Abstract
The epidemic of metabolic diseases has raised questions about the interplay between the human diet and the gut and its microbiota. The gut has two vital roles: nutrient absorption and intestinal barrier function. Gut barrier defects are involved in many diseases. Excess energy intake disturbs the gut microbiota and favors body entry of microbial compounds that stimulate chronic metabolic inflammation. In this context, the natural defense mechanisms of gut epithelial cells and the potential to boost them nutritionally warrant further study. One such important defense system is the activation of inducible heat-shock proteins (iHSPs) which protect the gut epithelium against oxidative stress and inflammation. Importantly, various microbial components can induce the expression of iHSPs. This review examines gut epithelial iHSPs as the main targets of microbial signals and nutrients and presents data on diseases involving disturbances of gut epithelial iHSPs. In addition, a broad literature analysis of dietary modulation of gut epithelial iHSPs is provided. Future research aims should include the identification of gut microbes that can optimize gut-protective iHSPs and the evaluation of iHSP-mediated health benefits of nutrients and food components.
Keywords: diet, gut, heat-shock protein, microbiota.
INTRODUCTION
The epidemics of several noncommunicable diseases, including insulin resistance syndrome, type-2 diabetes, cardiovascular disease, metabolic syndrome, and obesity, in Western and developing countries have raised questions about the interplay between the human diet, the gastrointestinal (GI) tract, and the microbiota of the GI tract. The GI tract has two vital functions, namely to absorb nutrients after food digestion and to act as a physical and immunological barrier against the entry of noxious compounds into the interior milieu. Both functions contribute to whole-body homeostasis and health preservation. Defects in gut barrier function are often involved in various GI and extra-GI diseases, including inflammatory bowel disease, irritable bowel syndrome, liver disease, metabolic syndrome, and obesity.1–3
It has become clear that excessive intake of unbalanced high-energy diets in the context of a sedentary lifestyle disturbs the gut microbiota and causally favors the entry of proinflammatory microbial compounds (e.g., lipopolysaccharide [LPS]) into the body, thus leading to chronic low-grade inflammation and metabolic disorders.4,5 In light of this, it is important to reconsider the natural defense mechanisms of gut epithelial cells (GECs) and the potential to boost them nutritionally, with an aim toward prevention of metabolic disorders.
The role of intestinal alkaline phosphatase as a major natural protective component has already been reviewed.6,7 Briefly, the functions of intestinal alkaline phosphatase include detoxification of LPS, control of gut microbiota composition, and anti-inflammation. One main conclusion of these reviews is that various nutrients and food components can stimulate the expression and activity of intestinal alkaline phosphatase, thus partially explaining their anti-inflammatory properties.
Inducible heat-shock proteins (iHSPs) constitute another important defense system at the GEC level. Incidentally, iHSPs may include intestinal alkaline phosphatase, as they are all dependently stimulated by heat stress.8 Inducible HSPs are highly conserved proteins involved in vital cellular functions such as protein folding, chaperoning, trafficking and addressing, apoptosis and proliferation, redox balance, inflammatory responses, and intercellular junctional integrity, and most underlying molecular mechanisms have been elucidated.9,10 Two iHSPs, namely HSP25 and HSP70, have been repeatedly demonstrated to protect GECs against inflammatory and oxidative stress in vitro and in vivo.10–12 Importantly, a variety of microbial components or products have been shown in recent years to induce the expression of iHSPs, demonstrating an important link with the gut microbiota.
This review focuses on intestinal and colonic iHSPs – HSP25 and HSP70 in rodents, which correspond to HSP27 and HSP72, respectively, in humans – as the main targets of nutrients and microbial signals. Of note, for clarity, the name HSP70 is used consistently, even for HSP72, throughout this review. A broad literature analysis of dietary modulation of iHSPs in GECs is also provided, as available reviews usually focus on a single iHSP and selected nutrients or food components (e.g., iHSP70 and functional nutrients, or iHSPs and fiber).13,14 Therefore, this review puts into perspective the host–microbiota interplay at the iHSP level in gut health and disease and shows the great potential of nutritional stimulation of these protective iHSPs. Maintenance of a healthy gut microbiota through dietary means is now recognized as a major approach to the preservation of host health.15
PHYSIOLOGICAL ROLES OF GUT EPITHELIAL INDUCIBLE HEAT-SHOCK PROTEINS
Though study of GECs has been limited, the mechanisms of iHSP action are likely to be common among different cell types. Many molecular mechanisms of iHSP action have been discovered in a wide variety of cells, especially cancer cells. These mechanisms have been reviewed repeatedly,9,16,17 and thus only the most prominent features of these mechanisms are summarized in this section. Current knowledge about GECs is also presented in this section.
Protein chaperoning
Both iHSPs (HSP27 and HSP70) are powerful protein chaperones.9 HSP27 has a central domain called α-crystallin, which is conserved across small HSPs, and a number of phosphorylation sites, both of which contribute to HSP27 functional properties. Direct binding of HSP27 to cellular proteins is independent of adenosine triphosphate but is determined by the degrees of HSP27 polymerization and phosphorylation; larger HSP27 oligomers undergo limited phosphorylation and exhibit (partially independently) chaperone, antiapoptotic, and antioxidant properties.17 HSP70 comprises one adenosine triphosphatase site and one peptide-binding site, and its chaperone activity is most often indirect (e.g., co-chaperone with c-terminal HSP70-interacting protein). Inducible HSPs are involved in the correct folding of functional proteins under normal conditions, and they participate in the renaturation or specific sorting and proteasomal degradation of specific proteins after cell stress.9 For example, client proteins for iHSP27 include structural (e.g., cytoskeleton) and functional (e.g., enzyme) proteins. Binding of iHSP27 to cytoskeletal F-actin depends on actin phosphorylation and iHSP27 oligomerization. Importantly, cytoskeleton regulation is impaired at low levels of HSP27 expression.
Cell proliferation and apoptosis
HSP27 stimulates cell proliferation by increasing nuclear factor-κB (NF-κB) activity, which regulates cell survival, proliferation, and differentiation, and by inhibiting NF-κB–dependent apoptotic pathways.9–17 The former effects lie in the capacity of HSP27 to bind NF-κB inhibitor (IκBα) protein and specifically promote its proteasomal degradation, thus increasing NF-κB release. HSP27 promotes both ubiquitin-dependent (e.g., through the proteasome) and -independent degradation of unfolded proteins after cellular stress. Both HSP27 and HSP70 exhibit antiapoptotic properties, though partially through different pathways (for details, see Figure 2 of Garrido et al.9). They act on many apoptotic pathways upstream and downstream of the mitochondria, e.g., by inhibiting early stages of stress cell signaling, by reducing the production of reactive oxygen species, by inhibiting proapoptotic proteins, and/or by protecting key prosurvival proteins like kinases (e.g., extracellular signal-regulated protein kinase [ERK] and Akt), all of which result in inhibited release of proapoptotic signals from mitochondria.9–17 For example, iHSP27 can prevent mitochondrial release of cytochrome c by various mechanisms, including direct cytochrome c sequestration and inhibition of certain kinases (e.g., c-Jun N-terminal kinase) or caspases (e.g., procaspase-3 activity). Regarding antioxidant properties, both HSPs can decrease the production of reactive oxygen species by activating antioxidant enzymes (e.g., glutathione reductase, peroxidase).9–17 In summary, both iHSP27 and iHSP70 promote cell survival and resistance to stress through multiple pathways that have been elucidated in various cell types.
Regulation of immune responses and inflammation
Membrane-bound and extracellular heat-shock proteins.
In addition to intracellular HSPs, there is evidence of membrane-bound (mHSPs) and, especially, extracellular HSPs (eHSPs), the latter resulting from passive cell leakage (e.g., after cell necrosis) or active secretion (free HSPs or mHSPs in exosomes).18–20 Membrane-bound HSP70 has been demonstrated in various studies, especially in solid tumor cells.21 Membrane-bound HSP70 is most often associated with lipid rafts and interacts with specific, negatively charged lipids such as phosphatidylserine and sphingolipids.20,21 However, the mechanisms of HSP70 insertion into the cell membrane and the biological roles of mHSP70, possibly related to cell signaling function, are poorly understood.20 The roles of eHSPs have been addressed experimentally, and data collectively suggest their involvement in immune cell modulation, though information is limited for gut immune cells (see below).
Heat-shock proteins as danger signals.
Heat-shock proteins and major histocompatibility complex (MHC) molecules are the two systems involved in peptide antigen presentation.19,21,22 Extracellular HSPs are able to interact directly with antigen-presenting cells (e.g., dendritic cells, macrophages) through the activation of various receptors (e.g., toll-like receptors 2 and 4; CD91) to produce danger signals, thus leading to innate immune responses.19
Extracellular HSP27 has been shown to be anti-inflammatory, with responses depending on immune cell types.23 Extracellular HSP27 stimulates the secretion of anti-inflammatory cytokines (e.g., interleukin [IL] 10]) by monocytes and inhibits their differentiation into mature dendritic cells and macrophages. Extracellular HSP27 also inhibits neutrophil apoptosis, but without increasing the secretion of inflammatory cytokines (contrary to, e.g., LPS). In contrast, eHSP70, also called “chaperokine,” is considered proinflammatory because it stimulates proinflammatory gene expression in monocytes, macrophages, and neutrophils.23 Extracellular HSP70 also stimulates chemotaxis of dendritic cells and neutrophils, primes monocytes, macrophages, dendritic cells, and natural killer cells, and enhances natural killer cell activity and phagocytic activity by macrophages. Importantly, eHSP70 uptake seems to increase tolerance to stress and possibly displays paracrine and autocrine activities. Speculatively, low levels of eHSP70 may contribute to the downregulation of host inflammation, while higher levels would potentiate it.23
Antigen presentation and adaptive immunity.
Heat-shock proteins, including iHSP70, have a role in peptide binding and intracellular peptide trafficking upstream of antigen presentation. For example, in the classical MHC class I pathway, which operates, e.g., for viral antigens and tumor-derived antigens,21 HSPs are required for peptide antigen transport from the proteasome to MHC class I molecules before being presented by antigen-presenting cells to T lymphocytes. Therefore, HSPs appear to be key regulators of antigen cross-presentation and priming of various T-cell responses.19
Current knowledge about gut epithelial cells
Apoptosis, autophagy.
Inducible HSPs have been reported to be antiapoptotic to GECs, e.g., in ischemia/reperfusion injury and L-threonine supplementation.24,25 Inducible HSP involvement in autophagy has been documented in various non-GEC lines, but only one article reported that HSP70 and its transcription factor, heat-shock factor (HSF) 1, mediated autophagy in GECs.26
Inflammation.
Gut iHSPs both impact the proinflammatory NF-κB pathway and are modulated by cytokines. Inducible HSP–dependent inhibition of the NF-κB pathway has also been documented in GECs.27,28 The underlying mechanisms could involve activation of IκBα gene expression and inhibition of phosphorylation and degradation of IκBα protein. Conversely, HSP27 is implicated in NF-κB activation by reactive oxygen species.29
Regarding cytokines, IL-2 is a strong inducer of both HSP27 and HSP70, while IL-1β, IL-10, IL-11, and tumor necrosis factor-α (TNF-α) are moderate inducers of HSP25/27 in GEC lines (Table 1).30–32 By contrast, IL-4 and interferon-γ do not seem to modulate the expression of iHSPs.30–32 Importantly, the combination of inflammatory cytokines interferon-γ and TNF-α was shown to strongly inhibit both HSP25/27 and HSP70.2 HSP70 translational inhibition by these cytokines involved the recruitment of HSP70 to stress granules.33 These data support the hypothesis that alterations in intestinal function in inflammatory situations may partially result from iHSP inhibition.32,33
Table 1.
