Abstract
Introduction
Previous studies have shown benefit not only from postoperative chemotherapy but also from a short interval to initiation of treatment after resection of primary colorectal cancer. The aim of this study was to determine difference in timing to postoperative chemotherapy for minimally invasive resection (MIR) vs. open resection (OR) of colorectal cancer liver metastases (CRCLM).
Methods
This is a retrospective review of 1:1 matched patients undergoing MIR (n=66) and OR (n=66) for CRCLM at a single institution.
Results
Patients undergoing MIR of CRCLM had significantly shorter length of hospital stay, fewer major complications, and shorter interval to postoperative chemotherapy (median 42 vs. 63 days, p<0.001). Univariable analysis showed that surgical approach, postoperative complications, blood loss, number of lesions, and length of stay were associated with timing to chemotherapy. On multivariable analysis, surgical approach was still associated with timing to chemotherapy, and postoperative complications resulted in a delay of chemotherapy among patients who underwent OR but not among those who underwent MIR. In addition, worse disease-free survival was seen among patients who received postoperative chemotherapy more than 60 days after surgery.
Conclusion
By modifying the deleterious effects of postoperative complications on timing of postoperative chemotherapy, patients undergoing MIR for CRCLM are treated with chemotherapy sooner after surgery compared to those undergoing OR.
Keywords: Minimally invasive surgery, Laparoscopic liver resection, Metastatic colorectal cancer, Postoperative chemotherapy, Hepatic resection
Introduction
Colorectal cancer is the third most common cancer and third leading cause of cancer deaths in the USA [1]. Approximately 30 % of patients with locally advanced colorectal cancer present with synchronous liver metastases (CRCLM) [2]. Despite the variety of neoadjuvant and adjuvant treatment protocols, surgical resection remains the only potential curative options in patients with CRCLM. Over the past two decades, survival of patients diagnosed with CRCLM has greatly improved, with recent data showing the 5-year survival rates to be 45–60 % [3–5]. These superior outcomes can be attributed to several factors, including improved surgical technique, perioperative care, and advancements in systemic chemotherapy [6, 7]. A major advancement in surgical technique is minimally invasive resection (MIR) of CRCLM. Minimally invasive resection, both laparoscopic- and robotic-assisted hepatectomy, has been shown to have comparable R0 resection proportions, safety, and efficacy to open resection (OR) for CRCLM [8–17]. Although the benefits of postoperative systemic chemotherapy remain controversial regarding overall survival, it is still routinely recommended after resection of CRCLM due to the well-established benefits in regards to increased disease-free survival [18–20].
Recent preclinical studies, mathematical models, and human molecular-based studies suggest that early initiation of chemotherapy after surgical resection improves long-term outcomes. Studies in various animal models reveal that surgery may activate dormant metastases, stimulate angiogenesis, increase the number of circulating tumor cells, and enhance the production of oncogenic growth factors [21–23]. Chemotherapy initiation soon after surgery may suppress these elements—thus preventing early cancer recurrence after surgery. A meta-analysis performed by Biagi et al. reported a decrease in overall survival by 14%for every 4-week delay in initiation of postoperative chemotherapy. Therefore, postoperative chemotherapy is recommended to be commenced within 4 to 6 weeks of surgical resection to optimize patient outcomes [24].
The purpose of this retrospective study was to determine whether patients undergoing MIR of CRCLM experience a shorter time interval between surgery and initiation of postoperative chemotherapy compared to those undergoing OR and whether it has an impact on long-term cancer-related outcomes. To achieve this aim, we performed a 1:1 case-matched comparison of patients undergoing MIR vs. OR based on demographics, comorbidities, and extent of liver resection with a primary study endpoint of timing of postoperative chemotherapy.
Material and Methods
This is a retrospective cohort study of patients with CRCLM who underwent resection at the University of Pittsburgh Medical Center (UPMC) between 2009 and 2013. A total of 508 liver resections for metastatic colorectal cancer were performed during this time period. Among these, 79 MIR and 432 OR were performed. MIR was defined as a pure laparoscopic, laparoscopic hand-assisted, robotically assisted laparoscopic approach or cases that were converted to open. Of the 79 patients who underwent MIR, seven did not have adequate follow-up data and in six patients the surgery of record was not their first resection of liver metastases and thus were excluded from the study. Each patient who underwent MIR was individually matched to one patient who underwent OR based on risk factors primarily associated with short-term postoperative complications [25, 26] (in descending order of priority): presence of background liver disease, extent of liver resection, simultaneous colon resection, preoperative CEA level, American Society of Anesthesiologists (ASA) score, age, gender, and body mass index (BMI). Extent of resection was classified as major if the procedure included the removal of four or more liver segments. Treatment recommendations were made through a multidisciplinary liver tumor conference, and the type of surgical resection that the patient received was based on lesion location and assessment of overall clinical status. The decision to perform either a robotic or laparoscopic procedure was surgeon-specific.
