Abstract
Background
In U.S. women, lifetime risk of ovarian cancer is 1.37%, but some women are at a substantially lower or higher risk than this average.
Methods
We have characterized the distribution of lifetime risk in the general population. Published data on the relative risks and their variances for five well-accepted risk and protective factors for ovarian cancer, oral contraceptive (OC) use, parity, tubal ligation, endometriosis and first degree family history of ovarian cancer in conjunction with a genetic risk score using genome-wide significant common, low penetrance variants were used. The joint distribution of these factors (i.e., risk/protective factor profiles) were derived using control data from four US population-based studies, providing a broad representation of women in the US.
Results
A total of 214 combinations of risk/protective factors were observed and the lifetime risk estimates ranged from 0.35% (95% CI 0.29-0.42) to 8.78% (95% CI 7.10-10.9). Among women with lifetime risk ranging from 4-9%, 73% had no family history of ovarian cancer; most of these women had a self-reported history of endometriosis.
Conclusions
Profiles including the known modifiable protective factors of OC use and tubal ligation were associated with a lower lifetime risk of ovarian cancer. OC use and tubal ligation were essentially absent among the women at 4-9% lifetime risk.
Impact
This work demonstrates that there are women in the general population who have a much higher than average lifetime risk of ovarian cancer. Preventive strategies are available. Should effective screening become available, higher than average risk women can be identified.
Keywords: Ovarian Cancer, Lifetime Risk, Epidemiology, United States, Risk Factors
INTRODUCTION
There were approximately 22,240 new cases of invasive epithelial ovarian cancer (ovarian cancer) and 14,030 deaths from the disease in the U.S. in 2013 (1). Ovarian cancer accounts for 5% of cancer-related deaths in women with overall five-year survival at less than 50%.
The average lifetime risk of ovarian cancer is ~1.37% in non-Hispanic (NH) White women in the U.S., but there are women at a substantially higher and lower risk. Approximately 10% of women who develop ovarian cancer carry high-penetrance alleles of major ovarian cancer genes, such as BRCA1, BRCA2, and others (2), that put them at a significantly higher lifetime risk of ovarian cancer.
There are also a number of personal and lifestyle factors as well as low-penetrance inherited genetic variants that affect a woman's risk for ovarian cancer. Parity, oral contraceptive pill (OCP) use, and tubal ligation are all associated with a substantial protective effect for ovarian cancer. Women with a history of endometriosis or a positive first degree family history of ovarian cancer are at increased risk (3); and, over the past several years, 11 common single nucleotide polymorphisms (SNPs) have been identified which have modest influences on risk (4-10). It is thus possible to identify groups of women in the general population at a much higher or lower than average lifetime risk of ovarian cancer by taking into account all of the aforementioned factors.
Secondary prevention efforts for ovarian cancer have so far been disappointing: results from the Prostate, Lung, Colorectal and Ovarian Cancer screening trial (PLCO) yielded no mortality benefit and substantial added morbidity due to increased surgical intervention (11). Whether the encouraging preliminary results from the U.K. Collaborative Trial of Ovarian Cancer Screening will have a mortality impact is not yet known (12).
OCP use provides substantial protection against ovarian cancer; use for five or more years cuts risk in half (3,13), and this protective effect extends for decades after use is discontinued (13). Tubal ligation also provides a substantial protective benefit (3). Salpingectomy at the time of pelvic surgery for other indications has also been considered as a strategy in low risk women (14) (https://www.sgo.org/clinical-practice/guidelines/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention/). An alternative preventive strategy that is commonly chosen by women who carry a high-risk mutation in one of the known ovarian cancer predisposing genes is risk-reducing bilateral salpingo-oophorectomy (RRSO).
