Abstract
Background:
Researchers have recently drawn attention to the analysis of gingival crevicular fluid (GCF) and peri-implant sulcus fluid (PISF) for the implementation of the diagnosis of periodontal and peri-implant disease. Nevertheless, the measurements of volume and biomarkers concentration can be critically biased when data collected from studies with parallel group design are compared, given the technical difficulties, methodological variables, as well as the variability of crevicular fluid characteristics among different individuals.
Objective:
The aim of the present study was to assess the GCF and PISF volumes in healthy and diseased sites belonging to the same patient.
Method:
Ten patients presenting a periodontally healthy tooth, a tooth with periodontitis, an implant with healthy peri-implant tissues and an implant with peri-implantitis were enrolled. Samples of GCF and PISF were collected from each site of interest and their volume measured with a Periotron 8000 device. Non-parametric statistical analysis was performed to test the significance of the differences in GCF and PISF volumes between i) sites of teeth and dental implants with the same condition of health or disease and ii) healthy and diseased sites of both teeth and dental implants subgroups. The correlation between probing pocket depth (PPD) and fluid production was also tested (p<0.05).
Results:
Healthy periodontal and peri-implant tissues produced comparable amounts of fluid that was significantly lower than in diseased sites (p<0.05). In the presence of diagnosed disease, the volumes of GCF and PISF were similar, too. The correlation between PPD and fluid production was significant only in healthy sites (PPD/GCF, ρ=0.890, p<0.001; PPD/PISF, ρ=0.810; p<0.005).
Conclusion:
The periodontal and peri-implant tissues behaved similarly in terms of fluid production in condition of both health and active disease.
Keywords: Dental implant, gingival crevicular fluid, inflammation, peri-implant sulcus fluid, peri-implantitis, periodontitis, Periotron 8000
INTRODUCTION
Gingivitis and periodontitis are some of the most widespread diseases among the worldwide populations of almost all ages and they are characterized by inflammation of the tissues surrounding the tooth as a result of infection by a mix of periodontal pathogenic bacteria [1]. The prevalence of severe forms of periodontitis, which can lead to increased tooth mobility and eventually tooth loss, is reported to be 5-20% of the adult population [1, 2]. Because of the spread and relevance of the periodontal disease, its prevention, diagnosis and treatment are of paramount importance and constitute routine activities in the dental clinical practice.
Dental implants are used to replace one or more teeth and have revolutionized the rehabilitation of partially and totally edentulous patients. They are a versatile, predictable and valid long-term treatment option [3], but are not immune from clinical complications due, above all, to an inflammation of peri-implant soft and hard tissues [3]. These lesions can appear in two forms, mucositis and peri-implantitis, which are both inflammatory reactions of the peri-implant tissues that correspond to the same pathological conditions of the periodontum, namely gingivitis and periodontitis [4]. With mucositis, the inflammation is reversible and limited to the soft tissues, with no clinical or radiological signs of supporting bone loss other than the initial remodelling that occurs during the first part of the osseointegration progress [5]. On the contrary, peri-implantitis has been described as inflammation of the soft tissues surrounding the implant associated with the evidence of progressive bone loss that exceeds the normal bone remodelling process [5]. These pathological processes are capable of causing substantial damage to the supporting tissues, ending to implant loss, so that prevention and treatment of peri-implant disease are important. Peri-implantitis and periodontitis share several features, but histopathological and structural differences between the two clinical conditions can considerably influence the response to the microbial insult [6].
The diagnostic assessment of periodontal disease by clinical methods is surely essential, but currently, available instruments and techniques are not always sufficient to locate a site where the inflammation is progressing [7]. In the 1960s, it was suggested that the gingival crevicular fluid (GCF) could be analysed to assess quantitatively the site-specific inflammatory status of the periodontal tissues [8]; since then, the interest in the diagnostic potential of GCF has increased and is still an up-to-date topic. GCF can be defined as an inflammatory exudate secreted by the inflamed gingiva inside the sulcus or periodontal pockets [9]. In the clinically healthy site, GCF is an interstitial fluid that appears in the sulcus because of an osmotic gradient. In case of inflamed periodontal tissues, GCF is similar to serum. It has been clinically and histometrically demonstrated that the production of GCF strongly increases when a gingiva is severely inflamed [10]. GCF flow appears to be directly related to the severity of the periodontal inflammation, and the flow increase depends on the greater vascular permeability and ulceration of the epithelium at inflamed sites [8]. With the aim of developing patient-specific diagnostic tests for periodontal disease, researchers have carried out GCF elution and searched for the presence of host response factors [11]. Nonetheless, this complex approach is still being refined and involves the understanding of a multitude of profiles of metabolites, whose interpretation can be misleading. Moreover, many other factors can increase the production of GCF, such as smoke [12], oral contraceptives and pregnancy [13], or orthodontic treatment [14], thus possibly affecting the delicate biomarkers concentration assessment.
