Abstract
Skin barrier defects play an important role in atopic dermatitis (AD). Involucrin, an important barrier protein suppressed in human AD, is downregulated by interleukin-4 (IL-4). However, the molecular mechanism for IL-4 downregulation of involucrin has not been delineated, and especially how Stat6, a transcriptional activator, represses involucrin expression is unknown. Since Stats usually recruit p300/CBP in the general transcription machinery of their target genes and involucrin expression also involves p300/CBP, we hypothesize that Stat6 activated by IL-4 may sequestrate p300/CBP from the involucrin transcription complex, thus suppressing involucrin expression in keratinocytes. Using IL-4 transgenic mice, an AD mouse model, we find that involucrin expression is similarly downregulated as in human AD. In HaCat cells, the Jak inhibitor and dominant negative studies indicate that the Jaks-Stat6 pathway is involved in IL-4 downregulation of involucrin. Next, we transfected HaCat cells with an involucrin promoter–luciferase construct and then treated them with IL-4. IL-4 greatly suppresses the promoter activity, which is totally abolished by cotransfecting the CREB-binding protein (CBP) expression vector, indicating that IL-4 cannot downregulate involucrin in the presence of excess CBP. Finally, chromatin immunoprecipitation assay demonstrates that IL-4 decreases CBP binding to the involucrin transcription complex. For the first time, we defined a molecular mechanism for IL-4 downregulation of involucrin in keratinocytes, which may play an important role in the pathogenesis of AD.
Introduction
Atopic dermatitis (AD), a chronic inflammatory skin disease, affects widely in developed countries. Although the etiology and pathogenesis of AD are not fully understood, it is generally accepted that skin barrier defects play an essential role (Chan 2008; Schittek 2011; Simon and Kernland Lang 2011). Genetically, several skin barrier protein polymorphisms have been linked to AD (Henry and others 2011; Lan and others 2011; Knuppel and others 2012; Margolis and others 2012; Paternoster and others 2012; Ring and others 2012). In addition, dysregulated immune responses may also affect skin barrier function, contributing to the development and progression of this disease (Segre 2006; Boguniewicz and Leung 2011; Eichenfield and others 2012). We and others have shown that interleukin-4 (IL-4), a Th2 cytokine significantly upregulated in AD, plays a central role in the pathophysiology of this disease (Chen and others 2004, 2008; Bao and others 2012b, 2013b; Shi and others 2012). In fact, overexpressing IL-4 in the basal epidermis generated a mouse AD model (Chan and others 2001). The IL-4 transgenic (Tg) mice spontaneously develop skin lesions, which meet the clinical and histological diagnostic criteria for human AD (Hanifin and Rajka 1980; Chen and others 2006). Importantly, the clinical relevance of IL-4 in AD was recently demonstrated in a clinical study, in which the AD patients had statistically significant clinical responses to an IL-4 receptor α antibody (Beck and others 2013).
Involucrin, which is an important barrier protein, is downregulated in AD (Kim and others 2008, 2013; Suarez-Farinas and others 2011; Theerawatanasirikul and others 2012). In addition, Kim and others (2008) reported that IL-4 downregulated involucrin expression in human keratinocytes, and they further reported that involucrin expression was suppressed in Stat6 Tg mice, suggesting that IL-4 suppression of involucrin is through a Stat6-dependent mechanism (Kim and others 2008). Recently, using organotypic skin models, van den Bogaard and others (2013) found that coal tar diminished IL-4 and IL-13 downregulation of involucrin. However, the detailed molecular mechanism for IL-4 downregulation of involucrin has not been delineated, and especially how Stat6, a transcriptional activator, represses involucrin expression is totally unknown.
Involucrin regulation in keratinocytes has been extensively investigated (Eckert and others 2004; Crish and others 2006; Crish and Eckert 2008; Sinitsyna and others 2010; Han and others 2012; Chew and others 2013). The transcription factors AP-1, SP1, and CCAAT-enhancer-binding protein (C/EBP), as well as the coactivator p300/CBP, were reported to play important roles in the upregulation of involucrin (Eckert and others 2004; Tran and Crowe 2004; Crish and Eckert 2008; Sinitsyna and others 2010). Stat6, the main transcription factor mediating the IL-4 signal pathway in keratinocytes (Bao and others 2013c), has not been shown to directly bind to the involucrin promoter. However, Stats proteins are known to recruit p300/CBP in the general transcription machinery of their target genes. These findings led us to hypothesize that IL-4-activated Stat6 may suppress involucrin expression indirectly by recruiting limited p300/CBP to IL-4 target genes, thus sequestrating the coactivator from the involucrin general transcription machinery.
In this study, we have demonstrated that involucrin is similarly suppressed in IL-4 Tg mice as in human AD. For the first time, we clearly delineate a molecular mechanism for IL-4 downregulation of involucrin in human keratinocytes, which may play an important role in the pathogenesis of AD.
Materials and Methods
Materials
Human recombinant IL-4, charcoal-stripped fetal bovine serum (FBS), Dulbecco's modified Eagle's medium (DMEM), FBS, penicillin/streptomycin, reverse transcriptase (RT), Alexa Fluor 594 secondary antibody, and Trizol reagent were from Invitrogen (Carlsbad, CA); anti-involucrin antibody was purchased from Sigma (St. Louis, MO); Jak inhibitor I was from Calbiochem (La Jolla, CA); SYBR Green PCR Master Mix and Effectene Transfection Reagent were obtained from Qiagen, Inc. (Valencia, CA); passive lysis buffer and Dual-Luciferase Reporter Assay were purchased from Promega (Madison WI); the involucrin promoter reporter construct was a gift from Dr. Robert H. Rice (University of California, Davis, Davis, CA) (Phillips and others 2000); and the dominant negative (DN)-Stat6, DN-Stat3, DN-Stat5a, and DN-Stat5b were kindly provided, respectively, by Dr. Steven M. Dubinett (University of California at Los Angeles, Los Angeles, CA) (Cui and others 2007), Dr. Toshio Hirano (Osaka University, Osaka, Japan) (Nakajima and others 1996), Dr. Alice Mui (University of British Columbia, British Columbia, Canada) (Mui and others 1996), and Dr. Li-yuan (Baylor College of Medicine, Houston, TX) (Luo and Yu-Lee 1997).