Influence of interleukins on inducible (iHSP25, iHSP72) and cognate (HSC73) heat-shock proteins (HSPs) in gut epithelial cell lines
| Interleukin | Concentration | GEC line | iHSP25/27a | iHSP72a | HSC73a | Reference |
|---|---|---|---|---|---|---|
| IL-1β | 50 ng/mL | YAMC | ++ | 0 | 0 | Kojima et al. (2003)30 |
| IL-2 | 0.01–50 ng/mL | YAMC | +++ | +++ | 0 | Kojima et al. (2003)30 |
| IL-4 | 50 ng/mL | YAMC | 0 | 0 | 0 | Kojima et al. (2003)30 |
| IL-10 | 50 ng/mL | YAMC | ++ | 0 | 0 | Kojima et al. (2003)30 |
| IL-11 | 0.1–100 ng/mL | Rat IEC-18 | ++ | 0 | 0 | Ropeleski et al. (2003)31 |
| IFN-γ | 100–200 U/mL | YAMC | 0 | 0 | 0 | Kojima et al. (2003)30; Hu et al. (2007)32 |
| TNF-α | 50–100 ng/mL | YAMC | ++ | 0 | 0 | Kojima et al. (2003)30; Hu et al. (2007)32 |
| IFN-γ + TNF-α | 200 U/mL, 100 ng/mL | YAMC | −−− | −−− | 0 | Hu et al. (2007)32 |
Abbreviations: IFN, interferon; IEC, intestinal epithelial cells; IL, interleukin; TNF, tumor necrosis factor; YAMC, nontransformed young adult mouse colonic cells.
aSymbols: +, stimulation (+++, strong; ++, moderate); -, inhibition (−−−, strong); 0, no effect.
Oxidative stress.
A major feature of gut epithelial iHSPs is their antioxidant properties, thus leading to enhanced gut epithelial protection against oxidative stress. This has been consistently demonstrated in various settings and GEC lines.27,31,34–44 However, this does not always translate to comparable results in vivo,45 possibly because of insufficient (though significant) increases in iHSP levels.
Gut barrier function
The integrity of gut barrier function is essential for health preservation throughout life.46,47 Inducible HSPs are prototypically induced by cells in response to heat shock. Heat stress has been demonstrated to increase intestinal tight junction permeability in Caco2 cells and in the proximal small intestine of mice.48,49 Heat stress also increases iHSP concentrations, and inhibition of iHSP response (e.g., using the polyphenol quercetin) results in further deterioration of intestinal permeability.48 The tight junction protein occludin was shown to be crucial in regulating gut permeability, and interactions between occludin and HSP70 were demonstrated.48,50 More precisely, an increase in occludin concentration after heat shock is mediated by HSF1 activation and binding to the occludin gene promoter.51 Even exogenous HSP70 added to cell cultures was able to prevent the heat stress–induced alteration in permeability.52 HSP70 was recently shown to be redistributed at the cytoskeleton level following Caco2 cell exposure to wheat gliadin, which induced cytoskeletal alterations, thus strengthening further the role of HSP70 in the maintenance of barrier function.53 Heat stress augmented the expression of HSP70 and occludin, thus probably limiting ileal permeability alterations in pigs.54 Conversely, decreased intestinal HSP70 and leaky intestine were reported in a model of necrotic enterocolitis in rats.55
In summary, iHSPs have many vital cellular functions. Expression of iHSPs is modulated by inflammatory and oxidative stress and is involved in major cellular physiological functions of GECs. Inducible HSPs also regulate gut barrier function, notably by controlling the expression of key tight junction proteins like occludin.
Regional, TISSUE, AND CELL DISTRIBUTION OF INDUCIBLE HEAT-SHOCK PROTEINS
Tanguay et al.56 first showed the presence of inducible HSP25 and HSP70 in epithelial cells along the GI tract of normal, nonstressed mice. Later, HSP70 protein was also reported in pig intestine.57 More detailed description of GI regional and tissue or cellular distribution was provided later. Inducible HSPs were reported to be either virtually absent or present at low levels in the small intestine, while they were strongly expressed in the colon of rodents.30,38,58 By contrast, pigs exhibited substantial iHSP concentrations along both the small and large intestines.45,59–63 An increasing proximal–distal gradient of iHSPs along the small intestine has been described.38,58 A proximal-distal gradient also exists along the large intestine of rodents, though with discrepancies between studies: decreasing vs increasing gradient.58,64 Interestingly, a gradient of iHSP proteins does not exist in the colon of germ-free mice.58 A higher relative concentration of iHSP proteins was recorded in the distal ileum than in the proximal colon in growing pigs, suggesting higher microbial stimulation in the former in this species.65 Data from humans are scant. One study reported immune detection of iHSP70 in the small intestine and the colon.66 Another study revealed that the ascending colon expressed more iHSP proteins than the descending colon.58
Tissue distribution of iHSP messenger RNA (mRNA) is essentially similar to and highly correlated with that of iHSP proteins in mice, thus indicating a strong transcriptional modulation of regional differences in iHSPs.38,58 However, correlations between iHSP mRNA and iHSP protein were not observed in pigs,45 suggesting alternate mechanisms of iHSP protein regulation.
Inducible HSP proteins are located almost exclusively in GECs, especially those in closer contact with the gut contents, with very little expression in the lamina propria.35,38,58,65 Thus, strong immunostaining of iHSP proteins is observed in villi of the small intestine and in upper-crypt epithelial cells in the colon, with virtually no staining along the crypts at either site. Of note, ileal Peyer’s patches stained strongly for iHSPs in the pig, but the precise identity of the iHSP-containing cells was not disclosed.65
In summary, iHSPs are expressed and are located in GECs in close contact with the lumen, and their tissue concentration essentially reflects the influence of the gut microbiota. Data on the expression of mHSPs and eHSPs in GECs are lacking. However, investigations with other cell types suggest anti- and proinflammatory roles for eHSP27 and eHSP70, respectively.
INDUCIBLE HEAT-SHOCK PROTEINS AND GUT- SPECIFIC MICROBIAL COMPONENTS AND METABOLITES
Microbial components
Deitch et al.67 were the first to report that Escherichia coli LPS stimulated HSP70 production in Caco2 cells. This was later confirmed by Kojima et al.36 in a murine GEC line (YAMC). However, in this latter work, it was iHSP25 (and not iHSP70 or HSC73) that was specifically induced (Table 2).27,33,35–38,41,58,68,69 Kojima et al.36 reported HSP25 induction to occur at very low doses of LPS (10 ng/mL; peak at 5 µg/mL) after 12 hours of treatment (peak at 24 hours). HSP25 mRNA increased from 6 hours onward and declined after 24 hours. However, the authors noted that the magnitude of HSP25 response was smaller than that observed after thermal stress.36 Both LPS and lipoteichoic acid were shown to induce the expression of colonic iHSP25 and iHSP70 in mice.68 Importantly, LPS added to drinking water at concentrations as low as 10 ng/mL and given to antibiotic-depleted mice was able to restore normal levels of colonic iHSPs and to rescue one-third of the animals from lethal inflammation.68 It was thus concluded that a certain level of gut stimulation by microbial products was necessary for conferring iHSP-mediated gut protection.
Table 2.
Influence of bacterial components and metabolites on inducible (iHSP25 and iHSP70) and cognate (HSC73) heat-shock proteins (HSPs) in gut epithelial cell lines or in vivo
| Bacterial component | Concentrationa | Model | iHSP25/27 | iHSP72 | HSC73 | Reference |
|---|---|---|---|---|---|---|
| LPS (origin unknown) | 1–10 µM | YAMC | Yes | Yes | No | Hu et al. (2010)33 |
| LPS (E. coli 055:B5) | 1 ng–10 µg/mL | YAMC | Yes | No | No | Kojima et al. (2004)36 |
| LPS (E. coli 026:B6) | 1 ng–10 µg/mL | Mouse | Yes | Yes | ND | Rakoff-Nahoum et al. (2004)68 |
| Flagellin Sb | 1–300 ng/mL | IEC-18, YAMC | Yes | ND | No | Petrof et al. (2008)41 |
| Flagellin Ec | ? | IEC-18, YAMC | No | ND | No | Petrof et al. (2008)41 |
| Toxin A (C. difficile) | 30 ng/mL | Caco2/bbe | ND | Yes | ND | Liu et al. (2003)69 |
| Secreted molecules | ||||||
| fMLP | 30–300 nM | Caco2/bbe | Yes | No | No | Carlson et al. (2007)27 |
| SEB | 0.1–100 µg/mL | MSIE | Yes | Yes | No | Musch et al. (2004)37 |
| Metabolites | ||||||
| n-Butyrate | 1–10 mM | IEC-18 | Yes | No | No | Ren et al. (2001)35 |
| n-Butyrate | 60 mM | RSITE | Yes | Yes | No | Arvans et al. (2005)38 |
| n-Butyrate | 1–10 mM | YAMC | Yes | Yes | No | Hu et al. (2010)58 |
| Isobutyrate | 5 mM | Rat IEC-18 | Yes | No | No | Ren et al. (2001)35 |
| Propionate | 5 mM | Rat IEC-18 | Yes | No | No | Ren et al. (2001)35 |
Abbreviations: Caco2/bbe, human colon carcinoma, brush-border-expressing cells; fMLP, formyl-methionyl-leucyl-phenylalanine; IEC, intestinal epithelial cells; LPS, lipopolysaccharide; MSIE, mouse small intestine epithelial cells; ND, not determined; RSITE, rat small intestinal tissue explants; SEB, Staphylococcus aureus enterotoxin B; YAMC, nontransformed young adult mouse colonic cells.
aConcentration in cell culture medium (in vitro), in drinking water (Rakoff-Nahoum et al.68), or in rat intestinal tissue explant culture (Arvans et al.38).
bFrom Salmonella enterica serovar Typhimurium.
cFrom nonpathogenic Escherichia coli F18 (dose not mentioned).
Other microbial components, such as the potent neutrophil chemoattractant formyl-methionyl-leucyl-phenylalanine (fMLP) tripeptide,27 enterotoxin B superantigen from Staphylococcus aureus,39 flagellin from Salmonella enterica serovar Typhimurium,43 and Clostridium difficile toxin A,69 are all inducers of iHSPs in various GEC lines (Table 2). Of note, the expression of HSP25 induced by fMLP at concentrations between 30 nM and 300 nM, but not at higher concentrations, suggests nonlinear responses of iHSPs to some microbial signals.27 Peptide formylation was crucial in the iHSP response, since nonformylated MLP had no effect. Blocking of fMLP transporter PepT1 also blocked HSP25 induction, demonstrating that fMLP must be internalized in order to induce iHSPs.27 Flagellin, also active at low doses (10–300 ng/mL) was shown to be a fast inducer of HSP25, starting at 6 hours and lasting for at least 48 hours.41
Microbial metabolites
Short-chain fatty acids result from microbial fermentation of undigested material in the distal gut lumen. Among these, n-butyrate, isobutyrate, and propionate were shown to induce expression of HSP25/27 and sometimes HSP70 in various rodent GEC lines and tissue explants (Table 2).35,38,58 The HSP response to butyrate (1–5 mM) was fast, starting at 6 hours, and reached a maximum after 12–24 hours.35 Butyrate-induced expression of iHSP was protective against oxidant stress.35 Importantly, Liu et al.65 observed in growing pigs that colonic iHSP was negatively correlated with propionic acid concentration and positively correlated with the percentage of isobutyrate in digesta. Surprisingly, however, they did not find correlations between iHSP and n-butyrate concentrations in this study.
Gut epithelial cell specificity in induction of heat-shock proteins by microbial products
Inducible HSP responses to microbial products often appeared specific for GECs, since, for instance, fibroblasts and macrophages did not respond to n-butyrate and E. coli LPS, respectively, by modulating iHSP expression.35,36 Thus, iHSP induction leading to subsequent protection against all kinds of stress appears to be a unique feature of the gut epithelium.
