Abstract
OBJECTIVES
This study investigates the effects of high glucose content on patients undergoing cold crystalloid versus cold blood cardioplegia in terms of early clinical results, functional myocardial recovery and ischaemia–reperfusion injury in patients undergoing repair of acyanotic cardiac lesions.
METHODS
Patients were randomly assigned to receive either crystalloid (n = 31) or blood cardioplegia (n = 31). Early clinical results were assessed. Changes in left ventricular fractional shortening, arterial blood lactate levels, central venous saturation, cardiac Troponin I release and blood glucose concentration were measured during the first 24 h after ischaemia.
RESULTS
There was no significant difference in clinical outcomes and postoperative complication rates between groups. The postoperative changes in left ventricular function, lactate levels, central venous saturation and Troponin I were not significantly different between groups. The use of crystalloid cardioplegia was associated with significant increases in serum glucose compared with blood cardioplegia.
CONCLUSIONS
A high glucose content blood cardioplegia does not show any advantage compared with crystalloid cardioplegia in terms of clinical outcomes, functional recovery and the degree of ischaemic injury in infants and children undergoing repair of acyanotic heart lesions. High glucose concentration of the cardioplegic solution might potentiate ischaemia–reperfusion injury and diminish the beneficial effects of blood cardioplegia.
Keywords: Myocardial protection, Blood cardioplegia, Paediatric cardiac surgery, High glucose concentration
INTRODUCTION
The prevention of postoperative heart failure is a major issue in cardiac surgery [1]. Along with hypothermia, cardioplegia (Cp) is the most important tool for protecting the heart from ischaemia–reperfusion injury and postoperative heart failure. It has been in widespread use for decades, initially as a crystalloid solution, which was then gradually replaced by blood Cp, which has been accepted by the majority of paediatric cardiac centres [2]. This trend can be mostly explained by theoretical considerations and by experimental evidence supporting the use of blood Cp [3]. Blood offers a more physiological buffer and has important oxygen-carrying capacity. However, few prospective, randomized clinical trials in paediatric cardiac surgery have been carried out comparing blood and crystalloid Cp using clinically meaningful endpoints and biochemical markers of ischaemia–reperfusion injury. The few studies that have been carried out have reported conflicting results [4, 5].
Furthermore, there has been no clinical trial comparing the effects of blood and crystalloid Cp in the setting of a coexisting high glucose concentration of the cardioplegic solution. A high physiological glucose concentration (11 mmol/l) in the cardioplegic solution might improve myocardial recovery because of increased glycolytic adenosine triphosphate production, whereas a profoundly high glucose content Cp might have a deleterious effect [6].
The aim of this study was to compare the effect of blood and crystalloid Cp on early clinical outcomes, myocardial functional recovery and myocardial ischaemic injury in acyanotic cardiac patients, especially in light of the high glucose content crystalloid Cp that has been used in our practice.
MATERIALS AND METHODS
Study design
The study included 62 of 64 consecutive infants and children undergoing elective repair of acyanotic cardiac lesions (arterial saturation ≥95%) between April 2013 and December 2013 at the University Children's Hospital, Belgrade. Patients with univentricular heart physiology, cyanotic heart lesions, obstructive left heart lesions, previous cardiac surgeries and those who required deep hypothermic circulatory arrest were excluded. Two patients were excluded from the study due to non-compliance. There was less than 1% missing at random data. All patients were in a stable condition without preoperative respiratory or inotropic support. The study was approved by the University Children's Hospital Ethical committee (26/203) and informed consent for all patients was obtained. This study has not been registered with a public trial registry because it represents the summaries of standard clinical treatments. The block randomization method was used prior to starting the study. By opening the next numbered envelope, a patient was randomly allocated to either the crystalloid cardioplegia (CCp) or the blood cardioplegia (BCp) group. The trial was not blinded for theatre staff due to obvious difference in two cardioplegic solutions; however, it was masked for the majority of outcome assessors [intensive care unit (ICU) staff, laboratory technicians and cardiac echocardiographer]. The CCp group received custom crystalloid Cp with the following content: Na+ 82 mmol/l, K+ 40 mmol/l, Glc 277 mmol/l, Ca2+ 2 mmol/l, Mg2+ 15 mmol/l, 5 mmol/l, Cl− 156 mmol/l, 577 mOsm/l and pH 7.35–7.65. The BCp group received a mixture of one part of potassium-enriched crystalloid Cp (60 ml of KCl added to 1000 ml of CCp) and four parts of autologous blood from the cardiopulmonary bypass (CPB) circuit, giving a final K+ concentration of ∼20 mmol/l and glucose concentration of ∼60 mmol/l (Supplementary Material). Both Cp were given using the CPB circuit with heat exchanger (the temperature was regulated to 4°C) and pressure control (up to 200 mmHg prior to the heat exchanger). The Cp delivery technique was uniform for all patients as follows: a 30 ml/kg dose of Cp solution was initially administered into the aortic root for 4–5 min, followed by a 2- to 3-min repeated dose (15 ml/kg) at 20–25 min intervals using the same pattern.
