Abstract
The purpose of this study was to investigate the effects of acute and chronic administration of anabolic–androgenic steroids (AAS) on nociception and morphine antinociception in acute pain models, as well as on chronic inflammatory nociception. In Experiment 1, adult, gonadally intact male rats were injected s.c. for 28 days with either 5 mg/kg testosterone (T), dihydrotestosterone (DHT), stanozolol (STAN), or safflower oil vehicle (N = 12–25/group). On day 28, rats in each group were tested on acute thermal and mechanical nociceptive assays, before and after morphine treatment. In Experiment 2, rats in each group (N = 8–10/group) were injected with mineral oil or complete Freund's adjuvant (CFA) into one hindpaw after 28 days of AAS treatment, and then tested for thermal hyperalgesia, mechanical allodynia, inflammation and locomotor suppression intermittently for 28 days. Experiment 3 replicated nociceptive measurements in Experiments 1 and 2, but with a single AAS or vehicle injection occurring 3 h prior to testing (N = 10–12/group). While chronic AAS administration tended to decrease body weight gain and alter reproductive organ weights in the expected manner, it did not significantly alter acute nociception nor attenuate the development of various chronic pain indices after CFA administration. Morphine antinociceptive potency was significantly decreased by chronic DHT on the hotplate test only. Acute AAS administration also did not significantly alter acute or chronic nociception, or morphine antinociceptive potency. Comparisons between acute and chronic AAS administration suggest that steroid tolerance did not occur in rats treated with AAS chronically. Taken together, these data do not support the hypothesis that AAS exposure alters nociception or morphine antinociception in gonadally intact males.
Keywords: Androgen, Anabolic steroid, Testosterone, Dihydrotestosterone, Stanozolol, Pain
1. Introduction
Following their chemical identification in 1929, anabolic–androgenic steroids (AAS) gained popularity as a means to improve athletic performance. Recent research suggests that AAS use has now extended beyond professional athletes to adolescents, college students and females (Berning et al., 2008; McCabe et al., 2007; U.S Department of Health and Human Services, 2007; van den Berg et al., 2007). AAS and exercise have a strong link, because anabolic gains are maximized through the combination of AAS with exercise. Individuals who exercise at high intensity often experience pain through injury or overtraining. Users report that AAS help them to recover faster and to resume training. Accordingly, AAS have been implicated in the modulation of pain.
Recent studies have tested the effects of androgens in animal models of acute and chronic pain. For example, in male rats, depletion of testosterone (T) by gonadectomy increased formalin-induced nociceptive responses (Aloisi and Ceccarelli, 2000), while replacement of T to physiological levels decreased nociception in the formalin test (Gaumond et al., 2005). When androgens are increased to supraphysiologic levels, as in AAS abuse, experimental findings reveal significant variability. Administration of T or its metabolites increased latency to respond on acute pain tests (Frye et al., 2007; Hau et al., 2004), and decreased formalin-induced nociceptive responses (Aloisi et al., 2004; Fischer et al., 2007), suggesting that AAS have analgesic properties. However, other studies using the same tests reported little or no effect of T (Negus et al., 2001).
In addition to their effects on nociception, androgens may modulate opioid antinociception. Androgens interact with opioids in the brain (Johansson et al., 1997; 2000), and AAS overdose produces symptoms resembling opioid intoxication, which can be reversed with an opioid antagonist (Peters and Wood, 2004). However, animal studies of androgen effects on opioid antinociception are conflicting. Castration decreased opioid antinociception in male rats (Borzan and Fuchs, 2006; Stoffel et al., 2003), and T replacement enhanced opioid antinociception (Cicero et al., 2002; Stoffel et al., 2003). In contrast, other studies report no significant T effect on opioid antinociception (Celerier et al., 2003; Negus et al., 2001; Sumner et al., 2006), and still others found an increase in opioid antinociception after castration (South et al., 2001) and a decrease in opioid antinociception after AAS treatment (Philipova et al., 2003). It is important to keep in mind that the mechanisms of action, and hence the behavioral effects, of steroids at pharmacologic doses may be fundamentally different from hormone replacement at more physiologic levels. In this regard, human AAS users can achieve circulating androgen concentrations up to 100× the physiologic range for an adult male (Brower et al., 1990). Accordingly, we hypothesized that the antinociceptive effects of AAS, alone and in combination with opioids, may be primarily evident at supraphysiologic doses.
The purpose of the first two experiments was to investigate the effects of chronic AAS exposure on nociception and morphine antinociception. A follow-up experiment was conducted to determine whether steroid tolerance developed after chronic AAS administration in the first two experiments. Steroid tolerance has been demonstrated both in humans (Brower et al., 1991) and in rodents following chronic use: 15 days of ICV infusion of T produced tolerance to the depressive and motoric effects of steroids (Peters and Wood, 2004). Tolerance to the antinociceptive effects of steroids has not yet been investigated.
To model the typical human AAS user, we tested gonadally intact, young adult male rats. To evaluate the generality of AAS effects on nociception, three AAS were tested: T, dihydrotestosterone (DHT) and stanozolol (STAN). Testosterone is a pro-hormone that is typically converted to DHT via 5α-reductase, and to estradiol via aromatase. We examined T as a naturally occurring androgen and the precursor to DHT. DHT was chosen because it is both a non-aromatizable and more potent androgen than T, binding with greater affinity than T to androgen receptors (Kicman, 2008). Because DHT is non-aromatizable, we can be more certain that any effects are due to DHT itself and not due to an estrogen metabolite. The third AAS examined, STAN, is a synthetic, 17α-alkylated AAS with weak androgen receptor binding affinity due to its inability to be reduced by 5α-reductase (Kicman, 2008). STAN is also incapable of being aromatized to estradiol, resulting in decreased estrogenic side effects; for these reasons it is popular among AAS users (Wood, 2004). Thus, we chose STAN because of its common use and the fact that we may be fairly certain that any effects are due to STAN itself and not due to an estrogen metabolite. Each steroid was given in a dose of 5 mg/kg/day, which is a substantial androgen dose in that it falls within the range of human AAS use when adjusted according to body surface area according to FDA guidelines [http://www.accessdata.fda.gov/scripts/cder/onctools/animalquery.cfm], as well as being above physiologic replacement levels (which are up to 30 ug/kg/day: Hernandez et al., 1994; Lima et al., 2000). Furthermore, testosterone at 5 mg/kg stimulates sexual and aggressive behavior in male rats (Cunningham and McGinnis, 2006; Wesson and McGinnis, 2006). The first hypothesis was that AAS would attenuate thermal and mechanical nociception in acute models of pain, as well as attenuate the development of thermal hyperalgesia, mechanical allodynia and inflammation in a chronic pain (complete Freund's adjuvant, CFA) model. The second hypothesis was that AAS would enhance morphine antinociception.
2. Methods
2.1. Subjects
Subjects were 70–90 day old Sprague–Dawley rats (offspring from breeders purchased from Taconic Farms (Germantown, NY)). Rats were pair-housed according to hormone treatment (n = 8–25/group) after initial body weights were balanced among groups in Experiments 1 and 2 (but not Experiment 3). Rats were maintained on a 12/12-hour light/dark cycle (lights on at 0600 h) in a vivarium room maintained at 21 ± 2 °C. Food (Teklad) and water were available ad libitum except during testing. Rats were housed in an AAALAC-accredited vivarium and all procedures were approved by the Washington State University IACUC.
2.2. Apparatus
To measure acute thermal nociception, a hotplate analgesia meter (Columbus Instruments, Columbus, OH) and 1-L water bath (Precision Scientific, Winchester, VA) were used. The hotplate was set to 50 (±0.1) °C and the water bath was set to 50 (±0.5) °C. To measure acute mechanical nociception, the Randall–Selitto paw pressure test was conducted with an analgesy-meter (Ugo Basile, Varese, Italy), which increases pressure on the paw at a rate of 48 g/s from 30 to 1230 g. An electric von Frey anesthesiometer (IITC Life Science, Woodland Hills, CA) was used to measure mechanical allodynia. To measure thermal hyperalgesia, an 85 × 40 × 35 cm Basile Plantar (Hargreaves) test (Ugo Basile) was used, with an infrared intensity of 32 mW/cm2. Locomotor activity was measured using a photobeam test chamber (Opto-varimex, Columbus Instruments) consisting of 15 photobeams that cross the width of a 20 cm × 40 cm × 23 cm clear Plexiglas rodent cage. Photobeams are 2.5 cm apart and 8 cm above the cage floor. Whole-paw inflammation was assessed as displacement of tap water in a beaker filled to the 20-ml mark. Tests were conducted on both the left and right paw, with half of the rats being tested on the left hindpaw first and half on the right hindpaw first.