Patients’ charts were retrospectively reviewed for history and demographics, liver pathology, and intraoperative as well as postoperative outcomes. operative variables such as estimated blood loss (EBL) and transfusion frequency were determined from anesthesia records and operative notes. Postoperative complications were obtained from discharge summaries and thorough review of inpatient and outpatient progress notes and graded according to the Clavien-Dindo Classification scale—major complications were defined as events requiring surgical, endoscopic, or radiological intervention (Clavien-Dindo classification grade≥3) [27]. Chemotherapy data was obtained from the electronic patient chart or by contacting offices where patients received treatment. This data included the date of initiation of postoperative chemotherapy and whether patients received chemotherapy after detection of liver disease and prior to hepatic resection. All patients in the MIR group were compared with all case-matched patients in the OR group to determine perioperative differences and differences in timing to chemotherapy. Our standard follow-up is liver imaging every 3 months for the first 2 years after surgery then every 6 months thereafter. Adherence to follow-up imaging was similar in both groups.
Data analysis was conducted using Stata, version 13.1 (StataCorp, College Station, TX). Groups were compared using the χ2 test for categorical variables, analysis of variance for continuous parametric variables, and the Wilcoxon-Mann-Whitney test for continuous nonparametric variables. Categorical variables are presented as whole numbers and percentages. Continuous variables are presented as means with standard deviation. Continuous nonparametric variables are presented as medians with interquartile range (IQR). Kaplan- Meier estimates of disease-free survival and initiation of postoperative chemotherapy were calculated, and differences in the probabilities were compared with the log-rank test. For the initiation of postoperative chemotherapy, patients were censored at last follow-up, death, or at time of disease recurrence. The impact of clinical, pathologic, or operative variables in the initiation of postoperative chemotherapy was also evaluated with Cox proportional hazard regression models. Variables that were significant at a significance level of 0.05 in the univariable analysis were included in the multivariable analysis, and the possibility of effect modification between the surgical technique and the presence of postoperative complications was explored. For all statistical measures, p<0.05 was considered significant.
This study was approved by the UPMC Institutional Review Board (IRB), and data acquisition and storage was compliant with the IRB’s guidelines.
Results
Patient Characteristics, Perioperative Parameters, and Timing to Postoperative Chemotherapy
There were no significant differences between patients undergoing MIR (n=66) and with the case-matched OR group (n=66) based on the variables chosen for matching (Table 1). In the 66 patients included in the MIR group, 23 (35 %) had a pure laparoscopic resection, 19 (29 %) had a hybrid or a hand-assisted approach, 21 (32 %) had a robotic-assisted surgery, and 3 (4 %) were converted to open. The number of tumors was significantly higher in the OR group. Forty-two percent of patients in the MIR group and 50 % in OR group presented with synchronous disease. The median disease-free interval for patients presenting with metachronous disease was 22 months for the MIR group and 26.8 months for the OR group. As for prior chemotherapy treatments, 38 and 41 % of patients in the MIR and OR groups, respectively, have received chemotherapy prior to the liver resection. For patients with synchronous lesions, median time between colon and liver resection was 4.9 months (4.8 and 4.9 months for the MIR and OR groups, respectively). For the metachronous lesions, median time between colon and liver resections was 25 months (21.7 and 26.9 months for the MIR and OR groups, respectively).
Table 1.
