Abstract
OBJECTIVES
One of the final treatments for end-stage heart failure is heart transplantation. However, a shortage of donor hearts has created a long waiting list and limited benefits. Our ultimate goal is to create a whole beating heart fabricated on an organ scaffold for human heart transplantation. Here, we successfully performed the first transplantation using a decellularized whole porcine heart with mesenchymal stem cells.
METHODS
A porcine heart was harvested following cardiac arrest induced by a high-potassium solution and stored at −80°C for 24 h. The porcine heart was completely decellularized with 1% sodium dodecyl sulphate and 1% Triton X-100 under the control of perfusion pressure (100 mmHg) and maintained at 37°C. A decellularized whole-heart scaffold was sterilized with gamma irradiation. Cultured mesenchymal stem cells were collected and either infused into the ascending aorta or injected directly into the left ventricular wall. Finally, recellularized whole-heart scaffolds were transplanted into pigs under systemic anticoagulation treatment with heparin. Coronary artery angiography of the transplanted heart graft was performed.
RESULTS
In our decellularization method, all cellular components were removed, preserving the heart extracellular matrix. Heterotopic transplantations were successfully performed using a decellularized heart and a recellularized heart. The scaffolds were well perfused, without bleeding from the surface or anastomosis site. Coronary angiography revealed a patent coronary artery in both scaffolds. The transplanted decellularized heart was harvested on Day 3. Haematoxylin and eosin staining showed thrombosis in the coronary arteries and migrated inflammatory cells. Haematoxylin and eosin staining of the transplanted recellularized heart showed similar findings, with the exception of injected mesenchymal stem cells.
CONCLUSIONS
To the best of our knowledge, this is the first report of heterotopic transplantation of a decellularized whole porcine heart with mesenchymal stem cells. The scaffolds endured surgical procedures. We detected short-term coronary artery perfusion in the transplanted scaffolds by angiography. Future studies should analyse the histological features of transplanted decellularized scaffolds and optimize the system for recellularization to apply this unique technology clinically.
Keywords: Organ engineering, Cell transplantation, Regenerative therapy, Decellularization, Heterotopic transplantation, Coronary artery angiography
INTRODUCTION
Heart failure affects ∼1–2% of the adult population, with a prevalence rate of 10% among persons 70 years of age [1]. The 1-year mortality rate for patients in end-stage heart failure exceeds 50% [2]. At present, heart transplantation and left ventricular assist device (VAD) implantation are final treatments for end-stage heart failure. However, the shortage of donor hearts has resulted in a long waiting list and limited the benefit of transplantation. The third-generation centrifugal VAD has achieved a good survival rate. However, reducing VAD-specific complications such as thrombosis, right heart failure and infection is necessary to improve long-term outcomes [3]. For this devastating situation, alternative treatments have been sought, including direct cell injection therapy, stem cell patches and three-dimensional printing of tissues and whole organs [4–6]. A bioengineered heart using a tissue decellularization technique has also been reported. The decellularization technique, which removes all cellular components and preserves the three-dimensional ultrastructure of the extracellular matrix, has been attempted for many organs [7]. Since Ott et al. [8] reported perfusion decellularization of the whole rat heart, the development of bioengineered hearts with decellularization has evolved. Our ultimate goal is to create a ‘whole transplantable heart for end-stage heart failure’ with the decellularization technique. Recently, many authors have described whole porcine heart decellularization [9–11]. Now, the investigation of an optimized recellularization technique for decellularized hearts is underway as a preliminary study for human clinical care [12, 13]. In this study, we performed porcine whole-heart decellularization, preserving the intact extracellular matrix and complex vascular network, with a simple system in a short amount of time. We believe that the scale of the porcine model could be clinically relevant for human treatment. To create the decellularized heart, we modified the detergent perfusion–decellularization method previously reported by Ott et al. [8]. Then, to confirm the integrity of the decellularized heart to endure surgical procedures, we transplanted it heterotopically into another pig with anastomosis to the abdominal aorta and inferior vena cava. A preserved vascular network was verified by coronary artery angiography in the transplanted heart. Moreover, we attempted cell seeding of the decellularized heart with a multistep infusion method and a muscular injection method, followed by heterotopic transplantation and coronary angiography.
MATERIALS AND METHODS
Animals
All experimental procedures and protocols were reviewed and approved by the Animal Ethics Committee of the Keio University School of Medicine. Animals were treated according to the guidelines of the Ministry of Education, Culture, Sports, Science and Technology, Japan. Specific pathogen-free female pigs weighing 15–20 kg were used for heart harvesting to prepare decellularized matrices (n = 9) and to undergo heterotopic transplantation (n = 3). The pigs were housed for 7 weeks under appropriate living conditions before either tissue harvesting or heterotopic heart transplantation. Pigs undergoing recellularized heart transplantation were administered 100 mg prednisone (Shionogi, Osaka, Japan) and 0.24 mg/kg tacrolimus (Santen Pharmaceutical Co. Ltd, Osaka, Japan) daily beginning 1 day before transplantation to 5 days after transplantation.
