlier stage of a progenitor which can differentiate into islets and acinar cells. These progenitors are active during regeneration. However, use of various ductal epithelium specific markers like human carbonic anhydrase II (CAII) promoter48 or HNF1 β49 or Sox 950 for lineage tracing studies to convincingly show involvement of ductal epithelium in pancreas regeneration has resulted in controversial data. Further studies are required to support the yet controversial ductal origin hypothesis. Kushner et al51 have discussed that if duct cells are not the origin for increased number of islets observed after ductal ligation then what other candidates can produce beta cells so quickly and why they have not yet been identified and reported. They concluded that data generated in next few years will have surprises in the exciting field of pancreatic regeneration.
Xiao et al4,52 have discussed various studies done in support of reduplication of islets as a means for regeneration versus neogenesis and associated artefacts that could have resulted in controversial results. They used tamoxifen independent INSCremTmG mice where all cells are Tomato+ (except insulin expressing cells) whereas beta cells are GFP+ (green fluorescent protein). They proposed that if any non-islet cell should give rise to the islets – a transient yellow colour will be observed and picked up in an objective manner by flow cytometry. They could not detect any yellow cell by flow cytometry and thus concluded that neogenesis of β cells in the pancreas was a rare event. These results are in agreement with earlier reports53,54 which also found no evidence of neo-genesis of islets. To conclude from the available literature, despite huge research efforts by several groups worldwide scientific community has not yet been able to throw any light on underlying cellular mechanisms responsible for adult pancreas regeneration. Such knowledge will be helpful to regenerate a pancreas in a patient with diabetes and will have tremendous clinical relevance20.
We have reported the presence of very small embryonic-like stem cells (VSELs) in adult mouse pancreas5. Flow cytometry analysis showed that 0.6 per cent of pancreatic cells are 3-5 µm LIN-/CD45-/SCA+ VSELs. We have further reported that extensive mobilization of octamer-binding transcription factor 4 (OCT-4) positive stem cells occurs into the pancreas after partial pancreatectomy and that these are involved in regeneration5. We report the presence of cells that co-express OCT-4 and PDX-1 suggesting that nuclear OCT-4 positive VSELs give rise to PDX-1 positive progenitors which regenerate both acinar and β-cells. We have recently discussed the cause for the existing confusion by various groups and how the VSELs have eluded the scientists because of their very small size6. Earlier, VSELs have been reported for the first time in mouse pancreas by Zuba-Surma et al55 and VSELs get mobilized after streptozotocin treatment in mice56 and also in patients with pancreatic cancer57.
Similarly, marginal trans-differentiation reported by various investigators while differentiating MSCs into islets could have been because of VSELs which always exist as a sub-group among MSCs58. The conclusion made after using 270 cord blood samples by Hardikar's group45 that a sub-set of ‘pancreas committed cells’ exists whose numbers increase after the mice undergo partial pancreatectomy was true but the VSELs remained elusive in their study. VSELs are the endogenous pluripotent stem cells responsible for adult pancreatic regeneration and have the potential to treat T2DM. OCT-4 and other pluripotent transcripts are also reported in normal human pancreas59,60 and during pancreatic cancers61.
Conclusions
To conclude, there are several available options to regenerate the diabetic pancreas including pluripotent ES/iPS cells, adult stem cells and VSELs. VSELs have an edge over others as these are pluripotent stem cells in adult pancreas; do neither have associated immune-rejection issues nor risk of teratoma formation. However, the controversy surrounding the very existence of VSELs needs to be settled first. These stem cells have remained elusive for decades because of their small size and inadvertently get discarded during processing6,62. It is seemingly difficult for scientific community to accept their presence. We have recently reported detailed characterization of cord blood VSELs and shown that these are normal, non-apoptotic, diploid and quiescent cells expressing pluripotent and primordial germ cells specific markers present in the red blood cells pellet after Ficoll-Hypaque centrifugation of cord blood63. Moreover, these stem cells regenerate adult pancreas (both acinar cells and islets) and there is no need to culture or expand them in a cyclic guanosine monophosphate (Current Good Manufacturing Practice) facility - rather we need to develop strategies to manipulate the endogenous VSELs to our advantage. There is a need to first arrive at a consensus on the definition of stem cells62 and then be ready for mid-course corrections to successfully exploit the potential of VSELs in the field of regenerative medicine. This requires extensive brainstorming and support from funding agencies and policymakers to make further progress in the field.
Acknowledgment
Part of work on embryonic stem cells is done by Dr Prasad Pethe as part of his Ph.D. work. This work was supported by the Indian Council of Medical Research (ICMR) and partly by the Department of Biotechnology (DBT), New Delhi, India.
