Abstract
An impaired ability to regulate microglia activation by fractalkine (CX3CL1) leads to microglia chronic sub-activation. How this condition affects outcome after acute brain injury is still debated, with studies showing contrasting results depending on the timing and the brain pathology. Here, we investigated the early and delayed consequences of fractalkine receptor (CX3CR1) deletion on neurological outcome and on the phenotypical features of the myeloid cells present in the lesions of mice with traumatic brain injury (TBI). Wild type (WT) and CX3CR1−/− C57Bl/6 mice were subjected to sham or controlled cortical impact brain injury. Outcome was assessed at 4 days and 5 weeks after TBI by neuroscore, neuronal count, and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. Compared with WT mice, CX3CR1−/− TBI mice showed a significant reduction of sensorimotor deficits and lower cellular damage in the injured cortex 4 days post-TBI. Conversely, at 5 weeks, they showed a worsening of sensorimotor deficits and pericontusional cell death. Microglia (M) and macrophage (μ) activation and polarization were assessed by quantitative immunohistochemistry for CD11b, CD68, Ym1, and inducible nitric oxide synthase (iNOS)—markers of M/μ activation, phagocytosis, M2, and M1 phenotypes, respectively. Morphological analysis revealed a decreased area and perimeter of CD11b+ cells in CX3CR1−/− mice at 4 days post-TBI, whereas, at 5 weeks, both parameters were significantly higher, compared with WT mice. At 4 days, CX3CR1−/− mice showed significantly decreased CD68 and iNOS immunoreactivity, while at 5 weeks post-injury, they showed a selective increase of iNOS. Gene expression on CD11b+ sorted cells revealed an increase of interleukin 10 and insulin-like growth factor 1 (IGF1) at 1 day and a decrease of IGF1 4 days and 5 weeks post-TBI in CX3CR1−/−, compared with WT mice. These data show an early protection followed by a chronic exacerbation of TBI outcome in the absence of CX3CR1. Thus, longitudinal effects of myeloid cell manipulation at different stages of pathology should be investigated to understand how and when their modulation may offer therapeutic chances.
Key words: : fractalkine receptor, inflammation, macrophages, microglia, traumatic brain injury
Introduction
Inflammation is a central component of traumatic brain injury (TBI).1 Microglia (M) and macrophages (μ) are the main cellular contributors to post-TBI inflammation. Both cell types can contribute either to amplification or resolution of brain injury, assuming different phenotype with specific spatiotemporal patterns depending on the different stimuli.
In vitro experiments have shown that M/μ become classically activated (M1) when stimulated by interferon (IFN) γ and release pro-inflammatory cytokines, such as tumor necrosis factor (TNF) α, interleukin (IL) 12, IL6, IL1β, chemokines, nitric oxide (NO), and superoxide free radicals. Conversely, when stimulated by IL4, they can assume an alternative activated phenotype (M2), which is characterized by the production of anti-inflammatory cytokine and neurotrophic factors such as IL10, insulin-like growth factor 1 (IGF1), glial cell–derived neurotrophic factor, and brain-derived neurotrophic factor, and by the expression of specific antigens such as Ym1 and Arginase1.2 These two polarized phenotypes should be regarded as the extremes of a sort of continuum in which a mixture of the two can be found in the injured tissue.3,4
For both M and μ, the local microenvironment is emerging as a major determinant driving their activation phenotypes and functions.5 A persistent M/μ activation was recently reported in the injured brain of TBI mice up to 2 years post-injury and was associated with progressive lesion expansion, hippocampal neurodegeneration, and loss of myelin.1,6 Moreover, clinical data from TBI brain autopsies or from in vivo analysis of TBI patients by positron emission tomography have shown chronic activation of microglia up to several years after injury7,8 and a correlation between intensity of thalamic ligand binding and outcome. However, whether acute and persistent M/μ activation drives or is a marker of ongoing neuronal loss and tissue damage is still unclear.
One of the key mechanisms of M/μ regulation in physiological conditions and in the injured brain involves the interaction between fractalkine (CX3CL1) and its receptor, CX3CR1. CX3CL1 is a chemokine expressed by neurons, and it exists as a transmembrane protein with an extracellular domain. In the healthy central nervous system (CNS), CX3CL1 is cleaved by cathepsin S and proteins of the “a disintegrin and metalloproteinase” (ADAM) family, leading to the formation of the soluble form of CX3CL1. Constitutive CX3CL1 levels correspond to 1500 ± 500 pg/mg protein in normal mouse brain and ensure the maintenance of microglia in a resting state.9 Binding to CX3CR1, the receptor constitutively present on microglia cell membrane and inducible on specific subsets of macrophages,10 CX3CL1 suppresses M/μ activation, maintaining them in a non-reactive state. Importantly, CX3CL1 reduces the release of pro-inflammatory factors from M/μ.11,12 Following an acute brain insult, affected neurons significantly reduce the release of CX3CL1, allowing microglial activation.13,14
An early protective effect of CX3CR1 deletion was reported in different models of acute brain injury, including ischemia or spinal cord injury.15–19 At variance, in neurodegenerative disorders, disruption of the CX3CL1:CX3CR1 axis led to different results. The deficiency of CX3CR1 in models of Parkinson's disease and amyotrophic lateral sclerosis induced a worsening of the neurological outcome.11 In models of active experimental autoimmune encephalomyelitis (EAE), CX3CR1 deletion (CX3CR1−/−) mice exhibited more severe neurologic deficiencies, compared with wild type (WT) mice.20 CX3CR1−/− mice infected with three different strains of prions showed a significant reduction of incubation time associated with reduced survival, compared with WT mice.21 In studies conducted in Alzheimer's disease models, both protective22 or worsening23 results were reported in CX3CR1−/− mice. Overall, the data obtained in acute and chronic disease models thus suggest that the consequences of CX3CR1 deficiency may change over time. The acute beneficial effect may be transient, and at longer times, other events may take place, thus changing the impact of CX3CR1 deficiency. At present, the role of the CX3CL1:CX3CR1 axis following TBI that is an acute condition that triggers long lasting neurodegenerative processes has not been described.
In this study, we first performed a longitudinal analysis of myeloid cells in their local microenvironment to select relevant time-points of their activation after TBI in mice. Then we focused on the consequences of CX3CL1:CX3CR1 disruption on acute and delayed functional, structural outcome and M/μ activation, using CX3CR1−/− mice.
Methods
Animals
C57BL/6 WT and CX3CR1−/− mice (Harlan Laboratories, Udine, Italy and Jackson Laboratories, Sacramento, CA) were housed in a specific pathogen-free vivarium at a constant temperature (21 ± 1°C), with a 12 h light–dark cycle and ad libitum access to food and water. The IRCCS-Istituto di Ricerche Farmacologiche Mario Negri adheres to the principles set out in the following laws, regulations, and policies governing the care and use of laboratory animals: Italian Governing Law (D.lgs 26/2014; Authorization n.19/2008-A issued March 6, 2008, by Ministry of Health); Mario Negri Institutional Regulations and Policies providing internal authorization for persons conducting animal experiments (Quality Management System Certificate—UNI EN ISO 9001:2008—Reg. N° 6121); the National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011 edition), and European Union directives and guidelines (EEC Council Directive 2010/63/UE). The Statement of Compliance (Assurance) with the Public Health Service (PHS) Policy on Human Care and Use of Laboratory Animals has been recently reviewed (9/9/2014) and will expire on September 30, 2019 (Animal Welfare Assurance #A5023-01).
Experimental design
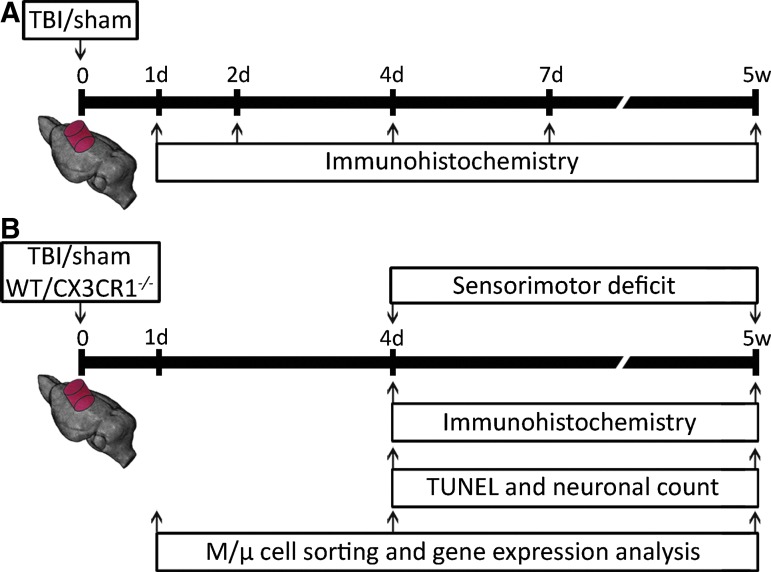
Experimental design 1 (Fig. 1A) was followed to characterize temporal M/μ activation after TBI in the pericontusional tissue obtained 1 day, 2 days, 4 days, 7 days, or 5 weeks after sham/TBI injury (n = 8 per time-point). Experimental design 2 (Fig. 1B) was followed to evaluate the consequences of CX3CR1 deletion on neurobehavioral and histopathological outcome, and on M/μ phenotypical features in TBI mice. Sensorimotor deficits were evaluated in WT/CX3CR1−/− mice 4 days and 5 weeks after sham/TBI injury (n = 8). Histological or immunohistochemical analysis was performed 4 days or 5 weeks after sham/TBI injury (n = 8). Real-time polymerase chain reaction (RT-PCR) analysis of CD11b+ cells sorted by MACS technology was performed 1 day, 4 days, or 5 weeks after sham/TBI injury.
