Abstract
In many social hierarchies, more subordinate individuals adjust their behaviour according to the presence or behaviour of more dominant individuals. In this study, it is shown that male mice form linear dominance hierarchies characterized by individuals attacking in bursts. Temporal pairwise-correlation analysis reveals that non-dominant individuals avoid behaving aggressively concurrently with an aggressively behaving alpha male. This anti-correlation is only found with alpha males and is greater for more despotic alpha males. It is concluded that less dominant individuals modulate their aggressive behaviour in response to their social context, resulting in an attentional group structure.
Keywords: social dominance, attention hierarchy, dominance hierarchy, temporal dynamics
1. Introduction
Within hierarchically organized social groups, the behaviour of subordinate individuals can be strongly modulated by dominant individuals. Less popular and less dominant preschool children give more visual attention to more popular and dominant children and modify their own aggression towards more subordinate individuals dependent upon the presence of a dominant child [1,2]. In non-human primates, subordinates direct more visual attention at more dominant individuals [3] and will forego rewards to view images of more dominant individuals [4]. In cichlid fish, non-territorial subordinate males avoid behaving aggressively when the dominant territorial male is present in the social group, whereas dominant males do not modify their behaviour according to social context [5]. It has been suggested that these behavioural changes in subordinates occur via social monitoring of higher-ranked individuals by subordinates, leading to an attention hierarchy [6]. More likely, such changes in group attentional structure are a consequence of social suppression of subordinates by more dominant individuals.
Observations from my laboratory have recently shown that groups of laboratory male mice living together in a laboratory-based burrow system that mimics the natural habitat of the ancestral Mus musculus rapidly form highly linear social dominance hierarchies with a high degree of directional consistency in aggression [7,8]. The hierarchy is established within 4 days of co-housing and in approximately 80% of these groups, rank order remains stable. Using behavioural data collected from these undisturbed group-living individuals, pairwise-correlation analyses of attacks made by dominant and more subordinate individuals were applied to test for the presence and consistency over time of hierarchies. These analyses revealed critical insights into the nature of attentional hierarchies and the role of contextual factors in the dynamics of these hierarchies.
2. Material and methods
(a). Animals, housing and behavioural observations
Outbred CD1 male mice aged seven weeks were purchased from Charles River Laboratories and habituated to the animal facility before use. All animals were individually marked with a nontoxic marker (Stoelting Co.) that remained for the duration of the study. At nine weeks of age, groups of 12 males were placed into large vivaria containing multiple nest-boxes, ramps and other physical enrichment objects (see the electronic supplementary material, figure S1). Standard chow and water were provided ad libitum at the top of the vivarium. Each cohort of animals remained in the vivarium for between 20 and 23 days (median = 22 days) and observed typically 1–3 h per day during the dark phase of the light cycle. Trained observers conducted all-occurrence behavioural observations of agonistic contests (attacks) occurring between individuals (see the electronic supplementary material, table S1) and inputted timestamped data live into an android device.
In this study, data from 13 stable social hierarchies (364 observation periods, 503 h of observations and 11 243 total contests) were obtained. Hierarchy linearity was evaluated by calculating the modified Landau's h’ value of each group [9]. The final social rank order of animals was determined using the Glicko pairwise-contest rating system [7,8,10]. For analyses of the pattern of aggressive behaviour, behavioural data were formatted into a temporal sequence of wins by each individual within each group (see the electronic supplementary material, Methods). These sequences are referred to as ‘event trains' analogous to ‘spike trains' in electrophysiology [11].
(b). Pairwise-correlation analysis
The recently developed spike time tiling coefficient (STTC) method was used to determine whether the aggressive events of alpha males in each cohort were temporally associated with the attacks made by other males in the cohort [11]. Specifically, this approach was used to test whether subordinate mice avoid behaving aggressively, when the dominant alpha male is actively aggressive in a social group. This method determines whether a higher or lower proportion of one individual's behavioural events co-occur within a given time-window (±Δt) of the events of another individual at rates significantly different from chance. Event trains were generated for times of attacks by the alpha male (‘alpha’), times of attacks by any other-male (‘other’) and times of attacks by every individual. From each event train (alpha, other, each male independently), the distribution of inter-event intervals (IEI) was calculated—the time in seconds between consecutive attacks—to determine whether attacks were non-normally distributed.