2.3. Drugs
Morphine sulfate (Sigma-Aldrich, Inc., St. Louis, MO) was dissolved in 0.9% physiological saline and administered s.c. in a volume of 1 ml/kg. T, DHT and STAN (Steraloids Inc., Newport, RI) were dissolved or suspended in safflower oil, and administered s.c. at a dose of 5 mg/kg, in a volume of 0.5 ml/kg. Paw inflammation was induced with 0.1-ml injection of a 5 mg/ml CFA solution (Mycobacterium butyricum suspended in mineral oil: Fisher Scientific, Pittsburgh, PA).
2.4. Procedure
For chronic hormone administration, either an AAS (T, DHT, or STAN) or vehicle (safflower oil) was administered s.c. daily at approximately 0900–1100 for 28–56 days. Body weight was recorded on the first day of treatment and thereafter at weekly intervals. Injection volume was adjusted weekly according to body weight. Acute nociception was tested on the 28th day of treatment, beginning approximately 3 h after the vehicle or AAS injection. Chronic nociception was tested starting on the 28th day of AAS treatment: immediately before and then 1, 3, 7, 10, 14, 21 and 28 days post-CFA or mineral oil injection; rats continued to receive daily AAS or vehicle injections during this testing period. Test days were chosen based on the methods and results of Cook and Nickerson (2005), as well as Nagakura et al.'s (2003) work, in which they found that thermal hyperalgesia and mechanical allodynia reached maximum by days 1 and 7, respectively, and gradually recovered over several weeks. For acute AAS administration (Experiment 3), rats were weighed at the start of the test day and a single injection of either AAS or vehicle was administered at approximately 0900. Acute nociception was tested 3 h following AAS administration. Chronic nociception was tested at four different time points: prior to AAS and CFA, 3 h post-AAS, 1 h post-CFA, and 24 h post-CFA to allow assessment of pain thresholds before both AAS and pain induction, after AAS in the absence of inflammatory pain, and after both AAS and induction of inflammatory pain, respectively. In all experiments, immediately following the last nociceptive test, rats were euthanized and seminal vesicles and testes were harvested. Testes were weighed wet. Seminal vesicles were fixed in Bouin's solution for approximately 2 weeks, after which they were trimmed, blotted and weighed.
3. Experiment 1: AAS effects on acute nociception and opioid antinociception
3.1. Procedure
On the 28th vehicle/AAS injection day, nociception was assessed in the following order: hotplate (latency to lick hindpaw or jump off plate, in s), tail withdrawal (latency to tail flick, in s), and paw pressure (latency to retract or attempt to retract foot, in s),with cutoff latencies of 60 s, 20 s and 25 s, respectively, to prevent tissue damage. The sequence of the acute nociceptive assays followed the order of no restraint to brief restraint, to decrease any effects of brief restraint stress on the subsequent test. Three baseline (non-drug) tests were conducted. Immediately following the second baseline test, saline (1.0 ml/kg) was administered s.c., and 20 min later the third baseline test was conducted. Cumulative dosing of morphine commenced immediately after completion of the third baseline test, with the following actual doses injected at 20-min intervals: 1.0 mg/kg, 0.8 mg/kg, 1.4 mg/kg, 2.4 mg/kg, 4.4 mg/kg and 8.0 mg/kg, reaching a total cumulative dose of up to 18 mg/kg. Cumulative dosing with doses spaced 1/4 to ½ log unit apart is often used in tests of acute antinociception to yield complete dose-effect curves in as few doses as possible (Manning et al., 2001; Stoffel et al., 2003; Morgan et al., 2006; Fischer et al., 2010). Twenty minutes after each injection, rats were re-tested on all 3 nociceptive tests in the order noted above. Testing continued until the rat reached cutoff on all three tests.
3.2. Statistical analyses
Body weight of vehicle- and AAS-treated groups over time was compared using a repeated-measures ANOVA with treatment (vehicle or AAS) as the between-subjects factor and week of AAS administration as the within-subjects factor. Nociceptive baseline for each rat was the mean of the second and third baseline tests; the first test is dropped due to the potential confoundment of exploration on the hotplate test (Craft and Bernal, 2001). Baseline nociception was compared between vehicle- and AAS-treated groups with a one-way ANOVA, followed by post-hoc analysis with Dunnett's t-test (2-sided). Because there were individual differences in baseline latencies, response latencies following each cumulative morphine dose for each rat were calculated as% Maximum Possible Effect (%MPE): [(post-injection latency − baseline latency)/(cutoff latency − baseline latency)]× 100. The point at which morphine antinociception reached 50%MPE (ED50) for each rat was calculated via log-linear interpolation using at least 1%MPE point falling under 50% and at least one point above 50%. Morphine ED50 values were compared between vehicle- and AAS-treated groups with a one-way ANOVA and post-hoc Dunnett's t-test, for each nociceptive test. Lastly, because organ weight increases with body weight, organ weight was adjusted by body weight: (organ weight (g)/BW (kg)), and then group differences were assessed with a one-way ANOVA and post-hoc Dunnett's t-test. Significance was set at p≤0.05.
4. Experiment 2: AAS effects on chronic inflammatory pain using the CFA test
4.1. Procedure
On the 28th day of vehicle/AAS treatment, baseline responses were measured on the von Frey and Hargreaves tests, as well as locomotor activity and paw inflammation (i.e., paw displacement). Immediately thereafter rats were lightly anesthetized with isoflurane and 0.1 ml of CFA or mineral oil was injected into the plantar surface of the right hindpaw. The same behavioral and inflammation measurements were taken on days 1, 3, 7, 10, 14, 21 and 28 following injection of CFA or mineral oil. Vehicle/AAS daily injections were continued throughout this testing period.
4.2. Statistical analyses
Body weight was compared over weeks of treatment as well as over test days with a repeated measures ANOVA, with week or test day as the within-subjects factor and AAS and CFA/mineral oil treatment as the between-subjects factors. Group differences in nociceptive thresholds and paw inflammation were determined using a repeated measures ANOVA with test day and foot (left vs. right) as the repeated factors, and AAS and CFA/mineral oil as the between-subjects factors. Paw displacement was determined by subtracting 20 ml (the initial volume) from the final volume. Locomotor activity (# of photobeam breaks) was compared among groups with a repeated measures ANOVA as well, with test day as the within-subjects factor and AAS and CFA/mineral oil treatment as the between-subjects factors. A difference statistic was also calculated by subtracting left foot responses from right foot responses in each rat, thereby simplifying data presentation and analysis. Lastly, organ weights in vehicle- and AAS-treated rats (in g/kg body weight) were compared with a one-way ANOVA and Student–Newman Keuls post-hoc.
5. Experiment 3: Acute AAS effects on acute and chronic nociception and opioid antinociception
5.1. Procedure
5.1.1. Acute pain
Rather than 28 days of AAS administration, a single injection of vehicle or an AAS (5 mg/kg) was given 3 h prior to baseline measurements on hotplate, tail withdrawal, and paw pressure. The same morphine cumulative dosing procedure was carried out as in Experiment 1 (with the exception of starting at a lower dose, 0.56 mg/kg).
5.1.2. Chronic pain
A separate group of rats was habituated for 15 min in hanging wire cages, followed by baseline measurements on the von Frey and Hargreaves tests, and then on locomotor activity and paw inflammation (i.e., paw displacement) tests. Rats were then treated with either vehicle or an AAS at 0900 and tested 3 h later on the same tests, followed by CFA injection. Testing was repeated 1 and 24 h after administration of CFA; a second injection of vehicle or an AAS was given 3 h prior to the test at 24 h.
5.2. Statistical analyses
For the acute pain testing, nociceptive baselines, ED50 values, locomotor activity scores and paw inflammation were analyzed with the same statistical tests used for Experiment 1 data. The measurements for the chronic pain model were compared with the same statistical tests used for Experiment 2 data.
