Abstract
Background & objectives:
Certain antiepileptic drugs (AEDs) such as valproic acid (VPA) are known to affect body weight, and lipid profile. However, evidences regarding effects of AEDs on the body composition are deficient. This cross-sectional study compared the body composition and lipid profile among patients with epilepsy on newer and conventional AEDs.
Methods:
The patients with epilepsy (n=109) on treatment with conventional and newer AEDs (levetiracetam, lamotrigine and clobazam) for > 6 months were enrolled. Of these, 70 were on monotherapy: levetiracetam (n=12), VPA (n=16), carbamazepine (n=20) and phenytoin (n=22) and the remaining on polytherapy. Their body composition [body fat mass, lean dry mass (LDM), total body water (TBW), intracellular water (ICW), extracellular water (ECW) and basal metabolic rate (BMR) was estimated and biochemical parameters were assessed.
Results:
Levetiracetam group had no significant difference with VPA, carbamazepine, phenytoin and control groups, except low LDM (17.8±2.4) than VPA groups (20.2±2.7, P<0.05). In comparison with control, AEDs monotherapy groups had no significant difference, except higher LDM and ECW in VPA group. Among groups based on conventional and newer AEDs, there was no significant difference in body composition parameters except for higher LDM (as % of BW) in conventional AEDs only treated group than control (P<0.01).
Interpretation & conclusions:
The alterations observed in body composition with valproic acid in contrast to other AEDs like levetiracetam, carbamazepine and phenytoin could affect treatment response in epilepsy especially in subjects with already altered body composition status like obese and thin frail patients, which needs to be established by prospective studies (CTRI/2013/05/003701).
Keywords: Antiepileptic drugs, body composition, body water, lean dry mass, levetiracetam, valproic acid
It has been estimated by World Health Organization (WHO) that around 50 million people worldwide have epilepsy and nearly 80 per cent of the people with epilepsy reside in developing regions1. The pattern of antiepileptic drugs (AEDs) use showed that nearly 50 per cent of the persons with epilepsy are on newer AEDs as monotherapy or add-on drug in polytherapy2. Several AEDs are associated with weight gain such as gabapentin, pregabalin, valproic acid (VPA), vigabatrin and to some extent carbamazepine (CBZ). Others are weight neutral such as lamotrigine (LTG), levetiracetam (LEV), and phenytoin (PHT) or associated with slight weight loss such as felbamate3. The studies done so far regarding the effect of AEDs on nutritional status have mostly concentrated upon the effect of VPA, particularly on body weight, body mass index (BMI) and in some studies on body fat mass (FM)4,5. The accuracy of the clinical screening of malnutrition based only on anthropometry measurements like weight, height, BMI could be limited; e.g. subjects may have the same BMI but a significantly decreased fat free mass (FFM) hidden by an expansion of the FM6. The FFM is sum of total body water (TBW) and lean dry mass (LDM). Body composition variation occurs due to imbalance between FM and FFM. The FFM loss is negatively correlated with survival in different disease conditions7,8.
Some studies have reported unfavourable effects of VPA on lipid profile like reduction in high density lipoprotein (HDL)-cholesterol and increase in triglyceride (TG) levels4,9. Reports on the effects of CBZ and PHT on lipid profile are contradictory4,10,11, and a few studies have demonstrated neutral effect of LEV and LTG on lipid profile11. Thus, the effect of chronic AEDs treatment on the lipid profile is inconclusively reported.
The newer AEDs are considered better alternative with similar efficacy to the classic first-line conventional AEDs, but sufficient data on the new AEDs to support this hypothesis are lacking. Therefore, the present study was done to compare body composition status and lipid profile among the patients with epilepsy on long term treatment of different AEDs including both conventional and newer AEDs.