STTC values were calculated using time-window values around behavioural events (Δt) of 60, 90 and 120 s. These values were derived from our observation that the majority of IEIs were smaller than 240 s (equivalent to the maximum value of 2Δt with Δt = 120 s; see the electronic supplementary material, figure S2). Three Δt values were used to demonstrate the robustness of the STTC value. Whether the STTC values generated when comparing alpha event trains with other-male event trains were significantly less than 0 for each Δt (indicating anti-correlation) was tested using a one-tailed one-sample T-test. This analysis was repeated for 1000 bootstrapped replicates of the raw data. Whether the observed STTC was lower than expected by chance was tested by performing a Monte Carlo randomization of raw data within each observation period (see the electronic supplementary material, Methods). The temporal change in STTC was examined by calculating its cumulative value from the beginning of observations to the end of each day. Whether the daily STTC values across cohorts were significantly less than 0 was calculated using one-tailed one-sample T-tests. For each cohort, the event trains of every unique individual were used to generate a matrix of STTC values for every relationship. The mean STTC value across all relationships was calculated for each animal. The mean STTC values over all relationships (or all relationships except the alpha male) were then determined for each rank of animal and determined if these were significantly less than 0 using a one-tailed one-sample T-test.
3. Results
All cohorts had significant linear hierarchies with significantly high Landau's modified h’ values (median = 0.78 IQR = [0.72,0.86]). The ranks of individuals in all groups were stable with individuals showing consistent directionality of behaviour towards other relatively more dominant or subordinate individuals. In particular, alpha males won on average 99.1% of all their contests across cohorts (median = 99.1, IQR = [98.4, 99.5], min = 93.8% and max = 100%). Although all alpha males had the highest rates of aggression in each group and rank-reversals (losing to lower-ranked opponents) were negligible for all alpha males, there did exist variation in how much each alpha male monopolized aggressive interactions within their hierarchy. The proportion of wins in each hierarchy that belonged to alpha males ranged from a minimum of 35.6% to a maximum of 79.5% (median = 60.2, IQR = [43.6, 68.4]), indicating variability in the despotic style of alpha males between groups.
(a). Bursting patterns of aggression
Whether the patterning of aggressive behaviour was non-random and non-uniformly distributed was addressed by assessing the intervals between aggressive acts (IEIs). Seventy-two per cent of alpha male IEIs (4216/5846 IEIs), 53% of non-alpha male IEIs (2002/3768 IEIs) and 69% of IEIs between any two non-alpha males (3258/4693 IEIs) were less than 4 min in duration (electronic supplementary material, figure S2). Using a generalized linear mixed-effects model, including rank as a predictor, individual nested within cohort as random factors, log-transformed IEI as the outcome variables and allowing random slopes to vary, social rank was a significant predictor of IEI (β = 0.036 ± 0.006, d.f. = 26.8, N = 9614 IEIs, t = 5.77, p < 0.001; electronic supplementary material, figure S3). The average IEI increases by almost 1 min with each decreasing rank. Across cohorts, the median and IQR of attacks made per hour by alpha and other males were 11.8[10.9, 13.8] and 8.1[5.9, 14.1], respectively. Comparing these values with the non-uniformity in IEI between attacks, it can be concluded that the general pattern of male mice attacks is bursts of several attacks made in relatively quick succession.