Footnotes
Conflicts of Interest: None.
References
- 1.Mohan V, Sandeep S, Deepa R, Shah DB, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217–30. [PubMed] [Google Scholar]
- 2.Misra A, Shrivastava U. Obesity and dyslipidemia in South Asians. Nutrients. 2013;5:2708–33. doi: 10.3390/nu5072708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li WC, Rukstalis JM, Nishimura W, Tchipashvili V, Habener JF, Sharma A, et al. Activation of pancreatic-duct-derived progenitor cells during pancreas regeneration in adult rats. J Cell Sci. 2010;123:2792–802. doi: 10.1242/jcs.065268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao X, Chen Z, Shiota C, Prasadan K, Guo P, El-Gohary Y, et al. No evidence for β cell neogenesis in murine adult pancreas. J Clin Invest. 2013;123:2207–17. doi: 10.1172/JCI66323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhartiya D, Mundekar A, Mahale V, Patel H. Very small embryonic-like stem cells are involved in regeneration of mouse pancreas post-pancreatectomy. Stem Cell Res Ther. 2014;5:106–17. doi: 10.1186/scrt494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhartiya D, Patel H. Very small embryonic-like stem cells are involved in pancreatic regeneration and their dysfunction with age may lead to diabetes and cancer. Stem Cell Res Ther. 2015;6:96–102. doi: 10.1186/s13287-015-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro AM. Immune antibody monitoring predicts outcome in islet transplantation. Diabetes. 2013;62:1377–8. doi: 10.2337/db13-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruni A, Gala-Lopez B, Pepper AR, Abualhassan NS, Shapiro AJ. Islet cell transplantation for the treatment of type 1 diabetes: recent advances and future challenges. Diabetes Metab Syndr Obes. 2014;7:211–23. doi: 10.2147/DMSO.S50789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.New encapsulated beta-cell replacement therapy for type 1 diabetes. [accessed on August 8, 2014]. Available from: http://www.diabetesincontrol.com/new-encapsulated-beta-cell-replacement-therapy-for-type-1-diabetes/
- 11.Thomson JA, Itskovitz-Eldor J, Shapiro, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 12.Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA. 1998;95:13726–31. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228–38. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, et al. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–53. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- 16.Schulz TC, Young HY, Agulnick AD, Babin MJ, Beatge EE, Bang AG, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS One. 2012;7:e37004. doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruin JE, Erener S, Vela J, Hu X, Johnson JD, Kurata HT, et al. Characterization of polyhormonal insulin-producing cells derived in vitro from human embryonic stem cells. Stem Cell Res. 2014;12:194–208. doi: 10.1016/j.scr.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Kirk K, Hao E, Lahmy R, Itkin-Ansari P. Human embryonic stem cell derived islet progenitors mature inside an encapsulation device without evidence of increased biomass or cell escape. Stem Cell Res. 2014;12:807–14. doi: 10.1016/j.scr.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Tabar V, Studer L. Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat Rev Genet. 2014;15:82–92. doi: 10.1038/nrg3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang FX, Morahan G. Pancreatic stem cells remain unresolved. Stem Cells Dev. 2014;23:2803–12. doi: 10.1089/scd.2014.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar N, Hinduja I, Nagvenkar P, Pillai L, Zaveri K, Mukadam L, et al. Derivation and characterization of two genetically unique human embryonic stem cell lines on in-house-derived human feeders. Stem Cells Dev. 2009;18:435–45. doi: 10.1089/scd.2008.0234. [DOI] [PubMed] [Google Scholar]
- 22.Nagvenkar P, Pethe P, Pawani H, Telang J, Kumar N, Hinduja I, et al. Evaluating differentiation propensity of in-house derived human embryonic stem cell lines KIND-1 and KIND-2. In Vitro Cell Dev Biol Anim. 2011;47:406–19. doi: 10.1007/s11626-011-9420-9. [DOI] [PubMed] [Google Scholar]
- 23.Pethe P, Nagvenkar P, Bhartiya D. Polycomb group protein expression during differentiation of human embryonic stem cells into pancreatic lineage in vitro. BMC Cell Biol. 2014;15:18–29. doi: 10.1186/1471-2121-15-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–52. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 25.Rajasekhar VK, Begemann M. Concise review: Roles of polycomb group proteins in development and disease: a stem cell perspective. Stem Cells. 2007;25:2498–510. doi: 10.1634/stemcells.2006-0608. [DOI] [PubMed] [Google Scholar]
