Abstract
In this study, we focused on the exceptionally large mammals inhabiting the Americas during the Quaternary period and the paramount role of body size in species ecology. We evaluated two main features of Pleistocene food webs: the relationship between body size and (i) trophic position and (ii) vulnerability to predation. Despite the large range of species sizes, we found a hump-shaped relationship between trophic position and body size. We also found a negative trend in species vulnerability similar to that observed in modern faunas. The largest species lived near the boundary of energetic constraints, such that any shift in resource availability could drive these species to extinction. Our results reinforce several features of megafauna ecology: (i) the negative relationship between trophic position and body size implies that large-sized species were particularly vulnerable to changes in energetic support; (ii) living close to energetic imbalance could favour the incorporation of additional energy sources, for example, a transition from a herbivorous to a scavenging diet in the largest species (e.g. Megatherium) and (iii) the interactions and structure of Quaternary megafauna communities were shaped by similar forces to those shaping modern fauna communities.
Keywords: food web theory, extinct mammals, metabolic demands, gape limitation, Quaternary
1. Introduction
Food web structure, dynamics and stability are related to the body size of the interacting species involved [1,2]. The extensively documented body size hierarchy in trophic relationships [3], with large animals consuming small ones, determines systematic trends, such as increase in the number of prey and reduced predation risk [4–6], the ability to feed in various local communities [7], the integration of different energy channels [8] and the trends in trophic position [9,10]. The central role of body size on food web structure sheds light on the processes that moulded extinct faunas, in which community size structure strongly departed from contemporary examples [11], even though ancient and modern faunas share remarkable similarities [12]. Nevertheless, exceptionally large body sizes of extinct species such as those observed in Pleistocene mammals could give rise to unusual interaction patterns [13].
The South American Pleistocene megafauna, a singular array of numerous giant mammals [11], is interesting from a food web perspective [13,14]. As these species became larger, their diet richness and range of prey body sizes consistently increased, as did their trophic positions [3,6,15]. However, the increasing energetic demands of body size will compromise the viability of large-sized species at higher trophic positions, where resources systematically dwindle through trophic interactions [9]. Thus, species of intermediate body size are expected to achieve higher trophic positions. Here we aim at elucidating diet–body size relationships and their ecological consequences for that peculiar megafauna [13] with lack of modern counterparts [16] and potential existence of a disproportionately low number of predators ([16–18], but see [19]), based on their exceptional body sizes and the emerging principles of trophic interactions observed in modern biotas. Specifically, we analyse the relationships between body size, and (i) the probability of being carnivorous and (ii) the trend in species vulnerability to predation. However, the existence of larger predators than those found in modern fauna, such as sabre-toothed cats, also suggests that the predation rate on Pleistocene herbivores was similar to contemporary rates [13]. Indeed, it has been argued that large mammals were subject to heavy predator control [19]. Here we intend to elucidate the trophic structure of large Quaternary mammals.
2. Material and methods
We analysed body size and feeding habits in Pleistocene mammal species found in Uruguay and Buenos Aires Province, Argentina, possibly conforming interacting communities [13,14,16–18]. Detailed information on feeding habitats is not available for Pleistocene mammals, thus species were classified according to their feeding habits into two trophic positions: carnivorous or non-carnivorous. We evaluated whether a linear increase or a humped relationship could better explain the association between trophic position and body size through three logistic regression models: (i) linear, (ii) quadratic and (iii) segmented, ranked using Akaike information criteria (AIC). The natural logarithm of body mass (M) was the continuous explanatory variable and the feeding categories were the dichotomous response (0–1). All models were fitted in the statistical program R [20,21].
A linear model for the relationship between the natural logarithm of extant predator body mass and prey body mass was constructed using data from terrestrial endothermic predators [3]. Large endothermic predators feeding on endothermic prey tend to consume larger prey at a given size [22]; we, therefore, chose to model the upper 95th conditional quantile distribution. We defined the predator–prey size range (PPR) as the distance between the 0.925 and the 0.975 conditional quantiles of prey size at a given predator size. We then evaluated the congruence between the PPR obtained in this manner and the PPR observed for African megafauna [5], the most (and nearly the only) comparable modern megafaunal assemblage [11]. To that end, we used data on the size range of prey eaten by modern mammal predators [5]. Finally, we calculated the species vulnerability of Pleistocene mammals as the number of potential predators expected for each species: we summed all predators for which prey size was inferior to the upper limit of the PPR. The sum of predators for which prey size was within PPR gives similar results (not shown), except to the smallest rodent (approx. 27 g). It should be noted that this statistical approach is robust to the trend in the decrease of the number of species with body size.