6. Results
6.1. Experiment 1
6.1.1. Body weight
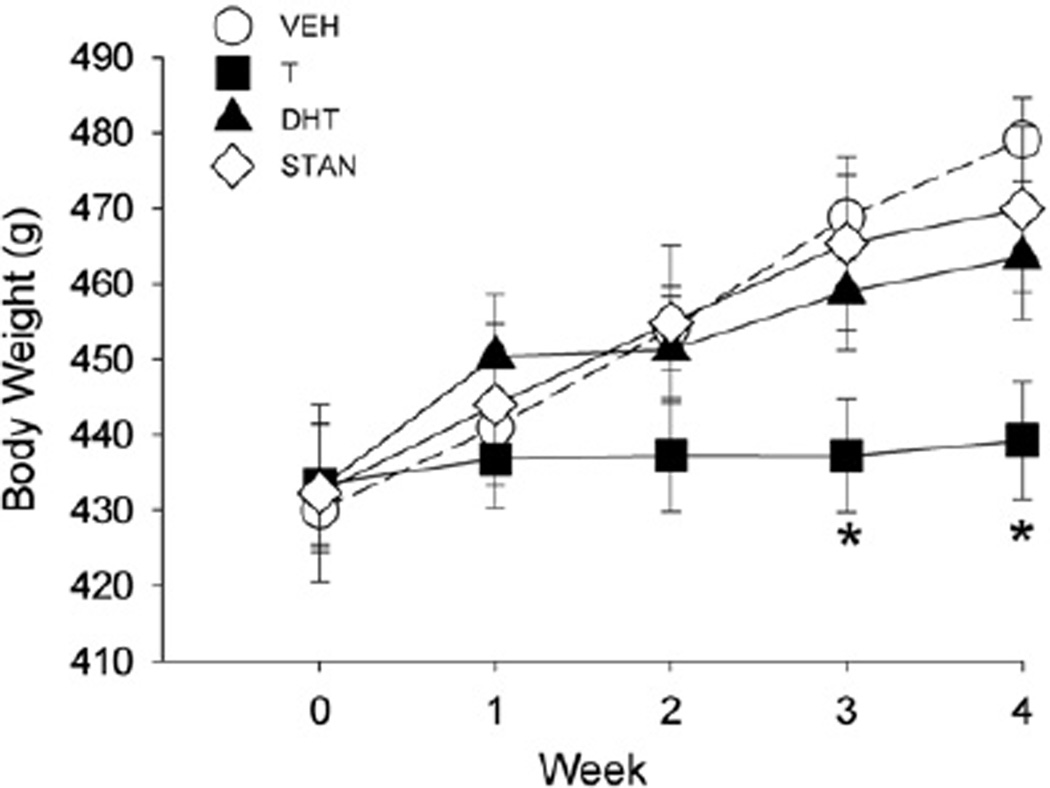
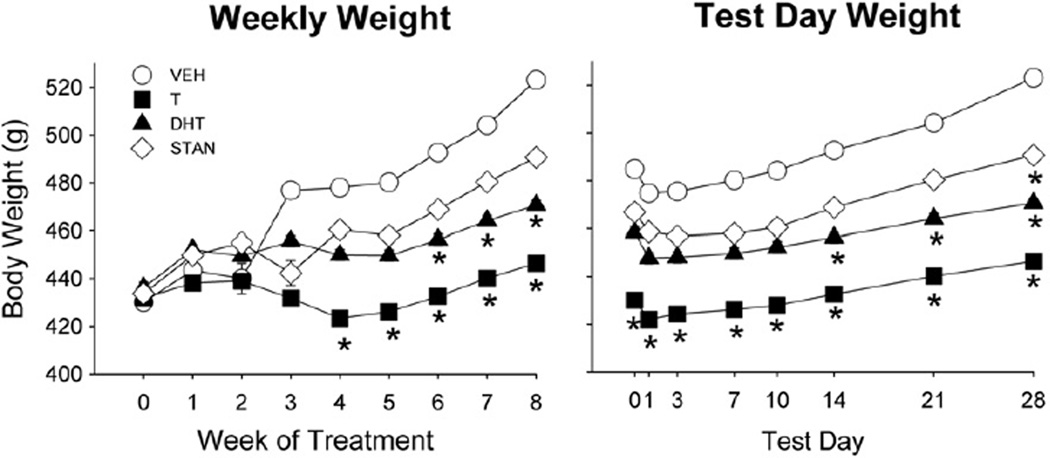
On day 1 of injections, mean body weight in all groups was approximately 430 g (Fig. 1). Body weight increased significantly over the 4 weeks of AAS or vehicle administration (Week: F(4,236) = 120.20, p<0.001). However, while the body weight of most groups increased steadily, the bodyweight of T-treated rats increased only slightly across the four weeks, such that T-treated rats were significantly lighter than vehicle-treated rats by weeks 3–4 (AAS × week: F(12,236) = 16.60, p<0.001; Fig. 1).
Fig. 1.
Body weight from week 0 (first day of AAS or oil vehicle administration) to week 4 in gonadally intact male rats treated for 28 days with vehicle (VEH), testosterone (T), dihydrotestosterone (DHT), or stanozolol (STAN) (5 mg/kg/day) (Experiment 1). Each point is the mean ± 1 S.E.M., N = 25 (vehicle group) or N = 12–14 rats (AAS groups). *significantly different from vehicle group, p≤0.05.
6.1.2. Nociceptive and antinociceptive behavior
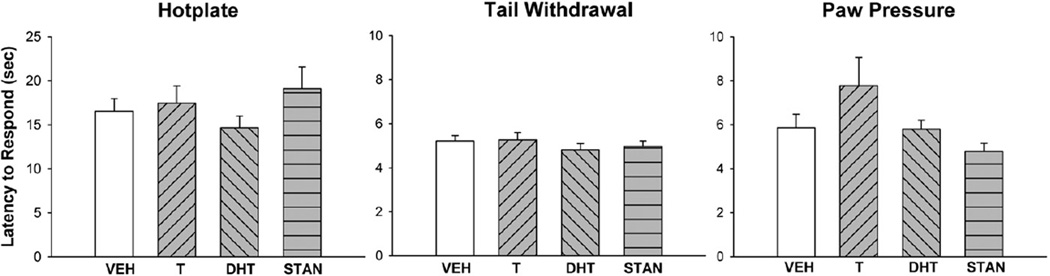
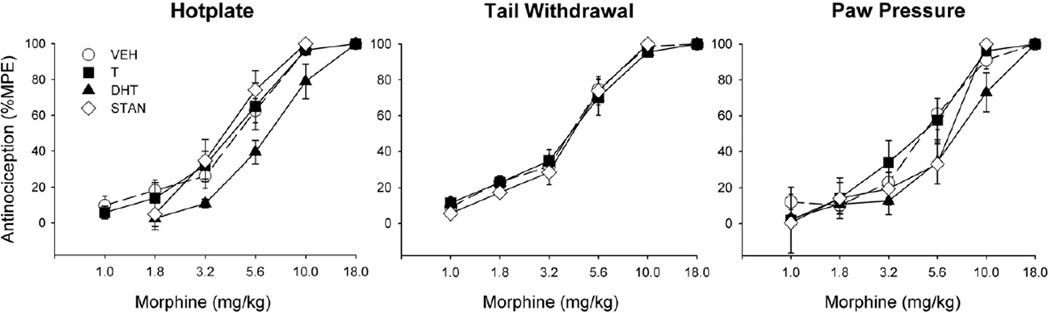
Fig. 2 shows nociceptive baselines for the hotplate, tail withdrawal and paw pressure tests. Administration of an AAS for 28 days did not significantly alter basal nociception on any test. However, Fig. 3 shows that AAS treatment significantly altered morphine's antinociceptive potency, on the hotplate test: specifically, ED50 values in DHT-treated rats were significantly higher than those in vehicle-treated controls (F(3,59) = 3.58, p = 0.02).
Fig. 2.
Nociceptive baselines on the 50 °C hotplate test (left panel), 50 °C tail withdrawal test (middle panel), and paw pressure test (right panel) on day 28 of daily treatment with vehicle (VEH), testosterone (T), dihydrotestosterone (DHT), or stanozolol (STAN). Nociceptive baselines were obtained immediately prior to administration of morphine. Each bar is the mean + 1 S.E.M., N = 25 (vehicle group) or N = 12–14 rats (AAS groups).
Fig. 3.
Morphine antinociception (%MPE) on the hotplate test (left panel), tail withdrawal test (middle panel) and paw pressure test (right panel) in gonadally intact male rats treated for 28 days with vehicle (VEH), testosterone (T), dihydrotestosterone (DHT), or stanozolol (STAN). Each point is the mean ± 1 S.E.M., N = 25 (vehicle group) and N = 12–14 rats (AAS groups). The morphine ED50 was significantly greater in the DHT group than in the vehicle group on the hotplate test only.
