Abstract
There is increasing recognition that low dissolved oxygen (DO) and low pH conditions co-occur in many coastal and open ocean environments. Within temperate ecosystems, these conditions not only develop seasonally as temperatures rise and metabolic rates accelerate, but can also display strong diurnal variability, especially in shallow systems where photosynthetic rates ameliorate hypoxia and acidification by day. Despite the widespread, global co-occurrence of low pH and low DO and the likelihood that these conditions may negatively impact marine life, very few studies have actually assessed the extent to which the combination of both stressors elicits additive, synergistic or antagonistic effects in marine organisms. We review the evidence from published factorial experiments that used static and/or fluctuating pH and DO levels to examine different traits (e.g. survival, growth, metabolism), life stages and species across a broad taxonomic spectrum. Additive negative effects of combined low pH and low DO appear to be most common; however, synergistic negative effects have also been observed. Neither the occurrence nor the strength of these synergistic impacts is currently predictable, and therefore, the true threat of concurrent acidification and hypoxia to marine food webs and fisheries is still not fully understood. Addressing this knowledge gap will require an expansion of multi-stressor approaches in experimental and field studies, and the development of a predictive framework. In consideration of marine policy, we note that DO criteria in coastal waters have been developed without consideration of concurrent pH levels. Given the persistence of concurrent low pH–low DO conditions in estuaries and the increased mortality experienced by fish and bivalves under concurrent acidification and hypoxia compared with hypoxia alone, we conclude that such DO criteria may leave coastal fisheries more vulnerable to population reductions than previously anticipated.
Keywords: ocean acidification, fisheries, hypoxia, eutrophication
1. Ecosystem occurrence of low oxygen and acidification
Low oxygen zones are a common feature within marine ecosystems. Human colonization of coastal zones has resulted in accelerated nutrient delivery to the sea that can have multiple negative consequences within coastal zones, including the degradation and destruction of critical marine habitats and the stimulation of algal blooms [1]. The respiration of the algal organic matter consumes oxygen, which can lead to low oxygen or hypoxic conditions in water bodies where respiration-driven oxygen consumption exceeds the rate at which oxygen is replenished. The upper threshold of hypoxic conditions typically ranges from 2 to 5 mg l−1 (62.5–157 µM), depending on the group defining the standard [2]. Spatially, hypoxic conditions can be common along coastal zones with elevated nutrient delivery and in deeper water bodies that are stratified owing to surface freshwater discharge from rivers and/or surface heating. Temporally, these conditions most commonly develop in temperate water bodies in summer when seasonal temperatures are elevated and thus saturated dissolved oxygen (DO) levels are already low, thermally induced vertical stratification is more likely to occur, wind-driven mixing is minimal, and microbial respiration rates are maximal. Hence, hypoxic zones within temperate latitudes are often seasonal features that dissipate when temperatures decrease, stratification is disrupted and respiration rates decline. Given this link to temperature, there is the expectation that the intensity, duration, frequency and number of hypoxic zones will expand with global warming [3]. More than 400 hypoxic zones have been identified in coastal zones across the planet [4], with this number increasing as nutrient loading, temperatures and scientific reporting of these regions increase with time [3,5]. Owing to the inhibitory effects that low oxygen has on marine life, hypoxic zones have been an intense area of focus for both coastal zone managers and scientists [2,4].
Low oxygen zones also develop within deeper waters of tropical and temperate oceans, typically at depths between 100 and 1000 m. These ‘oxygen minimum zones' (OMZ) are driven by many of the same processes that control coastal zone hypoxia; hence, ocean regions that experience larger nutrient inputs to euphotic zones (typically via upwelling) and strong thermal stratification are more likely to harbour extremely low oxygen levels at depth [5]. There is now a consensus that greenhouse-induced ocean warming will lead to a global expansion of OMZs in the coming decades [3,5,6].
Ocean acidification is a more recently studied phenomenon, but has garnered significant attention among scientists, policy-makers and the public during the past decade. While it had long been assumed that ocean alkalinity would provide the buffering capacity required to keep ocean pH levels stable through time, we now know that the rate at which CO2 is entering world oceans is too rapid for it to be buffered against [5,6]. As a consequence, CO2 entering the oceans is reducing the availability of carbonate ions and reducing pH, which is largely controlled by the ratio of carbonic acid to carbonate ions in seawater. This process also occurs in regions that experience low oxygen conditions, but at a greatly accelerated rate. The same nutrient-enhanced respiration of organic matter that creates hypoxic and OMZ in the ocean also produces high levels of CO2 that reacts in a manner identical to the atmospheric CO2 and thus similarly creates low pH and low carbonate ion availability [7–10]. The issue of coastal acidification is increasingly recognized, because seasonal levels of pCO2, pH and calcium carbonate saturation in many coastal zones (pH < 7.7T, pCO2 > 1000 µatm, Ωaragonite < 1 [9–11]) already exceed (i) the predicted extremes in the future open ocean [5] and (ii) the levels known to reduce the growth and survival of early-life stage bivalves [12] and fish [13].
