Abstract
OBJECTIVE
To evaluate the effect of structured physical activity on sleep-wake behaviors in sedentary community-dwelling elders with mobility limitations.
DESIGN
Multicenter, randomized trial of moderate-intensity physical activity versus health education, with sleep-wake behaviors pre-specified as a tertiary outcome over a planned intervention period ranging between 24 and 30 months.
SETTING
Lifestyle Interventions and Independence in Elder (LIFE) Study.
PARTICIPANTS
1635 community-dwelling persons, aged 70–89 years, who were initially sedentary with a Short Physical Performance Battery score <10.
MEASUREMENTS
Sleep-wake behaviors were evaluated by the Insomnia Severity Index (ISI) (≥8 defined insomnia), Epworth Sleepiness Scale (ESS) (≥10 defined daytime drowsiness), and Pittsburgh Sleep Quality Index (PSQI) (> 5 defined poor sleep quality) — administered at baseline and subsequently at 6, 18, and 30 months.
RESULTS
The randomized groups were similar on baseline demographic variables, including mean age (79 years) and sex (67% female). Relative to health education, structured physical activity significantly reduced the likelihood of having poor sleep quality (adjusted odds ratios [adjOR] for PSQI >5 of 0.80 [0.68, 0.94]), including a reduction in new cases (adjOR for PSQI >5 of 0.70 [0.54, 0.89]) but not in resolution of prevalent cases (adjOR for PSQI ≤5 of 1.13 [0.90, 1.43]). No significant intervention effects were observed for ISI or ESS.
CONCLUSION
Structured physical activity reduced the likelihood of developing poor sleep quality (PSQI >5) over the intervention period, when compared with health education, but had no effect on prevalent cases of poor sleep quality, or on sleep-wake behaviors evaluated by the ISI or ESS. These results suggest that the benefit of physical activity in this sample was preventive and limited to sleep-wake behaviors evaluated by the PSQI.
Keywords: physical activity, sleep-wake behaviors, insomnia, daytime drowsiness, poor sleep quality
INTRODUCTION
Sleep-wake disturbances are frequently noted in older Americans.1,2 In the Established Populations for Epidemiologic Studies of the Elderly (EPESE), involving 9,282 community-dwelling persons aged 65 years or older, 43% reported insomnia symptoms and 25% napped.1 These findings were confirmed in the National Sleep Foundation’s (NSF) survey of community-living older persons, which reported insomnia symptoms in 46% of persons aged 65–74 years and in 50% of those aged 75–84 years, with corresponding rates for napping of 39% and 46%.2 The occurrence of sleep-wake disturbances in older persons is associated with cognitive decline, depression, falls, cardiovascular disease, institutionalization, and death.1–4
Low physical activity and mobility limitations are also prevalent in older persons and may underlie the development of sleep-wake disturbances.1–6 In two large national surveys of older Americans, 82.6% did not have any regular physical activity,5 and 24.8% had difficulty or were unable to walk one-quarter mile.6 In the 2003 NSF poll,2 older Americans who were sedentary (exercised less than once per week) or had mobility disability (very difficult or unable to walk one-half mile, or up and down a flight of stairs without help) had a 2-fold or greater prevalence of insomnia and daytime drowsiness, respectively. In EPESE,3 the onset of physical disability (dependency in activities of daily living, or inability to walk up and down stairs or one half-mile without help) increased the likelihood of developing insomnia by 109%.
There is a strong rationale to promote physical activity in sedentary older persons with mobility limitations.7,8 Based on an extensive review of prior work, the U.S. Department of Health and Human Services has concluded that physical activity improves cardiovascular, musculoskeletal, and mental health.7 These benefits, in turn, can lead to improvements in the sleep-wake cycle and mobility,4,7–11 but the effect of exercise on sleep-wake behaviors remains to be established in sedentary older persons with mobility limitations. Although two prior studies of sedentary persons have shown, respectively, that a 16-week and 12-month moderate-intensity exercise program yielded modest improvements in subjective and objective measures of sleep,9,10 these studies included middle-aged persons and did not specifically evaluate individuals with mobility limitations.
The Lifestyle Interventions and Independence for Elders (LIFE) Study is a randomized controlled trial designed to compare a physical activity intervention with a health education intervention in 1,635 sedentary older persons with mobility limitations, over a planned intervention period ranging between 24 and 42 months.8,12 Although the primary outcome of the LIFE Study was major mobility disability, sleep-wake behaviors were also pre-specified as a tertiary outcome, with assessments at baseline and subsequently at 6, 18, and 30 months. Of note, at the baseline visit,13 LIFE participants frequently reported insomnia (32.8% had an Insomnia Severity Index14 ≥8), daytime drowsiness (18.1% had an Epworth Sleepiness Scale15 ≥10), and poor sleep quality (54.2% had a Pittsburgh Sleep Quality Index16 >5).
In a pre-specified LIFE Study analysis, we evaluated the effect of structured physical activity on sleep-wake behaviors, relative to health education, over a planned intervention period ranging between 24 and 30 months. Because structured physical activity has been found to reduce major mobility disability in the LIFE Study,8 we postulated that a physical activity intervention could improve sleep-wake behaviors.
METHODS
Trial design and participants (N=1635)
The LIFE Study is a multicenter, single-blind, parallel randomized trial involving 1,635 sedentary older persons with mobility limitations, conducted at 8 centers across the United States (on-line Appendix A).8,12,17 The study protocol was approved by the institutional review boards at all participating sites. Written informed consent was obtained from all study participants.
Details of the trial design were published previously.8,12,17 Eligibility criteria included: 1) age 70–89 years; 2) sedentary status, defined as <20 minutes/week of regular physical activity in the past month and <125 minutes/week of moderate physical activity;18 3) mobility limitations, defined as a Short Physical Performance Battery score <10,19,20 but able to walk 400 meters in ≤15 minutes (without sitting, leaning, or help of another person or walker); and 4) no major cognitive impairment, defined as a Modified Mini-Mental State Examination (3MSE)21 score of no more than 1.5 standard deviations below education- and race-specific norms.
Interventions
The physical activity intervention involved primarily walking with a goal of 150 minutes/week, as well as strength, flexibility, and balance training.8,12 The intervention included attendance at 2 center-based sessions per week and home-based activity 3–4 times per week for the duration of the study. The physical activity sessions progressed towards a goal of 30 minutes of walking 5-days a week at moderate intensity, 10 minutes of primarily lower extremity strength training (ankle weights), 10 minutes of balance training, and large muscle group flexibility exercises. Participants began with lighter intensity exercise and gradually increased intensity over the first 2–3 weeks of the intervention.