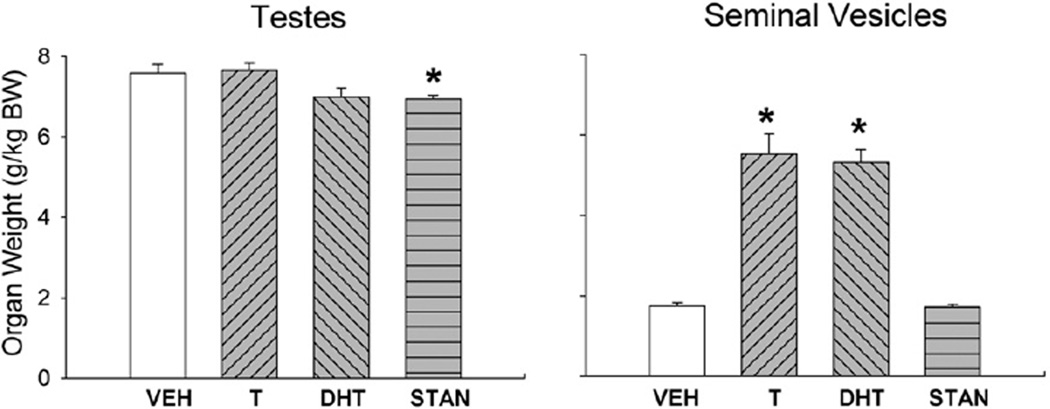
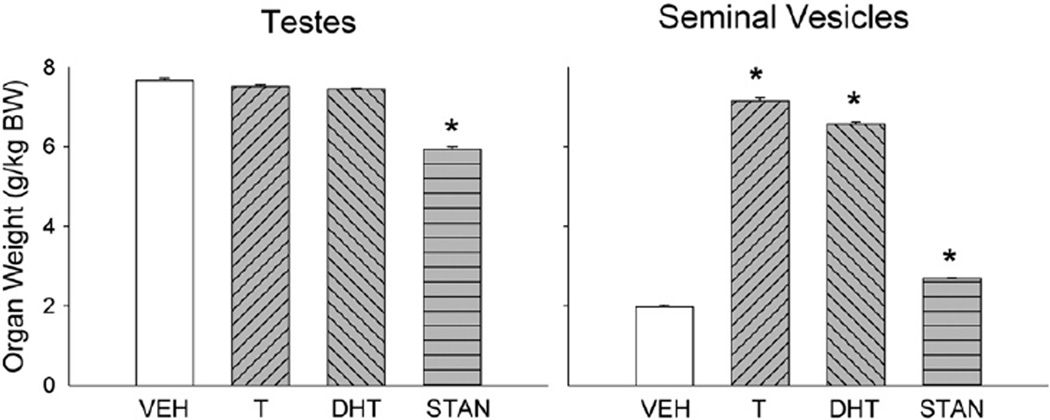
6.1.3. Reproductive organs
Fig. 4 (left panel) shows that testis weight was slightly lower in T- and DHT-treated rats, and significantly lower in STAN-treated rats when compared to controls (F(3,44) = 3.12, p = 0.03). Also shown in Fig. 4 (right panel), seminal vesicles in T- and DHT-treated rats were significantly heavier than those in controls, while seminal vesicle weight in STAN-treated rats was similar to that in controls (F(3,57) = 78.90, p<0.001).
Fig. 4.
Reproductive organ weights of gonadally intact males after 28 days of treatment with vehicle (VEH), testosterone (T), dihydrotestosterone (DHT), or stanozolol (STAN). Organs were harvested immediately following morphine antinociceptive testing. Organ weights were adjusted for individual differences in body weight (BW). Each bar is the mean + 1 S.E.M., N = 18 (vehicle group) and N = 6–12 rats (AAS groups). *significantly different from vehicle group, p≤0.05.
6.2. Experiment 2
6.2.1. Body weight
Treatment with CFA or mineral oil did not significantly influence body weight across weeks of treatment or test day, thus CFA- and mineral oil-injected rats were pooled into their respective steroid groups. Fig. 5 (left panel) shows body weight of all groups from the first week of AAS or vehicle injection through the end of testing at week 8 (CFA was administered into the right hindpaw at week 4). Group mean starting body weights ranged from approximately 425–440 g. Overall, body weights increased across the 8 weeks of vehicle/AAS treatment (week: F(8,560) = 31.60, p<0.001), but to a lesser extent in T- and DHT-treated rats than in controls (AAS × week: F(24,560) = 6.05, p<0.001).
Fig. 5.
Body weight at each week of AAS treatment (left panel) and on test days 0, 1, 3, 7, 10, 14, 21 and 28 for rats treated with vehicle (VEH), testosterone (T), dihydrotestosterone (DHT), or stanozolol (STAN) in Experiment 2. CFA was injected into the hindpaw at week 4 (i.e., test day 0). Each point is the mean ± 1 S.E.M., N = 8–10 rats/group. T-treated rats were significantly lighter than vehicle-treated rats by week 4 and DHT-treated rats were significantly lighter than vehicle groups after week 6 (left panel). Across various test days AAS groups gained less weight than vehicle-treated rats: T-treated rats across all days, DHT-treated rats on test days 14, 21, 28, and STAN-treated rats on day 28 (right panel). *significantly different from vehicle group, p≤0.05.
Fig. 5 (right panel) shows body weight gain during weeks 4–8 only, when rats were being tested at various time points after CFA injection into the hindpaw. In general, body weight decreased within the first few days after CFA injection, and then began to increase again (Time: F(7,490) = 198.80, p<0.001). T-treated rats weighed significantly less than vehicle-treated controls regardless of test day (AAS: F(3,70) = 5.32, p = 0.002); additionally, DHT-treated rats weighed less than controls on test days 14–28 (AAS × day: F(21,490) = 6.86, p<0.001).
6.2.2. Nociception and locomotor activity
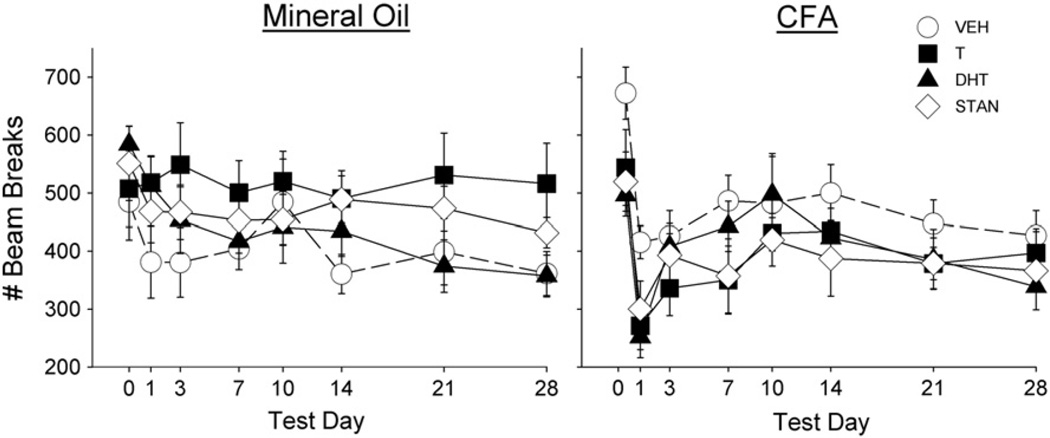
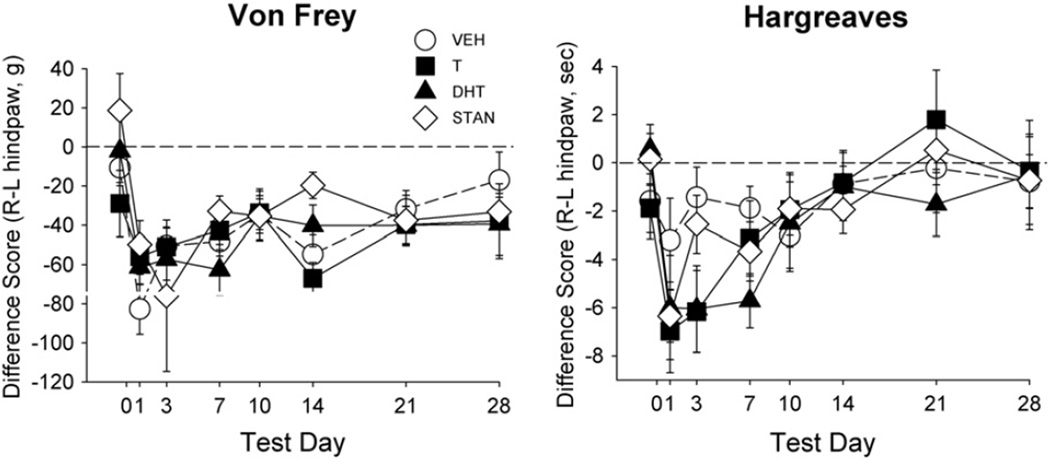
An initial assessment of the mineral oil-injected groups revealed no effects of AAS treatment on any nociceptive test. For example, in mineral oil-injected rats, the von Frey and Hargreaves tests yielded no significant response threshold differences between the right and left paw in any group (F(3,34) = 0.68, n.s.; data not shown), and no significant group differences in locomotor activity (Fig. 7, left panel). In contrast, Fig. 6 shows that in the CFA-injected groups, there was a dramatic decrease in mechanical and thermal thresholds on the von Frey (Day: F(7,238) = 8.34, p<0.001) and Hargreaves tests, respectively (Day: F(7,238) = 9.30, p<0.001), as well as a decrease in locomotor activity (Day: F(7,238) = 24.20, p<0.001; Fig. 7, right panel) immediately following administration of CFA. However, there were no statistically significant differences in allodynia, hyperalgesia or suppressed locomotion between AAS-treated rats and vehicle-treated controls.
