Abstract
The purpose of this study was to evaluate differences in vaginal immune cell populations, vaginal tissue gene expression, antimicrobial activity of the cervicovaginal (CV) lavage (CVL), vaginal flora, and p24 antigen production from CV tissues after ex vivo human immunodeficiency virus (HIV) infection between follicular (FOL) and luteal (LUT) phases of the menstrual cycle. CV tissue biopsies, CV secretions, and blood samples were obtained as part of two longitudinal clinical trials of healthy women (CONRAD D11-119 and A12-124 studies). Participants (n = 39) were HIV-seronegative women not using exogenous hormone supplementation, with normal menstrual cycles, who were screened to exclude sexually transmitted and reproductive tract infections. Serum levels of estradiol and progesterone were significantly higher in the LUT versus the FOL phase of the menstrual cycle. Controlling for race, reported contraceptive use/sexual practices, and clinical trial, we found no differences in vaginal tissue immune cell populations and activation status, transcriptomes, inhibition of HIV, herpes simplex virus type 2 and Escherichia coli by the CVL, vaginal pH or Nugent score, or production of p24 antigen after ex vivo infection by HIV-1BaL between CV samples obtained in the FOL phase versus the LUT phase of the menstrual cycle. There were no significant correlations between serum estradiol and progesterone levels and CV endpoints. The hypothesis that the LUT phase of the menstrual cycle represents a more vulnerable stage for mucosal infection with HIV was not supported by data from samples obtained from the lower genital tract (ectocervix and vagina) from these two clinical trials.
Introduction
Women are more susceptible than men to sexually acquire human immunodeficiency virus type 1 (HIV-1) infection.1–4 In regions of the world where HIV-1 incidence is highest, more women are newly infected per year than men.5 While social, behavioral, and economic conditions are certainly factors in the increasing incidence of HIV-1 in women, there has been an emphasis on the role of endogenous6 and exogenous7 hormones as cofactors in HIV-1 acquisition or infection and disease progression. Observational and prospective longitudinal studies support that pregnancy and lactation, characterized by high serum progesterone (4-pregnene-3,20-dione or P4) and low serum estradiol [1,3,5(10)-estratriene-3,17beta-diol or E2] levels, are also associated with an increased risk of HIV-1 acquisition compared with nonlactating nonpregnant controls.8–10 All women experience fluctuations of endogenous hormones as part of the normal menstrual cycle and menopause and therefore it is critical to understand the effect of these hormonal states on the early mucosal events of HIV-1 acquisition and transmission.
A general hypothesis that the luteal (LUT) phase of the menstrual cycle is a more vulnerable time for HIV-1 acquisition in women has been postulated based on the theory that components of the mucosal immune system in the female reproductive tract are suppressed or altered in the 7–10 days following ovulation to permit influx of paternal antigens, fertilization, and embryo implantation in the endometrium (reviewed in Wira and Fahey11). Studies demonstrated that rhesus12 and pigtail macaques13,14 were more vulnerable to Simian human immunodeficiency virus (SHIV) infection in the LUT phase of the menstrual cycle13 and this vulnerability to infection persisted during menstruation.14 This may be due to the temporary immune suppression by high P4 levels during the LUT phase or to the thinning of the cervicovaginal (CV) epithelium in macaques during the P4-dominant LUT phase.14
Much of the data supporting mucosal vulnerability to HIV infection in the LUT phase come from evidence of immune suppression, increases in HIV target cells,15 or other interactions between the immune and endocrine systems found in studies of endometrial explants15–17 (reviewed in Wira and Fahey11) or data showing increases in proinflammatory cytokines in endocervical mucus.18
However, there is a paucity of data regarding the effect of the menstrual cycle on immune cell recruitment and activation in the lower reproductive tract, including the ectocervix and vagina,19,20 with some studies showing no changes in CD4 and CCR5-positive cells, based on the menstrual cycle, in ectocervical explants.21
Previous studies regarding the effect of endogenous hormones on mucosal HIV targets in the lower genital tract have been done primarily with explants obtained from women undergoing elective surgeries such as hysterectomies21–25 or among small cohorts of women in a longitudinal19,26–28 or cross-sectional design.29 While surgical explants offer convenience and supply tissue without invasive biopsy procedures, women undergoing indicated surgery invariably have a host of medical comorbidities and concomitant medication use, which may affect study endpoints.30 In addition, all women undergoing hysterectomy receive preoperative antibiotics31 and a CV wash with either surfactants or 10% povidone–iodine32,33 as part of standard preoperative infection prevention procedures. Paired samples from various menstrual cycle phases obviously cannot be obtained from women undergoing removal of the uterus and cervix. Given these confounding factors, there is a paucity of human data that directly examine the impact of menstrual phase on HIV-1 susceptibility.
We present herein paired data from two clinical trials in which a well-screened population of healthy HIV-seronegative women, with normal menstrual cycles, no reproductive tract infections (RTIs), and no exposure to exogenous hormones, provided genital samples at baseline in follicular (FOL) and LUT phases of the menstrual cycle. The objective of this analysis was to determine if there were inherent mucosal differences between FOL and LUT phases of the menstrual cycle that could result in increased susceptibility to HIV by comparing vaginal immune cell populations, transcriptome, pH and Nugent score,34 antimicrobial activity of CV secretions, and p24 antigen production after ex vivo HIV-1BaL infection of CV tissues.
Methods
Clinical trials included
The CONRAD D11-119 study (S119) was designed to compare biologic endpoints in the lower genital tract involved in the early events of HIV-1 acquisition after in vivo exposure to proinflammatory (nonoxynol-9, imiquimod) and noninflammatory (hydroxyethyl cellulose placebo gel) vaginal products. The CONRAD A12-124 study (S124) was designed to compare biologic markers associated with HIV-1 acquisition in premenopausal and postmenopausal women at baseline and after exposure to tenofovir vaginal gel. The baseline visit samples for healthy premenopausal women are reported in this article.
S119 was approved by the Chesapeake Institutional Review Board (IRB) (No. Pro00006917) with a waiver of oversight from the Eastern Virginia Medical School (EVMS) IRB and was registered with ClinicalTrials.gov (No. NCT01593124). S124 was approved by the EVMS IRB (No. 13-02-FB-0017) and registered with ClinicalTrials.gov (No. NCT01810315). All S119 and S124 participants were seen and examined at the CONRAD Clinical Research Center at EVMS in Norfolk, VA.
Both studies enrolled healthy HIV-seronegative women, aged 21–45 years, who did not smoke or take exogenous hormones, with normal menstrual cycles, every 24–35 days. Dates of the participants' last three menstrual cycles were recorded at the screening visit to verify that the menstrual cycles were regular. The average menstrual cycle length for each participant was calculated based on her last three menstrual cycles. All participants were screened, at visit 1 (V1), for the presence of exclusionary RTIs, including bacterial vaginosis (BV), yeast vaginitis, Trichomonas vaginalis, Neisseria gonorrhoeae, Chlamydia trachomatis, and cervical dysplasia. In S119, participants were also screened for high-risk human papillomavirus subtypes and past exposure to antibodies (IgG) to herpes simplex virus type 2 (HSV-2), which were exclusionary. In S124, premenopausal participants had to demonstrate an LUT phase serum P4 level of 3 ng/ml or greater, to document ovulatory status, before baseline sampling in FOL and LUT phases.
Enrolled participants in both trials underwent two baseline sampling visits in FOL (menstrual cycle days 5–13) (visit 2, V2) and LUT (menstrual cycle days 18–26) (visit 3, V3) phases of the menstrual cycle. Genital sampling did not occur when menstrual blood was present in the vagina. Participants in S124 also had serum samples for E2 and P4 levels obtained at both baseline visits.