The health education intervention focused on weekly workshops during the first 26 weeks, and monthly sessions thereafter.8,12 Workshops included topics such as how to negotiate the health care system, how to travel safely, preventive services and screenings at different ages, and where to go for reliable health information and nutrition. Each workshop also included a 5–10 minute instructor-led program of gentle upper extremity stretching or flexibility exercises.
Demographics and Clinical Characteristics
The baseline characteristics included age, sex, ethnicity/race, living alone, body mass index (BMI, kg/meter2), cognition, depressive symptoms, smoking status, medical conditions, medications, use of caffeine/energy drinks, and health status.
Cognition was evaluated by the 3MSE,21 with a score <89 defining possible cognitive impairment; as per eligibility criteria, LIFE participants had a 3MSE score of no more than 1.5 standard deviations below education- and race-specific norms.12 Depressive symptoms were evaluated by the Center for Epidemiologic Studies Depression Scale (CES-D), with a score ≥16 defining high levels of depressive symptoms.22 Medical conditions were self-reported physician-diagnosed, and selected based on known associations with sleep-wake disturbances.1–4,23–26 Medications were defined as the total number of prescription medications and use of a prescription medication with potential central nervous system (CNS) effects (anticonvulsant, antidepressant, antihistamine, antipsychotic, barbiturate, benzodiazepine, muscle relaxant, opiate). Polypharmacy was defined by ≥4 prescription medications.27 Caffeine/energy drink use was established by daily consumption of ≥2 caffeinated beverages (soda, energy drinks, coffee, tea, iced coffee, iced tea).28 To assess health status, participants were asked, “Would you say your health in general is excellent, very good, good, fair, or poor?” Reduced health status was defined as a rating of “poor.”13
Mobility Limitation and Physical Inactivity
Baseline mobility was evaluated by the Short Physical Performance Battery (SPPB) and 400 meter walk test (400MWT) 8,12 The SPPB is a composite measure that consists of time to walk 4 meters at usual pace, time to complete five chair stands, and three increasingly difficult standing balance maneuvers.19,20 An SPPB score <8 identified moderate-to-severe mobility impairment.19,20 The 400MWT was completed at the participant’s usual walking pace over a 40-meter course, with a slow gait speed defined as <0.8 m/s.29
Physical inactivity was established by accelerometry (ActiGraph GT3X and ActiLife software [version 5]; Pensacola, FL), over a planned 7-day period.8,12 After dressing each morning, participants placed the accelerometer on their right hip (waistline belt), thereafter removing the monitor just prior to going to bed at night. Sedentary time was defined as percent of accelerometry wear time with activity <100 counts/minute (approximated sitting time),30 averaged across at least 5 days of monitoring, including 10-hours on each day (this amount of wear time correlates well with 3 weeks of wear time).31 Accelerometry was measured at baseline and 6, 12, and 24 months. In addition, the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire18 assessed self-reported physical activity at baseline and 6, 12, 24, and 36 months.
Sleep-Wake Behaviors
Sleep-wake behaviors were evaluated at baseline and 6, 18, and 30 months, using questionnaires that included the Insomnia Severity Index (ISI), Epworth Sleepiness Scale (ESS), and Pittsburgh Sleep Quality Index (PSQI). These were administered by blinded assessors during regularly scheduled visits at the field centers.
The ISI is a 7-item questionnaire based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for insomnia.14 The response to each item is scored on a 0–4 scale, using qualitative descriptors that include none, mild, moderate, severe, and very severe, respectively. The ISI score ranges from 0–28, with higher scores signifying more severe symptoms. A sleep-wake disturbance was established by an ISI≥8, defining insomnia.14
The ESS measures the chance of dozing during eight different activities, based on a scale of 0–3 that included the qualitative descriptors of none, slight, moderate, and high, respectively.15 The ESS score ranges from 0–24, with higher scores signifying more severe symptoms. Two frequently cited thresholds for defining daytime drowsiness include ESS scores of 10 and 11.15,32–36 To establish a clinically meaningful sleep-wake disturbance, daytime drowsiness was defined as an ESS≥10, based on prior NSF surveys and its association in older persons with other measures of daytime drowsiness, hypertension, stroke, frailty, and driving capacity.32–36
The PSQI provides a multidimensional evaluation of sleep-wake behaviors over the prior month.16 It includes seven subscales that include quantitative descriptors (timing, duration, or frequency) of subjective sleep quality, sleep latency, sleep duration, habitual sleep disturbances, use of sleep medications, and daytime dysfunction. Each subscale is weighted equally on a 0–3 scale, with the total PSQI score ranging from 0–21; the higher the score, the worse the sleep quality. A sleep-wake disturbance was established by a PSQI>5, defining poor sleep quality.16
Statistical Analysis
The baseline characteristics were first summarized by intervention group, using means and standard deviations (SD), or counts and percentages. The outcomes of interest were sleep-wake behaviors, evaluated across a 30-month follow-up period. The sleep-wake behaviors were evaluated as categorical variables (sleep-wake disturbances of insomnia [ISI≥8], daytime drowsiness [ESS≥10], and poor sleep quality [PSQI>5]), and as continuous variables (ISI [range 0–28], ESS [range 0–24], and PSQI [range 0–21]).
Next, using GEE marginal logistic regression models appropriate for repeated binary outcomes and pre-specified sleep-wake disturbance cut-points, the average odds ratios of having a sleep-wake disturbance in the physical activity group relative to the health education group were estimated across 30 months of follow-up.37 The models included terms for field center and gender (randomization was stratified on these factors). In addition, categorical terms for follow-up visit and intervention by follow-up visit interaction were included in the models; the interaction term was necessary to allow for separate estimation of intervention effects at each time point. An unstructured covariance matrix was used to account for the within-person correlation between repeated measures. The follow-up visit by intervention term was tested for all models and the average odds ratio across follow-up visits was estimated from a model without this interaction.
As a sensitivity analysis, using the same logistic regression models as previously described, the average odd ratios of having an incident sleep-wake disturbance relative to intervention group were estimated across the 30-month follow-up period, within two subgroups. Subgroup 1 consisted of participants reporting no sleep-wake disturbance at baseline and thus analyzed incident new sleep-wake disturbances as the outcome (“new cases”); whereas subgroup 2 consisted of participants with a baseline sleep-wake disturbance (“prevalent cases”) and analyzed incident resolution of the prevalent sleep-wake disturbance (“resolved cases”).