Anaesthetic and cardiopulmonary bypass techniques
Anaesthetic and CPB techniques were standardized for all patients. Invasive arterial and central venous pressures, electrocardiogram, rectal and/or nasopharyngeal temperature, inspiratory and expiratory gas concentrations, and pulse oximetry were continuously monitored. Anaesthesia was induced with sodium thiopental and Sevoflurane, and Vecuronium was used for muscle relaxation. For analgesia and maintenance of anaesthesia, Fentanyl was used in combination with Sevoflurane in either the breathing circuit or in the CPB sweep gas. Heparin (400 IU/kg) was administered and supplemented as required to maintain an active clotting time of 480 s or above. The CPB prime contained buffered Ringer-lactate solution, 20% human albumin (50 ml), 20% Mannitol (maximum 2.5 ml/kg), methylprednisolone (30 mg/kg) and heparin 4000 IU/l. Erythrocytes were added for a haematocrit of 35%. The CPB was established using standard aorto-bicaval cannulation with non-pulsatile flow at a minimum rate of 2.5 l/min/m2 under normothermia. The surgeries were performed under normothermia or mild hypothermia down to 30°C. pH stat acid–base management was used in all patients. Conventional ultrafiltration (CUF) was routinely used in our patients. A patient was weaned off CPB after reaching a core temperature of 36°C and electrolyte correction using inotropic support as appropriate. After weaning of CPB, modified ultrafiltration was utilized in patients less than 10 kg. Milrinone (0.5–0.7 mcg/kg/min) was routinely infused to discontinue CPB.
Postoperative management and assessment of clinical outcome
After surgery, all patients were admitted to the paediatric cardiac ICU. Decisions regarding inotropic support were based on haemodynamic parameters (e.g. arterial pressure, central venous saturation (ScvO2), blood gases and lactate level), and echocardiographic and clinical judgement. Postoperative inotropic support was considered to be either minimal (Dopamine ≤5 mcg/kg/min or/and Milrinone ≤0.7 mcg/kg/min) or significant (Dopamine >5 mcg/kg/min or any support which required adrenaline or noradrenaline). Standard glycaemic control was used in all patients [7]. Intraoperative and postoperative clinical variables were prospectively recorded to assess early clinical outcome. The former included the duration of CPB, aortic cross-clamp time, reperfusion time, minimal systemic temperature during CPB, CUF and modified ultrafiltration (MUF) filtrate volume, electrical activity during ischaemic time and ventricular fibrillation (VF) episodes after aortic declamping. Postoperative variables included the level of inotropic support for the first 24 h postoperatively, ventilation time, major non-fatal complications (bleeding requiring reopening, arrhythmia requiring treatment, respiratory insufficiency requiring reintubation and acute renal failure requiring peritoneal dialysis), ICU and hospital stay, and early (30-day) mortality. Postoperative transthoracic echocardiographic studies [left ventricular (LV) fractional shortening (FS) and atrioventricular valve regurgitation] were performed by one observer in the ICU, ∼1 h after termination of CPB and the next morning using an Acuson Cypress sonography system (Acuson Corp., Mountain View, CA, USA).
Blood samples
The cardiac Troponin I (cTnI) levels and ScvO2 were measured in central venous blood samples taken prior to surgery (baseline), at the end of the ischaemic time as well as at 1, 4, 12 and 24 h after surgery from the distal limb of a central venous line. Samples were labelled and stored at −70°C until the completion of the study when the cTnI analysis was carried out using an ARCHITECT immunoassay test (Abbott Laboratories, North Chicago, IL, USA). Lactate and glucose levels were measured in arterial blood samples taken at the same time intervals as central venous blood samples using a blood gas analyser (GEM Premier 3000, Instrumentation Laboratory, MA, USA). The ScvO2 were measured at the same time intervals using the same gas analyser. The ScvO2 values registered immediately after ischaemia while the patient was still on CPB support were excluded from the analysis.
Sample size and power calculation
The cTnI concentration was used as a primary endpoint for a post hoc power analysis. Our repeated-measures model had 31 subjects in each cardioplegic group. Each subject was measured six times. This model achieved 100% power when an F-test was used to test time–factor (within-subjects effect) and 65% power when an F-test was used to test group–time interactions (between-subjects effect) at a 5% significance level for both.
Statistical analysis
Variables were expressed as the mean ± SD, median (range) or frequency as appropriate. Lactate, ScvO2 and cTnI data were presented graphically as means ± 95% CI (‘error bars’). Continuous variables not following a normal Gaussian-shaped distribution such as age, weight, body surface area, days intubated and ICU stay were compared with the non-parametric Mann–Whitney U-test. Student's t-test was used for normally distributed data. The Shapiro–Wilk test was used to determine normality. Categorical data were compared with two-tailed Pearson χ2 test or Fisher's exact test. The latter was used when one or more cells had a frequency of five or less. The effects of Cp type and time on lactate level, ScvO2, glycaemia and cTnI, and the interaction of these factors were performed by a repeated-measures analysis of variance. A natural logarithmic transformation was applied in order to normalize the cTnI level distribution. The assumption for the covariance matrix was tested by Mauchly's test; degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity. All analyses were carried out using SPSS software (v 16.0; SPSS, Chicago, IL, USA).
RESULTS
A total of 62 children were randomly allocated to either the CCp (n = 31) or the BCp (n = 31) group. In the total cohort, the median age at repair was 9.8 months (2.4–132.8 months) and the median weight was 7.6 kg (3.4–46.0 kg). The two groups showed no statistically significant difference with respect to distribution of diagnosis, genetic anomalies, sex, age, preoperative haemoglobin concentration and LV function (Table 1). There were no differences in the ischaemic and CPB time between the groups as well as other intraoperative variables summarized in Table 2.