Material & Methods
The present cross-sectional study included ambulatory persons of either gender >18 yr of age, clinically diagnosed with epilepsy, receiving conventional and newer AEDs as monotherapy or polytherapy for at least six months from Neurology Out Patient Department- Epilepsy clinic, All India Institute of Medical Sciences (AIIMS), New Delhi. The AEDs considered were VPA, CBZ, PHT, LEV, LTG, and clobazam (CLB). Those receiving other than above mentioned AEDs, dietary/trace element supplements (excluding calcium or folic acid supplement), having signs of malnutrition, dietary restrictions, abnormal neurological findings, serious co-morbidities, other systemic diseases, subjects who had undergone exercise within eight hours, taken alcohol within 12 h prior to the study and unwillingness to provide informed consent were excluded from this study. Screening was done for consecutive subjects and they were enrolled after satisfying the inclusion and exclusion criteria and after obtaining written informed consent between August 2012 and January 2013. A total of 109 patients with epilepsy who were in interictal period and at least 24 h seizures free at the time of study, were enrolled. Apparently healthy age and gender matched individuals accompanying the patients and satisfying the eligibility criteria for healthy volunteers were recruited as healthy controls (n=42). The study protocol was approved by the Institute's Ethics Committee.
A detailed medical history including seizure history and compliance to therapy, geographical, socio-demographic background, dietary habits, available biochemical and radiological investigations were recorded. After body composition analysis, blood sample (3 ml) was collected for biochemical investigations.
Body composition analysis: Body weight was estimated by the Body Composition Analyzer (Tanita Corporation of America, Inc. Illinois, USA) and height was estimated by height measuring stature meter (San Surgicals, Delhi, India). Body composition analysis was performed by Bioelectrical Impedance Body composition Analyzer (BIA) Model: Quantum X (RJL Systems, Michigan 48035, USA). The analyzer was calibrated by using 500 ohm test resistor and attaching to the electrode clips. The resistance value was between 495 and 505 ohms and the reactance value was -003 to 003 ohms.
The subject lied supine with their arms 30 degrees from their body and legs not in contact with each other and adhesive electrodes with cables were placed on right side. After resistance and reactance readings were obtained, the electrodes were gently removed. Two consecutive measurements were taken on a single subject and the difference in values within one per cent was assumed as reliable reading. These readings along with subject's name, age, gender, height, weight, ID number, daily activity level were put on the RJL systems Body composition software (RJL Systems, Michigan, USA) to calculate the body composition based on the National Health and Nutrition Examination Survey (NHANES) criteria12. The parameters obtained through this calculation were: body FM in kg, FM, LDM, TBW, intracellular water (ICW), and extracellular water (ECW) as per cent of body weight, basal metabolic rate (BMR) in Kcal and BMI (kg/m2). FFM is the sum of TBW and LDM. TBW is the total amount of water present in the body, i.e. combination of ICW and ECW. LDM is the non-water portion of fat-free mass, which constitutes of bone mineral mass and visceral protein13. BMR is the number of calories that a person will use per day, and was calculated by the software using Harris-Benedict formula14. The control group was taken for comparison of the above parameters with the enrolled patients with epilepsy.
Biochemical investigations: Estimations of lipid profile, blood glucose level, liver and renal function parameters and electrolytes level were performed with an automated chemistry analyzer (Roche Hitachi 912 Chemistry Analyzer, GMI Inc., USA) in the Department of neuro-biochemistry, AIIMS, New Delhi, using kits from the analyzer manufacturer. Lipid profile parameters assessed were serum total cholesterol (TC), triglyceride (TG), low density lipoprotein (LDL)-C, HDL-C, and very low density lipoprotein (VLDL)-C.
Statistical analysis: Considering the BMI (mean 23.4±4.321 kg/m2) in Indian persons with epilepsy receiving six months of CBZ monotherapy in a previous study15; anticipating 20 per cent reduction in mean BMI in patients with new AEDs and with significance level (alpha) 5 and 80 per cent power, a sample size of 14 subjects per group was estimated for the study.
Data were analyzed using Statistical Package for the Social Sciences (SPSS) software ver. 16.0 (Chicago, IL, USA). The Shapiro-Wilk method was used to check the distribution of data. Study group variables with normal distribution were compared by using analysis of variance (ANOVA); for variables without normal distribution Kruskal Wallis and Mann-Whitney U tests were used. Chi square test was used for analysis of categorical variables. Data were expressed as mean ± SD for parametric data and as median and range (minimum-maximum) for non-parametric data.
Results
A total of 138 patients with epilepsy were screened for enrolment, 29 did not meet eligibility criteria; and a total of 109 eligible subjects were enrolled in the study. A total of 70 age-matched apparently healthy persons were screened, and of these, 42 were enrolled as healthy control.