Comparison of minimally invasive vs. open resection groups: demographic and pre-operative parameters
| Minimally invasive (n=66) |
Open (n=66) |
Overall p value | |
|---|---|---|---|
| Steatohepatitis, no. (%) | 10 (15) | 10 (15) | 1.00 |
| Extent of resection, no. (%) | 1.00 | ||
| Major | 15 (23) | 15 (23) | |
| Minor | 51 (77) | 51 (77) | |
| Combined colon resection, no. (%) | 3 (5) | 3 (5) | 1.00 |
| CEA > 100 µg, no. (%) | 2 (3 %) | 2 (3 %) | 1.00 |
| ASA, no. (%) | 1.00 | ||
| 1 | 0 (0) | 1 (2) | |
| 2 | 13 (20) | 7 (11) | |
| 3 | 49 (74) | 54 (82) | |
| 4 | 4 (6) | 4 (6) | |
| R0 margins, no. (%) | 58 (88) | 59 (89) | 1.00 |
| Cirrhosis, no. (%) | 0 (0) | 0 (0) | 1.00 |
| Age, mean (SD) | 62.1 (11.2) | 62.5 (12.3) | 0.84 |
| Prior chemotherapy, no. (%) | 25 (38) | 27 (41) | 0.77 |
| Synchronous disease at presentation, no. (%) | 27 (42) | 33 (50) | 0.33 |
| Male, no. (%) | 37 (56) | 43 (65) | 0.29 |
| Largest tumor sizea, cm | 2.2 (1.5, 3.0) | 2.6 (2.0, 3.5) | 0.18 |
| BMI, mean, kg/m2 | 0.07 | ||
| Underweight (<18.5) | 1 (2) | 1 (2) | |
| Normal (18.5–24.9) | 29 (44) | 17 (26) | |
| Overweight (25–29.9) | 24 (36) | 2 (39) | |
| Obese (>30) | 12 (18) | 22 (33) | |
| No. lesionsa | 1.0 (1.0, 2.0) | 2.0 (1.0, 3.0) | 0.02 |
CEA carcinoembryonic antigen, ASA American Society of Anesthesiologists, SD standard deviation, BMI body mass index
Reported as median (IQR)
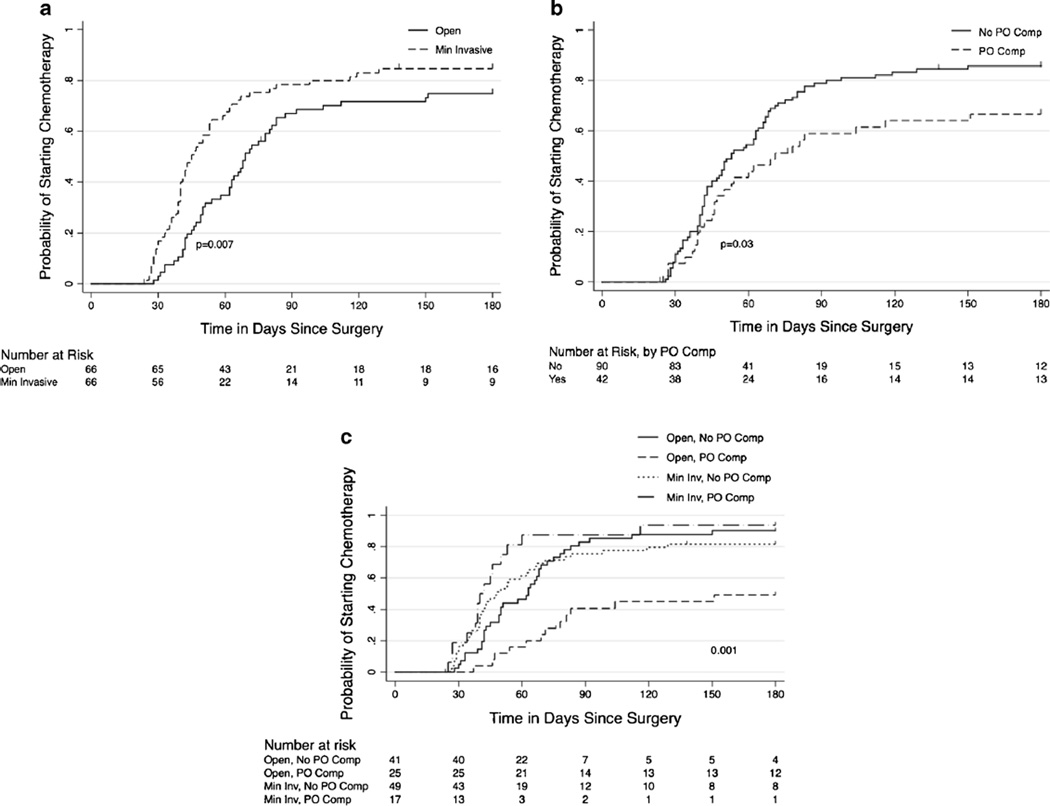
There were statistically significant differences in median estimated blood loss, hospital length of stay, Clavien grade 3 and 4 complications, and median days to postoperative chemotherapy (Table 2) between patients who underwent MIR compared to OR. Patients in the MIR group had a shorter median length of hospital stay (4 vs. 5 days, p<0.001). There were no differences in estimated blood loss, overall 30-day complications, intraoperative transfusions, or 30-day mortality. However, the proportion of major complications (Clavien grade 3 and 4) was lower in the minimally invasive group compared to the OR group (6 vs. 17 %, p=0.04). Similar proportions of patients were treated with postoperative chemotherapy in both groups (83 vs. 79 %, p=0.281). However, patients who underwent MIR started postoperative chemotherapy sooner after surgery compared to OR counterparts (median 42 vs. 63 days, p<0.001). Specifically, 67 % of those who underwent MIR started postoperative chemotherapy within 60 days after surgery compared to only 35 % of OR counterparts (Fig. 1a). Of note, 48.4 % of the patients in the OR group and 47.4 % of the patients in the MIR group received postoperative chemotherapy at our institution.