3. Results and discussion
The trophic position–body size relationship was better described by a hump-shaped relationship in which mammals of intermediate body size had a higher probability of being carnivorous (figure 1a). The quadratic and segmented logistic models fit this distribution better than the linear model (ΔAIC > 10; table 1). Indeed, large and small mammals had almost zero probability of being carnivorous, as expected due to gape and energy limitations [9] and also consistent with the empirical evidence for modern faunas [4,6,10,23]. Theoretically, given that metabolic rate scales with body size at a power of approximately 0.75 and a trophic transfer efficiency between 1% and 40%, the predicted slope of the transition at large sizes between carnivores and non-carnivores is between −0.16 and −0.82 (see the electronic supplementary material). The slope found (b3 = −0.78, 95% CI = −0.24 to −1.3), representing the transition from carnivorous to non-carnivorous, was consistent with theoretical expectations [9,10]. This use of first principles to infer trophic structure in past and present faunas [12,13] implies that Pleistocene large-sized mammal populations were limited by energetic constraints.
Figure 1.
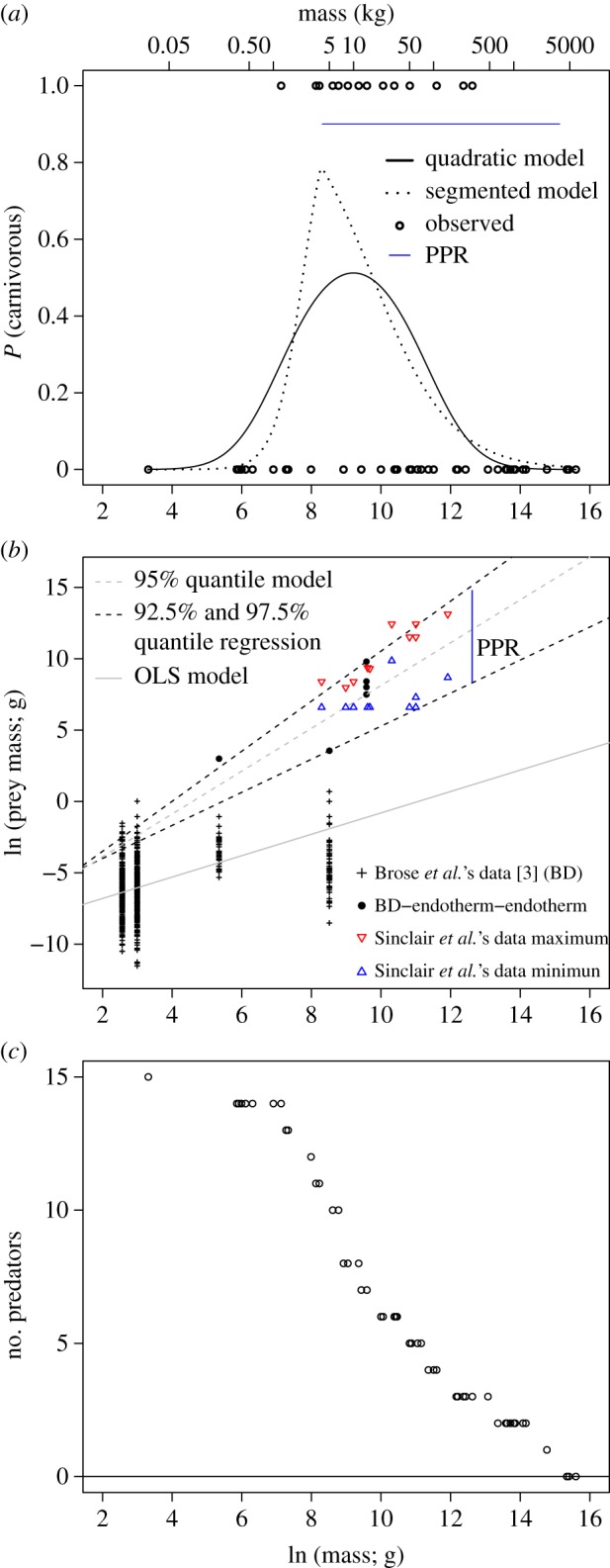
(a) Probability of being carnivorous as a function of natural logarithm of body mass (g) for Pleistocene mammals (fitted models in table 1). Blue line is an example of the range of potential prey for the sabre-toothed cat (M ∼ 304 kg) estimated by the predator–prey range (PPR) model. (b) Predator–prey body mass relationship for extant terrestrial endothermic organisms. Superimposed triangles are minimum and maximum prey sizes in modern African fauna [5], not used to construct the models. Vertical line exemplifies the PPR estimated for the sabre-toothed cat. (c) Number of potential Pleistocene predators as function of prey body size. (Online version in colour.)
