Abstract
Purified intermediate filament proteins can be reassembled in vitro to produce polymers closely resembling those found in cells, and these filament form viscoelastic gels. The crosslinks holding IFs together in the network include specific bonds between polypeptides extending from the filament surface and ionic interactions mediated by divalent cations. IF networks exhibit striking non-linear elasticity with stiffness, as quantified by shear modulus, increasing an order of magnitude as the networks are deformed to large stains resembling those that soft tissues undergo in vivo. Individual Ifs can be stretched to more than 2 or 3 times their resting length without breaking. At least ten different rheometric methods have been used to quantify the viscoelasticity of IF networks over a wide range of timescales and strain magnitudes. The mechanical roles of different classes of IF on mesenchymal and epithelial cells in culture have also been studied by an even wider range of microrheological methods. These studies have documented the effects on cell mechanics when IFs are genetically or pharmacologically disrupted or when normal or mutant IF proteins are exogenously expressed in cells. Consistent with in vitro rheology, the mechanical role of IFs is more apparent as cells are subjected to larger and more frequent deformations.
Keywords: Elastic modulus, Strain, Stiffness, Cytoskeleton, Vimentin, Desmin, Keratin, Neurofilaments, Viscoelastic
1. Introduction
Intermediate filaments provide the major structural support for many non-cellular materials such as hair, nails, and the slime surrounding hagfish. The mechanical properties of intracellular IFs are hypothesized to be essential for the normal function of many soft tissues, and mutations in distinct IF proteins lead to human diseases such as cardiomyopathies and skin blistering disorders that are characterized by a failure of affected tissues to withstand mechanical stress. The structures of IF proteins and the manner by which they assemble into filaments are highly distinct from those of the other cytoskeletal filaments F-actin and microtubules, and the mechanical properties of IF also diverge strongly from the rest of the cytoskeleton. The viscoelasticity of IF networks in vitro, and their contribution to the viscoelasticity of cells are increasing well characterized by a wide range of different techniques. These studies are beginning to show how the unusual structures of intermediate filaments contribute to the normal function of a large number of different cell types.
2. Viscoelasticity of purified IFs in vitro
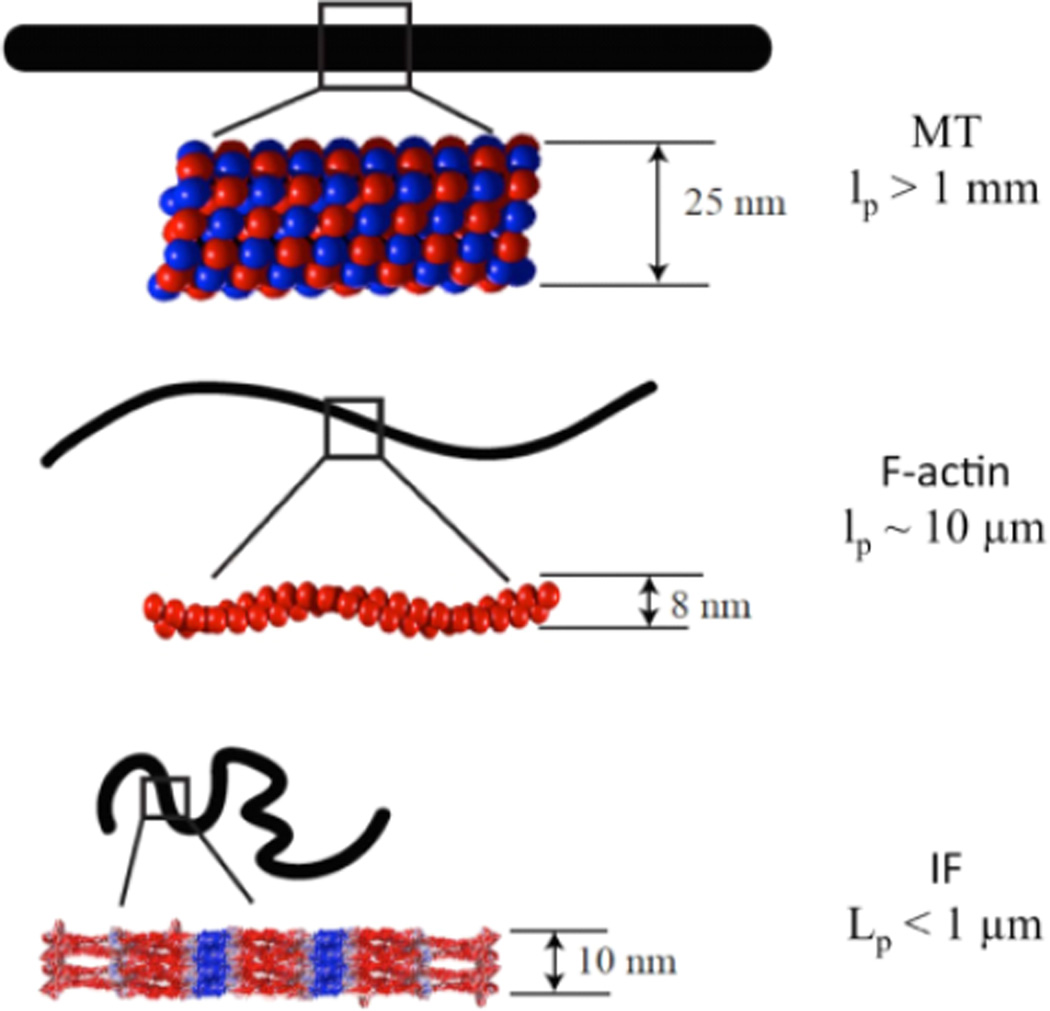
The mechanical properties of individual IF of different types have been measured directly by applying forces to them and imaging their deflection or have been inferred from images assuming that the polymer contours are deformed by thermal energy. The viscoelastic properties of IF networks constituted in vitro either as homogeneous networks or as composite network copolymerized with F-actin have been measured by a number of rheologic methods. The unique mechanical properties of intermediate filaments are related to two major structural differences between IFs and the other cytoskeletal polymers F-actin and microtubules. As shown in Figure 1, IFs are much more flexible than either microtubules or actin filaments. This flexibility differs from the other cytoskeletal polymers by orders of magnitude and is quantified by the persistence length lp, a measure of the distance over which a filament appears approximately straight.
Figure 1.
Schematic diagram of approximate diameter, subunit packing and filament configuration of each of the three cytoskeletal polymer types: microtubules (MT), F-actin, and intermediate filaments (IF). The black filament outline represents the configuration of each filament in solution at 37°C due to the thermal fluctuations acting on 10 micron long filaments with the persistence lengths lp listed on the right.
More precisely, lp is defined by the expression <cos θ(s)>=e−s/lp where <cos θ(s)> is an ensemble average of the angle θ formed by two tangents drawn at distances s along the contour. The persistence length is related to the elastic bending constant of the filament K by the expression K = λp/kBT where kBT is the thermal energy. This great flexibility is likely to be related to the greater degree of disorder and open hydrated space within intermediate filaments compared to actin or tubulin polymers. How precisely the subunit packing and higher-order structure of IFs allows them to be so flexible and resistant to breakage is not fully understood, but many different kinds of measurements reveal that IFs can potentially provide mechanical support to cells and tissues that cannot be achieved by the other polymer types. A representative, although not exhaustive, summary of the methods by which different types of IF have been characterized in vitro and the major findings of these studies are summarized in Table 1.