Peri-implant tissues are also capable of producing an inflammatory exudate that accumulates into the sulcus, namely the peri-implant sulcular fluid (PISF) [15]. Like GCF, PISF is considered a potential marker of the severity of the site inflammation and the study of its volume variations could be relevant for improving the diagnosis and treatment of peri-implant disease [16, 17]. Contrary to the GCF, a recent systematic review showed a moderate to limited evidence that cytokine levels in PISF are possible predictors of peri-implantitis. This depends on the fact that the majority of studies show methodological limitations [18].
To the best of our knowledge, no previous study investigated the diagnostic potential of GCF and PISF on the same individuals presenting healthy and diseased sites in teeth and implants. This approach would limit the risk of bias deriving from interpatient variability.
The aim of the present study is threefold: a) to quantitatively compare the volume of GCF and PISF in healthy periodontal and peri-implant sites to that of sites where active periodontitis or peri-implantitis has been diagnosed; b) to search for differences in fluid production between gingival and peri-implant tissues; and c) to assess the correlation between probing pocket depth (PPD) and sulcular fluid production.
MATERIALS AND METHODS
Selection of Patients
The study was conducted in accordance with the Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects, version 2002. The patients were sequentially enrolled among a pool of periodontal patients. All recruited patients were informed of the purpose of the present cross-sectional study and asked to express their consent to join the experimentation by signing a dedicated form. It was clearly expressed on the consent form that the participation to the study was voluntary and the patient could withdraw his/her consent at any time without the need of justification.
Ten patients were enrolled (6 females and 4 males, mean age 64.8 ±9.2 years, minimum 48 and maximum 78). The inclusion criteria were the presence of at least one single-rooted tooth and implant presenting buccal probing depth ≤ 3 mm and no bleeding on probing, and one diseased tooth and implant with diagnosis of periodontitis and peri-implantitis, respectively. For both lesions, diagnosis of active inflammatory status was made in case of PPD ≥ 5 mm, radiographic evidence of bone loss and bleeding on probing [19, 20]. In order to standardize the probing force (25 N), the operator was calibrated with repeated measurements of the imparted force with the aid of a precision balance [21, 22]. Table 1 reports anamnestic and site-specific PPD data of each patient. Smokers, patients with severe medical illness, physical or mental handicaps, and subjects that make use of drugs or medications with known effects on the gingival health and sulcular fluid flow were excluded from the experimentation. Furthermore, the presence of restoration on the designated tooth, occlusal trauma, hyperplastic soft tissue lesions, orthodontic appliances, incongruous implant-retained prosthesis or different implant systems in the same patient was a site-specific exclusion criterion.
Table 1.
Anagraphic data of the enrolled patients and values of probing pocket depth of the tooth and implant sites of interest.
| Patient | Sex | Age (y) | Probing pocket depth of the sites of interest (mm) | |||
|---|---|---|---|---|---|---|
| Teeth | Implants | |||||
| Periodontal health | Periodontitis | Peri-implant health | Peri-implantitis | |||
| 1 | F | 48 | 3 | 5 | 3 | 6 |
| 2 | F | 70 | 3 | 5 | 3 | 5 |
| 3 | F | 63 | 3 | 5 | 3 | 6 |
| 4 | M | 53 | 3 | 5 | 2 | 5 |
| 5 | F | 63 | 2 | 5 | 3 | 5 |
| 6 | M | 78 | 2 | 6 | 3 | 6 |
| 7 | F | 62 | 1 | 7 | 3 | 6 |
| 8 | M | 67 | 2 | 5 | 2 | 5 |
| 9 | M | 70 | 2 | 5 | 2 | 5 |
| 10 | F | 74 | 3 | 8 | 3 | 6 |
| Mean ±;SD | 2.4±0.7 | 5.6±1.1 | 2.7±0.5 | 5.5±0.5 | ||
SD, standard deviation
Measurement of Sulcular Fluid Volumes and Clinical Parameters
Three operators performed different tasks for data collection and analysis. An experienced dental hygienist took all the samples from the patients and gave them to a second blind operator, who was unaware of the study design and purposes and made all the laboratory assessment procedures. A third operator, who was kept blind too, handled the final data set for statistical analysis.