Mice
The IL-4 Tg mice were kept at 25°C in a pathogen-free environment. Skin tissues were collected from both the IL-4 Tg mice and wild-type mice. All experimental procedures were performed in accordance with the principles of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Illinois at Chicago.
Cell culture
HaCat cells, a human keratinocyte cell line, were grown in DMEM (calcium 1.8 mM) supplemented with FBS (10%), nonessential amino acids, and penicillin/streptomycin. The cells were treated with various concentrations of IL-4 in DMEM supplemented with 1% charcoal–dextran-treated FBS for 24 h. The cells were incubated in a humidified atmosphere of 5% CO2 at 37°C and media were replaced every 48 h.
RNA isolation and real-time RT-PCR analysis
Total RNA from cell culture and mouse skin was isolated using Trizol reagent according to the manufacturer's instructions. The RT and real-time polymerase chain reactions (PCRs) were performed as previously described (Bao and others 2007). Briefly, 1 μg of total RNA was reverse transcribed and the final volume was 100 μL. Five and 10 μL of cDNA was used for GAPDH and involucrin, respectively. For each real-time PCR (25 μL total volume), cDNA was mixed with 2× SYBR Green PCR Master Mix containing the following primers: the mouse involucrin primers used were 5′-CAT CTG AGA CAG CAC CAG GA-3′ and 5′-TGC TGC TTT TCT CCT GGA AT-3′; the mouse GAPDH primers used were 5′-AGA ACA TCA TCC CTG CAT CC-3′ and 5′-ACA CAT TGG GGG TAG GAA CA-3′; the human involucrin primers used were 5′-TGC CTG AGC AAG AAT GTG AG-3′ and 5′-TGC TCT GGG TTT TCT GCT TT-3′; and the human GAPDH primers used were 5′-ACA CCC ACT CCT CCA CCT TT-3′ and 5′-TGC TGT AGC CAA ATT CGT TG-3′. The PCR was carried out in duplicate, and the samples were analyzed on a Stratagene Mx3000 real-time PCR machine (Santa Clara, CA).
Immunofluorescence microscopy
Immunofluorescence microscopy was performed as described previously (Shi and others 2012). Briefly, mouse ear tissues were fixed in 4% paraformaldehyde solution followed by immersion overnight in 30% sucrose solution and then embedded in OCT compound (Fisher Scientific, Pittsburgh, PA). Cryosections were incubated with anti-involucrin antibody (Sigma) overnight at 4°C followed by incubation with Alexa Fluor 594-conjugated secondary antibody for 1 h at room temperature. The slides were mounted in the Vectashield medium (Vector Laboratories, Inc., Burlingame, CA) containing DAPI counterstain and examined using a Zeiss LSM 510 Meta Confocal Microscope (Oberkochen, Germany) equipped with a 63× water immersion objective lens.
Western blot analysis
Western blots were performed as described previously (Bao and others 2012b). Briefly, 8 μg of cellular proteins were separated on a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel under reducing condition and transferred to a PVDF membrane. The membrane was blocked by the Li-Cor blocking buffer (Li-Cor, Lincoln, NE) for an hour at room temperature. Membranes were then incubated with the primary antibody overnight at 4°C on a rocking platform. After a series of washes, membranes were incubated with Li-Cor secondary antibodies linked to infrared dyes (IRDye 680 or IRDye 800) for 1 h at room temperature. Finally, the blots were scanned by a Li-Cor Odyssey machine to obtain immunofluorescence images and quantification was done by ImageJ (NIH, Bethesda, MD).
Transfection of HaCat cell
Effectene transfection was performed according to the manufacturer's instructions. Briefly, HaCat cells were seeded in 12-well plates and cultured until 60%–80% confluency. An equal amount of DNA was transfected per well. Eighteen hours after transfection, the cells were treated with IL-4 (20 ng/mL) for another 24 h. Transient expression of the reporter gene was quantified by a standard luciferase assay and normalized against Renilla Luciferase. Luciferase activities were measured using a luminometer (Lumat LB 9507 luminometer; EG&G Berthold, Oak Ridge, TN).
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) was performed using the Sigma Imprint® ChIP kit according to the manufacturer's instructions. Briefly, 100-mm dishes were used. When cells were 60%–80% confluent, they were treated with IL-4 for 24 h (20 ng/mL). DNA was sheared into 500–1,000 bp pieces. An anti-CREB-binding protein (CBP) antibody (Cell Signaling Technology, Danvers, MA) was incubated with the samples at room temperature for 90 min on an orbital shaker. After cross-link reversal and DNA purification, real-time PCR was performed on the samples and inputs. The primers used are 5′-CCC TAA AGG GTT TGC TGC TT-3′ and 5′-GGT TGA AGG TGA TGG ACA GG-3′. PCR products were purified and sequenced.
Coimmunoprecipitation
HaCat cells were incubated in 1% charcoal–dextran-treated FBS for 24 h and then were treated with IL-4 (20 ng/mL) or vehicle in a serum-free medium for 20 min. Coimmunoprecipitation (Co-IP) was performed using Pierce™ Co-Immunoprecipitation Kit (Invitrogen) according to the the manufacturer's instructions. Briefly, 500 μg of protein was immunoprecipitated with the anti-CBP antibody and analyzed along with 10% input by an anti-Stat6 antibody (Cell Signaling Technology) in Western blotting.