(b). Anti-correlation in temporal pattern of attacks between alpha and other males
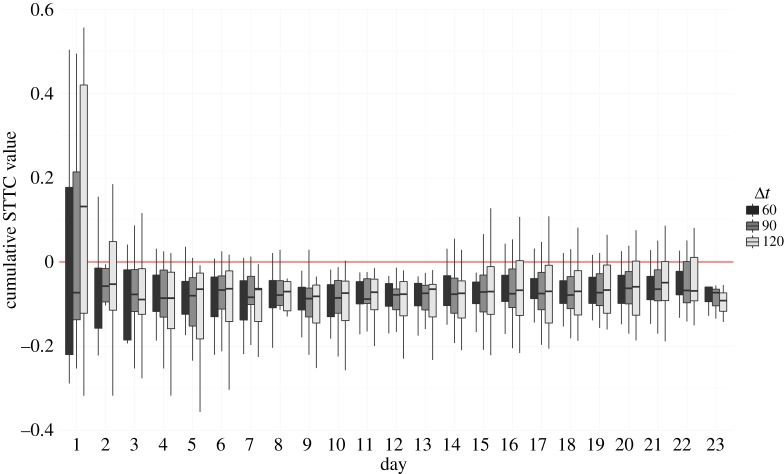
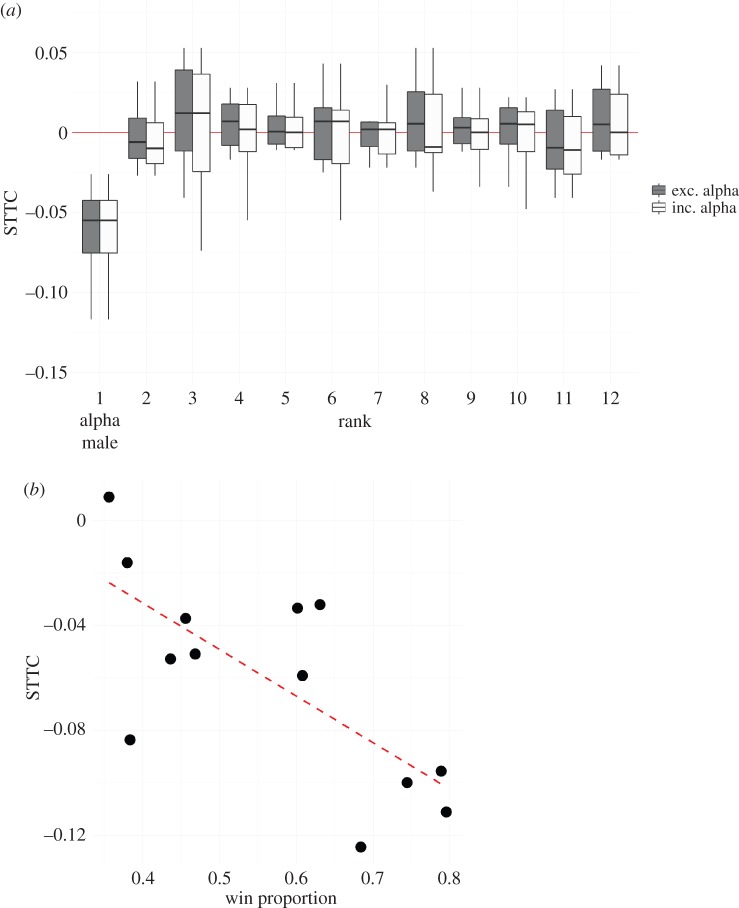
Whether burst-like patterns of aggression were correlated with each other was subsequently addressed. For all values of Δt, median STTC values across cohorts were significantly less than 0, indicating significant anti-correlation between the temporal patterning of alpha male attacks and those of other males (Δt = 60: STTC = −0.07 ± 0.01, t = −4.86, d.f. = 12, p < 0.001; Δt = 90: STTC = −0.06 ± 0.02, t = −3.61, d.f. = 12, p = 0.002; Δt = 120: STTC = −0.06 ± 0.02, t = −2.80, d.f. = 12, p = 0.008). This was confirmed for all 1000 bootstrapped resamples of the data (all p ≤ 0.001). Further, observed STTC values for all values of Δt were significantly smaller than those generated from 1000 Monte Carlo randomizations of the data (all p ≤ 0.001, electronic supplementary material, figure S4). The cumulative change in STTC is shown in figure 1. From day 5 onwards, the distribution of STTC values derived from comparing the temporal sequence of alpha male attacks with other-male attacks is significantly lower than 0 (all p-values less than 0.01). Next, the STTC values of each rank against all-other ranks individually for each cohort were compared. Figure 2a shows the distribution of mean STTC values by rank and indicates that only alpha male attacks are significantly anti-correlated with animals of other ranks (Δt = 90: STTC = −0.07 ± 0.01, t = −5.89, d.f. = 12, p < 0.001). Further, the STTC value of alpha males was negatively associated with the proportion of all contests in each cohort that were monopolized by the alpha males (i.e. how despotic individual alpha males were—Spearman rank correlations: Δt = 90: rs = −0.71, p = 0.008; figure 2b). The mean STTC value of males of all-other ranks was not significantly related to the degree of alpha male despotism in each cohort. Alpha male STTC values were not associated with each cohort's Landau's modified h’ linearity.