The measurement of GCF and PISF volumes was carried out with the aid of Periotron 8000 (Oralflow Inc., New York, NY, USA). The Periopaper devices (Oralflow Inc.) are 1.4 cm long paper strips that can be soaked to measure up to 1.2 µL. They are composed of an absorbent portion that has to be inserted into the sulcus and the other extremity is coated with plastic for better handling with forceps. The Periotron device allows for measurement of fluid volume by detecting the conductivity changes between a dry control Periopaper and a test strip that has been dipped in fluid. According to the manufacturer’s recommendations, the comprehensive and interim calibration schedule of the device was rigorously followed.
The clinical procedure for the collection of GCF and PISF started by isolating the tooth or implant of interest with cotton rolls and drying the external surface of the soft tissues with a gentle stream of air for 5 s. Visible supragingival plaque deposits were removed with the aid of a manual scaler. Afterwards, a sterile Periopaper strip was inserted into the buccal sulcus up to a maximal depth of 1 mm, regardless of PPD, and was kept in this position for 30 s. Trauma was carefully avoided during the insertion of the strip, in order to minimize the mechanical irritation of the sulcus [23]. All the strips contaminated by blood or saliva were discarded and the fluid collection repeated after 30 minutes. The Periotron values provided by the device were converted to actual volumes, expressed in µL, by referring to the correspondent calibration logarithmic curve.
At the end of the fluid collection procedures, PPD was measured in each site of interest and the value inserted into an electronic worksheet. PPD was defined as the distance, in mm, between the margin of the gingiva or peri-implant mucosa and the tip of the periodontal probe inserted into the sulcus. We made use of a PCP12 probe (Hu-Friedy, Chicago, IL, USA).
Statistical Analysis
Data analysis was carried out making use of statistical software (Statistical Package for Social Sciences version 15, SPSS® Inc., Chicago, IL, USA). The existence of the assumptions for the use of parametric tests was assessed by means of a Shapiro-Wilk test and a Levene test. Comparisons were made between GCF and PISF volumes of healthy teeth and dental implant sites and their counterparts with active inflammatory status, as well as of teeth and implants with similar condition, namely the presence or absence of active inflammation. For this purpose, a Friedman test was used, with multiple comparisons being made by means of Wilcoxon tests with Bonferroni correction. The correlation between probing depth and GCF/PISF volumes was evaluated by means of Spearman’s rank correlation coefficient. The value of α was set at 5%.
RESULTS
The mean values of GCF and PISF volumes are summarized in Fig. (1).
Fig. (1).
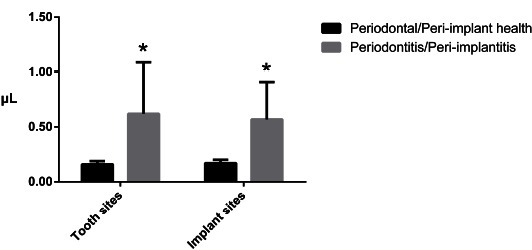
Mean values and standard deviations of gingival crevicular fluid and peri-implant sulcular fluid volumes. The asterisks indicate a statistically significant difference in the comparison between subgrups of healthy and diseased sites (p<0.05).
Similar amounts of sulcular fluid volumes were observed in teeth and implants with same condition of health or active disease. On the contrary, the difference in fluid volume between healthy sites and those affected by periodontitis/peri-implantitis was found to be statistically significant (p<0.05), with the latter producing on average approximately three times the amount of fluid of their healthy counterparts.
There was significant correlation between PPD and both GCF (ρ=0.890; p<0.001) and PISF (ρ=0.810; p<0.005) only in case of periodontal or peri-implant health, meaning that the greater the probing depth, the greater the fluid volume. In presence of periodontal or peri-implant inflammation, no significant correlation between PPD and volume of sulcular fluids was found. The relationship between crevicular fluid volume and PPD with the computed correlation lines relative to each subgroup is plotted in Fig. (2).