FIG. 1.
Experimental design. (A) Pattern of microglia/macrophage (M/μ) activation post–traumatic brain injury (TBI) in wild type (WT) mice. Mice were sacrificed 1 day, 2 days, 4 days, 7 days, or 5 weeks after TBI/sham injury for immunohistochemical analysis. (B) Consequences of fractalkine receptor deletion (CX3CR1−/−) on behavioral and histopathological outcome, and on M/μ activation post-TBI. Sensorimotor deficits evaluation and histological analysis were performed in WT and CX3CR1−/− mice 4 days and 5 weeks after TBI/sham injury. Mice were sacrificed at 4 days, 7 days, or 5 weeks for M/μ immunohistochemistry and morphological analysis or at 1 day, 4 days, or 5 weeks for CD11b+ cell sorting by MACS technology and gene expression analysis. Color image is available online at www.liebertpub.com/neu
Mice were randomly allocated to surgery (sham-operated or TBI) by a list randomizer (www.random.org/list), taking care to distribute them equally across experimental days. All surgeries were performed by the same investigator, blinded to the experimental groups. All behavioral, histological, and biochemical evaluations were done by investigators unaware of genotype or injury status of the animals.
Experimental TBI
Mice were anesthetized with sodium pentobarbital (65 mg/kg intraperitoneally) with rectal temperature maintained at 37° C and placed in a stereotaxic frame. Mice were then subjected to craniectomy followed by induction of controlled cortical impact brain injury (CCI) as previously described.24–26 Briefly, the injury was induced using a 3-mm rigid impactor driven by a pneumatic piston rigidly mounted at an angle of 20° from the vertical plane and applied vertically to the exposed dura mater, between bregma and lambda, over the left parieto-temporal cortex (antero-posteriority, −2.5 mm; laterality, −2.5 mm) at impactor velocity of 5 m/sec and deformation depth of 1 mm. The craniotomy was then covered with a cranioplasty and the scalp sutured. Sham-operated mice received identical anesthesia and surgery without brain injury.
Sensorimotor function assessment
Neurologic motor function was assessed by performing the well-established neuroscore test at 4 days and 5 weeks post-TBI. In our hands, the neuroscore has proven to be a sensitive paradigm for measuring post-traumatic neurologic deficits in mice up to 1 month post-injury.26–28 The neuroscore generates a score for each individual mouse from 4 (normal) to 0 (severely impaired) for each of the following indices: 1) forelimb function, 2) hindlimb function; 3) resistance to lateral right and left pulsion, as previously described.26–28 The best score per mouse is 12.
Tissue processing for histopathological analysis
At selected times after injury, mice were killed for histological analysis. Under deep anesthesia (equitensin 120 μL/mouse), animals were transcardially perfused with 20 mL of phosphate buffer saline (PBS) 0.1 mol/L, pH 7.4, followed by 50 mL of chilled paraformaldehyde (4%) in PBS. The brains were carefully removed from the skull and post-fixed for 6 h at 4°C, and then transferred to 30% sucrose in 0.1 mol/L phosphate buffer for 24 h until equilibration. The brains were frozen by immersion in isopentane at −45°C for 3 min before being sealed into vials and stored at −80°C until use. Twenty-μm thick serial sections were cut using a cryostat from bregma +1 mm to bregma −4 mm.
TUNEL staining
To assess the presence of injured cells showing deoxyribonucleic acid (DNA) damage, terminal deoxynucleotidyl transferase-Y-mediated dUTP nick end labeling (TUNEL) staining was performed on 20 μm sections using an in situ cell death detection kit (Roche, Mannheim, Germany) according to the manufacturer's instructions. One coronal section per mouse, located at −1.5 mm from bregma, was selected and adequate negative and positive controls were performed. After staining, the sections were visualized using fluorescent microscopy (Olympus IX70; Olympus, Tokyo, Japan). Images of the area of interest were acquired using appropriate software (Cell^F Olympus). For each mouse, nine fields at 20× magnification were analyzed over the injured cortex. TUNEL-positive cells were counted using ImageJ software (http://rsbweb.nih.gov/ij/) and expressed as the number per mm2 for subsequent statistical analysis.25
Neuronal count
Cresyl violet–stained brain sections were used for neuronal count. One coronal section per mouse, located at −1.5 mm from bregma, was chosen to assess the viability of neurons in the injured cortex. An Olympus BX61 microscope, inter-faced with Soft Imaging System Colorview video camera and AnalySIS software (all Olympus) was used. For each mouse, nine fields at 40× magnification were analyzed over the injured and the contralateral cortex. The degree of neuronal loss was calculated by pooling the number of stained neurons in the sections of each hemisphere and was expressed as a percentage of the contralateral hemisphere. Fields were analyzed using the open source platform software Fiji (http://fiji.sc/Fiji)29 and segmentation was used to discriminate neurons from glia on the basis of cell size.30,31
Immunohistochemistry
Immunohistochemistry was performed on 20-μm thick brain coronal sections using anti-mouse CD11b (1:1000, kindly provided by Dr. Doni), anti-mouse CD45 (1:800; BD Biosciences Pharmingen, San Jose, CA), anti-mouse CD68 (1:200; Serotec, Kidlington, UK), anti-mouse Ym1 (1:400, Stem Cell Technologies, Vancouver, Canada), anti-mouse inducible nitric oxide synthase (iNOS; 1:50; BD Biosciences Pharmingen to measure M/μ activation, recruitment, phagocytosis, M2, and M1 polarization respectively. Positive CD11b, CD45, CD68, Ym1, and iNOS cells were stained by reaction with 3,3-diaminobenzidine-tetrahydrochloride (Vector Laboratories, Burlingame, CA) as previously described.28 For each reaction, adequate negative controls were performed. For negative control staining, the primary antibodies were omitted, and no staining was observed. CD45-positive cells displayed two morphologies: a leukocyte-like shape corresponding to cells with a rounded cell body without branches and high expression of CD45 (CD45high), and a microglia-like shape having a small cell body and several branches and a fainter expression of CD45 (CD45low). Quantification was carried out only on CD45high cells.32,33
Three brain coronal sections per mouse (at 0.4, 1.6, and 2.8 mm posterior to bregma) were used to quantify CD11b-, CD68-, iNOS-, Ym1-, and CD45-stained areas. Quantitative analysis was thus performed in the pericontusional tissue defined by anatomic boundaries and by acquiring the same focal plan throughout the samples.26,28,34 An Olympus BX61 microscope equipped with a motorized stage and managed with AnalySIS software (Olympus) was used for image acquisition. For each section, nine fields (40× magnification) were selected for quantification. The pericontusional tissue was defined as the 2200 μm wide area surrounding the lesion edge. The first raw of fields was positioned at the contusion edge, spacing each field by 361.2 μm (distance between centers of the fields). A second and a third raw of fields were positioned further from the lesion and aligned to the first raw. Distance between each raw was 722.4 μm. An immunostained area for each marker was measured using Fiji software (http://fiji.sc/Fiji),29 expressed as positive pixels/total assessed pixels, and indicated as staining percentage area or as number per mm2 for subsequent statistical analysis.
Morphological analysis
Morphological analysis was carried out on CD11b-stained sections. Image processing was performed using Fiji software.29,35 An algorithm was created to segmentate and analyze stained cells.33 Briefly, images were first scaled into microns (pixel size = 0.172 × 0.172 μm). Background was subtracted and a math operation was applied so that all the gray values greater than a specified constant were replaced by the constant. The constant was defined by an operator on the basis of the best segmentation performance on pilot images and did not change throughout the experimental groups. Images were then binarized and smoothed to best fit cell shape and get rid of single positive pixels still present in the background. A further step of pixel erosion helped to achieve satisfactory cell shape fitting. To be sure to select only cells entirely present in the acquired field, cells with area > 25 μm2 were considered for analysis. Once segmented, the objects were measured for the following parameters: area, perimeter, Feret's diameter (max caliper), circularity, and solidity.36 Mean single cell values for each parameter were used for statistics.