Figure 1.
Cumulative change in STTC by day. The cumulative value by day from the beginning to end of observations of STTC calculated by comparing the temporal pattern of attack sequences for alpha versus all-other males for Δt = 60, 90 and 120 s. (Online version in colour.)
Figure 2.
Attacks of alpha males are anti-correlated with males of other ranks. (a) Mean STTC values for individuals of each rank against all-other ranks (including versus excluding alpha males). (b) Despotism of alpha males (the proportion of all attacks in each group that are made by alpha males) is associated with their STTC value (Δt = 90 s). (Online version in colour.)
4. Discussion
Using a novel approach for the characterization and quantification of aggressive social encounters, these findings indicate that male mice living in a social hierarchy modulate their aggressive behaviour in response to their current social context as defined by the concurrent aggressive behaviour of the most dominant alpha male. Aggressive behaviour occurs in bursts and non-alpha males modulate their attacking behaviour so as to avoid behaving aggressively within a window of up to at least 4 min around the times of alpha male attacks. This effect was consistent over the entire observation period. Further, this finding is specific to the alpha male and is not dependent upon having just received aggression. All males attenuate their aggressive behaviour only when the alpha male has been behaving aggressively to any other mice and do not at all modulate their behaviour when other males have recently been aggressive.
Given the complex three-dimensional environment used in this study, it is likely that males use multiple sensory systems to monitor the behaviour of the alpha male. Visual observation would be sufficient under conditions when individuals in the social group are in the same spatial location. Subordinate males may be able to detect ultrasonic calls emitted by dominant alpha males [12,13] and volatile and non-volatile odor cues may also be used by the alpha to signal their ongoing aggressive behaviour [14,15]. Perception of these sensory signals may allow for heightened social competence in subordinate males, an ability characterized by engaging in contextually appropriate social behaviour, by inhibiting aggression and avoiding conflict. Being socially competent may be critical to maintaining one's social rank or ascending in rank in the absence of the dominant and may be most critical in groups with more despotic alpha males. Indeed, the fact that the anti-correlation in attack times between alpha and other males increased in more despotic alpha males is consistent with this idea.
In children and non-human primates, subordinate individuals modify their behaviour according to the presence and behaviour of more dominant individuals [1–4]. Similarly, in highly territorial cichlid fish housed in mixed-sex groups, subordinate males almost never exhibit aggression when the dominant is present, only showing attack behaviour when the dominant male is not visible [5]. However, within these all-male mouse social hierarchies alpha males do not completely inhibit the aggression of more subordinate males. This discrepant finding may be related to variation in social hierarchies that emerge in all-male versus mixed-sex mouse social groups [16] or may be owing to species differences. Further, within our burrow system, there exist multiple locations where the dominant may not be able to constantly monitor agonistic contests between less dominant individuals. Regardless, these data suggest that social suppression of mice by the alpha male occurs following male mouse dominance hierarchy formation. Less dominant mice alter their aggressive behaviour dependent upon the concurrent social context, leading to the appearance of an attentional group structure that likely serves to foster the organization of social behaviour in such complex groups.
Supplementary Material
Acknowledgements
The author would like to thank Cait Williamson and Won Lee for their assistance in data collection.
Ethics
All procedures were conducted with approval from the Columbia University Institutional Animal Care and Use Committee (Protocol no: AC-AAAG0054).
Data accessibility
All raw data and code used in this paper are publically available at https://github.com/jalapic/attentionh.