The continuous measures of sleep-wake behaviors were evaluated using mixed effects analysis of covariance models appropriate for repeatedly measured outcomes. The different post-baseline mean levels of ISI, ESS, and PSQI were evaluated using a model containing the same terms as described for the repeated measures logistic regression analyses plus a continuous covariate containing the baseline, pre-randomization value of each outcome. Least squares means were obtained for each sleep-wake behavior and contrasts were used to obtain estimates and test the average intervention effect across follow-up visits.
Pre-specified subgroups included ethnicity/race (non-Hispanic white vs. other), sex, baseline physical performance (SPPB <8 vs. ≥8) and age (70–79 vs. ≥80).8 The effect of the intervention within pre-specified subgroups was investigated by entering the baseline subgroup and subgroup by intervention effects into each model (both GEE and mixed effects models) for any sleep-wake disturbance (3 outcomes X 4 subgroups). These additional terms allowed the use of contrasts to investigate homogeneity of average effects over time among subgroups. All subgroup hypothesis tests were considered to be hypothesis generating and conducted at the 0.05 level. Since we performed 24 tests of subgroup hypotheses each at the 0.05 level, there is a >70% chance that at least one test would be significant at an 0.05 level, assuming independence between tests.38
Finally, in both intervention groups, sleep-wake behaviors were evaluated across the trial period according to level of physical activity (≤ vs. >150 min/week of self-reported minutes of walking and weight training by CHAMPS,18 and ≤ vs. >200 min/week at >760 counts/min by accelerometry). Specifically, mean values for ISI, ESS, and PSQI were calculated at different levels of physical activity across the trial period, and correlation analyses (Spearman’s correlation coefficient) were performed between level of physical activity and the ISI, ESS, and PSQI.
RESULTS
Table 1 shows the baseline characteristics of the physical activity and health education groups. Both groups had a mean age of about 79 years and the majority was female and white. The frequency of living alone was similar in both groups, including a two-fold greater rate in females versus males. The mean BMI and frequency of non-smokers (never or former) were also similar in both groups. In addition, there were comparable 3MSE and CES-D scores; high rates of arthritis, cardiopulmonary risk factors and disease; frequent use of medications and caffeine/energy drinks; but low rates of reduced health status. For both groups, as expected given the eligibility criteria, the average SPPB score was moderate-to-severely reduced, and a large proportion of participants had slow gait speed and high sedentary time. Although sleep-wake disturbances (insomnia, daytime drowsiness, and poor sleep quality) were common, both groups had mean values for ISI, ESS, and PSQI that were less than one-third of the maximum available scores (28, 24, and 21, respectively).
Table 1.
Baseline characteristics of the physical activity and health education groups (N=1635)a
| Characteristics | Physical Activity (PA) | Health Education (HE) | ||
|---|---|---|---|---|
| NPAb | Mean (± SD) or No. (%) | NHEb | Mean (± SD) or No. (%) | |
| Age | 818 | 78.7 (± 5.2) | 817 | 79.1 (± 5.2) |
| Female | 547 (66.9%) | 551 (67.4%) | ||
| Non-white | 815 | 211 (25.9%) | 815 | 180 (22.1%) |
| Lived alone | ||||
| Female | 546 | 319 (58.4%) | 550 | 337 (61.3%) |
| Male | 271 | 69 (25.5%) | 262 | 83 (31.7%) |
| BMI, kg/m2 | 818 | 30.1 (± 5.7) | 817 | 30.3 (± 6.2) |
| Smoking status | ||||
| Never | 807 | 400 (49.6%) | 799 | 434 (54.3%) |
| Former | 381 (47.2%) | 341 (42.7%) | ||
| Current | 26 (3.2%) | 24 (3.0%) | ||
| 3MSEc | 818 | 91.5 (± 5.5) | 817 | 91.6 (± 5.3) |
| CES-Dd | 778 | 9.0 (± 8.2) | 775 | 9.5 (± 8.5) |
| Chronic conditionse | ||||
| Number of chronic conditions | 816 | 1.5 (± 1.0) | 815 | 1.5 (± 1.0) |
| Hypertension | 813 | 573 (70.5%) | 808 | 578 (71.5%) |
| Diabetes mellitus | 815 | 199 (24.4%) | 813 | 216 (26.6%) |
| Symptomatic arthritis | 812 | 153 (18.8%) | 165 (20.3%) | |
| Chronic lung diseasef | 815 | 130 (16.0%) | 812 | 123 (15.1%) |
| Coronary artery disease | 60 (7.4%) | 69 (8.5%) | ||
| Stroke | 814 | 57 (7.0%) | 814 | 52 (6.4%) |
| Heart failure | 813 | 26 (3.2%) | 809 | 45 (5.6%) |
| Medications (prescription) | ||||
| Number used | 817 | 5.3 (± 3.3) | 816 | 5.4 (± 3.3) |
| Polypharmacyg | 559 (68.4%) | 591 (72.4%) | ||
| CNS-basedh | 324 (39.7%) | 331 (40.6%) | ||
| Caffeine/energy drink use | 818 | 654 (80.0%) | 817 | 648 (79.3%) |
| Reduced health status | 816 | 131 (16.1%) | 813 | 140 (17.2%) |
| SPPB scorei | 818 | 7.4 (± 1.6) | 817 | 7.3 (± 1.6) |
| 400 meter walk time (minutes) | 8.4 (± 1.9) | 8.5 (± 1.9) | ||
| Slow gait speed (<0.8 m/sec)j | 348 (42.5%) | 364 (44.6%) | ||
| Sedentary time (%)k | 593 | 77.2 (± 7.9) | 581 | 76.9 (± 8.2) |
| Sleep-wake behaviorsl | ||||
| Insomnia Severity Index (ISI)m | 799 | 5.5 (± 5.0) | 779 | 6.0 (± 5.2) |
| Insomnia (ISI ≥8) | 809 | 250 (30.9%) | 801 | 282 (35.2%) |
| Epworth Sleepiness Scale (ESS)n | 793 | 6.1 (± 3.9) | 796 | 6.1 (± 4.0) |
| Daytime drowsiness (ESS ≥10) | 794 | 141 (17.8%) | 797 | 147 (18.4%) |
| Pittsburgh Sleep Quality Index (PSQI)o | 813 | 5.9 (± 3.8) | 807 | 5.9 (± 3.8) |
| Poor sleep quality (PSQI >5) | 816 | 383 (46.9%) | 813 | 395 (48.6%) |
BMI, body mass index; CES-D, Center for Epidemiologic Studies Depression Scale; CNS, central nervous system; COPD, chronic obstructive pulmonary disease; 3MSE, Modified Mini-Mental Status Exam; SD, standard deviation; SPPB, Short Physical Performance Battery.