Whole-heart harvesting
Pigs were anaesthetized with 0.2 mg/kg midazolam (Astellas, Tokyo, Japan) and 0.08 mg/kg medetomidine (Zenoaq, Fukushima, Japan) intramuscularly. Intravenous access was secured via a superficial ear vein. Midazolam (0.2 mg/kg) was administered intravenously, followed by intubation with a 6-mm endotracheal tube and mechanical ventilation with 20% FiO2. Isoflurane inhalation to maintain anaesthesia during the procedure was connected to a standard respiratory system. Muscle relaxation was achieved with vecuronium bromide (4 mg) (MSD, NJ, USA). Median sternotomy was performed to expose the heart. The pericardium between the ascending aorta and pulmonary artery and around the inferior vena cava and the superior vena cava was dissected. After clamping the ascending aorta with a vascular clamp, a cardioplegic solution (300 ml normal saline, 40 mEq KCl, 5000 IU heparin sodium) was infused into the coronary arteries through the ascending aorta with a 21-gauge needle. The superior vena cava, inferior vena cava, pulmonary vein and pulmonary artery were transected to evacuate blood and decompress the heart. After transecting the aorta distal to the vascular clamp, we excised the heart from the thoracic cavity.
Decellularization of the porcine heart
Excised hearts were frozen at −80°C for a minimum of 24 h prior to decellularization. After thawing the frozen heart at 4°C, a 6- to 10-mm connector was cannulated to the ascending aorta just distal to the brachiocephalic artery and tied with 1-0 silk to connect a perfusion circuit. A silicone tube for decompressing the heart was inserted through the pulmonary vein into the left ventricle during decellularization. The heart was washed with deionized water for 15 min at 100 ml/min. We perfused the heart at a constant perfusion pressure of 100 mmHg with a warm (37°C) solution of 1% sodium dodecyl sulphate (SDS; Sigma, St Louis, MO, USA) in deionized water at 200–1000 ml/min for 9 h, and we intermittently washed the heart with deionized water for 15 min every 3 h to remove any residual substances. We then perfused 1% Triton X-100 (Sigma) in deionized water at 1000 ml/min for 3 h, followed by deionized water for 1 h to remove any residual detergent and cell debris. Whole-heart decellularization was achieved by a freezing–thawing induction of cellular lysis followed by aortic perfusion with 1% SDS and 1% Triton X-100, which are ionic and anionic detergents that can simultaneously lyse cells and solubilize cytoplasmic components.
Histological analyses
Normal hearts, decellularized hearts, transplanted decellularized hearts and transplanted recellularized hearts were fixed in 4% paraformaldehyde, embedded in paraffin and sectioned into 4-μm sections. After rehydration, sections were stained with haematoxylin and eosin (H&E). To determine cellularity, 4′,6-diamidino-2-phynylindole (DAPI) was used. Moreover, paraffin-embedded sections from the decellularized scaffold and the normal heart were rehydrated and antigen retrieval was performed. The samples were permeabilized and incubated with mouse monoclonal anti-collagen type I, rabbit polyclonal anti-collagen type IV, rabbit polyclonal anti-laminin and mouse monoclonal anti-troponin T (Abcam, Cambridge, MA, USA). Secondary antibodies were goat anti-mouse IgG (Invitrogen, Tokyo Japan) and goat anti-rabbit IgG (Abcam). After all procedures, we counterstained specimens with DAPI.
Heterotopic transplantation of the decellularized scaffold
After decellularization, the scaffold was sterilized with gamma irradiation and stored at 4°C for further transplantation. To simplify the transplantable scaffold model, we chose the ascending aorta and the superior vena cava as an inlet and outlet of the blood stream, respectively. The inferior vena cava, pulmonary veins and pulmonary artery were closed with continuous 6-0 polypropylene sutures. However, the pathway of the blood stream was not physiologically normal in this model. The model might not be suitable for clinical application, but only in the experiment. The recipient pig was anaesthetized as described above. Following a midline incision, laparotomy was performed, and the abdominal aorta and inferior vena cava were dissected. A 16-gauge catheter was inserted into the left renal vein to infuse heparin and for later blood sampling. Heparin sodium (5000 IU) was administered and added appropriately to maintain an activated clotting time (ACT) >400 s. The decellularized scaffold was transplanted by surgical anastomosis using interposition artificial grafts; the ascending aorta was anastomosed to the abdominal aorta of the recipient pig, and the superior vena cava was anastomosed to the inferior vena cava of the recipient pig with continuous 6-0 polypropylene sutures. A 14-gauge catheter was inserted into the aortic graft for coronary angiography. The recipient pig received continued anticoagulation therapy (sodium heparin, 8000–12 000 IU/kg/day) to maintain an ACT of >200 s postoperatively. At Day 3, the transplanted scaffold was excised. Coronary artery angiography was performed with iopamidol 300 mg/ml (KONICA MINOLTA, Inc., Tokyo, Japan; diluted 1 : 4 with normal saline) on Days 0 and 3. Digital subtraction angiography was performed using a mobile C-arm imaging system (Arcadis Avantic, Siemens, Berlin, Germany).