Fig. 7.
Effect of CFA injection on locomotor activity in rats treated with vehicle (VEH), testosterone (T), dihydrotestosterone (DHT), or stanozolol (STAN). Locomotor activity differed across test days in comparison to baseline (day 0) activity. Each point is the mean ± 1 S.E.M., N = 8–10 rats/group.
Fig. 6.
Effect of CFA injection on mechanical response threshold (von Frey test, left panel) and thermal response threshold (Hargreaves test, right panel) in rats treated with vehicle (VEH), testosterone (T), dihydrotestosterone (DHT), or stanozolol (STAN). Data are presented as difference scores between the left (L—uninjected) and right (R—injected) paw; the dashed line indicates a difference score of 0 for the right paw compared to the left, meaning response thresholds were the same in each paw, whereas points below the dotted line indicate heightened sensitivity (lower threshold) in the injected paw compared to the uninjected paw. Nociceptive thresholds significantly differed across test days in comparison to baseline (day 0) responding. Each point is the mean ± 1 S.E.M., N = 8–10 rats/group.
6.2.3. Paw inflammation
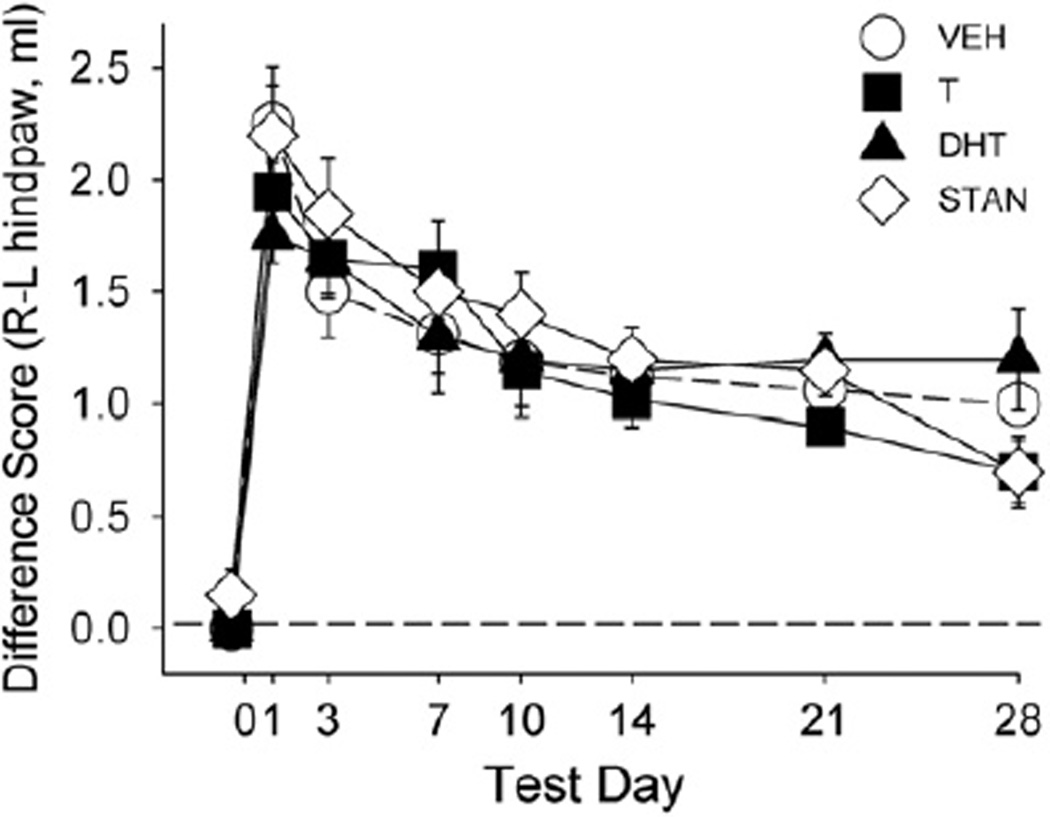
Inflammation was demonstrated by increases in paw volume in the right (injected) paw compared to the left (uninjected) paw, as shown in Fig. 8. In rats injected with mineral oil, right paw volume was no greater than left paw volume (data not shown). In CFA-injected rats, right paw volume increased immediately after CFA injection (F(7,238) = 65.00, p<0.001). However, no significant differences in paw volume were found between AAS-treated groups and controls.
Fig. 8.
Effect of CFA injection on paw inflammation as measured by paw displacement across test days. 0, 1, 3, 7, 10, 14, 21 and 28 for rats treated with vehicle (VEH), testosterone (T), dihydrotestosterone (DHT), or stanozolol (STAN). The dotted line indicates a difference score of 0 for the right (R) paw compared to the left (L), meaning volume was the same in each paw, whereas points above the dotted line indicate greater volume of the injected paw compared to the uninjected paw. Each point is the mean ± 1 S.E.M., N = 8–10 rats/group.
6.2.4. Reproductive organs
Fig. 9 shows the effect of AAS on reproductive organs after 56 days of AAS administration. Similar to Experiment 1, only STAN-treated rats had significantly lower testis weights in comparison to controls (F(3,74) = 14.00, p<0.001). Also similar to Experiment 1, T- and DHT-treated rats had substantially heavier seminal vesicles than controls; however, in addition, STAN-treated rats had significantly heavier seminal vesicles compared to controls (F(3,74) = 155.80, p<0.001).
Fig. 9.
Reproductive organ weights of gonadally intact males following the last test on day 56 for rats treated with vehicle (VEH), testosterone (T), dihydrotestosterone (DHT), or stanozolol (STAN). Organs weights were adjusted for individual differences in body weight (BW). Each bar is the mean + 1 S.E.M., N = 18–20 rats/group. *significantly different from vehicle group, p≤0.05.
6.3. Experiment 3
6.3.1. Acute pain
Acute administration of an AAS (single injection of T, DHT or STAN) did not significantly influence basal nociception on the hotplate, tail withdrawal or paw pressure tests (data not shown). ED50 values for morphine antinociception in AAS-treated rats tended to be higher than those in vehicle-treated controls on the hotplate test, however, this difference was not statistically significant (F(3,40) = 2.28, p = 0.09; data not shown). The single dose treatment with T, DHT or STAN also did not produce any significant changes in reproductive organ weights, either the testes or seminal vesicles.
To assess the effect of duration of AAS exposure on nociceptive measures, Experiment 1 (chronic AAS administration) and Experiment 3 (acute AAS administration) data were compared. Comparing acute nociception in Experiments 1 vs. 3, baseline response latencies on the hotplate (F(1,105) = 11.73, p = 0.001) and tail withdrawal (F (1,105) = 28.37, p<0.001) tests were longer in Experiment 3 than in Experiment 1. Additionally, ED50 values for morphine antinociception overall tended to be lower in Experiment 3 than in Experiment 1, regardless of nociceptive test (hotplate: F(1,35) = 17.37, p<0.001; tail withdrawal: F(1,35) = 20.80, p<0.001; paw pressure: F(1,35) = 13.77, p = 0.001; data not shown).
6.3.2. Chronic pain
Acute administration of an AAS (single injection) did not produce any changes in mechanical allodynia (F(3,44) = 1.79, n.s.) or thermal hyperalgesia (F(3,44) = 0.86, n.s.) that developed after CFA injection (data not shown). Acute AAS administration also did not exert an anti-inflammatory effect (F(3,44) = 0.57, n.s.) or change locomotor activity (F(3,44) = 0.28, p = 0.84) or organ weight (seminal vesicles: F(3,12) = 2.19, n.s.; testes: F(3,12) = 1.36, n.s.) compared to vehicle-treated controls (data not shown).
Comparing Experiment 2 (chronic AAS administration) and Experiment 3 (acute AAS administration) data, there were no significant differences overall on either the Von Frey (F(1,84) = 1.53, n.s.) or Hargreaves (F(1,84) = 1.24, n.s.) tests (data not shown). However, rats acutely treated with an AAS locomoted less than chronically treated rats (F(1,84) = 20.37, p<0.001; data not shown).
7. Discussion
7.1. Effect of chronic AAS administration on acute and chronic nociception
Chronically AAS-treated rats did not differ from controls in acute nociceptive thresholds on the hotplate, tail withdrawal and paw pressure tests. This result is consistent with findings from other studies showing that modulating androgen levels either through castration (Ali et al., 1995; Liu and Gintzler, 2000; Stoffel et al., 2003) or castration with T replacement via hormone capsules (Sumner et al., 2006) did not alter basal nociception. However, several studies have demonstrated an antinociceptive effect of T in tests of acute thermal nociception (Edinger and Frye, 2004; Frye et al., 2007; Hau et al., 2004).