Statistical analysis
Data were examined by t-test (Figs. 1, 2B, 4A and 5), one-way ANOVA followed by the Tukey test (Figs. 2A, 3A and 4B), or two-way ANOVA followed by Bonferroni post-tests (Fig. 3B) using Prism software (GraphPad Software, Inc., San Diego, CA). Values were considered statistically significant at P < 0.05.
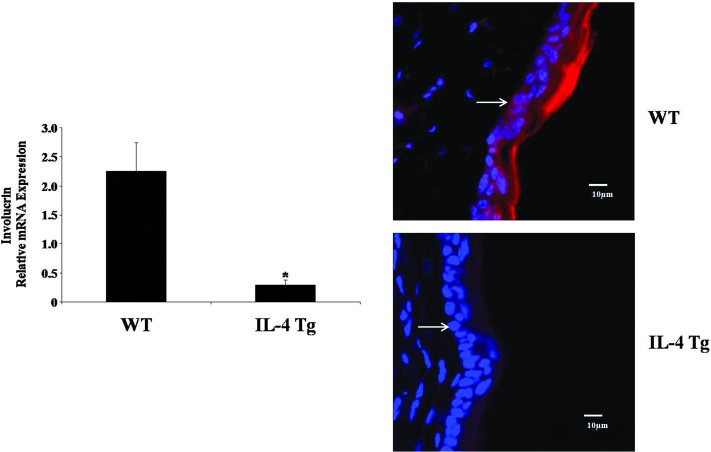
FIG. 1.
Involucrin expression is downregulated in the skin of interleukin-4 (IL-4) transgenic (Tg) mice. The expression of involucrin was compared between wild-type (WT) mice and IL-4 Tg mice at both mRNA and protein levels. Mouse GAPDH was used as the internal control. Values are expressed as the mean ± SEM (n = 3). *P < 0.05 versus WT mice. For immunofluorescence microscopy, involucrin, red; nucleus, blue. Arrow denotes the dermal–epidermal border. Experiments were repeated three times.
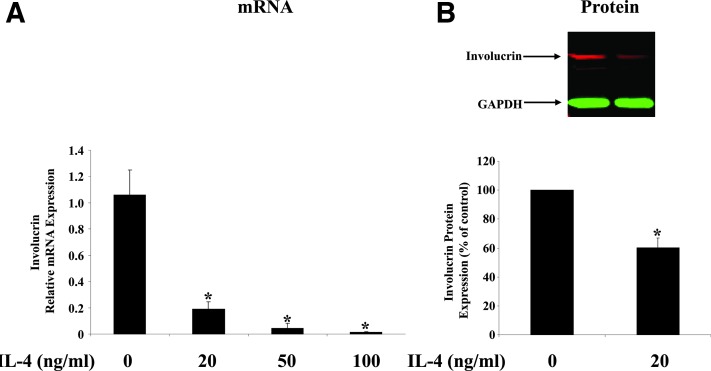
FIG. 2.
IL-4 downregulates the expression of involucrin in HaCat cells. HaCat cells, a human keratinocyte cell line, were treated with different doses of IL-4 for 24 h. Involucrin expression was analyzed by real-time reverse transcription polymerase chain reaction (RT-PCR) (A) and Western blot analysis (B). Human GAPDH was used as the internal control. Values are expressed as the mean ± SEM (n = 3). *P < 0.05 versus 0 ng/mL.
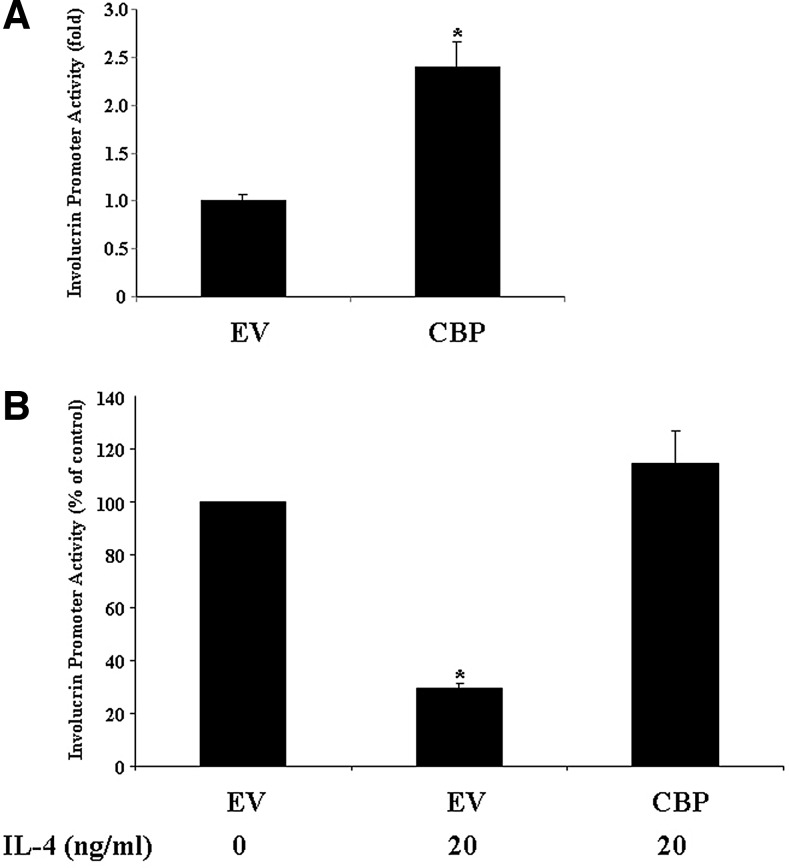
FIG. 4.