- 26.Sauvageau M, Sauvageau G. Polycomb group proteins multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell. 2010;7:299–312. doi: 10.1016/j.stem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dimmeler S, Ding S, Rando TA, Trounson A. Translational strategies and challenges in regenerative medicine. Nat Med. 2014;20:814–21. doi: 10.1038/nm.3627. [DOI] [PubMed] [Google Scholar]
- 28.Bhansali A, Upreti V, Khandelwal N, Marwaha N, Gupta V, Sachdeva N, et al. Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev. 2009;18:1407–16. doi: 10.1089/scd.2009.0164. [DOI] [PubMed] [Google Scholar]
- 29.Bhansali A. Cost of diabetes care: prevent diabetes or face catastrophe. J Assoc Physicians India. 2013;61:95. [PubMed] [Google Scholar]
- 30.Dave SD, Trivedi HL, Chooramani SG, Chandra T. Management of type 1 diabetes mellitus using in vitro autologous adipose tissue trans-differentiated insulin-making cells. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-200226. pii.bcr2013200226.doi:10.1136/bcr-2013-200226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dave SD, Trivedi HL, Gopal SC, Chandra T. Combined therapy of insulin-producing cells and haematopoietic stem cells offers better diabetic control than only haematopoietic stem cells’ infusion for patients with insulin-dependent diabetes. BMJ Case Rep. 2014 doi: 10.1136/bcr-2013-201238. pii.bcr2013201238.doi:10.1136/bcr-2013-201238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mesples A, Majeed N, Zhang Y, Hu X. Early immunotherapy using autologous adult stem cells reversed the effect of anti-pancreatic islets in recently diagnosed type 1 diabetes mellitus: preliminary results. Med Sci Monit. 2013;19:852–7. doi: 10.12659/MSM.889525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Zheng P, Wang X, Dai G, Cheng H, Zhang Z, et al. A preliminary evaluation of efficacy and safety of Wharton's jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cell Res Ther. 2014;5:57–65. doi: 10.1186/scrt446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nowbar AN, Mielewczik M, Karavassilis M, Dehbi HM, Shun-Shin MJ, Jones S, et al. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. BMJ. 2014;348:g2688. doi: 10.1136/bmj.g2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Dou Z. Under a non-adherent state, bone marrow mesenchymal stem cells can be efficiently induced into functional islet-like cell clusters to normalize hyperglycemia in mice: a control study. Stem Cell Res Ther. 2014;5:66–78. doi: 10.1186/scrt455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadam S, Govindasamy V, Bhonde R. Generation of functional islets from human umbilical cord and placenta derived mesenchymal stem cells. Methods Mol Biol. 2012;879:291–313. doi: 10.1007/978-1-61779-815-3_17. [DOI] [PubMed] [Google Scholar]
- 37.Phadnis SM, Joglekar MV, Dalvi MP, Muthyala S, Nair PD, Ghaskadbi SM, et al. Human bone marrow-derived mesenchymal cells differentiate and mature into endocrine pancreatic lineage in vivo. Cytotherapy. 2011;13:279–93. doi: 10.3109/14653249.2010.523108. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Shen W, Hua J, Lei A, Lv C, Wang H, et al. Pancreatic islet-like clusters from bone marrow mesenchymal stem cells of human first-trimester abortus can cure streptozocin-induced mouse diabetes. Rejuvenation Res. 2010;13:695–706. doi: 10.1089/rej.2009.1016. [DOI] [PubMed] [Google Scholar]
- 39.Gao F, Wu DQ, Hu YH, Jin GX, Li GD, Sun TW, et al. In vitro cultivation of islet-like cell clusters from human umbilical cord blood-derived mesenchymal stem cells. Transl Res. 2008;151:293–302. doi: 10.1016/j.trsl.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Gopurappilly R, Bhat V, Bhonde R. Pancreatic tissue resident mesenchymal stromal cell (MSC)-like cells as a source of in vitro islet neogenesis. J Cell Biochem. 2013;114:2240–7. doi: 10.1002/jcb.24572. [DOI] [PubMed] [Google Scholar]
- 41.Joglekar MV, Joglekar VM, Joglekar SV, Hardikar AA. Human fetal pancreatic insulin-producing cells proliferate in vitro. J Endocrinol. 2009;201:27–36. doi: 10.1677/JOE-08-0497. [DOI] [PubMed] [Google Scholar]