Pain measurements in Experiment 2 (chronic inflammatory pain) also did not reveal any significant differences between chronic AAS-treated groups and controls. As such, the hypothesis that AAS administration attenuates acute and chronic pain was not supported. This result contrasts with research findings that implicate a protective role of androgens in the development and severity of chronic inflammatory pain, as shown in formalin (Aloisi et al., 2004; Gaumond et al., 2002) and CFA-induced arthritis models. In one CFA study, castration caused the onset of arthritic symptoms to occur sooner and administration of T decreased inflammation as measured by paw volume (Harbuz et al., 1995). The failure to observe significant anti-allodynic, anti-hyperalgesic and anti-inflammatory effects of AAS in the present study is not likely due to a general failure of the inflammatory pain procedure. Immediately following administration of CFA, all rats showed a significant decrease in threshold on both Hargreaves and von Frey tests, and an increase in paw inflammation; furthermore, allodynia and inflammation waned but did not return to baseline during the 28-day experiment, very similar to what has been reported previously (Cook and Nickerson, 2005; Nagakura et al., 2003).
It is also unlikely that the chronic AAS treatment regimen we used was insufficient either in dosage or duration, as all three AAS significantly altered reproductive organ weight in either Experiment 1 or 2 (or both). It is also likely that administration of 5 mg/kg daily for 4–8 weeks is sufficient to observe an effect on nociception, as other studies using a shorter duration of T administration have demonstrated antinociceptive effects (Fischer et al., 2007) as well as an increase in aggression with an equivalent daily dose (Breuer et al., 2001). Together with the significant effects of AAS on body weight and reproductive organ weight observed in the present study, these previous studies suggest that the dose and duration of AAS administration in the present study were sufficient to produce behavioral effects.
A possible explanation for why antinociceptive effects of AAS were not observed may be that in the present study, gonadally intact males were used—to model the typical human AAS user with normal sex steroid levels—whereas in many previous studies, subjects were gonadectomized (e.g., Frye and Seliga, 2001; Gaumond et al., 2002; Pednekar and Mulgaonker, 1995). Gonadectomy results in a profound depletion of sex steroids and steroid production. Therefore comparing steroid-treated rats to gonadectomized rats would maximize group differences in behavior due to the drastic group difference in sex steroid levels, whereas a more modest difference would be expected when comparing intact rats to those treated with supraphysiological doses of AAS.
Because some previous studies used acute AAS administration (e.g., Celerier et al., 2003; Edinger and Frye, 2004, 2005), we also considered the possibility that acute rather than chronic AAS exposure may alter pain. However, our negative findings after a single injection of AAS (Experiment 3) do not support this hypothesis.
A final explanation for the conflicting findings of our study compared to anecdotal reports of AAS-induced attenuation of pain in humans may stem from the pain models used. Human athletes using AAS often incur damage to the lower back, knee and shoulders, and suffer specifically from significant muscle strain and joint pain resulting from intense physical activity (Calhoon and Fry, 1999). Thus the pain models we and others typically use in the rodent may not accurately reflect the musculoskeletal pain characteristic of human AAS users. The acute pain models used in this study demonstrate brief, superficial pain and the CFA regimen produces local tissue inflammation (restricted to the paw). A model in which the pain state is induced through high-intensity, high-resistance exercise may better represent the type of pain against which AAS may be antinociceptive.
7.2. Effect of chronic AAS on morphine antinociception
In Experiment 1 (chronic AAS administration), only minimal differences between AAS-treated groups and controls were found in regard to morphine antinociception. Specifically, only chronic DHT treatment significantly altered morphine potency, and only on the hotplate test. This finding does not support the hypothesis that AAS potentiate morphine antinociception, as antinociception in T- and STAN-treated groups did not differ from controls, and DHT-treated rats expressed a rightward shift in the dose response curve, thereby indicating a decrease in the potency of morphine. While this result was surprising, we previously found a similar DHT effect on morphine potency in gonadectomized male rats (chronic DHT-replaced rats were less sensitive than gonadally intact males to morphine: Stoffel et al., 2003).
AAS are known to modulate brain opioid peptides. For example, the AAS nandrolone administered daily for two weeks increased levels of kappa, mu and delta opioid agonist immunoreactivity in the hypothalamus, striatum and periaqueductal gray (Johansson et al., 2000). Likewise, chronic administration of nandrolone increased beta-endorphin levels in the ventral tegmental area (Johansson et al., 1997). In addition to the increase in opioid peptides, sex hormones may also stimulate an increase in mu-opioid receptor mRNA (Petersen and LaFlamme, 1997; Quinones-Jenab et al., 1997). Conversely, Hammer et al. (1993) found that hormone-depleted rats experienced reduced proenkephalin mRNA expression in various brain structures, and this reduction was not reversed with administration of DHT. Pluchino et al. (2009) also found that depletion of gonadal hormones decreased brain beta-endorphin levels, and T administration conversely increased beta-endorphin levels in various brain areas and in the plasma of gonadectomized rats. In contrast, DHT treatment—using a 5 mg/kg/day regimen similar to that used in the present study—failed to restore beta-endorphin levels. These studies suggest that the blunted morphine antinociceptive sensitivity we observed in chronic DHT-treated rats may be due to the failure of DHT (relative to other AAS) to increase brain opioid peptide levels.
How do we account for the differential effects of chronic DHT versus T and STAN? The classic concept of DHT as a “pure” androgen has been revised due to recent findings that 5α-androstane-3β, 17β-diol (3βdiol), a key DHT metabolite, can bind to the beta form of the estrogen receptor (Pak et al., 2005). It is possible that differential activation of androgen and estrogen receptors, including alpha and beta receptors, accounts for the unique effects of chronic DHT versus T and STAN in tests of opioid antinociception against acute pain. These unexpected findings indicate that further investigation on the effect of chronic DHT on opioid potency and antinociception is warranted.
7.3. Effect of chronic AAS on other pain indices and reproductive organs
7.3.1. Body weight
In both Experiments 1 (acute pain) and 2 (chronic inflammatory pain), chronic T-treated rats and to a lesser degree chronic DHT-treated rats gained less weight than controls, which is consistent with studies demonstrating that chronic AAS reduce fat mass (Hartgens and Kuipers, 2004) and T administration decreases weight gain (Clark et al., 1997). Not surprisingly, chronic STAN, being the weakest of the androgens (Kicman, 2008), inhibited weight gain the least in this study. In Experiment 2, temporary body weight decreases after CFA injection were also an indicator of pain severity. Differences in body weight among chronic AAS groups were independent of CFA injection, implying that the decreased weight gain in T and DHT groups primarily reflected AAS effects rather than indicating more pain.
7.3.2. Pain suppressed behavior
While pain may elicit nocifensive behaviors, it also suppresses normal behaviors such as feeding and locomotion (Negus et al., 2006). The locomotor test was used in Experiment 2 as an additional reflection of pain following CFA injection. The dramatic decrease in the number of beam breaks on the day immediately following CFA administration occurred in concert with the drop in thresholds on the von Frey and Hargreaves tests, suggesting that pain suppressed locomotion. Recovery of locomotor activity also roughly paralleled the recovery of mechanical and thermal response thresholds, as well as recovery from paw inflammation. However, chronic AAS administration failed to attenuate the decreases in locomotion resulting from CFA injection, again suggesting that AAS did not attenuate inflammatory pain in gonadally intact males.
7.3.3. Reproductive organs
Testes and seminal vesicles were harvested to confirm that the chronic AAS regimen used in this study was physiologically effective. Although T and DHT failed to decrease testis weight significantly across all experiments, all three AAS significantly increased seminal vesicle weight in Experiment 2, and T and DHT also increased seminal vesicle weight in Experiment 1. Seminal vesicles are highly sensitive to circulating androgens, and provide a bioassay of androgen exposure (Schmidt et al., 1972). By contrast, testis weight is due to activity of the seminiferous epithelium, which is sensitive to endogenous androgen production; with supplemental exogenous androgen exposure, endogenous steroid production is reduced due to negative feedback at the hypothalamus and pituitary (Bauman et al., 1988). Ultimately, when endogenous T is suppressed for an extended period, testis volume decreases. The duration of androgen treatment even in the chronic AAS experiments may have been insufficient to significantly reduce testis volume.