It is likely that some women who are at a substantially higher risk than the average lifetime risk for U.S. NH White women because of a combination of genetic and lifestyle factors may want to consider an RRSO, a tubal ligation, bilateral salpingectomy, or extended use of OCPs. The lifetime risk of ovarian cancer at which a woman might consider these preventive approaches is dependent on a risk-benefit analysis by the woman in consultation with her physician.
The aim of this study was to quantify the population distribution of lifetime risks of ovarian cancer among women in the U.S. based on combinations of known risk and protective factors in order to inform such discussions.
MATERIALS AND METHODS
Relative risk estimates and standard errors for the well-accepted risk/protective factors - family history of ovarian cancer, endometriosis, parity, OCP use, and tubal ligation - were recently published by the Ovarian Cancer Association Consortium (OCAC) based on more than 5000 cases and more than 7000 controls (3). These estimates were used in the calculations presented here (3) (Table 1). For presentation purposes first degree family history of ovarian cancer, personal history of endometriosis and tubal ligation were each treated as dichotomous variables, while parity was categorized as never, one birth, and two or more births (derived via meta-analysis from data presented in reference 3), and OCP use was categorized as never use, 1-4 years of use and 5+ years of use (also derived via meta-analysis from data presented in reference 3).
Table 1.
Relative risk estimates for the six variables used to estimate lifetime risk of ovarian cancer
| Risk Factor | OR | 95% CI | ||
|---|---|---|---|---|
| Oral contraceptive use | ||||
| Never | 1.00 | |||
| 1-4.99 years | 0.62 | 0.56 | - | 0.69 |
| 5+ years | 0.40 | 0.36 | - | 0.44 |
| Parity | ||||
| None | 1.00 | |||
| 1 birth | 0.76 | 0.66 | - | 0.88 |
| 2+ births | 0.58 | 0.51 | - | 0.65 |
| Tubal ligation | ||||
| No | 1.00 | |||
| Yes | 0.74 | 0.67 | - | 0.83 |
| Endometriosis | ||||
| No | 1.00 | |||
| Yes | 1.53 | 1.30 | - | 1.81 |
| First-degree family history | ||||
| No | 1.00 | |||
| Yes | 2.09 | 1.70 | - | 2.57 |
| Genetic risk score | ||||
| Quintile 1 | 1.00 | |||
| Quintile 2 | 1.25 | 1.22 | - | 1.28 |
| Quintile 3 | 1.44 | 1.38 | - | 1.49 |
| Quintile 4 | 1.66 | 1.58 | - | 1.75 |
| Quintile 5 | 2.12 | 1.99 | - | 2.27 |
Eleven confirmed common susceptibility alleles for ovarian cancer have been reported (4-10). The published risk estimates for these variants are shown in Table 2. Analysis of OCAC data suggests that the relative risks associated with these genetic variants are multiplicative and independent of family history of ovarian cancer; we were therefore able to calculate a genetic risk score by multiplying the relative risks associated with each SNP and then categorized these relative risk scores into quintiles with associated standard errors (details of these calculations are given in the Supplementary Materials and Methods). These quintile genetic risk score relative risks are shown in Table 1.
Table 2.