At each visit, point-of-care testing for prostate-specific antigen was performed to exclude recent vaginal semen exposure (ABAcard; Abacus Diagnostics). After confirming the absence of recent semen exposure, the following samples were obtained: (1) sterile swabs for vaginal pH and Nugent score, (2) a cervicovaginal lavage (CVL) with 4 cc of sterile saline, (3) vaginal tissue biopsies for gene expression and for immune cell population phenotype, and (4) ectocervical tissue (S124) or vaginal tissue (S119) for p24 antigen production after ex vivo HIV-1BaL infection. For all genital tissue biopsy sites, except the biopsy for p24 antigen production, 20% benzocaine gel (Topex; Sultan Healthcare) was applied to the ectocervix and vagina for pain control before obtaining the CV biopsies. Ferric subsulfate 20% (Monsel's solution) and pressure were applied to the biopsy sites to control bleeding. Women were instructed to place nothing in the vagina for 5 days after the biopsy procedures. Procedures for CV tissue biopsies in mucosal safety and clinical trials were followed as recently outlined.35
Vaginal pH and Nugent score
A Dacron swab was used to collect vaginal cells and secretions and rolled onto a glass slide. The pH was measured using pH paper with a range of 4.0–7.0 (MColorpHast ph Indicator strips; EMD Millipore). A Gram stain was performed and the Nugent score was assessed on site by one of the investigators (A.R.T. or T.K.) by previously described methods.34
Antimicrobial activity of the CVL
Within 30 min of collection, the CVL was centrifuged at 4°C for 10 min at 500 × g. Aliquots of CVL supernatant were stored at −80°C and shipped to the laboratory of B.Herold, MD, at Albert Einstein College of Medicine for testing of the antimicrobial activity of the CVL. The activity of CVL against HIV-1, herpes simplex type 2 virus (HSV-2), and Escherichia coli was measured within 12 months of collection, as previously described.20
For anti-HIV activity, TZM-bl cells were cultured in 96-well plates overnight. The cells were challenged with HIV-1BaL (∼103 TCID50) mixed 1:1 with CVL or control buffer (normal saline containing 200 μg/ml of bovine serum albumin). After 48 h of incubation at 37°C, the inoculum was removed by washing, cells were lysed with addition of luciferase cell culture lysis reagent (Promega), and the samples were stored at −80°C. Luciferase activity was assessed (Luciferase Assay System; Promega) and expressed in relative light units. Mock infected cells served as a negative control and wells in which tenofovir (100 μg/ml) was added served as an internal positive assay control. Inhibition of HIV infection was expressed as mean percent reduction compared with control. All samples were tested in triplicate.
To assess the anti-E. coli activity, bacteria (ATCC strain 43827) were grown overnight to stationary phase, and then 3 μl of culture (∼109 colony-forming units/ml) was mixed with 27 μl of CVL or control buffer (20 mmol/liter potassium phosphate, 60 mmol/liter sodium chloride, 0.2 mg/ml albumin, pH 4.5) and incubated at 37°C for 2 h. The mixtures were further diluted in genital tract buffer [806 mol/liter KH2PO4, 59.89 mmol/liter NaCl, enriched with 30% trypticase soy broth (TSB)] to yield 800–1,000 colonies on control plates and plated in duplicate on agar enriched with TSB. Colonies were counted using ImageQuant TL v2005 after an overnight incubation at 37°C. Results are presented as the mean percentage inhibition relative to colonies formed on control plates. Bacteria cultured with 10,000 U of penicillin/10,000 g/ml streptomycin served as an internal positive assay control and consistently showed 100% inhibition of colony formation.
For anti-HSV activity, Vero cells were challenged in duplicate with ∼50–200 plaque-forming units of HSV-2(G) mixed 1:1 with each CVL or control buffer (normal saline containing 200 μg/ml bovine serum albumin) and plaques were counted after 48 h, as previously described.20
Analysis of vaginal immune cell populations and histology
For the immunohistochemistry (IHC) analyses, one vaginal biopsy from each visit was placed in 10% neutral buffered formalin for 24–48 h. After the completion of fixation, the tissues were transferred to an embedding cassette and processed overnight in the Tissue-Tek V.I.P, Vacuum Infiltration Processor (E150 Series; Sakura Finetek). The infiltration program involves a scheduled sequence of solutions [from phosphate-buffered saline (PBS), through increasing percent grades of ethanol solutions, to xylene, and finally to paraffin at (58°C)]. After the completion of paraffin processing, tissue blocks of the vaginal biopsies were cut into 5-μm sections. IHC staining of tissue sections was performed using the ABC method (avidin:biotinylated enzyme complex from Vector labs). Briefly, the slides were deparaffinized, and rehydrated, followed by antigen retrieval in citrate buffer (pH 6.2; Dako) at high temperature. Nonspecific binding was blocked using specific protein block for 30 min at room temperature. After washing with PBS, the slides were incubated overnight with primary antibody at 4°C. Slides were then washed with PBS and subjected to biotinylated secondary antibody, followed by ABC reagent. The antigens were detected using the AEC chromogen substrate kit (SkyTek Labs) and mounted with Accergyl mounting media (Accurate Chemicals). Cell phenotype was identified using specific monoclonal antibodies against CD45, CD3, CD8, CD1a, CD68, CCR5, CD4, and HLA-DR. Positive stained cells were counted under the microscope (Nikon E-800). In brief, five to six fields were randomly selected using a Nikon E800 microscope from each section and these images were captured using a CCD camera (Spot Camera; Diagnostic Instruments). Cell density was expressed as cells/mm2.
RNA isolation
One vaginal biopsy from each visit was placed in RNA later solution (Ambion AM7021; Ambion Life Sciences) and frozen at −80°C. For RNA isolation, tissue was homogenized with TRIzol (Invitrogen Life Technologies) using OMNI international homogenizer and total RNA was extracted and then purified using RNeasy mini kit columns (Qiagen) according to the manufacturers' instructions.
Vaginal tissue microarray gene expression analysis
Gene expression analysis using Affymetrix U133 Plus 2 arrays and data processing using Biometric Research Branch (BRB)-ArrayTools version 4.4.0 (National Cancer Institute, available at http://linus.nci.nih.gov/BRB-ArrayTools.html) were performed as previously described.36 The U133 Plus 2 Affymetrix chip contains more than 56,000 probe sets that encompass 38,500 well-characterized human genes and expressed sequence tags. Genes with false discovery rate (FDR)-corrected p-values <.0537 and showing fold change differences ≥2 between groups were considered as differentially expressed. A total of 1,000 permutations were completed to identify a list of genes containing less than 5% false positives at a confidence of 80%.
Gene set expression analysis
Gene set expression comparison was performed using the BRB array tool as described in Simon and Lam BRB array tool user guide (http://linus.nci.nih.gov/brb). Annotated gene sets were from the collection of Broad Institute Molecular Signature Database (MSigDB). Tests used to find significant gene sets were Fisher (LS) and the Kolmogorov–Smirnov (KS) permutation tests. LS/KS permutation test finds gene sets that have more genes differentially expressed among the phenotype classes than expected by chance. The threshold of determining significant gene sets was 0.005.
HIV-1 p24 antigen production from ectocervical or vaginal tissue biopsies after ex vivo infection
In the D11-119 study, a vaginal tissue biopsy and, in the A12-124 study, an ectocervical tissue biopsy was obtained without the use of topical anesthetic. CV tissue biopsies were placed in a microcentrifuge tube containing complete Leibovitz 15 tissue culture media (cL15; Gibco) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco). Biopsy samples were kept on ice and shipped through overnight courier to the laboratory of Dr. Susana Asin (V.A. Medical Center). The following day, the biopsies were stabilized for 4 h at 37°C and infected with 104 TCID50 of HIVBAL in a final volume of 100 μl per well in a 96-well plate. After overnight infection, the tissues were extensively washed (five times) to eliminate residual input virus and cultured in cL15 medium for 21 days. A sample of the culture supernatants was collected after the final wash (day 0). At day 4 after infection, all of the supernatant (100 μl) was collected and replaced with an equivalent volume of cL15. Supernatants were also collected at days 7, 11, 14, 18, and 21 after infection. At each time point, 50% of tissue culture supernatants were collected and replaced with an equivalent volume of fresh cL15. On day 21, the tissues were weighed; genomic DNA was isolated and evaluated for HIV-1 reverse transcription (viral DNA) by real-time polymerase chain reaction. Day 0, 7, 14, and 21 supernatants were evaluated for HIV-1 p24 antigen expression by ELISA (Perkin Elmer).
Statistical analyses were performed with SAS version 9.3. Descriptive statistics included mean, median, standard deviation, and range for continuous variables and frequencies and percentages for categorical variables. Normality of the data from the separate (D11-119 and A12-124) and combined study populations was examined. Continuous endpoints from the two separate studies were compared using an independent samples t-test for normally distributed data or Wilcoxon–Mann–Whitney test for non-normally distributed data. For categorical variables, chi square statistic or Fisher's exact tests were used as indicated by expected cell size. Paired comparisons were performed to compare FOL phase versus LUT phase samples taken from the same participant. To achieve normality, data were log transformed, as indicated, for the analyses of p24 production and immune cell types as has been previously done.38,39 For the analyses of p24 antigen production, categorization of infected versus not infected samples was done using 0.1 log steps and a Boolean method, followed by comparison of infected versus not infected samples by Fisher's exact test or McNemar's statistic as appropriate.40 Log transformation of the antimicrobial data could not be performed because some of the data points were negative numbers.
Multiple regression analyses were performed to evaluate the differences in the FOL phase versus LUT phase, controlling for participant race, contraceptive/sexual practices, and study [a marker of tissue source (cervical or vaginal) for p24 comparisons].
Spearman or Pearson correlation coefficients were calculated between serum E2 and P4 and each CV endpoint (immune cell populations, vaginal epithelial thickness, number of vaginal epithelial cell layers, antimicrobial activity of the CVL, Nugent score, vaginal pH, and p24 antigen production) for participants enrolled in the S124 study. Linear regression analyses were performed to determine if the day of menstrual cycle sampling could predict any CV endpoint (noted above).