Acidification and low oxygen conditions are inextricably linked via the process of respiration and therefore display highly similar dynamics in ocean ecosystems [7–11]. In temperate coastal zones, both conditions appear during warmer months when respiration rates are maximal and thermal stratification is most likely. Further, they both occur at extreme levels in regions that receive excessive nutrient loads such as near large coastal cities [9,10] or within eutrophied river plumes [8]. Estuaries have long been known as net heterotrophic ecosystems owing to both allochthonous (imported) and autochtonous (self-produced) sources of organic carbon; hence, on a net annual basis they are producing CO2 and consuming O2 [14]. Beyond the role of excessive nutrient loads in driving this trend, specific estuarine habitats such as salt marshes and mangroves are naturally enriched in organic carbon that is respired and creates hypoxic and acidified conditions, particularly within warmer waters [11].
The persistence of hypoxia and acidification in coastal zones can vary from hours to months to years, depending on the intensity of respiration, the geomorphology of the region, tidal flushing, the depth and structure of the water column, and other factors related to the hydrodynamics of a given water body. In shallow, well-mixed coastal zones with high rates of respiration, hypoxia and acidification can occur diurnally, because maximal photosynthetic rates during the day result in high DO and pH levels, whereas the cumulative effects of respiration during night decrease DO and pH levels to a predawn minimum. The intensity of this process can be related to the depth of the water column, given that sediments are known to be strong sources of CO2 [15] and their influence is likely to be inversely proportional to the depth of the water column. Given that most manual measurements of pH and DO are made during daylight hours, it is likely that the occurrence of hypoxia and acidification has been underreported in many ecosystems. Superimposed upon diurnal changes in net metabolism in coastal zones are the actions of tides. In general, low tides are likely to maximize the influence of local metabolic rates while high tides may bring less intense metabolism if the water mass is originating from a region further from land with lowered amounts of organic matter, respiration and benthic influence [11,16]. Regardless, within deeper, stratified ecosystems including OMZs, hypoxic and acidified water can persist for weeks, months or longer, as benthic and/or deep water respiration continually consumes oxygen and produces CO2 faster than it is replenished via diffusion and mixing from surface waters [10,17].
Despite the strong coupling of hypoxia and acidification via respiration, there is a series of processes that likely make acidification more common and persistent in many coastal zones, both today and in the future. Beyond respiration, there is a series of coastal processes that promote acidification and high CO2 conditions but have minor effects on DO levels, including the discharge of acidified riverine water, acid deposition, sea ice melting and the lower alkalinity of coastal zones that results in a lower buffering capacity against acidification compared with ocean regimes. Furthermore, the differential diffusion and solubility of DO and CO2 cause oxygen levels in seawater to come to equilibrium with prevailing atmospheric conditions more rapidly than CO2 [18]. As a consequence, when deeper waters that are low in pH and DO are advected to the surface via upwelling, the signature of acidification can be persistent [7] even to the detriment of marine life [19], whereas oxygen levels are commonly more normal. Similarly, when the water column of temperate, coastal hypoxic zones cools and destratifies, whole water columns become normoxic, but low pH conditions can persist for several weeks [10]. An additional factor that likely enhances acid production (lower pH) during times when DO levels are increasing is the oxidation of anaerobic metabolites. The reduced constituents (e.g. NH4+, HS−, Fe2+, Mn2+) that build up in surface sediments during hypoxia oxidize seasonally when systems re-oxygenate [20]. These oxidation reactions produce strong acids that titrate alkalinity and lower pH [15]. For all of these reasons, acidification is likely more persistent than hypoxia in coastal zones.
As climate change progresses, the effects of atmospheric CO2 on coastal acidification will intensify in a nonlinear fashion, leading to acidification becoming an even more prominent stressor in coastal zones. Modelling efforts have shown that under future climate change scenarios, synergistic interactions may occur between CO2 emanating from atmospheric sources and from the respiration of organic matter. As a consequence, the buffer capacity of some ocean regions may be overwhelmed, resulting in a degree of acidification that is non-additive and greater than would have been predicted from the CO2 loading by either individual source [21]. This may make temperate and tropical estuaries even more vulnerable to coastal acidification, as they warm and experience the synergistic effects of acidification driven by both respiration and atmospheric CO2. While oceans are also expected to deoxygenate under climate change scenarios, the relative decrease in DO via this process is small compared with the expected changes in pH and CO2 [5,6] (figure 1).
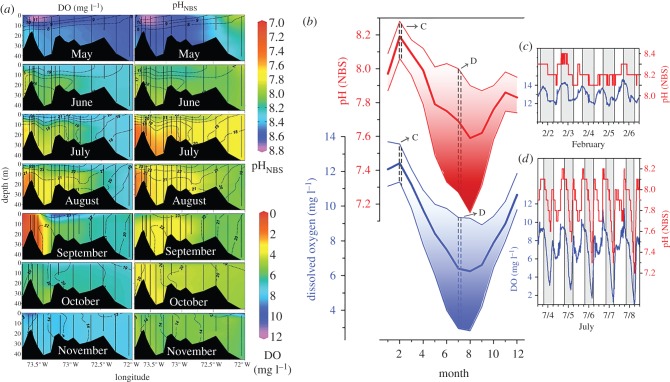
Figure 1.
