Abstract
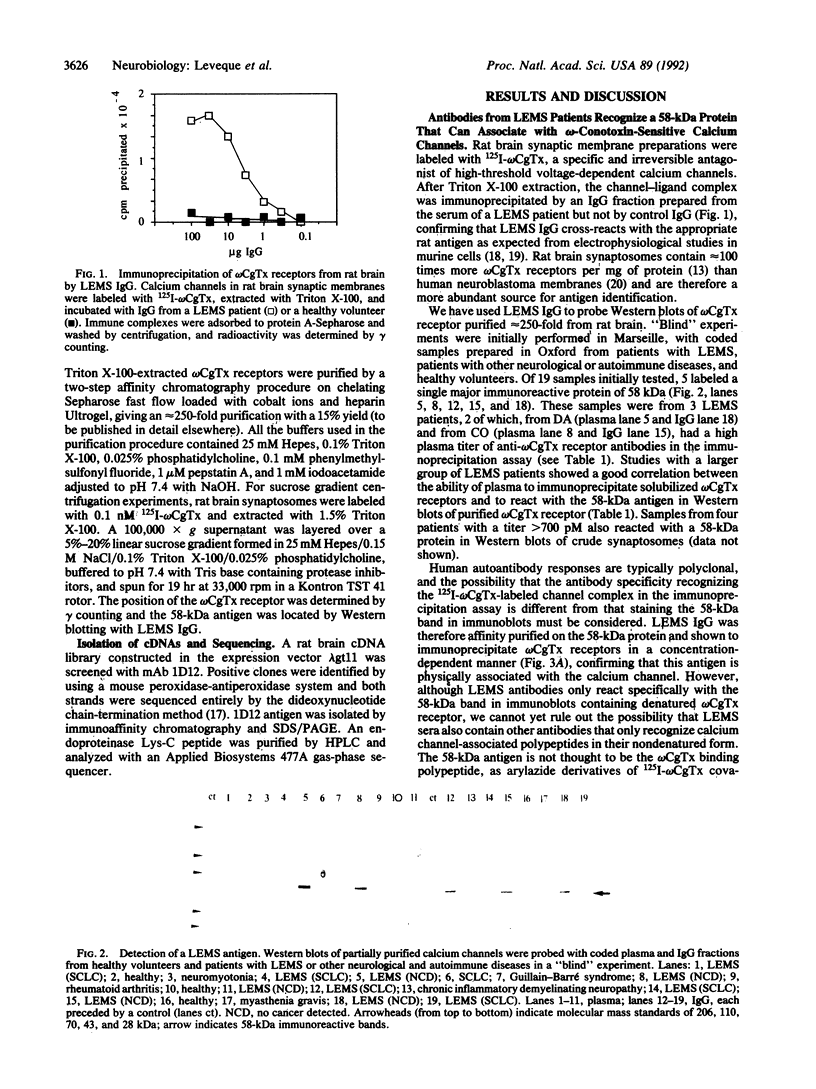
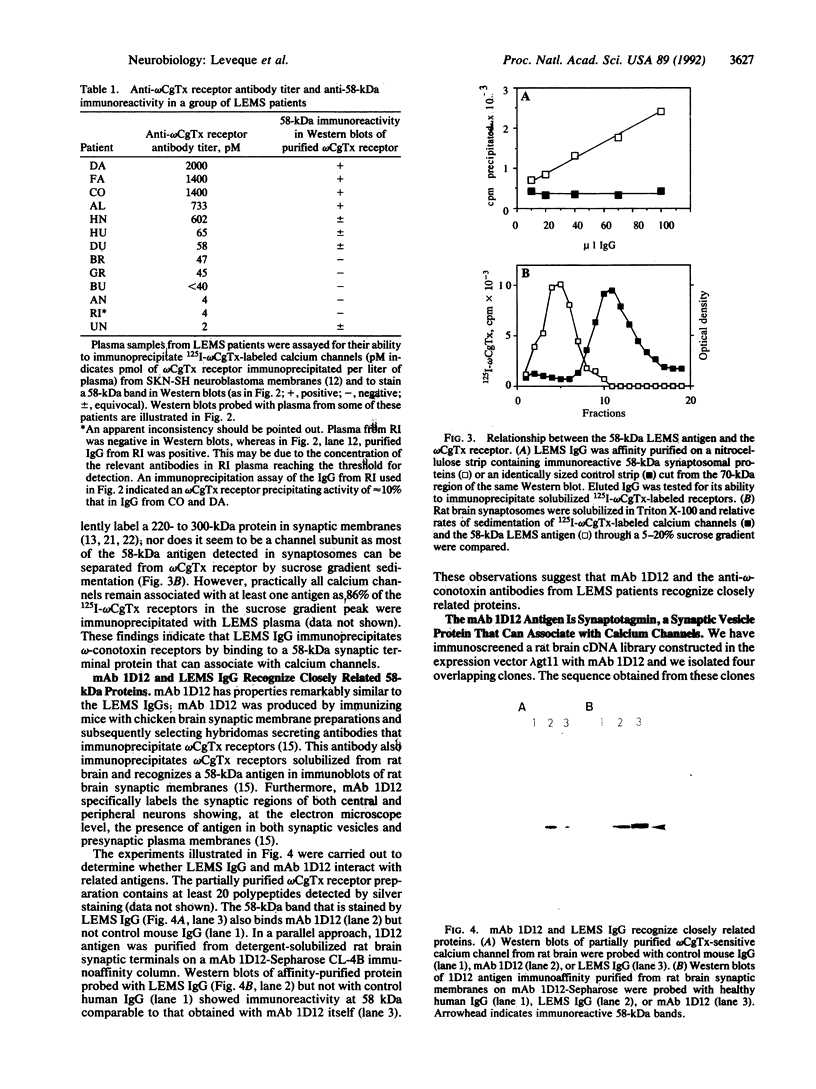
Immunoglobulin G fractions from patients with Lambert-Eaton myasthenic syndrome (LEMS), an autoimmune disease of neuromuscular transmission, immunoprecipitate 125I-labeled omega-conotoxin GVIA-labeled calcium channels solubilized from rat brain. A 58-kDa antigen was detected by probing Western blots of partially purified calcium channels with LEMS plasma and IgG and was shown to be the relevant antigen in omega-conotoxin receptor immunoprecipitation. Monoclonal antibody 1D12, produced by immunizing mice with synaptic membranes, has properties similar to these autoimmune IgGs in both immunoprecipitation and Western blotting assays. 1D12 antigen was purified by immunoaffinity chromatography and shown to bind LEMS IgG. The antigen was identified by screening a rat brain cDNA library with 1D12 and was found to have strong homology to the synaptic vesicle membrane protein synaptotagmin. Our results indicate therefore that these antibodies immunoprecipitate omega-conotoxin receptors by binding to synaptotagmin that is associated with calcium channels. We suggest that the interaction between synaptotagmin and the voltage-gated calcium channel plays a role in docking synaptic vesicles at the plasma membrane prior to rapid neurotransmitter release and that autoantibody binding to a synaptotagmin-calcium-channel complex may be involved in the etiology of LEMS.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Saisu H. Identification of the receptor for omega-conotoxin in brain. Probable components of the calcium channel. J Biol Chem. 1987 Jul 15;262(20):9877–9882. [PubMed] [Google Scholar]
- Ahlijanian M. K., Westenbroek R. E., Catterall W. A. Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord, and retina. Neuron. 1990 Jun;4(6):819–832. doi: 10.1016/0896-6273(90)90135-3. [DOI] [PubMed] [Google Scholar]
- Barhanin J., Schmid A., Lazdunski M. Properties of structure and interaction of the receptor for omega-conotoxin, a polypeptide active on Ca2+ channels. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1051–1062. doi: 10.1016/0006-291x(88)90736-x. [DOI] [PubMed] [Google Scholar]