INDUCIBLE HEAT-SHOCK PROTEINS AND THE GUT MICROBIOTA
Gut microbiota
Ren et al.35 initially observed that a diet enriched with fermentable material (pectin), when compared with a nonfiber diet, stimulated expression of HSP25 (but not HSP70 or HSC73) protein in the distal ileum and the colon of rats only after 3 days of feeding these diets. They also suggested that regional differences in iHSP expression along the gut might reflect differential microbial activities and local concentrations of small-chain fatty acids. Anaerobic bacteria (e.g., Bacteroides fragilis) and the gut microbiota in general were then reported to have important roles in inducing the expression of iHSPs.30,68 The cause-and-effect relationship between iHSP and the microbiota in rodents was elegantly demonstrated by Arvans et al.38 for the small intestine and by Hu et al.58 for the large intestine. Briefly, Arvans et al.38 made self-emptying vs self-filling blind intestinal loops and found that both iHSP25 and iHSP70 were stimulated in self-filling loops that all favored microbial overgrowth. Hu et al.58 reported no differences in iHSPs between germ-free and specific-pathogen-free mice in the jejunum or the distal colon, but 2-fold higher iHSP protein concentrations in the proximal colon of specific-pathogen-free mice. In addition, lysates from the cecum and the proximal colon were able to upregulate expression of the iHsp gene and iHSP in cultured GEC lines and in the distal colon mucosa of specific-pathogen-free mice.58 Finally, the herbal medicine Juzentaihoto (Shi-Quan-Da-Bu-Tang in Chinese) was clearly shown to require the gut microbiota for stimulating intestinal HSP70 mRNA expression in mice, as no response occurred in germ-free animals.70
Many gram-positive bacteria present in the human gut microbiota, such as Bifidobacterium breve, Lactobacillus paracasei, and Lactobacillus plantarum (but not Enterococcus faecalis), could induce expression of iHSP27 in Caco2 brush-border-expressing (Caco2-bbe) cells (Table 3).30,39,40,43,44,71,72 A synthetic pentapeptide, also known as competent and sporulating factor from Bacillus subtilis, was shown to induce expression of HSP27 and protect gut epithelial cell (GEC) lines from oxidant injury.42,72,73 Among gram-negative bacteria, E. coli can induce expression of gut epithelial iHSP, but some other bacteria, such as Enterobacter aerogenes, E. faecalis, and Proteus mirabilis, cannot.42 Lactobacillus johnsonii may be involved in increased intestinal iHSP70 response to Juzentaihoto in mice.70
Table 3.
Influence of probiotic or bacteria (live, dead) or cell culture supernatant on induction of gut inducible heat-shock proteins (iHSPs) in vitro, ex vivo, or in vivo
| Bacteria or probiotic | Cell line or model | Bacteria or probiotic |
Reference | ||
|---|---|---|---|---|---|
| Live | Dead (heat-killed) | Cell culture supernatant | |||
| In vitro | |||||
| Bacteroides fragilis | YAMC | iHSP25, iHSP72 | ND | iHSP25, iHSP72 | Kojima et al. (2003)30 |
| Escherichia coli DH5α (attenuated) | YAMC | 0 | ND | 0 | Kojima et al. (2003)30 |
| Lactobacillus rhamnosus GG | YAMC | iHSP25, iHSP72 | ND | iHSP25, iHSP72 | Tao et al. (2006)39 |
| Bacillus subtilis JH642 | Caco2/bbe | ND | ND | iHSP27 | Fujiya et al. (2007)40 |
| Lactobacillus rhamnosus GG | Caco2/bbe | ND | ND | iHSP27 | Fujiya et al. (2007)40 |
| Lactobacillus plantarum | Caco2/bbe | ND | ND | iHSP27 | Fujiya et al. (2007)40 |
| Bifidobacterium breve | Caco2/bbe | ND | ND | iHSP27 | Fujiya et al. (2007)40 |
| Enterococcus faecalis | Caco2/bbe | ND | ND | ∼ 0 | Fujiya et al. (2007)40 |
| Escherichia coli Nissle | Caco2/bbe | ND | ND | 0 | Fujiya et al. (2007)40 |
| Enterobacter aerogenes | Caco2/bbe | ND | ND | 0 | Fujiya et al. (2007)40 |
| Proteus mirabilis | Caco2/bbe | ND | ND | 0 | Fujiya et al. (2007)40 |
| Lactobacillus brevis SBC8803 (0.01%) | Caco2/bbe | ND | iHSP25 | iHSP25 | Ueno et al. (2011)43 |
| Lactobacillus brevis SBC8803 (0.1%) | Caco2/bbe | ND | iHSP25 | iHSP25 | Ueno et al. (2011)43 |
| Lactobacillus brevis SBC8803 (1%) | Caco2/bbe | ND | 0 | ND | Ueno et al. (2011)43 |
| Lactobacillus brevis SBC8013 | Caco2/bbe | ND | 0 | ND | Ueno et al. (2011)43 |
| Streptococcus faecalis | Caco2/bbe | ND | iHSP25 | ND | Ueno et al. (2011)43 |
| Lactobacillus plantarum 2142 | IPEC-J2 | ND | ND | Hsp70 mRNA | Paszti-Gere et al. (2012)44 |
| Lactobacillus paracasei | Caco2/IL-1β | iHSP27, iHSP70 | ND | 0 | Reilly et al. (2007)71 |
| Ex vivo | |||||
| Lactobacillus brevis SBC8803 | Mouse intestine | ND | iHSP27, iHSP70 | ND | Ueno et al. (2011)43 |
| Streptococcus faecalis | Mouse intestine | ND | iHSP27, iHSP70 | ND | Ueno et al. (2011)43 |
| In vivo | |||||
| Lactobacillus brevis SBC8803 | C57Bl/6 mice + ethanol | ND | Hsp25 mRNA | ND | Segawa et al. (2008)72 |
Abbreviations: Caco2/bbe, human colon carcinoma, brush border-expressing cells; IL-1β, interleukin 1β; IPEC-J2, swine nontransformed jejunal epithelial cells; ND, not determined; YAMC, nontransformed young adult mouse colonic cells.
Further links between the gut commensal microbiota and iHSPs in vivo were recently demonstrated in growing pigs.65 Ileal HSP27 was found to correlate negatively with particular luminal bacteria (Lactobacillus reuteri and Enterobacteriaceae) (Table 4).65 Ileal iHSP correlated positively with lactic acid–producing bacteria and L. johnsonii. Ileal iHSP25 and colonic iHSP70 correlated negatively with the diversity of the mucosa-associated microbiota and with the mucosa-associated, butyrate-producing Roseburia faecis. Finally, colonic iHSP70 correlated negatively with the Bacteroidetes Prevotella brevis but positively with Faecalibacterium prausnitzii, otherwise known for its anti-inflammatory properties and for being depleted in inflammatory bowel disease.74,75
Table 4.
Correlations between gut inducible heat-shock proteins (iHSPs) and luminal bacterial metabolites or luminal or mucosa-associated microbiota in pigsa,b
| Distal ileum |
Proximal colon |
||||
|---|---|---|---|---|---|
| iHSP27 | iHSP70 | iHSP27 | iHSP70 | ||
| Luminal bacterial metabolites or microbiota | |||||
| Propionic acid concentration | r < 0 | ||||
| Isobutyric acid percentage | r > 0 | ||||
| Enterobacteriaceae | r < 0 | ||||
| Lactic acid–producing bacteria | r > 0 | ||||
| Lactobacillus reuteri | r < 0 | ||||
| Lactobacillus johnsonii | r > 0 | ||||
| Prevotella brevis | r < 0 | ||||
| Mucosa-associated microbiota | |||||
| Microbiota diversity index | r < 0# | ||||
| Megasphaera elsdenii | r > 0 | ||||
| Roseburia faecis | r < 0 | r < 0 | |||
| Faecalibacterium prausnitzii | r > 0 | ||||
aAdapted from Liu et al.65
bCorrelation coefficients: r > 0, positive correlation (P < 0.05 or more); r < 0, negative correlation (P < 0.05 or more); #tendency (P = 0.065).
Probiotics
The protective action of probiotics on the GI tract may be mediated by different mechanisms, including iHSP induction in human and rodent GEC lines. This has been clearly demonstrated for a variety of lactobacilli and bifidobacteria (Table 3 and Table 5 76–79). This was also recently reported in a porcine intestinal cell line (IPEC-J2) for two probiotics (L. johnsonii strain P47-HY and L. reuteri strain P43-HUV).80 Such protective actions could be mediated through bacterial cell wall components or secreted molecules or metabolites. Various probiotics (e.g., B. subtilis JH642, Lactobacillus rhamnosus GG) were able to induce expression of iHSP in vitro, while others (E. coli Nissle) were not.40 Other studies have revealed the importance of secreted peptides of various lengths (Table 6).27,35–37,39–42,68 The probiotic mixture VSL#3, which contains various lactobacilli and bifidobacteria, was also shown to convey intestinal protection by inducing the expression of iHSP25 and iHSP70.81 Recently, Lactobacillus brevis SBC8803 was reported to operate through the release of polyphosphate chains that, when provided in a synthetic form, also conferred protection through iHSP induction both in vitro and in mice.42 Thus, induction of iHSPs may be one component in the protective action of probiotics.
Table 5.
Influence of probiotics on inducible (iHSP25, iHSP72) heat-shock proteins, inhibition of pathogens, suppression of interleukin-8 secretion, and protection in human gut epithelial cell lines
| Probiotic species/strain | iHSP25/27 | iHSP70a | Inhibition of pathogensb | Suppression of IL-8 secretionc | Protection in GEC linesd | Reference |
|---|---|---|---|---|---|---|
| Effects with the probiotic | ||||||
| Lactobacillus curvatus 2775 | ND | ++ | ND | Yes | ND | Nemeth et al. (2006)76 |
| Lactobacillus plantarum 2142 | ND | ++ | ND | Yes | ND | Nemeth et al. (2006)76 |
| Lactobacillus casei 2756 | ND | ++ | ND | Yes | ND | Nemeth et al. (2006)76 |
| Bifidobacterium infantis W52 | ND | +++ | Yes | Yes | Partial | Koninkx et al. (2010)77; Malago et al. (2010)78 |
| Lactobacillus casei W56 | ND | +++ | Yes | Yes | Partial | Koninkx et al. (2010)77; Malago et al. (2010)78 |
| Lactobacillus lactis W58 | ND | +++ | Yes | Yes | Partial | Koninkx et al. (2010)77; Malago et al. (2010)78 |
| Lactobacillus acidophilus W70 | ND | + | No | No | Partial | Koninkx et al. (2010)77; Malago et al. (2010)78 |
| Bifidobacterium bifidum W23e | ND | + | No | No | Partial | Koninkx et al. (2010)77; Malago et al. (2010)78 |
| Lactobacillus salivarius W24 | ND | + | No | No | Partial | Koninkx et al. (2010)77; Malago et al. (2010)78 |
| Lactobacillus casei Shirota | ND | 0 | 0 | 0 | 0 | Malago et al. (2010)79 |
| Lactobacillus plantarum 299v | ND | 0 | 0 | 0 | 0 | Malago et al. (2010)79 |
| Effects with probiotic culture supernatant | ||||||
| Lactobacillus casei 2756 | ND | ++ | Yes | Yes | ND | Nemeth et al. (2006)76 |
| Lactobacillus curvatus 2775 | ND | ++ | Yes | Yes | ND | Nemeth et al. (2006)76 |
| Lactobacillus plantarum 2142 | ND | ++ | Yes | Yes | ND | Nemeth et al. (2006)76 |
| Lactobacillus casei Shirota | ND | ++ | ND | Yes | ND | Malago et al. (2010)78 |
| Lactobacillus plantarum 299v | ND | ++ | ND | Yes | ND | Malago et al. (2010)78 |
Abbreviations: GEC, gut epithelial cells; ND, not determined.
aMeasured in Caco2 cells. +++ (strong) = 2- to 3-fold basal HSP70; ++ (moderate) = 1.5- to 2.5-fold basal HSP70; + (weak) = 1.5-fold basal HSP70; 0 = no change.
bMeasurement of inhibition of Salmonella enteritidis 857 growth.
cMeasurement of inhibition of Salmonella enterica–induced Caco2 IL-8 production.65–67
dMeasurement of transepithelial electrical resistance of Caco2 cells infected with Salmonella enterica serovar Enteritidis 857.68
Table 6.