Recellularization of the decellularized scaffold
We chose porcine mesenchymal stem cells (pMSCs), which were verified to provide a beneficial effect on cardiomyocytes, improving metabolism, synthesis, angiogenesis and cell proliferation [14]. We hypothesized that pMSCs would be strong candidates as supportive cells in transplantable whole-organ engineered grafts. pMSCs kindly provided by Dr Teratani (Jichii Medical University, Tochigi, Japan) were used for recellularization. The multipotent capacity of the cells has been analysed previously [15]. The cells were incubated at 37°C in 5% CO2 from the 15th passage. We used two methods of cell seeding for the decellularized heart. The first was the coronary perfusion method, in which the cell source was perfused into the coronary arteries through the ascending aorta. The decellularized scaffold was transferred to a customized large-size organ culture chamber. The cells were seeded in an intra-aortic multistep infusion method, as previously described by Yagi et al. [16]. The perfusion incubation system was placed into an incubator with the temperature controlled at 37°C and with 5% CO2. The method we applied for the second cell seeding was direct injection into the parenchymal space. The cells were injected by using 27-gauge needles with 1-mm syringes containing 200 μl of PBS in each [17]. For either method, a total of 1.5 × 107 pMSCs were used. To calculate engraftment efficiency, the perfusate was collected, and the viability of cells not retained in the heart was determined by trypan blue exclusion.
Heterotopic transplantation of the recellularized scaffolds
The recellularized scaffolds were transplanted using the same technique we used for the decellularized heart. Coronary angiography was performed. The scaffolds were excised and analysed histologically.
RESULTS
Whole-heart decellularization
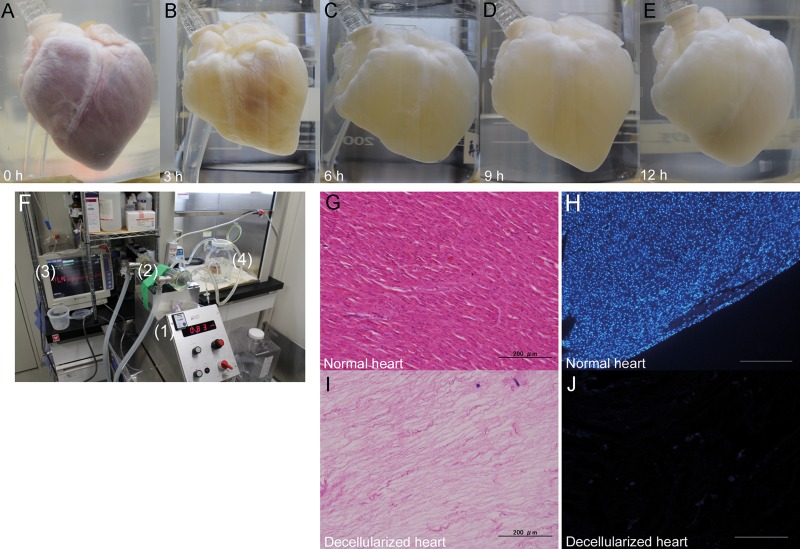
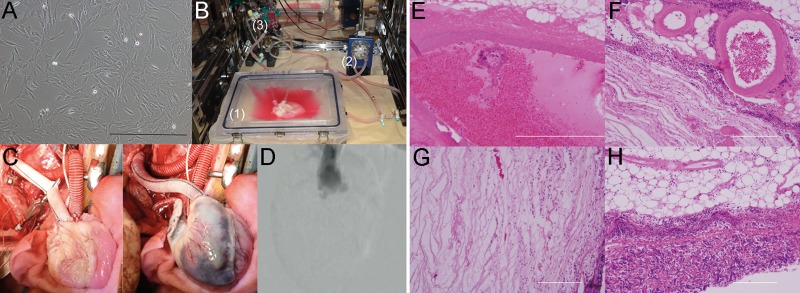
The decellularization protocol was based on the porcine liver decellularization protocol previously reported by Yagi et al. [16], and the procedure using rats reported by Ott et al. [8]. Figure 1A–E shows macroscopic images of the heart prior to decellularization and after various steps in the decellularization process. The decellularized scaffolds were translucent while preserving the three-dimensional architectures, including the coronary arteries and their branches. Our perfusion decellularization system is simple and easy to make, consisting of a silicone tube, peristaltic roller pump, heat exchanger and pressure monitor (Fig. 1F). In total, the process of heart decellularization took 13 h. H&E staining of the decellularized heart showed an intact extracellular matrix, but no visible nucleus when compared with the normal heart (Fig. 1G and I). There was no DAPI staining nucleus in the decellularized scaffold (Fig. 1H and J).
Figure 1:
Decellularization of the whole porcine heart. (A–E) Representative macroscopic images of a porcine heart during the decellularization process at 0, 3, 6, 9 and 12 h. (F) Representative photograph of the perfusion–decellularization system (1: roller pump, 2: heat exchanger, 3: pressure monitor, 4: heart chamber). (G–J) Haematoxylin and eosin staining of the normal heart (G) and decellularized heart (I). 4′,6-Diamidino-2-phynylindole staining of a normal heart (H) and a decellularized heart (J). Scale bar: 200 μm.
Immunohistochemical analysis of the extracellular matrix
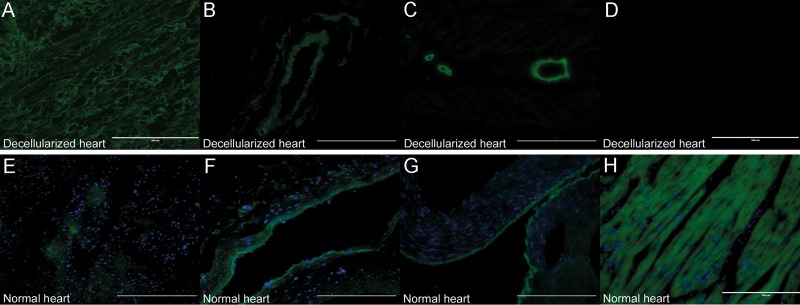
Immunohistochemical analysis of the decellularized scaffold revealed that collagen types I and IV and laminin (Fig. 2A–C) were well retained, indicating a structural composition of the matrix similar to that of the intact porcine heart (Fig. 2E–G), while cardiac troponin T had completely disappeared compared with that in the normal porcine heart (Fig. 2D and H). Importantly, these findings suggested complete removal of cellular components while maintaining the extracellular matrix composition.