Fig. (2).
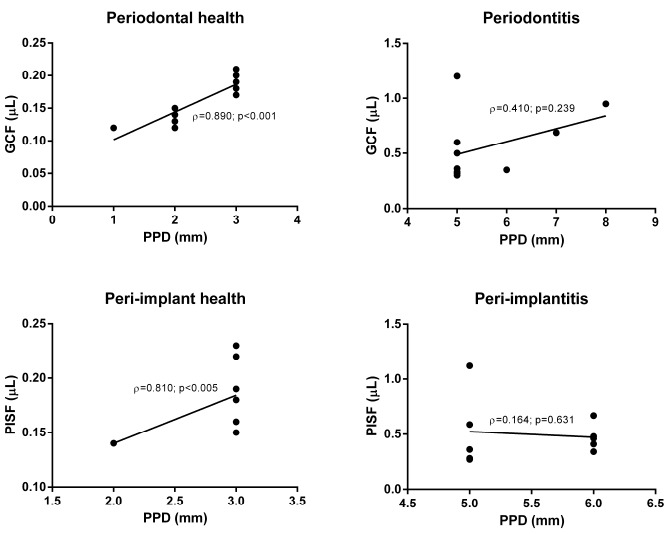
Diagrammatic representation of correlation between PPD and corresponding PISF and GCF volumes. PISF: peri-implant sulcular fluid, GCF: gingival crevicular fluid, PPD: probing pocket depth.
DISCUSSION
The present cross-sectional study found no difference in sulcular fluid production between periodontal and peri-implant tissues presenting the same condition of health or active disease. Our results are in accordance with the findings of previous studies reporting that peri-implant mucosa and tissues subjected to physiopathological and inflammatory stimuli can evoke quantitative and qualitative changes in PISF, similarly to what happens with inflamed periodontum and GCF [17, 24-29]. In a study with similar aim, Tözüm et al. [17] focused on the research of similarities between GCF and PISF production in teeth and implants in active inflammatory states. However, the determination of the experimental groups in the study by Tözüm et al. [17] appears questionable because not each recruited subject presented the four sites of interest (periodontal health, periodontitis, peri-implant health and peri-implantitis). This methodology might introduce bias as well as the use of tests for independent data. In an attempt to reduce this risk, Bhardwaj et al. [16] designed a study with similar purposes by choosing patients with healthy and diseased implants and age and sex matched control subjects with corresponding teeth for GCF determination. However, also this study design appears suboptimal because it does not guarantee absence of variability among test and control patients. Our findings are in agreement with the increased GCF and PISF volumes in diseased sites in comparison to healthy ones that these research groups have described. This confirmation is relevant because the study design we adopted (four sites of interest per patient) is less probable to be affected by a bias deriving from interpatient variability. Data management with tests for dependent data is appropriate, too. In light of these considerations, it could be speculated that the production of the sulcular fluid, regardless of its periodontal or peri-implant origin, is mainly related to the presence of an active inflammatory lesion rather than to the histological structure of the site. Specifically, the distribution of collagen fibres and composition of connective tissue in peri-implant soft tissues that differ from the periodontum seems not to affect the sulcular fluid production.
We did not choose to carry out a biomarker/mediator analysis also because it is hard to compare the findings among different studies, as the methods used to elute biomarkers from Periopapers are seldom reported in detail and it is hence difficult to understand the meaning of the datum or replicate the experimental set-up [30]. With regard to the methodological factors that can alter the measurement of the fluid volume, the collection of GCF and PISF samples was standardized as much as possible and aimed at reducing the risk of evaporation to minimum. Our sampling protocol consisted of placing the Periopaper 1 mm into the sulcus after drying and keeping it in place for 30 seconds, in accordance with the recommendations of a comprehensive technical review [30]. The measurement was carried out immediately after the removal of the Periopaper from the sulcus to avoid the risk of bias due to evaporation.