Of the total 109 patients with epilepsy, 70 were on monotherapy of AEDs: VPA (n=16), CBZ (n=20), PHT (n=22), and LEV (n=12) (Table I). None of them were on monotherapy of LTG and CLB. According to the type conventional or new drug use, the 109 patients were grouped as: Group A (one or more conventional AEDs: VPA, CBZ, and/or PHT) (n=60); Group B (one or more new AEDs: LEV, LTG, and/or CLB) (n=15); Group C (combination of conventional and new AEDs) (n=34). Eleven subjects were on LTG, of these, three were in combination with LEV and eight were in combination with conventional AEDs. Eleven were on CLB, and all were in combination with conventional AEDs.
Table I.
Demographic, clinical and treatment characteristics of patients on antiepileptic drugs (AEDs) monotherapy and control group
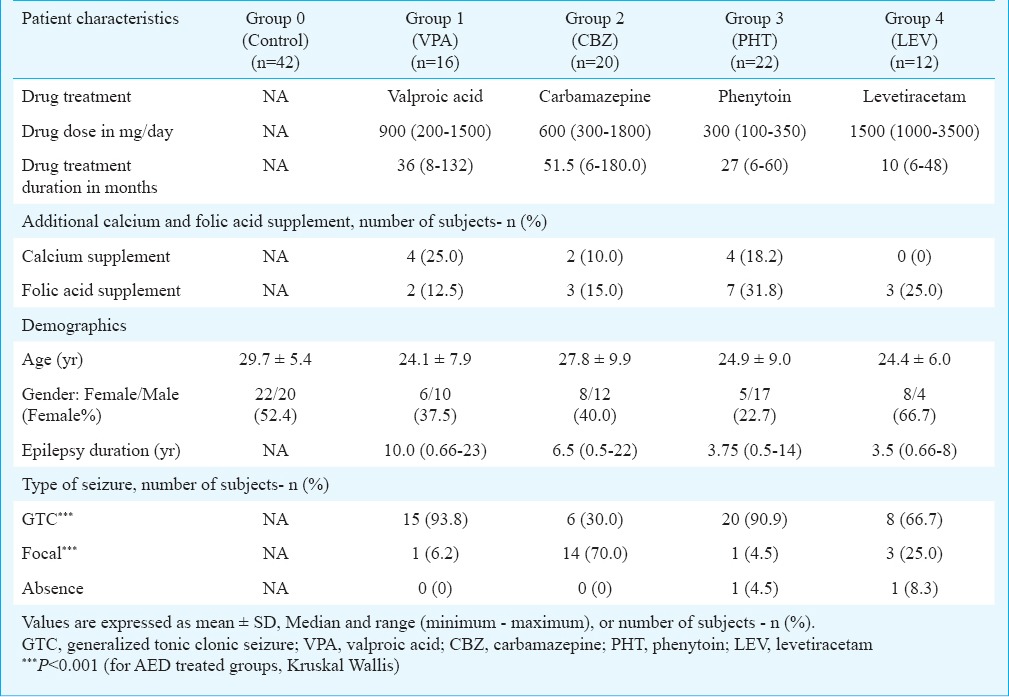
There was no significant difference among monotherapy groups in age and epilepsy duration. Number of female subjects was minimum in group 3 (PHT) (22.7%) and highest in group 4 (LEV) (66.7%). Subjects with generalized seizures were predominant in all monotherapy groups, except CBZ group having 70.0 per cent subjects with focal seizure (Table I).
The demographic characteristics of groups based on conventional and new AEDs were comparable in terms of age, gender distribution, height, weight, body mass index, epilepsy duration and type of seizure distribution without any significant difference. However, control group had higher mean age (29.7±5.4 yr) (P<0.05) and higher body weight (66.3±7.8 kg) (P<0.05) as compared to other groups. Mean ages in other groups were as: group A (conventional AEDs only) (25.5±9.0 yr), group B (newer AEDs only) (25.1±6.9 yr), and group C (conventional with newer AEDs) (25.7±8.4 yr). Mean body weights in other groups were as follows: group A (59.5±13.6 kg), group B (60.7±11.6 kg), and group C (60.6±11.6 kg).