- Chester K. A., Lang B., Gill J., Vincent A., Newsom-Davis J. Lambert-Eaton syndrome antibodies: reaction with membranes from a small cell lung cancer xenograft. J Neuroimmunol. 1988 May;18(2):97–104. doi: 10.1016/0165-5728(88)90058-6. [DOI] [PubMed] [Google Scholar]
- Cruz L. J., Olivera B. M. Calcium channel antagonists. Omega-conotoxin defines a new high affinity site. J Biol Chem. 1986 May 15;261(14):6230–6233. [PubMed] [Google Scholar]
- Geppert M., Archer B. T., 3rd, Südhof T. C. Synaptotagmin II. A novel differentially distributed form of synaptotagmin. J Biol Chem. 1991 Jul 25;266(21):13548–13552. [PubMed] [Google Scholar]
- HOREJSI J., SMETANA R. The isolation of gamma globulin from blood-serum by rivanol. Acta Med Scand. 1956 Jun 30;155(1):65–70. doi: 10.1111/j.0954-6820.1956.tb14351.x. [DOI] [PubMed] [Google Scholar]
- Kerr L. M., Yoshikami D. A venom peptide with a novel presynaptic blocking action. Nature. 1984 Mar 15;308(5956):282–284. doi: 10.1038/308282a0. [DOI] [PubMed] [Google Scholar]
- Kim Y. I., Neher E. IgG from patients with Lambert-Eaton syndrome blocks voltage-dependent calcium channels. Science. 1988 Jan 22;239(4838):405–408. doi: 10.1126/science.2447652. [DOI] [PubMed] [Google Scholar]
- Lang B., Newsom-Davis J., Peers C., Prior C., Wray D. W. The effect of myasthenic syndrome antibody on presynaptic calcium channels in the mouse. J Physiol. 1987 Sep;390:257–270. doi: 10.1113/jphysiol.1987.sp016698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon V. A., Lambert E. H. Autoantibodies bind solubilized calcium channel-omega-conotoxin complexes from small cell lung carcinoma: a diagnostic aid for Lambert-Eaton myasthenic syndrome. Mayo Clin Proc. 1989 Dec;64(12):1498–1504. doi: 10.1016/s0025-6196(12)65705-x. [DOI] [PubMed] [Google Scholar]
- Leys K., Lang B., Johnston I., Newsom-Davis J. Calcium channel autoantibodies in the Lambert-Eaton myasthenic syndrome. Ann Neurol. 1991 Mar;29(3):307–314. doi: 10.1002/ana.410290313. [DOI] [PubMed] [Google Scholar]
- Marqueze B., Martin-Moutot N., Levêque C., Couraud F. Characterization of the omega-conotoxin-binding molecule in rat brain synaptosomes and cultured neurons. Mol Pharmacol. 1988 Aug;34(2):87–90. [PubMed] [Google Scholar]