Table 2.
Comparison of minimally invasive vs. open resection groups: intraoperative and postoperative parameters
| Minimally invasive (n=66) |
Open (n=66) |
Overall p value | |
|---|---|---|---|
| EBLa (ml) | 150 (50–150) | 250 (100–350) | 0.06 |
| Intraoperative transfusion, no. (%) | 4 (12) | 3 (5) | 0.374 |
| Length of staya (days) | 4 (3–6) | 5 (4–6) | 0.001 |
| 30-day complication, no. (%) | 17 (26) | 25 (38) | 0.191 |
| Complication grade 3 or 4, no. (%) | 4 (6) | 11 (17) | 0.049 |
| 30-day mortality, no. (%) | 0 (0) | 0 (0) | 1.0 |
| Received postoperative chemotherapy, no. (%) | 55 (83) | 52 (79) | 0.281 |
| Time to chemotherapya (days) | 42 (34–54) | 63 (44–79) | <0.001 |
EBL estimated blood loss
Reported as median (IQR)
Fig. 1.
a Probability of starting chemotherapy for minimally invasive vs. open resection. b Probability of starting postoperative chemotherapy as a function of postoperative complication. c Probability of starting postoperative chemotherapy for minimally invasive vs. open resection +/− postoperative complications
Factors Associated with Timing of Postoperative Chemotherapy
Univariable analysis demonstrated that surgical technique (MIR vs. OR hazard ratio 1.77, p=0.009), postoperative complications (hazard ratio 0.62, p=0.027), number of lesions (solitary vs. multiple hazard ratio 1.7, p=0.007), EBL (hazard ratio 0.49, p=0.001), and length of hospital stay (hazard ratio 0.56, p=0.004) were significant factors affecting timing to chemotherapy (Table 3).
Table 3.
Univariable analysis of factors associated with initiation of postoperative chemotherapy
| Variable | Hazard ratio (95 % CI) | p value |
|---|---|---|
| Age (<75 vs. ≥75) | 0.61 (0.32–1.18) | 0.164 |
| Gender (M vs. F) | 1.05 (0.71–1.56) | 0.791 |
| BMI (<30 vs. ≥30) | 1.09 (0.72–1.67) | 0.678 |
| ASA (1–2 vs. 3–4) | 0.68 (0.41–1.12) | 0.144 |
| Number of lesions (solitary vs. multiple) | 1.7 (1.16–2.52) | 0.007 |
| Size of the largest lesion (≤2.5 vs. >2.5 cm) | 0.67 (0.44–1.01) | 0.006 |
| Steatohepatitis (no vs. yes) | 1.04 (0.62–1.76) | 0.865 |
| Synchronous resection (no vs. yes) | 0.91 (0.37–2.24) | 0.843 |
| CEA (≤100 vs. >100) | 1.82 (0.55–6.02) | 0.359 |
| Surgical technique (minimally invasive vs. open) | 1.77 (1.14–2.46) | 0.009 |
| Type of resection (minor vs. major) | 0.71 (0.44–1.14) | 0.146 |
| Margin (R0 vs. R1) | 1.02 (0.55–1.91) | 0.938 |
| Postoperative complications (yes vs. no) | 0.62 (0.40–0.96) | 0.027 |
| EBL (>200 vs. ≤200 ml) | 0.49 (0.31–0.77) | 0.001 |
| LOS (>4 vs. ≤4 days) | 0.56 (0.37–0.83) | 0.004 |
CI confidence interval, BMI body mass index, M male, F female, ASA American Society of Anesthesiologists, CEA carcinoembryonic antigen, EBL estimated blood loss, LOS length of stay
Of the 132 patients included in this study, 42 (32 %) suffered any grade postoperative complications. Overall, patients who did not suffer postoperative complications initiated postoperative chemotherapy sooner after surgery than those who did have postoperative complications (median 53 vs. 70 days, p=0.03). At 60 days after surgery, 55 % of those who did not have postoperative complications had initiated chemotherapy in comparison to only 42 % of those who did have postoperative complications (Fig. 1b). At 180 days after surgery, 87 % of those without postoperative complications had initiated chemotherapy in comparison to 69 % of those who did have postoperative complications (p=0.02). Of note, although the numbers are small, previous chemotherapy and having a combined procedure were not significantly associated with postoperative complications in our cohort.