Table 1:
Demographic and preoperative data and comparison between groups
| Variable analysed | CCp (N = 31) | BCp (N = 31) | Overall (N = 62) | P-value |
|---|---|---|---|---|
| Male/female | 14/17 | 15/16 | 29/33 | 0.61 χ2 |
| Down syndrome, n (%) | 5 (16) | 2 (6) | 7 (11) | 0.42F |
| Age (months), median (range) | 8.5 (3.2–132.8) | 10.7 (2.4–121.6) | 9.8 (2.4–132.8) | 0.88MW |
| Age ≤1 year, n (%) | 17 (55) | 17 (55) | 34 (55) | 1.00 χ2 |
| Weight (kg), median (range) | 7.5 (3.4–46) | 7.8 (3.6–42.0) | 7.6 (3.4–46.0) | 0.93MW |
| BSA (m2), median (range) | 0.37 (0.22–1.39) | 0.4 (0.23–1.21) | 0.39 (0.22–1.39) | 0.87MW |
| Hgb (g/l), mean ± SD | 10.4 ± 1.1 | 10.5 ± 1.2 | 10.42 ± 1.15 | 0.58T |
| Hct (%), mean ± SD | 33.2 ± 3.4 | 33.7 ± 3.7 | 33.5 ± 3.5 | 0.53T |
| Preoperative FS (%), mean ± SD | 39.8 ± 5.1 | 42.5 ± 6.4 | 41.1 ± 5.9 | 0.068T |
| ASD II/sinus venosus, n (%) | 5/2 (11) | 2/2 (7) | 7/4 (18) | 0.51F |
| pAVSD, n (%) | 2 (3) | 1 (2) | 3 (5) | 1.00F |
| TAPVC cardiac type | 0 | 2 (3) | 2 (3) | 0.49F |
| Cor triatriatum | 0 | 1 (2) | 1 (2) | 1.00F |
| VSD, n (%) | 18 (29) | 19 (31) | 45 (60) | 0.79 χ2 |
| cAVSD, n (%) | 4 (6) | 4 (6) | 8 (12) | 1.00F |
BSA: body surface area; Hgb: haemoglobin; Hct: haematocrit; FS: fractional shortening; ASD: atrial septal defect; pAVSD: partial atrioventricular septal defect; TAPVC: total anomalous pulmonary venous connection; VSD: ventricular septal defect; cAVSD: complete atrioventricular septal defect; MW: Mann–Whitney U-test; χ2: Pearson's χ2 test; F: Fisher's exact test; T: Student's t-test.
Table 2:
Intraoperative variables and comparison between groups
| Variable analysed | CCp (N = 31) | BCp (N = 31) | Overall (N = 62) | P-value |
|---|---|---|---|---|
| Ischaemia (min), mean ± SD | 49.2 ± 30.3 | 53.8 ± 27.8 | 51.4 ± 28.9 | 0.53T |
| Min temperature, median (range) | 32 (32–36) | 32 (30–36) | 32 (30–36) | 0.13MW |
| Ischaemia >40 min, n (%) | 17 (55) | 22 (71) | 39 (63) | 0.29 χ2 |
| CPB (min), mean ± SD | 75.0 ± 28.4 | 79.4 ± 32.2 | 77.2 ± 34.2 | 0.62T |
| Reperfusion (min), mean ± SD | 16.1 ± 7.8 | 14.8 ± 5.4 | 15.5 ± 6.7 | 0.44T |
| Maintenance dose I, n (%) | 15 (47) | 19 (61) | 34 (55) | 0.44 χ2 |
| Maintenance dose II, n (%) | 4 (13) | 3 (10) | 7 (13) | 1.00F |
| Indexed cardioplegia volume (ml/kg), mean ± SD | 35 ± 11 | 38 ± 13 | 36 ± 12 | 0.34T |
| Electrical activity between doses, n (%) | 1 (3) | 2 (6) | 3 (5) | 1.00F |
| DC | 1 | 1 | 2 | 1.00F |
| CUF (ml/kg), median (range) | 35 (11–103) | 31 (8–97) | 34 (8–103) | 0.47MW |
| MUF, n (%) | 19 (61) | 17 (55) | 36 (58) | 0.79 χ2 |
| MUF (ml/kg), median (range) | 25 (22–30) | 24 (13–42) | 25 (13–42) | 0.10MW |
| MUF + CUF (ml/kg), median (range) | 51 (11–128) | 47 (7–138) | 49 (7–138) | 0.24MW |
CPB: cardiopulmonary bypass; CUF: conventional ultrafiltration; DC: electrical cardioversion; MUF: modified ultrafiltration; MW: Mann–Whitney U-test; χ2: Pearson's χ2 test; F: Fisher's exact test; T: Student's t-test.
Clinical outcome
There was 1 early death in the CCp group in a child with trisomy 21 who underwent complete atrioventricular septal defect repair at the age of 6 months. The patient died of cardiac failure and multiorgan dysfunction on the first postoperative day. It was the patient with the longest ischaemic time of 130 min as well as peak postoperative cTnI level above 300 ng/ml, suggestive of severe ischaemic–reperfusion injury. The entire cohort had a median duration of mechanical ventilator support of 7 h (range, 1–692 h) and a median postoperative ICU stay of 2 days (range, 1–29 days) with no observed differences between groups. There was no statistically significant difference between the two cardioplegic groups in terms of level and duration of inotropic support, major postoperative complication rate and mean postoperative haemoglobin concentration (Table 3).