To estimate the day of ovulation, we determined each participant's average menstrual cycle length and subtracted 14 as the LUT phase of the menstrual cycle is typically the most constant phase of the cycle. Additional repeated-measures analyses were performed to evaluate differences in the endpoints based on whether samples were obtained in the early FOL phase versus preovulatory phase (up to 4 days preceding estimated ovulation) and early (up to 4 days post ovulation) versus late (5 days or more post ovulation) LUT phase.
Statistical significance was determined at the level of alpha = 0.05.
Results
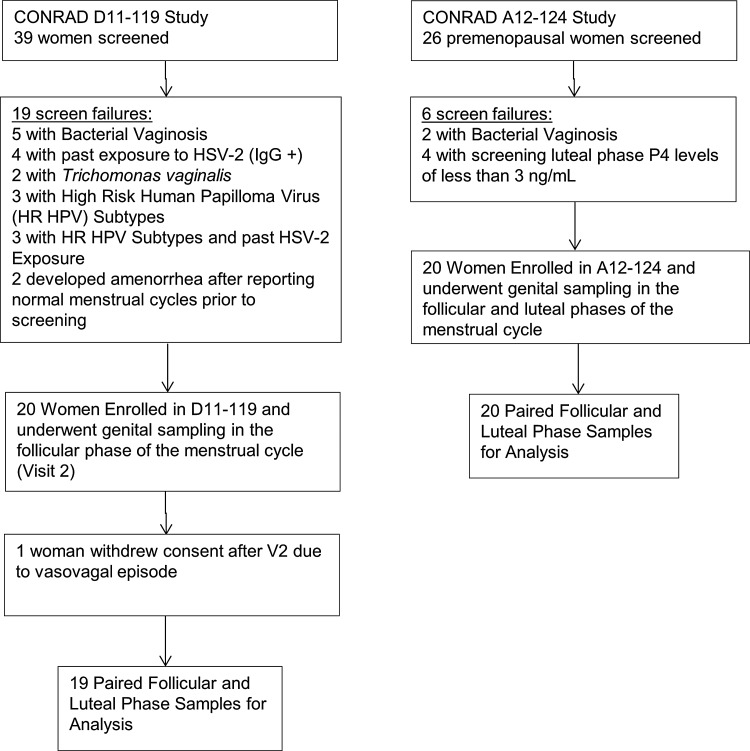
There were a total of 39 paired FOL and LUT phase samples obtained from both studies (Fig. 1). There were statistically significant differences in race of participants enrolled in the two studies (Table 1), with a higher proportion of African American women enrolled in the S119 study. Although no participant used exogenous hormones for contraception, there was a difference in contraceptive use and sexual practices reported by participants in the two studies, with a higher proportion of condom users and women in same-sex relationships in the S119 study. Women in the S124 study most commonly used a tubal ligation for contraception. We therefore controlled for race and contraceptive use/sexual practices in the regression analyses.
FIG. 1.
Screening, enrollment, and follow-up diagram for CONRAD D11-119 and CONRAD A12-124 studies.
Table 1.
Demographic Characteristics of Study Populations in the D11-119 and A12-124 Studies
| S119 | S124 | ||||||
|---|---|---|---|---|---|---|---|
| Continuous variable (units) | n | Mean | SD | n | Mean | SD | p-value |
| Age (years) | 19 | 32.3 | 6.6 | 20 | 35.2 | 6 | .10 |
| BMI (kg/m2) | 19 | 32.8 | 9.5 | 20 | 31.9 | 9.8 | .82 |
| Years of education | 19 | 14.1 | 1.9 | 20 | 14 | 1.5 | .85 |
| Gravidity | 19 | 2.2 | 2 | 20 | 3.1 | 1.6 | .07 |
| Menstrual cycle length | 19 | 29.1 | 1.4 | 20 | 29.4 | 1.7 | .95 |
| Day of FOL phase sampling | 19 | 9.7 | 2.4 | 20 | 7.7 | 1.9 | .10 |
| Day of LUT phase sampling | 19 | 22.3 | 2.7 | 20 | 22.3 | 3.1 | .56 |
| S119 | S124 | ||||
|---|---|---|---|---|---|
| Categorical variable | n | % in study | n | % in study | p-value |
| Ethnicity | .12 | ||||
| Hispanic | 0 | 0 | 3 | 15 | |
| Non-Hispanic | 19 | 100 | 17 | 85 | |
| Race | .01 | ||||
| Black/African American | 13 | 68 | 6 | 30 | |
| Native Indian/Alaska Native | 0 | 0 | 1 | 5 | |
| White | 6 | 32 | 13 | 65 | |
| Contraception/sexual practices | <.01 | ||||
| Abstinence | 4 | 21 | 3 | 15 | |
| Tubal ligation | 4 | 21 | 8 | 40 | |
| Male condoms | 8 | 42 | 7 | 35 | |
| Same-sex relationship | 2 | 11 | 0 | 0 | |
| Male vasectomy | 1 | 5 | 2 | 10 | |
FOL, follicular; LUT, luteal.
In both studies combined, FOL phase sampling (V2) was performed on menstrual cycle day 8.7 ± 2.3 (range 5–13), while LUT phase sampling (V3) was performed on menstrual cycle day 22.3 ± 2.9 (range 18–26). Serum E2 and P4 levels from participants in the S124 study were significantly higher in the late LUT phase compared with the early FOL phase (p < .05). In FOL and LUT phases, respectively, mean serum E2 levels were 76.1 ± 63.3 pg/ml and 121.3 ± 75.7 pg/ml and serum P4 levels were 0.4 ± 0.2 ng/ml and 7.2 ± 5.2 ng/ml.
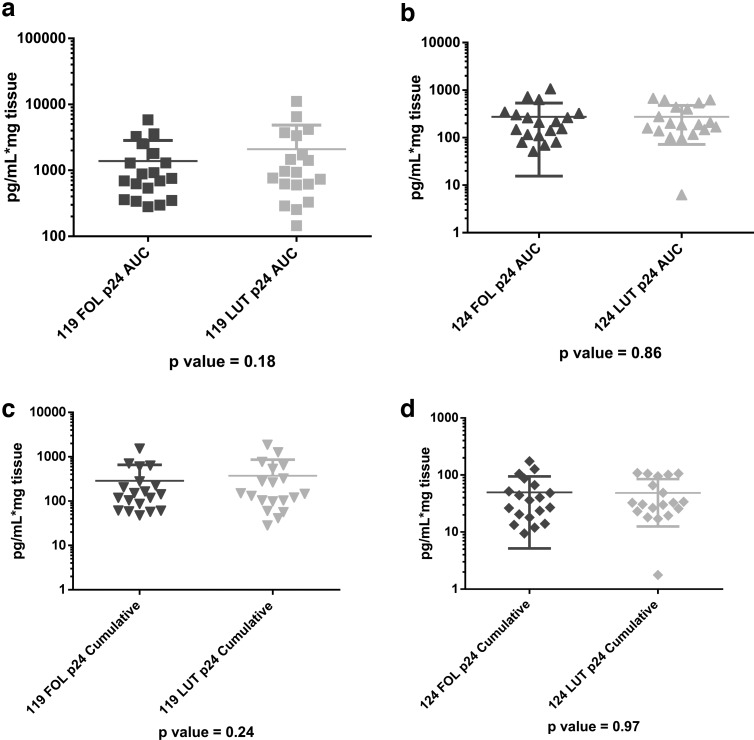
In the unadjusted paired comparisons, all dependent variables (antimicrobial activity of the CVL, vaginal pH, vaginal Nugent score, vaginal immune cell density, vaginal epithelial thickness, number of vaginal cell layers, and ectocervical or vaginal p24 antigen production) showed no significant differences between FOL and LUT phases for the combined data (Table 2, all unadjusted p-values >.14) and when the data were analyzed separately for each study (data not shown). Examples of the nonsignificant differences between p24 antigen production in the FOL phase versus LUT phase for vaginal (S119) and ectocervical (S124) samples, analyzed separately, are shown in Figure 2a–d.
Table 2.