Spatial and temporal covariance of hypoxia and acidification in coastal ecosystems. (a) Changes in DO and pH with season, depth and longitude along a west–east transect through Long island sound (LIS), where low DO and pH predominantly develop during late summer, at depth and in the urbanized western LIS (after [10]). (b) Seasonal evolution of pH (red) and DO (blue) in a temporal tidal saltmarsh adjacent to LIS, showing long-term (2008–2012) monthly averages of daily minima, means and maxima. Insets c and d illustrate the typical diel and tidal variability of DO and pH in February and July, respectively (after [11]). NBS, National Bureau of Standards.
2. Effects of low dissolved oxygen and low pH on marine life
Very few studies have assessed the sensitivity of marine organisms to the combination of low pH and low DO [22–24]. Pioneering work by Burnett [24] demonstrated that adult estuarine fish, shrimp or oysters possess astonishing regulatory capacities in the face of adverse pH and DO conditions, which, however, can make them more vulnerable to other toxins or parasite infections. An unfortunate limitation of previous laboratory hypoxia studies is that many administered only N2 gas to reduce oxygen levels in experimental vessels, which also reduces CO2 and therefore raises pH [23], contrary to the conditions observed in hypoxic zones [8–10]. Given the tendency for detrimental effects of low oxygen and low pH individually, the combination of both stressors is expected to elicit at least additive (no interaction) or synergistically negative (interaction) effects, likely depending on exposure time, strength, examined traits, species and life stages. To explore and predict organismal responses to multi-stressor environments, existing bioenergetic frameworks [25,26] provide a useful starting point, because they focus on aerobic scope as the affected, fundamental trait (i.e. a proxy for the surplus energy available for growth, reproduction, predator avoidance, etc.). Pörtner [25] suggested that an organism's capacity to sustain aerobic metabolism is limited to a species- and life-stage-specific thermal window (figure 2d). Low oxygen and elevated CO2 levels in the environment are both expected to reduce thermal windows, by directly depressing the oxygen content of blood and other body fluids or by decreasing the functional capacity of pH-sensitive tissues (e.g. pH-sensitive oxygen binding proteins such as haemoglobin and haemocyanin). Organisms may also need to spend additional energy on ventilation or acid/base regulation, or activate mechanisms for protection and damage repair, thereby increasing their basal metabolism and shrinking the aerobic scope. These negative effects could be compounded further by reduced assimilation of food and/or stress-induced impacts on the aerobic production of ATP [26]. With respect to acidification and hypoxia, however, these frameworks remain largely untested.
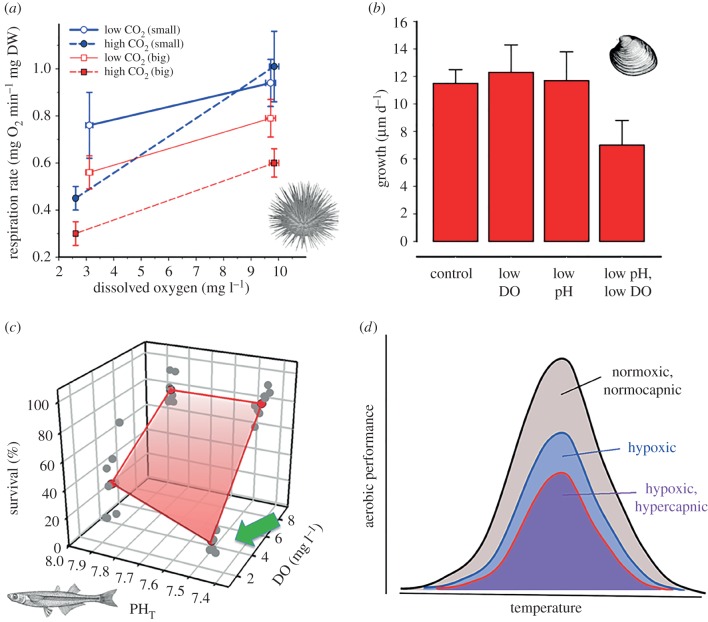
Figure 2.
Synergistic negative effects of low DO and low pH (high CO2) on different traits and marine taxa. (a) Synergistic decrease in respiration rate in small and big sea urchins [27]; (b) growth rate of juvenile quahog was unaffected by low DO or low pH individually, but decreased under combined stressor conditions [23]; (c) survival of Atlantic silverside larvae to 10 dph. Survival was robust against low pH and sensitive to low DO, but decreased synergistically under combined stressors (green arrow, [22]); (d) representation of Pörtners [25] ‘Oxygen- and capacity-limited thermal tolerance’ framework, adapted to the multiple stressor scenario of acidification and hypoxia. Error bars represent standard deviations.