Molecular sensors and intracellular signaling pathways involved in the induction of gut heat-shock proteins (iHSPs) by bacterial components, metabolites, or secreted molecules in gut epithelial cells in culture or in vivo
| GEC sensor | Bacterial trigger | Produced iHSP | Intracellular signaling pathway | Reference |
|---|---|---|---|---|
| Bacterial components | ||||
| TLR2 | LTA | iHSP25, iHSP70 | Unidentified | Rakoff-Nahoum et al. (2004)68 |
| TLR4 | LPS | iHSP25, iHSP70 | Unidentified | Rakoff-Nahoum et al. (2004)68 |
| TLR4 | LPS | iHSP25 | p38 and ERK1/2 | Kojima et al. (2004)36 |
| TLR5 | Flagellin Sa | iHSP25 | p38 | Petrof et al. (2008)41 |
| Bacterial metabolites | ||||
| Unidentified | n-Butyrate | iHSP25 | Unidentified | Ren et al. (2001)35 |
| Unidentified | Isobutyrate | iHSP25 | Unidentified | Ren et al. (2001)35 |
| Unidentified | Proprionate | iHSP25 | Unidentified | Ren et al. (2001)35 |
| Bacterially secreted molecules | ||||
| Unidentified | SEB | iHSP25, iHSP70 | ERK1/2 | Musch et al. (2004)37 |
| Unidentified | Peptides <10 kDa | iHSP25 | p38 and JNK | Tao et al. (2006)39 |
| PepT1 | fMLP peptide | iHSP25 | p38 | Carlson et al. (2007)27 |
| OCTN2 | ERGMT peptide | iHSP25 | p38 | Fujiya et al. (2007)40 |
| Integrin-β | Polyphosphate | iHSP25 | p38 | Segawa et al. (2011)42 |
Abbreviations: ERGMT, glutamyl-arginyl-glycyl-methionyl-threonine (secreted by Bacillus subtilis or synthesized); fMLP, formyl-methionyl-leucyl-phenylalanine (common to many Gram-negative bacteria); ERK, extracellular signal-regulated protein kinase; IEC, intestinal epithelial cells; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; LTA, lipoteichoic acid; OCTN2, organic cation transporter number 2; PepT1, peptide transporter 1; SEB, Staphylococcus aureus enterotoxin B; TLR, toll-like receptor; p38, protein 38 mitogen-activated protein kinase.
aFrom Salmonella enterica serovar Typhimurium.
Antibiotics
As described above, a variety of gut microbes and bacterial components are strong inducers of gut epithelial iHSPs. Conversely, some studies in vivo have disclosed that some, but not all, antibiotics can disturb epithelial iHSP induction and subsequent gut protection. For instance, Kojima et al.30 reported that metronidazole, an antibiotic targeting anaerobic bacteria, decreased both iHSP25 and iHSP70 concentrations in rat colonic mucosa and increased the permeability and susceptibility of colonic mucosa to C. difficile toxin A. Ciprofloxacin, a broad-spectrum antibiotic that notably targets gram-negative aerobic bacteria, displayed variable and inconsistent effects on colonic iHSP25 and had no effect on iHSP70 in rats.30 The combination of ampicillin, metronidazole, neomycin, and vancomycin, used experimentally to deplete the gut microbiota, reduced the expression of gut epithelial iHSP, thus resulting in higher mortality of chemically induced colitis in mice.68 In contrast, binary antibiotic associations of metronidazole and vancomycin or neomycin had no effects on iHSP.68
Contrasting effects were reported in preterm pigs: oral and systemic administration of three antibiotics (ampicillin, gentamicin, and metronidazole) for 5 days decreased HSP27 but increased iHSP70 in the small intestine.82 The latter effect may have resulted from the gentamicin-specific inhibition of HSP70’s chaperoning activity, thus promoting a positive feedback response.83,84 In normal pigs, oral administration of the broad-spectrum antibiotic amoxicillin to mothers around parturition was found to affect the mother’s fecal and the offspring’s gut microbiota as well as the offspring’s gut epithelial iHSPs: small intestinal iHSP27 remained unaffected, while iHSP70 decreased transiently, and both iHSP proteins increased in colonic mucosa.45,63 Data were interpreted as being essentially related to amoxicillin-induced changes in maternal microbiota and subsequent microbiota transfer to offspring after birth.
Mechanisms of inducible heat-shock protein induction by microbial products
Microbial molecules that stimulate expression of iHSPs by GEC lines use different signaling pathways (Table 6). These include specific membrane proteins or receptor complexes (e.g., integrin-β; toll-like receptors 2 and 4) as well as peptide transporters (e.g., the organic cation/carnitine transporter 2; peptide transporter 1).27,36,40,41,68 The intracellular signaling pathways most often activated are p38 mitogen-activated protein (MAP) kinases, alone or in association with other kinases (e.g., ERK1/2; c-Jun N-terminal kinase). Additional pathways, such as those involving reactive oxygen species, have been documented as well.29 In all cases, the final effect is the downregulation of the proinflammatory NF-κB pathway and the repression of proinflammatory cytokine gene activation and production. Of note, the protective effects of the probiotic mixture VSL#3 involved another mechanism, proteasome inhibition, thus leading to reduced degradation of IκBβ and p105 proteins and subsequently increased repression of NF-κB.81 Conversely, proteasome inhibitors are inducers of iHSP expression in GECs.85
Collectively, these data underline the complex relationships between iHSPs and the gut luminal or mucosa-associated bacteria or probiotics. They demonstrate the causal role of bacterial cell wall components and various secreted molecules in keeping tissue iHSP concentrations optimal for gut protection. Some of the underlying mechanisms have been disclosed. Finally, some antibiotics may contribute to the disruption of iHSP profiles and a reduction in gut epithelial resistance to stress.
INDUCIBLE HEAT-SHOCK PROTEINS AND GASTROINTESTINAL DISEASES
Intrauterine growth restriction and necrotizing enterocolitis
Necrotizing enterocolitis is a major cause of death in premature humans, and intrauterine growth restriction is a risk factor for necrotizing enterocolitis.86,87
Recent data indicate that expression of gut epithelial iHSPs is disturbed in intrauterine growth restriction and necrotizing enterocolitis. Messenger RNA and protein levels of iHSP70 were reported to be higher in the duodenum and jejunum (but not the colon) of intrauterine growth–restricted pig neonates, thus underlining the stressful nature of intrauterine growth restriction.88 This was associated with reduced tissue concentrations of NF-κB p65 protein and inhibition of the NF-κB developmental pathway, with detrimental effects on the development of immune function in intrauterine growth–restricted piglets.89
In a rat model of necrotizing enterocolitis induced by maternal separation and milk formula feeding, the expression of both ileal iHSP70 protein and gene was reduced.55 Mother’s milk was hypothesized to induce physiological levels of iHSP70 in normal rat pups, though precise mechanisms were not disclosed.55 Another group confirmed reduced intestinal iHSP70 in both human and mouse necrotizing enterocolitis.90 Of note, heat preconditioning of rat pups increased ileal iHSP, inhibited NF-κB activation, and reduced necrotizing enterocolitis–induced tissue alterations.91 This clearly demonstrates the contribution of iHSP in preventing necrotizing enterocolitis.
Inflammatory bowel disease and celiac disease
In humans, two genes, namely HSP70-1 and HSP70-2, encode the same protein, iHSP70.92 Importantly, various allelic polymorphisms in these genes have been related to inflammatory bowel disease.
Crohn’s disease.
Available genetic studies on possible links between HSP70-2 polymorphism and Crohn’s disease indicate that A or AA genotypes are associated with less severe, and BB genotype with more severe, forms of Crohn’s disease.93–96
Ulcerative colitis.
The AA genotype of the HSP70-2 gene was found to be associated with severe ulcerative colitis, while the BB genotype was associated with a lower risk of steroid-dependent and refractory types of colitis.96,97
Functionally, iHSP70 seems to be inhibited in active ulcerative colitis. Two studies concluded iHSP70 to be lower in active than in inactive ulcerative colitis, thus leading to possibly enhanced vulnerability of the former to inflammation.32,98 Furthermore, iHSP70 mRNA levels in active and inactive tissues were not different, suggesting post-transcriptional differences in iHSP70 protein handling.32 The 3′ untranslated region (3′-UTR)-dependent translational inhibition of HSP70 mRNA was then demonstrated in ulcerative colitis.99 However, one study reported tissue iHSP70 protein concentrations to be higher at the time of disease diagnosis and to decrease after treatment.100
Animal models of gut inflammation.
Chemically induced colitis (e.g., that induced by dextran sulfate sodium) is often used as a model for inflammatory bowel disease. One study demonstrated that colitis was more severe in hsf1 or hsp70 knockout mice and less severe in mice overexpressing the hsf1 or hsp70 gene.101 The protective role of iHSP70 was also observed after deletion of the macrophage migration inhibitory factor (MIF) gene in mice, thus documenting a suppressive effect of this factor on HSP70.102 Therefore, both HSF1 and iHSP70 play a protective role against colitis.
Celiac disease.
Celiac disease is an inflammatory enteropathy caused by gluten from wheat and other cereals in genetically predisposed individuals.103 A link between the HSP70-2 gene and celiac disease was first reported in 1993.104 Then, the C allele of the HSP70-1 gene was reported to be higher in Spanish celiac patients and was therefore considered an additional factor in celiac disease susceptibility.105 The C allele of the HSP70-1 gene was also higher in DRB1*03-negative patients and in DQB1*02-negative and DRB1*03-negative celiac patients. Elevated HSP70 mRNA and iHSP70 levels were observed in the duodenal mucosa of both untreated and treated children with celiac disease.106 Importantly, evidence of iHSP70 involvement and junctional redistribution in alterations induced by wheat gliadin in a Caco2 model for celiac disease has been reported recently.53 However, a 3-day challenge with wheat gluten in celiac and nonceliac patients was insufficient to demonstrate causal modulation of HSP70 gene expression or protein concentrations in duodenal biopsies.107
Other diseases
Intestinal ischemia/reperfusion injury.
Intestinal ischemia/reperfusion injury is a common, potentially fatal condition with a complex etiology and pathogenesis.108,109
Many earlier data have shown that preinduction of iHSPs confers protection against ischemia/reperfusion injury to the intestine.110–114 Induction of iHSP through a first ischemia/reperfusion episode was also found to protect partially against a second ischemic episode in a pig model.115 Protection by iHSP70 may involve decreased apoptosis through increased Bcl-2 expression, thus leading to the inhibition of mitochondrial release of cytochrome c.24
Alcohol abuse.
Alcoholic abuse initiates or increases susceptibility to many related diseases and is therefore a major public health problem worldwide.116 The gut plays a role in alcohol-related diseases, both directly, through alcohol absorption and alteration of the intestinal barrier, and indirectly, through the microbiota-mediated transformation of alcohol into toxic compounds (e.g., acetaldehyde) and alcohol-induced dysbiosis.116,117
Alcohol stimulated iHSP25 at concentrations between 10 and 40 g/L and iHSP70 at 10 g/L, with a loss of iHSP responses at higher concentrations (80 g/L) in Caco2 cells.118 Importantly, another study reported that alcohol (60 g/L) moderately stimulated the expression of HSF1 and iHSP70, but this was not protective.119 Finally, a dose-dependent decrease in iHSP70 along the small intestine, with the strongest effect associated with major ileal tissue injury, was reported in a rat model of intraperitoneal alcohol administration (0–8.25 g/kg bodyweight).120
Collectively, the available literature indicates reduced iHSP70 levels in patients with active ulcerative colitis, a finding supported by animal models. Genetic links between the HSP70-1 and HSP70-2 genes and chronic gut inflammatory diseases are established in humans, but underlying functional mechanisms are not yet fully understood, especially in celiac disease. Intestinal ischemia/reperfusion injury can be prevented by preinduction of iHSPs. Alcohol may induce the expression of gut epithelial iHSPs at low doses but inhibit it at higher doses.
DIETARY MODULATION OF INDUCIBLE HEAT-SHOCK PROTEINS IN THE GUT
Food intake
The pioneering work by Ehrenfried et al.121 revealed that chronic calorie restriction (−40%, compared with ad libitum intake) increased Hsp70 mRNA levels in the duodenal (but not ileal) mucosa of adult rats. The fasting-to-refeeding transition also seemed to stimulate iHSP70 concentrations in the small intestine, with no change in the colon of mice.122
A more systematic investigation was conducted in growing pigs submitted to feeding or fasting (1.5 days) or fasting (1.5 days) and refeeding (2.5 days).62 Fasting generated an increase in iHSP27 (but not iHSP70) along the small and large intestines. Conversely, refeeding essentially restored intestinal and colonic concentrations of iHSP27, still with no influence on iHSP70.62 Thus, besides interspecies variability, the availability of nutrients and specific food components (e.g., fiber) is a strong driver of iHSP variations along the intestines.