- Matthew W. D., Tsavaler L., Reichardt L. F. Identification of a synaptic vesicle-specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J Cell Biol. 1981 Oct;91(1):257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D. H., Cruz L. J., Olivera B. M., Tsien R. W., Yoshikami D. Omega-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J. H., Murray N. M., Newsom-Davis J. The Lambert-Eaton myasthenic syndrome. A review of 50 cases. Brain. 1988 Jun;111(Pt 3):577–596. doi: 10.1093/brain/111.3.577. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Peers C., Lang B., Newsom-Davis J., Wray D. W. Selective action of myasthenic syndrome antibodies on calcium channels in a rodent neuroblastoma x glioma cell line. J Physiol. 1990 Feb;421:293–308. doi: 10.1113/jphysiol.1990.sp017945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin M. S., Brose N., Jahn R., Südhof T. C. Domain structure of synaptotagmin (p65) J Biol Chem. 1991 Jan 5;266(1):623–629. [PubMed] [Google Scholar]
- Perin M. S., Fried V. A., Mignery G. A., Jahn R., Südhof T. C. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990 May 17;345(6272):260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- Perin M. S., Johnston P. A., Ozcelik T., Jahn R., Francke U., Südhof T. C. Structural and functional conservation of synaptotagmin (p65) in Drosophila and humans. J Biol Chem. 1991 Jan 5;266(1):615–622. [PubMed] [Google Scholar]
- Petrenko A. G., Perin M. S., Davletov B. A., Ushkaryov Y. A., Geppert M., Südhof T. C. Binding of synaptotagmin to the alpha-latrotoxin receptor implicates both in synaptic vesicle exocytosis. Nature. 1991 Sep 5;353(6339):65–68. doi: 10.1038/353065a0. [DOI] [PubMed] [Google Scholar]
- Roberts A., Perera S., Lang B., Vincent A., Newsom-Davis J. Paraneoplastic myasthenic syndrome IgG inhibits 45Ca2+ flux in a human small cell carcinoma line. Nature. 1985 Oct 24;317(6039):737–739. doi: 10.1038/317737a0. [DOI] [PubMed] [Google Scholar]