Immunofluorescence and confocal analysis
Immunofluorescence was performed on 20 μm coronal sections as previously described.15,32 Primary antibodies used were anti-mouse CD11b (1:30000; kindly provided by Dr. Doni) and anti-mouse CD68 (1:200; Serotec). Fluoro-conjugated secondary antibody used was Alexa 546 anti-rat (1:500; Invitrogen, Carlsbad, CA). Biotinylated anti-rat antibody (1:200; Vector Laboratories) also was used followed by fluorescent signal coupling with a streptavidin tyramide signal amplification kit (cyanine 5; Perkin Elmer, MA). Appropriate negative controls were run without the primary antibodies. None of the immunofluorescence reactions revealed unspecific fluorescent signal in the negative controls. Immunofluorescence was acquired using a scanning sequential mode to avoid bleed-through effects by an IX81 microscope equipped with a motorized stage and a confocal scan unit FV500 with three laser lines: Ar-Kr (488 nm), He-Ne red (646 nm), and He-Ne green (532 nm; Olympus) and an ultraviolet diode. Three-dimensional images were acquired over a 10 μm Z-axis with a 0.23 μm step size and processed using Imaris software (Bitplane, Zurich, Switzerland).
CD11b+ cell sorting and RT-PCR
Twenty-four hours, 4 days, and 5 weeks post-TBI, mice were killed and ipsilateral traumatized cortices were rapidly dissected out, weighted, and dissociated using MACS® neural dissociation kit (Miltenyi Biotech, Cambridge, MA). CD11b+ cells were isolated by a myelin removal and immunomagnetic cell separation kit (Myelin Removal Beads II, CD11b Microbeads; Miltenyi Biotech). CD11b+ cells were isolated according to the manufacturer's instructions, with the exception of increases in single runs of gentleMacs™ brain program #1 to three runs and brain program #3 to two runs in order to ensure complete tissue dissociation.
Total ribonucleic acid (RNA) was extracted from CD11b+ cells using the miRNeasy kit (Qiagen) according to the manufacturer's instructions. Samples of total RNA (100 ng) were treated with DNAse (Applied Biosystems, Foster City, CA) and reverse-transcripted with random hexamer primers using multiscribe reverse transcriptase (TaqMan reverse transcription reagents; Applied Biosystems). Primers were designed to span exon junctions in order to amplify only spliced RNA using PRIMER-3 software (http://frodo.wi.mit.edu/) based on GenBank accession numbers, and are indicated in Table 1. The same starting concentrations of complementary DNA template were used in all cases. RT-PCR was conducted using Power SYBR Green according to the manufacturer's instructions (Applied Biosystems). β-Actin was used as reference gene and relative gene expression levels were determined according to the ΔΔCt method (Applied Biosystems). Data are presented as fold change, compared with values of the respective control group.
Table 1.
Primers Used for RT-PCR
| Gene | NCBI refSequence | Forward primer | Reverse primer |
|---|---|---|---|
| β-actin | NM_007393.3 | GCCCTGAGGCTCTTTTCCAG | TGCCACAGGATTCCATACCC |
| IL1β | NM_008361.3 | AGTTGACGGACCCCAAAAGA | GGACAGCCCAGGTCAAAGG |
| TNFα | NM_013693.2 | AGACCCTCACACTCAGATCATCTTC | TTGCTACGACGTGGGCTACA |
| IGF1 | NM_001111274.1 | CACACTGACATGCCCAAGAC | CCTTTCCTTCTCCTTTGCAG |
| IL10 | NM_010548 | CCTGGTAGAAGTGATGCCCC | TCCTTGATTTCTGGGCCATG |
| TGFβ | NM_011577.1 | GGAGAGCCCTGGATACCAAC | CAACCCAGGTCCTTCCTAAA |
RT-PCR, real-time polymerase chain reaction; NCBI, National Center for Biotechnology Information; IL, interleukin; TNF, tumor necrosis factor, IGF, insulin-like growth factor; TGF, transforming growth factor.
Statistical analysis
The data are presented as mean ± standard deviation. Statistical analysis was performed using standard software package GraphPad Prism, version 4.0 (GraphPad Software, San Diego, CA). M/μ activation longitudinal analysis in WT mice was performed using a one-way analysis of variance (ANOVA) followed by appropriate post hoc test. The comparison between wild type and CX3CR1−/− groups was performed using two-way ANOVA followed by a proper post hoc test for all parameters considered except for the baseline gene expression of CD11b+ cells in WT and CX3CR1−/− mice, which was analyzed by Mann-Whitney t-test. A p value of 0.05 was considered statistically significant.
Results
Pattern of M/μ activation after TBI
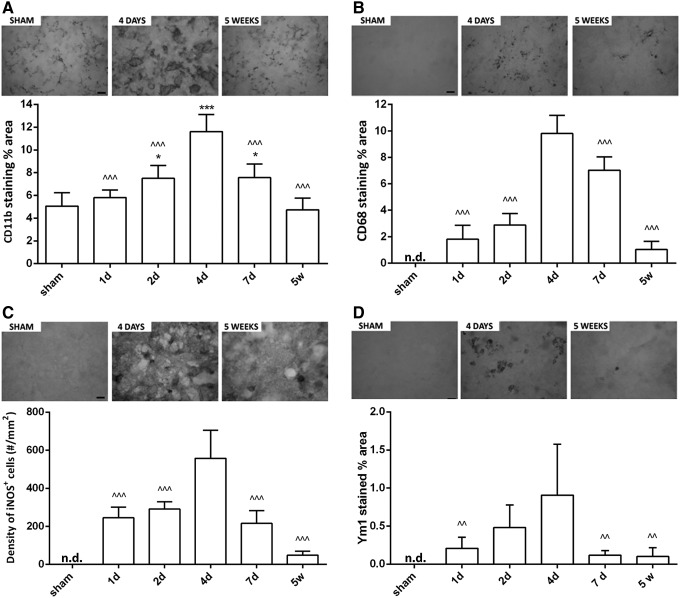
To gain insight into the post-TBI time-course of M/μ activation, a quantitative immunohistochemical analysis for CD11b, CD68, iNOS, and Ym1 was performed in WT sham and TBI mice at 1 day, 2 days, 4 days, 7 days, and 5 weeks (Fig. 1A). CD11b, a pan-marker of M/μ was present in sham-operated mice. Starting from 2 days post-TBI, CD11b immunoreactivity increased in the pericontusional tissue and was maximally expressed at 4 days. CD11b immunoreactivity was still elevated at 7 days and returned to baseline values 5 weeks after injury (Fig. 2A). CD68 staining was undetectable in sham-operated mice. Starting from 1 day post-TBI, CD68 immunoreactivity increased in the pericontusional tissue and was maximally expressed at 4 days. CD68 staining was still evident 5 weeks post-TBI (Fig. 2B). M1 and M2 polarization was investigated by measuring iNOS and Ym1 staining, respectively. These two markers were undetectable in sham-operated mice. Inducible NOS immunoreactivity was detectable starting from 1 day post-TBI. This protein was maximally expressed at 4 days, markedly decreased at 7 days and was still detectable 5 weeks after injury (Fig. 2C). Ym1 immunoreactivity appeared at 1 day and reached the maximal expression at 4 days. Seven days and 5 weeks post-TBI, Ym1 immunoreactivity was low but still present in the pericontusional tissue (Fig. 2D).
FIG. 2.
Quantitative immunohistochemical analysis of microglia/macrophage (M/μ) markers in wild type (WT) mice after traumatic brain injury (TBI)/sham injury. Quantification of CD11b (A), CD68 (B), inducible nitric oxide synthase (iNOS) (C), and Ym1 (D) immunostaining in WT mice 1 day, 2 days, 4 days, 7 days, and 5 weeks after TBI/sham injury. Above, representative microphotographs of each marker in sham and TBI mice at the time-point of marker maximal expression and at 5 weeks (bar = 20 μm). Below, quantification of the staining. Data are expressed as mean ± standard deviation of 27 frames/mouse, n = 8. *p < 0.05 vs sham; ^^p < 0.01, ^^^p < 0.001 vs. 4 days for all selected markers, one-way analysis of variance followed by Tukey's post hoc test. n.d., not detected.
Consequences of CX3CR1 deletion after TBI
To investigate the role of CX3CL1:CX3CR1 signaling post-TBI, we used CX3CR1−/− mice. Sensorimotor deficits, histopathological determinations (TUNEL count, neuronal count), and assessment of M/μ activation and polarization were performed over time post-TBI in WT and CX3CR1−/− mice (Fig. 1B).