3. Factorial low oxygen × low pH experiments on marine organisms
Factorial experiments are powerful approaches to study the effects of low DO and low pH environments on marine biota, because they apply stressors individually and in combination on the same set of individuals. This enables experimenters to quantify how effects of concurrent (i.e. environmentally realistic) low DO × low pH conditions differ from (i) the effects of each individual stressor and (ii) the hypothetical sum of the two stressors, thus testing for interactions. Of particular concern is the potential for synergistic negative effects of low DO and pH on a wide range of traits and species. At the time of writing, we found six such factorial experiments that are summarized in table 1. These studies employed various treatment levels, and measured different traits in different life stages of species across a broad taxonomic spectrum. They comprise starting points of a much needed research effort, while still allowing few generalizations.
Table 1.
Overview of published factorial experiments on species sensitivities to low oxygen and low pH individually and in combination. X: no effect, arrow: increase/decrease,+synergistic effects,−antagonistic effect, ± additive effect (no interaction). Traits: activity (A), growth (G), hatching success (HS), metabolism (Mb), metamorphosis (%, Mm), survival (S), time-to-hatch (TH).
| taxon | species | life stage | treatment level(s) |
exposure (days) | traits | main findings |
references | |||
|---|---|---|---|---|---|---|---|---|---|---|
| CO2 (ppm) | pHNBS | DO (mg l−1) | CO2/pH effect | DO effect | CO2 × DO (overall effect, interaction) | ||||||
| anthozoa | Anemonia alicemartinae | adults | 1000–1200 | 7.8–7.7 | 2.6–3.1 | 3–6 | S, Mb | S×, Mb ↑ | S×; Mb ↓ | S×; Mb ↓+ | Steckbauer et al. [27] |
| Phymactis papillosa | S×, Mb ↑ | S×; Mb ↓ | S×; Mb ↓± | |||||||
| gastropoda | Concholepas concholepas | S×, Mb× | S×; Mb ↓ | S×; Mb ↓± | ||||||
| Haliotis rufescens | juveniles | 1400 | 7.65 | 5.0 | 15 | S, G | n.a. | S ↓; G ↓ | S×; G ↓+ | Kim et al. [28] | |
| bivalvia | Argopecten irradians | larvae | 1400 | 7.70–7.65 | 1–1.6 | 30–40 | S, G, Mm | S ↓, G×, Mm× | S×, G ↓, Mm ↓ | S ↓±, G ↓±, Mm ↓− | Gobler et al. [23] |
| Mercenaria mercenaria | juveniles | 1700–2000 | 7.6–7.5 | S, G | S×, G ↓ | S ↓; G× | S ↓±; G ↓± | ||||
| Macoma balthica | juveniles | 2700 | 7.35 | 3.0 | 29 | S, G | S×, G× | S ↑; G ↑ | S ↑; G ↑+ | Jansson et al. [29] | |
| Mytilus edulis trossulus | adults | n.a. | 7.5; 7.0 | 2.2 | 0.5 | A, Mb | A×, Mb× | A×, Mb× | A×±, Mb×± | Jakubowska and Normant [30] | |
| echinoidea | Tetrapigus niger | adults | 1000–1200 | 7.8–7.7 | 2.6–3.1 | 3–6 | S, Mb | S×, Mb× | S×; Mb ↓ | S×; Mb ↓+ | Steckbauer et al. [27] |
| crustacea | Petrolisthes violaceus | S×, Mb× | S×; Mb ↓ | S×; Mb ↓− | ||||||
| Petrolisthes tuberculatus | S ×, Mb ↓ | S×; Mb× | S×; Mb ↓± | |||||||
| Allopetrolisthes angulosus | S×, Mb× | S×; Mb ↓ | S×; Mb ↓± | |||||||
| Pagurus edwardsi | S×, Mb× | S ×; Mb × | S×; Mb ↓± | |||||||
| pisces | Menidia beryllina | eggs/larvae | 2100–2200 | 7.53 | 1.6–2.5 | 15 | G, HS, S, TH | G ↓, HS×, S ↓, TH× | G ↓, HS ↓, S ↓, TH ↑ | G ↓±, HS ↓±, S ↓±, TH ↑± | Depasquale et al. [22] |
| Menidia menidia | G×, HS×, S, TH× | G ↓, HS ↓, S, TH ↑ | G ↓±, HS ↓±, S ↓+, TH ↑± | |||||||
| Cyprinodon variegatus | G×, HS×, S×, TH× | G ↓, HS ↓, S×, TH ↑ | G ↓±, HS ↓±, S×, TH ↑± | |||||||
(a). Molluscs
Five independent studies so far have assessed a total of six commercially and ecologically important mollusc species, including four bivalves [23,29,30] and two gastropods [27,28]. Measured traits included survival, metabolism, growth, activity and metamorphosis in either larvae/juveniles or adults. In larval bay scallops (Argopecten irradians) and juvenile hard clams (Mercenaria mercenaria) survival and growth were sensitive to low DO, low pH and low DO × low pH [23]. While low DO conditions (1.0–1.6 mg l−1) reduced larval scallop growth but not survival, low pH conditions (7.65–7.70) reduced survival but had no effect on growth. Both stressors combined elicited additive negative effects on both traits, while interacting antagonistically on scallop metamorphosis (table 1). For quahog juveniles (two months), the opposite sensitivity pattern emerged, i.e. low DO alone reduced survival but not growth, low pH conditions reduced growth but not survival, and the combined treatment again elicited additive reductions in both traits. However, growth in older quahog juveniles (four months) appeared robust to either low DO or low pH, but was synergistically reduced by both stressors combined (table 1 and figure 2b). The same study [23] also showed that larval scallops were highly sensitive to naturally hypoxic and acidified waters from a local eutrophic estuary, and that experimentally raising the pH or the pH × DO levels of these waters significantly improved larval scallop performance. This finding demonstrated that current conditions in some estuaries are already having harmful effects on marine life.