The sulcular fluid flow appeared to be in direct relation with GCF and PISF volumes of healthy teeth and implant, whereas this relationship was not present in case of active disease. This might indicate that in physiological conditions the sulcus produces small amounts of fluid that slowly drains out of the sulcus in a quantity that is proportionally related to the sulcus depth. A speculative explanation of the absence of proportionality between GCF and PISF in case of active lesions may be the fact that inflammation causes the affected site to react with an increase of fluid production. However, this effect is heterogeneous among patients and sites because each pathological pocket is an irregular environment characterized by unique structural, histological and microbiological features. It is conceivable that the deepest pocket is not necessarily the most inflamed and, consequently, its crevicular fluid production the most abundant. The fluid flow might possibly reach a plateau value that could express the maximum effort that periodontal and peri-implant tissues make to confront the attack of pathogenic bacteria. Probably, the plateau value is not a universal threshold suitable for every patient or periodontal/peri-implant lesion and can be variable. Therefore, the single datum of fluid volume is possibly insufficient and should be related to PPD in order to obtain a normalized ratio that could be used to score active and inactive lesions. The most challenging diagnostic assessment in periodontology concerns the “grey area” that characterizes the sites with PPD ranging from 3 to 5 mm, where the clinician cannot tell if the pathologic process is active. The present study enrolled patients with manifest conditions and was not designed to identify the border between health and disease. Our findings set the basis for future investigations regarding this specific problem.
One limitation of our study is the enrolment of patients with different implant systems, and it is still unknown whether this could introduce significant variability in sulcular fluid flow. Specifically, the connection type, implant surface, length of the implant perimeter, type of prosthetic appliance, surgical protocol and other factors might somehow alter the production of PISF. Even if under the conditions of the present pilot study these variables seemed to have little impact on fluid volume, this issue should be taken into account in future analyses. We must underline that in our study the inclusion criteria were strict since it is rather difficult to fulfil the requirement of having a healthy and diseased site for both tooth and implant. In future investigations, it would be appropriate to increase the sample size to reduce variability.
The diagnosis of peri-implant mucositis and peri-implantitis, especially in their early phases, is crucial in order to reduce the need of treating an active peri-implantitis, since non-surgical treatment protocols are ineffective and surgical techniques are difficult to perform and their outcome often not predictable [3]. Diagnosis of peri-implantitis is arduous, because probing an implant can surely detect the bleeding and monitor probing depth over time, but it may not determine the extent and pattern of bone loss without radiographs [3]. Even with the aid of conventional radiography, sensitivity is still an issue, meaning that not all peri-implantitis lesions may be observed on the periapical radiograph, particularly in case of lingual and buccal bony defects. As a solution, some authors have recently advocated the use of cone-beam computed tomography (CBCT) [31]; nonetheless, this approach does not eliminate the need of a baseline radiograph to detect bone changes [3]. Furthermore, CBCT is not exempt from drawbacks; for instance, prosthetic appliances can mask the area of interest creating artefacts and the patient is inevitably exposed to a certain radiation dose. For these reasons, the use of CBCT should probably be restricted only to those cases requiring surgical planning for the treatment of peri-implantitis and not extended to all cases where a diagnosis is pending. The analysis of GCF and PISF volumes is, on the contrary, harmless, rather simple, and quick to perform. To establish an early diagnosis and –if needed– an intervention, the integration of CGF and PISF volume analysis with clinical and periapical radiographic examination might be useful in the future, provided that a volumetric threshold value or a hallmark marker could be identified.
CONCLUSION
Under the conditions of the present study, GCF and PISF volumes were similar in healthy sites with PPD ≤ 3 mm as well as in those affected by periodontitis/peri-implantitis with PPD ≥ 5 mm. The volume of fluid was greater in case of periodontal or peri-implant disease but appeared to be directly related to PPD only in case of periodontal/peri-implant health.