Neurological outcome
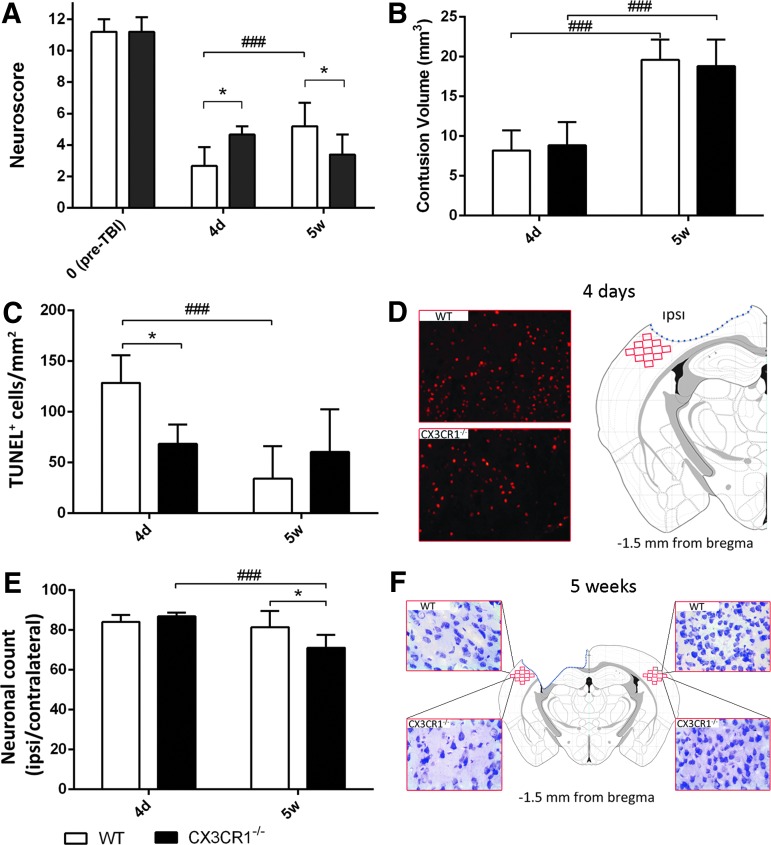
No difference in sensorimotor performance was observed between WT and CX3CR1−/− at baseline (Fig. 3A). All injured mice showed sensorimotor deficits that persisted for the entire duration of the study. As expected, the sensorimotor deficits of WT mice recovered during the observation period showing a significant amelioration at 5 weeks, compared with 4 days post-TBI (p < 0.05; Fig. 3A). CX3CR1−/− injured mice showed significantly attenuated sensorimotor deficits (p < 0.05), compared with WT mice 4 days post-TBI. Of note, no recovery over time was detected in these mice, thus leading to a significantly worst performance at 5 weeks, compared with WT mice (p < 0.05; Fig. 3A).
FIG. 3.
Early and late outcome in wild type (WT) and fractalkine receptor deletion (CX3CR1−/−) mice post–traumatic brain injury (TBI). (A) Functional damage assessed by neuroscore. Before TBI, CX3CR1−/− and WT mice showed a similar neuroscore. After TBI, CX3CR1−/− mice showed a significant reduction of sensorimotor deficits at an early time-point (4 days post-TBI), compared with WT mice. Recovery over time was detected in WT but not in CX3CR1−/− mice, thus leading to a significantly worst performance of CX3CR1−/− mice, compared with WT mice at 5 weeks. (B) Contusion volume. Over time, both WT and CX3CR1−/− mice showed progressive cortical tissue loss with no difference between genotypes. (C, D) DNA damage. WT but not CX3CR1−/− mice showed a decrease in the number of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)+ cells over time post-TBI. Compared with WT mice, CX3CR1−/− mice showed a significant decrease in TUNEL+ cells at 4 days (C) as shown in the representative microphotographs (D). (E, F) Neuronal count. CX3CR1−/− but not WT mice showed a cell loss over time in traumatized cortex. At 5 weeks post-TBI, CX3CR1−/− mice showed a significant lower number of neuronal cells, compared with WT mice (E), as shown in the representative microphotographs (F). Fields for TUNEL evaluation and quantification and for neuronal counts were positioned as depicted in the D and F, respectively. Data are expressed as mean ± standard deviation, n = 11. *p < 0.05, ###p < 0.001, two-way analysis of variance followed by Tukey's post hoc test. Color image is available online at www.liebertpub.com/neu
TBI, as expected, caused a cavity of which the volume increased over time. Traumatized WT and CX3CR1−/− mice displayed a similar lesion volume and no significant difference was found among the two genotypes over time (Fig. 3B).
Four days post-TBI, an intense TUNEL-positive labeling was evident in the injured cortex of WT mice, indicating the presence of damaged, dying cells. In WT mice, TUNEL-positive cells significantly decreased over time (p < 0.01; Fig. 3C). CX3CR1−/− mice displayed significantly less TUNEL-positive cells 4 days post-TBI, compared with WT mice (p < 0.01; Fig. 3C, 3D). In CX3CR1−/− mice, TUNEL-positive cells however did not decrease over time. The indication of an exacerbated cell death at delayed time-points in CX3CR1−/− mice was in line with the results obtained by neuronal count. Four days post-TBI, the neuronal count performed in the injured cortex of WT mice revealed a 20% neuronal loss, compared with the uninjured contralateral area (Fig. 3E), with no changes over time. At variance, CX3CR1−/− mice showed a progressive loss of neuronal cells over time (p < 0.001). Accordingly, CX3CR1−/− mice showed a significantly lower neuronal count 5 weeks post-TBI, compared with WT mice (p < 0.05; Fig. 3E, 3F).
Functional and histopathological data consistently indicate that the lack of a CX3CL1:CX3CR1 pair determines protection during an early phase, but it worsens neurological outcome at delayed time-points post-TBI.
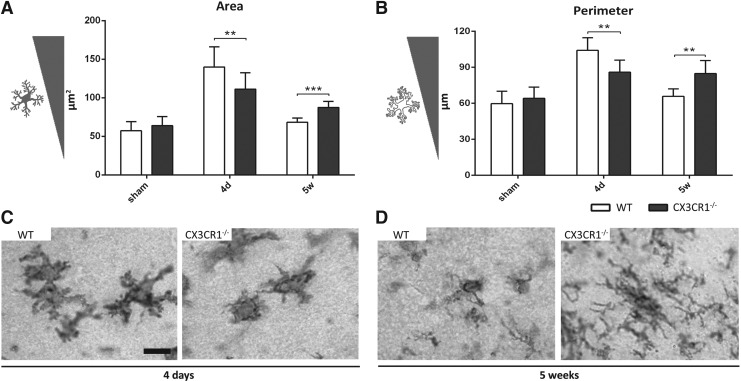
Myeloid cell morphological changes
Due to its presence on membrane surface and to its constitutive expression on myeloid cells, CD11b is particularly suitable to study M/μ morphology. In WT mice, the mean area and perimeter of CD11b+ cells were increased in the pericontusional cortex, compared with sham mice, reaching a peak 4 days post-injury and declining to sham levels at 5 weeks (Fig. 4A, 4B). M/μ cells in CX3CR1−/− mice displayed a significantly lower mean area and perimeter 4 days post-TBI, compared with WT mice (p < 0.01; Fig. 4A-C). Conversely, 5 weeks post-TBI, they displayed a significantly higher area and perimeter, compared with WT mice (p < 0.001 and p < 0.01, respectively; Fig. 4A, 4B, 4D). These data indicate that CX3CL1:CX3CR1 interaction controls M/μ hypertrophic shape.
FIG. 4.
Morphological analysis of microglia/macrophage (M/μ) in wild type (WT) and fractalkine receptor deletion (CX3CR1−/−) mice 4 days and 5 weeks after traumatic brain injury (TBI)/sham injury. CD11b+ cells showed an increase of the area (A) and the perimeter (B) at 4 days after TBI in WT mice, followed by a decrease at 5 weeks post-injury. Compared with WT mice, CX3CR1−/− mice showed a significant reduction of CD11b+ cell area (A) and perimeter (B) at 4 days followed by a significant increase of both parameters 5 weeks after TBI. Representative microphotographs show the typical morphology of M/μ cells in WT and CX3CR1−/− mice at 4 days (C) and 5 weeks (D). Data are reported as mean ± SD, n = 8. **p < 0.01, ***p < 0.001. Two-way analysis of variance followed by Tukey's post hoc test.
No differences in terms of circularity, solidity, Feret's diameter were detected among genotypes at the considered time-points (data not shown).
Quantitative immunohistochemical analysis of M/μ markers after TBI in WT and CX3CR1−/− mice
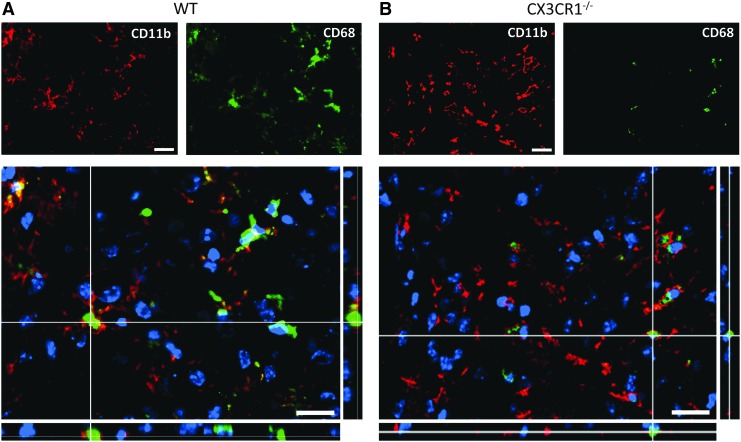
To assess if the different M/μ morphology observed in WT and CX3CR1−/− mice was associated to a different M/μ activation and polarization, we performed a quantitative immunohistochemical analysis of M/μ functional markers in the pericontusional tissue of WT and CX3CR1−/− mice. Similarly to what was observed in WT mice, Cd11b was present in CX3CR1−/− sham-operated mice. At 4 days after TBI, CD11b expression significantly increased in CX3CR1−/− mice, compared with sham mice (Fig. 5A), and returned to baseline values 5 weeks after injury. This pattern of expression was not different from WT mice (Fig. 5A). Similarly to what was observed in WT mice, CD68 staining was undetectable in CX3CR1−/− sham-operated mice. At 4 days post-injury, the expression of CD68 was greatly induced in CX3CR1−/− (Fig. 5B) and significantly decreased at 5 weeks after TBI. Compared with WT mice, CX3CR1−/− mice showed a higher expression of CD68 at 4 days (p < 0.01), while no difference was evident at 5 weeks after TBI (Fig. 5B). Qualitative analysis of CD11b and CD68 co-expression confirmed that in WT mice, CD68 was greatly increased in CD11b+ cells, displaying a hypertrophic morphology 4 days post-TBI (Fig. 6A, 6B). Of interest, CD68 often co-localized with the membrane marker CD11b in WT mice, while it remained mainly located in the cytoplasm in CX3CR1−/− mice, suggesting a lower degree of active phagocytosis in CX3CR1−/− mice, compared with WT mice (Fig. 6A, 6B).