Of 14831 participants who were screened, 6313 did not meet eligibility criteria and 6883 were excluded because they chose not to continue screening or refused participation (4125), planned to move out of the area within 24 months (2321), or had other reasons (437). This yielded a final sample of 1635 participants, with 818 randomized to physical activity and 817 to health education.
Varies as a consequence of participants being excluded because of poor testing performance, missing values, or a delayed start to data acquisition (i.e., accelerometry).
3MSE score <89 defined possible cognitive impairment.
CES-D score ≥ 16 defining high levels of depressive symptoms.
Self-reported, and selected on the basis of known associations with sleep-wake disturbances.
Asthma, chronic bronchitis, emphysema, or COPD.
Use of four or more medications.
Included: anticonvulsant, antidepressant, antihistamine, antipsychotic, barbiturate, benzodiazepine, muscle relaxant, or an opiate.
SPPB score <8 identified moderate-to-severe mobility impairment.
Measured during the 400 meter walk at the participant’s usual walking pace.
Percent of accelerometer wear time with activity <100 counts/minute, averaged across days. Limited to participants with at least 5 days of wear time and at least 10 hours on each day.
Sample sizes also varied according to the reported analysis. In particular, if the ISI, ESS, or PSQI score met criteria for a sleep-wake disturbance but the questionnaire was otherwise incomplete, a sleep-wake disturbance was still established (reported as a categorical variable), whereas the continuous score was considered missing (not included in the calculation of mean values).
ISI ranges from 0–28, with higher scores signifying more severe insomnia.
ESS ranges from 0–24, with higher scores signifying more severe daytime drowsiness
PSQI ranges from 0–21, with higher scores signifying a worse sleep quality.
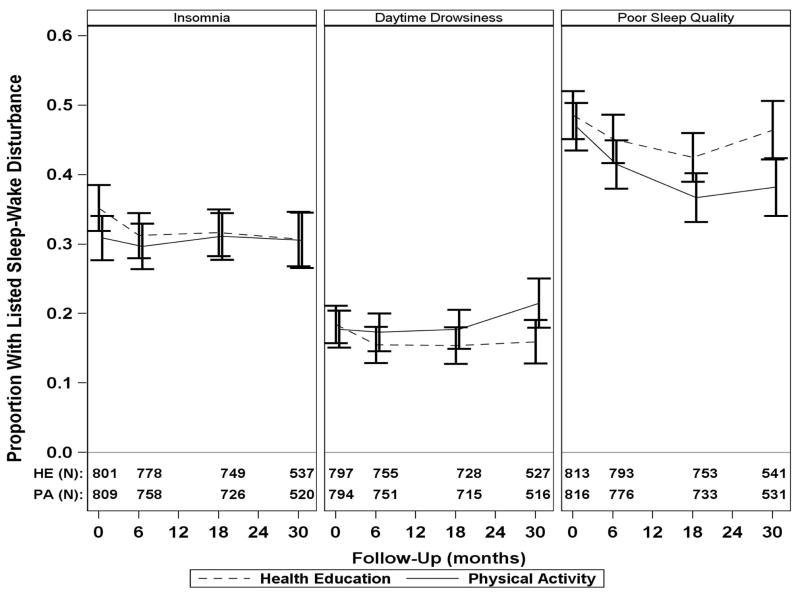
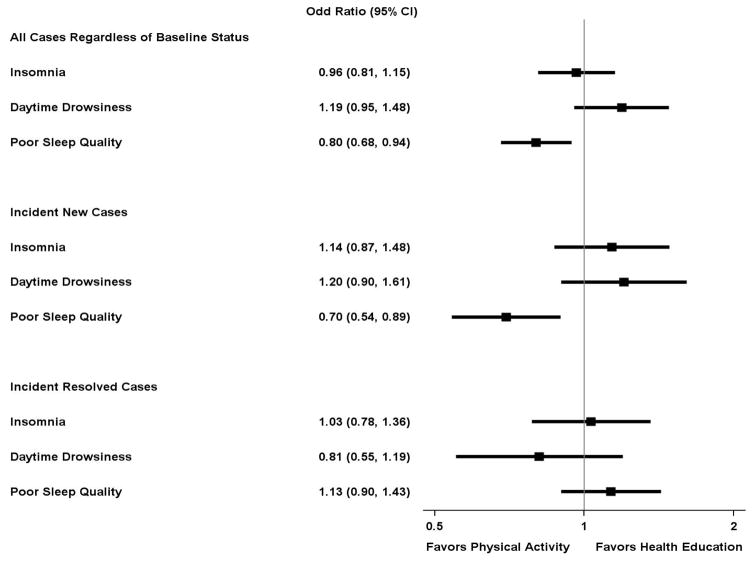
Figure 1 shows the prevalence of sleep-wake disturbances (categorical variables) over time in the physical activity and health education intervention groups. Appendix B provides the same information in tabular format (Table B1). The relative effects of the physical activity intervention on sleep-wake disturbances over time are shown in Figure 2. This figure includes three outcomes—all cases, new cases, and resolved cases of sleep-wake disturbances, respectively. For these outcomes, there was no statistical evidence of follow-up visit by intervention interaction (p>.20 for all). Relative to the health education group, the physical activity group had a lower likelihood of having poor sleep quality across the trial period, for all cases and new cases (adjusted odds ratios for PSQI>5 of 0.80 [0.68, 0.94] and 0.70 [0.54, 0.89], respectively), but not for resolution of prevalent cases (adjusted odds ratios for PSQI≤5 of 1.13 [0.90, 1.43]). No significant intervention effects were observed for insomnia or daytime drowsiness as defined by the ISI and ESS scores.
Figure 1.