Eleven common genetic risk variants used to create the genetic risk score
| Reference | SNP | OR | 95% CI | Chr. | Position | ||
|---|---|---|---|---|---|---|---|
| Bojesen | rs7725218a | 1.08 | 1.05 | - | 1.12 | 5 | 1282414 |
| Bolton | rs2363956 | 1.10 | 1.06 | - | 1.15 | 19 | 17394124 |
| Goode | rs2072590 | 1.16 | 1.12 | - | 1.21 | 2 | 177042633 |
| rs10088218 | 0.84 | 0.80 | - | 0.89 | 8 | 129543949 | |
| Permuth-Wey | rs12942666 | 1.11 | 1.07 | - | 1.15 | 17 | 43499839 |
| Pharoah | rs7651446 | 1.44 | 1.35 | - | 1.53 | 3 | 156406997 |
| rs11782652 | 1.19 | 1.12 | - | 1.26 | 8 | 82653644 | |
| rs1243180 | 1.10 | 1.06 | - | 1.13 | 10 | 21915619 | |
| rs9303542 | 1.12 | 1.08 | - | 1.16 | 17 | 46411500 | |
| Shen | rs3744763 | 1.06 | 1.03 | - | 1.10 | 17 | 36090885 |
| Song | rs3814113 | 0.82 | 0.79 | - | 0.86 | 9 | 16915021 |
Estimate provided by corresponding author
In order to determine the joint distribution of first degree family history of ovarian cancer, endometriosis, parity, OCP use, tubal ligation and the single genetic risk score combinations present in the U.S. population, control data from NH White women in four U.S. population-based studies (DOVE, HOPE, NCOCS, and USC) were used. The DOVE study was carried out in the Seattle, Washington area from 2002 to 2009 with 1391 controls. The HOPE study was carried out in the contiguous regions of western Pennsylvania, eastern Ohio and western New York from 2003 to 2008 with 1408 controls (15). The NCOCS study was carried out in North Carolina from 1999 to 2008 with 735 controls (16). The USC study was carried out in Los Angeles County from 1993 to 2008 (17,18) with 963 controls. Control participants from the four studies were interviewed in person using slightly different standardized questionnaires, but each contained the necessary information on first degree family history of ovarian cancer, endometriosis, parity, OCP use and tubal ligation. The risk factor combination frequencies were thus obtained from 4497 controls from four geographic regions of the U.S.
The variance associated with the log relative risk from each combination was obtained by summing the variances for each factor. This approach is based on the assumption of independence of the risk estimates for each factor, which was found to hold in the DOVE, HOPE, NCOCS, and USC data (Supplementary Methods). The relative risk for each combination of risk factors is relative to the specific combination of factors that comprise the reference group (no first degree family history, no endometriosis, nulliparous, never users of OCPs, no tubal ligation, and lowest genetic risk quintile). To convert relative risks to absolute lifetime risks, the frequency-weighted average of all the combination-specific relative risks (using the combined data observed in the NH Whites from the DOVE, HOPE, NCOCS, and USC studies) was first scaled to 1.37% (see below) and then this scaling factor was applied to each combination-specific relative risk and its 95% confidence interval.
The average lifetime risk of ovarian cancer by age 85 in a population of NH White women in the U.S. followed from birth was calculated by obtaining the age-specific all-cause mortality rates (CDC), ovarian cancer-specific mortality rates (SEER) and ovarian cancer incidence rates (SEER) following the AMP approach. The calculated average lifetime risk is 1.37%.
RESULTS
There were 360 possible combinations of risk factors based on the factors included in this analysis - family history (two levels), endometriosis (two levels), parity (three levels), OCP use (three levels), tubal ligation (two levels), and genetic risk score (five levels) – of which 214 were observed among the 4497 NH White controls in the DOVE, HOPE, NCOCS, and USC studies. Table 3 provides a demonstration of the multiplicative relative risks for the various combinations of risk factors.
Table 3.
Examples of multiplicative relative risks across genetic risk score and personal/lifetime risk/protective factors
| Genetic Risk Score | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| Referent for personal/lifestyle factors | 1.00 | 1.25 | 1.44 | 1.66 | 2.12 |
| OC use 1-4.99 years | 0.62 | 0.78 | 0.89 | 1.03 | 1.31 |
| OC use 5+ years | 0.40 | 0.50 | 0.58 | 0.66 | 0.85 |
| Parity 1 birth | 0.76 | 0.95 | 1.09 | 1.26 | 1.61 |
| Parity 2+ births | 0.58 | 0.73 | 0.84 | 0.96 | 1.23 |
| Tubal ligation | 0.74 | 0.93 | 1.07 | 1.23 | 1.57 |
| Endometriosis | 1.53 | 1.91 | 2.20 | 2.54 | 3.24 |
| Family history | 2.09 | 2.61 | 3.01 | 3.47 | 4.43 |
Note: The relative risk for various combinations can be obtained by multiplying the relevant cells in the table. For example, to obtain the relative risk for a 5+ OC user in Q1 genetic risk score who also has endometriosis, 0.40*1.53=0.61.