Comparison of levetiracetam monotherapy with other groups: Body composition parameters followed a normal distribution. No significant difference was found between VPA and LEV groups except in LDM (as % of BW) (20.2±2.7 vs. 17.8±2.4 respectively, P<0.05). There were no significant differences between LEV group in comparison with CBZ, PHT and control groups (Table II).
Table II.
Comparison of body composition among antiepileptic drugs monotherapy groups and control subjects
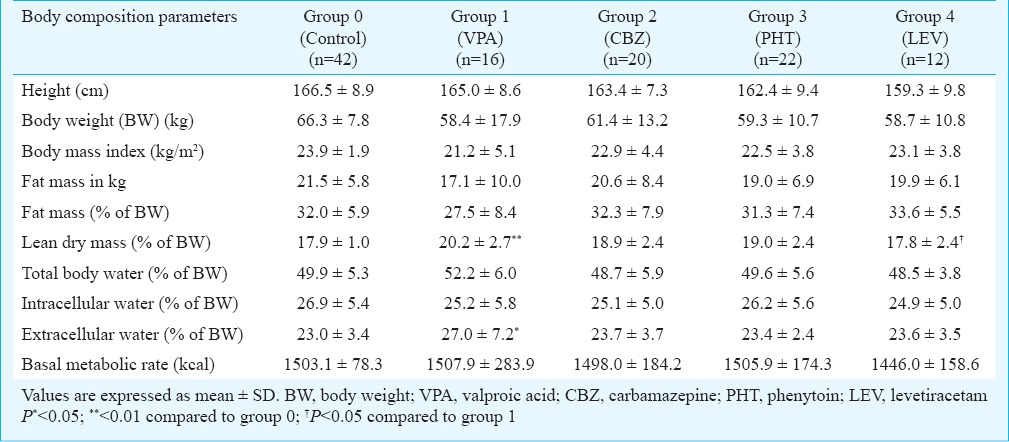
Comparison between monotherapy of conventional AED groups: The comparison among VPA, CBZ and PHT groups did not reveal any significant difference. There was a non-significant elevation of ECW (as % of BW) in VPA (27.0±7.2) than PHT group (23.4±2.4) (Table II).
Comparison of control group with AED monotherapy groups: Control group subjects had higher BW (66.3±7.8 kg), height (166.5±8.9 cm) and BMI (23.9±1.9 kg/m2) compared to other groups, but the differences were not significant. VPA group showed significantly higher LDM and ECW (as % of BW) (20.2±2.7 and 27.0±7.2, respectively) than that of control group (17.9±1.0 and 23.0±3.4, respectively) (P<0.01 and <0.05, respectively). There was no significant difference in body composition parameters between control group and CBZ, PHT and LEV monotherapy groups (Table II).
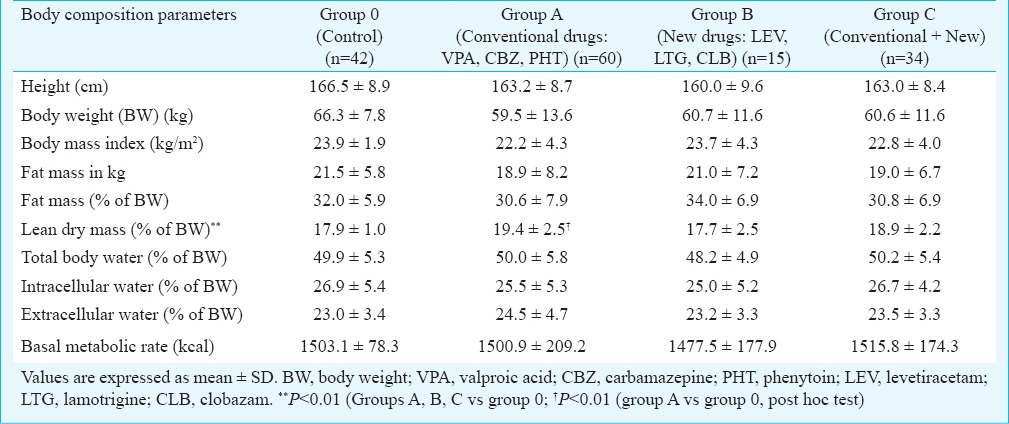
Body composition parameters: There was no significant difference in body composition parameters except for LDM. Overall difference was significant (P<0.01) for LDM (as % of BW) among Group A (conventional AEDs only) (19.4±2.5), group B (newer AEDs only) (17.7±2.5), group C (conventional with newer AEDs) (18.9±2.2) and control group (17.9±1.0). Post hoc test revealed significant difference (P<0.01) between control and group A (Table III).