Synergistic negative growth effects of low DO × low pH conditions were also reported by Kim et al. [28] for abalone (Haliotis rufescens), a mollusc that regularly experiences acidified and deoxygenated conditions during upwelling events. The authors exposed juveniles to several episodes of low DO (5 mg l−1) or concurrent low DO × pH conditions (7.65). Prolonged, repeated exposures (24 h) to low DO significantly increased mortality over the following few days, but in the low DO × pH treatment, survival was not significantly different from the controls (table 1). In contrast, concurrent low DO × pH conditions had a synergistic negative effect on juvenile abalone growth, whereas low DO alone did not reduce growth, possibly owing to the relatively short course of the experiment (15 days, table 1) and the intermittent nature of the exposure design.
Most marine species experience natural DO and pH variability; hence, exposure history likely influences the sensitivity of current and offspring generations. One study showed that Baltic clam (Macoma balthica) juveniles had higher growth and survival rates at low (3 mg l−1) compared with control DO conditions, whereas acidification (pH 7.35) alone had no effect on survival [29]. Clam growth was only reduced under combined low DO × low pH conditions—a significant interactive effect (table 1). Metabolism, survival and activity in adult Baltic blue mussels (Mytilus edulis trossulus) appeared to be robust even to severe acidification (pH = 7.5 and 7.0) and hypoxia (2.2 mg l−1), although the study was limited by short exposures and small sample sizes [30]. Similarly, concurrent low DO (2.8–3.3 mg l−1) × low pH (7.61–7.68) had no effects on early-life survival and veliger size in two mussel species (Mytilus californianus, M. galloprovincialis, individual low DO, low pH treatments were not tested [31]). All three studies [29–31] argued that local adaptation of bivalves to regular low DO × low pH conditions in the Baltic sea or the California upwelling region may partially explain these findings. In addition, some bivalves undertake anaerobic respiration as DO concentrations decline, an adaptation that may permit greater resistance to the combined effects of low DO and pH [32]. Importantly, however, the potential importance of anaerobiosis for bivalves increases with age and can only comprise 10% of total metabolism within early individuals [32], a fact that may further account for the higher vulnerability of early-life stages to hypoxia and acidification [23].
(b). Marine fish
One study tested the early-life sensitivities of three forage fish species from the Northwest Atlantic (Atlantic and Inland silverside Menidia menidia, M. beryllina, sheepshead minnow Cyprinodon variegatus, [22]) to low DO (1.6–2.5 mg l−1) and low pH (7.5). All three species exhibited different sensitivities. Low pH alone reduced post-hatch survival only in Inland, but not in Atlantic silversides nor in sheepshead minnows. Low DO alone reduced survival in both silverside species, but not in sheepshead minnows. The combined effects were additive in inland silversides, but synergistically negative in Atlantic silversides (figure 2c), whereas the survival of sheepshead minnows was resistant to the combination of both stressors. Larval growth, on the other hand, was negatively affected by low pH and DO individually only in inland silversides, with the combination causing an additive negative effect, whereas in Atlantic silversides and sheepshead minnows, growth was sensitive only to low DO, not to low pH, with the combination of both stressors resulting in additive negative effects.
(c). Other taxa
One study conducted a taxonomically broad, but short-term assessment of responses to low DO × pH conditions in benthic invertebrates from the central Chilean coast [27], including adults of two anemone species, a gastropod, a sea urchin (figure 2a) and four crustaceans. After 3–6 days of exposure to individual and combined conditions of low pH (7.7–7.8) and low DO (2.6–3.1 mg l−1), survival was unaffected; low DO alone generally resulted in metabolic depression, whereas low pH levels increased metabolism in anemones but had generally no effect in the other groups. Importantly, low DO × low pH elicited metabolic depression in every species, with either no (four species), synergistic (three species) or antagonistic (one species) stressor interactions (table 1). However, natural pH conditions at the time of specimen collection were more acidic (7.6) than the low pH treatment of the study, hence potentially contributing to the authors' conclusion that Chilean species are generally robust to acidification.