Effect of Follicular Versus Luteal Phase on Study Endpoints
| FOL phase in S119 and S124 studies (n = 39) | LUT phase in S119 and S124 studies (n = 39) | |||||
|---|---|---|---|---|---|---|
| Variable (units) | Mean | SD | Mean | SD | Unadjusted p-value | Adjusted p-value* |
| Antimicrobial activity of the CVL (% Inhibition) | ||||||
| HSV-2 inhibition | 42.6 | 33.5 | 51 | 31.2 | .24 | .24 |
| E. coli inhibition | 18.2 | 43.3 | 32.4 | 45.1 | .32 | .12 |
| HIV inhibition | 17.6 | 49.3 | 14.3 | 48.3 | .99 | .76 |
| p24 antigen production from vaginal (S119) or ectocervical (S124) tissue biopsies (pg.ml × mg tissue) | ||||||
| p24_0 | 1.7 | 2.2 | 1.9 | 2.7 | .32 | .71 |
| p24_7 | 27.6 | 33.4 | 54.8 | 123 | .42 | .17 |
| p24_14 | 41.7 | 51.1 | 72.5 | 133.1 | .61 | .15 |
| p24_21 | 97 | 254.1 | 81.8 | 155.1 | .63 | .74 |
| p24_Soft | 83.4 | 241.9 | 79.6 | 154.2 | .87 | .94 |
| p24_Cum | 168.1 | 287.1 | 211 | 379.4 | .77 | .53 |
| p24_Max | 105.9 | 253.5 | 95.2 | 168.7 | .85 | .82 |
| p24_AUC | 831 | 1176.7 | 1183.8 | 2140.8 | .70 | .32 |
| Immune cell populations (cells/mm2) in the EPI and LP and vaginal tissue histology | ||||||
| Epithelial thickness (μm) | 273 | 102.4 | 312.8 | 112.4 | .14 | .08 |
| Number of cell layers | 24.5 | 6.5 | 26.7 | 7.5 | .42 | .07 |
| CD45 EPI | 120.8 | 60.5 | 95.5 | 41.7 | .63 | .07 |
| CD45 LP | 75 | 39.2 | 69.4 | 35 | .41 | .55 |
| CD3 EPI | 87.3 | 44.1 | 70.2 | 33.2 | .99 | .15 |
| CD3 LP | 47.1 | 29.6 | 46.4 | 26.7 | .41 | .91 |
| CD8 EPI | 59.7 | 33.1 | 49.8 | 25.2 | .99 | .12 |
| CD8 LP | 31.3 | 24.7 | 29.7 | 17.7 | .61 | .78 |
| CD4 EPI | 0 | 0 | 0.1 | 0.3 | .50 | .17 |
| CD4 LP | 2.3 | 4 | 2.2 | 3.5 | .65 | .93 |
| CCR5 EPI | 0 | 0 | 0 | 0.2 | .99 | .30 |
| CCR5 LP | 3.3 | 4.9 | 3.4 | 5 | .84 | .91 |
| HLADR EPI | 45 | 26 | 51.1 | 33.3 | .62 | .35 |
| HLADR LP | 36.1 | 24.5 | 41.5 | 23.3 | .50 | .27 |
| Vaginal microflora | ||||||
| Vaginal pH | 4.3 | 0.4 | 4.4 | 0.3 | .82 | .85 |
| Nugent score | 2.5 | 2.8 | 2.8 | 3.0 | .85 | .66 |
p-Value of visit adjusted for race, study, and contraceptive use/sexual practices.
AUC, area under the curve; CVL, cervicovaginal lavage; EPI, epithelium; HIV, human immunodeficiency virus; LP, lamina propria.
FIG. 2.
No differences in p24 antigen production between follicular (FOL) phase versus luteal (LUT) phase. (a) Vaginal tissue (119 study) p24 area under the curve (AUC), FOL versus LUT. (b) Ectocervical tissue (124 study) p24 AUC, FOL versus LUT. (c) Vaginal tissue (119 study) p24 cumulative, FOL versus LUT. (d) Ectocervical tissue (124 study) p24 cumulative, FOL versus LUT.
HIV-1 reverse transcription at day 21 of tissue culture was set to 1 for the FOL phase and 1/LUT for the LUT phase. For all samples, HIV-1 reverse transcription at day 21 in the LUT phase (median 0.5) was not significantly different than 1 (p = .32)
We found that vaginal tissue biopsies obtained in S119 produced significantly higher levels of p24 antigen area under the curve (AUC), cumulative, maximum, soft endpoint, and p24 production at days 7, 14, and 21, and HIV-1 reverse transcription at day 21 than ectocervical tissue biopsies obtained in S124 (all p-values <.01, data not shown). We therefore controlled for tissue source (ectocervical vs. vaginal) in the adjusted analyses of the combined data by controlling for the study in which the participant was enrolled. p24 antigen production was normalized to tissue weight. The average weight of vaginal biopsies was 35 ± 17 mg, while the average weight of ectocervical biopsies was 25 ± 14 mg.
There were no significant differences in p24 antigen production, categorizing samples as infected versus noninfected, using Boolean categorization for p24 antigen production at days 0, 14, and 21, as well as cumulative, AUC, and soft endpoint p24 antigen production.38,39 Of the over 1,200 Boolean categorical comparisons generated, less than 1% showed statistically significant differences between FOL and LUT phases.
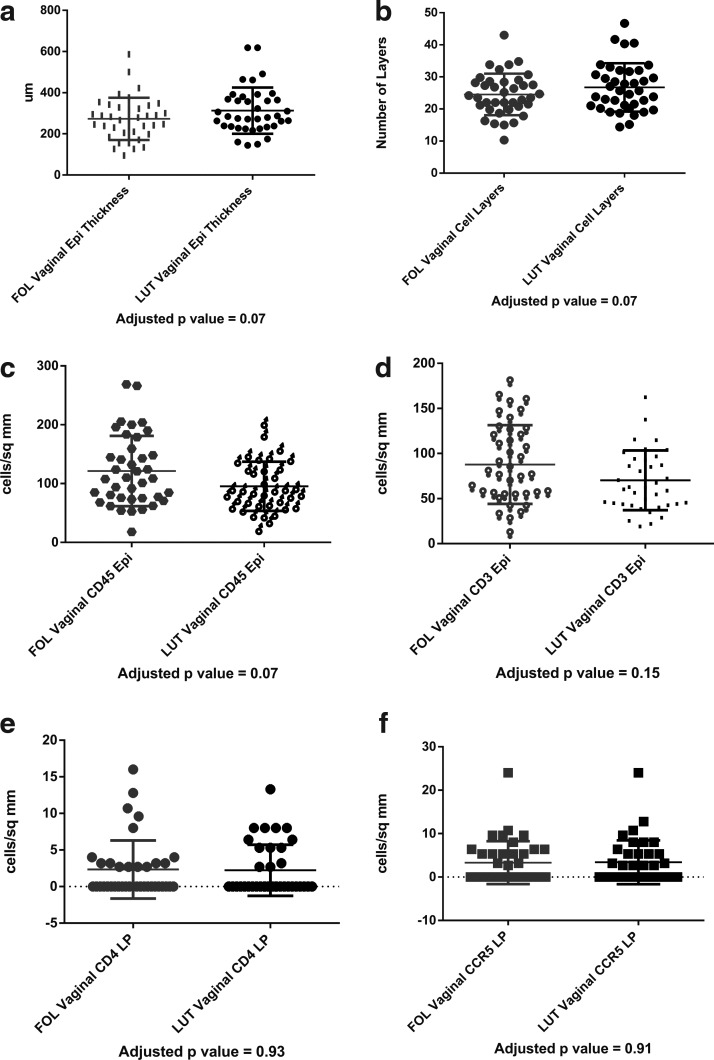
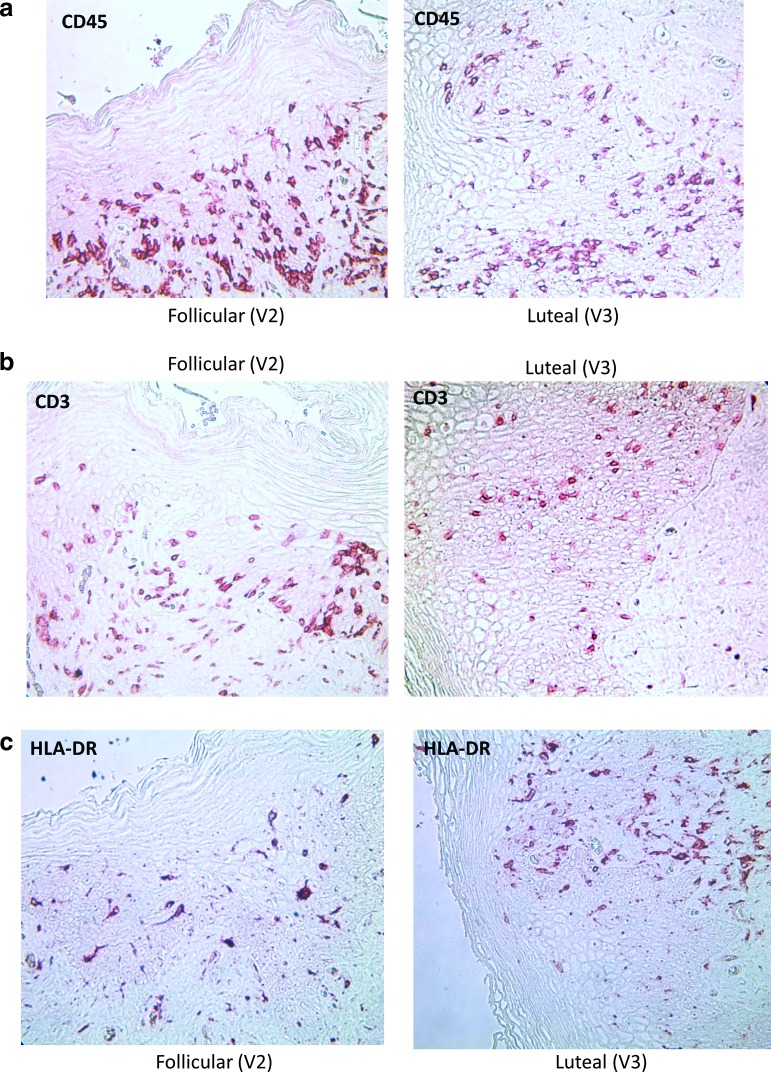
Table 2 demonstrates that there were no significant differences in vaginal immune cell populations, vaginal histology, vaginal pH, vaginal Nugent score, antimicrobial activity of the CVL, or p24 antigen production from vaginal or ectocervical tissues obtained in the FOL phase versus the LUT phase, adjusting for patient race, contraceptive/sexual practices, and study. Representative graphs of changes in vaginal thickness in micrometers (μm), number of vaginal cell layers, and CD45, CD3, CD4, and CCR5-positive cells in the vaginal epithelium are shown in Figure 4a–f and representative photographs of vaginal immune cells seen in the FOL phase versus LUT phase are shown in Figure 5a–c.