Prevalence of sleep-wake disturbancesa in the physical activity and health education intervention groups across 30 months of follow-upb,c
aCategorical variables, including insomnia (Insomnia Severity Index [ISI] ≥8), daytime drowsiness (Epworth Sleepiness Scale [ESS] ≥10), and poor sleep quality (Pittsburgh Sleep Quality Index [PSQI] >5), assessed at baseline and 6, 18, and 30 months. Of those who completed baseline sleep-wake questionnaires (Table 1), the percentage of participants with missing sleep-wake outcomes at each visit were: 6 months—ISI 5.8%, ESS 7.8%, PSQI 3.9%; 18 months—ISI 9.7%, ESS 11.3%, PSQI 9.1%; and 30 months—ISI 15.4%, ESS 16.6%, PSQI 14.3%. At each visit, for all sleep-wake outcomes, the absolute difference in percent missing between groups was always less than 3% (range of difference between intervention groups was 1.7% to 2.9%).
bOf the 818 participants who were randomized to physical activity, 118 discontinued the intervention after a median of 15.8 months. The physical activity group attended 63% of scheduled sessions after excluding medical leave (median [interquartile range] of 71% [50%–83%]) over a median of 28.5 months.
cOf the 817 participants who were randomized to health education, 160 discontinued the intervention after a median of 32.5 months. The health education group attended 73% of scheduled sessions (median [interquartile range] of 82% [63%–90%]) over a median of 32.5 months.
Figure 2.
Odds ratioa (95% confidence interval) for effect of physical activity intervention on sleep-wake disturbancesb over timec
aCalculated as the average odds ratio across the 30-month follow-up, adjusted for field center and gender.
bCategorical variables, including insomnia (Insomnia Severity Index ≥8), daytime drowsiness (Epworth Sleepiness Scale ≥10), and poor sleep quality (Pittsburgh Sleep Quality Index >5), and further stratified as: 1) all cases (regardless of baseline); 2) incident case of a new sleep-wake disturbance (in those who did not have the corresponding sleep-wake disturbance at baseline); and 3) incident case of a resolved sleep-wake disturbance (in those who had the corresponding sleep-wake disturbance at baseline).
cSee footnotes to Figure 1 regarding missing data on sleep-wake outcomes and adherence to the physical activity and health education interventions, across the 30-months of follow-up.
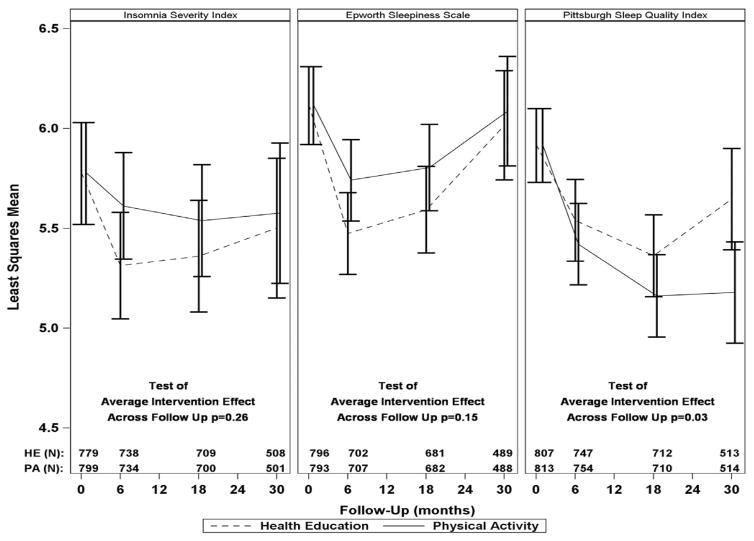
Figure 3 shows adjusted least squares mean values for the continuous measures of sleep-wake behaviors (ISI, ESS, and PSQI) over time in the physical activity and health education intervention groups. Appendix B provides the same information in tabular format (Table B2). For the three continuous measures of sleep-wake behaviors, there was no statistical evidence of follow-up visit by intervention interaction (p>0.19 for all). Relative to the health education group, the physical activity group had lower adjusted least squares mean values for the PSQI (p=.03 for average treatment effect across the follow-up period). As shown in Table 2, however, the magnitude of the average treatment effect on the PSQI was only 0.26 points lower in the physical activity than the health education group. No significant intervention effects were observed for the ISI and ESS scores.
Figure 3.
Adjusted least squares mean valuesa (95% confidence intervals) for sleep-wake behaviorsb over time in the physical activity and health education intervention groupsc
aAdjusted for field center and gender.
bContinuous variables, including Insomnia Severity Index (range 0–28), Epworth Sleepiness Scale (range 0–24), and Pittsburgh Sleep Quality Index (range 0–21)—assessed at baseline and 6, 18, and 30 months.
cSee footnotes to Figure 1 regarding missing data on sleep-wake outcomes and adherence to the physical activity and health education interventions, across the 30-months of follow-up.
Table 2.
Average effect of the physical activity and health education interventions based on least squares mean valuesa for sleep-wake behaviorsb across 30 months of follow-upc
| Intervention Group | Insomnia Severity Index | Epworth Sleepiness Scale | Pittsburgh Sleep Quality Index |
|---|---|---|---|
| Average Intervention Effect Across 30-Month Follow-Up Least Squares Means (95% Confidence Intervals) | |||
| Physical Activity | 5.58 (5.35, 5.80) | 5.88 (5.70, 6.05) | 5.26 (5.09, 5.42) |
| Health Education | 5.39 (5.17, 5.62) | 5.69 (5.52, 5.87) | 5.52 (5.35, 5.69) |
| Differenced | 0.18 (−0.13, 0.50) | 0.18 (−0.07, 0.43) | −0.26 (−0.50, −0.03) |
Adjusted for field center and gender.
Continuous variables, including Insomnia Severity Index (range 0–28), Epworth Sleepiness Scale (range 0–24), and Pittsburgh Sleep Quality Index (range 0–21).
See footnotes to Figure 1 regarding missing data on sleep-wake outcomes and adherence to the physical activity and health education interventions, across the 30-months of follow-up.
Physical activity minus health education.
In additional analyses, the average effect of physical activity and health education across the trial period on all cases of sleep-wake disturbances (categorical variables) did not differ statistically according to the pre-specified subgroups (p>.10), except for a borderline interaction between ethnicity/race and poor sleep quality (p=.06). Relative to health education, whites had a lower likelihood of having poor sleep quality (PSQI>5) in response to physical activity (adjusted odds ratio of 0.72 [0.60, 0.87], p<0.001), but this difference was not observed in non-whites (1.04 [0.75, 1.45], p=0.82). For the outcome of continuous measures of sleep-wake behaviors, the average effect of physical activity and health education across the trial period did not differ statistically according to the pre-specified subgroups (p≥.09), except for a significant interaction between sex and PSQI (p=.04). Relative to health education, women achieved better sleep quality (lower PSQI) in response to physical activity than men; specifically, the average intervention effect for the PSQI was 0.39 points lower in the physical activity versus health education group in women (p=0.007), but 0.13 points higher in men (p=0.52).