The suppression of involucrin promoter activity by IL-4 is abolished by a CREB-binding protein (CBP) expression vector in keratinocytes. HaCat cells were cotransfected with the involucrin promoter reporter construct and with an EV or the CBP expression vector for 48 h without IL-4 treatment (A). HaCat cells were cotransfected with the involucrin promoter reporter construct and with the EV or the CBP expression vector for 18 h. Cells were then treated with either IL-4 (20 ng/mL) or vehicle for another 24 h (B). Transient expression of the reporter gene was quantified by the firefly luciferase assay and normalized against Renilla Luciferase. The experiment was repeated three times with triplicate wells for each group. Values are expressed as the mean ± SEM. *P < 0.05 versus vehicle.
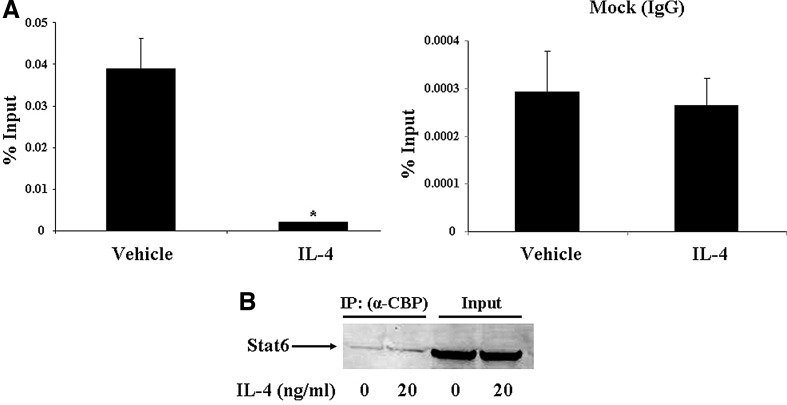
FIG. 5.
IL-4 decreases CBP binding to the involucrin promoter. HaCat cells were treated with IL-4 (20 ng/mL) or vehicle for 24 h. Chromatin immunoprecipitation assay was performed to study CBP binding to the involucrin promoter using an anti-CBP antibody (A). Data were quantified by real-time PCR and PCR products were sequenced. Values are expressed as the mean ± SEM (n = 3). *P < 0.05 versus vehicle. HaCat cells were incubated in 1% charcoal–dextran-treated fetal bovine serum (DMEM) for 24 h and then treated with IL-4 (20 ng/mL) or vehicle in the serum-free medium for 20 min. Coimmunoprecipitation was performed to study CBP interaction with Stat6 as described in Materials and Methods section (B). The experiment was repeated three times.
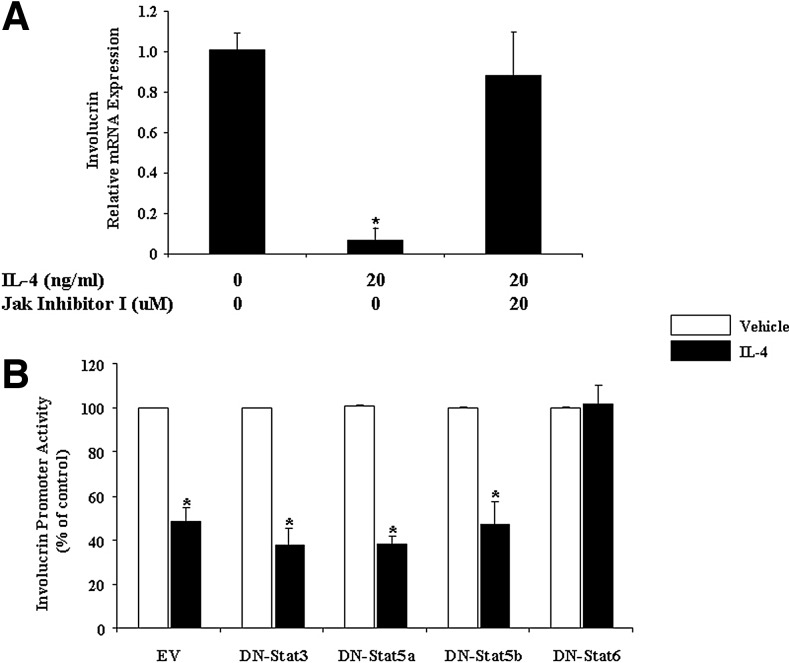
FIG. 3.
The Jak–Stat6 pathway is involved in IL-4 regulation of involucrin in HaCat Cells. (A) Total RNA obtained from HaCat cells treated with IL-4 and Jak inhibitor I (a pan-Jak inhibitor) was subjected to real-time RT-PCR analysis. Human GAPDH was used as the internal control. Values are expressed as the mean ± SEM (n = 3). *P < 0.05 versus control. (B) HaCat cells were cotransfected with an involucrin promoter reporter construct and an empty vector (EV), dominant negative (DN)-Stat3, DN-Stat5a, DN-Stat5b, or DN-Stat6 for 18 h. Cells were then treated with either IL-4 (20 ng/mL) or vehicle for another 24 h. Transient expression of the reporter gene was quantified by a firefly luciferase assay and normalized against Renilla Luciferase. The experiment was repeated three times with triplicate wells for each group. Values are expressed as the mean ± SEM. *P < 0.05 versus vehicle.
Results
Involucrin expression is downregulated in the skin of IL-4 Tg mice
Since involucrin expression is suppressed in human AD (Kim and others 2008; Suarez-Farinas and others 2011), we examined whether involucrin is also downregulated in IL-4 Tg mice, a well-established AD animal model (Chan and others 2001). As shown in Fig. 1, involucrin is downregulated at both the mRNA and protein levels in the IL-4 Tg mice compared with the wild-type mice, which is consistent with clinical findings.