Table 1.
Model statistics for the logistic regression model between carnivory (0–1) and the logarithm of body mass (M; g) for Pleistocene mammals. The general model P(M) = et/(1 + et) plus a Binomial error was fitted. bkp refers to break point estimated from segmented regression.
| model | structure | parameter values (s.e.) | ΔAIC |
|---|---|---|---|
| linear | t = b0 + b1 − M | b0 = 0.36 (1.1); b1 = −0.15 (0.1) | 12 |
| quadratic | t = b0 + b1 − M + b2M2 | b0 = −19.3 (8.1)*; b1 = 4.1 (1.7)*; b2 = −0.22 (0.09)* | 1.6 |
| segmented |
t = b0 + b1
M if M < bkp
t = b2 + b3 M if M ≥ bkp |
b0 = −18.3 (11.1); b1 = 5.8 (3.4)*
b2 = 8.7; b3 = −0.78 (0.3)*; bkp = 8.23 (0.5)* |
0 |
*p < 0.05.
The range of prey size predicted from predator size [3,6,15] fits the observed trend in modern African carnivores (figure 1b), whose data were not used for model construction. Based on the estimated PPR, we predicted the size range of prey consumed by each Pleistocene predator species (figure 1a). Our results agreed with previous estimates [13], in spite of their large size, few Pleistocene species were free from predation as adults (figure 1c). Thus, top-down forces were probably more important than previously thought [19].
The vulnerability of Pleistocene mammals to predation had a negative and nonlinear relationship with body size, similar to that in modern African mammals [5,24]. Although other mechanisms must be evaluated, this suggests that similar forces shaped the structure of extinct and modern mammal assemblages, with a consequentially decreasing role of predation in large mammals. First, even a single predator species could impact the entire prey assemblage [25]. Second, large predatory mammals tend to prefer large prey [22,26], which constitute a large proportion of their diet [24]. Third, reduced guild diversity could increase the predation rate experienced by prey because of a reduction in intraguild interference [27]. Fourth, the range of prey consumed could be a more relevant metric for local communities than the predator–prey richness [14]. Fifth, larger predators can dominate carnivorous guilds, accounting for most of the predation experienced by herbivorous mammals [24], a top-down pressure observed in modern faunas [19]. Finally, robust evidence supports the existence of top-down control by Pleistocene megafauna and resource limitation for their predators [19].
Congruently, recent analysis of Pleistocene food webs suggested a topological structure similar to modern food webs; however, Pleistocene communities were more vulnerable to disturbances caused by human invasion [13]. Our results further support the existence of two fundamental features of megafaunal ecology: (i) species involved in the negative trophic position–body size relationship were particularly vulnerable to any change in energetic support (productivity, area, fragmentation) and (ii) living close to energetic imbalance could favour the incorporation of additional energy sources in species' diets, such as a transition from a herbivorous to a scavenging diet in the largest species (e.g. Megatherium [28]). The increased vulnerability and the energetic constraint are congruent with the fact that Pleistocene extinctions were size selective, affecting only large-sized organisms [29]. The second feature is supported by the fact that the largest present-day terrestrial mammals tend to be non-animalivorous, with grizzly bears being the largest at over 500 kg. Given the size structure of this community, our results are congruent with this kind of habits being available for the much larger ground sloth.