4. A new frontier: effects of pH and dissolved oxygen fluctuations
How coastal organisms cope with the typically large diel and seasonal variability in pH and DO conditions [10,11] still remains largely unstudied. It is thus unclear whether pH and DO fluctuations primarily afford temporal relief from stressful conditions, or whether they compound environmental stress by requiring constant physiological adjustments. For larvae of two Californian mytilid mussels, Frieder et al.'s [31] study suggested the former, showing that constant low pH (7.48–7.51) during 8 days of exposure had a negative effect on development rate, whereas the fluctuating pH treatment (±0.15 pH units) was less detrimental. Keppel [33] recently observed that diel acidification cycles (pH 7.0–7.8 at control DO) severely reduced growth in oyster spat (Crassostrea virginica) only at low salinity conditions, whereas severe hypoxia cycles (0.5–7.2 mg l−1 at control pH) always reduced growth, and combinations of DO × pH fluctuations elicited additive negative effects. In contrast, Clark [34] found higher larval mortality in A. irradians and M. mercenaria in response to diurnally fluctuating DO and pH levels compared with individuals exposed to static DO and pH conditions. Hence, there might be significant metabolic costs to adapting to rapid diurnal changes in pH and DO, which traditional experiments using static exposures fail to observe.
Although still few, the reviewed studies already hint at the arduously complex task of trying to understand, let alone predict multi-stressor effects in marine organisms and ecosystems. The scarce evidence suggests that—individually—low DO is a greater stressor to most marine organisms than low pH conditions. Under environmentally realistic conditions, however, a real concern is that the addition of low pH to low DO has worse or even synergistically worse effects than what had been determined for hypoxia alone. While most traits under concurrent low DO and low pH appeared to be additively affected, every study so far also found synergistic interactions in at least one instance, i.e. when combined stressors elicited more extreme outcomes than anticipated from the sum of individual effects. Why such synergies occur in some traits, life stages or species, whereas being absent in others, is currently unclear but truly comprises an urgent research need.
Just as the interaction between pH and salinity in oyster growth, many other environmental variables have the ability to interact with organismal responses to concurrent DO × pH regimes. Of those, temperature and feeding conditions are likely the most important, as they can both exacerbate or mitigate acidification and hypoxia effects [25,35]. While interactive effects between temperature and acidification have already received considerable attention in several recent reviews and meta-analyses [36,37], potential interactions with shifting food sources, salinity or light have yet to be thoroughly explored and thus caution against predicting real-world consequences based on the still limited experimental evidence.
Given the common occurrence of low pH and DO conditions in global ocean ecosystems, the scarcity of multi-stressor research, and the potential for synergistic negative effects of low DO × pH (figure 2a–c), there is now an urgent need for expanding multi-stressor approaches in experimental, field and modelling studies. Experiments should first focus on organisms of high ecosystem or fishery importance and focus on fitness-relevant traits (e.g. survival, growth, fecundity). Factorial experiments will be the most useful to understand stressor interactions and should be designed to allow inferring reaction norms (i.e. three or more levels per stressor) rather than control versus treatment conclusions for a given trait. For organisms living in shallow habitats, examining effects of diurnal changes in low pH and low DO levels is warranted. In addition, existing spatial gradients in CO2 and O2 conditions (e.g. along the US Pacific coast) or field manipulations [38] should be explored further, as they will likely help translating laboratory to ecosystem responses and the potential for some species to adapt.
5. Policy implications for management of coastal ecosystems
In many coastal zones, ecosystem management plans are centred on maintaining DO levels above a target concentration below which adverse effects on marine life may occur [2]. In contrast, coastal zone management plans rarely, if ever, set target pH or pCO2 levels or base DO criteria on the concurrent effects of acidification, despite the fact that many estuaries experience acidification at levels and durations known to be harmful to marine life. Given that concurrent exposure to acidification and hypoxia reduces the survival of fish and shellfish below the survival observed from hypoxia alone [22,23], it is likely that water quality standards based on DO alone have made marine fisheries more vulnerable to population reductions than appreciated. While there is currently not enough information to make quantitative recommendations regarding the precise changes that should be made to existing DO criteria to better protect ocean animals against these two stressors, raising DO standards to higher minimums to accommodate concurrent low pH would be one managerial approach to address this issue. Were such criteria changes made, it would lead to an expansion in the criteria-defined number and spatial extent of hypoxia zones in regions where such changes were implemented.
Moving forward, ocean acidification from atmospheric CO2 will intensify and temporal refuges from acidification afforded by tides, diurnal cycles, or seasonal destratification will diminish. While managerial efforts to reduce nutrient loads may progressively alleviate respiration-driven oxygen consumption and acidification in some estuaries, the ability to manage acidification from the atmosphere, ice melt and weathering is far more complex and less likely. For all of these reasons, careful monitoring of pH, pCO2 and carbonate chemistry in coastal zones will be important for better understanding the threat of acidification to marine life and for setting independent managerial targets for these parameters as warranted.
Data accessibility
This work does not contain any new experimental or observational data.
Competing interests
We have no significant competing financial, professional or personal interests that might have influenced the materials presented in this manuscript.
Funding
This work was supported by NOAA's Ocean Acidification Programme through award no. NA12NOS4780148 from the National Centers for Coastal Ocean Science, the National Science Foundation (NSF no. 1129622), and the Chicago Community Trust.