FIG. 4.
No significant differences in vaginal tissue histology or immune cells between FOL and LUT phases. (a) Vaginal epithelial thickness, FOL phase versus LUT phase. (b) Vaginal epithelial cell layers Epi, FOL phase versus LUT phase. (c) Vaginal CD45Epi, FOL phase versus LUT phase. (d) Vaginal CD3 Epi, FOL phase versus LUT phase. (e) Vaginal CD4 LP, FOL phase versus LUT phase. (f) Vaginal CCR5 LP, FOL phase versus LUT phase.
FIG. 5.
Representative examples of paired samples, vaginal immune cells in the FOL phase versus LUT phase of the menstrual cycle. (a) CD45 cells in the epithelium. (b) CD3 cells in the epithelium. (c) HLA-DR+ cells in the epithelium.
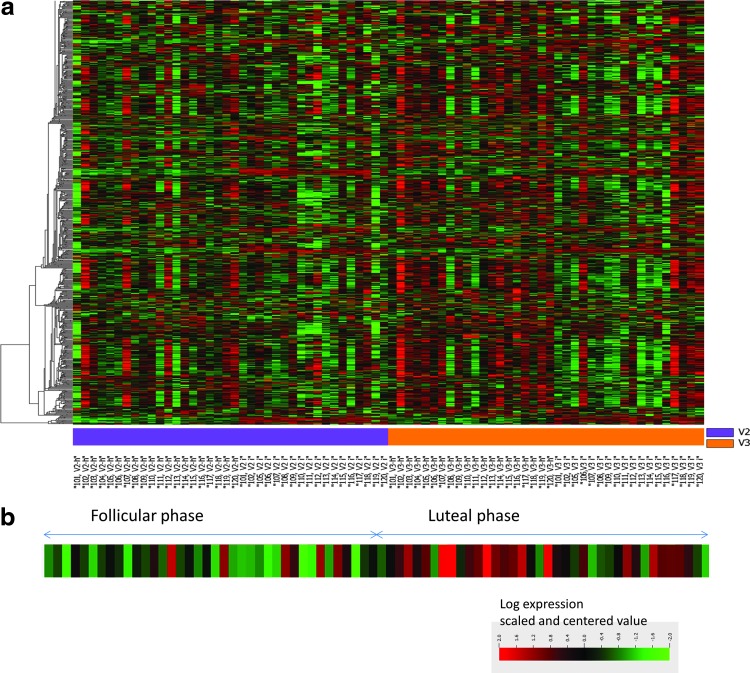
Comparison of gene expression profiles in vaginal tissues at FOL and LUT phases revealed only one gene to be differentially expressed (FDR-corrected p-value <.05, fold change ≥2 as described in the Methods section) (heat map for 2,295 genes shown in Fig. 3a). Gene expression of histidine ammonia lyase (HAL) was 2.4 times higher in the LUT phase compared with the FOL phase. There were no differences in up- or downregulation greater than twofold for any other genes analyzed using the U133 Plus 2 Affymetrix chip. A heat map demonstrating HAL expression in the 76 vaginal tissues from 38 study participants is shown in Figure 3b.
FIG. 3.
(a) Heat map expression of 2,295 genes in FOL phase versus LUT phase in vaginal tissues. (b) Heat map of expression of histidine ammonia lyase (HAL) in vaginal tissue obtained in FOL and LUT phases.
There were several gene sets and pathways, which had differential expression in the LUT phase versus the FOL phase, linked to interferon involvement and inflammation (Table 3 and Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/aid). The genes in these gene sets included several cytokines, chemokines, and interferon-induced genes, which were slightly upregulated (fold change <1.5, p-value >.005) in the LUT phase compared with the FOL phase.
Table 3.
Differentially Expressed Gene Sets Between Luteal and Follicular Phases
| Broad gene sets | Number of genes | LS permutation p-value upregulation in LUT compared with FOL phase | KS permutation p-value upregulation in LUT compared with FOL phase |
|---|---|---|---|
| BOSCO_INTERFERON_INDUCED_ANTIVIRAL_MODULE | 23 | .00001 | .00001 |
| BOYAULT_LIVER_CANCER_SUBCLASS_G6_UP | 8 | .00001 | .01047 |
| BROWNE_HCMV_INFECTION_4HR_UP | 20 | .00001 | .00001 |
| BROWNE_INTERFERON_RESPONSIVE_GENES | 26 | .00001 | .00001 |
| CHANG_CORE_SERUM_RESPONSE_DN | 60 | .00001 | .00005 |
| CHENG_IMPRINTED_BY_ESTRADIOL | 14 | .00001 | .00234 |
| DEBIASI_APOPTOSIS_BY_REOVIRUS_INFECTION_UP | 55 | .00001 | .00001 |
| FULCHER_INFLAMMATORY_RESPONSE_LECTIN_VS_LPS_DN | 124 | .00001 | .00001 |
| HECKER_IFNB1_TARGETS | 49 | .00001 | .00002 |
| HIRSCH_CELLULAR_TRANSFORMATION_SIGNATURE_UP | 58 | .00001 | .0001 |
KS, Kolmogorov–Smirnov; LS, Fisher.
We found no significant correlations between serum E2 or serum P4 and any CV endpoint (vaginal tissue immune cell counts, vaginal epithelial thickness, number of vaginal cell layers, antimicrobial activity of the CVL, vaginal pH, Nugent score, and ectocervical p24 antigen production) (all p-values >.35, data not shown).
There were also no significant linear models using menstrual cycle sampling day as a continuous variable and any of the CV endpoints (all p-values >.05, data not shown). There were no significant differences in CV endpoints when the menstrual cycle sampling phase was subcategorized into four groups (early FOL, preovulatory, early LUT, and late LUT) based on estimated day of ovulation (all p-values >.20, data not shown).
Discussion
The results of these studies represent a comprehensive characterization of biologic endpoints in the lower female reproductive tract, which are potentially related to HIV acquisition in a well-screened population of healthy, premenopausal HIV-seronegative women with normal menstrual cycles. We found no significant differences in vaginal immune cell populations, vaginal pH, Nugent score, the antimicrobial activity of CV secretions, or p24 antigen production after ex vivo HIV-1BaL infection of ectocervical and vaginal samples obtained in the FOL phase versus the LUT phase of the menstrual cycle. Of the ∼38,500 genes examined, only 1 gene, HAL, was significantly upregulated (greater than twofold) in vaginal tissues in the LUT phase compared with the FOL phase.