In summary, despite the unprecedented range in body size of Pleistocene mammals, a hump-shaped relationship between trophic position and body size was found, along with a food web structure that resembles that of modern faunas. The fact that the largest animals were close to energetic imbalance put these organisms in a delicate situation. Any shift in baseline conditions (e.g. resource availability) or the appearance of a novel predator able to hunt these organisms could lead this species into a highway to hell.
Supplementary Material
Acknowledgement
A.M.S. thanks Vito Muggeo and Gonzalo Perera for statistical advice and Tinto Rico for inspiring ideas.
Ethics
No ethics to declare.
Data accessibility
Data presented here were obtained from published sources or digitalized and are fully available for the scientific community. Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.5c409.
Authors' contributions
A.M.S., R.A.F. and M.A. designed research, R.A.F. generated megafaunal data. A.M.S. compiled data and generated statistical models. M.A. generated the first draft of the article and all authors substantially contributed to the writing of the manuscript and subsequent revisions. All authors agreed to be held accountable for the content therein and approved the final version of the manuscript.
Competing interests
Authors declare no competing interest.
Funding
This work was partially funded by ANII-FCE jovenes investigadores (FCE_3_2013_1_100394) to A.M.S. and (FCE 2014_1_104763) to M.A.
References
- 1.Brose U, Williams RJ, Martinez ND. 2006. Allometric scaling enhances stability in complex food webs. Ecol. Lett. 9, 1228–1236. ( 10.1111/j.1461-0248.2006.00978.x) [DOI] [PubMed] [Google Scholar]
- 2.Garay-Narváez L, Arim M, Flores JD. 2013. The more polluted the environment, the more important biodiversity is for food web stability. Oikos 122, 1247–1253. ( 10.1111/j.1600-0706.2012.00218.x) [DOI] [Google Scholar]
- 3.Brose U, et al. 2006. Consumer–resource body-size relationships in natural food webs. Ecology 87, 2411–2417. ( 10.1890/0012-9658(2006)87[2411:CBRINF]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 4.Arim M, Berazategui M, Barreneche JM, Ziegler L, Zarucki M, Abades SR. 2011. Determinants of density–body size scaling within food webs and tools for their detection. Adv. Ecol. Res. 45, 1–39. ( 10.1016/B978-0-12-386475-8.00001-0) [DOI] [Google Scholar]
- 5.Sinclair ARE, Mduma S, Brashares JS. 2003. Patterns of predation in a diverse predator–prey system. Nature 425, 288–290. ( 10.1038/nature01934) [DOI] [PubMed] [Google Scholar]
- 6.Arim M, Abades S, Laufer G, Loureiro M, Marquet PA. 2010. Food web structure and body size: trophic position and resource acquisition. Oikos 119, 147–153. ( 10.1111/j.1600-0706.2009.17768.x) [DOI] [Google Scholar]
- 7.McCann KS, Rasmussen JB, Umbanhowar J. 2005. The dynamics of spatially coupled food webs. Ecol. Lett. 8, 513–523. ( 10.1111/j.1461-0248.2005.00742.x) [DOI] [PubMed] [Google Scholar]
- 8.McCann KS. 2012. Food webs. Princeton, NJ: Princeton University Press. [Google Scholar]
- 9.Arim M, Bozinovic F, Marquet A. 2007. On the relationship between trophic position, body mass and temperature: reformulating the energy limitation hypothesis. Oikos 116, 1524–1530. ( 10.1111/j.0030-1299.2007.15768.x) [DOI] [Google Scholar]
- 10.Segura AM, Franco-Trecu V, Franco-Fraguas P. 2015. Gape and energy limitation determine a humped relationship between trophic position and body size. Can. J. Fish. Aquat. Sci. 72, 198–205. ( 10.1139/cjfas-2014-0093) [DOI] [Google Scholar]
- 11.Fariña RA, Vizcaìno SF, De Luliis G. 2013. Megafauna: giant beasts of Pleistocene South America. Bloomington, IN: Indiana University Press. [Google Scholar]