Dietary L-glutamine, ornithine, and polyamines
The vast majority of studies conducted both in vitro120,123–126 and in vivo127–131 indicate that L-glutamine is a strong inducer of iHSP25 and iHSP70 expressions in GECs in various stressful situations. This induction takes places in vitro at L-glutamine concentrations above 0.5 mM, with a plateau of iHSP response at 1–10 mM.126 The iHSP response to L-glutamine (8 mM) is rapid (i.e., 2 hours and 24 hours for Hsp70 mRNA and protein, respectively) and of a large magnitude (3- to 4-fold).121 Importantly, data on the role of L-glutamine in GEC autophagy are controversial: some support an inhibitory role through iHSP70 induction, and others support stimulation of autophagy through iHSP70 induction.26,132
L-Glutamine–mediated induction of iHSP expression involves polyamines, which do not modulate Hsf1 gene expression or HSF1 production directly but increase the binding between HSF1 and heat-shock element on Hsp genes.133 Putrescine (100 µM) and spermidine (50 µM), as well as their precursor ornithine (50–100 µM), were found to upregulate both iHSPs, while spermine (200 µM) elicited an iHSP25 response only.133 Consistent with this, a low spermine concentration (5 µM) was unable to induce iHSP70 expression in IEC-6 cells.134
In terms of mechanisms, L-glutamine stimulates Hsf1 mRNA and protein and Hsf1 gene promoter activity in GECs and the colonic epithelium.135 L-Glutamine downregulates the proinflammatory NF-κB pathway by reducing protein p65 nuclear translocation and cell apoptosis via the activation of phosphatidylinositol-3 kinase and MAP kinase and the reduction of cleaved caspase-3 activity.133,136
L-Glutamine protects against thermal stress by preventing heat-induced fibronectin degradation and by activating protective fibronectin-integrin signaling.137,138 Other mechanisms include dephosphorylation of p38 MAP kinase, activation of ERK1/2,137,138 and expression of the epithelial growth factor receptor pathway.139
L-Glutamine stimulated iHSP70 in an HSF1-dependent manner within 5 hours and protected intestinal barrier function in ethanol and heat-stress models of altered intestinal permeability.50,119 L-Glutamine also protected against diarrhea in rodents and pigs, which was related to an iHSP-dependent mechanism.129,131
Other amino acids and metabolic intermediates
L-Glutamate.
This dicarboxylic amino acid, reputed to be poorly absorbed by GECs, was actually shown to induce iHSP25 expression in rat IEC-18 cells.126 However, its potential to stimulate iHSP70 expression was much less than that of L-glutamine.126,140 A related product, N-carbamyl-glutamate (0.08%, in the diet), was also found to stimulate expression of iHSP70 in the intestine of pigs.140
L-Arginine.
This basic amino acid was first reported to have no effect on iHSP expression by IEC-18 cells.126 However, later investigations with another line (Caco2) revealed that L-arginine deficiency reduced, while L-arginine supplementation restored, physiological levels of iHSP70 by stimulating HSF4A and reducing oxidative stress.141 In vivo, L-arginine (0.6% in the diet for a week) increased Hsp70 mRNA and protein in the ileal mucosa of pigs.140
L-Threonine.
Recently, L-threonine was found to stimulate expression of iHSP25 and iHSP70, which led to decreased heat-induced apoptosis in GECs.25 A similar but smaller effect was also seen with the L-threonine nonmetabolizable analog α-amino isobutyric acid, but the induced iHSP response was not enough to confer protection.25 Effects of L-threonine and its analog may involve changes in cell osmolality.
Metabolic intermediates of amino acids.
Citrulline was able to restore iHSP70 concentrations in L-arginine–deficient Caco2 cells.141 A 1% α-ketoglutarate supplementation given to pigs for 10–16 days was able to prevent an LPS-induced increase in iHSP70 in the jejunum, with no significant effect on the ileum.142
Other amino acids.
Many other amino acids, including L-asparagine (an analog of L-glutamine), L-histidine, L-leucine, L-proline, and L-tyrosine, were tested in their free form for their ability to modulate iHSPs in GECs, but none of them was found to be active.126
Collectively, these data support the beneficial effect of L-glutamine supplementation in various physiological and stressful situations. The underlying molecular mechanisms have been essentially deciphered. Some other L-amino acids, precursors as well as derivatives, were also found to be active, though usually less so than L-glutamine. Finally, various amino acids seem to be inactive on gut epithelial iHSPs.
Proteins and peptides
Besides the effects of free L-amino acids on gut epithelial iHSPs, modulation of iHSPs by dietary proteins and peptides has been reported by several publications.
Animal products.
Rat milk may be responsible for the induction of intestinal iHSP70 in rat pup, though mechanisms were not disclosed.55 Wu et al.13 suggested that milk L-glutamine could be responsible for this effect. Conversely, a peptide mixture (n = 25 peptides; molecular weight, 1–5 kDa) from buffalo cheese acid whey was reported to decrease iHSP70 concentrations in Caco2 cells, making them more sensitive to oxidative stress.143 The bioactive peptides were identified as belonging to the opioid family. N-ε-carboxymethyl-lysine is a toxic product of Maillard reaction that appears on lysine residues of proteins and glucose under heat treatment. In its free form, it did not modulate iHSPs, but it increased (2.5-fold) iHSP27 expression when linked to casein in Caco2 cells.144 As a possible treatment for inflammatory bowel disease, a peptide extracted from gastric antrum was shown to strengthen the epithelial barrier by stabilizing tight junctions through iHSP25 expression in Caco2 cells.145 Feeding rats a diet supplemented with red meat heme (0.5 mM/kg diet), known as a risk factor for colorectal cancer, led to higher colonic mRNA (5-fold) and protein (3-fold) levels of iHSP25, with no effect on iHSP70 levels.146 However, despite reduced tissue inflammation, colitis did not improve. Finally, neither human colostrum proteins nor β-alanyl-histidine dipeptide (L-carnosine) influenced iHSP70 in IEC-6 cells or in rats submitted to acetic acid–induced colonic mucosa injury, respectively.134,147
Plant products.
Kidney bean (Phaseolus vulgaris) phytohemagglutinin and germ agglutinin from wheat grain (Triticum aestivum) are known as toxic compounds. They have been shown to decrease soluble iHSP70 in both Caco2 cells and intestinal tissues in rats.148 These data were interpreted as evidence of cytoplasmic iHSP70 reduction due to iHSP70 rescue migration to cell membranes. Exposure to gliadin, a wheat protein toxic to celiac patients, was recently demonstrated to induce iHSP70 redistribution to the cytoskeleton following junctional protein (ezrin and E-cadherin) disruption in Caco2 cells.53 In pigs, a diet supplemented with a melon (Cucumis melo LC.) pulp concentrate rich in the antioxidant enzyme superoxide dismutase was shown to decrease jejunal iHSP27 and colonic iHSP70 in weaned pigs, probably as a result of reduced oxidative stress.149 The protein Cry1Ab from Bacillus thuringiensis, found in transgenic corn (Zea mays) made resistant to insects, had no effect on cell viability but increased iHSP70 concentrations in the porcine intestinal IPEC-J2 cell line.150
In summary, various dietary proteins or peptides are able to modulate gut epithelial iHSPs. However, the underlying mechanisms of action and the consequences for GECs are not always documented.
Dietary fiber
Dietary pectin (6%), compared with no fiber or with nonfermentable fiber such as cellulose, increased iHSP25 and iHSP70 in the distal ileum and colon of rats.35,38 Chicory (Chichorium intybus L.) pectin also stimulated ileal and colonic iHSP27 in growing pigs.151 Ileal iHSP27 protein concentration was positively correlated with fiber intake.14 Importantly, chicory pectin is highly fermented in pig ileum and favors the growth of lactobacilli in the ileum and Clostridium species in the colon.151
A galacto-oligosaccharide/inulin mixture provided to the mother during gestation and lactation and to the offspring until weaning was shown to increase jejunal iHSP25 (but not iHSP70) protein in rat offspring.152 The underlying mechanisms of prebiotic action were not reported in this study.
Some polysaccharides may be detrimental to the gut. Highly sulfated saccharide carrageenans are used as thickening agents in foods but are also known for their proinflammatory properties. Carrageenans added to cultured GECs (1 µg/mL) had no effect on iHSP induction per se but were able to reduce the phosphorylation of iHSP27 and various kinases, thus leading to reactive oxygen species–mediated inflammation.153 This was also observed with the polysaccharide dextran sulfate sodium, often used experimentally to induce gut inflammation.154
In summary, fermentable fiber components stimulate the gut response to iHSPs, especially iHSP25, through microbial fermentation products. However, some proinflammatory saccharides can dephosphorylate iHSPs and promote oxidative stress and reduce gut cytoprotection.
Zinc
An organic form of zinc, polaprezinc ([N-(3-aminopropionyl-)-L-histidinato-zinc] used at 10–100 µM was demonstrated to stimulate iHSP25 and, more strongly, iHSP70 dose dependently and to protect Caco2 cells against oxidative stress in vitro.155
Zinc-L-carnosine complex was found to increase iHSP70, to suppress NF-κB activation, and, finally, to protect rats submitted to chemically induced (acetic acid) colonic mucosa injury.147 This was due to the zinc itself, potentiated by the presence of L-carnosine, because zinc sulfate, on a molar basis, displayed a lower effect, while L-carnosine alone had no effect at all.147
Zinc-mediated protection through iHSP induction was also observed in the colon of mice submitted to dextran sulfate sodium–induced colitis and supplemented (or not) with polaprezinc (15 mg/kg body weight) or zinc sulfate (7.5 mg/kg) for 7 days.156 In another study, zinc sulfate (25 mM, in water for 7 days) protected mice against TNF-induced lethal inflammation through an iHSP70-dependent mechanism.157 Zinc oxide (0.05–1 mM) can upregulate various heat-stress genes, including the Hsp70 gene in swine IPEC-J2 cells.158,159 However, high supplementation with zinc (as zinc oxide, 2200 mg of zinc per kilogram of diet for 1 week), despite its anti-inflammatory effects, did not influence gut epithelial iHSPs in pigs.160
These data suggest that zinc may have protective effects on the gut that are underpinned, at least partially, by iHSPs.
Exogenous n-butyrate
One study in veal calves reported that milk replacer supplementation with n-butyrate (3 g/kg milk powder), compared with flavomycin treatment, stimulated iHSPs in the colon but had no effects in the small intestine.161 However, it is not clear if the difference was due to the stimulatory effect of n-butyrate or the possible inhibitory effect of flavomycin on iHSPs, as there was no control group without these supplements. In pigs, many experiments with supplemental n-butyrate or its precursor tributyrin have been carried out, but none reported data on gut epithelial iHSPs.
Flavonoids
The anthocyanin cyanidin-3-O-β glucopyranoside and its aglycon form, cyanidin chloride, were reported to exert their antioxidant properties partly through induction of HSP70 expression in Caco2 cells.162 This was also observed with the flavonone naringenin (10–100 µM).163
By contrast, studies in vitro have shown that the polyphenol flavonoid quercetin (30–100 µM), as well as other flavonoids like flavone (150 µM), kaempferol (100 µM), and genistein (100 µM), are potent inhibitors of iHSPs (especially iHSP70).164–166 Luteolin (25 µM) was a weak inhibitor of iHSP70, and rutin had no effect.164 These flavonoids inhibit Hsp70 mRNA by acting upstream on HSF and inhibiting the interaction between HSF and heat-shock element.165 Since then, many studies have used quercetin experimentally as an inhibitor of iHSP70 induction in GECs under various conditions.51,85,102,123,155 Finally, grape seed extract rich in polyphenols was also found to inhibit iHSP70 in a bovine GEC line,167 thus confirming the negative effects of many polyphenols on iHSPs.