Author's contributions
I conceived and designed this study, collated data, conducted all analyses and wrote the article and agree to be accountable for all aspects of the work performed.
Competing interests
I have no competing interests.
Funding
I received funding from the Department of Psychology, Columbia University.
References
- 1.Freniere PL, Charlesworth WR. 1983. Dominance, attention, and affiliation in a preschool group: a nine-month longitudinal study. Ethol. Sociobiol. 4, 55–67. ( 10.1016/0162-3095(83)90030-4) [DOI] [Google Scholar]
- 2.Vaughn BE, Waters E. 1981. Attention structure, sociometric status, and dominance: interrelations, behavioral correlates, and relationships to social competence. Dev. Psychol. 17, 275–288. ( 10.1037/0012-1649.17.3.275) [DOI] [Google Scholar]
- 3.Pannozzo PL, Phillips KA, Haas ME, Mintz EM. 2007. Social monitoring reflects dominance relationships in a small captive group of brown capuchin monkeys (Cebus apella). Ethology 113, 881–888. ( 10.1111/j.1439-0310.2007.01392.x) [DOI] [Google Scholar]
- 4.Deaner RO, Khera AV, Platt ML. 2005. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Curr. Biol. 15, 543–548. ( 10.1016/j.cub.2005.01.044) [DOI] [PubMed] [Google Scholar]
- 5.Desjardins JK, Hofmann HA, Fernald RD. 2012. Social context influences aggressive and courtship behavior in a cichlid fish. PLoS ONE 7, e32781 ( 10.1371/journal.pone.0032781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chance MRA. 1976. The social structure of attention (eds Chance MRA, Larsen RR). New York, NY: John Wiley & Sons Ltd. [Google Scholar]
- 7.So N, Franks B, Lim S, Curley JP. 2015. A social network approach reveals associations between mouse social dominance and brain gene expression. PLoS ONE 10, e0134509 ( 10.1371/journal.pone.0134509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williamson CM, Lee W, Curley JP. 2016. Temporal dynamics of social hierarchy formation and maintenance in male mice. Anim. Behav . 115, 259–272. ( 10.1016/j.anbehav.2016.03.004) [DOI] [Google Scholar]
- 9.De Vries H. 1995. An improved test of linearity in dominance hierarchies containing unknown or tied relationships. Anim. Behav. 50, 1375–1389. ( 10.1016/0003-3472(95)80053-0) [DOI] [Google Scholar]
- 10.Glickman ME. 1999. Parameter estimation in large dynamic paired comparison experiments. Appl. Stat. 48, 377–394. ( 10.1111/1467-9876.00159) [DOI] [Google Scholar]
- 11.Cutts CS, Eglen SJ. 2014. Detecting pairwise correlations in spike trains: an objective comparison of methods and application to the study of retinal waves. J. Neurosci. Off. J. Soc. Neurosci. 34, 14 288–14 303. ( 10.1523/JNEUROSCI.2767-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assini R, Sirotin YB, Laplagne DA. 2013. Rapid triggering of vocalizations following social interactions. Curr. Biol. 23, R996–R997. ( 10.1016/j.cub.2013.10.007) [DOI] [PubMed] [Google Scholar]
- 13.Wesson DW. 2013. Sniffing behavior communicates social hierarchy. Curr. Biol. 23, 575–580. ( 10.1016/j.cub.2013.02.012) [DOI] [PubMed] [Google Scholar]
- 14.Nelson AC, Cunningham CB, Ruff JS, Potts WK. 2015. Protein pheromone expression levels predict and respond to the formation of social dominance networks. J. Evol. Biol. 28, 1213–1224. ( 10.1111/jeb.12643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stowers L, Kuo T-H. 2015. Mammalian pheromones: emerging properties and mechanisms of detection. Curr. Opin. Neurobiol. 34, 103–109. ( 10.1016/j.conb.2015.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zielinski WJ, Vandenbergh JG. 1993. Testosterone and competitive ability in male house mice, Mus musculus: laboratory and field studies. Anim. Behav. 45, 873–891. ( 10.1006/anbe.1993.1108) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data and code used in this paper are publically available at https://github.com/jalapic/attentionh.