The present study corroborates the evidence in favour of the diagnostic potential of volume analysis of both GCF and PISF. In future studies, effort should be made to verify the presence of a volumetric threshold value and assess its consistency among different sites and patients with the aim of solving the conundrum of treating or not the peri-implant sites in which the progression of the inflammatory process is uncertain.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
- 1.Albandar J.M. Epidemiology and risk factors of periodontal diseases. Dent. Clin. North Am. 2005;49(3):517–532. doi: 10.1016/j.cden.2005.03.003. v-vi. [DOI] [PubMed] [Google Scholar]
- 2.Haynes D.R. Emerging and future therapies for the treatment of bone loss associated with chronic inflammation. Inflammopharmacology. 2006;14(5-6):193–197. doi: 10.1007/s10787-006-0006-1. [DOI] [PubMed] [Google Scholar]
- 3.Peri-implant mucositis and peri-implantitis: a current understanding of their diagnoses and clinical implications. J. Periodontol. 2013;84(4):436–443. doi: 10.1902/jop.2013.134001. [DOI] [PubMed] [Google Scholar]
- 4.Mombelli A., Lang N.P. The diagnosis and treatment of peri-implantitis. Periodontol. 2000. 1998;17:63–76. doi: 10.1111/j.1600-0757.1998.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 5.Sanz M., Chapple I.L. Clinical research on peri-implant diseases: consensus report of Working Group 4. J. Clin. Periodontol. 2012;39(Suppl. 12):202–206. doi: 10.1111/j.1600-051X.2011.01837.x. [DOI] [PubMed] [Google Scholar]
- 6.Berglundh T., Zitzmann N.U., Donati M. Are peri-implantitis lesions different from periodontitis lesions? J. Clin. Periodontol. 2011;38(Suppl. 11):188–202. doi: 10.1111/j.1600-051X.2010.01672.x. [DOI] [PubMed] [Google Scholar]
- 7.Armitage G.C. Analysis of gingival crevice fluid and risk of progression of periodontitis. Periodontol. 2000. 2004;34:109–119. doi: 10.1046/j.0906-6713.2002.003427.x. [DOI] [PubMed] [Google Scholar]
- 8.Brill N. Gingival conditions related to flow of tissue fluid into gingival pockets. Acta Odontol. Scand. 1960;18:421–446. doi: 10.3109/00016356009043875. [DOI] [Google Scholar]
- 9.Cimasoni G. Crevicular fluid updated. Monogr. Oral Sci. 1983;12:III–VII, 1-152. [PubMed] [Google Scholar]
- 10.Armitage G.C. Clinical evaluation of periodontal diseases. Periodontol. 2000. 1995;7:39–53. doi: 10.1111/j.1600-0757.1995.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 11.Barros S.P., Williams R., Offenbacher S., Morelli T. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontol. 2000. 2016;70(1):53–64. doi: 10.1111/prd.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ustün K., Alptekin N.O. The effect of tobacco smoking on gingival crevicular fluid volume. Eur. J. Dent. 2007;1(4):236–239. [PMC free article] [PubMed] [Google Scholar]
- 13.Klinger G. Sulcus fluid flow rate in relation to hormonal influence. Stomatol. DDR. 1982;32(1):53–55. [PubMed] [Google Scholar]
- 14.Drummond S., Canavarro C., Perinetti G., Teles R., Capelli J., Jr The monitoring of gingival crevicular fluid volume during orthodontic treatment: a longitudinal randomized split-mouth study. Eur. J. Orthod. 2012;34(1):109–113. doi: 10.1093/ejo/cjq172. [DOI] [PubMed] [Google Scholar]
- 15.Lachmann S., Kimmerle-Müller E., Axmann D., Scheideler L., Weber H., Haas R. Associations between peri-implant crevicular fluid volume, concentrations of crevicular inflammatory mediators, and composite IL-1A -889 and IL-1B +3954 genotype. A cross-sectional study on implant recall patients with and without clinical signs of peri-implantitis. Clin. Oral Implants Res. 2007;18(2):212–223. doi: 10.1111/j.1600-0501.2006.01322.x. [DOI] [PubMed] [Google Scholar]