Table 1.
Methods to characterize IF mechanical properties in vitro
| IF type | method | concentration | time scale | main result |
|---|---|---|---|---|
| NF / glial IFs (Leterrier & Eyer, 1987) |
Falling ball viscometry |
1 to 5 mg/ml | Seconds to minutes |
NFs form gels by crossbridging divalent ions affect gelation |
| NF Vimentin (Leterrier et al., 1996) |
Oscillatory shear rheometry Parallel plate |
3 mg/ml | 10 ms to 1000 s |
NF networks strain- stiffen G' increases from <100 Pa to >kPa Modified by phosphorylation |
| Desmin Keratin NF (Kreplak et al., 2005) |
AFM | Single filaments |
Seconds to hours |
IFs withstand stretching to >200% without rupture |
| NF NF-F-actin (Wagner et al., 2007) |
Oscillatory shear rheometry |
4 mg/ml | Seconds to minutes |
NF gels rupture at high strain but rapidly reform. NF- F-actin composites lose recovery after large strain |
| Keratin (Leitner et al., 2012) |
Single bead thermal fluctuation microrheometry |
0.5 mg/ml | 0.5 ms to 1 s |
G' = 0.5 Pa with 2 mM Mg2+. Divalent ions stabilize networks |
| Vimentin (Janmey et al., 1991) |
Torsion pendulum |
0.3 to 10 mg/ml |
10 ms to 100 s |
Vimentin networks strain stiffen. Gels withstand >80 % strain |
| Keratin Vimentin (Pawelzyk et al., 2014) |
Macroscopic shear rheometry and optical microrheometry |
0.1 to 2 mg/ml | 50 ms to 10 s |
IF have attractive interactions due to hydrophobic and H bonds |
| Keratin Vimentin (Yamada, Wirtz, & Coulombe, 2003) |
Shear rheometry Couette and cone-plate geometries |
1 mg/ml | 50 ms to 10 s |
Apparent G' on order of 1–10 Pa affected by interfacial tensions. Weak frequency dependence |
| Vimentin (Lin, Broedersz, et al., 2010) |
Parallel plate shear rheometry |
0.2 to 1 mg/ml | 300 ms to 50 s |
Elastic response mainly entropic. Divalent ions act as crosslinkers. |
| Desmin (Schopferer et al., 2009) |
1. Oscillatory squeeze flow 2. Cone-plate shear rheometry |
1 to 2 mg/ml | 1. 50 μs to 1 s 2. 1 s |
Strain stiffening but not always initial gelation is altered by disease-causing mutations |
| Vimentin and NF (Lin, Yao, et al., 2010) |
Cone-plate shear rheometry |
0.3 to 3 mg/ml | 0.03 to 1000 s |
Elasticity and strain- stiffening fit by theory for semi- flexible polymer networks |
| Desmin Vimentin (Schopferer et al., 2009) |
1. Oscillatory squeeze flow 2. Cone-plate shear rheometry |
0.4 to 2.8 mg/ml |
50 μs to 1 s |
Desmin (lp≈900 nm) is stiffer than vimentin (lp ≈400 nm) both electrostatics and binding affects network stiffness |
| Vimentin Vimentin+actin (Esue et al., 2006) |
Cone-plate shear rheometry |
0.04 to 0.4 mg/ml |
1- ms to 5 s |
Vimentin C-terminal tail binds F-actin to increase elastic modulus |
| Vimentin (Guzman et al., 2006) |
AFM deflection | Single filaments |
seconds | Bending modulus of single IFs between 300 and 400 MPa |
| Vimentin (Mucke et al., 2004) |
EM and AFM imaging |
Single filaments |
static | Persistence length 1 μm |
| Keratin (Bousquet et al., 2001) |
Cone-plate shear rheometry |
0.5 to 1 mg/ml | seconds | K14 C-terminal tail binds filament side to form crosslink |
| Keratin (Chou & Buehler, 2012) |
Molecular dynamics |
Single dimer | <20 ns | All atom simulation predicts force- extension of keratin dimer |
| NF (Janmey et al., 2007) |
Parallel plate shear rheometry |
2 mg/ml | seconds | Shear deformations generate negative normal stress in NF networks |
Several clear features unique to IF network mechanics emerge from these studies, and some issues related to the magnitude of IF network stiffness and the nature of inter-filament links remains to be clarified. Unlike other elements of the cytoskeleton, individual IFs and the networks they form can withstand large deformations that would rupture F-actin or microtubules (Guzman et al., 2006; Janmey, Euteneuer, Traub, & Schliwa, 1991; Kreplak, Bar, Leterrier, Herrmann, & Aebi, 2005). Not only do IF networks not rupture at large strain, but their elastic moduli increase, so that the incremental stiffness of vimentin, neurofilament, and other IF types can be ten times larger at 100% strain that in the limit of low strain (Bertaud, Qin, & Buehler, 2010; Janmey et al., 1991; Leterrier, Kas, Hartwig, Vegners, & Janmey, 1996; Lin, Yao, et al., 2010; Pawelzyk, Mucke, Herrmann, & Willenbacher, 2014; Schopferer et al., 2009). The dependence of IF networks elastic moduli on protein connection is also different from that of other biopolymer gels. Whereas the shear moduli of fibrin and actin networks scales with at least the square of the protein concentration, the shear moduli of vimentin and desmin networks increase much more gradually with power law exponents as low as 0.5 (Janmey et al., 1991; Lin, Broedersz, et al., 2010; Schopferer et al., 2009) relating elastic modulus to concentration. The reason for this discrepancy between IF and other biopolymer gels is not known.
The molecular mechanisms that link IFs together so that they form mechanically resistant networks are also not well understood. Specific crosslinking proteins do not appear to be required for network formation, and several bonds between IF subunit C-terminal extensions and the sides of other filaments have been reported (Bousquet et al., 2001; Esue, Carson, Tseng, & Wirtz, 2006; Pawelzyk et al., 2014). Complementary attractive interactions between NF sidearms are also implicated in linking these IFs to each other (Gou, Gotow, Janmey, & Leterrier, 1998). The most common method to create IF networks in vitro is to add divalent cations, usually Mg2+, to several millimolar concentrations. The mechanisms by which divalent ions crosslink IFs is not fully characterized but has been hypothesized to involve either specific metal-binding bonds (Lin, Broedersz, et al., 2010) or polyelectrolyte effects that depend on the high surface charge of all IFs (Huisman et al., 2011; Janmey, Slochower, Wang, Wen, & Cebers, 2014). Identifying the molecular mechanisms for IF crosslinking and bundle formation remains a major challenge to defining this system with the same detail as currently available for network formation by other biopolymers.
