Abstract
During collective movement, bolder individuals often emerge as leaders. Here, we investigate whether this reflects a greater propensity of bold individuals to initiate movement, or a preference for shy individuals to follow a bolder leader. We set up trios of stickleback fish comprising a focal individual who was either bold or shy, and one other individual of each personality. We then recorded the movements of all individuals in and out of cover in a foraging context to determine how assiduously the focal fish followed the movements of each other partner. We found that a shy focal fish preferred to follow a leader whose personality matched its own, but we did not detect such a difference in bold fish. Despite this preference, however, the greater propensity of bold individuals to initiate movements out of cover meant that they successfully led more joint trips. Thus, when offered a choice of leaders, sticklebacks prefer to follow individuals whose personality matches their own, but bolder individuals may, nevertheless, be able to impose their leadership, even among shy followers, simply through greater effort.
Keywords: animal personality, followership, Gasterosteus aculeatus
1. Introduction
In collective movement, leadership is often correlated with personality [1]. Notably, bold individuals are more likely to lead, and shy individuals to follow [2–4]. At first glance, this might suggest that bolder individuals are more willing to initiate movement [5–7]. However, it is unclear to what extent leadership reflects the choices of leaders versus those of followers. To successfully lead a group, one individual must first initiate a movement, and then be followed by others. Consequently, leadership might reflect either leader effort or follower preference.
Moreover, when individuals can choose whom to follow among group members with different personalities, it is far from clear that they should always favour bolder leaders. Similarity in personality may instead be more important for group dynamics. Individuals with similar personality are more likely to share similar physiological states [8] and life-history traits [9], thus making the actions of similar individuals more relevant to the potential follower with less conflict. Intriguingly, in studies on decision-making in humans, individuals are more susceptible to the opinions of similar others [10], but we do not know if this is also the case in other animals.
In this study, we used small groups of three-spined sticklebacks (Gasterosteus aculeatus) to study the interaction between leader and follower personality. Specifically, we asked whether, within a heterogeneous group, focal individuals preferentially follow bolder group members or those of similar personality, and whether these preferences determine who emerges as a leader within the group.
2. Methods
From individual boldness scores (the proportion of time out of cover when alone in the experimental tank), we created groups of three individuals made up of either a bold focal fish with a bold and a shy partner (19 trios), or a shy focal fish with a bold and a shy partner (18 trios). Fish with a boldness score greater than 0.3 were classed as ‘bold’, otherwise ‘shy’. Variance in personality scores did not differ between bold and shy fish (F-test for the equal variance, p = 0.157). Individuals were matched so that, within each trio, the difference in boldness score between bold and shy individuals was greater than 0.25, and between individuals of similar personality smaller than 0.05. Detailed information on animal maintenance and boldness assessment are found in the electronic supplementary material.
The experimental tank was covered with opaque plastic and lined with gravel to create a slope (2–12 cm water depth), and partitioned lengthwise into three long lanes by transparent plastic barriers (90 × 10 cm each) so that fish could see each other. Plastic plants were placed at the deep end of each lane, and a white plastic feeding tile (1.5 cm2) was placed at the other end. A vertical white plastic screen (8 × 8 cm) in front of each feeding tile prevented fish from seeing food from the deep end. Each fish was randomly put in each lane of an experimental tank, with the focal in the middle. After 5 min of acclimation, we recorded their movements for 2 h. The experiment was conducted over 2 days for each trio, swapping the two fish in the side lanes on the second day. Fish were not re-used in multiple trios.