- 42.Parekh VS, Joglekar MV, Hardikar AA. Differentiation of human umbilical cord blood-derived mononuclear cells to endocrine pancreatic lineage. Differentiation. 2009;78:232–40. doi: 10.1016/j.diff.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Hashemian SJ, Kouhnavard M, Nasli-Esfahani E. Mesenchymal stem cells: Rising concerns over their application in treatment of type one diabetes mellitus. J Diabetes Res 2015. 2015:675103. doi: 10.1155/2015/675103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes. 1993;42:1715–20. doi: 10.2337/diab.42.12.1715. [DOI] [PubMed] [Google Scholar]
- 45.Hardikar AA, Karandikar MS, Bhonde RR. Effect of partial pancreatectomy on diabetic status in BALB/c mice. J Endocrinol. 1999;162:189–95. doi: 10.1677/joe.0.1620189. [DOI] [PubMed] [Google Scholar]
- 46.Finegood DT, Weir GC, Bonner-Weir S. Prior streptozotocin treatment does not inhibit pancreas regeneration after 90% pancreatectomy in rats. Am J Physiol. 1999;276:E822–7. doi: 10.1152/ajpendo.1999.276.5.E822. [DOI] [PubMed] [Google Scholar]
- 47.Bonner-Weir S, Inada A, Yatoh S, Li WC, Aye T, Toschi E, et al. Transdifferentiation of pancreatic ductal cells to endocrine beta-cells. Biochem Soc Trans. 2008;36:353–6. doi: 10.1042/BST0360353. [DOI] [PubMed] [Google Scholar]
- 48.Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA. 2008;105:19915–9. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solar M, Cardalda C, Houbracken I, Martin M, Maestro MA, De Medts N, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17:849–60. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653–65. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kushner JA, Weir GC, Bonner-Weir S. Ductal origin hypothesis of pancreatic regeneration under attack. Cell Metab. 2010;11:2–3. doi: 10.1016/j.cmet.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao X. How do β cells regenerate?. A critique. J Cytol Histol. 2014;5:2–3. [Google Scholar]
- 53.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–6. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 54.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–26. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Zuba-Surma EK, Kucia M, Wu W, Klich I, Lillard JW, Jr, Ratajczak J, et al. Very small embryonic-like stem cells are present in adult murine organs: Image Stream-based morphological analysis and distribution studies. Cytometry A. 2008;73A:1116–27. doi: 10.1002/cyto.a.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang Y, Kucia M, Hussain LR, Wen Y, Xu H, Yan J, et al. Bone marrow transplantation temporarily improves pancreatic function in streptozotocin-induced diabetes: potential involvement of very small embryonic-like cells. Transplantation. 2010;89:677–85. doi: 10.1097/TP.0b013e3181c9dc7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Starzyńska T, Dąbkowski K, Błogowski W, Zuba-Surma E, Budkowska M, Sałata D, et al. An intensified systemic trafficking of bone marrow-derived stem/progenitor cells in patients with pancreatic cancer. J Cell Mol Med. 2013;17:792–9. doi: 10.1111/jcmm.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhartiya D. Are mesenchymal cells indeed pluripotent stem cells or just stromal cells?. OCT-4 and VSELs biology has led to better understanding. Stem Cells Int 2013. 2013:547501. doi: 10.1155/2013/547501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao M, Amiel SA, Christie MR, Muiesan P, Srinivasan P, Littlejohn W, et al. Evidence for the presence of stem cell-like progenitor cells in human adult pancreas. J Endocrinol. 2007;195:407–14. doi: 10.1677/JOE-07-0436. [DOI] [PubMed] [Google Scholar]
- 60.White MG, Al-Turaifi HR, Holliman GN, Aldibbiat A, Mahmoud A, Shaw JA. Pluripotency-associated stem cell marker expression in proliferative cell cultures derived from adult human pancreas. J Endocrinol. 2011;211:169–76. doi: 10.1530/JOE-11-0123. [DOI] [PubMed] [Google Scholar]
- 61.Herreros-Villanueva M, Bujanda L, Billadeau DD, Zhang JS. Embryonic stem cell factors and pancreatic cancer. World J Gastroenterol. 2014;20:2247–54. doi: 10.3748/wjg.v20.i9.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhartiya D. Stem cells, progenitors and regenerative medicine: A retrospection. Indian J Med Res. 2015;141:154–61. doi: 10.4103/0971-5916.155543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shaikh A, Nagvenkar P, Pethe P, Hinduja I, Bhartiya D. Molecular and phenotypic characterization of CD133 and SSEA4 enriched very small embryonic-like stem cells in human cord blood. Leukemia. 2015;29:1909–17. doi: 10.1038/leu.2015.100. [DOI] [PubMed] [Google Scholar]