Finally, sleep-wake behaviors did not differ substantially over time according to levels of physical activity. In particular, mean values for the PSQI were similar across levels of physical activity, and no statistically significant correlations were observed between physical activity levels (either absolute levels or change from baseline) and the PSQI (data not shown).
DISCUSSION
In this clinical trial involving sedentary older persons with mobility limitations,8,12,17 we found that structured physical activity when compared with health education reduced the likelihood of having poor sleep quality (PSQI>5) over the course of 30 months by 20%. The benefit of physical activity was observed for new cases, with an odds reduction of 30%, but not for resolution of prevalent cases. The physical activity intervention also yielded a statistically significant improvement in sleep quality (lower PSQI), although this benefit was small and limited to women. Finally, physical activity offered no observed benefit for sleep-wake behaviors as evaluated by the ISI and ESS.
These results suggest that structured physical activity is an effective preventive strategy for reducing the burden of poor sleep quality in older persons. This preventive effect is especially noteworthy because the PSQI evaluates multiple sleep-wake behaviors, is sensitive to change, and has been validated in older persons.16,39 In addition, LIFE participants had many risk factors for developing poor sleep quality, including: 1) older age with predominant female and white representation (may predispose to disturbances in sleep continuity and structure); 2) high rates of obesity, diabetes, cardiopulmonary disease, and arthritis (may lead to sleep-disruptive symptoms [pain, dyspnea, bronchitis, nocturia], sleep-disordered breathing, and sleep-related movement disorders); 3) frequent use of medications and caffeine/energy drinks (may lead to disturbed sleep continuity and structure); and 4) sedentary status and mobility limitations (may lead to reduced homeostatic drive to nighttime sleep or loss of circadian zeitgebers, which include environmental and social cues that synchronize the sleep-wake cycle).1–4,23–26,40–42 Based on the observed 30% odds reduction and event rate as low as 0.20, we estimate that 20 older persons would need to undergo a physical activity intervention to prevent an incident case of poor sleep quality.
The mechanisms underlying the benefit of physical activity on poor sleep quality cannot be established by the current study. One modifiable mechanism could include restoration of circadian zeitgebers.4,40,41 By requiring attendance at a center twice a week and home-based activity 3–4 times per week, the physical activity intervention may have restored important circadian zeitgebers, including improved socialization, more structured daytime routines, and greater time spent outside the home (exposure to sunlight). Moreover, by lowering the risk of developing mobility disability,8 the physical activity intervention may have prevented the progressive loss of circadian zeitgebers. Older women are especially vulnerable to the loss of circadian zeitgebers, given their high likelihood of living alone (two-fold greater rate in females versus males in the LIFE Study) and reduced sleep homeostasis (decreased amount of normalized slow wave sleep, concurrent with increased alpha activity [EEG pattern of wakefulness] and lower sleep-onset release of growth hormone, relative to men).43,44 These differences could explain why the benefit of physical activity on the PSQI (continuous variable) was observed in women but not men.
An effective preventive strategy for reducing the burden of poor sleep quality in older persons could lead to important clinical benefits.1–4 First, a lower rate of incident poor sleep quality may alter prescription practices regarding hypnotics and, in turn, potentially reduce the rate of corresponding adverse drug events. Second, a lower rate of incident poor sleep quality may be associated with a lower rate of incident depression. Third, the age-related decline in cognitive function may be attenuated by a lower rate of incident poor sleep quality. Hence, future work should test the hypothesis that the physical activity intervention in the LIFE Study may lower the incidence rate of hypnotic use, depression, and cognitive impairment, as a consequence of a lower rate of incident poor sleep quality.
Nonetheless, the current study has also shown that physical activity did not benefit prevalent cases of poor sleep quality. The high burden of multimorbidity and polypharmacy, which often contribute to chronic sleep-wake disturbances in older persons,1–4,24,45,46 might not have been reduced by the physical activity intervention. The multimorbidity may include cardiopulmonary disease and primary sleep disorders (sleep apnea and movement disorders), while polypharmacy may include CNS-active medications. Accordingly, future work should test the hypothesis that the physical activity intervention in the LIFE Study did not have a beneficial effect on prevalent cases of poor sleep quality because of continued multimorbidity and polypharmacy.
Similarly, we postulate that the high prevalence of multimorbidity and polypharmacy may underlie the lack of benefit of physical activity on the ISI and ESS, for two related reasons. First, the ISI and ESS are symptom-specific, focused on insomnia and daytime drowsiness, respectively.14,15 Second, multimorbidity is often associated with chronic sleep-disruptive symptoms, whereas polypharmacy may disrupt sleep and impair daytime alertness.1–4 Hence, future work should test the hypothesis that the physical activity intervention in the LIFE Study did not have a beneficial effect on the symptoms of insomnia (ISI) and daytime drowsiness (ESS) because of continued multimorbidity and polypharmacy.
The LIFE Study did not enroll participants based on the presence of sleep-wake disturbances, which could also explain the limited benefit of the physical activity intervention. Sleep-wake disturbances were generally mild at baseline, including mean values for PSQI, ISI, and ESS that were less than one-third of maximum available scores (21, 28, and 24, respectively).14–16 The mild sleep-wake disturbances might be attributable to the low rate of poor self-rated health (16.6%)13 and the exclusion of persons with mobility disability at study entry.12 Prior work has shown that reduced health status and physical disability are associated with sleep-wake disturbances.1–4
A few additional study limitations warrant comment. First, because aging may be associated with reduced symptom-awareness,47–49 it is possible that the PSQI, ISI, and ESS may have misclassified sleep-wake disturbances. Of these questionnaires, the PSQI may have been the least likely to misclassify sleep-wake disturbances because it additionally quantifies the timing and duration of sleep-wake behaviors, as well as the frequency of hypnotic medication use.16 In contrast, the ISI and ESS only included qualitative descriptors of symptoms (e.g. mild, moderate, severe, etc).14,15 Misclassification of sleep-wake disturbances may have also occurred because of the age-related “paradox of well-being.”50 The latter refers to the high level of life satisfaction in older persons, and can also include lower health expectations.50 Second, the sample size of subgroups was modest, and may have especially limited the evaluation of ethnicity/race as a modifying factor (borderline interaction between ethnicity/race and poor sleep quality [p=.06]). To address these limitations, future work will need to evaluate the effect of physical activity based on sleep-wake disturbances as the primary outcome, including severe forms that are objectively confirmed by wrist actigraphy and polysomnography, as well as a larger non-white representation.