3. IFs and the mechanical properties of cells
The unique mechanical properties of IFs in vitro, characterized by strain-stiffening of networks and the capacity of IFs to withstand very large extensions, have motivated recent studies to determine the roles of IFs in the mechanical properties of cells. Diverse studies have shown the effects of specific IF types in cell migration, adhesion, and mechanotransduction (Chung, Rotty, & Coulombe, 2013; Ivaska, Pallari, Nevo, & Eriksson, 2007; Pallari & Eriksson, 2006; Sakamoto, Boeda, & Etienne-Manneville, 2013; Wang & Stamenovic, 2000). The large diversity of cellular IF types, which are often integrated with actin and microtubules networks, lead to a range of cellular effects when different IF types are genetically or pharmacologically disrupted or when they are overexpressed. Biochemical and genetic methods used to alter IF expression or assembly in cells are summarized in Table 2. Table 3 summarizes the methods used to characterize IF impact on cell mechanical properties and the main conclusions about their contribution to cell mechanics.
Table 2.
Biological tools allowing to modify the properties of If networks used for biomechanical studies
| Type of IF |
Cell type | Tools for modifying network |
Effect on IF network morphology |
|---|---|---|---|
| Vimentin (Haudenschild et al., 2011; Wang & Stamenovic, 2000) |
Endothelial cells and primary human articular chondrocytes |
Acrylamide targets directly IF network, but has other effects than can obscure interpretation |
Perinuclear condensation of the vimentin network |
| Vimentin (Gladilin et al., 2014) |
Natural killer cells | Withaferin A targets directly vimentin network |
Disruption of the vimentin network and aggregates formation |
| Vimentin (Brown et al., 2001) |
T lymphocytes | Calyculin A targets vimentin phosphatases, but also other enzymes that can indirectly affect IFs |
Formation of a condensed juxtanuclear aggregate of vimentin |
| Vimentin (Rathje et al., 2014) |
Immortalized human skin fibroblasts |
Simian virus 40 large T antigen |
Condensation of the vimentin network in the perinuclear area and retraction of thin peripheral filaments |
| Vimentin (Plodinec et al., 2011) |
Rat-2 fibroblasts | Mutated desmin L345P targets directly vimentin or desmin network |
Perinuclear aggregation of the vimentin inducing network total disruption |
| Desmin (Bonakdar et al., 2012) |
Primary human myoblasts from patients carrying desmin mutations |
Mutated desmin targets directly vimentin or desmin network |
Not described |
| Keratin (Beil et al., 2003) |
Human pancreatic epithelial tumor cells |
Sphingosylphosphorylcholine to induce keratins phosphorylation |
Perinuclear reorganization of the keratin network |
Table 3.
Summary of the investigations of the mechanical role of IF networks at the cellular level
| Type of IF | Cell type | Technique | Cellular elements probed |
Effect of the lack or the disruption of the vimentin network at the cellular scale |
|---|---|---|---|---|
| Vimentin (Eckes et al., 1998) |
Primary fibroblasts from vimentin KO rats |
Rotational force magnetic twisting cytometer (Wang & Ingber, 1994) |
Cell cortex submitted to large strains |
Cortical rigidity lower of 40% |
| Collagen lattice contraction (Mendez et al., 2014) |
Cells contractile machinery |
Contractions forces developed by vim - /-cells significantly reduced |
||
| Vimentin (Wang & Stamenovic, 2000) |
Primary fibroblasts from vimentin KO rats Primary fibroblasts from WT rats and endothelial cells acrylamide treated |
Rotational force magnetic twisting cytometer (Wang & Ingber, 1994) |
Cell cortex submitted to different ranges of strain |
Reduce ability to stiffen the cortex in response to applied forces and global cortex stiffness lower, at large strains. These effects are amplified when the magnitude of the cell strain increase. |
| Vimentin (Guo et al., 2013) |
Primary fibroblasts from vimentin KO mice |
Optical magnetic twisting cytometry (Fabry et al., 2001) |
Cell cortex submitted to low strains |
No effect on cells cortical rigidity |
| Optical tweezers | Cytoplasm | Intracytoplasmic rigidity of cell reduced by about a factor 2 |
||
| Vimentin (Gladilin et al., 2014) |
Natural killer cells treated with withaferin A |
Microfluidic optical stretcher (Guck et al., 2001) |
Whole cell submitted to large strain |
Global cell softening of about 20% |
| Vimentin (Brown et al., 2001) |
T lymphocytes treated with Calyculin A |
High G-force centrifugation (Mege, Capo, Benoliel, Foa, & Bongrand, 1985) |
Whole cell submitted to large strain |
Whole cell deformability increased by about 40% |
| Vimentin (Haudenschild et al., 2011) |
Primary human articular chondrocytes |
Straining of cells embedded in alginate gels |
Whole cell submitted to large strain |
Softening of the entire cell by a factor 3 |
| Vimentin (Rathje et al., 2014) |
Immortalized human skin fibroblasts expressing simian virus 40 large T antigen |
Colloidal probe force-mode AFM (Ducker et al., 1991) |
Local cortex or cytoplasm in function of indentation depth |
Cytoplasmic Young's modulus increased locally by 2 times |
| Vimentin (Plodinec et al., 2011) |
Rat-2 fibroblasts expressing L345P mutated desmin |
AFM | Local cortex or cytoplasm in function of indentation depth |
Perinuclear stiffening of the cytoplasm |
| Desmin (Bonakdar et al., 2012) |
Primary human fibroblasts from patients carrying the R350P desmin mutation |
Magnetic tweezers (Kollmannsberger & Fabry, 2007) |
Cell cortex locally submitted to different ranges of strain |
Cortical stiffness increased by 2 times Cortical stiffening 3 times lower after repeated straining of the cell |
| Keratins (Beil et al., 2003) |
Human pancreatic epithelial tumor cells treated with sphingosylphosphorylcholine |
Parallel microplate cell stretcher |
Whole cell | Cells elastic moduli decreased by 40% |
| Migration through size-limited pores |
Whole cell | Cells deformability significantly increased |
||
| Keratins (Seltmann et al., 2013) |
Primary keratinocytes from KO mice lacking all keratins |
Optical stretcher (Lincoln et al., 2007) |
Whole cell | Cells deformability increased by about 60% |
| Keratins (Sivaramakrishnan et al., 2008) |
Keratinocyte cell line KtyII−/− |
AFM | Cytoplasm | Cytoplasmic Young modulus above cell nucleus is lowered by about 40 % |
| Magnetic tweezers | Cytoplasm | Cytoplasmic viscosity is 40% weaker |
||
| Neuro- filaments (Grevesse et al., 2015) |
Primary rat cortical neurons |
Magnetic tweezers | Neurites vs. soma |
NF-rich neurites are both stiffer and more viscous than the soma |
3.1 Type III IF: vimentin and desmin
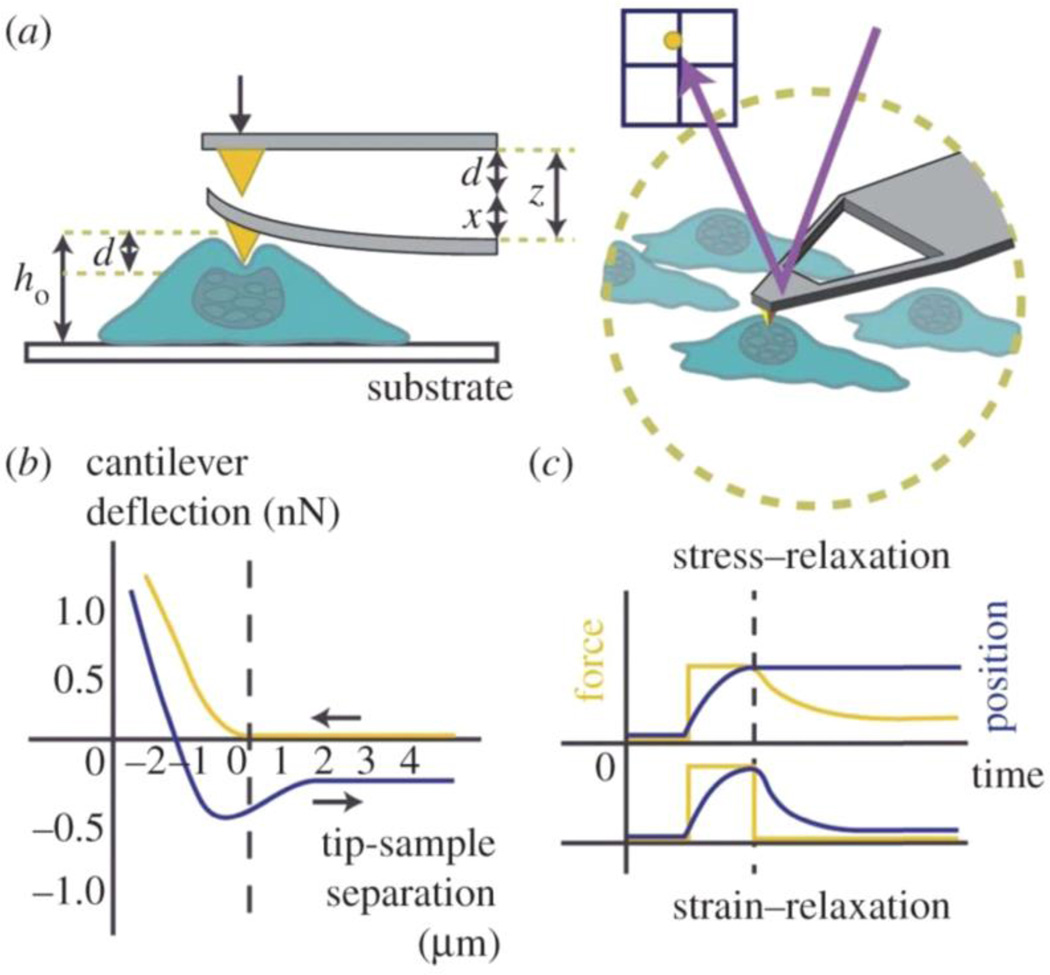