Figure 2:
Immunohistochemical analysis of the extracellular matrix and cardiac troponin T. (A–D) Immunohistochemical staining of a decellularized porcine heart for collagen type I (A), collagen type IV (B), laminin (C) and cardiac troponin T (D). (E–H) Immunohistochemical staining of a normal porcine heart for collagen type I (E), collagen type IV (F), laminin (G) and cardiac troponin T (H). Scale bar: 200 μm.
Heterotopic transplantation of the decellularized scaffold
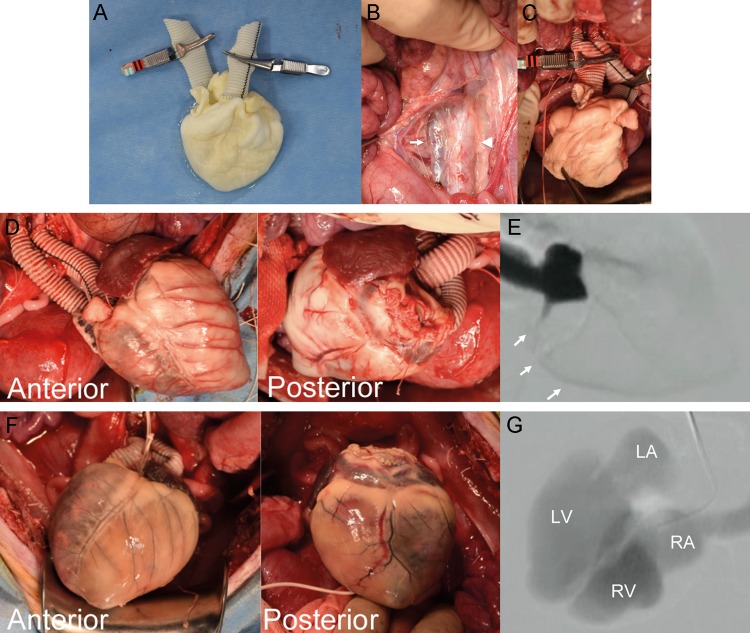
Heterotopic transplantation of the decellularized scaffold to the recipient's abdominal aorta and inferior vena cava was successfully performed. Woven polyethylene terephthalate grafts were anastomosed to the ascending aorta and superior vena cava preoperatively (Fig. 3A). The grafts were anastomosed to the recipient's abdominal aorta and inferior vena cava intraoperatively (Fig. 3B). No blood leakage from the decellularized heart surface was detected (Fig. 3C and D). There was no blood leakage at the closure sites of the inferior vena cava, pulmonary veins and pulmonary artery. Macroscopic findings demonstrated that blood-perfused coronary arteries and their branch vessels retained their luminal diameter and shape. The integrity of the scaffold was sufficient to endure surgical procedures and blood pressure. Coronary artery angiography revealed the total length of the right coronary artery during the systolic phase (Fig. 3E). An orifice of the left coronary artery, with the exception of a distal lesion, and an aortic valve regurgitation jet were observed. On Day 3, the transplanted heart scaffold was harvested. Macroscopically, the coronary arteries were occluded by thrombus formation (Fig. 3F). Coronary artery angiography did not reveal coronary artery perfusion. However, contrast medium went through the cardiac chambers, including the left ventricle, left atrium, right atrium and the superior vena cava (Fig. 3G).
Figure 3:
Heterotopic transplantation of a decellularized heart. (A) Representative photograph of a decellularized heart with anastomosed artificial grafts. (B) Intraoperative findings. White arrow: inferior vena cava. Arrowhead: ascending aorta. (C and D) The decellularized heart before (C) and after (D) reperfusion. (E) Digital subtraction angiography of the coronary artery. White arrows: right coronary artery. (F) The transplanted heart on the seventh postoperative day. (G) Digital subtraction angiography of the decellularized heart on the seventh postoperative day. LA: left atrium; LV: left ventricle; RA: right atrium; RV: right ventricle.
