Abstract
The use of skeletal stem cells (SSCs) for cell-based therapies is currently one of the most promising areas for skeletal disease treatment and skeletal tissue repair. The ability for controlled modification of SSCs could provide significant therapeutic potential in regenerative medicine, with the prospect to permanently repopulate a host with stem cells and their progeny. Currently, SSC differentiation into the stromal lineages of bone, fat and cartilage is assessed using different approaches that typically require cell fixation or lysis, which are invasive or even destructive. Raman spectroscopy and coherent anti-Stokes Raman scattering (CARS) microscopy present an exciting alternative for studying biological systems in their natural state, without any perturbation. Here we review the applications of Raman spectroscopy and CARS imaging in stem-cell research, and discuss the potential of these two techniques for evaluating SSCs, skeletal tissues and skeletal regeneration as an exemplar.
Keywords: coherent anti-Stokes Raman scattering, coherent, Raman, skeletal, stem cells
1. Introduction
Throughout the last century, medical breakthroughs have led to a tremendous increase in life expectancy. However, as a consequence, an increasing ageing population has resulted in an increase in age-related diseases, as well as associated reductions in quality of life, leading to a dramatic impact on healthcare. Indeed, skeletal tissue loss due to injury or disease results in a significantly reduced quality of life at significant socio-economic cost. Fractures alone cost the European economy €17 billion and the US economy $20 billion annually [1]. In the USA, there are around 8 million bone fractures per year, of which approximately 5–10% are associated with delayed healing or non-union [2]. Each year in the UK there are approximately 150 000 wrist, vertebral and hip fractures due to osteoporosis, with an estimated healthcare cost of £2.1 billion per annum. Thus, novel and effective medical approaches are essential to fulfil the current demographic challenges [3–5].
The use of stem cells for cell-based therapies is one of the most promising and exciting areas for tissue repair and disease treatment, including those affecting brain, skeletal muscle and heart [6,7]. In fact, the unique properties of stem cells, with their ability to self-renew and potential to differentiate into several different specialized cell types, present an ideal tool for reparative medicine [3,8].
The bone marrow, which is the major site of haematopoiesis (the process which leads to the formation of all blood cells), serves as a reservoir for a variety of cells, including haematopoietic cells as well as cells of the non-haematopoietic stroma [4,9–11]. The bone-marrow stroma constitutes the scaffold that supports the regulation of haematopoiesis, establishing and maintaining the haematopoietic microenvironment necessary for growth and blood-cell maturation [9,12]. Within the bone-marrow stroma reside a rare multipotent stem-cell population called skeletal stem cells (SSCs). The term ‘skeletal stem cell’ is all too frequently confused with ‘mesenchymal stem cell’ (MSC) in the literature (reviewed in [12]). However, MSCs are developmentally distinct from skeletal lineages, and it is important to recognize that the various extra-skeletal tissues and organs noted to retain MSCs are developmentally distinct from skeletal lineages and, critically, are not generated by skeletal progenitors present in bone marrow. The term ‘skeletal stem cell’, as eloquently detailed by Bianco & Robey [13], denotes specifically the rare population of postnatal non-haematopoietic stromal cells found in the bone marrow with the capacity to regenerate bone and bone-marrow stroma (reviewed in [3]), and the term SSC will be used throughout this review to refer to this select population.
The ability to isolate SSCs from human bone marrow, together with their capability to differentiate into skeletal tissues, has attracted significant attention within the medical community, and promises new opportunities for the use of SSCs in clinical applications [3,10,14]. For example, SSCs could be applied for bone/cartilage tissue reparation for a range of musculoskeletal conditions, or as key components of the haematopoietic microenvironment for microvessel assembly guidance, or even as conceptual/modelling instruments for stem-cell biology and mechanistic studies [12]. However, pivotal in the development of successful therapies for bone augmentation will be the design of well defined and reproducible protocols for SSC differentiation. SSCs can be induced to form bone, cartilage and fat depending on their microenvironment (figure 1) [4,15,16], and the differentiation into osteoblasts, chondrocytes and adipocytes, both in vitro and in vivo, is plastic (reversible) [11]. Controlled differentiation of stem cells offers significant therapeutic potential in skeletal regeneration, with the prospective to permanently repopulate a host with stem cells and their progeny, an objective that could be achieved by careful monitoring and characterization of the differentiation process of SSCs.
Figure 1.
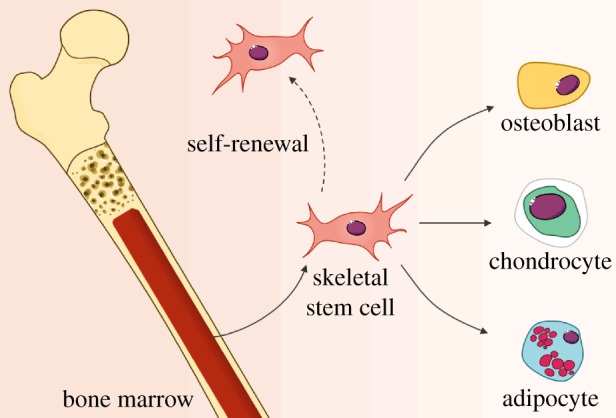
The differentiation potential of skeletal stem cells. A population of skeletal stem cells can be isolated and enriched from the human bone marrow, and expanded in vitro. Under appropriate conditions these skeletal stem cells have the ability to self-renew and to differentiate towards bone, cartilage and fat lineages. (Online version in colour.)
Currently, SSC differentiation can be evaluated through a number of assays including colorimetric assays [17], real-time quantitative polymerase chain reaction (qPCR) [17,18], histochemical analysis [18] and immunohistochemical assays [19]. These methods reveal the detailed biochemical features of SSCs and provide important information regarding their state of differentiation. However, current approaches are invasive, require cell fixation or lysis, and/or are destructive [20–22], indicating current monitoring techniques are unsuitable for time-course studies, as well as unsuitable for SSC characterization prior to therapeutic use. For example, photobleaching of fluorophores/fluorescent dyes limits the temporal availability for imaging the sample of interest. A further important issue is the non-specific binding of dyes or labels, and the staining process may modify the functionality of the target molecule and affect cell biochemical phenomena [21,23].
The current limitations in the characterization of SSC differentiation have led to the need to find new alternatives. Prospective approaches seek to identify molecules at subcellular level without using any dye or label, i.e. ‘label-free’, but by using the intrinsic properties of the molecules. Label-free techniques which offer chemical or molecular selectivity are particularly attractive. Methods which rely on the measurement of vibrational information such as Raman spectroscopy and coherent anti-Stokes Raman scattering (CARS) microscopy are inherently non-invasive (as no introduction of labels is required), non-destructive, as well as chemically selective. It is noted that as with all optical techniques power and exposure to light need to be within a threshold to prevent any damage and phototoxic effects on cells. In this review, we present a detailed overview of the recent applications of Raman spectroscopy and CARS microscopy in stem-cell research, and discuss the potential of label-free techniques to study SSCs for skeletal regeneration.
2. Characterization of skeletal stem cells using label-free approaches
Raman spectroscopy provides information from the intrinsic vibrations of molecules in their native state, without the need for an exogenous label and has been explored as a label-free method for biological and biomedical applications [24–26]. Fourier transform infrared (FTIR) spectroscopy, a technique which provides complementary information to Raman spectroscopy, has also been applied to biological samples in order to study their vibrational profile. However, FTIR is not ideal for biomedical imaging because water strongly absorbs infrared light [21,23,27]. Raman spectroscopy is typically carried out with visible or near-infrared laser sources, and is ideal for measurement of biological samples in the natural state, as water is a very poor Raman scatterer. Both FTIR and Raman spectra can reveal a molecular fingerprint of SSCs, from single molecules to complex structures, providing broad chemical information on their content (lipids, proteins, nucleic acids, etc.). A comparison of the key features from different characterization methods for SSCs is presented in table 1 [23,25,28–31], and it can be seen that Raman spectroscopy and CARS microscopy offer some advantages over traditional SSC characterization techniques.
Table 1.