In conclusion, using data from the largest and longest duration randomized trial of physical activity in sedentary elders with functional limitations (LIFE Study), we found that structured physical activity reduces the likelihood of new cases of poor sleep quality (PSQI>5) by a statistically significant 30%, relative to health education. No significant benefits of physical activity were shown for prevalent cases of poor sleep quality, or sleep-wake behaviors that were evaluated by the ISI and ESS. These results suggest that the beneficial effect of physical activity in this population sample is preventive and limited to sleep-wake behaviors that are evaluated by the PSQI.
Supplementary Material
Acknowledgments
Research investigators for the LIFE Study group are listed in the on-line Appendix A.
Funding: This work was supported by a National Institutes of Health/National Institute on Aging Cooperative Agreement #U01AG22376 and a supplement from the National Heart, Lung and Blood Institute 3U01AG022376, and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH. Clinicaltrials.gov identifier NCT01072500. Dr. Vaz Fragoso is the recipient of a Department of Veterans Affairs Merit Award. Dr. Gill is the recipient of an Academic Leadership Award (K07AG043587) from the National Institute on Aging. The research is partially supported by the Claude D. Pepper Older Americans Independence Centers at the University of Florida (P30AG028740), Wake Forest University (P30AG21332), Tufts University (P30AG031679), University of Pittsburgh (P30AG024827), and Yale University (P30AG021342) and the NIH/NCRR CTSA at Stanford University (UL1RR025744), University of Florida (U54RR025208) and Yale University (UL1TR000142). Tufts University is also supported by the Boston Rehabilitation Outcomes Center (R24HD065688).
Conflict of Interest Disclosures
| Financial/Personal Conflicts | Fragoso | Miller | King | Kritchesky | Liu |
|---|---|---|---|---|---|
| No | No | No | No | No | |
| Employment/Affiliation | X | X | X | X | X |
| Grants/Funds | X | X | X | X | X |
| Honoraria | X | X | X | X | X |
| Speaker Forum | X | X | X | X | X |
| Consultant | X | X | X | X | X |
| Stocks | X | X | X | X | X |
| Royalties | X | X | X | X | X |
| Expert Testimony | X | X | X | X | X |
| Board Member | X | X | X | X | X |
| Patents | X | X | X | X | X |
| Personal Relationship | X | X | X | X | X |
| Financial/Personal Conflicts | Myers | Nadkarni | Pahor | Spring | Gill |
|---|---|---|---|---|---|
| No | No | No | No | No | |
| Employment/Affiliation | X | X | X | X | X |
| Grants/Funds | X | X | X | X | X |
| Honoraria | X | X | X | X | X |
| Speaker Forum | X | X | X | X | X |
| Consultant | X | X | X | X | X |
| Stocks | X | X | X | X | X |
| Royalties | X | X | X | X | X |
| Expert Testimony | X | X | X | X | X |
| Board Member | X | X | X | X | X |
| Patents | X | X | X | X | X |
| Personal Relationship | X | X | X | X | X |
Footnotes
Author Contributions: Dr. Vaz Fragoso had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors made substantial contributions to study concept and design, to data acquisition, analysis and interpretation, and to drafting the submitted article.
Sponsor’s Role: The investigators retained full independence in the conduct of this research and report no conflicts of interest. The NIH sponsor was a voting member (1 vote out of 12 votes) of the LIFE Steering Committee, which approved the design and conduct of the study; collection, management, analysis, and interpretation of the data.
Clinicaltrials.gov identifier NCT01072500.
References
- 1.Foley DJ, Monjan AA, Brown SL, et al. Sleep complaints among elderly persons: An epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 2.National Sleep Foundation. Sleep in America Poll (Executive Summary) National Sleep Foundation; 1522 K Street NW, Suite 500, Washington, DC 20005: 2003. [Accessed 10 August 2014]. at http://sleepfoundation.org/media-center. [Google Scholar]
- 3.Foley DJ, Monjan AA, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: An epidemiologic study of 6,800 persons over three years. Sleep. 1999;22:S366–S372. [PubMed] [Google Scholar]
- 4.Vaz Fragoso CA, Gill TM. Sleep complaints in community-living older persons: a multifactorial geriatric syndrome. J Am Geriatr Soc. 2007;55:1853–1866. doi: 10.1111/j.1532-5415.2007.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoenborn CA, Adams PF. Data from the National Health Interview Survey. Health Behaviors of Adults: United States, 2005–2007. DHHS Publication No. (PHS) 2010–1573. [PubMed] [Google Scholar]
- 6.Kaye HS, Kang T, LaPlante MP. Disability Statistics Report. 14. Washington, D.C: U.S. Department of Education, National Institute on Disability and Rehabilitation Research; 2000. Mobility Device Use in the United States. [Google Scholar]
- 7.Physical Activity Guidelines for Americans. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Office of Disease Prevention and Health Promotion; 2008. [Google Scholar]
- 8.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE Study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King AC, Oman RF, Brassington GS, et al. Moderate-intensity exercise and self-rated quality of sleep in older adults: a randomized controlled trial. JAMA. 1997;277:32–37. [PubMed] [Google Scholar]
- 10.King AC, Pruitt LA, Woo S, et al. Effects of moderate-intensity exercise on polysomnographic and subjective sleep quality in older adults with mild to moderate sleep complaints. J Gerontol Med Sci. 2008;63A(9):997–1004. doi: 10.1093/gerona/63.9.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloom HG, Ahmed I, Alessi CA, et al. Evidence-based recommendations for the assessment and management of sleep disorders in older persons. J Am Geriatr Soc. 2009;57:761–789. doi: 10.1111/j.1532-5415.2009.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fielding RA, Rejeski WJ, Blair S, et al. The lifestyle interventions and independence for elders study: design and methods. J Gerontol A Biol Sci Med Sci. 2011;66A(11):1226–1237. doi: 10.1093/gerona/glr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaz Fragoso CA, Miller ME, Fielding RA, et al. on behalf of the LIFE investigators. Sleep-wake disturbances in sedentary community-dwelling elders with functional limitations. J Am Geriatr Soc. 2014;62:1064–1072. doi: 10.1111/jgs.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bastien CH, Vallieres A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Medicine. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 15.Johns M. Sleepiness in different situations measured by the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/17.8.703. [DOI] [PubMed] [Google Scholar]