Histological analysis of the transplanted decellularized scaffold
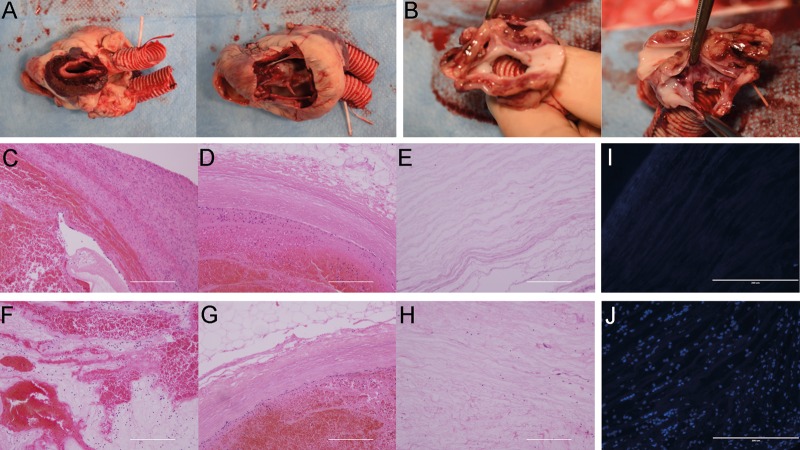
The inner surface of the extracellular matrix was smooth, and no thrombosis existed in the cardiac chamber. Meanwhile, a haematoma filled the parenchymal space, especially the left atrium (Fig. 4A). There was no stenosis at the anastomosis sites (Fig. 4B). H&E staining showed massive inflammatory cell infiltration in the parenchymal space in the left and right atrium (Fig. 4C and F). The left and right coronary arteries and their branch vessels were obstructed by thrombi (Fig. 4D and G). A few inflammatory cells migrating into the left and right ventricular muscle were detected (Fig. 4E and H). DAPI staining also revealed cell infiltration into the right and left ventricles (Fig. 4I and J).
Figure 4:
Histological analysis of the transplanted decellularized heart. (A) Representative photograph of the excised scaffold. (B) The anastomosis site. Ascending aorta (left); inferior vena cava (right). (C–H) Haematoxylin and eosin staining of the excised scaffold of the left atrium (C), left coronary artery (D), left ventricle (E), right atrium (F), right coronary artery (G) and right ventricle (H). (I and J) 4′,6-Diamidino-2-phynylindole staining of the left ventricle (I) and right ventricle (J). Scale bar: 200 μm.
Recellularization of the decellularized scaffold and heterotopic transplantation
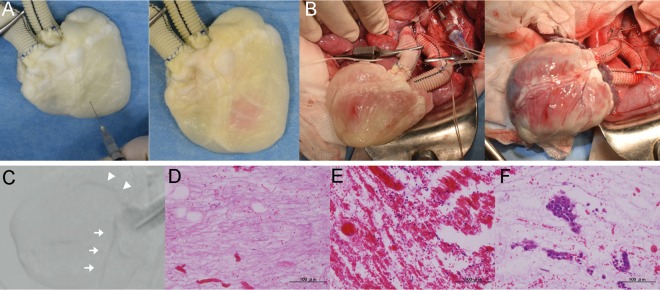
pMSCs incubated at 37°C in 5% CO2 from the 15th passage were used for recellularization (Fig. 5A). In the coronary perfusion method described above, pMSCs were infused through the ascending aorta using the perfusion chamber (Fig. 5B). Two days after multistep infusion, <104 infused cells remained in the perfusate. The recellularized bioengineered whole-heart scaffold was then transferred for heart transplantation (Fig. 5C). Although, macroscopically, the coronary artery appeared to be perfused with blood after reperfusion, intraoperative coronary angiography did not show any blood flow in the artery (Fig. 5D), suggesting that the coronary arteries had been occluded immediately after reperfusion. H&E staining showed that the coronary artery was filled with thrombi and inflammatory cells in several locations throughout the vessel. However, there were no pMSCs detected in the vessel lumen (Fig. 5E–H).
Figure 5:
Heterotopic transplantation of the recellularized heart with the coronary perfusion method. (A) Pig mesenchymal stem cells incubated at 37°C in 5% CO2, 15th passage. Scale bar: 200 μm. (B) Perfusion incubation system (1: closed large-size chamber; 2: roller pump; 3: bubble trap). (C) Operative findings of the scaffold, before (left) and after (right) de-clamping. (D) Digital subtraction angiography of the coronary artery. (E–H) Haematoxylin and eosin staining of the recellularized transplanted heart. Thrombosis in the parenchymal space (E) and the coronary artery (F). Migrated inflammatory cells in the parenchymal space (G) and epicardial layer (H). Scale bar: 200 μm.
With the muscular injection method, pMSCs were injected into the left ventricular muscle just before transplantation surgery (Fig. 6A). The bioengineered heart transplantation was successfully performed (Fig. 6B). Intraoperative angiography of the coronary arteries was performed, which demonstrated blood flow through almost the entire length of the right and left coronary arteries (Fig. 6C). Although H&E staining showed thrombosis and inflammatory cells in the parenchymal space, clusters of intact pMSCs were observed to be engrafted in the parenchyma (Fig. 6D–F).
Figure 6:
Heterotopic transplantation of the recellularized heart with the muscular injection method. (A) Representative photography of the muscular injection of pig mesenchymal stem cells in the decellularized heart. (B) Operative findings of the scaffold, before (left) and after (right) de-clamping. (C) Digital subtraction angiography of the coronary artery. White arrow: left coronary artery. Arrowhead: right coronary artery. (D–F) Haematoxylin and eosin staining of the recellularized transplanted heart. Nearly intact ventricular muscle at the apex (D). Thrombosis in the parenchymal space (E). Injected porcine mesenchymal stem cells (F). Scale bar: 100 μm.