FIG. 5.
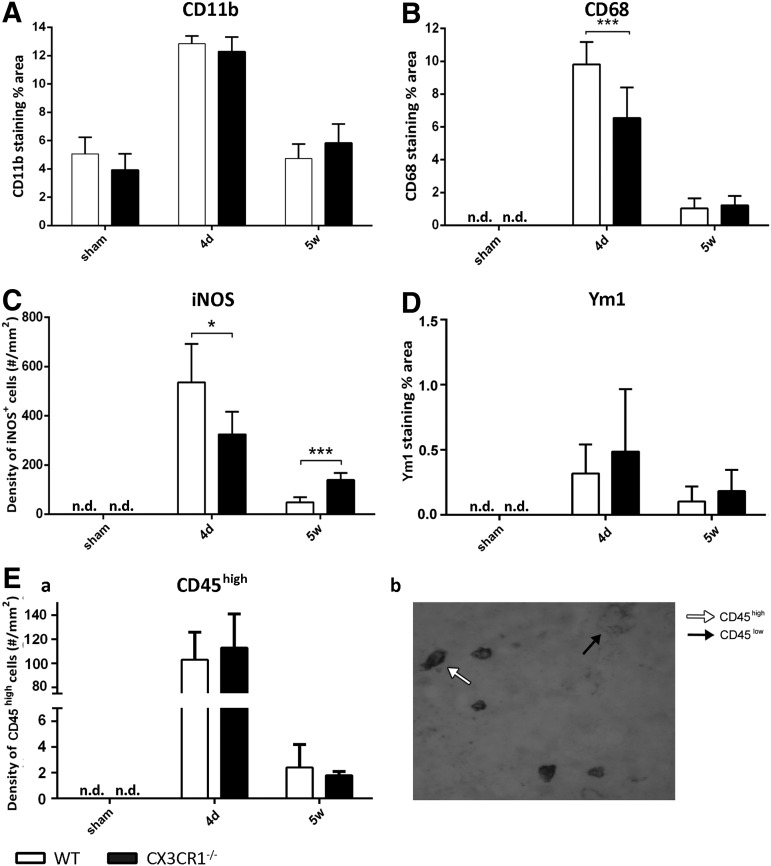
Quantitative immunohistochemical analysis of microglia/macrophage (M/μ) markers in wild type (WT) and fractalkine receptor deletion (CX3CR1−/−) mice 4 days and 5 weeks after traumatic brain injury (TBI)/sham injury. (A) No difference between genotypes was detected for CD11b+ immunostaining. (B) Compared with WT mice, CX3CR1−/− mice showed a reduction of lysosomal activity (CD68+ positivity) at 4 days post-TBI. (C) Compared with WT mice, CX3CR1−/− mice showed a significant decrease (4 days) followed by a significant increase (5 weeks) in the density of inducible nitric oxide synthase (iNOS)+ cells. (D, E-a). No differences between genotypes were detected for Ym1 and high expression of CD45 (CD45high) immunostaining. (E-b) Representative microphotograph showing the typical appearance of round-shaped CD45high cells (white arrow) selectively counted. Data are reported as mean ± standard deviation (n = 8). *p < 0.05, ***p < 0.001, two-way analysis of variance followed by Tukey's post hoc test. n.d., not detected.
FIG. 6.
Coexpression of CD11b (red) and CD68 (green) 4 days post–traumatic brain injury in wild type (WT) and fractalkine receptor deletion (CX3CR1−/−) mice. CD68 was greatly increased in CD11b+ cells displaying a hypertrophic morphology in WT mice. CD68 often co-localized with the membrane marker CD11b in WT mice, while it remained mainly located in the cytoplasm in CX3CR1−/− mice. Data are representative of three independent experiments. Bars: 20 μm. Color image is available online at www.liebertpub.com/neu
Similarly to what was observed in WT mice, iNOS and Ym1 were undetectable in sham-operated CX3CR1−/− mice. At 4 days post-TBI, the expression of the M1 marker iNOS was greatly induced in CX3CR1−/− mice and significantly decreased at 5 weeks. Compared with WT mice, CX3CR1−/− mice showed a lower degree of expression at 4 days (p < 0.05; Fig. 5C). Notably, iNOS decreased from 4 days to 5 weeks in both strains; however it was reduced to a lower extent in knockout mice, remaining more elevated, compared with WT mice at 5 weeks (p < 0.001; Fig. 5C). At 4 days post-TBI, the expression of the M2 marker Ym1 was induced in CX3CR1−/− mice and significantly decreased at 5 weeks. No difference among genotypes was detected for Ym1 expression (Fig. 5D). CD45high-positive cells displayed a rounded morphology and corresponded to recruited macrophages, neutrophils, and lymphocytes (Fig. 5E-b). CD45high cells were undetectable in sham-operated WT and CX3CR1−/− mice. At 4 days post-TBI, the immunoreactivity of CD45high cells was clearly evident in WT mice and significantly decreased at 5 weeks. The immunoreactivity of CD45high cells in CX3CR1−/− over time was similar to what was observed in WT mice, revealing no differences between genotypes in terms of recruited cells (Fig. 5E).
Gene expression analysis of CD11b+ cells
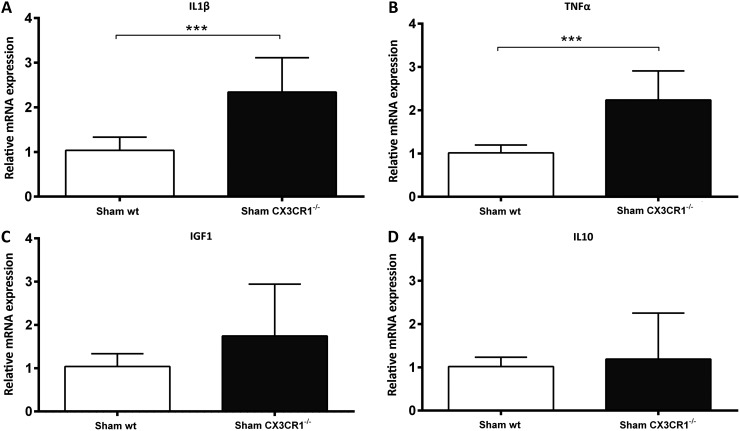
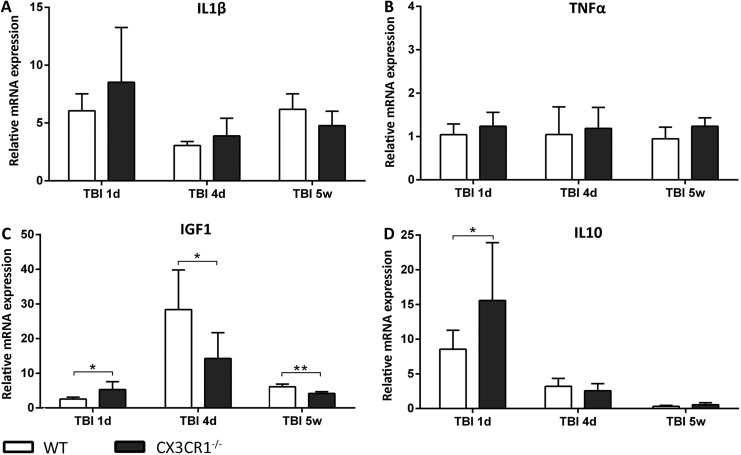
In order to better characterize myeloid cells post-TBI in WT and CX3CR1−/− mice, we isolated CD11b+ cells by MACS technology and analyzed gene expression by RT-PCR. First, we compared the genotype-dependent gene expression differences in M/μ isolated from sham animals. M/μ from sham CX3CR1−/− mice displayed higher IL1β and TNFα messenger RNA (mRNA) expression, compared with sham WT mice (p < 0.001; Fig. 7A, 7B), while no differences were detected for IGF1 and IL10 (Fig. 7C, 7D). Then, we compared WT and CX3CR1−/−M/μ gene expression post-TBI (Fig. 8). M/μ obtained from WT and CX3CR1−/− TBI mice displayed similar IL1β and TNFα mRNA expression at all time-points considered (Fig. 8A, 8B). Twenty-four hours post-TBI, CX3CR1−/− M/μ displayed a higher expression of the M2 marker IGF1 and the anti-inflammatory IL10, compared with WT (both p < 0.05; Fig. 8C, 8D). Conversely, 4 days and 5 weeks post-injury, IGF1 expression was significantly lower in CX3CR1−/− mice, compared with WT mice (p < 0.05 and p < 0.01, respectively; Fig. 8C).