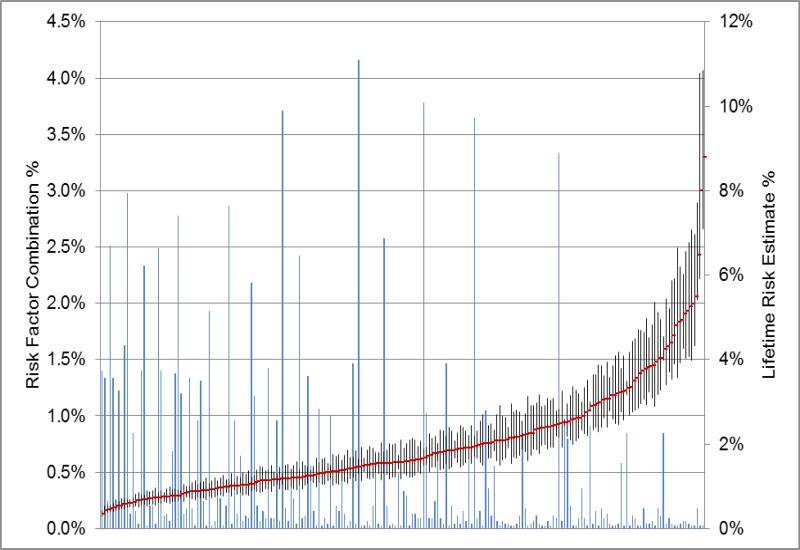
The combination of risk factors with the lowest lifetime risk, 0.35% (95% CI 0.29% - 0.42%), was present in 1.4% of women , and 0.02% of women were in the highest risk group and have an estimated lifetime risk of 8.78% (95% CI 7.10% - 10.85%) (Figure 1). There were five combinations of risk factors, comprising 6.8% of women, with a lifetime risk of 0.5% or lower; the combinations of these risk factors are shown in Table 4. All of these combinations included women who used OCPs for 5+ years and had had at least one child.
Figure 1. Distribution of the lifetime risks of ovarian cancer based on the observed combinations of the risk factors.
The blue lines along the X axis represent the 214 combinations of risk factors and the height of the line the frequency of the group; the frequencies are on the left Y axis. The right Y axis gives the lifetime risk scale; the lifetime risks are indicated by the red points. The vertical black bars are the 95% confidence intervals around the lifetime risk estimate.
Table 4.
Frequencies of and lifetime risks for the combination of risk factors that result in a lifetime risk of ovarian cancer of 0.50% or less
| ≤0.5% Lifetime Risk | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OC Use | Parity | Tubal Ligation | Endometriosis | Family History | Genetic Risk Quintile | Population Frequency | Lifetime Risk | Lifetime Risk CI | ||
| 5+ years | 2+ births | Yes | No | No | 1 | 1.40% | 0.35% | 0.29% | - | 0.42% |
| 5+ years | 2+ births | Yes | No | No | 2 | 1.33% | 0.43% | 0.36% | - | 0.52% |
| 5+ years | 1 birth | Yes | No | No | 1 | 0.24% | 0.45% | 0.37% | - | 0.56% |
| 5+ years | 2+ births | No | No | No | 1 | 2.51% | 0.47% | 0.40% | - | 0.54% |
| 5+ years | 2+ births | Yes | No | No | 3 | 1.33% | 0.50% | 0.41% | - | 0.60% |
There were 17 combinations of risk factors among the 1.85% of women at a 4% or higher lifetime risk of ovarian cancer (Table 5). The path to this high lifetime risk of ovarian cancer included either a family history of ovarian cancer or a self-reported history of endometriosis (16/17 groups). More than one-half of these risk groups included women in the highest genetic risk quintile (9/17). No OCP use was common among women at the highest lifetime risk (13/17). There was only one combination that did not include history of endometriosis or family history of ovarian cancer; women in this group had no protective factors and fell into the highest genetic risk quintile, resulting in a lifetime risk of 4.25% among 0.85% of the female population in the US. Also, women without a first degree family history of ovarian cancer made up 73% of women in the 4-9% lifetime risk category.