Patients in each cohort, MIR and OR, were stratified by postoperative complications. On univariable analysis, patients who underwent OR and suffered postoperative morbidity had a longer interval to start of postoperative chemotherapy compared to patients who underwent OR and did not experience postoperative morbidity (median 151 vs. 63 days, p=0.015). In contrast, postoperative complications did not affect the interval to start of postoperative chemotherapy for patients who underwent MIR (median 48 vs. 40 days, p=0.34) (Fig. 1c).
On multivariable analysis, surgical technique, number of lesions, and length of stay were independently associated with timing of postoperative chemotherapy (Table 4). Multivariable analysis demonstrated that postoperative complications modified the effect of surgical technique on timing of postoperative chemotherapy. For patients who underwent OR, patients who suffered postoperative complications had a longer interval to start of postoperative chemotherapy compared to those who did not suffer postoperative complications (hazard ratio 0.45, p=0.017). In contrast, postoperative complications did not significantly affect timing of postoperative chemotherapy for patients who underwent MIR (hazard ratio 2.05, p=0.052).
Table 4.
Multivariable analysis associated with initiation of postoperative chemotherapy
| Variable | Hazard ratio | p value |
|---|---|---|
| Surgical technique | ||
| Open | 1.0 (referent) | – |
| Minimally invasive | 2.23 (1.16–4.31) | 0.017 |
| Surgical technique +/− complication | ||
| Minimally invasive, no complication | 1.0 (referent) | – |
| Minimally invasive, complication | 2.05 (0.96–3.97) | 0.052 |
| Open, no complication | 0.89 (0.57–1.42) | 0.647 |
| Open, complication | 0.45 (0.23–0.86) | 0.017 |
| EBL | ||
| ≤200 ml | 1.0 (referent) | – |
| >200 ml | 0.70 (0.45–1.09) | 0.113 |
| Number of lesions | ||
| Multiple | 1.0 (referent) | – |
| Solitary | 1.71 (1.14–2.54) | 0.009 |
| LOS | ||
| ≤4 days | 1.0 (referent) | – |
| >4 days | 0.64 (0.41–0.99) | 0.043 |
EBL estimated blood loss, LOS length of stay
Timing to Postoperative Chemotherapy and Long-Term Outcomes
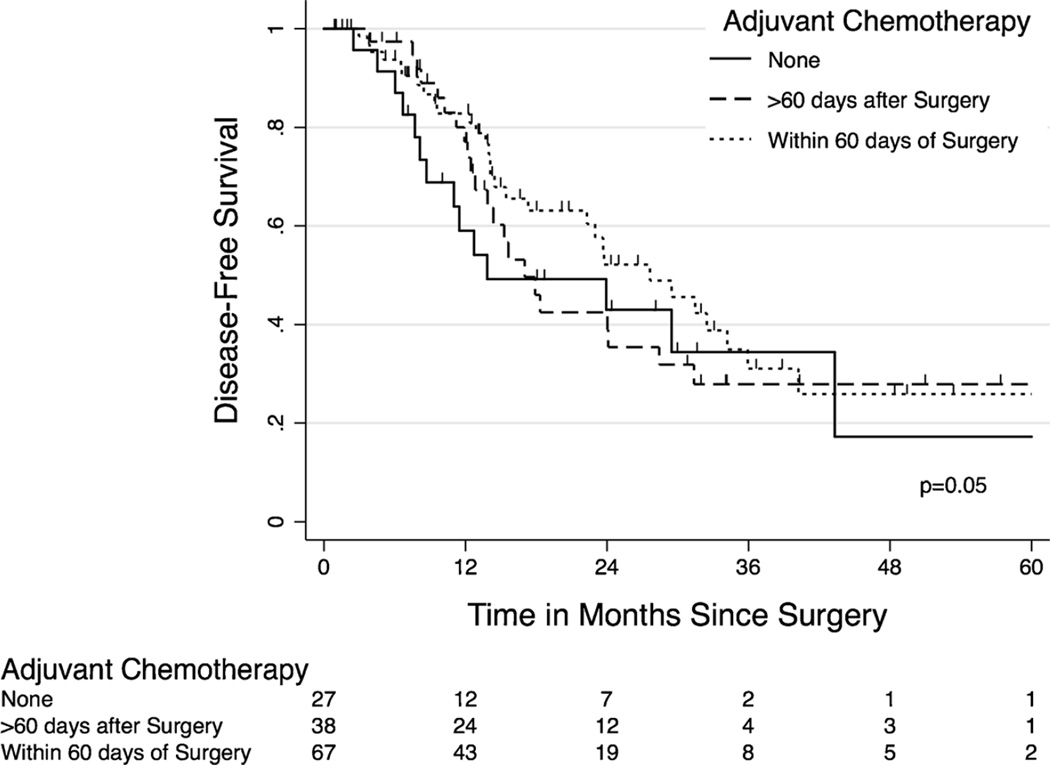
The influence of postoperative chemotherapy timing on recurrence-free survival for all study patients was examined. Median length of follow-up for all patients was 34 months (IQR 18–48 months). Kaplan-Meir estimates showed that patients who received postoperative chemotherapy within 60 days of surgery had a trend towards longer recurrence-free survival compared to those with a longer interval to chemotherapy and to those not treated with postoperative chemotherapy (median 29 vs. 14 months, p=0.05) as shown in Fig. 2. These results extended to overall survival with median overall survival for the whole cohort being 53 months with 3-year overall survival 60 % for OR vs. 74.4 % for MIR and 5-year overall survival 38.6 % for OR vs. 51.3 % for MIR (p=0.06), which was close to approaching significance.