References
- 1.Valiela I. 2006. Global coastal change. Malden, MA: Blackwell Publishing Ltd. [Google Scholar]
- 2.Vaquer-Sunyer R, Duarte CM. 2008. Thresholds of hypoxia for marine biodiversity. Proc. Natl Acad. Sci. USA 105, 15 452–15 457. ( 10.1073/pnas.0803833105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keeling RF, Körtzinger A, Gruber N. 2010. Ocean deoxygenation in a warming world. Annu. Rev. Mar. Sci. 2, 199–229. ( 10.1146/annurev.marine.010908.163855) [DOI] [PubMed] [Google Scholar]
- 4.Diaz RJ, Rosenberg R. 2008. Spreading dead zones and consequences for marine ecosystems. Science 321, 926–929. ( 10.1126/science.1156401) [DOI] [PubMed] [Google Scholar]
- 5.Doney SC, et al. 2012. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 4, 11–37. ( 10.1146/annurev-marine-041911-111611) [DOI] [PubMed] [Google Scholar]
- 6.Gruber N. 2011. Warming up, turning sour, losing breath: ocean biogeochemistry under global change. Phil. Trans. R. Soc A 369, 1980–1996. ( 10.1098/rsta.2011.0003) [DOI] [PubMed] [Google Scholar]
- 7.Feely RA, Alin SR, Newton J, Sabine CL, Warner M, Devol A, Krembs C, Maloy C. 2010. The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary. Est. Coast. Shelf Sci. 88, 442–449. ( 10.1016/j.ecss.2010.05.004) [DOI] [Google Scholar]
- 8.Cai W-J, et al. 2011. Acidification of subsurface coastal waters enhanced by eutrophication. Nat. Geosci. 4, 766–770. ( 10.1038/ngeo1297) [DOI] [Google Scholar]
- 9.Melzner F, Thomsen J, Koeve W, Oschlies A, Gutowska M, Bange H, Hansen H, Körtzinger A. 2012. Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar. Biol. 160, 1875–1888. ( 10.1007/s00227-012-1954-1) [DOI] [Google Scholar]
- 10.Wallace RB, Baumann H, Grear JS, Aller RC, Gobler CJ. 2014. Coastal ocean acidification: the other eutrophication problem. Est. Coast. Shelf Sci. 148, 1–13. ( 10.1016/j.ecss.2014.05.027) [DOI] [Google Scholar]
- 11.Baumann H, Wallace R, Tagliaferri T, Gobler CJ. 2015. Large natural pH, CO2 and O2 fluctuations in a temperate tidal salt marsh on diel, seasonal and interannual time scales. Estuar. Coast. 38, 220–231. ( 10.1007/s12237-014-9800-y) [DOI] [Google Scholar]
- 12.Talmage SC, Gobler CJ. 2010. Effects of past, present, and future ocean carbon dioxide concentrations on the growth and survival of larval shellfish. Proc. Natl. Acad. Sci. USA 107, 17 246–17 251. ( 10.1073/pnas.0913804107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumann H, Talmage SC, Gobler CJ. 2012. Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nat. Clim. Change 2, 38–41. ( 10.1038/nclimate1291) [DOI] [Google Scholar]
- 14.Del Giorgio PA, Williams PJLB. 2005. Respiration in aquatic ecosystems. Oxford, NY: Oxford University Press. [Google Scholar]
- 15.Green MA, Aller RC. 1998. Seasonal patterns of carbonate diagenesis in nearshore terrigenous muds: relation to spring phytoplankton bloom and temperature. J. Mar. Res. 56, 1097–1123. ( 10.1357/002224098765173473) [DOI] [Google Scholar]
- 16.Waldbusser GG, Salisbury JE. 2014. Ocean acidification in the coastal zone from an organism's perspective: multiple system parameters, frequency domains, and habitats. Annu. Rev. Mar. Sci. 6, 221–247. ( 10.1146/annurev-marine-121211-172238) [DOI] [PubMed] [Google Scholar]
- 17.Paulmier A, Ruiz-Pino D, Garçon V. 2011. CO2 maximum in the oxygen minimum zone (OMZ). Biogeosciences 8, 239–252. ( 10.5194/bg-8-239-2011) [DOI] [Google Scholar]
- 18.Millero FJ, Graham TB, Huang F, Bustos-Serrano H, Pierrot D. 2006. Dissociation constants of carbonic acid in seawater as a function of salinity and temperature. Mar. Chem. 100, 80–94. ( 10.1016/j.marchem.2005.12.001) [DOI] [Google Scholar]
- 19.Barton A, Hales B, Waldbusser GG, Langdon C, Feely RA. 2012. The Pacific oyster, Crassostrea gigas, shows negative correlation to naturally elevated carbon dioxide levels: implications for near-term ocean acidification effects. Limnol. Oceanogr. 57, 689–710. ( 10.4319/lo.2012.57.3.0698) [DOI] [Google Scholar]
- 20.Soetaert K, Hofmann AF, Middelburg JJ, Meysman FJR, Greenwood J. 2007. The effect of biogeochemical processes on pH. Mar. Chem. 105, 30–51. ( 10.1016/j.marchem.2006.12.012) [DOI] [Google Scholar]