Mycotoxins
Mycotoxins are natural food contaminants present worldwide and that constitute a threat to human and animal health. The GI tract is the first target for mycotoxins, which deplete both barrier function and enteric immunity.168 Highly oxidant mycotoxins such as zearalenone or citrinin, and moderate oxidant mycotoxins like fumonisins, may increase cellular iHSP70, while those with low oxidant activity (e.g., ochratoxin A or deoxinivalenol) may not.169 In fact, there are few data on the effects of food mycotoxins on gut epithelial iHSPs. One study reported no effect of deoxinivalenol on iHSP70 induction in HT-29 cells.170 Pigs fed fumonisin B1 (1.5 mg/kg body weight for 9 days) displayed a slight but significant increase in iHSP70 (but not iHSP27) protein concentrations in the jejunum, while concentrations in the colon were unchanged.171 These data are in broad agreement with findings suggesting the oxidant potential of mycotoxins.169
CONCLUSION
Inducible HSPs display fundamental functions in GECs and, importantly, can be modulated through microbial components and metabolites and specific nutrients or food components (Figure 1). Increased expression of iHSP is most often synonymous with improved gut protection in normal epithelial cells, while the opposite is detrimental and may favor the development of chronic gut diseases. Evidence of mHSPs and eHSPs has also been demonstrated, with eHSPs displaying immune-modulatory roles. Future research should aim to better define which microbes and what levels of microbial components can optimize the expression of gut-protective iHSPs. Likewise, parallel efforts should continue to examine, especially in vivo, the potential of nutrients, food components (e.g., fiber types, polyphenols), and vegetables and fruits in complex diets to modulate health through iHSPs.
Figure 1.
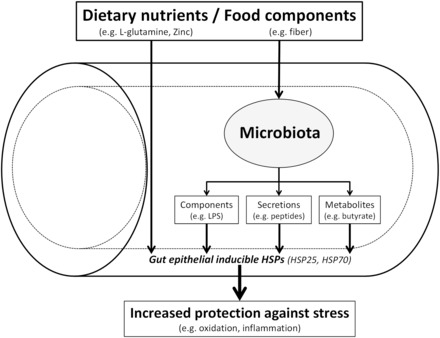
Dietary nutrients and beneficial food components stimulate inducible heat-shock proteins (iHSP25 and/or iHSP70) in the gut epithelium either directly or indirectly through the microbiota (e.g., microbial components, secretions, or metabolites), thus leading to increased epithelial protection against stress (e.g., oxidation or inflammation).
Acknowledgments
The authors Professor Hermann-Josef Rothkötter (Institute of Anatomy, Otto-von-Guericke University, Magdeburg, Germany) for critically reading this review.
Funding. This work was financially supported by the Institut National de la Recherche Agronomique, France, and the European Union (Interplay project no. 227549) (J.P.L.). Funders had no role in the preparation of the present review.
Declaration of interest. The authors have no relevant interests to declare.
References
- 1.Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189 doi:10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pastorelli L, De Salvo C, Mercado JR, et al. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front Immunol. 2013;4:280 doi:10.3389/fimmu.2013.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnabl B. Linking intestinal homeostasis and liver disease. Curr Opin Gastroenterol. 2013;29:264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cani PD, Delzenne NM. Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opin Pharmacol. 2009;9: 737–743. [DOI] [PubMed] [Google Scholar]
- 5.Cani PD, Osto M, Geurts L, et al. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lallès JP. Intestinal alkaline phosphatase: multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr Rev. 2010;68:323–332. [DOI] [PubMed] [Google Scholar]
- 7.Lallès JP. Intestinal alkaline phosphatase: novel functions and protective effects. Nutr Rev. 2014;72:82–94. [DOI] [PubMed] [Google Scholar]
- 8.Harada T, Koyama I, Kasahara T, et al. Heat shock induces intestinal-type alkaline phosphatase in rat IEC-18 cells. Am J Physiol Gastrointest Liver Physiol. 2003;284:G255–G262. [DOI] [PubMed] [Google Scholar]
- 9.Garrido C, Brunet M, Didelot C, et al. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5: 2592–2601. [DOI] [PubMed] [Google Scholar]
- 10.Otaka M, Odashima M, Watanabe S. Role of heat shock proteins (molecular chaperones) in intestinal mucosal protection. Biochem Biophys Res Commun. 2006;348:1–5. [DOI] [PubMed] [Google Scholar]
- 11.Petrof EO, Ciancio MJ, Chang EB. Role and regulation of intestinal epithelial heat shock proteins in health and disease. Chin J Dig Dis. 2004;5:45–50. [DOI] [PubMed] [Google Scholar]
- 12.Mizushima T. HSP-dependent protection against gastrointestinal diseases. Curr Pharm Des. 2010;16:1190–1196. [DOI] [PubMed] [Google Scholar]
- 13.Wu X, Zhang Y, Yin Y, et al. Roles of heat-shock protein 70 in protecting against intestinal mucosal damage. Front Biosci (Landmark Ed). 2013;18: 356–365. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Dicksved J, Lundh T, et al. Heat shock proteins: intestinal gatekeepers that are influenced by dietary components and the gut microbiota. Pathogens. 2014;3:187–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doré J, Blottière H. The influence of diet on the gut microbiota and its consequences for health. Curr Opin Biotechnol. 2015;32:195–199. [DOI] [PubMed] [Google Scholar]
- 16.Arrigo AP, Simon S, Gibert B, et al. Hsp27 (HspB1) and αB-crystallin (HspB5) as therapeutic targets. FEBS Lett. 2007;581:3665–3674. [DOI] [PubMed] [Google Scholar]
- 17.Acunzo J, Andrieu C, Baylot V, et al. Hsp27 as a therapeutic target in cancers. Curr Drug Targets. 2014;15:423–431. [DOI] [PubMed] [Google Scholar]
- 18.Asea A. Mechanisms of HSP72 release. J Biosci. 2007;32:579–584. [DOI] [PubMed] [Google Scholar]
- 19.Tamura Y, Torigoe T, Kukita K, et al. Heat-shock proteins as endogenous ligands building a bridge between innate and adaptive immunity. Immunotherapy. 2012;4:841–852. [DOI] [PubMed] [Google Scholar]
- 20.De Maio A. Extracellular Hsp70: export and function. Curr Protein Pept Sci. 2014;15:225–231. [DOI] [PubMed] [Google Scholar]
- 21.Multhoff G, Pockley AG, Streffer C, et al. Dual role of heat shock proteins (HSPs) in anti-tumor immunity. Curr Mol Med. 2012;12:1174–1182. [DOI] [PubMed] [Google Scholar]
- 22.Binder RJ. Functions of heat shock proteins in pathways of the innate and adaptive immune system. J Immunol. 2014;193:5765–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giuliano JS, Jr, Lahni PM, Wong HR, et al. Pediatric sepsis – part V: extracellular heat shock proteins: alarmins for the host immune system. Open Inflamm J. 2011;4:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan ZQ, Zhang Y, Li XL, et al. HSP70 protects intestinal epithelial cells from hypoxia/reoxygenation injury via a mechanism that involves the mitochondrial pathways. Eur J Pharmacol. 2010;643:282–288. [DOI] [PubMed] [Google Scholar]
- 25.Baird CH, Niederlechner S, Beck R, et al. L-Threonine induces heat shock protein expression and decreases apoptosis in heat-stressed intestinal epithelial cells. Nutrition. 2013;29:1404–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dokladny K, Zuhl MN, Mandell M, et al. Regulatory coordination between two major intracellular homeostatic systems: heat shock response and autophagy. J Biol Chem. 2013;288:14959–14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlson RM, Vavricka SR, Eloranta JJ, et al. fMLP induces Hsp27 expression, attenuates NF-κB activation, and confers intestinal epithelial cell protection. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1070–G1078. [DOI] [PubMed] [Google Scholar]
- 28.Dokladny K, Lobb R, Wharton W, et al. LPS-induced cytokine levels are repressed by elevated expression of HSP70 in rats: possible role of NF-κB. Cell Stress Chaperones. 2010;15:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharyya S, Dudeja PK, Tobacman JK. Lipopolysaccharide activates NF-κB by TLR4-Bcl10-dependent and independent pathways in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G784–G790. [DOI] [PubMed] [Google Scholar]
- 30.Kojima K, Musch MW, Ren H, et al. Enteric flora and lymphocyte-derived cytokines determine expression of heat shock proteins in mouse colonic epithelial cells. Gastroenterology. 2003;124:1395–1407. [DOI] [PubMed] [Google Scholar]
- 31.Ropeleski MJ, Tang J, Walsh-Reitz MM, et al. Interleukin-11-induced heat shock protein 25 confers intestinal epithelial-specific cytoprotection from oxidant stress. Gastroenterology. 2003;124:1358–1368. [DOI] [PubMed] [Google Scholar]
- 32.Hu S, Ciancio MJ, Lahav M, et al. Translational inhibition of colonic epithelial heat shock proteins by IFN-γ and TNF-α in intestinal inflammation. Gastroenterology. 2007;133:1893–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu S, Claud EC, Musch MW, et al. Stress granule formation mediates the inhibition of colonic Hsp70 translation by interferon-γ and tumor necrosis factor-α. Am J Physiol Gastrointest Liver Physiol. 2010;298:G481–G492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musch MW, Sugi K, Straus D, et al. Heat-shock protein 72 protects against oxidant-induced injury of barrier function of human colonic epithelial Caco2/bbe cells. Gastroenterology. 1999;117:115–122. [DOI] [PubMed] [Google Scholar]
- 35.Ren H, Musch MW, Kojima K, et al. Short-chain fatty acids induce intestinal epithelial heat shock protein 25 expression in rats and IEC 18 cells. Gastroenterology. 2001;121:631–639. [DOI] [PubMed] [Google Scholar]
- 36.Kojima K, Musch MW, Ropeleski MJ, et al. Escherichia coli LPS induces heat shock protein 25 in intestinal epithelial cells through MAP kinase activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G645–G652. [DOI] [PubMed] [Google Scholar]
- 37.Musch MW, Petrof EO, Kojima K, et al. Bacterial superantigen-treated intestinal epithelial cells upregulate heat shock proteins 25 and 72 and are resistant to oxidant cytotoxicity. Infect Immun. 2004;72:3187–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arvans DL, Vavricka SR, Ren H, et al. Luminal bacterial flora determines physiological expression of intestinal epithelial cytoprotective heat shock proteins 25 and 72. Am J Physiol Gastrointest Liver Physiol. 2005;288:G696–G704. [DOI] [PubMed] [Google Scholar]
- 39.Tao Y, Drabik KA, Waypa TS, et al. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C1018–C1030. [DOI] [PubMed] [Google Scholar]
- 40.Fujiya M, Musch MW, Nakagawa Y, et al. The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell Host Microbe. 2007;1:299–308. [DOI] [PubMed] [Google Scholar]
- 41.Petrof EO, Musch MW, Ciancio M, et al. Flagellin is required for salmonella- induced expression of heat shock protein Hsp25 in intestinal epithelium. Am J Physiol Gastrointest Liver Physiol. 2008;294:G808–G818. [DOI] [PubMed] [Google Scholar]
- 42.Segawa S, Fujiya M, Konishi H, et al. Probiotic-derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin-p38 MAPK pathway. PLoS One. 2011;6:e23278 doi:10.1371/journal.pone.0023278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ueno N, Fujiya M, Segawa S, et al. Heat-killed body of Lactobacillus brevis SBC8803 ameliorates intestinal injury in a murine model of colitis by enhancing the intestinal barrier function. Inflamm Bowel Dis. 2011;17:2235–2250. [DOI] [PubMed] [Google Scholar]
- 44.Paszti-Gere E, Szeker K, Csibrik-Nemeth E, et al. Metabolites of Lactobacillus plantarum 2142 prevent oxidative stress-induced overexpression of proinflammatory cytokines in IPEC-J2 cell line. Inflammation. 2012;35:1487–1499. [DOI] [PubMed] [Google Scholar]
- 45.Arnal ME, Zhang J, Messori S, et al. Early changes in microbial colonization selectively modulate intestinal enzymes, but not inducible heat shock proteins in young adult swine [published correction appears in PLoS One. 2014;9:e98730 doi:10.1371/journal.pone.0098730] PLoS One. 2014;9:e87967. doi:10.1371/journal.pone.0087967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camilleri M, Madsen K, Spiller R, et al. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viggiano D, Ianiro G, Vanella G, et al. Gut barrier in health and disease: focus on childhood. Eur Rev Med Pharmacol Sci. 2015;19:1077–1085. [PubMed] [Google Scholar]