Comparison of the main features of skeletal stem-cell characterization techniques.
| detection sensitivity | quantitative analysis | molecular specificity | sample preparation | non-invasive analysis | time consumption | in vivo relevance | |
|---|---|---|---|---|---|---|---|
| biochemical assays | low | semi | medium | yes | no | medium | medium |
| histological assays | low | no | medium | yes | no | high | high |
| qPCR | high | yes | high | yes | no | high | low |
| IR spectroscopy | medium | yes | high | yes | yes | low | low |
| Raman spectroscopy | low | yes | high | no | yes | low | high |
| CARS | medium | semi | medium | no | yes | low | high |
3. Raman spectroscopy
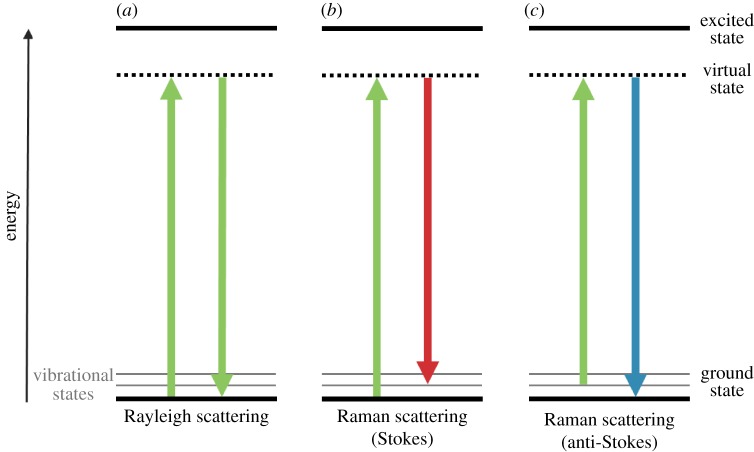
When a beam of light interacts with a molecule, the energy is mostly scattered elastically (no change in energy) with the same frequency as the incident light, known as Rayleigh scattering (figure 2a). A few incident photons interact and exchange energy with the molecular bond vibration, resulting in inelastically scattered light, also known as Raman scattering [31]. A molecule that absorbs a photon enters into a virtual excited state and, almost immediately, another photon is emitted at a slightly different wavelength. When the incident photon loses energy to a molecular bond vibration a red-shifted Stokes photon, i.e. a photon with longer wavelength, is generated and the molecule ends up in a higher vibrational state (figure 2b). A blue-shifted anti-Stokes scattering can also occur, if the molecule of interest is already in a higher vibrational state, resulting in the emission of a lower wavelength photon (figure 2c). The difference in frequency between incident and scattered photons corresponds to the vibrational energy level of the molecule [23,31–33], and is typically called Raman shift (as the shift in wavelength from the incident radiation is measured).
Figure 2.
Schematic diagram of the energy transitions involved in Rayleigh scattering (a) and Raman scattering (b,c). Raman scattering occurs through the interaction of an incident photon with a molecular vibration mode, gaining (anti-Stokes scattering, blue-shifted) or losing (Stokes scattering, red-shifted) an amount of energy equal to that vibrational mode. (Online version in colour.)
As each molecule is unique with its own set of characteristic bonds and therefore vibrational modes, Raman spectroscopy provides a molecular ‘fingerprint’. Given that cells and tissues are composed of different molecules, Raman spectroscopy offers, potentially, a powerful tool to generate a characteristic signature of SSCs and skeletal tissues.
Furthermore, Raman spectroscopy offers other advantages to study living cells including: (i) high spatial resolution, (ii) qualitative and quantitative spectral information, (iii) ability to detect at the subcellular level, and (iv) the ability to analyse cells in real time without altering cell function, as a laser operating at the visible/near-infrared region is applied to prevent significant damage to proteins, DNA, RNA and other present biomolecules [30,34–37].
The last decade has witnessed the use of Raman spectroscopy in the search for prospective spectral markers for characterizing stem cells, including murine embryonic stem cells [20,37–39], human embryonic stem cells [21,40–43], MSCs [21,26,35], SSCs [36,44,45], adipose-derived stem cells (ADSCs) [21,46], as well as the monitoring of stem-cell differentiation into skeletal tissues [21,35,36,45,46]. Table 2 summarizes the current applications of Raman spectroscopy for stem-cell research and the major outcomes. We discuss in detail those studies relevant for skeletal regeneration.
Table 2.
Raman spectroscopy applications in stem cells.
| reference (year) | cell type | study | main findings |
|---|---|---|---|
| Notingher et al. (2004) [37,39,47] | murine embryonic stem cells | characterization of murine embryonic stem cells | changes in the Raman spectra in the RNA peak region can be used as a differentiation marker |
| Ichimura et al. (2014) [38] | murine embryonic stem cells | spontaneous differentiation of embryonic stem cells | differences between Raman spectra of embryonic stem cells before and after spontaneous differentiation |
| Downes et al. (2011) [21] | human embryonic stem cells | characterization of human embryonic stem cells | differences in the Raman spectra between nucleus (higher levels of RNA) and cytoplasm (higher levels of protein and glycogen) |
| Chan et al. (2009) [40] | human embryonic stem cells | embryonic stem-cell differentiation into cardiomyocytes | changes in the RNA and DNA Raman peaks, before and after differentiation |
| Schulze et al. (2010) [42] | human embryonic stem cells | differentiation status of human embryonic stem cells | identification of Raman bands and ratios (e.g. RNA/proteins) to indicate embryonic stem-cell state of differentiation |
| Pascut et al. (2013) [41] | human embryonic stem cells | embryonic stem-cell differentiation into cardiomyocytes | changes in the Raman spectra of carbohydrate and lipid chemical shifts, increasing during differentiation process |
| Tan et al. (2012) [43] | human embryonic stem cells and human-induced pluripotent stem cells | differences between embryonic stem cells and induced pluripotent stem cells | very similar Raman spectra, with small changes in the glycogen bands |
| Pijanka et al. (2010) [26] | human embryonic stem cells and human mesenchymal stem cells | differences between human embryonic stem cells and MSCs | Raman scattering allowed one to distinguish an increase in the DNA band when comparing the embryonic stem cells with the MSCs nuclei |
| Chiang et al. (2009) [35] | human mesenchymal stem cells | MSC differentiation into osteoblasts | changes in the Raman spectra in the hydroxyapatite characteristic peak region during the osteogenic differentiation |
| Downes et al. (2011) [21] | human mesenchymal stem cells | MSC differentiation into osteoblasts | changes in the Raman spectra in the hydroxyapatite, collagen and carbonate chemical shifts during the osteogenic differentiation |
| McManus et al. (2011) [45] | human skeletal stem cells | SSC differentiation into osteoblasts | changes in the spectra in the hydroxyapatite Raman shift during osteogenic differentiation; measurement of carbonate-to-phosphate and mineral-to-matrix ratios at different stages of development |
| Hung et al. (2013) [36] | human skeletal stem cells | SSC differentiation into osteoblasts | changes in the spectra in the octacalcium phosphate, β-tricalcium phosphate and hydroxyapatite Raman shifts, able to detect the extent of maturation during osteogenic differentiation |
| James et al. (2015) [44] | human skeletal stem cells | analysis of functional markers in SSCs using immortalized SSC clonal lines | different SSC clones were identified by Raman spectroscopy, presenting the same biomolecular profile as human SSC fractions |
| Downes et al. (2011) [21] | human adipose-derived stem cells | ADSC differentiation into osteoblasts and adipocytes | changes in the Raman spectra in the hydroxyapatite, collagen and carbonate chemical shifts after osteogenic differentiation; Raman peaks from lipids/proteins are sharper after adipogenic differentiation |
| Ojansivu et al. (2015) [46] | human adipose-derived stem cells | ADSC differentiation into osteoblasts, using different bioactive glasses | similarities in the hydroxyapatite, octacalcium and β-tricalcium phosphate Raman chemical shifts between different cell-culture conditions |
| Mitchell et al. (2015) [48] | human adipose-derived stem cells | ADSC differentiation into adipocytes | characterization of ADSC differentiation into adipocytes at early stages of differentiation |
In 2009, Chiang et al. [35] studied osteogenic differentiation of MSCs applying Raman spectroscopy, with the purpose to monitor the production of hydroxyapatite throughout the osteogenic process. Chiang and colleagues found changes in the hydroxyapatite characteristic ‘chemical’ shift, over the period of 7–21 days following the commencement of differentiation. The state of differentiation of MSCs was confirmed by the use of alizarin red S staining for calcium. Chiang et al. also detailed a novel marker in MSC-derived osteoblasts by monitoring hydroxyapatite with Raman spectroscopy, providing the first indication that this technique could be a promising tool for the study of skeletal tissue development. Downes et al. [21] also induced MSC osteogenic differentiation for 7 days, and observed characteristic peaks in the osteoblast spectra related to phosphate in hydroxyapatite, collagen and carbonate.