No significant differences in vaginal immune cells between FOL and LUT phases
Previous studies indicated that there were significant alterations in endometrial immune cells in response to endogenous reproductive hormonal fluctuations.16,17 In studies of the upper reproductive tract, it was hypothesized that high levels of serum E2 and P4 in the LUT phase downregulated cytotoxic T lymphocyte activity in the uterus.41 We did not study immune cell function or activity. Our findings of no significant changes in vaginal tissue immune cells between FOL and LUT phases of the menstrual cycle are consistent with previous studies of rhesus macaques42 and in human studies using vaginal and ectocervical explants,21–24 classified as FOL or LUT phase based on endometrial histology.43 Specifically, semiquantitative levels of HIV target cells CD4, CCR5, and CXCR4 and GalCer in leukocyte populations in the ectocervix did not vary significantly with phase of the menstrual cycle.21 Although the concentration of leukocytes was found to be generally higher in upper reproductive tract explants (fallopian tubes, endometrium, endocervix) compared with lower reproductive tract (ectocervical and vaginal) explants, there were no significant differences in immune cell concentrations of ectocervical and vaginal explants obtained in the FOL phase versus LUT phase of the menstrual cycle.24
Patton et al. reported that the absolute number of Langerhans cells (CD1a), macrophages (KP1), CD4 and CD8 lymphocytes, and neutrophil cell populations in vaginal biopsies obtained from naturally cycling women (n = 11) was not significantly different between the early FOL, periovulatory, and late LUT phases.44
In a small cross-sectional study, including men, postmenopausal, and pregnant and nonpregnant premenopausal women, serum P4 levels were significantly associated with CCR5 expression on peripheral blood mononuclear cells (PBMCs).45 Vaginal tissue biopsies were obtained from a subset of the pregnant women (n = 12), including three women in active labor. Pregnant and laboring women had significantly higher proportions of vaginal CD3− and CD14− cells expressing CCR5 compared with nonpregnant women and women in the first trimester of pregnancy, and serum P4 levels were significantly associated with an increased percentage of vaginal tissue cells expressing CCR5.45 Although this study accounts for a range of endogenous hormonal states, it is likely that the inflammatory state of the genital tract and other covariates differed significantly among cohorts.45
No significant differences in the antimicrobial activity of the CVL
The secretion of cervical mucus is significantly influenced by endogenous reproductive hormones, with cervical mucus volume peaking with surges in preovulatory serum E2.46–48
The biologic rationale for differences in antimicrobial activity of the CVL is based on data from small cohorts showing that secreted cytokines, chemokines, and other cationic antimicrobial polypeptides in the vagina have significant alterations in concentrations based on the phase of the menstrual cycle with immune factors such as IL-6, IL-1β, IL-1RA, and MIP-1β being significantly higher in the FOL phase versus the LUT phase,19,27 while IL-1α and β-defensin were significantly elevated in the LUT phase.27 Keller et al. found that immune mediators, SLPI, α and β defensins, lysozyme, and lactoferrin, were significantly lower at mid-cycle ovulation compared with both the FOL and LUT phases.20 Secretion of mannose-binding lectin, a molecule in the complement system that plays a critical role in host protection, from vaginal epithelial cells is increased in the LUT phase of the menstrual cycle.49
Other investigations found that CV immune mediators did not change based on the menstrual cycle (e.g., IL-10, TNF-α, IL-8, RANTES, and TNFR II),19,26,50 but were higher during menstruation.26,50 Genital tract cytokines have also been shown to vary based on clinical factors, such as cervical ectopy, inflammation, and sexual activity.27,51
An important functional assay, which may incorporate host contributions of cytokines, chemokines, and vaginal microflora, is the antimicrobial activity of the CVL as this helps to model how these factors may potentially work in concert to either protect or enhance infection. Our data are consistent with a cross-sectional study of premenopausal women, sampled either in the FOL (n = 26) or LUT (n = 27) phase of the menstrual cycle, showing no difference between HIV inhibition of the CVL and the menstrual cycle phases.29 However, between 18%–30% of the women sampled in this study had BV at the time of sampling,29 which has been shown to alter the antimicrobial activity of the CVL.52,53 In a longitudinal study, Patel et al. recovered vaginal secretions from seven women in the FOL, periovulatory, and LUT phases and found no significant difference in HIV inhibition in vitro by the vaginal secretions based on the phase of the menstrual cycle, although several cytokine and chemokine levels varied based on the cycle.54 Finally, Shust et al. found no difference in the anti- HSV activity of the CVL among nine women sampled in the FOL phase versus LUT phase of the menstrual cycle.28 We report the largest sample of a well-defined group of healthy HIV-negative women, without evidence of RTIs, to have paired CVL samples collected in the early FOL phase versus late LUT phase and confirm the smaller study findings28,29,54 of no difference in the antimicrobial activity against HIV, HSV-2, and E. coli based on the menstrual cycle.
No differences in vaginal pH or Nugent score between FOL and LUT phases
It is well supported that alterations in the vaginal microflora may affect endpoints measured in these studies (reviewed inThurman and Doncel55). Participants in both clinical studies were screened to exclude BV (Nugent 7–10), although intermediate vaginal flora was not exclusionary. Given that the vaginal microbiota is dynamic and may change on a daily basis in healthy women,56 we assessed vaginal flora at each sampling in both studies. We found that the Nugent score and vaginal pH were similar in FOL and LUT phases among healthy women. Our data are in accordance with a previous study, using pyrosequencing to detail the vaginal microbiome in 32 healthy US women over a 16-week period.56 They found that menstruation was the factor associated with the highest rate of disturbances in vaginal microbiota and that smaller proportions of women with Lactobacilli-dominated microflora transitioned to non-Lactobacilli-dominated (category 4a or 4b) flora.56
No difference in p24 antigen production between FOL and LUT phases
p24 antigen production from tissue biopsies or explants after ex vivo infection by HIV-1BaL has been used as a model for early mucosal HIV infection in women and as an exploratory safety endpoint in phase I studies of potential vaginal or rectal HIV microbicides.38,40,57,58 In contrast to other investigators, we found that vaginal tissue produced significantly more p24 antigen than ectocervical tissue.40 When the samples were analyzed separately, in combination (unadjusted p-value, Table 2), or in combination controlling for race, contraceptive/sexual practices, and tissue source (adjusted p-value, Table 2), we found no significant differences in p24 antigen production between FOL and LUT phases. We also found that HIV-1 reverse transcriptase at day 21 was not significantly different in the FOL phase versus the LUT phase. In many phase I clinical studies, CV tissue biopsies have been timed in the menstrual cycle to make sure that the phase of the menstrual cycle is not a confounding variable. Our data support that the menstrual cycle phase does not affect p24 antigen production, but tissue source (ectocervical vs. vaginal) is a factor. We recognize that there are data to support that in PBMCs, sex hormone concentrations in FOL phase conditions increased and LUT phase conditions decreased HIV-1 replication.59 These findings also demonstrated that E2 and P4 regulate HIV-1 replication most likely by altering HIV-1 transcriptional activation.59 Thus, the effect of sex hormones in PBMCs does not correlate with our results in cervical and vaginal tissue biopsies.
While a pivotal study of macaques (n = 19) supported enhanced susceptibility to SHIV infection in the late LUT phase,13 the follow-on study (n = 46), with a more detailed delineation of the macaque menstrual cycle, estimated that 40% of infections were acquired during the premenstrual (late LUT) phase and 49% were acquired during the menstrual (early FOL) phase.14 Furthermore, the timing of infection was estimated by a 7-day seroconversion eclipse period, which the authors acknowledge has inherent variability and potential error when used in a repeated low-dose challenge model.14
No difference in vaginal tissue gene expression (greater than twofold) between FOL and LUT phases
Using microarray, we found that gene expression in vaginal tissue was not significantly dysregulated by more than twofold in the FOL phase versus the LUT phase of the menstrual cycle. Only one gene, HAL, was significantly upregulated (2.4-fold) in the LUT phase compared with the FOL phase. HAL is the enzyme that converts histidine into urocanic acid (UCA). The highest level of HAL expression was observed in the epidermis during keratinocyte differentiation, along with the presence of high amounts of UCA.60 In the skin, histidine is produced predominantly from filaggrin, a histidine-rich protein specifically expressed in differentiated keratinocytes.61 Of note, it has previously been reported that lower genital tract explants contain little or no filaggrin.62 One of the functions proposed for UCA in the skin is its contribution to the acidification of the stratum corneum. To the best of our knowledge, expression of HAL in the female genital tract has not been reported, and the difference in its expression between FOL and LUT phases warrants further study.
We determined that there was a trend toward upregulation of some pathways involving inflammatory and interferon regulation in the LUT phase. Although deregulation of these gene subsets did not reach the statistical significance that we set for differentially expressed genes, their common activation may impact the state of the vaginal epithelium in terms of susceptibility to infections. Others, using a proteomic approach, have shown a significant difference in serpin A1 levels in the CVL based on menstrual cycle phase among highly exposed seronegative women.63 In addition, proteomic data support that CV epithelial adhesion proteins and antiproteases were reduced, and leukocyte recruitment and extravasation proteins are elevated in the CVL of healthy HIV-negative women during the LUT phase, which may support reduced CV epithelial defenses in the LUT phase.64
The two clinical studies reported here have several strengths, foremost being that we screened participants to exclude factors such as RTIs and other medical comorbidities, which may confound endpoints when using explants or samples obtained from a wide variety of donors undergoing indicated surgery. Our findings of no differences in the FOL phase versus LUT phase can be used to guide clinical protocol design for phase I studies, which sometimes attempt to restrict sampling and product use to the LUT phase of the menstrual cycle, which may be inconvenient to participants and clinic staff.