- 48.Dokladny K, Moseley PL, Ma TY. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol Gastrointest Liver Physiol. 2006;290:G204–G212. [DOI] [PubMed] [Google Scholar]
- 49.Novosad VL, Richards JL, Phillips NA, et al. Regional susceptibility to stress-induced intestinal injury in the mouse. Am J Physiol Gastrointest Liver Physiol. 2013;305:G418–G426. [DOI] [PubMed] [Google Scholar]
- 50.Zuhl MN, Lanphere KR, Kravitz L, et al. Effects of oral glutamine supplementation on exercise-induced gastrointestinal permeability and tight junction protein expression. J Appl Physiol. 2014;116:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dokladny K, Ye D, Kennedy JC, et al. Cellular and molecular mechanisms of heat stress-induced up-regulation of occludin protein expression: regulatory role of heat shock factor-1. Am J Pathol. 2008;172:659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang PC, He SH, Zheng PY. Investigation into the signal transduction pathway via which heat stress impairs intestinal epithelial barrier function. J Gastro Hepatol. 2007;22:1823–1831. [DOI] [PubMed] [Google Scholar]
- 53.Bidmon-Fliegenschnee B, Lederhuber HC, Csaicsich D, et al. Overexpression of Hsp70 confers cytoprotection during gliadin exposure in Caco-2 cells [published online June 18, 2015]. Pediatr Res. 2015;78:358–364. [DOI] [PubMed] [Google Scholar]
- 54.Pearce SC, Mani V, Weber TE, et al. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J Anim Sci. 2013;91:5183–5193. [DOI] [PubMed] [Google Scholar]
- 55.Liedel JL, Guo Y, Yu Y, et al. Mother's milk-induced Hsp70 expression preserves intestinal epithelial barrier function in an immature rat pup model. Pediatr Res. 2011;69:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanguay RM, Wu Y, Khandjian EW. Tissue-specific expression of heat shock proteins of the mouse in the absence of stress. Dev Genet. 1993;14:112–118. [DOI] [PubMed] [Google Scholar]
- 57.McComb MA, Spurlock ME. Expression of stress proteins in porcine tissues: developmental changes and effect of immunological challenge. J Anim Sci. 1997;75:195–201. [DOI] [PubMed] [Google Scholar]
- 58.Hu S, Wang Y, Lichtenstein L, et al. Regional differences in colonic mucosa-associated microbiota determine the physiological expression of host heat shock proteins. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1266–G1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.David JC, Grongnet JF, Lallès JP. Weaning affects the expression of heat shock proteins in different regions of the gastrointestinal tract of piglets. J Nutr. 2002;132:2551–2561. [DOI] [PubMed] [Google Scholar]
- 60.Sepponen K, Pösö AR. The inducible form of heat shock protein 70 in the serum, colon and small intestine of the pig: comparison to conventional stress markers. Vet J. 2006;171:519–524. [DOI] [PubMed] [Google Scholar]
- 61.Lallès JP, Lessard M, Oswald IP, et al. Consumption of fumonisin B1 for 9 days induces stress proteins along the gastrointestinal tract of pigs. Toxicon. 2010;55:244–249. [DOI] [PubMed] [Google Scholar]
- 62.Lallès JP, David JC. Fasting and refeeding modulate the expression of stress proteins along the gastrointestinal tract of weaned pigs. J Anim Physiol Anim Nutr (Berl). 2011;95:478–488. [DOI] [PubMed] [Google Scholar]
- 63.Arnal ME, Zhang J, Erridge C, et al. Maternal antibiotic-induced early changes in microbial colonization selectively modulate colonic permeability and inducible heat shock proteins, and digesta concentrations of alkaline phosphatase and TLR-stimulants in swine offspring. PLoS One. 2015;10:e0118092 doi:10.1371/journal.pone.0118092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beck SC, Paidas CN, Mooney ML, et al. Presence of the stress-inducible form of hsp-70 (hsp-72) in normal rat colon. Shock. 1995;3:398–402. [PubMed] [Google Scholar]
- 65.Liu HY, Dicksved J, Lundh T, et al. Expression of heat shock proteins 27 and 72 correlates with specific commensal microbes in different regions of porcine gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2014;306:G1033–G1041. [DOI] [PubMed] [Google Scholar]
- 66.Scieglinska D, Piglowski W, Chekan M, et al. Differential expression of HSPA1 and HSPA2 proteins in human tissues; tissue microarray-based immunohistochemical study. Histochem Cell Biol. 2011;135:337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deitch EA, Beck SC, Cruz NC, et al. Induction of heat shock gene expression in colonic epithelial cells after incubation with Escherichia coli or endotoxin. Crit Care Med. 1995;23:1371–1376. [DOI] [PubMed] [Google Scholar]
- 68.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. [DOI] [PubMed] [Google Scholar]
- 69.Liu TS, Musch MW, Sugi K, et al. Protective role of HSP72 against Clostridium difficile toxin A-induced intestinal epithelial cell dysfunction. Am J Physiol Cell Physiol. 2003;284:C1073–C1082. [DOI] [PubMed] [Google Scholar]
- 70.Kato M, Ishige A, Anjiki N, et al. Effect of herbal medicine Juzentaihoto on hepatic and intestinal heat shock gene expression requires intestinal microflora in mouse. World J Gastroenterol. 2007;13:2289–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reilly N, Poylin V, Menconi M, et al. Probiotics potentiate IL-6 production in IL-1β-treated Caco-2 cells through a heat shock-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1169–R1179. [DOI] [PubMed] [Google Scholar]
- 72.Segawa S, Wakita Y, Hirata H, et al. Oral administration of heat-killed Lactobacillus brevis SBC8803 ameliorates alcoholic liver disease in ethanol-containing diet-fed C57BL/6N mice. Int J Food Microbiol. 2008;128:371–377. [DOI] [PubMed] [Google Scholar]
- 73.Okamoto K, Fujiya M, Nata T, et al. Competence and sporulation factor derived from Bacillus subtilis improves epithelial cell injury in intestinal inflammation via immunomodulation and cytoprotection. Int J Colorectal Dis. 2012;27:1039–1046. [DOI] [PubMed] [Google Scholar]
- 74.Miquel S, Martín R, Rossi O, et al. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013;16:255–261. [DOI] [PubMed] [Google Scholar]
- 75.Cao Y, Shen J, Ran ZH. Association between Faecalibacterium prausnitzii reduction and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Gastroenterol Res Pract. 2014;2014:872725 doi:10.1155/2014/872725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nemeth E, Fajdiga S, Malago J, et al. Inhibition of Salmonella-induced IL-8 synthesis and expression of Hsp70 in enterocyte-like Caco-2 cells after exposure to non-starter lactobacilli. Int J Food Microbiol. 2006;112:266–274. [DOI] [PubMed] [Google Scholar]
- 77.Koninkx JFJG, Tooten PCJ, Malago JJ. Probiotic bacteria induced improvement of the mucosal integrity of enterocyte-like Caco-2 cells after exposure to Salmonella enteritidis 857. J Function Food. 2010;2:225–234. [Google Scholar]
- 78.Malago JJ, Tooten PC, Koninkx JF. Anti-inflammatory properties of probiotic bacteria on Salmonella-induced IL-8 synthesis in enterocyte-like Caco-2 cells. Benef Microbes. 2010;1:121–130. [DOI] [PubMed] [Google Scholar]
- 79.Malago JJ, Nemeth E, Koninkx JF, et al. Microbial products from probiotic bacteria inhibit Salmonella enteritidis 857-induced IL-8 synthesis in Caco-2 cells. Folia Microbiol (Praha). 2010;55:401–408. [DOI] [PubMed] [Google Scholar]
- 80.Liu HY, Roos S, Jonsson H, et al. Effects of Lactobacillus johnsonii and Lactobacillus reuteri on gut barrier function and heat shock proteins in intestinal porcine epithelial cells. Physiol Rep. 2015;3:pii:e12355 doi:10.14814/phy2.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petrof EO, Kojima K, Ropeleski MJ, et al. Probiotics inhibit nuclear factor-κB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology. 2004;127:1474–1487. [DOI] [PubMed] [Google Scholar]
- 82.Jiang P, Jensen ML, Cilieborg MS, et al. Antibiotics increase gut metabolism and antioxidant proteins and decrease acute phase response and necrotizing enterocolitis in preterm neonates. PLoS One. 2012;7:e44929 doi:10.1371/journal.pone.0044929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miyazaki T, Sagawa R, Honma T, et al. 73-kDa molecular chaperone HSP73 is a direct target of antibiotic gentamicin. J Biol Chem. 2004;279:17295–31700. [DOI] [PubMed] [Google Scholar]
- 84.Yamamoto S, Nakano S, Owari K, et al. Gentamicin inhibits HSP70-assisted protein folding by interfering with substrate recognition. FEBS Lett. 2010;584: 645–651. [DOI] [PubMed] [Google Scholar]
- 85.Pritts TA, Hungness ES, Hershko DD, et al. Proteasome inhibitors induce heat shock response and increase IL-6 expression in human intestinal epithelial cells. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1016–R1026. [DOI] [PubMed] [Google Scholar]
- 86.Dominguez KM, Moss RL. Necrotizing enterocolitis. Clin Perinatol. 2012;39: 387–401. [DOI] [PubMed] [Google Scholar]
- 87.Choi YY. Necrotizing enterocolitis in newborns: update in pathophysiology and newly emerging therapeutic strategies. Korean J Pediatr. 2014;57:505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhong X, Wang T, Zhang X, et al. Heat shock protein 70 is upregulated in the intestine of intrauterine growth retardation piglets. Cell Stress Chaperones. 2010;15:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhong X, Li W, Huang X, et al. Impairment of cellular immunity is associated with overexpression of heat shock protein 70 in neonatal pigs with intrauterine growth retardation. Cell Stress Chaperones. 2012;17:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Afrazi A, Sodhi CP, Good M, et al. Intracellular heat shock protein-70 negatively regulates TLR4 signaling in the newborn intestinal epithelium. J Immunol. 2012;188:4543–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim EK, Lee KY, Lee HJ, et al. Heat shock pretreatment reduces intestinal injury in a neonatal rat model of early necrotizing enterocolitis. Neonatology. 2013;103:1–6. [DOI] [PubMed] [Google Scholar]
- 92.Milner CM, Campbell RD. Structure and expression of the three MHC-linked HSP70 genes. Immunogenetics. 1990;32:242–251. [DOI] [PubMed] [Google Scholar]
- 93.Esaki M, Furuse M, Matsumoto T, et al. Polymorphism of heat-shock protein gene HSP70-2 in Crohn disease: possible genetic marker for two forms of Crohn disease. Scand J Gastroenterol. 1999;34:703–707. [DOI] [PubMed] [Google Scholar]
- 94.Debler J, Schiemann U, Seybold U, et al. Heat-shock protein HSP70-2 genotypes in patients with Crohn's disease: a more severe clinical course with intestinal complications in presence of the PstI-polymorphism. Eur J Med Res. 2003;8: 120–124. [PubMed] [Google Scholar]
- 95.Klausz G, Molnár T, Nagy F, et al. Polymorphism of the heat-shock protein gene Hsp70-2, but not polymorphisms of the IL-10 and CD14 genes, is associated with the outcome of Crohn's disease. Scand J Gastroenterol. 2005;40:1197–1204. [DOI] [PubMed] [Google Scholar]
- 96.Nam SY, Kim N, Kim JS, et al. Heat shock protein gene 70-2 polymorphism is differentially associated with the clinical phenotypes of ulcerative colitis and Crohn's disease. J Gastro Hepatol. 2007;22:1032–1038. [DOI] [PubMed] [Google Scholar]