IL-4 downregulates the expression of involucrin in HaCat cells
Next, we asked the question of whether the suppression is due to the direct action of IL-4 in the epidermis using an in vitro method. We treated HaCat cells with increasing doses of IL-4 for 24 h, and we found that IL-4 downregulates involucrin expression in a dose-dependent manner (Fig. 2A). The data are consistent with a previous report in primary human keratinocytes (Kim and others 2008). Since primary keratinocytes are very refractory to transfection, we did the transfection studies on HaCat cells. This cell line has been widely used to study signal transduction pathways in keratinocytes. IL-4 also suppresses involucrin expression at the protein level in HaCat cells (Fig. 2B). A similar suppressive effect of IL-4 on involucrin protein expression was reported recently in human esophageal epithelial cells (Shan and others 2014).
The Jak-Stat6 pathway is involved in IL-4 downregulation of involucrin in keratinocytes
Kim and others (2008) reported that involucrin is downregulated in the Stat6 Tg mice, suggesting indirectly that IL-4 downregulation of involucrin could be mediated by the Jak–Stat pathway. To further investigate whether this pathway is indeed involved, we treated HaCat cells with IL-4 plus Jak inhibitor I, a pan-Jak inhibitor. As shown in Fig. 3A, Jak inhibitor I totally eliminates the IL-4 effect. This finding suggests that Jak(s) may play roles in IL-4 downregulation of involucrin. Since the selectivity of the commercially available “individual Jak-specific inhibitors” is limited, now we still cannot definitively determine which Jak(s) is the main player. Next, we tested which Stat is involved in involucrin regulation by cotransfecting HaCat cells with an involucrin promoter–luciferase construct and with an empty vector, DN-Stat3, DN-Stat5a, DN-Stat5b, or DN-Stat6 (Fig. 3B). The data from this experiment clearly showed that only DN-Stat6 eliminates IL-4 downregulation of involucrin (Fig. 3B). Previously, we used Western blot to demonstrate that IL-4 stimulates the phosphorylation of both Stat3 and Stat6 among different Stat family members in keratinocytes (Bao and others 2012b), it appears that only Stat6 is functionally involved in IL-4 regulation of involucrin in keratinocytes. Put together, the Jak inhibitor and DN studies allow us to determine clearly that IL-4 downregulation of involucrin is through the Jaks–Stat6 pathway.
The downregulation of involucrin by IL-4 is due to Stat6 sequestration of the coactivator CBP from the involucrin transcription complex
p300/CBP is essential for involucrin transcription. As shown in Fig. 4A, CBP stimulates the involucrin promoter activity significantly. Since Stat6 has not been shown to directly bind to the involucrin promoter, which lacks a perfect Stat6 response element, we tested our hypothesis that IL-4 downregulation of involucrin may be caused by IL-4-activated Stat6 sequestrating p300/CBP from the involucrin transcription complex. We transfected HaCat cells with the involucrin promoter–luciferase construct. We also contransfected the cells with either an empty vector or a CBP expression vector. Eighteen hours after transfection, we treated the cells with IL-4 or vehicle. As shown in Fig. 4B, IL-4 treatment dramatically decreases the involucrin promoter activity, which is consistent with the real-time PCR data. However, in the presence of excess CBP (due to CBP transfection), the IL-4 suppressive effect on involucrin promoter activity was abolished. This suggests that the downregulation of involucrin by IL-4 is through a competitively inhibitory process, whereby IL-4-activated Stat6 may recruit CBP to the general transcription machinery of IL-4 target genes, thus preventing involucrin transcription factors from using this key coactivator for involucrin transcription.
Previously, it was reported that p300/CBP is recruited to the AP-1 site in the involucrin promoter (Eckert and others 2004). Next, we used a CBP antibody to immunoprecipitate all the endogenous CBP protein and used a pair of primers surrounding the AP-1 site of the involucrin promoter. We found that IL-4 indeed decreases the amount of CBP recruited to the involucrin promoter (Fig. 5A). As a negative control, IgG has no effect. DNA sequencing of the PCR products proved that we successfully amplified a piece of the involucrin promoter containing the AP-1 site (Phillips and others 2000; Sinitsyna and others 2010). Finally, we used Co-IP to demonstrate that CBP physically interacts with Stat6 in HaCat cells (Fig. 5B). Interestingly, IL-4 treatment does not increase this interaction. These data are consistent with what Gingras and others (1999) reported in COS7 cells. Although Stat6 activation is required for nuclear translocation, unactivated Stat6 can be coimmunoprecipitated with p300/CBP, which suggests that the Stat6/CBP interaction per se is independent of Stat6 activation (Gingras and others 1999).
Discussion
Skin barrier defects play essential roles in the pathogenesis of AD. Extensive studies on filaggrin have indicated that the defects may be caused by hereditary polymorphisms as well as dysregulated immune responses. Compared with filaggrin, studies on involucrin are relatively scarce. It was only known that involucrin expression is suppressed in AD (Eckert and others 2004; Crish and others 2006; Crish and Eckert 2008; Sinitsyna and others 2010; Han and others 2012; Chew and others 2013) and IL-4 was reported to downregulate its expression in keratinocytes, but the molecular mechanism for the downregulation was not clear.