Table 3:
Clinical outcomes and comparison between groups
| Variable analysed | CCp (N = 31) | BCp (N = 31) | Overall (N = 62) | P-value |
|---|---|---|---|---|
| Early mortality, n (%) | 1 (3) | 0 (0) | 1 (1.6) | 1.00F |
| Respiratory insufficiency, n (%) | 3 (10) | 3 (10) | 6 (10) | 1.00F |
| Rhythm disturbances, n (%) | 3 (10) | 5 (16) | 8 (13) | 0.71F |
| AV block II and III, temporary PM, n (%) | 3 (10) | 2 (6) | 5 (8) | 1.0F |
| Tachyarrhythmias, n (%) | 0 (0) | 3 (10) | 3 (5) | 0.24F |
| Peritoneal dialysis, n (%) | 1 (3) | 0 (0) | 1 (1.6) | 1.00F |
| Significant residual L-R shunt, n (%) | 1 (3) | 1 (3) | 2 (3) | 1.00F |
| MR ≥ + 2/4, n (%) | 2 (6) | 1 (3) | 3 (5) | 1.00F |
| TR ≥ + 2/4, n (%) | 2 (6) | 1 (3) | 3 (5) | 1.00F |
| Major bleeding, n | 0 | 0 | 0 | |
| Reoperation, n | 0 | 0 | 0 | |
| Inotropic support | ||||
| None, n (%) | 4 (13%) | 7 (23) | 11 (18) | 0.51F |
| Minimal, n (%) | 21 (68) | 23 (74) | 44 (71) | 0.78 χ2 |
| Significant, n (%) | 6 (19) | 1 (3) | 7 (11) | 0.10F |
| Significant inotropic support (h), median (range) | 16 (1–44) | 21 | 21 (1–44) | |
| FS1 (%), mean ± SD | 37.5 ± 9.0 | 35.7 ± 7.5 | 36.6 ± 8.3 | 0.40T |
| FS2 (%), mean ± SD | 40.6 ± 7.9 | 39.3 ± 6.0 | 40.0 ± 7.0 | 0.44T |
| Hgb (g/l), mean ± SD (24 h) | 11.4 ± 0.83 | 11.19 ± 0.91 | 11.28 ± 0.87 | 0.45T |
| MV support (h), median (range) | 7 (1–121) | 7 (1–692) | 7 (1–692) | 0.73MW |
| ICU stay (days), median (range) | 2 (1–16) | 2 (1–29) | 2 (1–29) | 0.12MW |
| Hospitalization (days), median (range) | 7 (1–24) | 8 (6–40) | 7 (1–40) | 0.15MW |
AV: atrioventricular; PM: pacemaker; L-D: left to right; MR: mitral regurgitation; TR: tricuspid regurgitation; FS1: fractional shortening within 1 h after surgery; FS2: fractional shortening on the first postoperative morning; Hgb: haemoglobin; MV: mechanical ventilation; ICU: intensive care unit; MW: Mann–Whitney U-test; χ2: Pearson's χ2 test; F: Fisher's exact test.
Functional myocardial recovery
Although the trend was for the LV function to be more impaired in the BCp group, no significant differences in the FS between groups were detected following surgery as well as on the first postoperative morning (Fig. 1).
Figure 1:
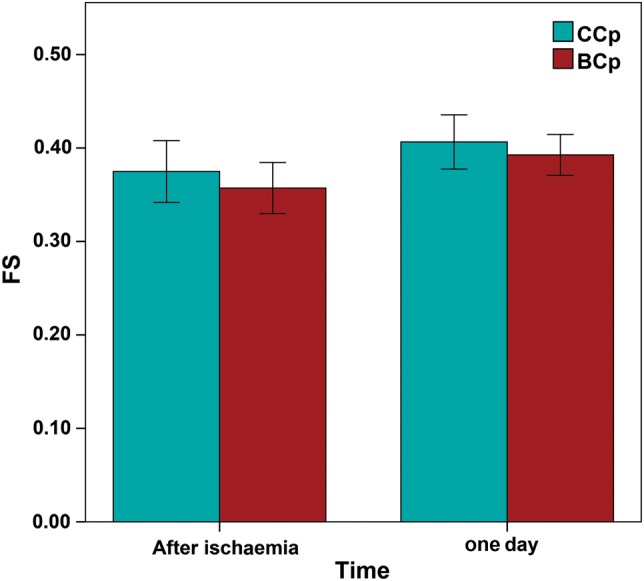
FS between the two Cp groups measured within 1 h after surgery and on the first postoperative day. FS: fractional shortening; Cp: cardioplegia.
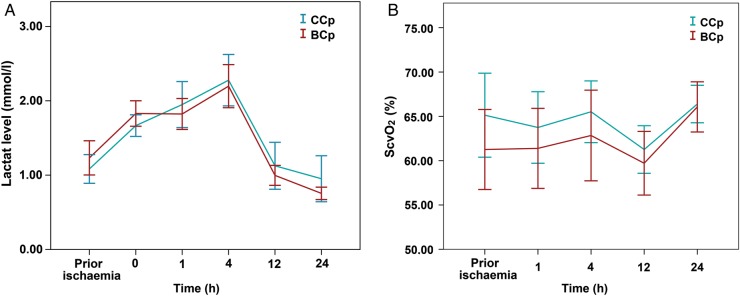
Fig. 2A depicted a significant time effect on lactate level in both groups (F = 43.206, P < 0.001). However, the time-dependent postoperative lactate level was similar between the two Cp groups (F = 1.430, P = 0.228) with no difference at any time point. The difference increased, but still remained insignificant after controlling for effects of age and cross-clamp time as covariates (F = 2.240, P = 0.063).