Table III.
Body composition analysis of subjects on conventional and new AEDs
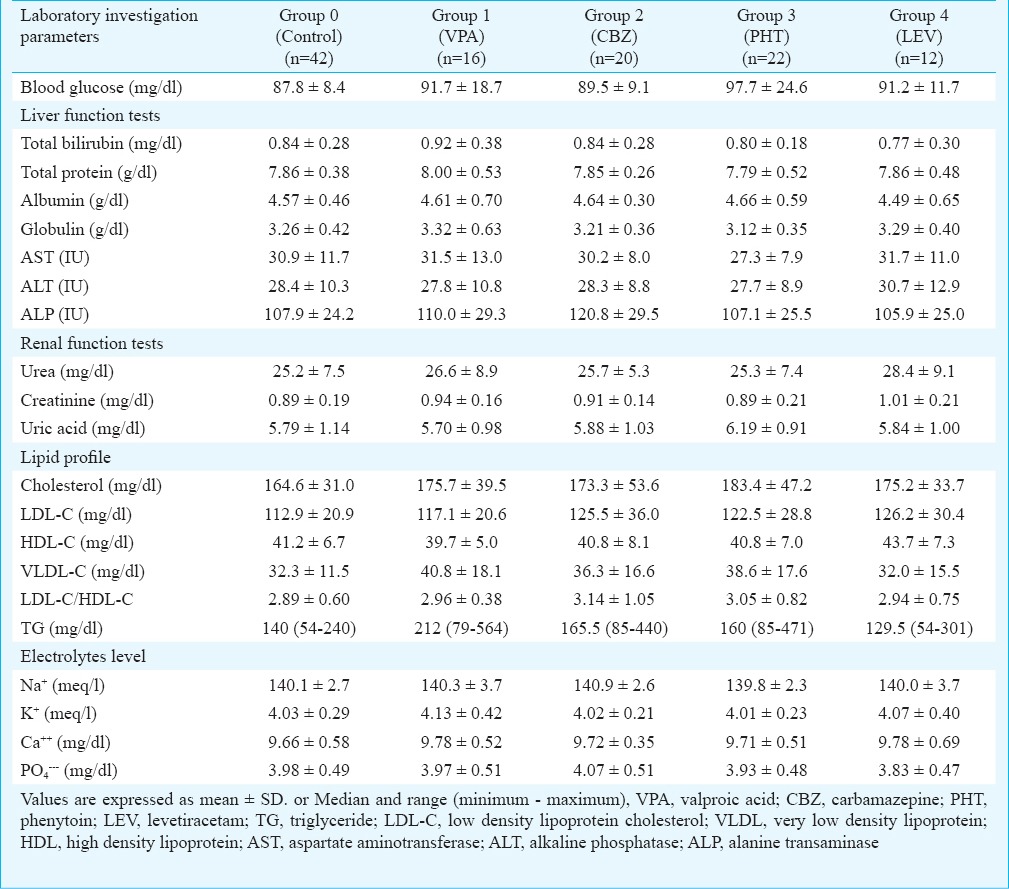
Biochemical parameters in AED monotherapy groups: There was no significant difference among AEDs monotherapy groups and control group in the blood glucose level, liver and renal function tests, electrolytes level and lipid profile. There was apparently higher triglyceride level in the VPA group [median (range): (212 (79-564) mg/dl] as compared to LEV group (129.5 (54-301) mg/dl), though the difference was not significant (Table IV).
Table IV.