Vimentin and desmin are the most represented filaments in this subgroup. Investigating the mechanical influence of type III IF on cell mechanical properties has led to divergent results largely because the results of mechanical measurements are strongly dependent on the way of probing the cell and the magnitude of the strain the cell is submitted to during the experiments (Table 3). The most commonly employed current method to characterize the cortical stiffness of cells is by indenting their surface with a conical tip or a colloidal bead attached to an AFM cantilever, as depicted in Figure 2. The principle of AFM and its application to reveal the mechanical affects of IFs in cells are summarized in Figure 2 and Table 4. This method allows stiffness differences between cell types to be measured at various degrees of deformation, often with simultaneous imaging. Calculation of the absolute magnitude of elastic moduli from AFM as well as other microrheological techniques is difficult because numerous assumptions about contact geometry, material homogeneity and volume conservation need to be made.
Figure 2.
AFM modes of measurement. (a) AFM can be used to precisely apply compressive strains apically to cells within their aqueous environment. A laser deflected from the back of the AFM cantilever is measured by a photo sensitive detector (PSD) to quantify cantilever deflection. (b) AFM force-indentation curves are often used to measure cellular elasticity, by fitting the approach curve (yellow) to the Hertz model of contact mechanics. The retraction curve (blue) often shows a hysteresis and can be used to analyze adhesion and dissipation. (c) Stress and strain relaxation curves are often used to measure time-dependent cellular response. Following an applied strain on a cell, the cantilever can be kept at a constant height, and measurements of cellular force onto the cantilever can be measured. Alternatively, following an initial strain, changes in the height of the cantilever as the cell relaxes can be measured. Modified cantilevers are also useful for measuring binding/unbinding forces between ligands and receptors (Haase & Pelling, 2015).
Table 4.
Using Atomic Force Microscopy to determine cell stiffness, with emphasis on methods to detect mechanical effects of IFs
|
1. AFM cantilever calibration. Before each experiment, the cantilever is moved down onto a rigid surface such as the bottom of the glass or plastic plate while measuring the bending of the cantilever, as assessed by the laser beam deflected from its surface and the vertical displacement of the base of the cantilever as determined by the piezoelectric device. Since the rigid surface cannot be deformed by the relatively soft AFM cantilevers used for cells, any difference between the vertical displacement of the cantilever tip and base is due to deflection of the cantilever. The measured deflection is calibrated by taking the slope of the cantilever deflection vs. piezo displacement curve. The spring constant of the cantilever is then determined by measuring its resonance frequency in liquid, as discussed in detail elsewhere (Levy & Maaloum, 2002). | |
|
2. Culture cells on standard glass or plastic dishes or on substrates of adjustable stiffness. The substrates need to be rigidly held in a container that is large enough in diameter and deep enough to allow the AFM column (often call the head) which holds the cantilever, to be immersed into medium above the cell. Typically the width of the dish is >20 mm and the depth of liquid above the cell is several mm. The piezoelectric devices that move the AFM probe vertically have limited range, so the depth of liquid cannot be much larger than mm. | |
|
3. Identifying the point of contact between AFM probe and cell surface. Usually, the probe is moved near the cell surface using the microscope stage and imaging the focal plain of the AFM probe relative to that of the cell's apical surface. Once near enough to allow the piezoelectric drive to span the remaining distance, (generally several microns) the final movements are made by the AFM software and hardware. The probe can be moved slowly until the deflection of the laser beam indicates that the cantilever is beginning to bend, presumably because it has touched the tip of the cell. Other methods based on changes in resonance can also determine the point at which contact is made. | |
|
4. Indentation of the cell surface. As the AFM tip descends farther into the cell or gel, the cantilever will become increasingly bent (unless something breaks or slips) and the result is a force-extension curve where force is calculated from the measured bending of the cantilever, and extension from the vertical displacement of the AFM tip. | |
|
5. Force-displacement measurements. The force-displacement data derived from the initial indentation into the sample are generally limited to a few hundred nms, over a time on the order of a second, depending on the shape of the probe, the material properties of interest, and the capabilities of the instrument hardware and software. Indentation is usually followed immediately by retraction. Perfect superposition of the indentation and retraction curves is expected for a purely elastic material to which the probe does not adhere. In reality, there is usually a difference between the indentation and retraction curves. The area between the curves is a measure of energy dissipated during the deformation, and often termed the plasticity index, but it is not simply related to a material constant such as a viscosity. This quantity is particularly dependent on the depth to which cells are indented and changes as a result of differences in IF expression. | |
|
6. Calculation of elastic modulus. Quantifying the cell stiffness requires calculating an elastic modulus (usually the Young's modulus, a material property) from the force-extension curve (an experimental system-specific set of values). Conversion of force-indentation curves to absolute values of stiffness is perhaps the most challenging aspect of AFM measurements and the one most likely to lead to errors. Generally, a formula like that derived by Hertz is used to calculate elastic moduli from force measurements by accounting for the size and shape of the AFM probe and making assumptions about the nature of the sample's surface. For a spherical or hemi-spherical shape AFM tip, the Hertz relation is: | |
|
| |
| where f is the force applied to the cell, k is the spring constant of the cantilever, d is the deflection of the cantilever, E is the Young’s modulus of the cell or other sample, R is the radius of the bead, δ is the indentation into the cell and ν is the Poisson’s ratio of the cell (a value related to the extent to which the sample maintains constant volume when deformed and often assumed to be near 0.5 for full volume conservation). For different geometries of the AFM tip the form of the Hertz relation varies, as detailed in several recent reports (Guz, Dokukin, Kalaparthi, & Sokolov, 2014; Melzak & Toca-Herrera, 2015; Thomas, Burnham, Camesano, & Wen, 2013). | |