Table 5.
Frequencies and lifetime risks for the combination of risk factors that result in a lifetime risk of ovarian cancer of 4% or higher
| 4%-9% Lifetime Risk | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OC Use | Parity | Tubal Ligation | Endometriosis | Family History | Genetic Risk Quintile | Population Frequency | Lifetime Risk | Lifetime Risk CI | ||
| Never | 2+ births | No | No | Yes | 4 | 0.18% | 4.03% | 3.17% | - | 5.11% |
| 1-4 years | None | No | Yes | No | 5 | 0.11% | 4.03% | 3.29% | - | 4.94% |
| Never | None | No | No | No | 5 | 0.85% | 4.25% | 3.98% | - | 4.54% |
| 1-4 years | None | No | No | Yes | 4 | 0.04% | 4.30% | 3.40% | - | 5.44% |
| Never | None | No | Yes | No | 3 | 0.09% | 4.40% | 3.71% | - | 5.20% |
| Never | 1 birth | No | No | Yes | 3 | 0.04% | 4.56% | 3.54% | - | 5.87% |
| Never | None | Yes | Yes | No | 5 | 0.02% | 4.81% | 3.91% | - | 5.91% |
| 1-4 years | 2+ births | No | Yes | Yes | 5 | 0.02% | 4.88% | 3.58% | - | 6.65% |
| Never | 1 birth | No | Yes | No | 5 | 0.04% | 4.93% | 3.93% | - | 6.19% |
| Never | None | No | Yes | No | 4 | 0.07% | 5.07% | 4.27% | - | 6.02% |
| Never | 2+ births | No | No | Yes | 5 | 0.07% | 5.14% | 4.03% | - | 6.55% |
| Never | 1 birth | No | No | Yes | 4 | 0.04% | 5.26% | 4.08% | - | 6.78% |
| Never | 2+ births | No | Yes | Yes | 3 | 0.02% | 5.32% | 3.99% | - | 7.08% |
| 1-4 years | None | No | No | Yes | 5 | 0.02% | 5.49% | 4.33% | - | 6.96% |
| Never | None | No | Yes | No | 5 | 0.18% | 6.47% | 5.42% | - | 7.71% |
| Never | 1 birth | No | Yes | Yes | 4 | 0.02% | 7.99% | 5.92% | - | 10.78% |
| Never | None | No | No | Yes | 5 | 0.02% | 8.78% | 7.10% | - | 10.85% |
DISCUSSION
We have used the joint distribution of confirmed, well-accepted risk and protective factors for ovarian cancer to estimate lifetime risk of ovarian cancer by age 85 for NH White women in the U.S. Although the average lifetime risk of ovarian cancer among these women is ~1.37%, we have shown that there are many women at a substantially lower risk, and there is a group of women at much higher lifetime risk (Figure 1). For example, a nulliparous woman in the highest genetic risk quintile, who has no family history and no history of endometriosis, but has not used an OCP and not had a tubal ligation, has a lifetime risk of 4.25% (95% CI 3.98%-4.54%). If this same woman had used OCPs for five years or more, her lifetime risk would have been reduced to 1.71% (95% CI 1.52%-1.92%).