DISCUSSION
To the best of our knowledge, this was the first successful heterotopic transplantation of a bioengineered heart that was fabricated by decellularization and recellularization of a whole porcine heart scaffold. We also showed reperfusion of the coronary artery by intraoperative angiography. Before this study, heterotopic transplantation of the decellularized heart had not yet been reported in a large animal model. In a rat model, Ott et al. [8] first performed successful heterotopic transplantation of a recellularized heart scaffold. They emphasized that one of the most inevitable problems in transplantation of decellularized tissue is thrombosis. Kasimir et al. [18] reported that decellularized matrices induce human platelet activation and cause thrombosis and inflammation. In whole decellularized hearts, Momtahan et al. [13] studied static blood thrombosis in vitro by comparing decellularized and normal porcine hearts. They concluded that the lack of endothelial cells in decellularized hearts induced platelet activation and adhesion. Robertson et al. [19] reported heterotopic transplantation of hearts re-endothelialized with rat neonatal cardiomyocytes. In that report, re-endothelialization diminished the thrombogenicity of the scaffolds. The most important factor in preventing thrombosis for whole-organ engineering is recellularization with endothelial cells attached to the blood-contacting surfaces of the extracellular matrix. However, we used pMSCs, which were verified to provide a beneficial effect on cardiomyocytes, improving metabolism, synthesis, angiogenesis and cell proliferation [14]. Moreover, Kadota et al. [20] reported that MSCs have the possibility to be transdifferentiated into the endothelial lineage in the extracellular matrix. We hypothesized that MSCs would be strong candidates as supportive cells in transplantable whole-organ engineered grafts. The differentiation of MSCs is mediated by the surrounding environment [21]. In this mediation, the extracellular matrix plays an important role. We used MSCs that have a potential to differentiate into cardiac cells as well as those of endothelial lineage, expecting that this decellularized heart-specific extracellular matrix effectively influences their differentiation capacity [22]. However, the observation period was not long enough for its evaluation in this study; therefore, it was not observed whether MSCs had differentiated into these lineages after implantation. On the other hand, there was a report describing a recellularized heart with cardiomyocytes having electrical activity in a rat model [8]. However, in this work, we applied MSCs but not cardiomyocytes which were activated electrically. Therefore, the electrical activity of the transplanted heart was not observed. In the future, we are planning to recellularize the scaffold with different cell types that are expected to be part of the component of cardiac construction; e.g. cardiomyocytes, MSCs and endothelial cells, via direct injection or coronary infusion technique, to optimize functional correlation between those cells. In our study, we showed patent coronary arteries in the decellularized heart in vivo controlled by the systemic administration of an anticoagulant, and confirmed that the blood flow reached the distal end of the artery during the systolic phase. However, blood flow was short-lived due to thrombosis. The same phenomena occurred even in the recellularized heart. We attempted two recellularization methods. The coronary perfusion method was modified from a previously reported method [16]. This method can deliver the cells evenly throughout the whole heart. The other method was direct injection of pMSCs. Although this method ensures that cell insertion in the optimal place is certain, it may result in a higher number of technical errors. In the present study, neither coronary perfusion nor muscular injection of pMSCs contributed to preventing thrombosis. As we could not compare the efficacy of these two methods, further study is needed to determine the optimum recellularization method for reducing thrombosis in the long term in order to adapt this unique technology for clinical applications.
Since Ott et al. first reported a whole-heart decellularization technique, several methods have been developed and reported [9–11]. Their decellularization techniques took about from 12 h to 18 days for a whole heart. In these reports, SDS was the preferred decellularization detergent. We added Triton X-100 to remove any cell debris and remaining SDS, similar to the methods reported by Momtahan et al. [13]. However, the previously reported decellularization methods were not simple and were time-consuming. In this study, we efficiently removed cellular components while preserving the extracellular matrix in a shorter time (13 h) compared with the time required in the other studies. Moreover, as the macroscopic findings of the decellularized heart did not change after 6 h, we may be able to perform whole-heart decellularization in less time. Although we are concerned with the cytotoxic side effects of decellularized scaffold and remnant of DNA contents, we did not perform any evaluation at this time, since the decellularization protocol we applied here was based on the previous work in which there was no cytotoxic issue reported due to the remnant detergents or DNA fragments [13]. Also, the cytotoxic side effects due to the possible remnant content have not been reported as an issue for cell repopulation or implantation for now [11]. However, in the stage of clinical application, these issues should be clarified. Terminal sterilization by gamma irradiation could effectively reduce the time course without disrupting morphological organ construction. However, it has been reported that gamma irradiation may alter and reduce extracellular matrix components [23]. The conditions to minimize destruction of extracellular components by gamma irradiation need to be defined.
The native extracellular matrix scaffold is constructed primarily of networks of collagens and other structural proteins and has the potential to provide a proper microenvironment for the heart. In addition, the three-dimensional structure of the native extracellular matrix has been shown to determine the structural arrangement of cardiomyocytes. In the bioengineering field, the extracellular matrix is a crucial factor for cell–cell interaction, cell proliferation and differentiation [24]. The principal extracellular matrix component in the heart is collagen type I. We verified the preservation of the heart extracellular matrix, including collagen type I, collagen type IV and laminin (a basement membrane) after our modified decellularization method. To generate a transplantable heart scaffold, it is critical to maintain the integrity of the surface to avoid leakage of blood flow in case of vascularization of the heart graft. In terms of integrity, we verified that the decellularized heart could endure surgical procedures, including handling with forceps, surgical stitches and blood pressure.