FIG. 7.
Gene expression analysis of CD11b+ sorted cells in wild type (WT) and fractalkine receptor deletion (CX3CR1−/−) non-injured (sham) mice. (A, B) Compared with WT sham, CX3CR1−/− sham mice had higher interleukin (IL) 1β and tumor necrosis factor (TNF) α messenger RNA expression. (C, D) No differences between genotypes were detected for sham expression of insulin-like growth factor 1 (IGF1) and IL10. Data are expressed as fold of induction, compared with the WT sham group, and are reported as mean ± standard deviation (n = 8). ***p < 0.001, Mann Whitney t-test.
FIG. 8.
Gene expression analysis of CD11b+ sorted cells in wild type (WT) and fractalkine receptor deletion (CX3CR1−/−) mice 1 day, 4 days, and 5 weeks after traumatic brain injury (TBI). Compared with WT mice, CX3CR1−/− mice displayed significantly higher insulin-like growth factor 1 (IGF1) and interleukin (IL) 10 messenger ribonucleic acid (mRNA) expression 1 day post-TBI (C, D). At 4 days and 5 weeks, however, a switch in IGF1 mRNA expression was observed, being IGF1 significantly lower in CX3CR1−/− mice. Data are expressed as fold of induction, compared with the WT sham group, and are reported as mean ± standard deviation (n = 8). *p < 0.05 vs. WT, two-way analysis of variance followed by Tukey's post hoc test.
Discussion
This study shows that CX3CR1 deletion in TBI mice induces early protection and late neuro-worsening, which are associated to specific M/μ polarization. This suggests that CX3CL1:CX3CR1 signaling exacerbates the toxic cascades at early time-points, whereas it is needed for long-term recovery in TBI. This is the first evidence of a dual role of the CX3CL1:CX3CR1 pair after acute brain injury, thus providing a unifying interpretation of the apparently controversial literature on the consequences of CX3CR1 deletion in different brain conditions when assessed at acute or chronic stages.
We initially performed a longitudinal analysis of myeloid cells in their local microenvironment by selecting antigens associated with pan M/μ activation (CD11b), phagocytosis (CD68), M2 activation (Ym1), and M1 activation (iNOS), and identified the time-points relevant for their functional commitment. These markers were selected based on our own previous data15,28,30,34 and on other published data37–39 showing that iNOS (for M1) and Ym1 (for M2), more than other markers (such as CD206), are highly responsive to genetic15,34 or stem cell–mediated28 manipulation of inflammatory cells after brain injury. We revealed an early and sustained M/μ activation and phagocytosis, reaching maximal levels in the first week post-TBI and showing a prolonged presence of CD68 positivity up to 5 weeks. Ym1 immunoreactivity was maximal 4 days after TBI, being drastically reduced at 7 days. At 5 weeks post-TBI, a small amount of Ym1-positive cells were still present in pericontusional tissue, in agreement with previous observations.6 Inducible NOS+ cells peaked 4 days post-TBI and gradually decreased thereafter, being present up to 5 weeks post-TBI. The results show an intense M/μ activation in the first week following TBI with characteristics of mixed M1 and M2 phenotypes with temporal kinetics of M/μ polarization in gray matter that are consistent with that reported by others using a similar model of CCI.40–42
Having identified 4 days as the critical time-point for M/μ functional polarization in the pericontusional tissue, we assessed the consequences of CX3CL1:CX3CR1 signaling in acute (4 days) and delayed (5 weeks) M/μ activation and its relation with functional and structural outcome using CX3CR1−/− mice. At 4 days post-injury, CX3CR1−/− mice displayed a significant improvement in sensorimotor performance associated with significant decrease of pericontusional TUNEL-positive cells, compared with WT mice. At 5 weeks post-TBI, CX3CR1−/− mice displayed significantly worse neurological performance, together with reduced neuronal count, in spite of no effects on contusion volume. The lesion volume measurements represent the macroscopic assessment of tissue disruption at the injury site, whereas cell counts quantify cellular death or survival in the remaining pericontusional cortex. Our results indicate that the absence of CX3CR1 does not affect the lesion progression by 5 weeks but it significantly worsens cellular viability in the tissue surrounding the injury, thus hampering neurological recovery. Whether macroscopic effects on contusion volume progression may appear at later time-points cannot be excluded.
This is the first evidence of a dual role of the CX3CL1:CX3CR1 pair after acute brain injury, and specifically for TBI exacerbating toxic cascades at early times but being needed for reparative/protective events later on. Our data allow the reinterpretation of the available literature that provides apparently controversial results on the consequences of CX3CR1 deletion when assessed either at acute or delayed time-points after injury. Specifically, in three independent studies,15,19,43 ischemic stroke modeled by transient middle cerebral artery occlusion (tMCAo) delivered to CX3CR1−/− mice has been associated with reduced histological damage evaluated at early time-points (within 4 days). Conversely, the only study evaluating the delayed (but not the acute) effects of CX3CR1 deficiency following tMCAo showed a worsening of cognitive outcome associated with an increase in M1 polarized microglial cells.14 After spinal cord injury (SCI), when CX3CL1:CX3CR1 signaling was abolished, neurological recovery was improved, spinal cord pathology reduced, and the phenotype and function of M/μ were different from that normally present after SCI18 at both 5 days and 35 days. A follow up study showed, however, an impaired locomotor function associated with an increase in infiltrating M/μ cells when assessed at 42 days post-injury.44
The results obtained in our study allow the proposal of a unifying interpretation of the literature data, highlighting a dual role for CX3CL1 and its receptor regulating microglial function at different time-points after acute brain injury. We report that the loss of CX3CL1:CX3CR1 interaction is associated with a different neuroinflammatory response in TBI, mediated by M/μ. The homeostatic role of CX3CL1 provides inhibitory signals to microglia, keeping them in a non-reactive state.45,46 The disruption of the CX3CL1:CX3CR1 axis abrogates this inhibitory effect, leading to a state of M/μ sub-activation. Accordingly, we have documented an increased expression of IL1β and TNFα mRNA levels in CX3CR1−/− sham mice, compared with WT mice. This sub-activation profile is in accordance with the results by Rogers and co-workers showing increased protein levels of hippocampal IL1β and cerebellar TNFα in CX3CR1−/− mice, compared with WT mice.47 Post-TBI, CX3CR1−/− mice showed an increased expression of IGF1 and IL10 mRNA, with a similar expression of pro-inflammatory markers, compared with WT mice, at 1 day. At 4 days post-TBI, WT but not CX3CR1−/− mice showed a M/μ with hypertrophic morphology (area and perimeter significant increase), together with a high expression of the phagocytic marker CD68 and iNOS. The data thus suggest that M/μ chronic sub-activation may favor a protective M/μ reaction to injury at early time-points and show that CX3CL1 action may contribute to M/μ hypertrophic shape.
Conversely, at chronic stages post-TBI, the lack of a functional CX3CL1:CX3CR1 axis led to detrimental M/μ hyper-activation. Indeed, at 5 weeks post-injury, we observed an opposite phenotype in CX3CR1−/− mice, characterized by a worsening of neurological deficits, an increased neuronal loss and a more active M/μ (as shown by the increased values of area and perimeter parameters) associated with an increased protein expression of the marker iNOS and a decreased expression of IGF1 mRNA. This suggests that the lack of a functional CX3CL1:CX3CR1 pair induced a less plastic M/μ activation with toxic effects at chronic stages. Of note, in experimental and clinical neurodegenerative conditions, fractalkine restrains chronic unremitting neuroinflammation by mobilizing nuclear factor (erythroid-derived 2)-like 2 (NRF2).48 NRF2 is a crucial player in the antioxidant protection of microglial cells, thus avoiding the pernicious effects of oxidative stress and long lasting over-activation of the M1 phenotype.49 An additional potential mechanism to be mentioned involves the recruitment of different macrophage subsets over time. Macrophages belonging to the CX3CR1-dependent pathway (nonclassical monocyte subset) possess reparative features, such as tissue healing function.10 CX3CR1 deficiency would impair the recruitment of this protective population, thus resulting in a chronic detrimental phenotype.50
Our study did not dissect a specific role for M and μ but rather focused on myeloid cells as a whole, considering that the local environment is a major determinant of their polarization state and effectors functions.36,51,52 The immunohistochemical approaches detailed in the study allowed us to carefully describe the activation state of the myeloid cells in their local environment, so as to keep the information of cell location relative to the lesion and on their morphology. We focused on the pericontusional area, where myeloid cell modifications typically appear as a consequence of treatments or manipulations.26,28 Notably, our approaches also allowed us to gain insight on cell morphological changes, which are key to myeloid cell activity.36,53,54 We acknowledge that full information on the activation state of M/μ subsets requires analysis of several markers. In this study, we focused on a few well-accepted M/μ markers and provided their detailed longitudinal characterization. To gain insight into the functional meaning of M polarization, we sorted CD11b-positive cells from the injured area and focused on critical factors produced by microglia in relation to the activation states. IL1β and TNFα, typically associated with the M1 phenotype, were selected to describe the pro-inflammatory action.55–59 Among candidate survival factors, the rationale for selecting IGF1, commonly associated with M2 polarization state, was supported by the data by Ueno and colleagues,60 who showed how microglia are an important source of IGF1 that is specific for M, abundantly expressed, and linked to neuronal survival. IL10, which is part of the M2 or M2-like repertoire, was selected as a prototype of anti-inflammatory activity.37,61,62
Our data, in line with the early observation by Scherbel and colleagues63 of a dual role of TNFα at different stages after TBI, highlight the controversial action of cellular and molecular inflammatory cascades at different times after injury. Inhibiting inflammation after TBI is a challenge since it possesses both detrimental and beneficial properties.61 For each target, the definition of the therapeutic window is therefore a priority. Recently, a selective, high-affinity small molecule inhibitor of CX3CR1 (AZD8797) has been developed.64 This molecule has the potential to be administered as an oral drug in humans, and in a model of EAE in rats has shown promising results reducing paralysis, CNS pathology, and incidence of relapses in treated, compared with untreated rats.65 The possibility to selectively target CX3CR1 acutely after injury opens new therapeutic perspectives whose potential should be tested in experimental TBI.