|
7. IF-specific AFM methods and results. Detecting the mechanical effects of changes in IF expression by AFM depends on the way the cell is deformed. For example, loss of keratin leads to softening detected by small amplitude deformation of the cell surface, but often loss of vimentin does not. However, when cells are repeatedly deformed or deformed to greater depths, loss of vimentin becomes evident by changes in elastic modulus or plasticity index. |
3.1.1 Knockout models of vimentin
Several biomechanical studies have been performed on mesenchymal cells with a disrupted or no vimentin network (Holwell, Schweitzer, & Evans, 1997; Klymkowsky, 1981). The first demonstration of a role of vimentin in cells mechanical properties employed a rotational force magnetic twisting cytometer (Wang & Ingber, 1994) to study primary fibroblasts from vimentin KO mice (Eckes et al., 1998). Vimentin null cells exhibit a lower cortical rigidity than WT cells and disturbed migratory abilities suggesting a role of vimentin in the stabilization of actin and microtubules networks in cells. A subsequent study of fibroblasts from vimentin KO mice quantified the mechanical impact of vimentin on cells (Wang & Stamenovic, 2000). This study, using the same magnetic twisting device, showed that the mechanical alteration of those fibroblasts due to the lack of vimentin, i.e. softer cortex and reduced ability to stiffen, is detectable only when cells are submitted to large strain. Studies using AFM stiffness mapping shoed that the cortical stiffness of vimentin null cells measured at small strains, is not altered compared to WT cells, but that the absence of vimentin becomes apparent as cells are deformed more strongly or repeatedly. Loss of vimentin also increases the viscous loss during a cycle of cell deformation (Mendez, Restle, & Janmey, 2014). Another study on fibroblasts from KO vimentin mice, probed by optical magnetic twisting cytometry, demonstrated that the cortical rigidity of cells is not affected by the lack of vimentin, but the strain intensity applied to the cells during experiments was not reported (Guo et al., 2013). However, the same study showed that the interior cytoplasmic rigidity of fibroblasts null for vimentin, measured by active bead microrheometry, is reduced by a factor of 2, leading to an increased velocity of vesicular trafficking, suggesting that vimentin filaments are important to stabilize organelles position in the cytoplasm (Guo et al., 2013).
3.1.2 Drugs and proteins disrupting vimentin
The contribution of vimentin network to cells mechanical properties can also be assessed by using drugs specifically targeting vimentin or the enzymes that alter its phosphorylation, and inducing the disruption, aggregation or depolymerization of its network. Withaferin A treatment induces disruption of the vimentin network and leads to its aggregation (Thaiparambil et al., 2011). Incubation of suspended natural killer (NK) cells with withaferin A induces a global cell softening of about 20 %, when probed at large strains with a microfluidic optical stretcher (Gladilin, Gonzalez, & Eils, 2014). Calyculin A is a drug targeting vimentin phosphatases and inducing the disruption of the vimentin network (Eriksson et al., 1992; Eriksson, Toivola, Sahlgren, Mikhailov, & Harmala-Brasken, 1998). In T lymphocytes, the vimentin network is organized as a cortical cage maintaining the mechanical integrity of the cell since the collapse of this network, with calyculin A, increases cell deformability by about 40% and presumably softens the cell, as quantified by high g-force centrifugation onto adhesive substrate followed by morphological analysis (Brown, Hallam, Colucci-Guyon, & Shaw, 2001). Calyculin A is an inhibitor of vimentin protein phosphatases, but this molecule can also inhibit other cellular phosphatases like the one affecting myosin light chain and thus alter acto-myosin contractility of the cell. Acrylamide can also be used to induce the depolymerization and aggregation of vimentin IFs (Durham, Pena, & Carpenter, 1983; Eckert, 1985). Characterization of acrylamide-treated primary human articular chondrocytes shows that the loss of the vimentin network integrity induce a 3 fold softening of the entire cells as evaluated by applying large strains to cells embedded in alginate gels (Haudenschild et al., 2011). Acrylamide is used at low concentration is this study (4mM) to limit its effect on other cytoskeletal components but 4mM of acrylamide is enough to affect nuclear lamina architecture and mitochondrial homeostasis (Hay & De Boni, 1991). Moreover at higher concentration acrylamide disrupt actin and microtubules networks (Sager, 1989) so an effect of acrylamide on other cellular elements than vimentin cannot be excluded.
The over-expression of an oncogene protein (simian virus 40 large T antigen) is another way to induce a perinuclear reorganization of the vimentin network in human fibroblasts. This spatial reorganization of the network induces a 2 fold increase of the cytoplasmic young modulus, when quantified with colloidal probe force-mode AFM (Ducker, Senden, & Pashley, 1991), in the region where the vimentin density is increased (Rathje et al., 2014).
3.1.3 Mutant desmin to disrupt vimentin
Another possible tool to disrupt the vimentin network is the expression of wild type or mutated desmin in cells that originally express only vimentin. Desmin can copolymerize with vimentin and so some dominant negative desmin mutants can induce the collapse of the vimentin network. The expression of the L345P desmin mutant in rat fibroblasts induces a perinuclear aggregation of the vimentin network correlated to a local stiffening of the cytoplasm in those areas as measured by AFM (Plodinec et al., 2011) (Figure 3). The effect of exogenous expression of three different desmin mutants on the elastic moduli of fibroblasts is shown in Figure 3D. Expression of GFP-fused WT desmin has a small softening effect on the cell, possibly due to destabilization of the entire IF network by the GFP-desmin fusion protein. In contrast, the desA213V point mutant of desmin, which can form filaments, stiffens the cell possibly by increasing total IF content but also potentially by altering the mechanics of the IFs into which this mutant incorporates. The non-filament forming desmin mutant desL345P has a complex effect on cell stiffness. It collapses the endogenous vimentin network around the nucleus, thereby strongly stiffening the perinuclear region, leaving the rest of the cell slightly less stiff than normal.