Similar approaches were used, where SSCs derived from human bone marrow, and subsequent differentiation into osteoblasts, were characterized and monitored [36,45]. For example, McManus et al. [45] used Raman spectroscopy as a biochemical characterization tool for SSC differentiation into osteoblasts, and compared the results with immunocytochemistry and qPCR analysis (figure 3). McManus et al. determined carbonate-to-phosphate and mineral-to-matrix ratios using specific peaks in Raman spectra at different stages of osteogenic development, and observed an increase of the two ratios with time. Hung's research group identified new spectral markers for osteogenic differentiation in SSCs [36]. In this case, the characteristic chemical shift of octacalcium phosphate was present before differentiation, and the peak decreased throughout the assay period. By contrast, the hydroxyapatite signal increased during SSC differentiation into osteoblasts, and, in addition, a new peak belonging to the β-tricalcium phosphate appeared following differentiation. Hung et al. further corroborated their results using histochemical and gene expression analyses.
Figure 3.
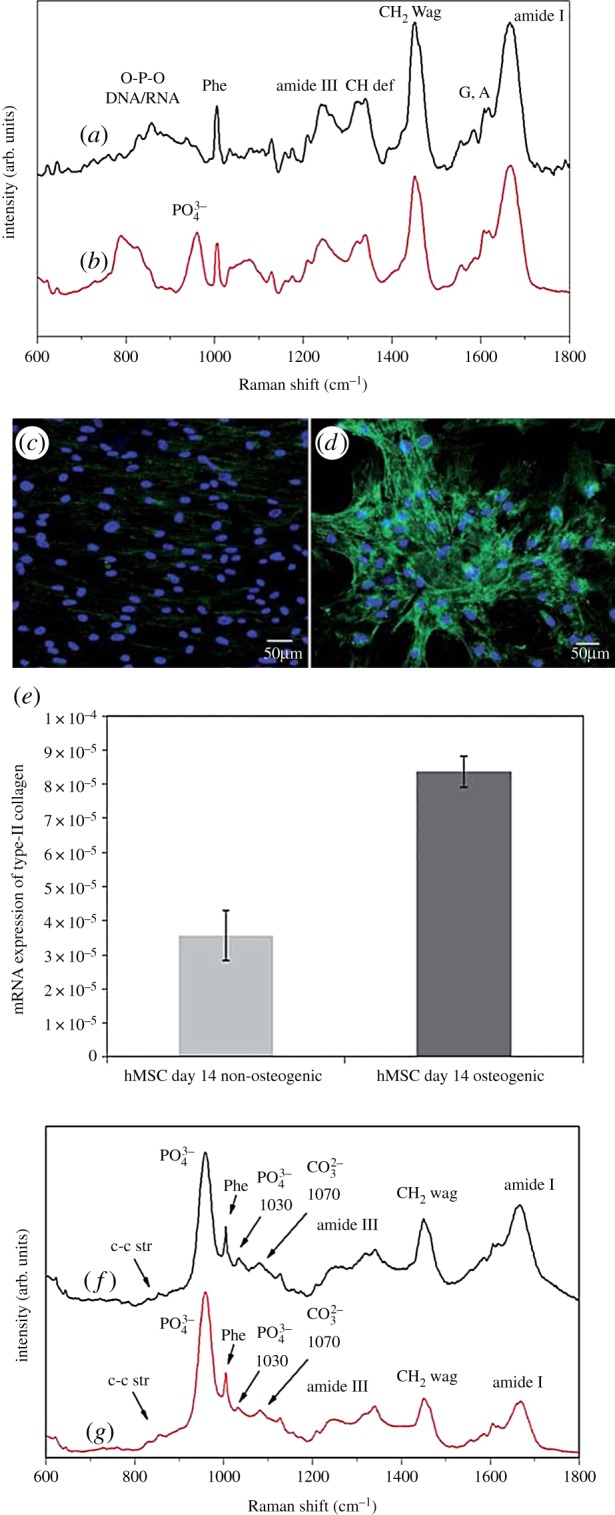
Raman spectra of pre-mineralized SSCs cultured in osteogenic media at day 7 (a), day 14 (b), day 21 (f) and day 28 (g). Immunocytochemical localization of type II collagen in SSCs cultured for 14 days in basal media (c) and osteogenic media (d). Relative expression of type II collagen gene in SSCs cultured for 14 days in basal/osteogenic media, determined by qPCR (e). Adapted from [45]. Copyright Royal Society of Chemistry. (Online version in colour.)
Other groups have reported on ADSCs for skeletal regeneration and their characterization by Raman spectroscopy [21,46]. Downes et al. [49] differentiated ADSCs into osteoblasts and adipocytes, and characterized the different populations using Raman spectroscopy. Similar to Hung's work [36], Ojansivu et al. [46] recently used octacalcium phosphate, hydroxyapatite and β-tricalcium phosphate as specific markers for osteogenic differentiation, in order to compare culture conditions of ADSCs with different bioactive glasses. More recently, Mitchell et al. [48] demonstrated that Raman spectroscopy can be used to detect biochemical changes associated with adipogenic differentiation of ADSCs in a non-invasive and aseptic manner. Mitchell and colleagues were able to monitor the adipogenic differentiation of live ADSCs during 14 days and found significant differences from day 7.
An interesting possible application of Raman spectroscopy in SSC research is the identification of different single-cell-derived clones, which could guide the search for new strategies to analyse the differentiation potential of SSCs, or even SSC isolation from human bone marrow. James et al. [44] studied distinct subtypes of human bone marrow stromal cells, and Raman spectroscopy was applied to identify the molecular fingerprint of the stromal cells subtypes together with the biomolecular profile of human bone-marrow CD317+ fractions. Peak intensity ratios were obtained, and the main difference in the Raman shift was found at 1088.6 cm−1, which is related to the symmetric phosphate stretch of the DNA backbone, indicating a fundamental difference in the DNA of the stromal cell subtypes.
Raman spectroscopic analysis is frequently applied in conjunction with multivariate statistical analysis, for example, principal component analysis and hierarchical clustering analysis [26,37,45], due to the huge amount of chemical information embedded in Raman spectra. These methods provide an approach and are often necessary to extract information on different constituents which exist in varying proportions in a heterogeneous sample such as bone. In addition, a large amount of spectral data can be generated from a tissue map or from different populations (healthy versus diseased, etc.). Identification of compositional changes, classification and quantification of concentrations require the use of such statistical methods as has been shown in several studies with stem cells [40,42,45], and other diseases such as cancer [50]. Indeed, multivariate statistical methods for spectra analysis have contributed to the increase in Raman spectroscopy applications in the examination of living cells, and could be a helpful and vital tool for the comparison of biological samples.
4. Coherent anti-Stokes Raman scattering imaging
While extremely powerful in terms of characterization ability, the efficiency of Raman scattering is extremely small (approximately 1 in 107 scattered photons [51]). Thus for imaging Raman scattering is rather limited as acquisition times per pixel can be up to several seconds. The use of alternative Raman-based techniques that can significantly boost and enhance Raman signal levels, such as CARS or surface-enhanced Raman scattering (SERS), offer exciting alternatives. SERS is highly sensitive but involves using an exogenous material to facilitate the read-out, usually functionalized metal nanoparticles, which may not be desirable. CARS provides the same label-free chemical contrast as Raman spectroscopy, although, and importantly from the current perspective, with improved sensitivity [52].