TBI is a leading cause of death and disability in the world. After initial injury, complex and variable pathophysiological mechanisms participate to determine the final outcome. Among these, one key mechanism is neuroinflammation, whose delayed nature sparks the interest on inflammatory cells—local modulators of pathology—as potential cellular targets for reducing disease burden. In this study, we document a dual role of the CX3CL1:CX3CR1 axis being toxic early on but protective at later stages in TBI. These findings globally suggest that M/μ signaling pathways may yield opposite outcomes depending on the time or disease stages. Thus, longitudinal effects of M/μ manipulation at different stages of pathology should always be carefully investigated to understand how and when M/μ modulation may offer therapeutic chances.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kumar A. and Loane D.J. (2012). Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain. Behav. Immun. 26, 1191–1201 [DOI] [PubMed] [Google Scholar]
- 2.Colton C.A. (2009). Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 4, 399–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosser D.M. and Edwards J.P. (2008). Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsieh C.L., Kim C.C., Ryba B.E., Niemi E.C., Bando J.K., Locksley R.M., Liu J., Nakamura M.C., and Seaman W.E. (2013). Traumatic brain injury induces macrophage subsets in the brain. Eur. J. Immunol. 43, 2010–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fumagalli S., Perego C., Pischiutta F., Zanier E.R., and De Simoni M.G. (2015). The ischemic environment drives microglia and macrophage function. Front. Neurol. 6, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loane D.J., Kumar A., Stoica B.A., Cabatbat R., and Faden A.I. (2014). Progressive neurodegeneration after experimental brain trauma: association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 73, 14–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramlackhansingh A.F., Brooks D.J., Greenwood R.J., Bose S.K., Turkheimer F.E., Kinnunen K.M., Gentleman S., Heckemann R.A., Gunanayagam K., Gelosa G., and Sharp D.J. (2011). Inflammation after trauma: microglial activation and traumatic brain injury. Ann. Neurol. 70, 374–383 [DOI] [PubMed] [Google Scholar]
- 8.Johnson V.E., Stewart J.E., Begbie F.D., Trojanowski J.Q., Smith D.H., and Stewart W. (2013). Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain J. Neurol. 136, 28–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardona A.E., Sasse M.E., Liu L., Cardona S.M., Mizutani M., Savarin C., Hu T., and Ransohoff R.M. (2008). Scavenging roles of chemokine receptors: chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood 112, 256–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nahrendorf M., Swirski F.K., Aikawa E., Stangenberg L., Wurdinger T., Figueiredo J.L., Libby P., Weissleder R., and Pittet M.J. (2007). The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardona A.E., Pioro E.P., Sasse M.E., Kostenko V., Cardona S.M., Dijkstra I.M., Huang D., Kidd G., Dombrowski S., Dutta R., Lee J.C., Cook D.N., Jung S., Lira S.A., Littman D.R., and Ransohoff R.M. (2006). Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 9, 917–924 [DOI] [PubMed] [Google Scholar]
- 12.Denes A., Vidyasagar R., Feng J., Narvainen J., McColl B.W., Kauppinen R.A., and Allan S.M. (2007). Proliferating resident microglia after focal cerebral ischaemia in mice. J. Cereb. Blood Flow Metab. 27, 1941–1953 [DOI] [PubMed] [Google Scholar]
- 13.Tarozzo G., Campanella M., Ghiani M., Bulfone A., and Beltramo M. (2002). Expression of fractalkine and its receptor, CX3CR1, in response to ischaemia-reperfusion brain injury in the rat. Eur. J. Neurosci. 15, 1663–1668 [DOI] [PubMed] [Google Scholar]
- 14.Briones T.L., Woods J., and Wadowska M. (2014). Chronic neuroinflammation and cognitive impairment following transient global cerebral ischemia: role of fractalkine/CX3CR1 signaling. J. Neuroinflammation 11, 13. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Fumagalli S., Perego C., Ortolano F., and De Simoni M.G. (2013). CX3CR1 deficiency induces an early protective inflammatory environment in ischemic mice. Glia 61, 827–842 [DOI] [PubMed] [Google Scholar]
- 16.Soriano S.G., Amaravadi L.S., Wang Y.F., Zhou H., Yu G.X., Tonra J.R., Fairchild-Huntress V., Fang Q., Dunmore J.H., Huszar D., and Pan Y. (2002). Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. J. Neuroimmunol. 125, 59–65 [DOI] [PubMed] [Google Scholar]
- 17.Cipriani R., Villa P., Chece G., Lauro C., Paladini A., Micotti E., Perego C., De Simoni M.G., Fredholm B.B., Eusebi F., and Limatola C. (2011). CX3CL1 is neuroprotective in permanent focal cerebral ischemia in rodents. J. Neurosci. 31, 16327–16335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnelly D.J., Longbrake E.E., Shawler T.M., Kigerl K.A., Lai W., Tovar C.A., Ransohoff R.M., and Popovich P.G. (2011). Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. J. Neurosci. 31, 9910–9922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dénes A., Ferenczi S., Halász J., Környei Z., and Kovács K.J. (2008). Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J. Cereb. Blood Flow Metab. 28, 1707–1721 [DOI] [PubMed] [Google Scholar]
- 20.Garcia J.A., Pino P.A., Mizutani M., Cardona S.M., Charo I.F., Ransohoff R.M., Forsthuber T.G., and Cardona A.E. (2013). Regulation of adaptive immunity by the fractalkine receptor during autoimmune inflammation. J. Immunol. 1950 191, 1063–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grizenkova J., Akhtar S., Brandner S., Collinge J., and Lloyd S.E. (2014). Microglial Cx3cr1 knockout reduces prion disease incubation time in mice. BMC Neurosci. 15, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuhrmann M., Bittner T., Jung C.K.E., Burgold S., Page R.M., Mitteregger G., Haass C., LaFerla F.M., Kretzschmar H., and Herms J. (2010). Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer's disease. Nat. Neurosci. 13, 411–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho S.H., Sun B., Zhou Y., Kauppinen T.M., Halabisky B., Wes P., Ransohoff R.M., and Gan L. (2011). CX3CR1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer disease. J. Biol. Chem. 286, 32713–32722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longhi L., Perego C., Ortolano F., Zanier E.R., Bianchi P., Stocchetti N., McIntosh T.K., and De Simoni M.G. (2009). C1-inhibitor attenuates neurobehavioral deficits and reduces contusion volume after controlled cortical impact brain injury in mice. Crit. Care Med. 37, 659–665 [DOI] [PubMed] [Google Scholar]
- 25.Ortolano F., Colombo A., Zanier E.R., Sclip A., Longhi L., Perego C., Stocchetti N., Borsello T., and De Simoni M.G. (2009). c-Jun N-terminal kinase pathway activation in human and experimental cerebral contusion. J. Neuropathol. Exp. Neurol. 68, 964–971 [DOI] [PubMed] [Google Scholar]
- 26.Zanier E.R., Montinaro M., Vigano M., Villa P., Fumagalli S., Pischiutta F., Longhi L., Leoni M.L., Rebulla P., Stocchetti N., Lazzari L., and De Simoni M.G. (2011). Human umbilical cord blood mesenchymal stem cells protect mice brain after trauma. Crit. Care Med. 39, 2501–2510 [DOI] [PubMed] [Google Scholar]
- 27.Pischiutta F., D'Amico G., Dander E., Biondi A., Biagi E., Citerio G., De Simoni M.G., and Zanier E.R. (2014). Immunosuppression does not affect human bone marrow mesenchymal stromal cell efficacy after transplantation in traumatized mice brain. Neuropharmacology 79, 119–126 [DOI] [PubMed] [Google Scholar]
- 28.Zanier E.R., Pischiutta F., Riganti L., Marchesi F., Turola E., Fumagalli S., Perego C., Parotto E., Vinci P., Veglianese P., D'Amico G., Verderio C., and De Simoni M.G. (2014). Bone marrow mesenchymal stromal cells drive protective M2 microglia polarization after brain trauma. Neurother. J. Am. Soc. Exp. Neurother. 11, 679–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.Y., White D.J., Hartenstein V., Eliceiri K., Tomancak P., and Cardona A. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perego C., Fumagalli S., and De Simoni M.G. (2011). Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J. Neuroinflammation 8, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longhi L., Orsini F., De Blasio D., Fumagalli S., Ortolano F., Locatelli M., Stocchetti N., and De Simoni M.G. (2014). Mannose-binding lectin is expressed after clinical and experimental traumatic brain injury and its deletion is protective. Crit. Care Med. 42, 1910–1918 [DOI] [PubMed] [Google Scholar]