Figure 3.
AFM indentation method for analyzing desmin IF nanomechanics in cells. (A) When indenting a cell, the AFM tip first encounters the actin cytoskeleton (blue) below the plasma membrane and then (B) the intermediate filament network (red). (C) The retracting AFM force curve specifies the cell’s response to the force F applied to indent the cell to a depth hc. The force curve can be divided into two main segments. The lower segment corresponds to the response of the actin cytoskeleton beneath the plasma membrane, whereas the upper segment of the curve predominantly represents the response of the deeper intermediate filament network. A linear fit to the upper 50% of the force curve (red) is used to determine the elastic modulus. (D) Elastic modulus (Es) of untransfected cells (rat-2 fibroblasts), cells transfected with WT desmin-GFP fusions (DesWT) and cells expressing two types of desmin point mutants, DesA213V, which forms filaments and DesL345P, which does not. Solid bars denote the average stiffness of the whole cell or the region away from the nucleus, and the cross-hatched bar denotes the perinuclear area. The statistical analysis shows mean values of Es and standard deviation (* p < 0.05, ** p < 0.0001) (Plodinec et al., 2011).
3.1.4 Mutated desmin to disrupt desmin
Desmin is a type III IF specifically expressed in muscle cells. The study of primary myoblasts from a patient carrying the desmin mutation R350P by magnetic tweezers shows that the cortical rigidity of these cells is twice that of cells from a WT patient, and their cortical stiffening under repeated stretching is 3 fold lower than for healthy human myoblasts. (Bonakdar et al., 2012).
3.2 Keratins
Epithelial cells specifically express keratin belonging to type I (keratins 9–20) and II (keratins 1–8) IFs. The shear modulus of the keratin component of the isolated epithelial cell cytoskeleton ranges from approximately 34 Pa near the perinuclear area to 10 Pa near the cell edge (Sivaramakrishnan, DeGiulio, Lorand, Goldman, & Ridge, 2008). This finding is consistent with studies of vimentin listed above that report a larger effect of disrupting IFs when cells are indented closer to the perinuclear region.
The involvement of keratins in cell mechanical properties has also been studied by drug-induced reorganization of the keratin network using sphingosylphosphorylcholine (SPC) to induce a perinuclear reorganization of keratin filaments in human pancreatic epithelial tumor cells (Beil et al., 2003). SPC treatment reduced the elastic moduli of treated cells, characterized with a parallel microplate cell stretcher, by 40%, and enhanced these cells ability to squeeze through small pores in a size-limited migration assay.
Double optical trapping experiments performed on suspended murine keratinocytes without keratin filaments show that deformability of these cells is increased about 60 % (Seltmann, Fritsch, Kas, & Magin, 2013). Another study on keratinocytes lacking keratin networks showed that the cell body Young’s modulus of these cells is lowered by 40% as measured with AFM, and their intracytoplasmic viscosity is 40% lower as assessed with magnetic tweezers (Ramms et al., 2013).
3.3 Neurofilaments
Because NFs are localized to thin projections of neurons such as the axons of mature cells or neurite of developing cells or those reported in vitro, direct measurements of NF contributions to cell mechanics are less extensive than those of keratins or Type III IFs. A recent study used magnetic tweezers to apply force to magnetic beads attached either to the cell body or the neurites of neurons cultured on adhesion lines patterned on a substrate. Creep curves similar to those on Figure 2C showed that the high concentration of NFs in neurites caused them to be both stiffer than the cell body but also more viscous (Grevesse, Dabiri, Parker, & Gabriele, 2015). The increased viscosity is attributed to the many transient interactions between NF sidearms that provide resistance to abrupt deformation but that can reorganize in response to prolonged forces, consistent with the rapidly reforming gels of NFs in vitro after they are disrupted by large strains (Wagner et al., 2007).
4. Conclusion
Macrorheological methods applied to purified networks formed by multiple types of IFs as well as AFM imaging and deformation of single intermediate filaments have revealed viscoelastic properties that differ from other biopolymers and that have potential to many biological functions. Measurements of the effects of IF disruption or deletion in cultured cells has revealed significant changes in cell mechanics, but thus far, usually more modest effects on cell stiffness than are produced, for example, but disrupting the actin network. In some cases such as measured of cortical stiffness in cells devoid of vimentin has shown no significant effect. The largest effects of IF disruption appear to be in keratin-containing cells and suspended cells such as lymphocytes. Future studies of systems that are closer to the in vivo context, such as confluent monolayers and cells in 3-D culture might reveal additional mechanical effects of IF in many different cell types.
Acknowledgments
We are grateful to Fitzroy Byfield for advice on AFM methods. This work was supported by grant GM096971 from the US National institutes of Health.
Abbreviations
- AFM
atomic force microscope
- GFP
green fluorescent protein
- IF
intermediate filament
- KO
knockout
- MT
microtubule
- NF
neurofilament
- Pa
Pascal = Newton/m2
- SPC
sphingosylphosphorylcholine
- WT
wild-type
References
- Beil M, Micoulet A, von Wichert G, Paschke S, Walther P, Omary MB, Seufferlein T. Sphingosylphosphorylcholine regulates keratin network architecture and visco-elastic properties of human cancer cells. Nat Cell Biol. 2003;5:803–811. doi: 10.1038/ncb1037. [DOI] [PubMed] [Google Scholar]
- Bertaud J, Qin Z, Buehler MJ. Intermediate filament-deficient cells are mechanically softer at large deformation: A multi-scale simulation study. Acta Biomaterialia. 2010;6:2457–2466. doi: 10.1016/j.actbio.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Bonakdar N, Luczak J, Lautscham L, Czonstke M, Koch TM, Mainka A, Fabry B. Biomechanical characterization of a desminopathy in primary human myoblasts. Biochem Biophys Res Commun. 2012;419:703–707. doi: 10.1016/j.bbrc.2012.02.083. [DOI] [PubMed] [Google Scholar]
- Bousquet O, Ma LL, Yamada S, Gu CH, Idei T, Takahashi K, Coulombe PA. The nonhelical tail domain of keratin 14 promotes filament bundling and enhances the mechanical properties of keratin intermediate filaments in vitro. Journal of Cell Biology. 2001;155:747–753. doi: 10.1083/jcb.200104063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MJ, Hallam JA, Colucci-Guyon E, Shaw S. Rigidity of circulating lymphocytes is primarily conferred by vimentin intermediate filaments. Journal of Immunology. 2001;166:6640–6646. doi: 10.4049/jimmunol.166.11.6640. [DOI] [PubMed] [Google Scholar]