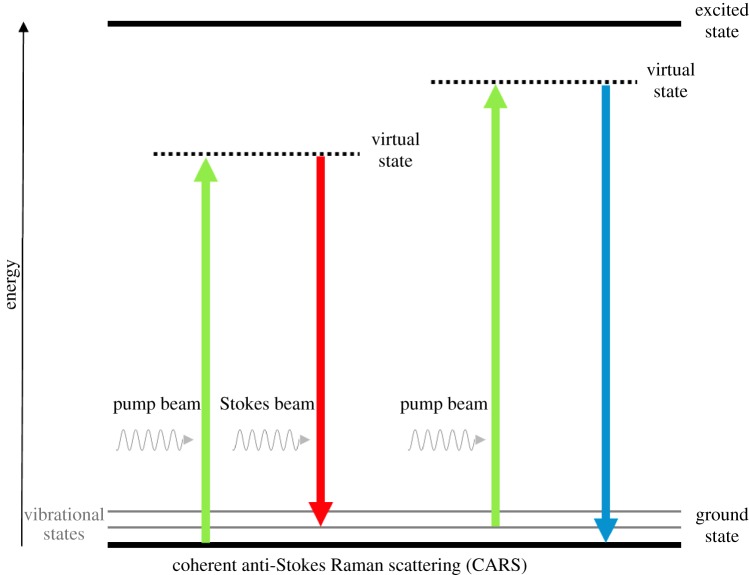
CARS occurs when a target molecule is irradiated using two laser beams simultaneously at different frequencies, a pump beam ωP and a Stokes beam ωS (figure 4) [53]. In essence, when the difference between the higher frequency (pump beam) and the lower frequency (Stokes beam) equals the vibrational frequency of the target bond of the molecule, a CARS signal is generated [32,33,52,54], with an angular frequency equal to ωCARS = 2ωP − ωS. By tuning the frequencies of the two beams to match a particular vibration, a coherent signal with much higher intensity than the signal from spontaneous Raman scattering (up to five orders of magnitude [21,55]) is emitted, providing vibrational contrast in the subsequent CARS image [32,52]. Acquisition times per pixel in CARS imaging are typically between 1 and 10 µs.
Figure 4.
Schematic diagram of the energy transitions involved in coherent anti-Stokes Raman scattering (CARS). In CARS, molecular vibrational modes are coherently populated through optical pumping, by the combination of the pump and the Stokes. The vibration coherence is probed by the pump to generate the CARS signal. Thus when the energy difference between the pump beam (higher frequency) and the Stokes beam (lower frequency) equals a particular molecular vibration of the target, the CARS signal is maximized. (Online version in colour.)
CARS is a multiphoton (four-wave mixing) process with no net deposition of energy within the molecule (as illustrated in figure 4), and is most often carried out with near-infrared laser sources. Besides being non-invasive, non-destructive and label-free, CARS provides relevant benefits over other imaging techniques: (i) the sample photo-damage is minimized, as no net energy is transferred to the sample [54], (ii) CARS provides inherent three-dimensional sectioning ability and video-rate imaging [23,54,56,57], due to the nonlinear multiphoton nature of CARS process, which is of paramount importance for studying cells or tissues, and (iii) fluorescence does not interfere in CARS, as anti-Stokes Raman scattering occurs at a different (blue-shifted) wavelength from fluorescence [32,52].
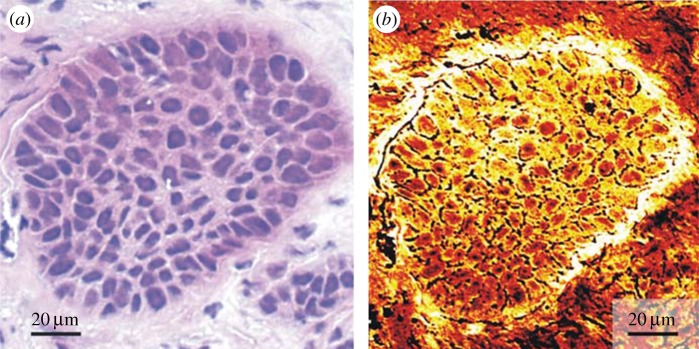
CARS microscopy has been applied in the imaging of living cells and ex vivo tissues, using diverse vibrational contrasts such as for DNA, lipids and proteins [10,52,54,58]. Figure 5, as an example, shows the CARS imaging of a human squamous-cell carcinoma metastasis in the brain. Meyer et al. analysed the same tissue sample with CARS microscopy (targeting lipids) without any staining, and with brightfield microscopy after staining the sample with the gold standard haematoxylin and eosin (H&E).
Figure 5.
Comparison of H&E staining (a) and CARS imaging (b) of a human squamous-cell carcinoma metastasis in the brain. The bright components indicate the intensity of the CARS signal from lipids. Adapted from [59]. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. (Online version in colour.)
The abundance and the essential role of lipids in the human body, combined with the fact that lipids have strong CH2 and CH3 stretching vibration signals at 2853 cm−1 and 2935 cm−1 [24,52,57], respectively, have made lipids the target of choice for demonstrating CARS applications. CARS applications in cell studies are being expanded—for instance, in order to develop an adipose-tumour epithelial cell co-culture system designed to reproduce the in vivo mammary environment, Salameh et al. [60] required a non-destructive and non-invasive technique to access the viability of adipocytes in co-culture. In this study, CARS analysis demonstrated the sustained viability of adipocytes, and an ex vivo co-culture model system to evaluate stromal–epithelial interactions in breast cancer was established. Another example is the application of CARS to study the effect of chemotherapeutic drugs in colon tumour cells. As demonstrated by Steuwe et al. [24], CARS was a useful tool to rapidly image the subcellular accumulation of lipids in cancer cells undergoing cell death induced by different chemotherapeutic drugs.
The last few years have seen emergent data of in vitro cell studies for skeletal regeneration that have applied CARS imaging. The principal findings regarding CARS applications for stem-cell characterization are summarized in table 3. One of the first examples, shown in 2007 by Konorov et al. [56], used CARS microscopy to analyse murine embryonic stem cells. Although image quality did not allow the identification of individual cells, this approach provided a first step towards the use of CARS imaging in stem-cell research. The following year, Schie et al. induced and characterized MSC differentiation into adipocytes, which is commonly assessed by identifying the development of lipid droplets. As the CARS signal for lipids is particularly strong, Schie and colleagues successfully imaged the lipid droplets after 21 days of adipogenic differentiation [61]. Recently, Smus et al. [66] used CARS imaging to assess adipogenic differentiation of SSCs, in conjunction with molecular analysis of gene expression and conventional oil red O staining methodology. The authors demonstrated that CARS microscopy was a valuable technique to detect early stages of SSC differentiation (24 h and 72 h after adipogenesis induction), with enhanced resolution and definition of lipid droplets. Furthermore, CARS microscopy provided an alternative method to monitor changes in SSCs as a result of chemical modulation of adipogenic differentiation.
Table 3.