Laboratory investigation parameters among AED monotherapy groups
Biochemical parameters in groups with conventional and new AEDs: The comparison between group A (conventional AEDs only), group B (newer AEDs only) and group C (conventional with newer AEDs) with respect to biochemical parameters did not reveal any significant difference, except LDL-C and triglyceride level. Overall difference (P<0.05) in LDL-C was due to higher level in group B (131.6±36.4 mg/dl), than control (112.9±20.9 mg/dl), group C (113.4±24.3 mg/dl) and group A (122.5±29.3 mg/dl). But post hoc test did not reveal any significant difference. Similarly, overall difference in triglyceride level (P<0.05) was due to higher level in group C [median (range): 188.5 (68-581) mg/dl] and group B [175 (54-565) mg/dl] than control [140 (54-240) mg/dl] and group A [166 (79-564) mg/dl]. Post hoc test revealed significant difference (P<0.01) between control and group C.
Discussion
Body composition evaluation has important contribution in medical management, especially in chronic diseases with long term treatment. It reduces the negative impact of malnutrition on the clinical outcome and quality of life, thereby increasing the overall medico-economic benefits16. Weight gain is a frequently occurring side effect of VPA, the real incidence and magnitude of which is unknown4,5,17-19. The present study having a limited sample size could not find any difference in body weight among AED treatment groups. In a study in 427 Indian women on different AEDs, it was found that 50 per cent subjects on VPA had weight gain5. Torrent et al18 have shown that VPA but not CBZ and LTG causes weight gain. However, there are some studies with contradictory results where weight gains were similar in both VPA and placebo group17,20. Espinosa et al21 reported significant weight gain with CBZ treatment rather than with VPA treatment in similar socio-economic group. Unfortunately, definite predictors of weight gain due to VPA therapy have not yet been identified, and this has been ascribed to leptin activation and insulin resistance, thereby increasing body fat mass and weight19. Some studies have reported weight neutral effect of CBZ5,22, while others suggested an increase in body weight21. There are not many studies regarding the effect of LEV on body weight, most have shown it as weight neutral3,22 and a few studies have shown that it causes weight reduction23,24.
In this study, all the study groups had mean BMI within the normal range, i.e. below 25 kg/m2 and there was no significant difference. Previous studies5,15,25 have reported increase in BMI in VPA treated subjects in a range of 2 to 4 kg/m2, however, de Vries et al26 have reported no increase in BMI after VPA treatment. There are previous studies demonstrating neutral effect of CBZ4,10,19, PHT5,15 and LTG4,19. However, some studies reported that women on CBZ and LTG had a greater BMI when compared with the control group15,27.
In this study, body FM analysis did not reveal any significant difference between LEV group compared to CBZ, PHT and control groups. However, VPA group had lower FM than LEV group, which could be probably explained by the lower percentage of female subjects (37.5%) in VPA group compared to LEV group (66.7%).
One of the explanation for body composition parameter deviation in VPA group in our study may be that VPA group patients had comparatively lower mean body weight, BMI and FM than those in the other groups, though the difference was not significant. The low percentage of FM in the VPA group may lead to high percentage of FFM in body, which can lead to high percentage of LDM and ECW. According to literature ECW occupies approximately 25 per cent of the total body mass28. A 40 per cent increase in ECW would result in only a ten per cent increase in TBW, which could be of considerable clinical importance, because excessive fluid retention in the extracellular space is known to cause increased morbidity in acutely ill patients28. However, in this study, VPA and PHT groups had mean ECW close to the reference range, and the apparent difference between them may not be clinically relevant.
In our study, lipid profile estimation among the monotherapy groups did not reveal any significant difference. Only triglyceride level was high in the VPA monotherapy group and in subjects on combination of conventional and newer AEDs. Further studies need to be done to find out the exact mechanism.
In conclusion, the results of this study showed no significant differences in body weight, BMI in any of the study groups. Body composition parameters did not differ significantly among new AEDs like LEV, conventional AEDs like CBZ and PHT and control group. However, VPA group showed significant alteration in body composition, i.e. low percentage of fat mass and high LDM than LEV group; more ECW as compared to PHT group; and more LDM and ECW as compared to control group. Lipid profile derangement (increased triglyceride) was evident in the combined conventional and new AEDs treated groups and VPA monotherapy group. These alterations in body composition with valproic acid in contrast to other AEDs could affect treatment response in epilepsy especially in subjects with already altered body composition status like obese and thin frail patients. The cross-sectional nature of the study and limited sample size preclude definite conclusion and requires larger prospective studies.