- 21.Sunda WG, Cai W-J. 2012. Eutrophication induced CO2-acidification of subsurface coastal waters: interactive effects of temperature, salinity, and atmospheric pCO2. Environ. Sci. Technol. 46, 10 651–10 659. ( 10.1021/es300626f) [DOI] [PubMed] [Google Scholar]
- 22.Depasquale E, Baumann H, Gobler CJ. 2015. Variation in early life stage vulnerability among Northwest Atlantic estuarine forage fish to ocean acidification and low oxygen. Mar. Ecol. Prog. Ser. 523, 145–156. ( 10.3354/meps11142) [DOI] [Google Scholar]
- 23.Gobler CJ, Depasquale E, Griffith A, Baumann H. 2014. Hypoxia and acidification have additive and synergistic negative effects on the growth, survival, and metamorphosis of early life stage bivalves. PLoS ONE 9, e83648 ( 10.1371/journal.pone.0083648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnett LE. 1997. The challenges of living in hypoxic and hypercapnic aquatic environments. Am. Zool. 37, 633–640. ( 10.2307/3884140) [DOI] [Google Scholar]
- 25.Pörtner HO. 2012. Integrating climate-related stressor effects on marine organisms: unifying principles linking molecule to ecosystem-level changes. Mar. Ecol. Prog. Ser. 470, 273–290. ( 10.3354/meps10123) [DOI] [Google Scholar]
- 26.Sokolova IM. 2013. Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integr. Comp. Biol. 53, 597–608. ( 10.1093/icb/ict028) [DOI] [PubMed] [Google Scholar]
- 27.Steckbauer A, Ramajo L, Hendriks IE, Fernandez M, Lagos N, Prado L, Duarte CM. 2015. Synergistic effects of hypoxia and increasing CO2 on benthic invertebrates of the central Chilean coast. Front. Mar. Sci. 2, 49, ( 10.3389/fmars.2015.00049) [DOI] [Google Scholar]
- 28.Kim T, Barry J, Micheli F. 2013. The effects of intermittent exposure to low-pH and low-oxygen conditions on survival and growth of juvenile red abalone. Biogeosciences 10, 7255–7262. ( 10.5194/bg-10-7255-2013) [DOI] [Google Scholar]
- 29.Jansson A, Norkko J, Dupont S, Norkko A. 2015. Growth and survival in a changing environment: combined effects of moderate hypoxia and low pH on juvenile bivalve Macoma balthica. J. Sea Res. 102, 41–47. ( 10.1016/j.seares.2015.04.006) [DOI] [Google Scholar]
- 30.Jakubowska M, Normant M. 2014. Metabolic rate and activity of blue mussel Mytilus edulis trossulus under short-term exposure to carbon dioxide-induced water acidification and oxygen deficiency. Mar. Freshw. Behav. Physiol. 48, 25–39. ( 10.1080/10236244.2014.986865) [DOI] [Google Scholar]
- 31.Frieder CA, Gonzalez JP, Bockmon EE, Navarro MO, Levin LA. 2014. Can variable pH and low oxygen moderate ocean acidification outcomes for mussel larvae? Glob. Change Biol. 20, 754–764. ( 10.1111/gcb.12485) [DOI] [PubMed] [Google Scholar]
- 32.Wu RSS. 2002. Hypoxia: from molecular responses to ecosystem responses. Mar. Pollut. Bull. 45, 35–45. ( 10.1016/S0025-326X(02)00061-9) [DOI] [PubMed] [Google Scholar]
- 33.Keppel AG. 2014. The effects of co-varying diel-cycling hypoxia and pH on disease susceptibility, growth, and feeding in Crassostrea virginica. MS thesis, University of Maryland.
- 34.Clark HR. 2015. Response of early life stage bivalves to diurnal changes in carbon dioxide and dissolved oxygen concentrations. Stony Brook, NY: Stony Brook University. [Google Scholar]
- 35.Thomsen J, Casties I, Pansch C, Körtzinger A, Melzner F. 2013. Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: laboratory and field experiments. Glob. Change Biol. 19, 1017–1027. ( 10.1111/gcb.12109) [DOI] [PubMed] [Google Scholar]
- 36.Harvey BP, Gwynn-Jones D, Moore PJ. 2013. Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol. Evol. 3, 1016–1030. ( 10.1002/ece3.516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byrne M, Przeslawski R. 2013. Multistressor impacts of warming and acidification of the ocean on marine invertebrates’ life histories. Integr. Comp. Biol. 53, 582–596. ( 10.1093/icb/ict049) [DOI] [PubMed] [Google Scholar]
- 38.Arnold T, Mealey C, Leahey H, Miller AW, Hall-Spencer JM, Milazzo M, Maers K. 2012. Ocean acidification and the loss of phenolic substances in marine plants. PLoS ONE 7, e35107 ( 10.1371/journal.pone.0035107) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This work does not contain any new experimental or observational data.