CARS imaging applications in stem cells.
| reference (year) | cell type | study | main findings |
|---|---|---|---|
| Konorov et al. (2007) [56] | murine embryonic stem cells | distinguish differentiated and undifferentiated embryonic stem cells | preliminary images of embryonic stem cells; need improvements in spatial resolution and image contrast |
| Schie et al. (2008) [61] | human mesenchymal stem cells | MSC differentiation into adipocytes | lipid droplets with high CARS contrast in 21 days differentiated adipocytes |
| Jo et al. (2011) [62] | human adipose-derived stem cells | ADSC differentiation into adipocytes | characterization of ADSC differentiation into adipocytes; lipid droplets with high CARS contrast in differentiated adipocytes |
| Downes et al. (2011) [21] | human adipose-derived stem cells | ADSC differentiation into osteoblasts and adipocytes | preliminary CARS imaging of differentiated osteoblasts, with frequency tuned to hydroxyapatite (960 cm−1); lipid droplets with high CARS contrast in differentiated adipocytes |
| Mouras et al. (2012) [63] | human adipose-derived stem cells | ADSC differentiation into osteoblasts and adipocytes | collagen fibres detected using SHG, and osteoblasts imaged using CARS and two-photon excitation fluorescence; lipid droplets with high CARS contrast in differentiated adipocytes |
| Mortati et al. (2012) [64] | human skeletal stem cells | collagen production of SSCs in fibrin hydrogel scaffolds | collagen fibres of SSCs in fibrin hydrogel scaffolds were imaged using CARS and SHG |
| Di Napoli et al. (2014) [65] | human adipose-derived stem cells | ADSC differentiation into adipocytes | lipid droplets with high contrast in adipocytes, comparing standard CARS and broadband hyperspectral CARS imaging; hyperspectral CARS was more quantitative than the usual single-frequency CARS |
| Smus et al. (2015) [66] | human skeletal stem cells | SSC differentiation into adipocytes | lipid droplets with high contrast, assessed at early stages of differentiation; modulation of human SSC differentiation using different chemical compounds assessed by CARS |
Different research groups have worked with ADSCs and monitored cell differentiation with CARS microscopy. Jo et al. [62] selectively imaged lipid droplets with high contrast in differentiated adipocytes, and it was possible to observe a chronological differentiation of ADSCs, comparing oil red O staining with CARS images. In turn, Downes et al. [21] not only used CARS microscopy to characterize adipocytes, but also tried to establish the use of CARS to image osteoblasts. By imaging at the Raman frequency of hydroxyapatite (960 cm−1), Downes obtained preliminary results with some vibrational contrast although with limited resolution.
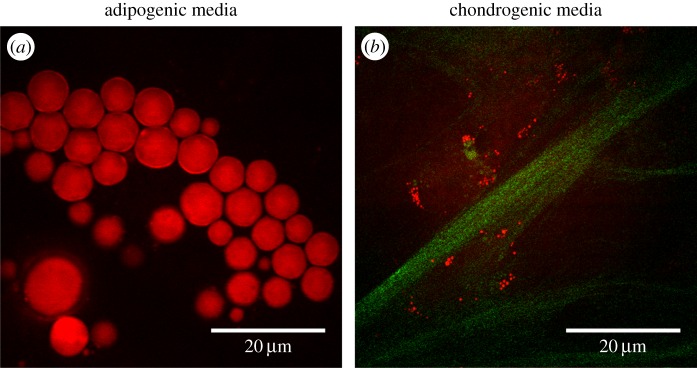
Undoubtedly, CARS imaging has emerged as an important alternative technique for monitoring the development of lipid droplets in SSCs. The combination of CARS microscopy with complementary imaging techniques, namely two-photon excitation fluorescence (TPEF) and second harmonic generation (SHG), can be highly advantageous and give an important insight on the development of SSCs from different angles. TPEF and SHG are also nonlinear imaging modalities which usually can be carried out with the same laser excitation sources thus allowing multimodal read-out. While TPEF allows mapping fluorophores (or auto-fluorophores) with near-infrared excitation, SHG is particularly sensitive to non-centrosymmetric structures such as collagen. CARS together with SHG and/or TPEF could give a more holistic insight into the development of SSCs. Figure 6 shows an exciting application of CARS and SHG microscopy as used by our research group to assess the state of differentiation of fetal femur-derived SSCs. Simultaneous imaging of lipids (CARS) and collagen fibres (SHG) in human fetal femur-derived SSCs cultured in adipogenic (figure 6a) and chondrogenic (figure 6b) media is shown.
Figure 6.
(a) CARS image demonstrating the formation of lipid droplets (red) in human fetal femur-derived SSCs cultured in adipogenic in vitro conditions; (b) multimodal imaging, using simultaneously CARS (red) and SHG (green), shows the presence of lipids (more prominent in (a)) and collagen fibres (more prominent in (b)), respectively, in human fetal femur-derived SSCs cultured in chondrogenic media. (Online version in colour.)
CARS combined with SHG and TPEF imaging has been applied by Mouras et al. to assess ADSC differentiation into adipocytes and osteoblasts [63]. In the same year, Mortati et al. [64] followed collagen production in living SSCs within a fibrin hydrogel scaffold in a three-dimensional culture system using SHG and CARS, clearly demonstrating the potential of multimodal CARS imaging. Applications of multimodal CARS imaging in SSCs are anticipated to be an expanding field.
Most CARS imaging applications map cellular components at a single vibration frequency and do not have the same chemical selectivity as Raman spectroscopy. Broadband or hyperspectral CARS imaging overcome this limitation—the hyperspectral CARS set-up provides CARS images at several vibrational frequencies and generates the corresponding spectra as in Raman spectroscopy. Di Napoli et al. [65] recently reported a comparison between CARS and broadband hyperspectral CARS imaging. After inducing adipogenesis in human ADSCs, CARS provided a qualitative contrast imaging while broadband hyperspectral CARS was used to determine the concentration of unsaturated lipids.
5. Outlook
SSCs show great capacity for bone and cartilage repair and regeneration; however, current techniques for SSC characterization studies prior to cell therapeutic use are invasive and destructive. The studies discussed in this review demonstrate the potential of Raman spectroscopy and CARS imaging for enhanced characterization of SSC differentiation in a non-invasive and non-destructive way, as neither sample preparation nor dyes/labels and other imaging contrast agents are required. We foresee the use of Raman spectroscopy and CARS imaging as standard methodologies for SSC characterization in research studies, or even for application in regenerative medicine, evaluating and monitoring the formation of new bone/cartilage.
Raman spectroscopy has enjoyed considerable exploitation in medical diagnostics (reviewed in Kong et al. [67]). The translation of Raman spectroscopy from bench to clinic is currently in progress, and considerable studies are directed towards the development of optical fibre Raman probes for endoscopic applications [68]. One exciting example is the intraoperative probe created by Leblond and co-workers (using optical fibres and Raman spectroscopy) for detecting cancer cells during brain surgery [69]. Frequently, it is difficult or even impossible to distinguish cancer from non-cancer brain tissue; consequently, after surgery, malignant cells remain in the brain leading to recurrence. This probe accurately identifies invasive cancer cells in the brain, based on Raman signal, guiding the surgeon in real time in the operating room. A related development is the incorporation of a fibre-optic Raman probe in a hypodermic needle, in order to achieve subcutaneous tissue measurements for in vivo diagnostics [70]. This non-invasive probe has huge potential for evaluating bone composition through the skin. Buckley et al. [71] have applied spatially offset Raman spectroscopy, also known as SORS, to detect a compositional abnormality in the bones of a patient suffering from osteogenesis imperfecta, a genetic bone disorder that affects type I collagen. The exemplar developments discussed here and others are being followed by clinical trials to validate the use of non-invasive Raman spectroscopy probes in the clinic for objective diagnostics, with the aim to improve patient outcomes and extend patient survival time.
As previously discussed in this review, in spontaneous Raman scattering there are few inelastically scattered photons, resulting in a weak Raman signal [29,32,54,58] due to which it is used primarily for point measurements especially in clinical applications. In order to circumvent this, instruments for Raman spectroscopy systems could be improved by including more powerful detectors and advanced algorithms to enhance signal to noise. However, in CARS microscopy the Raman signal is enhanced, becoming more suitable for real-time imaging applications. For CARS imaging to be used as a routine technique several technological hurdles still need to be overcome. The majority of CARS applications involve imaging at a single vibrational frequency, and to date only a couple of biomolecules are typically visualized using CARS microscopy (including lipids and proteins) given the limitations related to the availability of broadband laser sources. To surmount this limitation, broadband CARS techniques are being developed, where multiple Raman frequencies are imaged simultaneously [51]. It should also be mentioned that in CARS the signal is proportional to the square of the concentration of vibrational oscillators, hence for low quantities of biomolecules, CARS sensitivity considerably decreases and imaging becomes more challenging [21,52]. Another issue is the non-resonant background observed in CARS imaging which can decrease signal-to-noise ratio. Potentially, these disadvantages can be addressed by using a similar coherent Raman technique named stimulated Raman scattering (reviewed in [72] and [73]), though it still faces many similar challenges as CARS [73,74]. Furthermore, CARS microscopy remains relatively expensive in comparison to other characterization techniques, and requires sophisticated instrumentation. CARS microscopy is now commercialized by Leica Microsystems [75], but further developments are still required to make the technique more accessible. High-quality imaging systems will need to be built using affordable components, to enable and enhance uptake and application of this technique. On a different note, while Raman probes are in advanced stage of translation, application of CARS through optical fibres is still in its infancy. The development of special fibres that can handle laser pulses used in CARS that have large bandwidth and low distortion in the required wavelength range is an active area of research [76].