Acknowledgment
Authors acknowledge the laboratory technicians of Department of Neuro-Biochemistry, AIIMS, New Delhi, for assistance in biochemical investigations.
Footnotes
Conflicts of Interest: None.
References
- 1.World Health Organization. Epilepsy Fact Sheet N0999 October 2012. [accessed on January 10, 2014]. Available from: http://www.who.int/mediacentre/factsheets/fs999/en/index.html .
- 2.Sigamani A, Roy AK, Yeragani VK, Kulkarni C. Profile of pharmacotherapy and pharmacoeconomics of epilepsy treatment at a tertiary care hospital. Ann Neurosci. 2006;13:103–12. [Google Scholar]
- 3.Antel J, Hebebrand J. Weight-reducing side effects of the antiepileptic agents topiramate and zonisamide. Handb Exp Pharmacol. 2012;209:433–66. doi: 10.1007/978-3-642-24716-3_20. [DOI] [PubMed] [Google Scholar]
- 4.Ding MP, Bao YY, Chen Z, Liu ZR, Xu LL. Insulin resistance in epileptic patients during treatment of valproic acid. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2004;33:216–8. doi: 10.3785/j.issn.1008-9292.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Sahota P, Prabhakar S, Kharbanda PS, Bhansali A, Jain V, Das CP, et al. Seizure type, antiepileptic drugs, and reproductive endocrine dysfunction in Indian women with epilepsy: a cross-sectional study. Epilepsia. 2008;49:2069–77. doi: 10.1111/j.1528-1167.2008.01676.x. [DOI] [PubMed] [Google Scholar]
- 6.Kyle UG, Morabia A, Slosman DO, Mensi N, Unger P, Pichard C. Contribution of body composition to nutritional assessment at hospital admission in 995 patients: a controlled population study. Br J Nutr. 2001;86:725–31. doi: 10.1079/bjn2001470. [DOI] [PubMed] [Google Scholar]
- 7.Fürstenberg A, Davenport A. Assessment of body composition in peritoneal dialysis patients using bioelectrical impedance and dual-energy X-ray absorptiometry. Am J Nephrol. 2011;33:150–6. doi: 10.1159/000324111. [DOI] [PubMed] [Google Scholar]
- 8.Marin B, Desport JC, Kajeu P, Jesus P, Nicolaud B, Nicol M, et al. Alteration of nutritional status at diagnosis is a prognostic factor for survival of amyotrophic lateral sclerosis patients. J Neurol Neurosurg Psychiatry. 2011;82:628–34. doi: 10.1136/jnnp.2010.211474. [DOI] [PubMed] [Google Scholar]
- 9.Fang J, Chen S, Tong N, Chen L, An D, Mu J, et al. Metabolic syndrome among Chinese obese patients with epilepsy on sodium valproate. Seizure. 2012;21:578–82. doi: 10.1016/j.seizure.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Uludag IF, Kulu U, Sener U, Kose S, Zorlu Y. The effect of carbamazepine treatment on serum leptin levels. Epilepsy Res. 2009;86:48–53. doi: 10.1016/j.eplepsyres.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Mintzer S, Skidmore CT, Abidin CJ, Morales MC, Chervoneva I, Capuzzi DM, et al. Effects of antiepileptic drugs on lipids, homocysteine, and C-reactive protein. Ann Neurol. 2009;65:448–56. doi: 10.1002/ana.21615. [DOI] [PubMed] [Google Scholar]
- 12.Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnson CL, Heymsfield SB, et al. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26:1596–609. doi: 10.1038/sj.ijo.0802167. [DOI] [PubMed] [Google Scholar]
- 13.Jebb SA, Elia M. Techniques for the measurement of body composition: a practical guide. Int J Obes Relat Metab Disord. 1993;17:611–21. [PubMed] [Google Scholar]
- 14.Roza AM, Shizgal HM. The Harris Benedict equation reevaluated: resting energy requirements and the body cell mass. Am J Clin Nutr. 1984;40:168–82. doi: 10.1093/ajcn/40.1.168. [DOI] [PubMed] [Google Scholar]