- Chou CC, Buehler MJ. Structure and Mechanical Properties of Human Trichocyte Keratin Intermediate Filament Protein. Biomacromolecules. 2012;13:3522–3532. doi: 10.1021/bm301254u. [DOI] [PubMed] [Google Scholar]
- Chung BM, Rotty JD, Coulombe PA. Networking galore: intermediate filaments and cell migration. Curr Opin Cell Biol. 2013;25:600–612. doi: 10.1016/j.ceb.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducker WA, Senden TJ, Pashley RM. Direct measurement of colloidal forces using an atomic force microscope. Nature. 1991;353:239–241. [Google Scholar]
- Durham HD, Pena SD, Carpenter S. The neurotoxins 2,5-hexanedione and acrylamide promote aggregation of intermediate filaments in cultured fibroblasts. Muscle Nerve. 1983;6:631–637. doi: 10.1002/mus.880060903. [DOI] [PubMed] [Google Scholar]
- Eckert BS. Alteration of intermediate filament distribution in PtK1 cells by acrylamide. European Journal of Cell Biology. 1985;37:169–174. [PubMed] [Google Scholar]
- Eckes B, Dogic D, Colucci-Guyon E, Wang N, Maniotis A, Ingber D, Krieg T. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. Journal of Cell Science. 1998;111:1897–1907. doi: 10.1242/jcs.111.13.1897. [DOI] [PubMed] [Google Scholar]
- Eriksson JE, Brautigan DL, Vallee R, Olmsted J, Fujiki H, Goldman RD. Cytoskeletal integrity in interphase cells requires protein phosphatase activity. Proc Natl Acad Sci U S A. 1992;89:11093–11097. doi: 10.1073/pnas.89.22.11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JE, Toivola DM, Sahlgren C, Mikhailov A, Harmala-Brasken AS. Strategies to assess phosphoprotein phosphatase and protein kinase-mediated regulation of the cytoskeleton. Methods Enzymol. 1998;298:542–569. doi: 10.1016/s0076-6879(98)98044-2. [DOI] [PubMed] [Google Scholar]
- Esue O, Carson AA, Tseng Y, Wirtz D. A direct interaction between actin and vimentin filaments mediated by the tail domain of vimentin. Journal of Biological Chemistry. 2006;281:30393–30399. doi: 10.1074/jbc.M605452200. [DOI] [PubMed] [Google Scholar]
- Gladilin E, Gonzalez P, Eils R. Dissecting the contribution of actin and vimentin intermediate filaments to mechanical phenotype of suspended cells using high-throughput deformability measurements and computational modeling. Journal of Biomechanics. 2014;47:2598–2605. doi: 10.1016/j.jbiomech.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Gou JP, Gotow T, Janmey PA, Leterrier JF. Regulation of neurofilament interactions in vitro by natural and synthetic polypeptides sharing Lys-Ser-Pro sequences with the heavy neurofilament subunit NF-H: neurofilament crossbridging by antiparallel sidearm overlapping. Med Biol Eng Comput. 1998;36:371–387. doi: 10.1007/BF02522486. [DOI] [PubMed] [Google Scholar]
- Grevesse T, Dabiri BE, Parker KK, Gabriele S. Opposite rheological properties of neuronal microcompartments predict axonal vulnerability in brain injury. Sci Rep. 2015;5:9475. doi: 10.1038/srep09475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guck J, Ananthakrishnan R, Mahmood H, Moon TJ, Cunningham CC, Kas J. The optical stretcher: a novel laser tool to micromanipulate cells. Biophysical Journal. 2001;81:767–784. doi: 10.1016/S0006-3495(01)75740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Ehrlicher AJ, Mahammad S, Fabich H, Jensen MH, Moore JR, Weitz DA. The Role of Vimentin Intermediate Filaments in Cortical and Cytoplasmic Mechanics. Biophysical Journal. 2013;105:1562–1568. doi: 10.1016/j.bpj.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guz N, Dokukin M, Kalaparthi V, Sokolov I. If cell mechanics can be described by elastic modulus: study of different models and probes used in indentation experiments. Biophys J. 2014;107:564–575. doi: 10.1016/j.bpj.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman C, Jeney S, Kreplak L, Kasas S, Kulik AJ, Aebi U, Forro L. Exploring the mechanical properties of single vimentin intermediate filaments by atomic force microscopy. Journal of Molecular Biology. 2006;360:623–630. doi: 10.1016/j.jmb.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Haase K, Pelling AE. Investigating cell mechanics with atomic force microscopy. J R Soc Interface. 2015;12:20140970. doi: 10.1098/rsif.2014.0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudenschild DR, Chen JF, Pang NN, Steklov N, Grogan SP, Lotz MK, D'Lima DD. Vimentin Contributes to Changes in Chondrocyte Stiffness in Osteoarthritis. Journal of Orthopaedic Research. 2011;29:20–25. doi: 10.1002/jor.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay M, De Boni U. Chromatin motion in neuronal interphase nuclei: changes induced by disruption of intermediate filaments. Cell Motil Cytoskeleton. 1991;18:63–75. doi: 10.1002/cm.970180107. [DOI] [PubMed] [Google Scholar]
- Holwell TA, Schweitzer SC, Evans RM. Tetracycline regulated expression of vimentin in fibroblasts derived from vimentin null mice. Journal of Cell Science. 1997;110(Pt 16):1947–1956. doi: 10.1242/jcs.110.16.1947. [DOI] [PubMed] [Google Scholar]
- Huisman EM, Wen Q, Wang YH, Cruz K, Kitenbergs G, Erglis K, Janmey PA. Gelation of semiflexible polyelectrolytes by multivalent counterions. Soft Matter. 2011;7:7257–7261. doi: 10.1039/C1SM05553D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska J, Pallari HM, Nevo J, Eriksson JE. Novel functions of vimentin in cell adhesion, migration, and signaling. Experimental Cell Research. 2007;313:2050–2062. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Janmey PA, Euteneuer U, Traub P, Schliwa M. Viscoelastic Properties of Vimentin Compared with Other Filamentous Biopolymer Networks. Journal of Cell Biology. 1991;113:155–160. doi: 10.1083/jcb.113.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey PA, McCormick ME, Rammensee S, Leight JL, Georges PC, MacKintosh FC. Negative normal stress in semiflexible biopolymer gels. Nat Mater. 2007;6:48–51. doi: 10.1038/nmat1810. [DOI] [PubMed] [Google Scholar]
- Janmey PA, Slochower DR, Wang YH, Wen Q, Cebers A. Polyelectrolyte properties of filamentous biopolymers and their consequences in biological fluids. Soft Matter. 2014;10:1439–1449. doi: 10.1039/c3sm50854d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymkowsky MW. Intermediate filaments in 3T3 cells collapse after intracellular injection of a monoclonal anti-intermediate filament antibody. Nature. 1981;291:249–251. doi: 10.1038/291249a0. [DOI] [PubMed] [Google Scholar]
- Kollmannsberger P, Fabry B. High-force magnetic tweezers with force feedback for biological applications. Rev Sci Instrum. 2007;78:114301. doi: 10.1063/1.2804771. [DOI] [PubMed] [Google Scholar]
- Kreplak L, Bar H, Leterrier JF, Herrmann H, Aebi U. Exploring the mechanical behavior of single intermediate filaments. Journal of Molecular Biology. 2005;354:569–577. doi: 10.1016/j.jmb.2005.09.092. [DOI] [PubMed] [Google Scholar]