In summary, while promising, CARS imaging still does not provide the simplicity offered by many established characterization techniques for skeletal regeneration for routine use. For adoption of Raman spectroscopy and CARS imaging in the clinic, in addition to overcoming the technological hurdles, it will also be necessary to: (i) standardize protocols (sample preparation, data analysis and presentation, etc.), (ii) perform multicentre studies, (iii) provide cost rationale/justification for the national health systems, and (iv) provide specific training to clinicians (reviewed in Sulé-Suso et al. [29]). Nevertheless, only by acknowledgement of the relative strengths/weaknesses and current challenges facing Raman spectroscopy and CARS imaging can a step change occur in methodologies to monitor the differentiation of SSCs in their natural state, and indeed other stem and progenitor populations in other tissues.
The research findings discussed in this review provide a strong case for the use of Raman-based techniques for SSC characterization. The label-free, non-destructive and non-invasive nature of Raman spectroscopy and CARS microscopy present an exciting prospective alternative to dynamically monitor SSC development for skeletal regeneration, with widespread potential in other hard and soft tissues.
Competing interests
The authors declare no competing interests.
Funding
Financial support from the Institute for Life Sciences, University of Southampton, BBSRC (BB/L021072/1), ERC grant Nano-ChemBioVision 638258 and Wessex Medical Research is gratefully acknowledged.
References
- 1.Dawson JI, Kingham E, Evans NR, Tayton E, Oreffo ROC. 2012. Skeletal regeneration: application of nanotopography and biomaterials for skeletal stem cell based bone repair. Inflamm. Regen. 32, 72–89. ( 10.2492/inflammregen.32.072) [DOI] [Google Scholar]
- 2.Panteli M, Pountos I, Jones E, Giannoudis PV. 2015. Biological and molecular profile of fracture non-union tissue: current insights. J. Cell. Mol. Med. 19, 685–713. ( 10.1111/jcmm.12532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson JI, Kanczler J, Tare R, Kassem M, Oreffo ROC. 2014. Concise review: bridging the gap: bone regeneration using skeletal stem cell-based strategies—where are we now? Stem Cells 32, 35–44. ( 10.1002/stem.1559) [DOI] [PubMed] [Google Scholar]
- 4.Lovell-Badge R. 2001. The future for stem cell research. Nature 414, 88–91. ( 10.1038/35102150) [DOI] [PubMed] [Google Scholar]
- 5.Tare RS, Kanczler J, Aarvold A, Jones AM, Dunlop DG, Oreffo RO. 2010. Skeletal stem cells and bone regeneration: translational strategies from bench to clinic. Proc. Inst. Mech. Eng. H 224, 1455–1470. ( 10.1243/09544119JEIM750) [DOI] [PubMed] [Google Scholar]
- 6.Oreffo RO. 2014. Centre for human development, stem cells & regeneration. Regen. Med. 9, 563–567. ( 10.2217/rme.14.48) [DOI] [PubMed] [Google Scholar]
- 7.Srivastava D, Ivey KN. 2006. Potential of stem-cell-based therapies for heart disease. Nature 441, 1097–1099. ( 10.1038/nature04961) [DOI] [PubMed] [Google Scholar]
- 8.Chidgey AP, Layton D, Trounson A, Boyd RL. 2008. Tolerance strategies for stem-cell-based therapies. Nature 453, 330–337. ( 10.1038/nature07041) [DOI] [PubMed] [Google Scholar]
- 9.Wickramasinghe SN, Porwit A, Erber WN. 2011. Normal bone marrow cells: development and cytology. In Blood and bone marrow pathology, 2nd edn (eds Porwit A, McCullough J, Erber WN), ch. 2, pp. 19–44. Edinburgh, UK: Churchill Livingstone. [Google Scholar]
- 10.Bianco P. 2014. ‘Mesenchymal’ stem cells. Annu. Rev. Cell Dev. Biol. 30, 677–704. ( 10.1146/annurev-cellbio-100913-013132) [DOI] [PubMed] [Google Scholar]
- 11.Bianco P, Riminucci M, Gronthos S, Robey PG. 2001. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19, 180–192. ( 10.1634/stemcells.19-3-180) [DOI] [PubMed] [Google Scholar]
- 12.Bianco P, Robey PG. 2015. Skeletal stem cells. Development 142, 1023–1027. ( 10.1242/dev.102210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bianco P, Robey PG. 2004. Use of embryonic stem cells to treat heart disease. In Handbook of stem cells (eds Lanza R, Gearhart J, Hogan B, Melton D, Pedersen R, Thomson J, West M), ch. 36, pp. 415–424. Burlington, MA: Academic Press. [Google Scholar]
- 14.Aarvold A, Smith JO, Tayton ER, Jones AM, Dawson JI, Lanham S, Briscoe A, Dunlop DG, Oreffo RO. 2014. From bench to clinic and back: skeletal stem cells and impaction bone grafting for regeneration of bone defects. J. Tissue Eng. Regen. Med. 8, 779–786. ( 10.1002/term.1577) [DOI] [PubMed] [Google Scholar]
- 15.Chamberlain G, Fox J, Ashton B, Middleton J. 2007. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25, 2739–2749. ( 10.1634/stemcells.2007-0197) [DOI] [PubMed] [Google Scholar]
- 16.Murray IR, et al. 2014. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell. Mol. Life Sci. 71, 1353–1374. ( 10.1007/s00018-013-1462-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause U, Seckinger A, Gregory CA. 2011. Assays of osteogenic differentiation by cultured human mesenchymal stem cells. Methods Mol. Biol. 698, 215–230. ( 10.1007/978-1-60761-999-4_17) [DOI] [PubMed] [Google Scholar]
- 18.Fink T, Zachar V. 2011. Adipogenic differentiation of human mesenchymal stem cells. Methods Mol. Biol. 698, 243–251. ( 10.1007/978-1-60761-999-4_19) [DOI] [PubMed] [Google Scholar]
- 19.Solchaga LA, Penick KJ, Welter JF. 2011. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: tips and tricks. Methods Mol. Biol. 698, 253–278. ( 10.1007/978-1-60761-999-4_20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Notingher I, Jell G, Lohbauer U, Salih V, Hench LL. 2004. In situ non-invasive spectral discrimination between bone cell phenotypes used in tissue engineering. J. Cell. Biochem. 92, 1180–1192. ( 10.1002/jcb.20136) [DOI] [PubMed] [Google Scholar]
- 21.Downes A, Mouras R, Bagnaninchi P, Elfick A. 2011. Raman spectroscopy and CARS microscopy of stem cells and their derivatives. J. Raman Spectrosc. 42, 1864–1870. ( 10.1002/jrs.2975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robey PG. 2011. Cell sources for bone regeneration: the good, the bad, and the ugly (but promising). Tissue Eng. B 17, 423–430. ( 10.1089/ten.teb.2011.0199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel II, Steuwe C, Reichelt S, Mahajan S. 2013. Coherent anti-Stokes Raman scattering for label-free biomedical imaging. J. Opt. 15, 094006 ( 10.1088/2040-8978/15/9/094006) [DOI] [Google Scholar]
- 24.Steuwe C, Patel II, Ul-Hasan M, Schreiner A, Boren J, Brindle KM, Reichelt S, Mahajan S. 2013. CARS based label-free assay for assessment of drugs by monitoring lipid droplets in tumour cells. J. Biophotonics 7, 906–913. ( 10.1002/jbio.201300110) [DOI] [PubMed] [Google Scholar]