Figure 2:
Lactate level (A) and central venous saturation ScvO2 (B) for each of the two cardioplegia groups during the first 24 h after ischaemia. Data represent the mean values and error bars denote standard errors. ScvO2: central venous saturation.
Repeated-measures ANOVA showed that the ScvO2 was comparable between the two Cp groups during the first 24 h after ischaemia (F = 0.327, P = 0.895), and no significant differences were detected at any time point (Fig. 2B). Furthermore, there was no statistically significant difference in haemoglobin levels between the two study arms (F = 1.685, P = 0.173).
Ischaemic myocardial injury
Repeated-measures ANOVA showed that only the main effect of time was significant (P < 0.001), with no difference in cTnI levels between the two cardioplegic techniques (F = 0.769, P = 0.576) (Fig. 3). After adjustment for ischaemic time and diagnosis, two-way ANOVA showed that the difference in the cTn level between the Cp groups remained non-significant (F = 0.971, P = 0.444 and F = 0.881, P = 0.415, respectively). Nineteen of 62 (31%) patients had a maximum postoperative cTnI level above 35 ng/ml, with no difference in patients' distribution between the Cp groups (BCp: 10 vs CCp: 9, χ2, P = 0.783). Two patients had cTnI concentration above 100 ng/ml, of whom 1 died.
Figure 3:
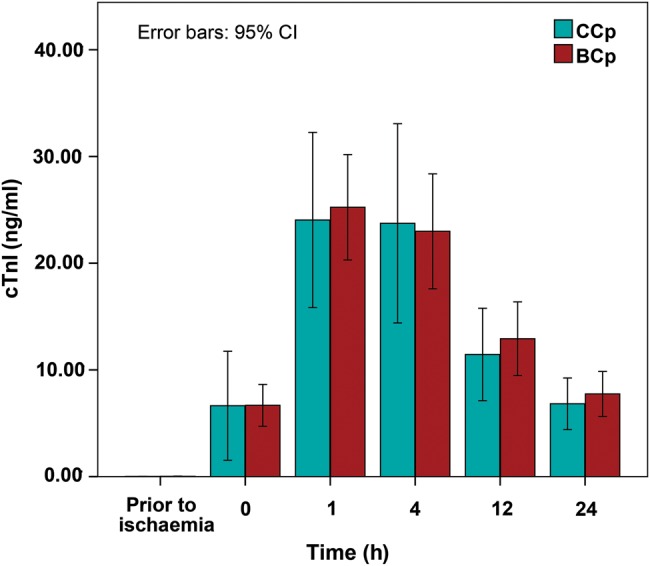
Cardiac Troponin I for each of the two cardioplegia groups during the first 24 h after ischaemia. Data represent the mean values and error bars denote standard errors. cTnI: cardiac Troponin I.
Glucose level
Serum glucose declined immediately after cross-clamp release and thereafter decreased (Fig. 4), remaining significantly higher than preoperative values 24 h postoperatively (T-test, P < 0.001) in both Cp groups. Although the glucose level was significantly higher in the CCp group only at the beginning of reperfusion (T-test, P = 0.02), repeated-measures ANOVA showed that the overall effect of the cardioplegic method on glucose levels during the first 24 postoperative hours reached statistical significance (F = 2.820, P = 0.024).
Figure 4:
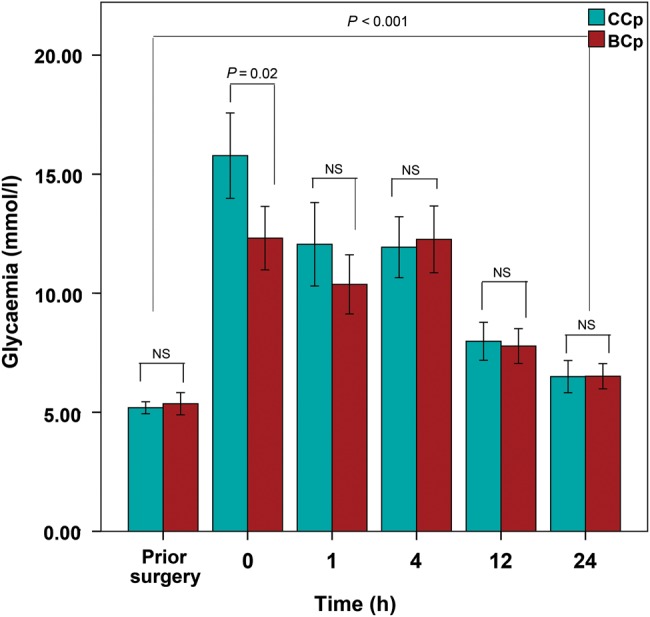
Serum glucose (mmol/l) for each of the two cardioplegia groups during the first 24 h after ischaemia. Data represent the mean values and error bars denote standard errors.
DISCUSSION
This study compares two cardioplegic techniques using standard clinical outcomes, postoperative LV function assessment by measuring FS, lactate level and ScvO2, and degree of myocardial ischaemic–reperfusion injury by measuring cTnI release. The changes in serum glucose were investigated particularly due to high glucose content of the custom Cp solution used in our practice.