Women who carry genetic variants that put them at an exceptionally high lifetime risk of ovarian cancer (>10%) are currently offered an RRSO. However, the precise lifetime risk at which a woman would consider an RRSO is a matter to be decided by the woman in consultation with her physician. For women who are at, for example, three times the average lifetime risk of ovarian cancer (4.11% versus 1.37%), such a discussion might be warranted. This type of discussion might be particularly relevant for a higher than average ovarian cancer risk woman who is having a hysterectomy and considering also undergoing a salpingectomy and/or oophorectomy. Further research on the impact of both of these procedures among peri- and post-menopausal women is clearly warranted in light of some findings favoring ovarian preservation (19) , but there is little evidence against including a salpingectomy (but see below).
There are other prevention strategies available for younger women. OCP use provides substantial protection against ovarian cancer with 5+ years of use reducing risk by half. This effect attenuates in the decades following cessation of use, but a strong association remains over time (13). There are several issues that need to be considered related to OCP use as chemoprevention of ovarian cancer. The first is the impact on risk of other conditions such as breast cancer and venous thrombosis. Secondly, the mechanism underlying the protective effect of OCPs is unclear and a key question is whether newer generation, extended- or continuous-cycle OCPs afford the same (or less or greater) level of protection as those that were in use when this association was established.
Tubal ligation is another preventive measure that offers an approximate 25% reduction in risk; this protection is particularly strong for endometriosis-associated ovarian cancer, namely clear cell and endometrioid subtypes (20). It has recently been reported that the age at which the procedure is performed does not affect the extent of the protection (21). The mechanism of protection for tubal ligation is also not understood.
Also, as evidence has mounted that many high-grade serous ovarian cancers, the most deadly subtype, likely arise from fallopian tube precursor lesions (22), pre-menopausal women having a hysterectomy could elect to have a salpingectomy. Also, salpingectomy could be considered as an alternative to a standard tubal ligation. The risks from this approach appear low (14). How this will impact a woman's ovarian cancer risk is uncertain and dependent on several factors including the portion of ovarian cancers that are derived from the fallopian tube, and the latency period of precursor lesions.
Only recently have confirmed (genome-wide significant) common susceptibility alleles been identified for ovarian cancer. The risk associated with these alleles appears to be independent of a first-degree family history of ovarian cancer (3). Armed with this SNP information, it is now possible to better define an at-risk group for ovarian cancer who might be appropriate for screening should an effective modality be identified. Calculating a woman's lifetime risk of ovarian cancer based on the factors included here is fairly straightforward as all of the variables except genetic risk score are known by the patient (endometriosis is based on self-report). An individual's relevant SNP information will soon be obtainable at reasonable cost and permit the use of the model presented here to be used.
Risk modeling has been used in breast cancer as well as among BRCA1/2 mutation carriers. Another important future application of these results would be identifying women for whom surveillance would be appropriate. Currently, there are not effective screening strategies for ovarian cancer, however when effective screening measures become available, this work could be used to identify the high risk women in the general population who should be encouraged to undergo screening.
This analysis has some limitations. To calculate the probabilities of the joint distribution of these risk factors, we used the control subjects from four geographically dispersed population-based case-controls studies to represent the U.S. Also, the relative risk estimates used in these calculations were derived in part from the four case-control studies utilized in this analysis. The estimates are derived from a larger base of studies and are also in line with other published data. Because we based this analysis on published data and carried out simulations to derive the genetic risk quintiles, the variance may be underestimated, but our simulations suggest that the standard errors are reasonable. We also did not remove women who had had an oophorectomy our US population counts and thus our denominators are slightly inflated and the lifetime risks are underestimated. The data presented here relate to lifetime risk to age 85 for NH Whites. Risk at younger ages is of course lower and estimates for other racial/ethnic groups are needed. Lastly, there are likely other risk factors that contribute to lifetime risk of ovarian cancer which would influence these estimates, including menopausal estrogen therapy use, aspirin use and possibly others that have not been described.