Even if heart transplantation is achieved, the patient must endure lifelong immunosuppression and the risk of chronic rejection. One of the benefits of transplantation using a decellularized scaffold is less induction of immunogenic reaction compared with that with cell-composed tissue. However, we previously observed the migration of many inflammatory cells into the parenchymal space of the decellularized heart. In this experiment, we recellularized the scaffold with allogeneic pMSCs. The extracellular matrix component of the scaffold itself also might have minimal antigenicity, which could raise the immunogenic reaction. Therefore, we have administered immunosuppressive drugs preoperatively based on a previous report to reduce immunogenic reactions [25]. In addition, it is important to adjust an appropriate dose of steroids and immunosuppressive medicine to prevent acute inflammatory reaction after implantation. We consider that the reason of coronary thrombosis was multifactorial and the inflammatory response might partly affect this issue. In the near future, stem cell-derived cardiomyocytes will be used in recellularization as a cell source. The cell covers the decellularized tissue, resulting in fewer immunogenic reactions and the termination of immunosuppressive drug use.
In conclusion, we successfully performed heterotopic transplantation of a bioengineered porcine heart using decellularization and recellularization of the whole-organ scaffold. The scaffolds were capable of enduring surgical procedures. We detected short-term coronary artery perfusion by intraoperative angiography. Although the separate stages of the experiments should be verified more specifically, we believe that the report of a successful transplantation with seamless experimental procedures from efficient decellularizion of the whole-heart scaffold with multipotent stem cells to surgical implantation is scientifically important, which was achieved only by a tight collaboration of cardiothoracic surgeons with bio-engineers in our group. Further study is needed to optimize re-endothelialization and prevent thrombosis in the long term.
Funding
This work was supported by Japan Society for the Promotion of Science, grant-in-aid for Scientific Research (C) (grant no. 15K10223).
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
The authors thank Toshio Otake, Koji Matsumoto and Keiko Kumagai from the Laboratory Animal Center; Kenta Inomata, Kana Sugiyama and Makiko Hieda from the Department of Surgery and Shima and Nakane from the Department of Cardiovascular Surgery, School of Medicine, Keio University.
APPENDIX. CONFERENCE DISCUSSION
Dr M. Andreas (Vienna, Austria): First, you already pointed out that the endothelial cells may prevent thrombosis. What other methods are possible to prevent thrombosis and to enable the organ to be correctly perfused? The first question.
The second question is: You have a very short washout period for these decellularizations procedure. Have you seen any toxic effects to the pig or are you afraid of that?
Dr Kitahara: The first question, we find many reports about prevention of the thrombosis, but the other authors said that the most important thing is endothelialization to prevent the thrombosis. I think so. We strictly manage the anticoagulation with heparin infusion, but the thrombosis occurs. The endothelialization of the coronary artery is very important. I'm sorry, what is the second question?
Dr Andreas: The second question was, if you have seen any toxic effects? Because your washout period is very short.
Dr Kitahara: I have never seen, so I can't answer this question. But 13 hours is too short to decellularize the heart. But I think I can shorten this time to 6 or 7 because microscopically the decellularized scaffold was not changing from the 6 hours to the 12 hours, so we could shorten the time of the decellularization to diminish the damage of the toxic effect or something.
Dr P. Akhyari (Dusseldorf, Germany): I have two questions. First, did you use freezing as a component of decellularization, or did you use it just for the storage of the organ?
Dr Kitahara: You mean the freezing at −80°C for 24 hours, after thawing for decellularization for 24 hours? We used pathogen decellularization. I'm sorry, I don't understand.
Dr Akhyari: If you don't freeze the heart, is the decellularization the same?
Dr Kitahara: I attempt that. But it took more time to decellularize the heart, it takes about 24 hours or more.
Dr Akhyari: The second question is: Have you analysed the effect of this freezing on the matrix? Because there is a lot of debate on freezing or not freezing.
Dr Kitahara: I have never done that. I want to do that.
Dr M. Shchatsinka (Minsk, Belarus): Maybe I didn't catch it, but what was the reason for freezing the heart before decellularization?
Dr Kitahara: The freezing can life the cell so it makes it easy to decellularize with the pathogen.
Dr Shchatsinka: Did you use DNases and RNases to remove genetic material from the heart?
Dr Kitahara: I'm sorry?
Dr Shchatsinka: Decellularization techniques often involve using of DNase and RNase, enzymes to digest nucleotides in the tissue; did you use it?
Dr Kitahara: No, never. I use only the detergent, the sodium dodecyl sulphate and the Triton X-100.
Dr Shchatsinka: So, the last question: Why did you explant the heart at the third day, why such a short period?
Dr Kitahara: Shortly after the implantation, there was thrombus formation in the coronary artery, so we think that we can't observe 7 or more days. We didn't think that we couldn't, so I'm sorry I can't answer exactly.
Dr D. Chambers (London, UK): Can I just ask you, when did your thrombus occur? Did it occur earlier than 3 days?
Dr Kitahara: Yes, earlier than 3 days.
Dr Chambers: So your heart was not perfused for many minutes?
Dr Kitahara: Almost 30 or 40 minutes later, after.
Dr Chambers: So very soon after you implanted the heart?
Dr Kitahara: Yes.
Dr H. Ankersmit (Vienna, Austria): So there was no possibility to see any regenerative effects that maybe cells were homing in the matrix? Because you have shown us histology data. There is a strong inflammation. Can you go back to the histology data? So where did you get this? So this is thrombus formation and this is the matrix here, that's the matrix without any cells populating; is that correct?
Dr Kitahara: Yes, that's correct.
Dr Ankersmit: So basically the heart was not perfused?