In our study, neither cardioplegic solution appeared to have an effect on early mortality and incidence of major postoperative complications. These findings appear comparable with other studies that have investigated the effects of crystalloid and blood Cp in patients with non-cyanotic cardiac defects of similar complexity [8, 9]. Furthermore, our study failed to demonstrate any advantage of blood Cp in terms of lower inotropic support, shorter time to extubation and ICU stay. These findings are consistent with the works of other authors who have used these variables as clinical surrogates of myocardial protective efficacy [10, 11]. On the other hand, Caputo et al. [9] have showed that patients with ventricular septal defect (VSD) who received crystalloid Cp more frequently required significant and prolonged inotropic support. However, in the same study, the authors acknowledged that they did not detect any other significant differences in other clinical outcomes.
In our study, the postoperative ventricular function, as assessed by FS, arterial lactate level and ScvO2, showed no statistically significant difference between the two Cp groups. These conclusions are in keeping with the works of other authors who have demonstrated no significant difference in myocardial functional recovery in children between the two Cp strategies [10]. Furthermore, the largest randomized controlled study in an adult population did not find significant differences in the LV ejection fraction after CABG [12]. On the other hand, Amark et al. [8] in their study found that blood Cp was associated with improved LV function in children after complete AVSD repair. However, instead of quantitative assessment used in our study, they used qualitative echocardiographic methods for the assessment of LV function according to a five-grade scale.
Modi et al. [13] have investigated the effect of three different cardioplegic techniques on lactate level (CCp, BCp, BCp + terminal hot shot). In the acyanotic group of patients who received CCp, the lactate level increased, whereas in patients who received BCp, the lactate level decreased without statistical significance. This finding correlates with lactate level changes in our study where the arterial lactate 1 h after ischaemia increased in the CCp group and slightly decreased in the BCp group. However, Caputo et al. [9] in their study have found a significantly higher lactate level in the CCp group within 2 h after ischaemia. An additional factor specific to our study which might contribute to a more significant increase in lactate level immediately after ischaemia in the CCp group is high glucose concentration (277 mmol/l) of the crystalloid Cp solution. Owen et al. [6] in their experimental study of isolated rat heart has showed that supraphysiological glucose concentrations (>50 mmol/l) inhibit glycogenolysis, thus decreasing total glycolysis and related ATP production during cardiac arrest, which might result in higher lactate release upon reperfusion.
We measured the ScvO2 as an equivalent to mixed venous saturation, which has been shown to be a valuable surrogate of cardiac index [14]. In our study, the lowest mean ScvO2 values were recorded 4 h after ischaemia with no significant difference between the Cp groups at each time point. Sinha et al. [15] also have shown the most severe drop in cardiac index between 4 and 12 h and no significant difference between the two cardioplegic groups in infants. However, in the same study, they have shown significantly higher cardiac index among neonatal patients who received BCp.
In summary, each of the three variables that correlate with myocardial functional recovery (FS, lactate level and ScvO2) were affected by the type of Cp in our study. Differences in mean values at particular time points might be explained by uneven starting values measured before ischaemia. Non-significant intergroup trend differences in our study most likely have been a result of different confounders, where the age at surgery and ischaemic time have been considered as the most powerful. However, we could not demonstrate significant differences between Cp groups in any of those variables after controlling for effects of age and cross-clamp time as covariates.
Plasma concentration of cTnI has already been confirmed as a specific and sensitive marker of myocardial injury [16]. We did not find any significant differences between the CBp and CCp groups in terms of postoperative cTnI concentration. Modi et al. [13] in their study also have shown no differences in the postoperative cTnI level between three cardioplegic techniques in the acyanotic subgroup of patients. On the other hand, Caputo et al. [9] showed 43% less mean postoperative cTnI values in the blood cardioplegic group who underwent VSD repair compared with the CCp group. In our study, we showed strong positive correlation between ischaemic time and overall mean cTnI level. This finding correlates with other studies that have investigated the relationship between ischaemic time and postoperative cTnI values. Also, there was a strong positive correlation between postoperative cTnI release and early clinical outcomes in our study. Imura et al. [17] in their study have shown strong correlation between peak cTnI values 48 h postoperatively and duration of inotropic support, intubation and ICU stay time. Furthermore, the peak postoperative cTnI value above 35 ng/ml has been confirmed as a strong predictive factor of early mortality and morbidity [18]. This is in agreement with our study in which patients with cTnI values above 35 ng/ml experienced both longer ventilation support and ICU stay time.