- 12.Dunne JA, Williams RJ, Martinez ND, Wood RA, Erwin DH. 2008. Compilation and network analyses of Cambrian food webs. PLoS Biol. 6, e102 ( 10.1371/journal.pbio.0060102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pires MM, Koch PL, Fariña RA, de Aguiar MAM, dos Reis FS, Guimarães PR. 2015. Pleistocene megafaunal interaction networks became more vulnerable after human arrival. Proc. R. Soc. B 282, 20151367 ( 10.1098/rspb.2015.1367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valkenburgh BV, Janis CM. 1993. Historical diversity patterns in North American large herbivorous and carnivorous. In Species diversity in ecological communities (eds Ricklefs RE, Schluter D), pp. 330–340. Chicago, IL: University of Chicago Press. [Google Scholar]
- 15.Otto SB, Rall BC, Brose U. 2007. Allometric degree distributions facilitate food-web stability. Nature 450, 1226–1229. ( 10.1038/nature06359) [DOI] [PubMed] [Google Scholar]
- 16.Hummel J, Clauss M. 2008. Megaherbivores as pacemakers of carnivore diversity and biomass: distributing or sinking trophic energy? Evol. Ecol. Res. 10, 925–930. [Google Scholar]
- 17.Fariña RA. 1996. Trophic relationships among Lujanian mammals. Evol. Theory 11, 125–134. [Google Scholar]
- 18.Fariña RA, Czerwonogora A, Di Giacomo M. 2014. Splendid oddness: the curious palaeoecology of South American Pleistocene mammals revisited. An. Acad. Bras. Cienc. 86, 315–335. [DOI] [PubMed] [Google Scholar]
- 19.Ripple WJ, Van Valkenburgh B. 2010. Linking top-down forces to the pleistocene megafaunal extinctions. BioScience 60, 516–526. ( 10.1525/bio.2010.60.7.7) [DOI] [Google Scholar]
- 20.R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 21.Muggeo VMR. 2003. Estimating regression models with unknown break-points. Stat. Med. 22, 3055–3071. ( 10.1002/sim.1545) [DOI] [PubMed] [Google Scholar]
- 22.Carbone C, Mace GM, Roberts SC, Macdonald DW. 1999. Energetic constraints on the diet of terrestrial carnivores. Nature 402, 286–288. ( 10.1038/46266) [DOI] [PubMed] [Google Scholar]
- 23.Burness GP, Diamond J, Flannery T. 2001. Dinosaurs, dragons, and dwarfs: the evolution of maximal body size. Proc. Natl Acad. Sci. USA 98, 14 518–14 523. ( 10.1073/pnas.251548698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owen-Smith N, Mills MG. 2008. Predator–prey size relationships in an African large-mammal food web. J. Anim. Ecol. 77, 173–183. ( 10.1111/j.1365-2656.2007.01314.x) [DOI] [PubMed] [Google Scholar]
- 25.Croll DA, Maron JL, Estes JA, Danner EM, Byrd GV. 2005. Introduced predators transform subarctic islands from grassland to tundra. Science 307, 1959–1961. ( 10.1126/science.1108485) [DOI] [PubMed] [Google Scholar]
- 26.Carbone C, Codron D, Scofield C, Clauss M, Bielby J. 2014. Geometric factors influencing the diet of vertebrate predators in marine and terrestrial environments. Ecol. Lett. 17, 1553–1559. ( 10.1111/ele.12375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arim M, Marquet PA. 2004. Intraguild predation: a widespread interaction related to species biology. Ecol. Lett. 7, 557–564. ( 10.1111/j.1461-0248.2004.00613.x) [DOI] [Google Scholar]
- 28.Fariña RA, Blanco RE. 1996. Megatherium, the stabber. Proc. R. Soc. Lond. B 263, 1725–1729. ( 10.1098/rspb.1996.0252) [DOI] [PubMed] [Google Scholar]
- 29.Lyons SK, Smith FA, Brown JH. 2004. Of mice, mastodons and men: human-mediated extinctions on four continents. Evol. Ecol. Res. 6, 339–358. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data presented here were obtained from published sources or digitalized and are fully available for the scientific community. Data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.5c409.