Dr Kitahara: The heart was not perfused, yes. No, no.
REFERENCES
- 1.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007;93:1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Califf RM, Adams KF, McKenna WJ, Gheorghiade M, Uretsky BF, McNulty SE et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: the Flolan Internaional Randomized Survival Trial (FIRST). Am Heart J 1997;134:44–54. [DOI] [PubMed] [Google Scholar]
- 3.Özalp F, Bhagra S, Bhagra C, Butt T, Ramesh B, Robinson-Smith N et al. Four-year outcomes with third-generation centrifugal left ventricular assist devices in an era of restricted transplantation. Eur J Cardiothorac Surg 2014;46:e35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stempien-Otero A, Helterline D, Plummer T, Farris S, Prouse A, Polissar N et al. Mechanisms of bone marrow-derived cell therapy in ischemic cardiomyopathy with left ventricular assist device bridge to transplant. J Am Coll Cardiol 2015;65:1424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coulombe KL, Bajpai VK, Andreadis ST, Murry CE. Heart regeneration with engineered myocardial tissue. Annu Rev Biomed Eng 2014;16:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu Rev Biomed Eng 2014;16:247–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials 2011;32:3233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med 2008;14:213–21. [DOI] [PubMed] [Google Scholar]
- 9.Wainwright JM, Czajka CA, Patel UB, Freytes DO, Tobita K, Gilbert TW et al. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods 2010;16:525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weymann A, Loganathan S, Takahashi H, Schies C, Claus B, Hirschberg K et al. Development and evaluation of a perfusion decellularization porcine heart model—generation of 3-dimensional myocardial neoscaffolds. Circ J 2011;75:852–60. [DOI] [PubMed] [Google Scholar]
- 11.Momtahan N, Sukavaneshvar S, Roeder BL, Cook AD. Strategies and processes to decellularize and recellularize hearts to generate functional organs and reduce the risk of thrombosis. Tissue Eng Part B Rev 2015;21:115–32. [DOI] [PubMed] [Google Scholar]
- 12.Weymann A, Patil NP, Sabashnikov A, Jungebluth P, Korkmaz S, Li S et al. Bioartificial heart: a human-sized porcine model—the way ahead. PLoS ONE 2014;9:e111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Momtahan N, Poornejad N, Struk JA, Castleton AA, Herrod BJ, Vance BR et al. Automation of pressure control improves whole porcine heart decellularization. Tissue Eng Part C Methods 2015;21:1148–61. [DOI] [PubMed] [Google Scholar]
- 14.Kawamura M, Miyagawa S, Fukushima S, Saito A, Toda K, Daimon T et al. Xenotransplantation of bone marrow-derived human mesenchymal stem cell sheets attenuates left ventricular remodeling in a porcine ischemic cardiomyopathy model. Tissue Eng Part A 2015;21:2272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto K, Yokoo T, Matsunari H, Iwai S, Yokote S, Teratani T et al. Xenotransplanted embryonic kidney provides a niche for endogenous mesenchymal stem cell differentiation into erythropoietin-producing tissue. Stem Cells 2012;30:1228–35. [DOI] [PubMed] [Google Scholar]
- 16.Yagi H, Fukumitsu K, Fukuda K, Kitago M, Shinoda M, Obara H et al. Human-scale whole-organ bioengineering for liver transplantation: a regenerative medicine approach. Cell Transplant 2013;22:231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Zhang F, Song G, Gu W, Chen M, Yang B et al. Intramyocardial injection of pig pluripotent stem cells improves left ventricular function and perfusion: a study in a porcine model of acute myocardial infarction. PLoS ONE 2013;8:e66688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasimir MT, Rieder E, Seebacher G, Nigisch A, Dekan B, Wolner E et al. Decellularization does not eliminate thrombogenicity and inflammatory stimulation in tissue-engineered porcine heart valves. J Heart Valve Dis 2006;15:278–86; discussion 286. [PubMed] [Google Scholar]
- 19.Robertson MJ, Dries-Devlin JL, Kren SM, Burchfield JS, Taylor DA. Optimizing recellularization of whole decellularized heart extracellular matrix. PLoS ONE 2014;9:e90406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadota Y, Yagi H, Inomata K, Matsubara K, Hibi T, Abe Y et al. Mesenchymal stem cells support hepatocyte function in engineered liver grafts. Organogenesis 2014;10:268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010;466:829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikhapoh IA, Pelham CJ, Agrawal DK. Sry-type HMG box 18 contributes to the differentiation of bone marrow-derived mesenchymal stem cells to endothelial cells. Differentiation 2015;89:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matuska AM, McFetridge PS. The effect of terminal sterilization on structural and biophysical properties of a decellularized collagen-based scaffold: implications for stem cell adhesion. J Biomed Mater Res B Appl Biomater 2015;103:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eitan Y, Sarig U, Dahan N, Machluf M. Acellular cardiac extracellular matrix as a scaffold for tissue engineering: in vivo cell support, remodeling, and biocompatibility. Tissue Eng Part C Methods 2010;16:671–83. [DOI] [PubMed] [Google Scholar]
- 25.Kelly DM, Demetris AJ, Fung JJ, Marcos A, Zhu Y, Subbotin V et al. Porcine partial liver transplantation: a novel model of the ‘small-for-size’ liver graft. Liver Transpl 2004;10:253–63. [DOI] [PubMed] [Google Scholar]