This model does not take into account high penetrance mutations in ovarian cancer genes such as BRCA1, BRCA2, RAD51C, RAD51D. This information is not available in these data, but less than 1% of the population carry mutations in these genes and some of this risk is captured by family history of ovarian cancer. Our goal was to estimate lifetime risk in the general population and these results are applicable to women with unknown BRCA status. This body of work has provided a framework by which to incorporate both non-genetic and common genetic factors into estimating lifetime risk. There are women at a substantially elevated lifetime risk of ovarian cancer; several preventive strategies as well as modifiable risk factors that could result in substantially reduced lifetime risk of the disease are available now.
Supplementary Material
Acknowledgments
Financial Support
This work was supported by donations from by the family and friends of Kathryn Sladek Smith to the Ovarian Cancer Research Fund (A Berchuck). It was also supported by the National Institutes of Health (P30 CA14089, R01 CA61132 [MC Pike], P01 CA17054 [CL Pearce, MC Pike, AH Wu], N01 PC67010, R03 CA113148 [CL Pearce, MC Pike, AH Wu], R01 CA141154 [CL Pearce, MC Pike, AH Wu, DA Stram, DO Stram, AW Lee], R03 CA115195 [CL Pearce, AH Wu], R01 CA112523 [M Rossing, JA Doherty], R01 CA87538 [M Rossing, JA Doherty], R01 CA76016 [JM Schildkraut, A Berchuck], R01 CA95023 [RB Ness, F Modugno], R01 CA126841 [RB Ness, F Modugno], K07-CA80668 [RB Ness, F Modugno], NIH/National Center for Research Resources/General Clinical Research Center grant MO1- RR000056 [RB Ness, F Modugno], P50-CA159981 [RB Ness, F Modugno]); California Cancer Research Program (0001389V20170 and 2110200 [CL Pearce, AH Wu, MC Pike]); Eve Appeal (U Menon); Oak Foundation (U Menon); Women's Health Theme of the UK National Institute of Health Research supported University College London Hospital/University College London Comprehensive Biomedical Research Centre (U Menon); US Army Medical Research and Materiel Command (DAMD17-02-1-0669 [ RB Ness, F Modugno] and DAMD17-02-1-0666 [JM Schildkraut, A Berchuck]); Roswell Park Alliance Foundation (RB Ness, F Modugno); The Barbara Thomason Ovarian Cancer Professorship from the American Cancer Society (A Berchuck); National Institute of Environmental Health Sciences (T32ES013678 [AW Lee]); and Cancer Research UK (C490/A10124 [PDP Pharoah] and C490/A16561 [PDP Pharoah]).
Footnotes
Conflicts of Interest: The authors disclose no potential conflicts of interest.
REFERENCES
- 1.Cancer Facts & Figures 2013. American Cancer Society; Atlanta: 2013. [Google Scholar]
- 2.Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A. 2011;108(44):18032–18037. doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearce CL, Rossing MA, Lee AW, Ness RB, Webb PM, Chenevix-Trench G, et al. Combined and interactive effects of environmental and GWAS-identified risk factors in ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(5):880–890. doi: 10.1158/1055-9965.EPI-12-1030-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bojesen SE, Pooley KA, Johnatty SE, Beesley J, Michailidou K, Tyrer JP, et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat Genet. 2013;45(4):371–384. 384e371–372. doi: 10.1038/ng.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goode EL, Chenevix-Trench G, Song H, Ramus SJ, Notaridou M, Lawrenson K, et al. A genome-wide association study identifies susceptibility loci for ovarian cancer at 2q31 and 8q24. Nat Genet. 2010;42(10):874–879. doi: 10.1038/ng.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Permuth-Wey J, Lawrenson K, Shen HC, Velkova A, Tyrer JP, Chen Z, et al. Identification and molecular characterization of a new ovarian cancer susceptibility locus at 17q21.31. Nat Commun. 