7.4. Acute versus chronic AAS administration
A number of studies demonstrating antinociceptive effects of AAS, or potentiation of morphine antinociception by AAS have implemented much shorter hormone regimens than our chronic treatment regimen: 1 h prior (s.c. and i.m.), 6 days prior (s.c.), crystalline hormone-filled cannula implants 7 days prior, and intra-hippocampal infusion immediately prior to testing (Aloisi et al., 2004; Celerier et al., 2003; Edinger and Frye, 2004, 2005). To address the possibility of tolerance development following chronic AAS administration in Experiments 1 and 2, we repeated these studies using an acute AAS regimen in which only one injection was given 3 h prior to acute nociceptive testing, or two injections were given for the chronic pain model (one injection on each of the two test days). Nociceptive baselines were higher overall in Experiment 3 (acute AAS administration) in comparison to Experiment 1 (chronic AAS administration). In addition, morphine tended to be more potent when AAS were administered acutely rather than chronically, suggesting that rats treated chronically with AAS in Experiment 1 may have developed steroid tolerance. However, these trends also occurred in rats receiving vehicle—thus, it is more likely that repeated handling for 28–56 consecutive days in the chronic AAS experiments led to differences in nociception and morphine antinociceptive potency between rats treated chronically vs. acutely with AAS. Rats treated acutely were not handled prior to testing and were therefore more likely to be stressed upon testing than rats handled repeatedly. Repeated handling attenuates stress-induced activation of the HPA axis (Gadek-Michalska and Bugajski, 2003), thereby preventing stress-induced potentiation of morphine antinociception, which typically results from the release of endogenous opioids in response to stressful stimuli (Fleetwood and Holtzman, 1989; Parikh et al., 2011). Lastly, nociceptive thresholds in the chronic inflammatory pain model did not differ between treatment groups in the acute AAS experiment (Experiment 3), suggesting that steroid tolerance resulting from chronic AAS administration is an unlikely explanation for the lack of steroid effect on nociception in Experiment 2.
8. Conclusion
The AAS examined in this study did not significantly alter acute nociception nor did they significantly attenuate various chronic pain indices after administration of CFA. Furthermore, morphine antinociception was only modulated by chronic DHT on the hotplate test; rather than potentiating morphine antinociception, DHT decreased morphine potency. The findings of this study conflict with those of previous experiments that primarily used gonadectomized subjects. Thus it can be concluded that there are no consistent effects of AAS on pain, particularly in gonadally intact subjects. However, because there are very few AAS studies to date in gonadally intact animals and no controlled studies in humans, particularly under conditions of chronic pain, it may be too early to draw firm conclusions. As such, further study in gonadally intact animals, that better represent the typical human AAS user, is needed. Lastly, a model in which pain is induced through prolonged high-intensity, high-resistance exercise may be better suited for examining the effects of AAS on pain and opioid sensitivity.
Acknowledgments
This research was supported by funds provided for medical and biological research by the State of Washington Initiative Measure No. 171. The authors thank Michelle Cyr and Brittany Navarre for excellent technical assistance.
References
- Ali BH, Sharif SL, Elkadi A. Sex differences and the effect of gonadectomy on morphine-induced antinociception and dependence in rats and mice. Clin Exp Pharmacol Physiol. 1995;22:342–344. doi: 10.1111/j.1440-1681.1995.tb02012.x. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Ceccarelli I. Role of gondal hormones in formalin-induced pain responses of male rats: modulation by estradiol and naloxone administration. Neuroscience. 2000;95:559–566. doi: 10.1016/s0306-4522(99)00445-5. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, Ceccarelli I, Fiorenzani P, De Padova AM, Massafra C. Testosterone affects formalin-induced responses differently in male and female rats. Neuroscience. 2004;361:262–264. doi: 10.1016/j.neulet.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Bauman DH, Richerson JT, Britt AL. A comparison of body and organ weights, physiologic parameters, and pathologic changes in the target organs of rats given combinations of exercise, anabolic hormone, and protein supplementation. Am J Sports Med. 1988;16:397–402. doi: 10.1177/036354658801600416. [DOI] [PubMed] [Google Scholar]
- Berning JM, Adams KJ, DeBeliso M, Stamford BA, Newman IM. Anabolic androgenic steroids: use and perceived use in non-athlete college students. J Am Coll Health. 2008;56:499–503. doi: 10.3200/JACH.56.5.499-504. [DOI] [PubMed] [Google Scholar]
- Borzan J, Fuchs PN. Organizational and activational effects of testosterone on carrageenan-induced inflammatory pain and morphine analgesia. Neuroscience. 2006;143:885–893. doi: 10.1016/j.neuroscience.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Breuer ME, McGinnis MY, Lumia AR. Aggression in male rats receiving anabolic androgenic steroids: effects of social and environmental provocation. Horm Behav. 2001;40:409–418. doi: 10.1006/hbeh.2001.1706. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Blow FC, Young JP, Hill EM. Symptoms and correlates of anabolic–androgenic steroid dependence. Br J Addict. 1991;86:759–768. doi: 10.1111/j.1360-0443.1991.tb03101.x. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Eliopulos GA, Blow FC, Catlin DH, Beresford TP. Evidence for physical and psychological dependence on anabolic androgenic steroids in eight weight lifters. Am J Psychiatry. 1990;147:510–520. doi: 10.1176/ajp.147.4.510. [DOI] [PubMed] [Google Scholar]
- Calhoon G, Fry A. Injury rates and profiles of elite competitive weightlifters. J Athl Train. 1999;34:232–238. [PMC free article] [PubMed] [Google Scholar]
- Celerier E, Yazdi MT, Castane A, Ghozland S, Nyberg F, Maldonado R. Effects of nandrolone on acute morphine responses, tolerance and dependence in mice. Eur J Pharmacol. 2003;465:69–81. doi: 10.1016/s0014-2999(03)01462-6. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, O'Connor L, Meyer ER. Role of steroids in sex differences in morphine-induced analgesia: activational and organizational effects. J Pharmacol Exp Ther. 2002;300:695–701. doi: 10.1124/jpet.300.2.695. [DOI] [PubMed] [Google Scholar]
- Clark A, Harrold E, Fast A. Anabolic–androgenic steroid effects on the sexual behavior of intact male rats. Horm Behav. 1997;31:35–46. doi: 10.1006/hbeh.1997.1355. [DOI] [PubMed] [Google Scholar]
- Cook CD, Nickerson MD. Nociceptive sensitivity and opioid antinociception and antihyperalesia in Freund's Adjuvant induced arthritic male and female rats. J Pharmacol Exp Ther. 2005;313:449–459. doi: 10.1124/jpet.104.077792. [DOI] [PubMed] [Google Scholar]
- Craft RM, Bernal SA. Sex differences in opioid antinociception: kappa and ‘mixed action’ agonists. Drug Alcohol Depend. 2001;63:215–228. doi: 10.1016/s0376-8716(00)00209-x. [DOI] [PubMed] [Google Scholar]
- Cunningham RL, McGinnis MY. Physical provocation of pubertal anabolic androgenic steroid exposed male rats elicits aggression towards females. Horm Behav. 2006;50:410–416. doi: 10.1016/j.yhbeh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone's analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5a-reduced metabolites in the hippocampus. Behav Neurosci. 2004;118:1352–1364. doi: 10.1037/0735-7044.118.6.1352. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone’s anti-anxiety and analgesic effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2005;30:418–430. doi: 10.1016/j.psyneuen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Fischer BD, Ward SJ, Henry FE, Dykstra LA. Attenuation of morphine antinociceptive tolerance by a CB1 receptor agonist and an NMDA receptor antagonist: Interactive effects. Neuropharmacology. 2010;58:544–550. doi: 10.1016/j.neuropharm.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer L, Clemente JT, Tambeli CH. The protective role of testosterone in the development of temporomandibular joint pain. J Pain. 2007;8:437–442. doi: 10.1016/j.jpain.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Fleetwood SW, Holtzman SG. Stress-induced potentiation of morphine-induced analgesia in morphine-tolerant rats. Neuropharmacology. 1989;28:563–567. doi: 10.1016/0028-3908(89)90134-2. [DOI] [PubMed] [Google Scholar]