Fig. 2.
Kaplan-Meier curve of recurrence-free survival as a function of postoperative chemotherapy within 60 days
Discussion
Hepatic resection is the cornerstone of curative treatment for CRCLM. While advancements in surgical technique have dramatically lessened postoperative mortality [28–31], postoperative morbidity still occurs in 20–40 % of patients. Many studies have demonstrated that a minimally invasive approach to resection of CRCLM results in better postoperative pain control, decreased length of stay, and no compromise to oncologic principles relative to an open approach [15]. However, the oncologic-specific outcomes for MIR of CRCLM are less clear. A theoretical but not yet established advanced to MIR is earlier start to postoperative chemotherapy due to quicker recovery and better postoperative performance status. This study suggests that outcomes after minimally invasive approach are improved in many aspects compared with open approaches. Suggestions of improved recovery in the MIR group are evident by the shorter hospital stay and the reduced major complication rates. Important oncologic findings in this study are the earlier onset of initiating chemotherapy and shorter delay of treatment due to postoperative complications. In addition, an improved recurrence-free survival was observed in the MIR group that is reflective of the differences in the timing to chemotherapy.
The key finding of this study is that a minimally invasive approach to resection of CRCLM is an effect modifier for the influence of postoperative complications on timing of postoperative chemotherapy. Postoperative complications are still common after resection of CRCLM and occur at a higher rate after open resection. The combination of surgical technique and postoperative complications together affects timing to postoperative chemotherapy. This is true of even grade 1 or 2 complications as these include wound infections which can cause significant delay in initiation of chemotherapy, particularly in open resection [27]. Our study suggests that minimal invasion will allow for timely postoperative chemotherapy treatment. While postoperative complications resulted in a delay in start of postoperative chemotherapy for patients who underwent OR, postoperative morbidity did not influence timing of postoperative chemotherapy for patients who underwent MIR. Possible explanation of these findings include (1) lower proportion of severe complications after MIR, (2) reduced impact of postoperative complications on performance status after MIR, and (3) faster wound healing after MIR relative to OR. Advantages to our study include matching of OR and MIR patients based on factors established to be associated with postoperative outcomes and receipt of postoperative chemotherapy.