We have extensively studied IL-4-regulated genes in kerantiocytes. We found that IL-4 plays an essential role in the pathogenesis of AD by upregulating epidermal chemotactic, angiogenic, and proinflammatory genes and downregulating antimicrobial and barrier function genes (Bao and others 2012a, 2012b, 2013a, 2013b). The Jaks–Stat6 pathway appears to be the dominant pathway for IL-4 action in keratinocytes (Bao and others 2012a, 2012b, 2013a, 2013c). Interestingly, Gingras and others (1999) have shown that p300/CBP is required for the Stat6-mediated IL-4 signal transduction pathway. Using 2-hybrid protein interaction assay and coimmunoprecipitation, they also demonstrated that p300/CBP becomes a part of the general transcription machinery by binding to Stat6 (Gingras and others 1999).
Previously, it was reported that an AP-1 site and a C/EBP site in the proximal regulatory region are essential for the involucrin promoter activity (Eckert and others 2004). Among various transcription factors, AP-1 appears to play a central role (Eckert and Welter 1996). When c-Fos, junB, junD, C/EBP, and other transcription factors bind to the regulatory region, they recruit various coactivators with p300/CBP being a critical one (Eckert and others 2004; Tran and Crowe 2004; Crish and Eckert 2008; Sinitsyna and others 2010). The coactivator p300/CBP plays an essential role in gene regulation by connecting different transcription factors in the general transcription machinery, acetylating transcription factors, and acetylating conserved lysine amino acids on histones (Chan and La Thangue 2001). It was reported that arsenite suppression of involucrin involved substantial reduction of c-Fos and p300 binding at the AP-1 site in the involucrin promoter (Sinitsyna and others 2010). In addition, E6 oncoprotein from human papillomaviruses interacts directly with p300, leading to its degradation and thus the downregulation of involucrin expression (Howie and others 2011).
Since p300/CBP is critical for the expression of both involucrin and IL-4 target genes, IL-4 signaling in keratinocytes may lead to activated Stat6 competitively recruiting this coactivtor, making it unavailable to the involucrin transcription machinery. It is interesting to note that, while Stat6 transactivates its target genes by directly binding to its response elements in the promoter area (Bao and others 2012a, 2012b; Qiu and others 2013), Stat6 downregulates involucrin expression indirectly by sequestrating its essential coactivator CBP.
In this study, we focused on IL-4 downregulation of involucrin at the transcriptional level. Our preliminary data also found that IL-4 may regulate involucrin expression posttranscriptionally, probably through miRNA regulation. We are investigating the detailed mechanisms.
Taken together, results of this investigation delineate, for the first time, a molecular mechanism for IL-4 suppression of involucrin in keratinocytes, which may play an important role in the pathogenesis of AD.
Acknowledgments
We would like to thank Dr. Robert H. Rice for the involucrin promoter reporter construct, Dr. Steven M. Dubinett for the DN-Stat6, Dr. Toshio Hirano for the DN-Stat3, Dr. Alice Mui for the DN-Stat5a, Dr. Li-yuan Yu-Lee for the DN-Stat5b, and Dr. Ke Ma for technical support in confocal microscopy. This work is, in part, supported by the Albert H. and Mary Jane Slepyan Endowed Fellowship Fund (L.B.) and the Dr. Orville J. Stone Endowed Professorship in Dermatology Fund (L.S.C.).
Author Disclosure Statement
No competing financial interests exist.
References
- Bao L, Alexander J, Shi VY, Chan LS. 2012a. IL-4 up-regulates epidermal IL-19 expression in atopic dermatitis. J Invest Dermatol 132 (Suppl. 1):S3–S3 [Google Scholar]
- Bao L, Alexander J, Zhang H, Chan LS. 2013a. IL-4 down-regulation of involucrin expression in atopic dermatitis involves competitive binding of Stat6 to the coactivator CBP. J Invest Dermatol 133:S39–S39 [Google Scholar]
- Bao L, Shi VY, Chan LS. 2012b. IL-4 regulates chemokine CCL26 in keratinocytes through the Jak1, 2/Stat6 signal transduction pathway: implication for atopic dermatitis. Mol Immunol 50(1–2):91–97 [DOI] [PubMed] [Google Scholar]
- Bao L, Shi VY, Chan LS. 2013b. IL-4 up-regulates epidermal chemotactic, angiogenic, and pro-inflammatory genes and down-regulates antimicrobial genes in vivo and in vitro: relevant in the pathogenesis of atopic dermatitis. Cytokine 61(2):419–425 [DOI] [PubMed] [Google Scholar]
- Bao L, Tessier C, Prigent-Tessier A, Li F, Buzzio OL, Callegari EA, Horseman ND, Gibori G. 2007. Decidual prolactin silences the expression of genes detrimental to pregnancy. Endocrinology 148(5):2326–2334 [DOI] [PubMed] [Google Scholar]
- Bao L, Zhang H, Chan LS. 2013c. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. Jak-Stat 2(3):e24137-1–e24137-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck LA, Thaci D, Hamilton JD, Ren H, Rocklin R, Ming J, Graham N, Radin A. 2013. Systemic treatment of patients with severe atopic dermatitis (AD) with an anti IL-4Rα mAb (REGN668/SAR231893) results in rapid and sustained improvements in disease signs and symptoms. J Invest Dermatol 133 (Suppl. 1):S178 [Google Scholar]