From the video, the times when each individual left or returned to cover were recorded, and the trio was categorized at any given time as one of eight possible ‘states’ (figure 1). The rates of switching between states (i.e. transition rate from state i to state j, qij) were estimated by fitting a continuous-time Markov model (‘msm’ v. 1.2 [11] in R). To assess whether focal fish preferred to follow one partner over another, we used a likelihood ratio test to compare the fit of the model in which each fish's transition intensities were allowed to vary only with its own boldness score as a covariate (thus ruling out any discrimination by the focal fish between its two partners according to their personality similarity), to the alternative model in which partner's personality (matched or mismatched) was additionally included as a covariate (thus allowing for the focal's ‘preference’ for one partner over another). We further compared transition intensities within a given model by bootstrapping (10 000 repeats). Lastly, we investigated how many times focal fish actually followed each partner by fitting a generalized linear mixed-effects model to the number of following events, specifying a negative binomial error distribution with a log link and trio identity as a random effect (‘glmmADMB’ v. 0.8.3.3 [12] in R).
Figure 1.
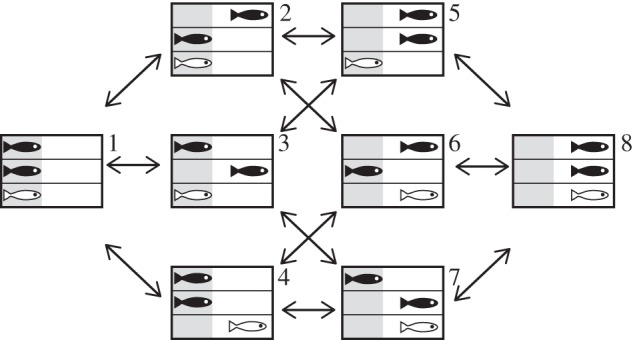
The possible ‘states’ (numbers on the top right corners) of a group. Fish can either be under cover (grey area) or out of cover (white area). A focal fish was placed in the central lane between a partner with matching personality (black) and mismatching personality (white).
3. Results
We found that a model in which the focal individual could distinguish between and respond differently to the other two partners, according to whether or not its personality matched theirs, gave a better fit to the data than a simpler model in which each individual's movements reflected only its own boldness score as a covariate (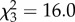 , p = 0.001). This shows that fish follow partners differently depending on their personality similarity, so we continued our analysis by fitting separate models for bold and shy focal fish.
, p = 0.001). This shows that fish follow partners differently depending on their personality similarity, so we continued our analysis by fitting separate models for bold and shy focal fish.
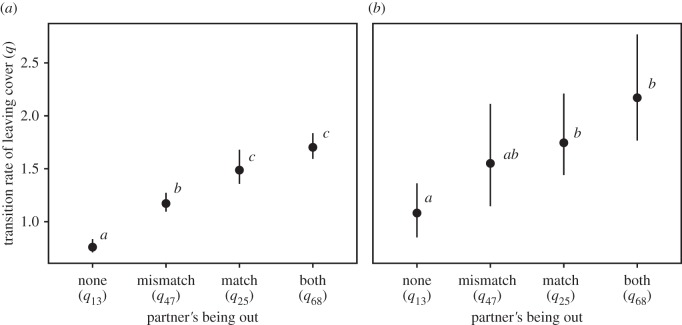
Shy focals (figure 2a) were more likely to leave cover when either partner was already out compared with when both partners were under cover (q13 < q47, q13 < q25, p < 0.001), but the presence of a matched partner out of cover led to a higher propensity to join compared with a mismatched partner (q25 < q47, p < 0.001). Shy focals were more likely to leave cover when both partners were out of cover compared with when just a mismatched partner was out of cover (q47 < q68, p < 0.001), but not when just a matched partner was out of cover (q25 = q68, p = 0.059). That is, the presence of a mismatched partner out of cover did not increase the focal's tendency to leave when a matched partner is already out of cover.
Figure 2.
Tendency of the focal fish to leave cover in relation to a partner's position (no partner out, a partner with mismatching personality out, a partner with matching personality out, both partners out). (a) Shy focal fish and (b) bold focal fish. Points and vertical lines represent best estimates and 95% CIs from bootstrapping, respectively. Different letters indicate significant differences.