Previous studies have similarly shown worse oncologic outcomes with increasing time to chemotherapy in patients with primary colorectal cancer and in patients with pancreatic cancer [32–34]. In addition, in cases of CRCLM, previous evidence demonstrates that timely initiation of postoperative chemotherapy improves progression-free survival as shown in the EORTC trial in 2008. The most recent update to the EORTC trial showed no benefit in overall survival with the addition of perioperative chemotherapy for the management of resectable CRCLM. However, this is attributed to the fact that the study was not initially powered to detect a difference in overall survival. Of note, this study also could not demonstrate improved overall survival which may be secondary to the sample size or the need for longer follow-up. Additionally, Aloia et al. have recently proposed the importance of return to intended oncologic treatment (RIOT) as a quality measure for hepatic resection of CRCLM [19, 20, 35]. In as much as RIOT rates may serve as a quality measure, we further suggest that based on our study, the time to RIOT is another important metric to assess outcomes and compare MIR and OR approaches.
Limitations of this study include the retrospective, single-center design, potential for undetected bias, and length of follow-up. In addition, the initiation of chemotherapy is in general the prerogative of primarily the medical oncologist; the mere perception of a “minimal” vs. “a more invasive open resection” may be the dominant factor to determine the start date of the chemotherapy, as this aspect could not be blinded. Because of our small sample sizes, multivariable analysis to look at all possible confounding factors was limited. Our subjects were selected from a single institution with high volumes of complex hepatobiliary cases—thus, results may not be generalizable to all centers. These biases could be corrected by the initiation of larger scale prospective multicenter databases that enroll patients at the time of their preoperative clinic visit on an “intention-to-treat” basis. Ideally, these databases would reflect synchronous cases of colorectal cancer with liver metastases only. Prospectively maintained multicenter registries of hepatic resections for CRCLM, perioperative outcomes, and administration of postoperative chemotherapy should be started to more accurately record patient characteristics, selection criteria, as well as intra- and postoperative outcomes including timing to chemotherapy. These studies may also reveal additional long-term benefits of prompt postoperative chemotherapy in stage IV colorectal cancer as well as differences in these long-term outcomes in the minimally invasive vs. open resections.
In conclusion, MIR is not only technically feasible and safe in the setting of CRCLM but may also provide long-term oncologic benefits. By modifying the deleterious effects of postoperative complications on timing of postoperative chemotherapy, patients undergoing MIR for CRCLM are treated with postoperative chemotherapy sooner after surgery compared to those undergoing OR.
Footnotes
Conflict of Interest The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254–259. doi: 10.1097/01.sla.0000217629.94941.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1271–1280. doi: 10.1245/s10434-006-9045-5. [DOI] [PubMed] [Google Scholar]
- 4.Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, Wei SH, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. 2006;141:460–466. doi: 10.1001/archsurg.141.5.460. discussion 466–7. [DOI] [PubMed] [Google Scholar]
- 5.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24:3347–3353. doi: 10.1200/JCO.2006.06.1317. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250:831–841. doi: 10.1097/SLA.0b013e3181b0c4df. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen KT, Geller DA. Outcomes of laparoscopic hepatic resection for colorectal cancer metastases. J Surg Oncol. 2010;102:975–977. doi: 10.1002/jso.21655. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen KT, Laurent A, Dagher I, Geller DA, Steel J, Thomas MT, et al. Minimally invasive liver resection for metastatic colorectal cancer: a multi-institutional, international report of safety, feasibility, and early outcomes. Ann Surg. 2009;250:842–848. doi: 10.1097/SLA.0b013e3181bc789c. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen KT, Marsh JW, Tsung A, Steel JJ, Gamblin TC, Geller DA. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg. 2011;146:348–356. doi: 10.1001/archsurg.2010.248. [DOI] [PubMed] [Google Scholar]