- 32.Gesuete R., Storini C., Fantin A., Stravalaci M., Zanier E.R., Orsini F., Vietsch H., Mannesse M.L.M., Ziere B., Gobbi M., and De Simoni M.G. (2009). Recombinant C1 inhibitor in brain ischemic injury. Ann. Neurol. 66, 332–342 [DOI] [PubMed] [Google Scholar]
- 33.Perego C., Fumagalli S., and De Simoni M.G. (2011). Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J. Neuroinflammation 8, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longhi L., Perego C., Ortolano F., Aresi S., Fumagalli S., Zanier E.R., Stocchetti N., and De Simoni M.G. (2013). Tumor necrosis factor in traumatic brain injury: effects of genetic deletion of p55 or p75 receptor. J. Cereb. Blood Flow Metab. 33, 1182–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor S.E., Morganti-Kossmann C., Lifshitz J., and Ziebell J.M. (2014). Rod microglia: a morphological definition. PloS One 9, e97096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zanier E.R., Fumagalli S., Perego C., Pischiutta F., and De Simoni M.G. (2015). Shape descriptors of the “never resting” microglia in three different acute brain injury models in mice. Intensive Care Med. Exp. 3, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.David S. and Kroner A. (2011). Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 12, 388–399 [DOI] [PubMed] [Google Scholar]
- 38.Sica A. and Mantovani A. (2012). Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar A., Stoica B.A., Sabirzhanov B., Burns M.P., Faden A.I., and Loane D.J. (2013). Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol. Aging 34, 1397–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turtzo L.C., Lescher J., Janes L., Dean D.D., Budde M.D., and Frank J.A. (2014). Macrophagic and microglial responses after focal traumatic brain injury in the female rat. J. Neuroinflammation 11, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang G., Zhang J., Hu X., Zhang L., Mao L., Jiang X., Liou A.K.F., Leak R.K., Gao Y., and Chen J. (2013). Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J. Cereb. Blood Flow Metab. 33, 1864–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bedi S.S., Smith P., Hetz R.A., Xue H., and Cox C.S. (2013). Immunomagnetic enrichment and flow cytometric characterization of mouse microglia. J. Neurosci. Methods 219, 176–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang Z., Gan Y., Liu Q., Yin J.X., Liu Q., Shi J., and Shi F.D. (2014). CX3CR1 deficiency suppresses activation and neurotoxicity of microglia/macrophage in experimental ischemic stroke. J. Neuroinflammation 11, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blomster L.V., Brennan F.H., Lao H.W., Harle D.W., Harvey A.R., and Ruitenberg M.J. (2013). Mobilisation of the splenic monocyte reservoir and peripheral CX3CR1 deficiency adversely affects recovery from spinal cord injury. Exp. Neurol. 247, 226–240 [DOI] [PubMed] [Google Scholar]
- 45.Wolf Y., Yona S., Kim K.W., and Jung S. (2013). Microglia, seen from the CX3CR1 angle. Front. Cell. Neurosci. 7, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biber K., Neumann H., Inoue K., and Boddeke H.W.G.M.(2007). Neuronal “On” and “Off” signals control microglia. Trends Neurosci. 30, 596–602 [DOI] [PubMed] [Google Scholar]
- 47.Rogers J.T., Morganti J.M., Bachstetter A.D., Hudson C.E., Peters M.M., Grimmig B.A., Weeber E.J., Bickford P.C., and Gemma C. (2011). CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J. Neurosci. Off. J. Soc. Neurosci. 31, 16241–16250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lastres-Becker I., Innamorato N.G., Jaworski T., Rábano A., Kügler S., Van Leuven F., and Cuadrado A. (2014). Fractalkine activates NRF2/NFE2L2 and heme oxygenase 1 to restrain tauopathy-induced microgliosis. Brain J. Neurol. 137, 78–91 [DOI] [PubMed] [Google Scholar]
- 49.Rojo A.I., McBean G., Cindric M., Egea J., López M.G., Rada P., Zarkovic N., and Cuadrado A. (2014). Redox control of microglial function: molecular mechanisms and functional significance. Antioxid. Redox Signal. 21, 1766–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prinz M. and Priller J. (2010). Tickets to the brain: role of CCR2 and CX3CR1 in myeloid cell entry in the CNS. J. Neuroimmunol. 224, 80–84 [DOI] [PubMed] [Google Scholar]
- 51.Cohen M., Matcovitch O., David E., Barnett-Itzhaki Z., Keren-Shaul H., Blecher-Gonen R., Jaitin D.A., Sica A., Amit I., and Schwartz M. (2014). Chronic exposure to TGFβ1 regulates myeloid cell inflammatory response in an IRF7-dependent manner. EMBO J. 33, 2906–2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gosselin D., Link V.M., Romanoski C.E., Fonseca G.J., Eichenfield D.Z., Spann N.J., Stender J.D., Chun H.B., Garner H., Geissmann F., and Glass C.K. (2014). Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cunningham C.L., Martínez-Cerdeño V., and Noctor S.C. (2013). Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 33, 4216–4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McWhorter F.Y., Wang T., Nguyen P., Chung T., and Liu W.F. (2013). Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. U. S. A. 110, 17253–17258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barone F.C., Arvin B., White R.F., Miller A., Webb C.L., Willette R.N., Lysko P.G., and Feuerstein G.Z. (1997). Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke 28, 1233–1244 [DOI] [PubMed] [Google Scholar]
- 56.Rothwell N., Allan S., and Toulmond S. (1997). The role of interleukin 1 in acute neurodegeneration and stroke: pathophysiological and therapeutic implications. J. Clin. Invest. 100, 2648–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lambertsen K.L., Meldgaard M., Ladeby R., and Finsen B. (2005). A quantitative study of microglial-macrophage synthesis of tumor necrosis factor during acute and late focal cerebral ischemia in mice. J. Cereb. Blood Flow Metab. 25, 119–135 [DOI] [PubMed] [Google Scholar]
- 58.Amantea D., Bagetta G., Tassorelli C., Mercuri N.B., and Corasaniti M.T. (2010). Identification of distinct cellular pools of interleukin-1beta during the evolution of the neuroinflammatory response induced by transient middle cerebral artery occlusion in the brain of rat. Brain Res. 1313, 259–269 [DOI] [PubMed] [Google Scholar]
- 59.Amantea D., Micieli G., Tassorelli C., Cuartero M.I., Ballesteros I., Certo M., Moro M.A., Lizasoain I., and Bagetta G. (2015). Rational modulation of the innate immune system for neuroprotection in ischemic stroke. Front. Neurosci. 9, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ueno M., Fujita Y., Tanaka T., Nakamura Y., Kikuta J., Ishii M., and Yamashita T. (2013). Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci. 16, 543–551 [DOI] [PubMed] [Google Scholar]
- 61.Ziebell J.M. and Morganti-Kossmann M.C. (2010). Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurother. J. Am. Soc. Exp. Neurother. 7, 22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vitkovic L., Maeda S., and Sternberg E. (2001). Anti-inflammatory cytokines: expression and action in the brain. Neuroimmunomodulation 9, 295–312 [DOI] [PubMed] [Google Scholar]
- 63.Scherbel U., Raghupathi R., Nakamura M., Saatman K.E., Trojanowski J.Q., Neugebauer E., Marino M.W., and McIntosh T.K. (1999). Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc. Natl. Acad. Sci. U. S. A. 96, 8721–8726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karlström S., Nordvall G., Sohn D., Hettman A., Turek D., Åhlin K., Kers A., Claesson M., Slivo C., Lo-Alfredsson Y., Petersson C., Bessidskaia G., Svensson P.H., Rein T., Jerning E., Malmberg Å., Ahlgen C., Ray C., Vares L., Ivanov V., and Johansson R. (2013). Substituted 7-amino-5-thio-thiazolo[4,5-d]pyrimidines as potent and selective antagonists of the fractalkine receptor (CX3CR1). J. Med. Chem. 56, 3177–3190 [DOI] [PubMed] [Google Scholar]
- 65.Ridderstad Wollberg A., Ericsson-Dahlstrand A., Juréus A., Ekerot P., Simon S., Nilsson M., Wiklund S.J., Berg A.L., Ferm M., Sunnemark D., and Johansson R. (2014). Pharmacological inhibition of the chemokine receptor CX3CR1 attenuates disease in a chronic-relapsing rat model for multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 111, 5409–5414 [DOI] [PMC free article] [PubMed] [Google Scholar]