- Frye CA, Babson A, Walf AA. Self-administration of 3α-androstanediol increases locomotion and analgesia and decreases aggressive behavior of male hamster. Pharmacol Biochem Behav. 2007;86:415–421. doi: 10.1016/j.pbb.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Frye CA, Seliga EM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn Affect Behav Neurosci. 2001;1:371–381. doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- Gadek-Michalska A, Bugajski J. Repeated handling, restraint, or chronic crowding impair the hypothalamic-pituitary-adrenocortical response to acute restraint stress. J Physiol Pharmacol. 2003;54:449–459. [PubMed] [Google Scholar]
- Gaumond I, Arsenault P, Marchand S. The role of sex hormones on formalin-induced nociceptive responses. Brain Res. 2002;958:139–145. doi: 10.1016/s0006-8993(02)03661-2. [DOI] [PubMed] [Google Scholar]
- Gaumond I, Arsenault P, Marchand S. Specificity of female and male sex hormones on excitatory and inhibitory phases of formalin-induced nociceptive responses. Brain Res. 2005;1052:105–111. doi: 10.1016/j.brainres.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Hammer RP, Bogic L, Handa RJ. Estrogenic regulation of proenkephalin mRNA expression in the ventromedial hypothalamus of the adult male rat. Mol Brain Res. 1993;19:129–134. doi: 10.1016/0169-328x(93)90157-k. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Perveen-Gill Z, Lightman SL, Jessop DS. A protective role for testosterone in adjuvant-induced arthritis. Br J Rheumatol. 1995;34:1117–1122. doi: 10.1093/rheumatology/34.12.1117. [DOI] [PubMed] [Google Scholar]
- Hartgens F, Kuipers H. Effects of androgenic–anabolic steroids in athletes. Sports Med. 2004;34:513–554. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- Hau M, Dominguez OA, Evrard HC. Testosterone reduces responsiveness to nociceptive stimuli in a wild bird. Horm Behav. 2004;46:165–170. doi: 10.1016/j.yhbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Gonzalez L, Murzi E, et al. Testosterone modulates mesolimbic dopaminergic activity in male rats. Neurosci. Lett. 1994;171:172–174. doi: 10.1016/0304-3940(94)90632-7. [DOI] [PubMed] [Google Scholar]
- Johansson P, Halberg M, Kindlundh A, Nyberg F. The effect of opioid peptides in the rat brain, after chronic treatment with the anabolic androgenic steroid, nandrolone decanoate. Brain Res Bull. 2000;50:413–418. doi: 10.1016/s0361-9230(99)00263-4. [DOI] [PubMed] [Google Scholar]
- Johansson P, Ray A, Zhou Q, Huang W, Karlsson K, Nyberg F. Anabolic androgenic steroids increase β-endorphin levels in the ventral tegmental area in the male rat brain. Neurosci Res. 1997;27:185–189. doi: 10.1016/s0168-0102(96)01141-8. [DOI] [PubMed] [Google Scholar]
- Kicman AT. Pharmacology of anabolic steroids. Br J Pharmacol. 2008;154:501–521. doi: 10.1038/bjp.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima AP, Lunardi LO, Rosa E, Silva AA. Effects of castration and testosterone replacement on peritoneal histamine concentration and lung histamine concentration in pubertal male rats. J. Endocrinol. 2000;167:71–75. doi: 10.1677/joe.0.1670071. [DOI] [PubMed] [Google Scholar]
- Liu NJ, Gintzler AR. Prolonged ovarian sex steroid treatment of male rats produces antinociception: identification of sex-based divergent analgesic mechanisms. Pain. 2000;85:273–281. doi: 10.1016/s0304-3959(99)00278-x. [DOI] [PubMed] [Google Scholar]
- Manning BH, Merin NM, Meng ID, Amaral DG. Reduction in opioid- and cannabinoid-induced antinociception in rhesus monkeys after bilateral lesions of the amygdaloid complex. J. Neurosci. 2001;21:8238–8246. doi: 10.1523/JNEUROSCI.21-20-08238.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Brower KJ, West BT, Nelson TF, Wechsler H. Trends in non-medical use of anabolic steroids by U.S. college students: results from four national surveys. Drug Alcohol Depend. 2007;90:243–251. doi: 10.1016/j.drugalcdep.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M, Fossum E, Stalding B, King M. Morphine Antinociceptive Potency on Chemical, Mechanical, and Thermal Nociceptive Tests in the Rat. The Journal of Pain. 2006;7:358–366. doi: 10.1016/j.jpain.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Nagakura Y, Okada M, Kohara A, Kiso T, Toya T, Iwai A, et al. Allodynia and hyperalgesia in adjuvant-induced arthritic rats: time course of progression and efficacy of analgesics. J Pharmacol Exp Ther. 2003;306:490–497. doi: 10.1124/jpet.103.050781. [DOI] [PubMed] [Google Scholar]
- Negus SS, Pope HG, Kanayama G, Wines JD, Fischer BD. Lack of evidence for opioid tolerance or dependence in rhesus monkeys following high-dose anabolic–androgenic steroid administration. Psychoneuroendocrinology. 2001;26:789–796. doi: 10.1016/s0306-4530(01)00028-2. [DOI] [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther. 2006;319:507–5s14. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- Pak TR, Chung WC, Lund TD, Hinds LR, Clay CM, Handa RJ. The androgen metabolite, 5alpha-androstane-3beta, 17beta-diol, is a potent modulator of estrogen receptor-beta1-mediated gene transcription in neuronal cells. Endocrinology. 2005;146:147–155. doi: 10.1210/en.2004-0871. [DOI] [PubMed] [Google Scholar]
- Parikh D, Hamid A, Friedman TC, et al. Stress-induced analgesia and endogenous opioid peptides: the importance of stress duration. Eur J Pharmacol. 2011;650:563–567. doi: 10.1016/j.ejphar.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pednekar JR, Mulgaonker VK. Role of testosterone on pain threshold in rats. Indian J Physiol Pharmacol. 1995;39:423–424. [PubMed] [Google Scholar]
- Peters KD, Wood RI. Androgen dependence in hamsters: overdose, tolerance, and potential opioidergic mechanisms. Neuroscience. 2004;130:971–981. doi: 10.1016/j.neuroscience.2004.09.063. [DOI] [PubMed] [Google Scholar]
- Petersen SL, LaFlamme KD. Progesterone increases levels of mu-opioid receptor mRNA in the preoptic area and arcuate nucleus of ovariectomized, estradiol-treated female rats. Mol Brain Res. 1997;52:32–37. doi: 10.1016/s0169-328x(97)00194-0. [DOI] [PubMed] [Google Scholar]
- Philipova T, Ivanova T, Kasakov L, Vlaskovska M. Nandrolone modulates the non-opioid and opioid analgesia and tolerant/dependence: role of sexual dimorphism. Arch Physiol Biochem. 2003;111:429–436. doi: 10.3109/13813450312331342283. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Ninni D, Casarosa E, Giannini A, Merlini S, Cubeddu A, et al. Sex differences in brain and plasma β-endorphin content following testosterone, dihydrotestosterone and estradiol administration to gonadectomized rats. Neuroendocrinology. 2009;89:411–423. doi: 10.1159/000209506. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Jenab S, Ogawa S, Inturrisi C, Pfaff DW. Estrogen regulation of muopioid receptor mRNA in the forebrain of female rats. Mol Brain Res. 1997;47:134–138. doi: 10.1016/s0169-328x(97)00041-7. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Noack I, Voigt KD. Metabolism and mode of action of androgens in target tissues of male rats. II. Mode of action of testosterone and 5-dihydrotestosterone at a cellular level on seminal vesicles and prostates of rats. Acta Endocrinol. 1972;69:165–173. doi: 10.1530/acta.0.0690165. [DOI] [PubMed] [Google Scholar]
- South SM, Wright AW, Lau M, Mather LE, Smith MT. Sex-related differences in antinociception and tolerance development following chronic intravenous infusion of morphine in the rat: modulatory role of testosterone via morphine clearance. J Pharmacol Exp Ther. 2001;207:446–457. [PubMed] [Google Scholar]
- Stoffel EC, Ulibarri CM, Craft RM. Gonadal steroid hormone modulation of nociception, morphine antinociception and reproductive indices in male and female rats. Pain. 2003;103:285–302. doi: 10.1016/s0304-3959(02)00457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JE, Ulibarri C, Craft RM. Testosterone modulation of reproductive indices vs. morphine antinociception in male rats. Life Sci. 2006;79:2119–2127. doi: 10.1016/j.lfs.2006.07.007. [DOI] [PubMed] [Google Scholar]
- U.S Department of Health and Human Services. Monitoring the future study: overview of key findings. [Accessed January 22, 2008];2007 http://www.monitoringthefuture.org. [Google Scholar]
- van den Berg P, Neumark-Sztainer D, Cafri G, Wall M. Steroid use among adolescents: longitudinal findings from Project EAT. Pediatrics. 2007;119:476–486. doi: 10.1542/peds.2006-2529. [DOI] [PubMed] [Google Scholar]
- Wesson DW, McGinnis MY. Stacking anabolic androgenic steroids (AAS) during puberty in rats: a neuroendocrine and behavioral assessment. Pharmacol Biochem Behav. 2006;83:410–419. doi: 10.1016/j.pbb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Wood RI. Reinforcing aspects of androgens. Physiol Behav. 2004;83:279–289. doi: 10.1016/j.physbeh.2004.08.012. [DOI] [PubMed] [Google Scholar]