Different studies have shown up to 90% incidence of hyperglycaemia (>7 mmol/l) after cardiac surgery procedure in infants and children [19]. Apart from the cardiac surgery procedure per se, the significant increase in serum glucose immediately after cross-clamp release in our patients additionally might be explained by high glucose concentration of both the BCp (∼60 mmol/l) and CCp solutions (277 mmol/l). Nevertheless, we have applied the protocol of standard postoperative glycaemic control to all our patients irrespective of the severity of postoperative glycaemia according to results of the randomized study from Boston Childrens' Hospital [7]. The same study has shown that ‘tight’ control does not decrease the incidence of postoperative infection, mortality, morbidity and ICU stay, but increases the chance of severe hypoglycaemia. We also were not able to demonstrate a higher morbidity rate in the CCp subgroup. Although our primary objective did not to investigate the effect of the high glucose content Cp solution on myocardial protection, this appeared as the most important reason for reconsidering our intraoperative cardioprotection policy. It is a well-known fact that a primary substrate for normal myocardium is free fatty acids; however, glucose appears to be a dominant myocardial substrate in the immature heart and during periods of myocardial ischaemia [20]. It has been suggested that the greater capacity for glucose metabolism and, in particular, anaerobic glycolysis confers a greater resistance to ischaemic injury on the immature myocardium [5]. Conversely, others have suggested that the greater the anaerobic glycolytic activity, the greater will be the accumulation of lactate during ischaemia, and the more rapid will be the development of tissue acidosis and the onset of irreversible damage [6]. Roe's Cp is one of the few crystalloid solutions that have been used in clinical practice with similar high concentration of glucose (278 mmol/l) as used in our study (Supplementary Material). Mei et al. [21] in their study on isolated rabbit hearts have shown that Roe's solution provides significantly less satisfactory protection compared with St. Thomas 1 and Tyer's solutions, and this may be attributed, in part, to high glucose concentration. These results are in line with those of Wilson et al. [22], who found that the Roe's solution did not confer good protection on dogs undergoing CPB, as indicated by poor functional recovery. However, it is important to address that Roe's cardioplegic solution represents an intracellular type of Cp with low natrium and zero calcium content which might affect protection capacity as well.
Reports that have investigated optimal glucose concentration of cardioplegic solutions have been contradictory and the most of them were published a few decades ago. However, most of them have shown that low glucose concentration (in the range between 7 and 11 mmol/l) might be beneficial for decreasing infarct size, increasing ATP and creatinine phosphate levels, and improving ventricular function [23]. Moreover, results of several experimental studies have suggested that high glucose concentration of the cardioplegic solution attenuates myocardial protection of Cp or even might have a deleterious effect [6, 24].
In addition to these, the results of our study, which have shown a severe postoperative hyperglycaemia in the first 4 h irrespective of the Cp method, higher arterial blood lactate level immediately after aortic de-clamping in the CCp group and absence of expected beneficial effect of blood Cp, resulted in abolishing the use of high glucose Cp solution in our practice either as solely crystalloid or as a component of blood Cp, which was replaced by a Buckberg-type custom-made blood Cp solution due to unavailability of commercial Cp solutions at the time of completion of the study.
LIMITATIONS
There is considerable variability in terms of age at surgery and ischaemic time which might contribute to myocardial resistance to ischaemic–reperfusion injury. Ischaemic time was rather short and in most patients did not exceed 60 min. The FS could be profoundly affected by volume load of the heart, paradoxical septal movement and the size of the VSD patch. ScvO2 correlates with cardiac index and cardiac output after a rough approximation and neglect of numerous variables that affect the both oxygen delivery (e.g. haemoglobin level and arterial blood oxygen saturation) and oxygen consumption. To the best of our knowledge, there has been no clinical study in children or adults comparing different cardioplegic strategies using cardioplegic solution with a high glucose concentration (277 mmol/l) without insulin. This study was not designed to investigate the effect of high glucose content Cp on myocardial protection or the potential negative effect of high postoperative hyperglycaemia on early clinical outcomes. Using the cTnI concentration as a primary endpoint, the sample size of our study provided 65% power for between-group comparison, which might be considered as the major limitation of the study.
CONCLUSION
In summary, blood Cp does not show any advantage compared with crystalloid Cp in terms of clinical outcomes, functional recovery and the degree of ischaemic injury in acyanotic patients. One might expect the benefits of BCp to be more evident in patients undergoing more complex procedures with longer cross-clamp times and preoperative cyanosis. A high glucose concentration in the cardioplegic solution might aggravate ischaemia–reperfusion injury and attenuate the potential beneficial effect of blood Cp.
SUPPLEMENTARY MATERIAL
Funding
This work was supported by The International Organisation for Migration (IOM).
Conflict of interest: none declared.
Supplementary Material
ACKNOWLEDGEMENTS
We thank J. Mitrovic for expert biochemical analysis assistance, perfusionists D. Vukadinovic and M. Pajic for their technical assistance, and the staff of the cardiac theatre, paediatric intensive care unit and biochemistry laboratory of the University Children's Hospital, Belgrade.