- Leitner A, Paust T, Marti O, Walther P, Herrmann H, Beil M. Properties of intermediate filament networks assembled from keratin 8 and 18 in the presence of Mg(2)+ Biophysical Journal. 2012;103:195–201. doi: 10.1016/j.bpj.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier JF, Eyer J. Properties of highly viscous gels formed by neurofilaments in vitro. A possible consequence of a specific inter-filament cross-bridging. Biochem J. 1987;245:93–101. doi: 10.1042/bj2450093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier JF, Kas J, Hartwig J, Vegners R, Janmey PA. Mechanical effects of neurofilament cross-bridges - Modulation by phosphorylation, lipids, and interactions with F-actin. Journal of Biological Chemistry. 1996;271:15687–15694. doi: 10.1074/jbc.271.26.15687. [DOI] [PubMed] [Google Scholar]
- Levy R, Maaloum M. Measuring the spring constant of atomic force microscope cantilevers: thermal fluctuations and other methods. Nanotechnology. 2002;13:33–37. [Google Scholar]
- Lin YC, Broedersz CP, Rowat AC, Wedig T, Herrmann H, MacKintosh FC, Weitz DA. Divalent Cations Crosslink Vimentin Intermediate Filament Tail Domains to Regulate Network Mechanics. Journal of Molecular Biology. 2010;399:637–644. doi: 10.1016/j.jmb.2010.04.054. [DOI] [PubMed] [Google Scholar]
- Lin YC, Yao NY, Broedersz CP, Herrmann H, MacKintosh FC, Weitz DA. Origins of Elasticity in Intermediate Filament Networks. Physical Review Letters. 2010;104 doi: 10.1103/PhysRevLett.104.058101. [DOI] [PubMed] [Google Scholar]
- Lincoln B, Schinkinger S, Travis K, Wottawah F, Ebert S, Sauer F, Guck J. Reconfigurable microfluidic integration of a dual-beam laser trap with biomedical applications. Biomed Microdevices. 2007;9:703–710. doi: 10.1007/s10544-007-9079-x. [DOI] [PubMed] [Google Scholar]
- Mege JL, Capo C, Benoliel AM, Foa C, Bongrand P. Study of cell deformability by a simple method. J Immunol Methods. 1985;82:3–15. doi: 10.1016/0022-1759(85)90219-4. [DOI] [PubMed] [Google Scholar]
- Melzak KA, Toca-Herrera JL. Atomic force microscopy and cells: Indentation profiles around the AFM tip, cell shape changes, and other examples of experimental factors affecting modeling. Microsc Res Tech. 2015;78:626–632. doi: 10.1002/jemt.22522. [DOI] [PubMed] [Google Scholar]
- Mendez MG, Restle D, Janmey PA. Vimentin Enhances Cell Elastic Behavior and Protects against Compressive Stress. Biophysical Journal. 2014;107:314–323. doi: 10.1016/j.bpj.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke N, Kreplak L, Kirmse R, Wedig T, Herrmann H, Aebi U, Langowski J. Assessing the flexibility of intermediate filaments by atomic force microscopy. J Mol Biol. 2004;335:1241–1250. doi: 10.1016/j.jmb.2003.11.038. [DOI] [PubMed] [Google Scholar]
- Pallari HM, Eriksson JE. Intermediate filaments as signaling platforms. Sci STKE. 2006;2006:pe53. doi: 10.1126/stke.3662006pe53. [DOI] [PubMed] [Google Scholar]
- Pawelzyk P, Mucke N, Herrmann H, Willenbacher N. Attractive Interactions among Intermediate Filaments Determine Network Mechanics In Vitro. Plos One. 2014;9 doi: 10.1371/journal.pone.0093194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plodinec M, Loparic M, Suetterlin R, Herrmann H, Aebi U, Schoenenberger CA. The nanomechanical properties of rat fibroblasts are modulated by interfering with the vimentin intermediate filament system. Journal of Structural Biology. 2011;174:476–484. doi: 10.1016/j.jsb.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Ramms L, Fabris G, Windoffer R, Schwarz N, Springer R, Zhou C, Hoffmann B. Keratins as the main component for the mechanical integrity of keratinocytes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18513–18518. doi: 10.1073/pnas.1313491110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathje LSZ, Nordgren N, Pettersson T, Ronnlund D, Widengren J, Aspenstrom P, Gad AKB. Oncogenes induce a vimentin filament collapse mediated by HDAC6 that is linked to cell stiffness. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1515–1520. doi: 10.1073/pnas.1300238111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager PR. Cytoskeletal effects of acrylamide and 2,5-hexanedione: selective aggregation of vimentin filaments. Toxicol Appl Pharmacol. 1989;97:141–155. doi: 10.1016/0041-008x(89)90063-x. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Boeda B, Etienne-Manneville S. APC binds intermediate filaments and is required for their reorganization during cell migration. Journal of Cell Biology. 2013;200:249–258. doi: 10.1083/jcb.201206010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopferer M, Bar H, Hochstein B, Sharma S, Mucke N, Herrmann H, Willenbacher N. Desmin and Vimentin Intermediate Filament Networks: Their Viscoelastic Properties Investigated by Mechanical Rheometry. Journal of Molecular Biology. 2009;388:133–143. doi: 10.1016/j.jmb.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Seltmann K, Fritsch AW, Kas JA, Magin TM. Keratins significantly contribute to cell stiffness and impact invasive behavior. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18507–18512. doi: 10.1073/pnas.1310493110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan S, DeGiulio JV, Lorand L, Goldman RD, Ridge KM. Micromechanical properties of keratin intermediate filament networks. Proc Natl Acad Sci U S A. 2008;105:889–894. doi: 10.1073/pnas.0710728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiparambil JT, Bender L, Ganesh T, Kline E, Patel P, Liu Y, Marcus AI. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int J Cancer. 2011;129:2744–2755. doi: 10.1002/ijc.25938. [DOI] [PubMed] [Google Scholar]
- Thomas G, Burnham NA, Camesano TA, Wen Q. Measuring the mechanical properties of living cells using atomic force microscopy. J Vis Exp. 2013 doi: 10.3791/50497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner OI, Rammensee S, Korde N, Wen Q, Leterrier JF, Janmey PA. Softness, strength and self-repair in intermediate filament networks. Exp Cell Res. 2007;313:2228–2235. doi: 10.1016/j.yexcr.2007.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Ingber DE. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophysical Journal. 1994;66:2181–2189. doi: 10.1016/S0006-3495(94)81014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Stamenovic D. Contribution of intermediate filaments to cell stiffness, stiffening, and growth. American Journal of Physiology-Cell Physiology. 2000;279:C188–C194. doi: 10.1152/ajpcell.2000.279.1.C188. [DOI] [PubMed] [Google Scholar]
- Yamada S, Wirtz D, Coulombe PA. The mechanical properties of simple epithelial keratins 8 and 18: discriminating between interfacial and bulk elasticities. Journal of Structural Biology. 2003;143:45–55. doi: 10.1016/s1047-8477(03)00101-1. [DOI] [PubMed] [Google Scholar]