- Boguniewicz M, Leung DY. 2011. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev 242(1):233–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan HM, La Thangue NB. 2001. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci 114 (Pt 13):2363–2373 [DOI] [PubMed] [Google Scholar]
- Chan LS. 2008. Atopic dermatitis in 2008. Curr Dir Autoimmun 10:76–118 [DOI] [PubMed] [Google Scholar]
- Chan LS, Robinson N, Xu L. 2001. Expression of interleukin-4 in the epidermis of transgenic mice results in a pruritic inflammatory skin disease: an experimental animal model to study atopic dermatitis. J Invest Dermatol 117(4):977–983 [DOI] [PubMed] [Google Scholar]
- Chen L, Lin SX, Agha-Majzoub R, Overbergh L, Mathieu C, Chan LS. 2006. CCL27 is a critical factor for the development of atopic dermatitis in the keratin-14 IL-4 transgenic mouse model. Int Immunol 18(8):1233–1242 [DOI] [PubMed] [Google Scholar]
- Chen L, Marble DJ, Agha R, Peterson JD, Becker RP, Jin T, Li J, Chan LS. 2008. The progression of inflammation parallels the dermal angiogenesis in a keratin 14 IL-4-transgenic model of atopic dermatitis. Microcirculation 15(1):49–64 [DOI] [PubMed] [Google Scholar]
- Chen L, Martinez O, Overbergh L, Mathieu C, Prabhakar BS, Chan LS. 2004. Early up-regulation of Th2 cytokines and late surge of Th1 cytokines in an atopic dermatitis model. Clin Exp Immunol 138(3):375–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew YC, Adhikary G, Xu W, Wilson GM, Eckert RL. 2013. Protein kinase C delta increases kruppel-like factor 4 protein, which drives involucrin gene transcription in differentiating keratinocytes. J Biol Chem 288(24):17759–17768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crish JF, Eckert RL. 2008. Synergistic activation of human involucrin gene expression by Fra-1 and p300—evidence for the presence of a multiprotein complex. J Invest Dermatol 128(3):530–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crish JF, Gopalakrishnan R, Bone F, Gilliam AC, Eckert RL. 2006. The distal and proximal regulatory regions of the involucrin gene promoter have distinct functions and are required for in vivo involucrin expression. J Invest Dermatol 126(2):305–314 [DOI] [PubMed] [Google Scholar]
- Cui X, Zhang L, Luo J, Rajasekaran A, Hazra S, Cacalano N, Dubinett SM. 2007. Unphosphorylated STAT6 contributes to constitutive cyclooxygenase-2 expression in human non-small cell lung cancer. Oncogene 26(29):4253–4260 [DOI] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Efimova T, Dashti SR, Deucher A, Bone F, Adhikary G, Huang G, Gopalakrishnan R, Balasubramanian S. 2004. Regulation of involucrin gene expression. J Invest Dermatol 123(1):13–22 [DOI] [PubMed] [Google Scholar]
- Eckert RL, Welter JF. 1996. Epidermal keratinocytes—genes and their regulation. Cell Death Differ 3(4):373–383 [PubMed] [Google Scholar]
- Eichenfield LF, Ellis CN, Mancini AJ, Paller AS, Simpson EL. 2012. Atopic dermatitis: epidemiology and pathogenesis update. Semin Cutan Med Surg 31 (3 Suppl.):S3–S5 [DOI] [PubMed] [Google Scholar]
- Gingras S, Simard J, Groner B, Pfitzner E. 1999. p300/CBP is required for transcriptional induction by interleukin-4 and interacts with Stat6. Nucleic Acids Res 27(13):2722–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Rorke EA, Adhikary G, Chew YC, Xu W, Eckert RL. 2012. Suppression of AP1 transcription factor function in keratinocyte suppresses differentiation. PLoS One 7(5):e36941. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hanifin JM, Rajka G. 1980. Diagnostic features of atopic-dermatitis. Acta Derm Venereol (Stockh) 92(suppl):44–47 [Google Scholar]
- Henry J, Hsu CY, Haftek M, Nachat R, de Koning HD, Gardinal-Galera I, Hitomi K, Balica S, Jean-Decoster C, Schmitt AM, Paul C, Serre G, Simon M. 2011. Hornerin is a component of the epidermal cornified cell envelopes. FASEB J 25(5):1567–1576 [DOI] [PubMed] [Google Scholar]
- Howie HL, Koop JI, Weese J, Robinson K, Wipf G, Kim L, Galloway DA. 2011. Beta-HPV 5 and 8 E6 promote p300 degradation by blocking AKT/p300 association. PLoS Pathog 7(8):e1002211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BE, Leung DY, Boguniewicz M, Howell MD. 2008. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol 126(3):332–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Choi YS, Cheong KA, Lee AY. 2013. Mechanism underlying the effect of combined therapy using glucosamine and low-dose cyclosporine A on the development of atopic dermatitis-like skin lesions in NC/Nga mice. Int Immunopharmacol 15(2):424–432 [DOI] [PubMed] [Google Scholar]
- Knuppel S, Esparza-Gordillo J, Marenholz I, Holzhutter HG, Bauerfeind A, Ruether A, Weidinger S, Lee YA, Rohde K. 2012. Multi-locus stepwise regression: a haplotype-based algorithm for finding genetic associations applied to atopic dermatitis. BMC Med Genet 13:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan CC, Tu HP, Wu CS, Ko YC, Yu HS, Lu YW, Li WC, Chen YC, Chen GS. 2011. Distinct SPINK5 and IL-31 polymorphisms are associated with atopic eczema and non-atopic hand dermatitis in Taiwanese nursing population. Exp Dermatol 20(12):975–979 [DOI] [PubMed] [Google Scholar]