- 16.Bhardwaj S., Prabhuji M.L. Comparative volumetric and clinical evaluation of peri-implant sulcular fluid and gingival crevicular fluid. J. Periodontal Implant Sci. 2013;43(5):233–242. doi: 10.5051/jpis.2013.43.5.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tözüm T.F., Akman A.C., Yamalik N., Tulunoglu I., Turkyilmaz I., Karabulut E., Kilinc K., Cehreli M.C. Analysis of the inflammatory process around endosseous dental implants and natural teeth: myeloperoxidase level and nitric oxide metabolism. Int. J. Oral Maxillofac. Implants. 2007;22(6):969–979. [PubMed] [Google Scholar]
- 18.Duarte P.M., Serrão C.R., Miranda T.S., Zanatta L.C., Bastos M.F., Faveri M., Figueiredo L.C., Feres M. Could cytokine levels in the peri-implant crevicular fluid be used to distinguish between healthy implants and implants with peri-implantitis? A systematic review. J. Periodontal Res. doi: 10.1111/jre.12354. [DOI] [PubMed] [Google Scholar]
- 19.Zitzmann N.U., Berglundh T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008;35(8) Suppl.:286–291. doi: 10.1111/j.1600-051X.2008.01274.x. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira S.D., Silva G.L., Cortelli J.R., Costa J.E., Costa F.O. Prevalence and risk variables for peri-implant disease in Brazilian subjects. J. Clin. Periodontol. 2006;33(12):929–935. doi: 10.1111/j.1600-051X.2006.01001.x. [DOI] [PubMed] [Google Scholar]
- 21.Eickholz P., Grotkamp F.L., Steveling H., Mühling J., Staehle H.J. Reproducibility of peri-implant probing using a force-controlled probe. Clin. Oral Implants Res. 2001;12(2):153–158. doi: 10.1034/j.1600-0501.2001.012002153.x. [DOI] [PubMed] [Google Scholar]
- 22.Andrade R., Espinoza M., Gómez E.M., Espinoza J.R., Cruz E. Intra- and inter-examiner reproducibility of manual probing depth. Braz. Oral Res. 2012;26(1):57–63. doi: 10.1590/S1806-83242012000100010. [DOI] [PubMed] [Google Scholar]
- 23.Atici K., Yamalik N., Eratalay K., Etikan I. Analysis of gingival crevicular fluid intracytoplasmic enzyme activity in patients with adult periodontitis and rapidly progressive periodontitis. A longitudinal study model with periodontal treatment. J. Periodontol. 1998;69(10):1155–1163. doi: 10.1902/jop.1998.69.10.1155. [DOI] [PubMed] [Google Scholar]
- 24.Hultin M., Gustafsson A., Hallström H., Johansson L.A., Ekfeldt A., Klinge B. Microbiological findings and host response in patients with peri-implantitis. Clin. Oral Implants Res. 2002;13(4):349–358. doi: 10.1034/j.1600-0501.2002.130402.x. [DOI] [PubMed] [Google Scholar]
- 25.Behneke A., Behneke N., d’Hoedt B., Wagner W. Hard and soft tissue reactions to ITI screw implants: 3-year longitudinal results of a prospective study. Int. J. Oral Maxillofac. Implants. 1997;12(6):749–757. [PubMed] [Google Scholar]
- 26.Boutros S.M., Michalowicz B.S., Smith Q.T., Aeppli D.M. Crevicular fluid enzymes from endosseous dental implants and natural teeth. Int. J. Oral Maxillofac. Implants. 1996;11(3):322–330. [PubMed] [Google Scholar]
- 27.Niimi A., Ueda M. Crevicular fluid in the osseointegrated implant sulcus: a pilot study. Int. J. Oral Maxillofac. Implants. 1995;10(4):434–436. [PubMed] [Google Scholar]
- 28.Salcetti J.M., Moriarty J.D., Cooper L.F., Smith F.W., Collins J.G., Socransky S.S., Offenbacher S. The clinical, microbial, and host response characteristics of the failing implant. Int. J. Oral Maxillofac. Implants. 1997;12(1):32–42. [PubMed] [Google Scholar]
- 29.Sakallıoğlu U., Lütfioğlu M., Sakallıoğlu E.E., Sert S., Ceylan G. Osmotic pressure of periimplant sulcular and gingival crevicular fluids: a split-mouth, randomized study of its measurement and clinical significance. Clin. Oral Implants Res. 2011;22(7):706–710. doi: 10.1111/j.1600-0501.2010.02044.x. [DOI] [PubMed] [Google Scholar]
- 30.Wassall R.R., Preshaw P.M. Clinical and technical considerations in the analysis of gingival crevicular fluid. Periodontol. 2000. 2016;70(1):65–79. doi: 10.1111/prd.12109. [DOI] [PubMed] [Google Scholar]
- 31.Golubovic V., Mihatovic I., Becker J., Schwarz F. Accuracy of cone-beam computed tomography to assess the configuration and extent of ligature-induced peri-implantitis defects. A pilot study. Oral Maxillofac. Surg. 2012;16(4):349–354. doi: 10.1007/s10006-012-0320-2. [DOI] [PubMed] [Google Scholar]