- 25.Lee YJ, Vega SL, Patel PJ, Aamer KA, Moghe PV, Cicerone MT. 2014. Quantitative, label-free characterization of stem cell differentiation at the single-cell level by broadband coherent anti-Stokes Raman scattering microscopy. Tissue Eng. C 20, 562–569. ( 10.1089/ten.TEC.2013.0472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pijanka JK, et al. 2010. Vibrational spectroscopy differentiates between multipotent and pluripotent stem cells. Analyst 135, 3126–3132. ( 10.1039/c0an00525h) [DOI] [PubMed] [Google Scholar]
- 27.Freudiger CW, Min W, Saar BG, Lu S, Holtom GR, He C, Tsai JC, Kang JX, Xie XS. 2008. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 322, 1857–1861. ( 10.1126/science.1165758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schie IW, Huser T. 2013. Label-free analysis of cellular biochemistry by Raman spectroscopy and microscopy. Compr. Physiol. 3, 941–956. ( 10.1002/cphy.c120025) [DOI] [PubMed] [Google Scholar]
- 29.Sulé-Suso J, Forsyth NR, Untereiner V, Sockalingum GD. 2014. Vibrational spectroscopy in stem cell characterisation: is there a niche? Trends Biotechnol. 32, 254–262. ( 10.1016/j.tibtech.2014.03.002) [DOI] [PubMed] [Google Scholar]
- 30.Tsai TH, Short MA, McLean DI, Zeng H, McElwee K, Lui H. 2014. Label-free identification and characterization of murine hair follicle stem cells located in thin tissue sections with Raman micro-spectroscopy. Analyst 139, 2799–2805. ( 10.1039/c4an00155a) [DOI] [PubMed] [Google Scholar]
- 31.Wachsmann-Hogiu S, Weeks T, Huser T. 2009. Chemical analysis in vivo and in vitro by Raman spectroscopy—from single cells to humans. Curr. Opin. Biotechnol. 20, 63–73. ( 10.1016/j.copbio.2009.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folick A, Min W, Wang MC. 2011. Label-free imaging of lipid dynamics using coherent anti-Stokes Raman scattering (CARS) and stimulated Raman scattering (SRS) microscopy. Curr. Opin. Genet. Dev. 21, 585–590. ( 10.1016/j.gde.2011.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez LG, Lockett SJ, Holtom GR. 2006. Coherent anti-Stokes Raman scattering microscopy: a biological review. Cytometry A 69, 779–791. ( 10.1002/cyto.a.20299) [DOI] [PubMed] [Google Scholar]
- 34.Chan JW, Lieu DK. 2009. Label-free biochemical characterization of stem cells using vibrational spectroscopy. J. Biophotonics 2, 656–668. ( 10.1002/jbio.200910041) [DOI] [PubMed] [Google Scholar]
- 35.Chiang HK, Peng F-Y, Hung S-C, Feng Y-C. 2009. In situ Raman spectroscopic monitoring of hydroxyapatite as human mesenchymal stem cells differentiate into osteoblasts. J. Raman Spectrosc. 40, 546–549. ( 10.1002/jrs.2161) [DOI] [Google Scholar]
- 36.Hung PS, Kuo YC, Chen HG, Chiang HH, Lee OK. 2013. Detection of osteogenic differentiation by differential mineralized matrix production in mesenchymal stromal cells by Raman spectroscopy. PLoS ONE 8, e65438 ( 10.1371/journal.pone.0065438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Notingher I, Bisson I, Bishop AE, Randle WL, Polak JM, Hench LL. 2004. In situ spectral monitoring of mRNA translation in embryonic stem cells during differentiation in vitro. Anal. Chem. 76, 3185–3193. ( 10.1021/ac0498720) [DOI] [PubMed] [Google Scholar]
- 38.Ichimura T, Chiu LD, Fujita K, Kawata S, Watanabe TM, Yanagida T, Fujita H. 2014. Visualizing cell state transition using Raman spectroscopy. PLoS ONE 9, e84478 ( 10.1371/journal.pone.0084478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Notingher I, Jell G, Notingher PL, Bisson I, Tsigkou O, Polak JM, Stevens MM, Hench LL. 2005. Multivariate analysis of Raman spectra for in vitro non-invasive studies of living cells. J. Mol. Struct. 744–747, 179–185. ( 10.1016/j.molstruc.2004.12.046) [DOI] [Google Scholar]
- 40.Chan JW, Lieu DK, Huser T, Li RA. 2009. Label-free separation of human embryonic stem cells and their cardiac derivatives using Raman spectroscopy. Anal. Chem. 81, 1324–1331. ( 10.1021/ac801665m) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pascut FC, Kalra S, George V, Welch N, Denning C, Notingher I. 2013. Non-invasive label-free monitoring the cardiac differentiation of human embryonic stem cells in-vitro by Raman spectroscopy. Biochim. Biophys. Acta 1830, 3517–3524. ( 10.1016/j.bbagen.2013.01.030) [DOI] [PubMed] [Google Scholar]
- 42.Schulze HG, Konorov SO, Caron NJ, Piret JM, Blades MW, Turner RFB. 2010. Assessing differentiation status of human embryonic stem cells noninvasively using Raman microspectroscopy. Anal. Chem. 82, 5020–5027. ( 10.1021/ac902697q) [DOI] [PubMed] [Google Scholar]
- 43.Tan Y, Konorov SO, Schulze HG, Piret JM, Blades MW, Turner RFB. 2012. Comparative study using Raman microspectroscopy reveals spectral signatures of human induced pluripotent cells more closely resemble those from human embryonic stem cells than those from differentiated cells. Analyst 137, 4509–4515. ( 10.1039/C2AN35507H) [DOI] [PubMed] [Google Scholar]
- 44.James S, et al. 2015. Multiparameter analysis of human bone marrow stromal cells identifies distinct immunomodulatory and differentiation-competent subtypes. Stem Cell Rep. 4, 1004–1015. ( 10.1016/j.stemcr.2015.05.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McManus LL, Burke GA, McCafferty MM, O'Hare P, Modreanu M, Boyd AR, Meenan BJ. 2011. Raman spectroscopic monitoring of the osteogenic differentiation of human mesenchymal stem cells. Analyst 136, 2471–2481. ( 10.1039/c1an15167c) [DOI] [PubMed] [Google Scholar]
- 46.Ojansivu M, Vanhatupa S, Björkvik L, Häkkänen H, Kellomäki M, Autio R, Ihalainen JA, Hupa L, Miettinen S. 2015. Bioactive glass ions as strong enhancers of osteogenic differentiation in human adipose stem cells. Acta Biomater. 21, 190–203. ( 10.1016/j.actbio.2015.04.017) [DOI] [PubMed] [Google Scholar]
- 47.Notingher I, Bisson I, Polak JM, Hench LL. 2004. In situ spectroscopic study of nucleic acids in differentiating embryonic stem cells. Vib. Spectrosc. 35, 199–203. ( 10.1016/j.vibspec.2004.01.014) [DOI] [Google Scholar]
- 48.Mitchell A, Ashton L, Yang XB, Goodacre R, Smith A, Kirkham J. 2015. Detection of early stage changes associated with adipogenesis using Raman spectroscopy under aseptic conditions. Cytometry A 87, 1012–1019. ( 10.1002/cyto.a.22777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Downes A, Mouras R, Elfick A. 2009. A versatile CARS microscope for biological imaging. J. Raman Spectrosc. 40, 757–762. ( 10.1002/jrs.2249) [DOI] [Google Scholar]