- Luo G, Yu-Lee L. 1997. Transcriptional inhibition by Stat5. Differential activities at growth-related versus differentiation-specific promoters. J Biol Chem 272(43):26841–26849 [DOI] [PubMed] [Google Scholar]
- Margolis DJ, Apter AJ, Gupta J, Hoffstad O, Papadopoulos M, Campbell LE, Sandilands A, McLean WH, Rebbeck TR, Mitra N. 2012. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol 130(4):912–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mui AL, Wakao H, Kinoshita T, Kitamura T, Miyajima A. 1996. Suppression of interleukin-3-induced gene expression by a C-terminal truncated Stat5: role of Stat5 in proliferation. EMBO J 15(10):2425–2433 [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Yamanaka Y, Nakae K, Kojima H, Ichiba M, Kiuchi N, Kitaoka T, Fukada T, Hibi M, Hirano T. 1996. A central role for Stat3 in IL-6-induced regulation of growth and differentiation in M1 leukemia cells. EMBO J 15(14):3651–3658 [PMC free article] [PubMed] [Google Scholar]
- Paternoster L, Standl M, Chen CM, Ramasamy A, Bonnelykke K, Duijts L, Ferreira MA, Alves AC, Thyssen JP, Albrecht E, Baurecht H, Feenstra B, Sleiman PM, Hysi P, Warrington NM, Curjuric I, Myhre R, Curtin JA, Groen-Blokhuis MM, Kerkhof M, Saaf A, Franke A, Ellinghaus D, Folster-Holst R, Dermitzakis E, Montgomery SB, Prokisch H, Heim K, Hartikainen AL, Pouta A, Pekkanen J, Blakemore AI, Buxton JL, Kaakinen M, Duffy DL, Madden PA, Heath AC, Montgomery GW, Thompson PJ, Matheson MC, Le Souef P, St Pourcain B, Smith GD, Henderson J, Kemp JP, Timpson NJ, Deloukas P, Ring SM, Wichmann HE, Muller-Nurasyid M, Novak N, Klopp N, Rodriguez E, McArdle W, Linneberg A, Menne T, Nohr EA, Hofman A, Uitterlinden AG, van Duijn CM, Rivadeneira F, de Jongste JC, van der Valk RJ, Wjst M, Jogi R, Geller F, Boyd HA, Murray JC, Kim C, Mentch F, March M, Mangino M, Spector TD, Bataille V, Pennell CE, Holt PG, Sly P, Tiesler CM, Thiering E, Illig T, Imboden M, Nystad W, Simpson A, Hottenga JJ, Postma D, Koppelman GH, Smit HA, Soderhall C, Chawes B, Kreiner-Moller E, Bisgaard H, Melen E, Boomsma DI, Custovic A, Jacobsson B, Probst-Hensch NM, Palmer LJ, Glass D, Hakonarson H, Melbye M, Jarvis DL, Jaddoe VW, Gieger C, Strachan DP, Martin NG, Jarvelin MR, Heinrich J, Evans DM, Weidinger S. 2012. Meta-analysis of genome-wide association studies identifies three new risk loci for atopic dermatitis. Nat Genet 44(2):187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MA, Qin Q, Rice RH. 2000. Identification of an involucrin promoter transcriptional response element with activity restricted to keratinocytes. Biochem J 348 (Pt 1):45–53 [PMC free article] [PubMed] [Google Scholar]
- Qiu R, Yang Y, Zhao H, Li J, Xin Q, Shan S, Liu Y, Dang J, Yu X, Gong Y, Liu Q. 2013. Signal transducer and activator of transcription 6 directly regulates human ORMDL3 expression. FEBS J 280(9):2014–2026 [DOI] [PubMed] [Google Scholar]
- Ring J, Mohrenschlager M, Weidinger S. 2012. Molecular genetics of atopic eczema. Chem Immunol Allergy 96:24–29 [DOI] [PubMed] [Google Scholar]
- Schittek B. 2011. The antimicrobial skin barrier in patients with atopic dermatitis. Curr Probl Dermatol 41:54–67 [DOI] [PubMed] [Google Scholar]
- Segre JA. 2006. Epidermal barrier formation and recovery in skin disorders. J Clin Invest 116(5):1150–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J, Oshima T, Farre R, Fukui H, Watari J, Miwa H. 2014. IL-4 induces columnar-like differentiation of esophageal squamous epithelium through JAK/PI3K pathway: possible role in pathogenesis of Barrett's esophagus. Am J Physiol Gastrointest Liver Physiol 306(8):G641–G649 [DOI] [PubMed] [Google Scholar]
- Shi VY, Bao L, Chan LS. 2012. Inflammation-driven dermal lymphangiogenesis in atopic dermatitis is associated with CD11b+ macrophage recruitment and VEGF-C up-regulation in the IL-4-transgenic mouse model. Microcirculation 19(7):567–579 [DOI] [PubMed] [Google Scholar]
- Simon D, Kernland Lang K. 2011. Atopic dermatitis: from new pathogenic insights toward a barrier-restoring and anti-inflammatory therapy. Curr Opin Pediatr 23(6):647–652 [DOI] [PubMed] [Google Scholar]
- Sinitsyna NN, Reznikova TV, Qin Q, Song H, Phillips MA, Rice RH. 2010. Arsenite suppression of involucrin transcription through AP1 promoter sites in cultured human keratinocytes. Toxicol Appl Pharmacol 243(3):275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Farinas M, Tintle SJ, Shemer A, Chiricozzi A, Nograles K, Cardinale I, Duan S, Bowcock AM, Krueger JG, Guttman-Yassky E. 2011. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol 127(4):954–964.e1–e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theerawatanasirikul S, Sailasuta A, Thanawongnuwech R, Suriyaphol G. 2012. Alterations of keratins, involucrin and filaggrin gene expression in canine atopic dermatitis. Res Vet Sci 93(3):1287–1292 [DOI] [PubMed] [Google Scholar]
- Tran NQ, Crowe DL. 2004. Regulation of the human involucrin gene promoter by co-activator proteins. Biochem J 381 (Pt 1):267–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaard EH, Bergboer JG, Vonk-Bergers M, van Vlijmen-Willems IM, Hato SV, van der Valk PG, Schroder JM, Joosten I, Zeeuwen PL, Schalkwijk J. 2013. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest 123(2):917–927 [DOI] [PMC free article] [PubMed] [Google Scholar]