Sample size and the possibility of a type II or beta error that is failing to detect an effect that is present must always be acknowledged when reporting nonsignificant findings between endpoints obtained at various sampling times. Both studies were not specifically designed to test differences between FOL and LUT phases, and all enrolled participants underwent other treatments, which are not reported in this article. Our data are based on paired comparisons, which strengthen the statistical power of the analysis. In addition, many of our findings are in accordance with previously reported data from ectocervical and vaginal explants21–24 and smaller longitudinal and cross-sectional studies, which obtained tissue biopsies from women.28,29,44,54
The FOL phase of the menstrual cycle is often characterized as an E2-dominant phase, while the LUT phase of the menstrual cycle is characterized as a P4-dominant phase.65,66 Serum E2 levels are low in the early FOL phase and then peak just before the lutenizing hormone (LH) surge in the 2–4 days before ovulation.66 To perform genital sampling at the peak of E2 production, we would need participants to check daily urinary LH and E2 levels and time visits based on the surge in these hormones. This was not feasible in these 2 studies. Therefore, we based the sampling, as is often done in clinical trials, on the menstrual cycle day, which resulted in women being sampled, on average, in the early FOL phase (day 8.7 ± 2.3) when mean serum E2 levels are expected to be significantly lower than those of the LUT phase.66 It would have been ideal to have a third periovulatory sampling to capture an E2-dominant phase to compare with the FOL and late LUT data. We attempted to control for differences in the FOL, preovulatory, early LUT, and late LUT phases by subdividing the samples obtained in the early FOL, preovulatory, early LUT, and late LUT phases based on estimations of the day of ovulation, calculated from the average length of each participant's menstrual cycle. This secondary analysis, however, did not yield any significant differences in the endpoints. We acknowledge that without data on the urinary LH or E2 surge and without knowing the date of the subsequent menstrual cycle, samples could be misclassified as early or late in respective menstrual cycle phases. In addition, subdividing the data from two categories (FOL vs. LUT) into four categories reduces the cell size of each cohort.
Categorizing the phase of the menstrual cycle based on menstrual cycle day is commonly done in clinical studies. Our results are in accordance with several other groups that have attempted to understand differences in the lower genital tract based on endogenous hormones. We obtained serum hormone levels at each genital sampling in the S124 study. However, we were unable to demonstrate significant relationships between systemic hormonal state and CV endpoints in healthy premenopausal women.
In conclusion, we report the results from two longitudinal studies of healthy, nonpregnant HIV-seronegative women with genital samples obtained in FOL and LUT phases of the menstrual cycle. Although we could not study all factors that change or are influenced by the phases of the menstrual cycle, our findings show no significant differences between FOL and LUT phases of the menstrual cycle in CV mucosal parameters associated with HIV susceptibility in women. These parameters are relevant as they are often assessed in HIV prevention, microbicide, and product safety trials.
Supplementary Material
Acknowledgments
This work was funded by an intra-agency agreement between the NIH/NIAID/DAIDS and USAID/OHA (NIAID Y1-AI-1756-01) and CONRAD (GPO-A-00-08-00005-00) and an RO1 from the NICHD (5R01HD072705). The views expressed by the authors do not necessarily reflect those of the funding agency or CONRAD.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Boily MC, Baggaley RF, Wang L, et al. : Heterosexual risk of HIV-1 infection per sexual act: Systematic review and meta-analysis of observational studies. Lancet Infect Dis 2009;9:118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray RH, Wawer MJ, Brookmeyer R, et al. : Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 2001;357:1149–1153 [DOI] [PubMed] [Google Scholar]
- 3.Wawer MJ, Gray RH, Sewankambo NK, et al. : Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis 2005;191:1403–1409 [DOI] [PubMed] [Google Scholar]
- 4.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J: Estimating per-act HIV transmission risk: A systematic review. AIDS 2014;28:1509–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS. The Gap Report. 2014. www.unaids.org/en/resources/campaigns/2014/2014gapreport/gapreport
- 6.Reichelderfer PS, Coombs RW, Wright DJ, et al. : Effect of menstrual cycle on HIV-1 levels in the peripheral blood and genital tract. WHS 001 Study Team. AIDS 2000;14:2101–2107 [DOI] [PubMed] [Google Scholar]
- 7.Polis CB, Phillips SJ, Curtis KM, et al. : Hormonal contraceptive methods and risk of HIV acquisition in women: A systematic review of epidemiological evidence. Contraception 2014;90:360–390 [DOI] [PubMed] [Google Scholar]
- 8.Gray RH, Li X, Kigozi G, et al. : Increased risk of incident HIV during pregnancy in Rakai, Uganda: A prospective study. Lancet 2005;366:1182–1188 [DOI] [PubMed] [Google Scholar]
- 9.Taha TE, Dallabetta GA, Hoover DR, et al. : Trends of HIV-1 and sexually transmitted diseases among pregnant and postpartum women in urban Malawi. AIDS 1998;12:197–203 [DOI] [PubMed] [Google Scholar]
- 10.Leroy V, Van de Perre P, Lepage P, et al. : Seroincidence of HIV-1 infection in African women of reproductive age: A prospective cohort study in Kigali, Rwanda, 1988–1992. AIDS 1994;8:983–986 [DOI] [PubMed] [Google Scholar]
- 11.Wira CR, Fahey JV: A new strategy to understand how HIV infects women: Identification of a window of vulnerability during the menstrual cycle. AIDS 2008;22:1909–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sodora DL, Gettie A, Miller CJ, Marx PA: Vaginal transmission of SIV: Assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res Hum Retroviruses 1998;14 Suppl 1:S119–S123 [PubMed] [Google Scholar]
- 13.Vishwanathan SA, Guenthner PC, Lin CY, et al. : High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr 2011;57:261–264 [DOI] [PubMed] [Google Scholar]
- 14.Kersh EN, Henning T, Vishwanathan SA, et al. : SHIV susceptibility changes during the menstrual cycle of pigtail macaques. J Med Primatol 2014;43:310–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeaman GR, Howell AL, Weldon S, et al. : Human immunodeficiency virus receptor and coreceptor expression on human uterine epithelial cells: Regulation of expression during the menstrual cycle and implications for human immunodeficiency virus infection. Immunology 2003;109:137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeaman GR, Guyre PM, Fanger MW, et al. : Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J Leukoc Biol 1997;61:427–435 [PubMed] [Google Scholar]
- 17.White HD, Crassi KM, Givan AL, et al. : CD3+ CD8+ CTL activity within the human female reproductive tract: Influence of stage of the menstrual cycle and menopause. J Immunol 1997;158:3017–3027 [PubMed] [Google Scholar]
- 18.Kutteh WH, Moldoveanu Z, Mestecky J: Mucosal immunity in the female reproductive tract: Correlation of immunoglobulins, cytokines, and reproductive hormones in human cervical mucus around the time of ovulation. AIDS Res Hum Retroviruses 1998;14 Suppl 1:S51–S55 [PubMed] [Google Scholar]
- 19.Al-Harthi L, Wright DJ, Anderson D, et al. : The impact of the ovulatory cycle on cytokine production: Evaluation of systemic, cervicovaginal, and salivary compartments. J Interferon Cytokine Res 2000;20:719–724 [DOI] [PubMed] [Google Scholar]
- 20.Keller MJ, Madan RP, Torres NM, et al. : A randomized trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity following application of 1% tenofovir gel. PLoS One 2011;6:e16475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeaman GR, Asin S, Weldon S, et al. : Chemokine receptor expression in the human ectocervix: Implications for infection by the human immunodeficiency virus-type I. Immunology 2004;113:524–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poppe WA, Drijkoningen M, Ide PS, Lauweryns JM, Van Assche FA: Lymphocytes and dendritic cells in the normal uterine cervix. An immunohistochemical study. Eur J Obstet Gynecol Reprod Biol 1998;81:277–282 [DOI] [PubMed] [Google Scholar]
- 23.Pudney J, Quayle AJ, Anderson DJ: Immunological microenvironments in the human vagina and cervix: Mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod 2005;73:1253–1263 [DOI] [PubMed] [Google Scholar]
- 24.Givan AL, White HD, Stern JE, et al. : Flow cytometric analysis of leukocytes in the human female reproductive tract: Comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol 1997;38:350–359 [DOI] [PubMed] [Google Scholar]