- 50.Lloyd GR, Orr LE, Christie-Brown J, McCarthy K, Rose S, Thomas M, Stone N. 2013. Discrimination between benign, primary and secondary malignancies in lymph nodes from the head and neck utilising Raman spectroscopy and multivariate analysis. Analyst 138, 3900–3908. ( 10.1039/C2AN36579K) [DOI] [PubMed] [Google Scholar]
- 51.Camp CH Jr, Cicerone MT. 2015. Chemically sensitive bioimaging with coherent Raman scattering. Nat. Photon. 9, 295–305. ( 10.1038/nphoton.2015.60) [DOI] [Google Scholar]
- 52.Pezacki JP, Blake JA, Danielson DC, Kennedy DC, Lyn RK, Singaravelu R. 2011. Chemical contrast for imaging living systems: molecular vibrations drive CARS microscopy. Nat. Chem. Biol. 7, 137–145. ( 10.1038/nchembio.525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tolles WM, Nibler JW, McDonald JR, Harvey AB. 1977. A review of the theory and application of coherent anti-Stokes Raman spectroscopy (CARS). Appl. Spectrosc. 31, 253–271. ( 10.1366/000370277774463625) [DOI] [Google Scholar]
- 54.Evans CL, Xie XS. 2008. Coherent anti-Stokes Raman scattering microscopy: chemical imaging for biology and medicine. Annu. Rev. Anal. Chem. 1, 883–909. ( 10.1146/annurev.anchem.1.031207.112754) [DOI] [PubMed] [Google Scholar]
- 55.Ohulchanskyy TY, Pliss AM, Prasad PN. 2011. Biophotonics: harnessing light for biology and medicine. In Biophotonics: spectroscopy, imaging, sensing, and manipulation (eds Bartolo DB, Collins J), pp. 3–17. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 56.Konorov SO, Glover CH, Piret JM, Bryan J, Schulze HG, Blades MW, Turner RF. 2007. In situ analysis of living embryonic stem cells by coherent anti-Stokes Raman microscopy. Anal. Chem. 79, 7221–7225. ( 10.1021/ac070544k) [DOI] [PubMed] [Google Scholar]
- 57.Krafft C, Dietzek B, Popp J. 2009. Raman and CARS microspectroscopy of cells and tissues. Analyst 134, 1046–1057. ( 10.1039/B822354H) [DOI] [PubMed] [Google Scholar]
- 58.Evans CL, Potma EO, Puoris'haag M, Cote D, Lin CP, Xie XS. 2005. Chemical imaging of tissue in vivo with video-rate coherent anti-Stokes Raman scattering microscopy. Proc. Natl Acad. Sci. USA 102, 16 807–16 812. ( 10.1073/pnas.0508282102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meyer T, Bergner N, Medyukhina A, Dietzek B, Krafft C, Romeike BFM, Reichart R, Kalff R, Popp J. 2012. Interpreting CARS images of tissue within the C–H-stretching region. J. Biophotonics 5, 729–733. ( 10.1002/jbio.201200104) [DOI] [PubMed] [Google Scholar]
- 60.Salameh TS, Le TT, Nichols MB, Bauer E, Cheng J, Camarillo IG. 2013. An ex vivo co-culture model system to evaluate stromal-epithelial interactions in breast cancer. Int. J. Cancer 132, 288–296. ( 10.1002/ijc.27672) [DOI] [PubMed] [Google Scholar]
- 61.Schie IW, Weeks T, McNerney GP, Fore S, Sampson JK, Wachsmann-Hogiu S, Rutledge JC, Huser T. 2008. Simultaneous forward and epi-CARS microscopy with a single detector by time-correlated single photon counting. Opt. Express 16, 2168–2175. ( 10.1364/OE.16.002168) [DOI] [PubMed] [Google Scholar]
- 62.Jo SJ, Choi WW, Lee ES, Lee JY, Park HS, Moon DW, Eun HC, Chung JH. 2011. Temporary increase of PPAR-γ and transient expression of UCP-1 in stromal vascular fraction isolated human adipocyte derived stem cells during adipogenesis. Lipids 46, 487–494. ( 10.1007/s11745-011-3525-5) [DOI] [PubMed] [Google Scholar]
- 63.Mouras R, Bagnaninchi PO, Downes AR, Elfick AP. 2012. Label-free assessment of adipose-derived stem cell differentiation using coherent anti-Stokes Raman scattering and multiphoton microscopy. J. Biomed. Opt. 17, 116011 ( 10.1117/1.jbo.17.11.116011) [DOI] [PubMed] [Google Scholar]
- 64.Mortati L, Divieto C, Sassi MP. 2012. CARS and SHG microscopy to follow collagen production in living human corneal fibroblasts and mesenchymal stem cells in fibrin hydrogel 3D cultures. J. Raman Spectrosc. 43, 675–680. ( 10.1002/jrs.3171) [DOI] [Google Scholar]
- 65.Di Napoli C, Pope I, Masia F, Watson P, Langbein W, Borri P. 2014. Hyperspectral and differential CARS microscopy for quantitative chemical imaging in human adipocytes. Biomed. Opt. Express 5, 1378–1390. ( 10.1364/boe.5.001378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smus JP, Moura CC, McMorrow E, Tare R, Oreffo RO, Mahajan S. 2015. Tracking adipogenic differentiation of skeletal stem cells by label-free chemically selective imaging. Chem. Sci. 6, 7089–7096. ( 10.1039/C5SC02168E) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kong K, Kendall C, Stone N, Notingher I. 2015. Raman spectroscopy for medical diagnostics—from in-vitro biofluid assays to in-vivo cancer detection. Adv. Drug Deliv. Rev. 89, 121–134. ( 10.1016/j.addr.2015.03.009) [DOI] [PubMed] [Google Scholar]
- 68.Bergholt MS, Zheng W, Ho KY, Teh M, Yeoh KG, So JB, Shabbir A, Huang Z. 2013. Fiber-optic Raman spectroscopy probes gastric carcinogenesis in vivo at endoscopy. J. Biophotonics 6, 49–59. ( 10.1002/jbio.201200138) [DOI] [PubMed] [Google Scholar]
- 69.Jermyn M, et al. 2015. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci. Transl. Med. 7, 274ra219. ( 10.1126/scitranslmed.aaa2384) [DOI] [PubMed] [Google Scholar]
- 70.Iping Petterson IE, Day JC, Fullwood LM, Gardner B, Stone N. 2015. Characterisation of a fibre optic Raman probe within a hypodermic needle. Anal. Bioanal. Chem. 407, 8311–8320. ( 10.1007/s00216-015-9021-7) [DOI] [PubMed] [Google Scholar]
- 71.Buckley K, Kerns JG, Gikas PD, Birch HL, Vinton J, Keen R, Parker AW, Matousek P, Goodship AE. 2014. Measurement of abnormal bone composition in vivo using noninvasive Raman spectroscopy. IBMS BoneKEy 11, 602 ( 10.1038/bonekey.2014.97) [DOI] [Google Scholar]
- 72.Zhang D, Wang P, Slipchenko MN, Cheng J-X. 2014. Fast vibrational imaging of single cells and tissues by stimulated Raman scattering microscopy. Acc. Chem. Res. 47, 2282–2290. ( 10.1021/ar400331q) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng JX, Xie XS. 2015. Vibrational spectroscopic imaging of living systems: an emerging platform for biology and medicine. Science 350, aaa8870. ( 10.1126/science.aaa8870) [DOI] [PubMed] [Google Scholar]
- 74.Ozeki Y, Umemura W, Otsuka Y, Satoh S, Hashimoto H, Sumimura K, Nishizawa N, Fukui K, Itoh K. 2012. High-speed molecular spectral imaging of tissue with stimulated Raman scattering. Nat. Photon. 6, 845–851. ( 10.1038/nphoton.2012.263) [DOI] [Google Scholar]
- 75.Leica Microsystems. 2016. CARS microscope: label free imaging Leica TCS SP8 CARS. See http://www.leica-microsystems.com/products/confocal-microscopes/details/product/leica-tcs-sp8-cars/.
- 76.Latka I, Dochow S, Krafft C, Dietzek B, Popp J. 2013. Fiber optic probes for linear and nonlinear Raman applications—current trends and future development. Laser Photonics Rev. 7, 698–731. ( 10.1002/lpor.201200049) [DOI] [Google Scholar]