- 16.Buysse DJ, Reynolds CF, III, Monk TH, et al. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatr Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 17.Marsh AP, Lovato LC, Glynn NW, et al. Lifestyle interventions and independence for elders study: recruitment and baseline characteristics. J Gerontol A Biol Sci Med Sci. 2013;68(12):1549–58. doi: 10.1093/gerona/glt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart AL, Verboncoeur CJ, McLellan BY, et al. Physical activity outcomes of CHAMPS II: a physical activity promotion program for older adults. J Gerontol A Biol Sci Med Sci. 2001;56(8):M465–M470. doi: 10.1093/gerona/56.8.m465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guralnik JM, Ferrucci K, Simonnick EM, et al. Lower extremity function over the age of 70 years as a predictor of subsequent disability. NEJM. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guralnik JM, Simonsick EM, Ferucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 21.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 22.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 23.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 24.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults. Results of the 2003 national sleep foundation sleep in America survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 25.National Sleep Foundation. Sleep in America Poll (Executive Summary) National Sleep Foundation; 1522 K Street NW, Suite 500, Washington, DC 20005: 2002. [Accessed 10 August 2014]. at http://sleepfoundation.org/media-center. [Google Scholar]
- 26.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang ES, Karter AJ, Danielson KK, et al. The association between the number of prescription medications and incident falls in a multi-ethnic population of adult type-2 diabetes patients: the diabetes and aging study. J Gen Intern Med. 2009;25(2):141–146. doi: 10.1007/s11606-009-1179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Sleep Foundation. Sleep in America Poll (Executive Summary) National Sleep Foundation; 1522 K Street NW, Suite 500, Washington, DC 20005: 2006. [Accessed 10 August 2014]. at http://sleepfoundation.org/media-center. [Google Scholar]
- 29.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167(7):875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews CE, Ainsworth BE, Thompson RW, Bassett DRJ. Sources of variance in daily physical activity levels as measured by an accelerometer. Med Sci Sports Exerc. 2002;34(8):1376–1381. doi: 10.1097/00005768-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 32.National Sleep Foundation. Sleep in America Poll (Executive Summary) National Sleep Foundation; 1522 K Street NW, Suite 500, Washington, DC 20005: 2000. [Google Scholar]
- 33.Goldstein IB, Ancoli-Israel S, Shapiro D. Relationship between daytime sleepiness and blood pressure in healthy older adults. Am J Hypertens. 2004;17:787–792. doi: 10.1016/j.amjhyper.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Davies DP, Rodgers H, Walshaw D, et al. Snoring, daytime sleepiness and stroke: a case-control study of first ever stroke. J Sleep Res. 2003;12:313–318. doi: 10.1046/j.0962-1105.2003.00371.x. [DOI] [PubMed] [Google Scholar]
- 35.Vaz Fragoso CA, Gahbauer E, Van Ness P, Gill T. Sleep-wake disturbances and frailty in community-living older persons. J Am Geriatr Soc. 2009;57:2094–2100. doi: 10.1111/j.1532-5415.2009.02522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitney CW, Enright PL, Newman AB, et al. Correlates of daytime sleepiness in 4578 elderly persons: The Cardiovascular Health Study. Sleep. 1998;21:27–36. doi: 10.1093/sleep/21.1.27. [DOI] [PubMed] [Google Scholar]
- 37.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 38.Wang R, Lagakos SW, Ware JH, et al. Statistics in medicine – reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 39.Omachi TA. Measuring sleep in rheumatologic diseases: the ESS, FOSQ, ISI, and PSQI. Arthritis Care Res. 2011;63(S11):S287–S296. doi: 10.1002/acr.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zisberg A, Gur-Yaish N, Shochat T. Contribution of routine to sleep quality in community elderly. Sleep. 2010;33:509–514. doi: 10.1093/sleep/33.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monk TH. Enhancing circadian zeitgebers. Sleep. 2010;33(4):421–422. doi: 10.1093/sleep/33.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Sleep Foundation. Sleep in America Poll (Executive Summary) National Sleep Foundation; 1522 K Street NW, Suite 500, Washington, DC 20005: 2010. [Accessed 10 August 2014]. at http://sleepfoundation.org/media-center. [Google Scholar]
- 43.Latta F, Leproult R, Tasali E, et al. Sex differences in delta and alpha EEG activities in older adults. Sleep. 2005;28:1525–34. doi: 10.1093/sleep/28.12.1525. [DOI] [PubMed] [Google Scholar]
- 44.Latta F, Leproult R, Tasali E, et al. Sex differences in nocturnal growth hormone and prolactin secretion in healthy older adults: Relationships with sleep EEG variables. Sleep. 2005;28:1519–24. doi: 10.1093/sleep/28.12.1519. [DOI] [PubMed] [Google Scholar]
- 45.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5):S7–S10. [PMC free article] [PubMed] [Google Scholar]
- 46.Budhiraja R, Roth T, Hudgel DW, et al. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. Sleep. 2011;34(7):859–867. doi: 10.5665/SLEEP.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Connolly MJ, Crowley JJ, Charan NB, et al. Reduced subjective awareness of bronchoconstriction provoked by methacholine in elderly asthmatic and normal subjects as measured on a simple awareness scale. Thorax. 1992;47:410–413. doi: 10.1136/thx.47.6.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alexander KP, Newby LK, Canno CP, et al. Acute coronary care in the elderly, part I: non–ST-segment–elevation acute coronary syndromes. Circulation. 2007;115:2549–2569. doi: 10.1161/CIRCULATIONAHA.107.182615. [DOI] [PubMed] [Google Scholar]
- 49.Zammitt NN, Frier BM. Hypoglycemia in type 2 diabetes: pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care. 2005;28:2948–2961. doi: 10.2337/diacare.28.12.2948. [DOI] [PubMed] [Google Scholar]
- 50.Levy BR. Mind matters: cognitive and physical effects of aging self-stereotypes. J Gerontol Psychol Sci. 2003;58B(4):203–211. doi: 10.1093/geronb/58.4.p203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.