Bold focals showed similar responses, but the trend was weaker (figure 2b). A bold focal was more likely to leave cover when a matched partner was already out compared with when both partners were under cover (q13 < q25, p < 0.001). The effect of a mismatched partner was intermediate, giving non-significant differences (q13 = q47, p = 0.067; q25 = q47, p = 0.471). Compared with when either partner was already out of cover alone, having both partners out did not affect the tendency to leave cover (q25 = q68, p = 0.151; q47 = q68, p = 0.082).
Despite the preferences described above, bold partners always led more joint trips than did shy partners, regardless of whether the focal fish was bold or shy, owing to their stronger tendency to leave cover. A shy focal fish followed the bold partner 5.1 ± 0.5 times (mean ± s.e.), whereas it followed the shy partner 2.5 ± 0.3 times (likelihood ratio test, 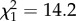 , p < 0.001). Similarly, a bold focal fish followed the bold partner 4.8 ± 0.6 times, whereas it followed the shy partner 1.9 ± 0.3 times (
, p < 0.001). Similarly, a bold focal fish followed the bold partner 4.8 ± 0.6 times, whereas it followed the shy partner 1.9 ± 0.3 times (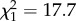 , p < 0.001).
, p < 0.001).
4. Discussion
This is the first study that demonstrates an influence of personality similarity on choice between potential leaders. When stickleback fish were grouped with two partners of different personalities, they were more likely to follow the partner of similar personality out of cover. Both bold and shy fish showed the same trend, but it was more pronounced in shy fish. The weaker trend in bold fish may have resulted from their own strong tendency to leave cover, which might obscure any preference for one potential leader over another. In line with this suggestion, previous work has shown that both bold and shy fish increased their tendency to follow when rewarded for doing so, but that bold fish were unable to suppress their tendency to initiate trips even in the absence of reward [7].
Our previous work on stickleback pairs found that in a group of two, leader and follower roles were more pronounced the greater the difference in personality between the fish [2]. In that simple setting, where there was no possibility of choice between alternative leaders, shyer fish were actually more likely to follow a bolder partner. Our current results, by contrast, suggest that as soon as such choice becomes possible, even in a group of three, individuals show a preference for partners of similar personality, and the preference was stronger in shy individuals. We speculate that in larger groups, this tendency might lead to formation of sub-groups of similar individuals that associate preferentially with one another. Empirical studies suggest that personality heterogeneity may often enhance the performance of a group [13,14]. But the kind of assortment we observed here might potentially counteract this effect by leading to group fission when personality differences become too great, just as preferential association between individuals of the same sex can lead to group breakup through sexual segregation [15].
Our current findings also suggest that, while bolder individuals are more likely to emerge as leaders [2–4], this may not always reflect a preference on the part of followers. Instead, in our study, bold partners were followed more often than shy partners despite the preference of a shy focal fish for a leader of similar personality, simply because they initiated more trips. It appears that differences in the propensity of bold and shy individuals to initiate movement can overwhelm the preferences of followers, even when the latter opposes the former. We therefore suggest that, just as variation in male courtship intensity may obscure female preference in studies of mate choice [16], future investigations of leadership will need to beware the possibility that variation in leader effort can mask follower preference.
In summary, our results suggest that the personalities of group members can interact in complex ways to determine the emergence of social roles, beyond the simple view that the bold lead and the shy follow. Future work will have to take into account both leader effort and follower choice, and how these two factors relate to personality.
Supplementary Material
Acknowledgements
We thank anonymous reviewers for their feedback.
Ethics
Experiments were approved by the Animal Users Management Committee of the University of Cambridge as ‘Unregulated Procedures’, as they were not invasive and did not put the fish in any stressful situation.
Data accessibility
Data are found in Dryad: http://dx.doi.org/10.5061/dryad.41db8.
Authors' contributions
J.L.H. conducted the experiment; S.N. and A.M. analysed the data; S.N. drafted the manuscript; all authors contributed the study design and writing the final manuscript. All authors approved the final version and accept accountability for the work.
Competing interests
We have no competing interests.
Funding
The work was funded by a fellowship from JSPS to S.N., a grant from ASAB to A.M. and a studentship from NERC to J.H.