- 12.Castaing D, Vibert E, Ricca L, Azoulay D, Adam R, Gayet B. Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centers. Ann Surg. 2009;250:849–855. doi: 10.1097/SLA.0b013e3181bcaf63. [DOI] [PubMed] [Google Scholar]
- 13.Kazaryan AM, Marangos IP, Rosok BI, Rosseland AR, Villanger O, Fosse E, et al. Laparoscopic resection of colorectal liver metastases: surgical and long-term oncologic outcome. Ann Surg. 2010;252:1005–1012. doi: 10.1097/SLA.0b013e3181f66954. [DOI] [PubMed] [Google Scholar]
- 14.Schiffman SC, Kim KH, Tsung A, Marsh JW, Geller DA. Laparoscopic versus open liver resection for metastatic colorectal cancer: A metaanalysis of 610 patients. Surgery. 2015;157(2):211–222. doi: 10.1016/j.surg.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 15.Tsung A, Geller DA, Sukato DC, Sabbaghian S, Tohme S, Steel J, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg. 2014;259:549–555. doi: 10.1097/SLA.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 16.Berber E, Akyildiz HY, Aucejo F, Gunasekaran G, Chalikonda S, Fung J. Robotic versus laparoscopic resection of liver tumours. HPB (Oxford) 2010;12:583–586. doi: 10.1111/j.1477-2574.2010.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic liver resection for hepatocellular carcinoma: short-term outcome. Am J Surg. 2013;205:697–702. doi: 10.1016/j.amjsurg.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976–4982. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 19.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–1215. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 21.Gunduz N, Fisher B, Saffer EA. Effect of surgical removal on the growth and kinetics of residual tumor. Cancer Res. 1979;39:3861–3865. [PubMed] [Google Scholar]
- 22.Fisher B, Gunduz N, Coyle J, Rudock C, Saffer E. Presence of a growth-stimulating factor in serum following primary tumor removal in mice. Cancer Res. 1989;49:1996–2001. [PubMed] [Google Scholar]
- 23.Baum M, Demicheli R, Hrushesky W, Retsky M. Does surgery unfavourably perturb the “natural history” of early breast cancer by accelerating the appearance of distant metastases? Eur J Cancer. 2005;41:508–515. doi: 10.1016/j.ejca.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 24.Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305:2335–2342. doi: 10.1001/jama.2011.749. [DOI] [PubMed] [Google Scholar]
- 25.Reddy SK, Marsh JW, Varley PR, Mock BK, Chopra KB, Geller DA, et al. Underlying steatohepatitis, but not simple hepatic steatosis, increases morbidity after liver resection: a case-control study. Hepatology. 2012;56:2221–2230. doi: 10.1002/hep.25935. [DOI] [PubMed] [Google Scholar]
- 26.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 27.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frankel TL, D’Angelica MI. Hepatic resection for colorectal metastases. J Surg Oncol. 2014;109:2–7. doi: 10.1002/jso.23371. [DOI] [PubMed] [Google Scholar]
- 29.House MG, Ito H, Gonen M, Fong Y, Allen PJ, DeMatteo RP, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210(744–52):752–755. doi: 10.1016/j.jamcollsurg.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 30.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 31.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 32.Croome KP, Farnell MB, Que FG, Reid-Lombardo KM, Truty MJ, Nagorney DM, et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg. 2014;260:633–638. doi: 10.1097/SLA.0000000000000937. discussion 638–40. [DOI] [PubMed] [Google Scholar]
- 33.Day AR, Middleton G, Smith RV, Jourdan IC, Rockall TA. Time to adjuvant chemotherapy following colorectal cancer resection is associated with an improved survival. Colorectal Dis. 2014;16:368–372. doi: 10.1111/codi.12570. [DOI] [PubMed] [Google Scholar]
- 34.Wang JH, King TM, Chang MC, Hsu CW. Comparison of the feasibility of laparoscopic resection of the primary tumor in patients with stage IV colon cancer with early and advanced disease: the short- and long-term outcomes at a single institution. Surg Today. 2013;43:1116–1122. doi: 10.1007/s00595-012-0398-z. [DOI] [PubMed] [Google Scholar]
- 35.Aloia TA, Zimmitti G, Conrad C, Gottumukalla V, Kopetz S, Vauthey JN. Return to intended oncologic treatment (RIOT): a novel metric for evaluating the quality of oncosurgical therapy for malignancy. J Surg Oncol. 2014;110:107–114. doi: 10.1002/jso.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]