- Robitaille R., Adler E. M., Charlton M. P. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990 Dec;5(6):773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- Ruth P., Röhrkasten A., Biel M., Bosse E., Regulla S., Meyer H. E., Flockerzi V., Hofmann F. Primary structure of the beta subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1989 Sep 8;245(4922):1115–1118. doi: 10.1126/science.2549640. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher E., Gotti C., Canal N., Scoppetta C., Piccolo G., Evoli A., Clementi F. Specificity of calcium channel autoantibodies in Lambert-Eaton myasthenic syndrome. Lancet. 1989 Sep 16;2(8664):640–643. doi: 10.1016/s0140-6736(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Sher E., Pandiella A., Clementi F. Omega-conotoxin binding and effects on calcium channel function in human neuroblastoma and rat pheochromocytoma cell lines. FEBS Lett. 1988 Aug 1;235(1-2):178–182. doi: 10.1016/0014-5793(88)81258-4. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Arimatsu Y., Fujita S., Fujimoto Y., Kondo S., Hama T., Miyamoto E. Protein kinase C and Ca2+/calmodulin-dependent protein kinase II phosphorylate a novel 58-kDa protein in synaptic vesicles. Brain Res. 1991 Jun 14;551(1-2):279–292. doi: 10.1016/0006-8993(91)90942-o. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Seagar M. J., Jones J. F., Reber B. F., Catterall W. A. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifaró J. M., Fournier S., Novas M. L. The p65 protein is a calmodulin-binding protein present in several types of secretory vesicles. Neuroscience. 1989;29(1):1–8. doi: 10.1016/0306-4522(89)90327-8. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Ellinor P. T., Horne W. A. Molecular diversity of voltage-dependent Ca2+ channels. Trends Pharmacol Sci. 1991 Sep;12(9):349–354. doi: 10.1016/0165-6147(91)90595-j. [DOI] [PubMed] [Google Scholar]
- Vincent A., Lang B., Newsom-Davis J. Autoimmunity to the voltage-gated calcium channel underlies the Lambert-Eaton myasthenic syndrome, a paraneoplastic disorder. Trends Neurosci. 1989 Dec;12(12):496–502. doi: 10.1016/0166-2236(89)90109-4. [DOI] [PubMed] [Google Scholar]
- Wendland B., Miller K. G., Schilling J., Scheller R. H. Differential expression of the p65 gene family. Neuron. 1991 Jun;6(6):993–1007. doi: 10.1016/0896-6273(91)90239-v. [DOI] [PMC free article] [PubMed] [Google Scholar]