2013;4:1627. doi: 10.1038/ncomms2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pharoah PD, Tsai YY, Ramus SJ, Phelan CM, Goode EL, Lawrenson K, et al. GWAS meta-analysis and replication identifies three new susceptibility loci for ovarian cancer. Nat Genet. 2013;45(4):362–370. 370e361–362. doi: 10.1038/ng.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen H, Fridley BL, Song H, Lawrenson K, Cunningham JM, Ramus SJ, et al. Epigenetic analysis leads to identification of HNF1B as a subtype-specific susceptibility gene for ovarian cancer. Nat Commun. 2013;4:1628. doi: 10.1038/ncomms2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song H, Ramus SJ, Tyrer J, Bolton KL, Gentry-Maharaj A, Wozniak E, et al. A genome-wide association study identifies a new ovarian cancer susceptibility locus on 9p22.2. Nat Genet. 2009;41(9):996–1000. doi: 10.1038/ng.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolton KL, Tyrer J, Song H, Ramus SJ, Notaridou M, Jones C, et al. Common variants at 19p13 are associated with susceptibility to ovarian cancer. Nat Genet. 2010;42(10):880–884. doi: 10.1038/ng.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. J Am Med Assoc. 2011;305(22):2295–2303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 12.Menon U, Gentry-Maharaj A, Hallett R, Ryan A, Burnell M, Sharma A, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol. 2009;10(4):327–340. doi: 10.1016/S1470-2045(09)70026-9. [DOI] [PubMed] [Google Scholar]
- 13.Collaborative Group on Epidemiological Studies of Ovarian C. Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371(9609):303–314. doi: 10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- 14.McAlpine JN, Hanley GE, Woo MM, Tone AA, Rozenberg N, Swenerton KD, et al. Opportunistic salpingectomy: uptake, risks, and complications of a regional initiative for ovarian cancer prevention. Am J Obstet Gynecol. 2014;210(5):471, e471–471, e411. doi: 10.1016/j.ajog.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Lo-Ciganic WH, Zgibor JC, Bunker CH, Moysich KB, Edwards RP, Ness RB. Aspirin, nonaspirin nonsteroidal anti-inflammatory drugs, or acetaminophen and risk of ovarian cancer. Epidemiology. 2012;23(2):311–319. doi: 10.1097/EDE.0b013e3182456ad3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moorman PG, Calingaert B, Palmieri RT, Iversen ES, Bentley RC, Halabi S, et al. Hormonal risk factors for ovarian cancer in premenopausal and postmenopausal women. Am J Epidemiol. 2008;167(9):1059–1069. doi: 10.1093/aje/kwn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pike MC, Pearce CL, Peters R, Cozen W, Wan P, Wu AH. Hormonal factors and the risk of invasive ovarian cancer: a population-based case-control study. Fertil Steril. 2004;82(1):186–195. doi: 10.1016/j.fertnstert.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Wu AH, Pearce CL, Tseng CC, Templeman C, Pike MC. Markers of inflammation and risk of ovarian cancer in Los Angeles County. Int J Cancer. 2009;124(6):1409–1415. doi: 10.1002/ijc.24091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon JS, Tinker A, Pansegrau G, McAlpine J, Housty M, McCullum M, et al. Prophylactic salpingectomy and delayed oophorectomy as an alternative for BRCA mutation carriers. Obstet Gynecol. 2013;121(1):14–24. doi: 10.1097/aog.0b013e3182783c2f. [DOI] [PubMed] [Google Scholar]
- 20.Sieh W, Salvador S, McGuire V, Weber RP, Terry KL, Rossing MA, et al. Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies. Int J Epidemiol. 2013;42(2):579–589. doi: 10.1093/ije/dyt042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sieh W, Salvador S, McGuire V, Weber RP, Terry KL, Rossing MA, et al. Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies. Int J Epidemiol. 2013;42(2):579–589. doi: 10.1093/ije/dyt042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19(1):3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.