- 25.Saba E, Origoni M, Taccagni G, et al. : Productive HIV-1 infection of human cervical tissue ex vivo is associated with the secretory phase of the menstrual cycle. Mucosal Immunol 2013;6:1081–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Harthi L, Kovacs A, Coombs RW, et al. : A menstrual cycle pattern for cytokine levels exists in HIV-positive women: Implication for HIV vaginal and plasma shedding. AIDS 2001;15:1535–1543 [DOI] [PubMed] [Google Scholar]
- 27.Kyongo JK, Jespers V, Goovaerts O, et al. : Searching for lower female genital tract soluble and cellular biomarkers: Defining levels and predictors in a cohort of healthy Caucasian women. PLoS One 2012;7:e43951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shust GF, Cho S, Kim M, et al. : Female genital tract secretions inhibit herpes simplex virus infection: Correlation with soluble mucosal immune mediators and impact of hormonal contraception. Am J Reprod Immunol 2010;63:110–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chappell CA, Isaacs CE, Xu W, et al. : The effect of menopause on the innate antiviral activity of cervicovaginal lavage. Am J Obstet Gynecol 2015;213:204.e1–e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patterson BK, Landay A, Andersson J, et al. : Repertoire of chemokine receptor expression in the female genital tract: Implications for human immunodeficiency virus transmission. Am J Pathol 1998;153:481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ACOG Committee on Practice Bulletins–Gynecology: ACOG practice bulletin No. 104: Antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol 2009;113:1180–1189 [DOI] [PubMed] [Google Scholar]
- 32.Culligan PJ, Kubik K, Murphy M, Blackwell L, Snyder J: A randomized trial that compared povidone iodine and chlorhexidine as antiseptics for vaginal hysterectomy. Am J Obstet Gynecol 2005;192:422–425 [DOI] [PubMed] [Google Scholar]
- 33.Markham SM, Rock J: Preoperative care. In: TeLinde's Operative Gynecology, Volume 10, 10th edition (Rock J, Jones H, eds.) Lippincott Williams and Wilkins, New York, 2011, pp. 103–163 [Google Scholar]
- 34.Nugent RP, Krohn MA, Hillier SL: Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991;29:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thurman A, Kimble T, Nel A, et al. : Safety of cervical and vaginal biopsies in microbicide and contraceptive research. Paper presented at International AIDS Society 2015, Vancouver, BC [Google Scholar]
- 36.Zalenskaya IA, Joseph T, Bavarva J, et al. : Gene expression profiling of human vaginal cells in vitro discriminates compounds with pro-inflammatory and mucosa-altering properties: Novel biomarkers for preclinical testing of HIV microbicide candidates. PLoS One 2015;10:e0128557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benjamini Y, Hochberg Y: Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995;57:289–300 [Google Scholar]
- 38.Richardson-Harman N, Mauck C, McGowan I, Anton P: Dose-response relationship between tissue concentrations of UC781 and explant infectibility with HIV type 1 in the RMP-01 rectal safety study. AIDS Res Hum Retroviruses 2012;28:1422–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richardson-Harman N, Lackman-Smith C, Fletcher PS, et al. : Multisite comparison of anti-human immunodeficiency virus microbicide activity in explant assays using a novel endpoint analysis. J Clin Microbiol 2009;47:3530–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dezzutti CS, Uranker K, Bunge KE, Richardson-Harman N, Macio I, Hillier SL: HIV-1 infection of female genital tract tissue for use in prevention studies. J Acquir Immune Defic Syndr 2013;63:548–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beagley KW, Gockel CM: Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol 2003;38:13–22 [DOI] [PubMed] [Google Scholar]
- 42.Ma Z, Lu FX, Torten M, Miller CJ: The number and distribution of immune cells in the cervicovaginal mucosa remain constant throughout the menstrual cycle of rhesus macaques. Clin Immunol 2001;100:240–249 [DOI] [PubMed] [Google Scholar]
- 43.Noyes RW, Hertig AT, Rock J: Dating the endometrial biopsy. Am J Obstet Gynecol 1975;122:262–263 [DOI] [PubMed] [Google Scholar]
- 44.Patton DL, Thwin SS, Meier A, Hooton TM, Stapleton AE, Eschenbach DA: Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am J Obstet Gynecol 2000;183:967–973 [DOI] [PubMed] [Google Scholar]
- 45.Sheffield JS, Wendel GD, Jr., McIntire DD, Norgard MV: The effect of progesterone levels and pregnancy on HIV-1 coreceptor expression. Reprod Sci 2009;16:20–31 [DOI] [PubMed] [Google Scholar]
- 46.Rarick LD, Shangold MM, Ahmed SW: Cervical mucus and serum estradiol as predictors of response to progestin challenge. Fertil Steril 1990;54:353–355 [DOI] [PubMed] [Google Scholar]
- 47.Adamopoulos DA, Kapolla N, Abrahamian A, Dessypris A, Nicopoulou S, Giannacodemos G: Sex steroids in cervical mucus of spontaneous or induced ovulatory cycles. Steroids 2000;65:1–7 [DOI] [PubMed] [Google Scholar]
- 48.Katz DF, Slade DA, Nakajima ST: Analysis of pre-ovulatory changes in cervical mucus hydration and sperm penetrability. Adv Contracept 1997;13:143–151 [DOI] [PubMed] [Google Scholar]
- 49.Bulla R, De Seta F, Radillo O, et al. : Mannose-binding lectin is produced by vaginal epithelial cells and its level in the vaginal fluid is influenced by progesterone. Mol Immunol 2010;48:281–286 [DOI] [PubMed] [Google Scholar]
- 50.Gargiulo AR, Fichorova RN, Politch JA, Hill JA, Anderson DJ: Detection of implantation-related cytokines in cervicovaginal secretions and peripheral blood of fertile women during ovulatory menstrual cycles. Fertil Steril 2004;82 Suppl 3:1226–1234 [DOI] [PubMed] [Google Scholar]
- 51.Hwang LY, Scott ME, Ma Y, Moscicki AB: Higher levels of cervicovaginal inflammatory and regulatory cytokines and chemokines in healthy young women with immature cervical epithelium. J Reprod Immunol 2011;88:66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valore EV, Wiley DJ, Ganz T: Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infect Immun 2006;74:5693–5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thurman AR, Kimble T, Herold B, et al. : Bacterial vaginosis and subclinical markers of genital tract inflammation and mucosal immunity. AIDS Res Hum Retroviruses 2015;31:1139–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel MV, Ghosh M, Fahey JV, Ochsenbauer C, Rossoll RM, Wira CR: Innate immunity in the vagina (Part II): Anti-HIV activity and antiviral content of human vaginal secretions. Am J Reprod Immunol 2014;72:22–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thurman AR, Doncel GF: Innate immunity and inflammatory response to Trichomonas vaginalis and bacterial vaginosis: Relationship to HIV acquisition. Am J Reprod Immunol 2011;65:89–98 [DOI] [PubMed] [Google Scholar]
- 56.Gajer P, Brotman RM, Bai G, et al. : Temporal dynamics of the human vaginal microbiota. Sci Transl Med 2012;4:132ra152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang KH, Hendrix C, Bumpus N, et al. : A multi-compartment single and multiple dose pharmacokinetic comparison of rectally applied tenofovir 1% gel and oral tenofovir disoproxil fumarate. PLoS One 2014;9:e106196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Richardson-Harman N, Hendrix CW, Bumpus NN, et al. : Correlation between compartmental tenofovir concentrations and an ex vivo rectal biopsy model of tissue infectibility in the RMP-02/MTN-006 phase 1 study. PLoS One 2014;9:e111507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asin SN, Heimberg AM, Eszterhas SK, Rollenhagen C, Howell AL: Estradiol and progesterone regulate HIV type 1 replication in peripheral blood cells. AIDS Res Hum Retroviruses 2008;24:701–716 [DOI] [PubMed] [Google Scholar]
- 60.Eckhart L, Schmidt M, Mildner M, et al. : Histidase expression in human epidermal keratinocytes: Regulation by differentiation status and all-trans retinoic acid. J Dermatol Sci 2008;50:209–215 [DOI] [PubMed] [Google Scholar]
- 61.Lynley AM, Dale BA: The characterization of human epidermal filaggrin. A histidine-rich, keratin filament-aggregating protein. Biochim Biophys Acta 1983;744:28–35 [DOI] [PubMed] [Google Scholar]
- 62.Dinh MH, Okocha EA, Koons A, Veazey RS, Hope TJ: Expression of structural proteins in human female and male genital epithelia and implications for sexually transmitted infections. Biol Reprod 2012;86:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahman S, Rabbani R, Wachihi C, et al. : Mucosal serpin A1 and A3 levels in HIV highly exposed sero-negative women are affected by the menstrual cycle and hormonal contraceptives but are independent of epidemiological confounders. Am J Reprod Immunol 2013;69:64–72 [DOI] [PubMed] [Google Scholar]
- 64.Birse K, Arnold KB, Novak RM, et al. : Molecular signatures of immune activation and epithelial barrier remodeling are enhanced during the luteal phase of the menstrual cycle: Implications for HIV susceptibility. J Virol 2015;89:8793–8805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fritz MA, Speroff L: The endocrinology of the menstrual cycle: The interaction of folliculogenesis and neuroendocrine mechanisms. Fertil Steril 1982;38:509–529 [DOI] [PubMed] [Google Scholar]
- 66.Speroff L, Vande Wiele RL: Regulation of the human menstrual cycle. Am J Obstet Gynecol 1971;109:234–247 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.