REFERENCES
- 1.Bull C, Cooper J, Stark J. Cardioplegic protection of the childs heart. J Thorac Cardiovasc Surg 1984;88:287–93. [PubMed] [Google Scholar]
- 2.Kotani Y, Tweddell J, Gruber P, Pizarro C, Austin EH, Woods RK et al. Current cardioplegia practice in pediatric cardiac surgery: a North American multiinstitutional survey. Ann Thorac Surg 2013;96:923–9. [DOI] [PubMed] [Google Scholar]
- 3.Barner HB. Blood cardioplegia: a review and comparison with crystalloid cardioplegia. Ann Thorac Surg 1991;52:1354–67. [DOI] [PubMed] [Google Scholar]
- 4.Raja SG. Is blood cardioplegia superior to crystalloid cardioplegia in pediatric cardiac surgery? Interact CardioVasc Thorac Surg 2008;7:498–9. [DOI] [PubMed] [Google Scholar]
- 5.Doenst T, Schlensak C, Beyersdorf F. Cardioplegia in pediatric cardiac surgery: do we believe in magic? Ann Thorac Surg 2003;75:1668–77. [DOI] [PubMed] [Google Scholar]
- 6.Owen O, du Toit EF, Opie LH. The optimal glucose concentration for intermittent cardioplegia in isolated rat heart when added to St. Thomas Hospital cardioplegic solution. J Thorac Cardiovasc Surg 1993;105:995–1006. [PubMed] [Google Scholar]
- 7.Agus M, Steil G, Wypij D, Costello J, Laussen P, Langer M et al. Tight glycemic control versus standard care after pediatric cardiac surgery. N Engl J Med 2012;37:1208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amark K, Berggren H, Bjork K, Ekroth A, Ekroth R, Nilsson K et al. Blood cardioplegia provides superior protection in infant cardiac surgery. Ann Thorac Surg 2005;80:989–94. [DOI] [PubMed] [Google Scholar]
- 9.Caputo M, Modi P, Imura H, Pawade A, Parry AJ, Suleiman MS et al. Cold blood versus cold crystalloid cardioplegia for repair of ventricular septal defect in pediatric cardiac surgery: a randomized controlled trial. Ann Thorac Surg 2002;74:530–4. [DOI] [PubMed] [Google Scholar]
- 10.Young JN, Choy IO, Silva NK, Obayashi DY, Barkan HE. Antegrade cold blood cardioplegia is not demonstrably advantageous over cold crystalloid cardioplegia in surgery for congenital heart disease. J Thorac Cardiovasc Surg 1997;114:1002–8. [DOI] [PubMed] [Google Scholar]
- 11.Imura H, Caputo M, Parry A, Pawade A, Angelini GD, Suleiman MS. Age-dependent and hypoxia related differences in myocardial protection during pediatric open heart surgery. Circulation 2001;103:1551–5. [DOI] [PubMed] [Google Scholar]
- 12.Ovrum E, Tangen G, Tollosfrud S, Oystese R, Ringdal MA, Istad R. Cold blood cardioplegia versus cold crystalloid cardioplegia: a prospective randomized study of 1440 patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 2004;128:860–5. [DOI] [PubMed] [Google Scholar]
- 13.Modi P, Suleiman MS, Reeves B, Pawade A, Parry AJ, Angelini GD et al. Myocardial metabolic changes during pediatric cardiac surgery: a randomized study of 3 cardioplegic technique. J Thorac Cardiovasc Surg 2004;128:67–75. [DOI] [PubMed] [Google Scholar]
- 14.Walley KR. Use of central venous oxygen saturation to guide therapy. Am J Respir Crit Care Med 2011;184:514–20. [DOI] [PubMed] [Google Scholar]
- 15.Sinha P, Zurakowski D, Jonas R. Comparison of two cardioplegia solution using thermodilution cardiac output in neonates and infants. Ann Thorac Surg 2008;86:1613–9. [DOI] [PubMed] [Google Scholar]
- 16.The Joint European Society of Cardiology/American College of Cardiology Committee. Myocardial infarction redefined: a consensus document of the Join European Society/American College of Cardiology for the redefinition of myocardial infarction. Eur Heart J 2000;21:1502–13. [DOI] [PubMed] [Google Scholar]
- 17.Imura H, Modi P, Pawade A, Parry AJ, Suleiman MS, Angelini GD et al. Cardiac troponin I in neonates undergoing the arterial switch operation. Ann Thorac Surg 2002;74:1998–2002. [DOI] [PubMed] [Google Scholar]
- 18.Bottio T, Vida V, Padalino M, Gerosa G, Stellin G. Early and long-term prognostic value of Troponin-I after cardiac surgery in newborns and children. Eur J Cardiothorac Surg 2006;30:250–5. [DOI] [PubMed] [Google Scholar]
- 19.Polito A, Thiagarajan RR, Laussen PC, Gauvreau K, Agus MS, Scheurer MA et al. Association between intraoperative and early postoperative glucose levels and adverse outcomes after complex congenital heart surgery. Circulation 2008;118:2235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopaschuk GD, Spafford MA, Marsh DR. Glycolysis is predominant source of ATP production immediately after birth. Am J Physiol 1991;261:H1698–705. [DOI] [PubMed] [Google Scholar]
- 21.Mei Y, Liu H, Long C, Cheng B, Gao S, Hu D. Effect of four crystalloid cardioplegias on immature rabbit hearts during global ischaemia. Asian J Surg 2006;29:79–85. [DOI] [PubMed] [Google Scholar]
- 22.Wilson GJ, Axford-Gatley RA, Bush BG, Romaschin AD, Mickle DA. European versus North American cardioplegia: comparison of Bretschneider's and Roe's cardioplegic solutions in a canine model of cardiopulmonary bypass. Thorac Cardiovasc Surg 1990;38:10–4. [DOI] [PubMed] [Google Scholar]
- 23.Van Oppell UO, Du Toit EF, King LM, Owen P, Dunne T, Reichart B et al. St. Thomas’ Hospital cardioplegic solution: beneficial effect of glucose and multidose reinfusions of cardioplegic solution. J Thorac Cardiovasc Surg 1991;102:405–12. [PubMed] [Google Scholar]
- 24.Hearse DL, Stewart DA, Braimbridge MV. Myocardial protection during ischemic cardiac arrest: possible deleterious effects of glucose and mannitol in coronary infusates. J Thorac Cardiovasc Surg 1978;76:16–23. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.