- 15.Ayyagari M, Chitela SR, Kolachana V. Obesity, polycystic ovarian syndrome and thyroid dysfunction in women with epilepsy. Ann Indian Acad Neurol. 2012;15:101–5. doi: 10.4103/0972-2327.94992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amaral TF, Matos LC, Tavares MM, Subtil A, Martins R, Nazaré M, et al. The economic impact of disease-related malnutrition at hospital admission. Clin Nutr. 2007;26:778–84. doi: 10.1016/j.clnu.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Freitag FG, Collins SD, Carlson HA, Goldstein J, Saper J, Silberstein S, et al. A randomized trial of divalproex sodium extended-release tablets in migraine prophylaxis. Neurology. 2002;58:1652–9. doi: 10.1212/wnl.58.11.1652. [DOI] [PubMed] [Google Scholar]
- 18.Torrent C, Amann B, Sánchez-Moreno J, Colom F, Reinares M, Comes M, et al. Weight gain in bipolar disorder: pharmacological treatment as a contributing factor. Acta Psychiatr Scand. 2008;118:4–18. doi: 10.1111/j.1600-0447.2008.01204.x. [DOI] [PubMed] [Google Scholar]
- 19.Hamed SA, Fida NM, Hamed EA. States of serum leptin and insulin in children with epilepsy: risk predictors of weight gain. Eur J Paediatr Neurol. 2009;13:261–8. doi: 10.1016/j.ejpn.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Caksen H, Deda G, Berberoğlu M. Does long-term use of valproate cause weight gain in prepubertal epileptic children? Int J Neurosci. 2002;112:1183–9. doi: 10.1080/00207450290026148. [DOI] [PubMed] [Google Scholar]
- 21.Espinosa PS, Salazar JC, Yu L, Mendiondo MS, Robertson WC, Baumann RJ. Lack of valproic acid-associated weight gain in prepubertal children. Pediatr Neurol. 2008;39:177–80. doi: 10.1016/j.pediatrneurol.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Menachem E. Weight issues for people with epilepsy - a review. Epilepsia. 2007;48(Suppl 9):42–5. doi: 10.1111/j.1528-1167.2007.01402.x. [DOI] [PubMed] [Google Scholar]
- 23.Hadjikoutis S, Pickersgill TP, Smith PE. Drug points: Weight loss associated with levetiracetam. BMJ. 2003;327:905. doi: 10.1136/bmj.327.7420.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gelisse P, Juntas-Morales R, Genton P, Hillaire-Buys D, Diaz O, Coubes P, et al. Dramatic weight loss with levetiracetam. Epilepsia. 2008;49:308–15. doi: 10.1111/j.1528-1167.2007.01273.x. [DOI] [PubMed] [Google Scholar]
- 25.Rauchenzauner M, Haberlandt E, Scholl-Bürgi S, Karall D, Schoenherr E, Tatarczyk T, et al. Effect of valproic acid treatment on body composition, leptin and the soluble leptin receptor in epileptic children. Epilepsy Res. 2008;80:142–9. doi: 10.1016/j.eplepsyres.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 26.de Vries L, Karasik A, Landau Z, Phillip M, Kiviti S, Goldberg-Stern H. Endocrine effects of valproate in adolescent girls with epilepsy. Epilepsia. 2007;48:470–7. doi: 10.1111/j.1528-1167.2006.00953.x. [DOI] [PubMed] [Google Scholar]
- 27.Svalheim S, Luef G, Rauchenzauner M, Mørkrid L, Gjerstad L, Taubøll E. Cardiovascular risk factors in epilepsy patients taking levetiracetam, carbamazepine or lamotrigine. Acta Neurol Scand Suppl. 2010;122:30–3. doi: 10.1111/j.1600-0404.2010.01372.x. [DOI] [PubMed] [Google Scholar]
- 28.Cornish BH, Bunce IH, Ward LC, Jones LC, Thomas BJ. Bioelectrical impedance for monitoring the efficacy of lymphoedema treatment programmes. Breast Cancer Res Treat. 1996;38:169–76. doi: 10.1007/BF01806671. [DOI] [PubMed] [Google Scholar]