References
- 1.King AJ, Johnson DDP, Van Vugt M. 2009. The origins and evolution of leadership. Curr. Biol. 19, R911–R916. ( 10.1016/j.cub.2009.07.027) [DOI] [PubMed] [Google Scholar]
- 2.Harcourt JL, Ang TZ, Sweetman G, Johnstone RA, Manica A. 2009. Social feedback and the emergence of leaders and followers. Curr. Biol. 19, 248–252. ( 10.1016/j.cub.2008.12.051) [DOI] [PubMed] [Google Scholar]
- 3.Kurvers RHJM, Eijkelenkamp B, van Oers K, van Lith B, van Wieren SE, Ydenberg RC, Prins HHT. 2009. Personality differences explain leadership in barnacle geese. Anim. Behav. 78, 447–453. ( 10.1016/j.anbehav.2009.06.002) [DOI] [Google Scholar]
- 4.Schuett W, Dall SRX. 2009. Sex differences, social context and personality in zebra finches, Taeniopygia guttata. Anim. Behav. 77, 1041–1050. ( 10.1016/j.anbehav.2008.12.024) [DOI] [Google Scholar]
- 5.Johnstone RA, Manica A. 2011. Evolution of personality differences in leadership. Proc. Natl Acad. Sci. USA 108, 8373–8378. ( 10.1073/pnas.1102191108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ioannou CC, Singh M, Couzin ID. 2015. Potential leaders trade off goal-oriented and socially oriented behavior in mobile animal groups. Am. Nat. 186, 284–293. ( 10.1086/681988) [DOI] [PubMed] [Google Scholar]
- 7.Nakayama S, Stumpe MC, Manica A, Johnstone RA. 2013. Experience overrides personality differences in the tendency to follow but not in the tendency to lead. Proc. R. Soc. B 280, 20131724 ( 10.1098/rspb.2013.1724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sih A, Mathot KJ, Moirón M, Montiglio P-O, Wolf M, Dingemanse NJ. 2015. Animal personality and state–behaviour feedbacks: a review and guide for empiricists. Trends Ecol. Evol. 30, 50–60. ( 10.1016/j.tree.2014.11.004) [DOI] [PubMed] [Google Scholar]
- 9.Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio P-O. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063. ( 10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suls J, Martin R, Wheeler L. 2002. Social comparison: why, with whom, and with what effect? Curr. Dir. Psychol. Sci. 11, 159–163. ( 10.1111/1467-8721.00191) [DOI] [Google Scholar]
- 11.Jackson CH. 2011. Multi-state models for panel data: the msm package for R. J. Stat. Softw. 38, 1–29. ( 10.18637/jss.v038.i08) [DOI] [Google Scholar]
- 12.Fournier DA, Skaug HJ, Ancheta J, Ianelli J, Magnusson A, Maunder M, Nielsen A, Sibert J. 2012. AD model builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim. Methods Softw. 27, 233–249. ( 10.1080/10556788.2011.597854) [DOI] [Google Scholar]
- 13.Burns JG, Dyer AG. 2008. Diversity of speed–accuracy strategies benefits social insects. Curr. Biol. 18, R953–R954. ( 10.1016/j.cub.2008.08.028) [DOI] [PubMed] [Google Scholar]
- 14.Pruitt JN, Riechert SE. 2011. How within-group behavioural variation and task efficiency enhance fitness in a social group. Proc. R. Soc. B 278, 1209–1215. ( 10.1098/rspb.2010.1700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruckstuhk K, Neuhaus P. 2005. Sexual segregation in vertebrates: ecology of the two sexes. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 16.Shamble PS, Wilgers DJ, Swoboda KA, Hebets EA. 2009. Courtship effort is a better predictor of mating success than ornamentation for male wolf spiders. Behav. Ecol. 20, 1242–1251. ( 10.1093/beheco/arp116) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are found in Dryad: http://dx.doi.org/10.5061/dryad.41db8.