- 97.Tahara T, Shibata T, Okubo M, et al. Heat-shock protein 70-2 BB genotype is associated with reduced risks of the steroid-dependent and refractory phenotypes of ulcerative colitis. Biomed Rep. 2014;2:555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vlachos II, Barbatis C, Tsopanomichalou M, et al. Correlation between depression, anxiety, and polymorphonuclear cells' resilience in ulcerative colitis: the mediating role of heat shock protein 70. BMC Gastroenterol. 2014;14:77 doi:10.1186/1471-230X-14-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu S, Zhu X, Triggs JR, et al. Inflammation-induced, 3′UTR-dependent translational inhibition of Hsp70 mRNA impairs intestinal homeostasis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1003–G1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tomasello G, Sciumé C, Rappa F, et al. Hsp10, Hsp70, and Hsp90 immunohistochemical levels change in ulcerative colitis after therapy. Eur J Histochem. 2011;55:e38 doi:10.4081/ejh.2011.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tanaka K, Namba T, Arai Y, et al. Genetic evidence for a protective role for heat shock factor 1 and heat shock protein 70 against colitis. J Biol Chem. 2007;282:23240–23252. [DOI] [PubMed] [Google Scholar]
- 102.Ohkawara T, Nishihira J, Ishiguro Y, et al. Resistance to experimental colitis depends on cytoprotective heat shock proteins in macrophage migration inhibitory factor null mice. Immunol Lett. 2006;107:148–154. [DOI] [PubMed] [Google Scholar]
- 103.Lundin KE, Sollid LM. Advances in coeliac disease. Curr Opin Gastroenterol. 2014;30:154–162. [DOI] [PubMed] [Google Scholar]
- 104.Partanen J, Milner C, Campbell RD, et al. HLA-linked heat-shock protein 70 (HSP70-2) gene polymorphism and celiac disease. Tissue Antigens. 1993;41: 15–19. [DOI] [PubMed] [Google Scholar]
- 105.Ramos-Arroyo MA, Feijoó E, Sánchez-Valverde F, et al. Heat-shock protein 70-1 and HLA class II gene polymorphisms associated with celiac disease susceptibility in Navarra (Spain). Hum Immunol. 2001;62:821–825. [DOI] [PubMed] [Google Scholar]
- 106.Sziksz E, Veres G, Vannay A, et al. Increased heat shock protein 72 expression in celiac disease. J Pediatr Gastroenterol Nutr. 2010;51:573–578. [DOI] [PubMed] [Google Scholar]
- 107.Brottveit M, Beitnes AC, Tollefsen S, et al. Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am J Gastroenterol. 2013;108:842–850. [DOI] [PubMed] [Google Scholar]
- 108.Lenaerts K, Ceulemans LJ, Hundscheid IH, et al. New insights in intestinal ischemia-reperfusion injury: implications for intestinal transplantation. Curr Opin Organ Transplant. 2013;18:298–303. [DOI] [PubMed] [Google Scholar]
- 109.Gonzalez LM, Moeser AJ, Blikslager AT. Animal models of ischemia-reperfusion-induced intestinal injury: progress and promise for translational research. Am J Physiol Gastrointest Liver Physiol. 2015;308:G63–G75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stojadinovic A, Kiang J, Smallridge R, et al. Induction of heat-shock protein 72 protects against ischemia/reperfusion in rat small intestine. Gastroenterology. 1995;109:505–515. [DOI] [PubMed] [Google Scholar]
- 111.Tsuruma T, Yagihashi A, Matsuno T, et al. The heat-shock protein 70 family reduces ischemia/reperfusion injury in small intestine. Transplant Proc. 1996;28: 2629–2630. [PubMed] [Google Scholar]
- 112.Tsuruma T, Yagihashi A, Watanabe N, et al. Heat-shock protein-73 protects against small intestinal warm ischemia-reperfusion injury in the rat. Surgery. 1999;125:385–395. [PubMed] [Google Scholar]
- 113.Fleming SD, Starnes BW, Kiang JG, et al. Heat stress protection against mesenteric I/R-induced alterations in intestinal mucosa in rats. J Appl Physiol. 2002;92:2600–2607. [DOI] [PubMed] [Google Scholar]
- 114.Sakamoto N, Kokura S, Okuda T, et al. Heme oxygenase-1 (Hsp32) is involved in the protection of small intestine by whole body mild hyperthermia from ischemia/reperfusion injury in rat. Int J Hyperthermia. 2005;21:603–614. [DOI] [PubMed] [Google Scholar]
- 115.Juel IS, Solligård E, Tvedt KE, et al. Post-ischaemic restituted intestinal mucosa is more resistant to further ischaemia than normal mucosa in the pig. Scand J Clin Lab Invest. 2008;68:106–116. [DOI] [PubMed] [Google Scholar]
- 116.Rocco A, Compare D, Angrisani D, et al. Alcoholic disease: liver and beyond. World J Gastroenterol. 2014;20:14652–14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhong W, Zhou Z. Alterations of the gut microbiome and metabolome in alcoholic liver disease. World J Gastrointest Pathophysiol. 2014;5:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park SC, Lim JY, Jeen YT, et al. Ethanol-induced DNA damage and repair-related molecules in human intestinal epithelial Caco-2 cells. Mol Med Rep. 2012; 5:1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Akagi R, Ohno M, Matsubara K, et al. Glutamine protects intestinal barrier function of colon epithelial cells from ethanol by modulating Hsp70 expression. Pharmacology. 2013;91:104–111. [DOI] [PubMed] [Google Scholar]
- 120.Lee SW, Choi DW, Park SC, et al. Expression of heat shock proteins and cytokines in response to ethanol induced damage in the small intestine of ICR mice. Intest Res. 2014;12:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ehrenfried JA, Chen J, Li J, et al. Glutamine-mediated regulation of heat shock protein expression in intestinal cells. Surgery. 1995;118:352–356; discussion 356–357. [DOI] [PubMed] [Google Scholar]
- 122.Katsuki K, Fujimoto M, Zhang XY, et al. Feeding induces expression of heat shock proteins that reduce oxidative stress. FEBS Lett. 2004;571:187–191. [DOI] [PubMed] [Google Scholar]
- 123.Wischmeyer PE, Musch MW, Madonna MB, et al. Glutamine protects intestinal epithelial cells: role of inducible HSP70. Am J Physiol. 1997;272:G879–G884. [DOI] [PubMed] [Google Scholar]
- 124.Chow A, Zhang R. Glutamine reduces heat shock-induced cell death in rat intestinal epithelial cells. J Nutr. 1998;128:1296–1301. [DOI] [PubMed] [Google Scholar]
- 125.Lindemann G, Grohs M, Stange EF, et al. Limited heat-shock protein 72 induction in Caco-2 cells by L-glutamine. Digestion. 2001;64:81–86. [DOI] [PubMed] [Google Scholar]
- 126.Phanvijhitsiri K, Musch MW, Ropeleski MJ, et al. Heat induction of heat shock protein 25 requires cellular glutamine in intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;291:C290–C299. [DOI] [PubMed] [Google Scholar]
- 127.Wischmeyer PE, Kahana M, Wolfson R, et al. Glutamine induces heat shock protein and protects against endotoxin shock in the rat. J Appl Physiol. 2001;90:2403–2410. [DOI] [PubMed] [Google Scholar]
- 128.Singleton KD, Wischmeyer PE. Oral glutamine enhances heat shock protein expression and improves survival following hyperthermia. Shock. 2006;25: 295–299. [DOI] [PubMed] [Google Scholar]
- 129.Xue H, Sawyer MB, Field CJ, et al. Bolus oral glutamine protects rats against CPT-11-induced diarrhea and differentially activates cytoprotective mechanisms in host intestine but not tumor. J Nutr. 2008;138:740–746. [DOI] [PubMed] [Google Scholar]
- 130.Xue H, Sufit AJ, Wischmeyer PE. Glutamine therapy improves outcome of in vitro and in vivo experimental colitis models. JPEN J Parenter Enteral Nutr. 2011;35:188–197. [DOI] [PubMed] [Google Scholar]
- 131.Zhong X, Zhang XH, Li XM, et al. Intestinal growth and morphology is associated with the increase in heat shock protein 70 expression in weaning piglets through supplementation with glutamine. J Anim Sci. 2011;89:3634–3642. [DOI] [PubMed] [Google Scholar]
- 132.Sakiyama T, Musch MW, Ropeleski MJ, et al. Glutamine increases autophagy under basal and stressed conditions in intestinal epithelial cells. Gastroenterology. 2009;136:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Iwashita Y, Sakiyama T, Musch MW, et al. Polyamines mediate glutamine-dependent induction of the intestinal epithelial heat shock response. Am J Physiol Gastrointest Liver Physiol. 2011;301:G181–G187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shoji H, Shimizu T. Antioxidative properties of human milk and spermine are not related to expression of Hsp 70. Acta Paediatr. 2008;97:81–84. [DOI] [PubMed] [Google Scholar]
- 135.Xue H, Slavov D, Wischmeyer PE. Glutamine-mediated dual regulation of heat shock transcription factor-1 activation and expression. J Biol Chem. 2012;287: 40400–40413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kallweit AR, Baird CH, Stutzman DK, et al. Glutamine prevents apoptosis in intestinal epithelial cells and induces differential protective pathways in heat and oxidant injury models. JPEN J Parenter Enteral Nutr. 2012;36:551–555. [DOI] [PubMed] [Google Scholar]
- 137.Niederlechner S, Klawitter J, Baird C, et al. Fibronectin-integrin signaling is required for L-glutamine's protection against gut injury. PLoS One. 2012;7:e50185 doi:10.1371/journal.pone.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Niederlechner S, Baird C, Wischmeyer PE. P38MAP kinase, but not phosphoinositol-3 kinase, signal downstream of glutamine-mediated fibronectin-integrin signaling after intestinal injury. Nutr J. 2013;12:88 doi:10.1186/1475-2891-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Niederlechner S, Baird C, Petrie B, et al. Epidermal growth factor receptor expression and signaling are essential in glutamine's cytoprotective mechanism in heat-stressed intestinal epithelial-6 cells. Am J Physiol Gastrointest Liver Physiol. 2013;304:G543–G552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu X, Ruan Z, Gao Y, et al. Dietary supplementation with L-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean meal-based diet. Amino Acids. 2010;39:831–839. [DOI] [PubMed] [Google Scholar]
- 141.Lenaerts K, Renes J, Bouwman FG, et al. Arginine deficiency in preconfluent intestinal Caco-2 cells modulates expression of proteins involved in proliferation, apoptosis, and heat shock response. Proteomics. 2007;7:565–577. [DOI] [PubMed] [Google Scholar]
- 142.Hou Y, Wang L, Ding B, et al. Dietary α-ketoglutarate supplementation ameliorates intestinal injury in lipopolysaccharide-challenged piglets. Amino Acids. 2010;39:555–564. [DOI] [PubMed] [Google Scholar]
- 143.De Simone C, Ferranti P, Picariello G, et al. Peptides from water buffalo cheese whey induced senescence cell death via ceramide secretion in human colon adenocarcinoma cell line. Mol Nutr Food Res. 2011;55:229–238. [DOI] [PubMed] [Google Scholar]
- 144.Schmid K, Haslbeck M, Buchner J, et al. Induction of heat shock proteins and the proteasome system by casein-N ε-(carboxymethyl)lysine and N ε-(carboxymethyl)lysine in Caco-2 cells. Ann N Y Acad Sci. 2008;1126: 257–261. [DOI] [PubMed] [Google Scholar]
- 145.Chen P, Kartha S, Bissonnette M, et al. AMP-18 facilitates assembly and stabilization of tight junctions to protect the colonic mucosal barrier. Inflamm Bowel Dis. 2012;18:1749–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schepens MA, Vink C, Schonewille AJ, et al. Dietary heme adversely affects experimental colitis in rats, despite heat-shock protein induction. Nutrition. 2011;27:590–597. [DOI] [PubMed] [Google Scholar]
- 147.Odashima M, Otaka M, Jin M, et al. Zinc L-carnosine protects colonic mucosal injury through induction of heat shock protein 72 and suppression of NF-κB activation. Life Sci. 2006;79:2245–2250. [DOI] [PubMed] [Google Scholar]
- 148.Ovelgönne JH, Koninkx JF, Pusztai A, et al. Decreased levels of heat shock proteins in gut epithelial cells after exposure to plant lectins. Gut. 2000;46: 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lallès JP, Lacan D, David JC. A melon pulp concentrate rich in superoxide dismutase reduces stress proteins along the gastrointestinal tract of pigs. Nutrition. 2011;27:358–363. [DOI] [PubMed] [Google Scholar]
- 150.Bondzio A, Lodemann U, Weise C, et al. Cry1Ab treatment has no effects on viability of cultured porcine intestinal cells, but triggers Hsp70 expression. PLoS One. 2013;8:e67079 doi:10.1371/journal.pone.0067079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu HY, Lundh T, Dicksved J, et al. Expression of heat shock protein 27 in gut tissue of growing pigs fed diets without and with inclusion of chicory fiber. J Anim Sci. 2012;90(suppl 4):25–27. [DOI] [PubMed] [Google Scholar]
- 152.Gourbeyre P, Desbuards N, Grémy G, et al. Exposure to a galactooligosaccharides/inulin prebiotic mix at different developmental time points differentially modulates immune responses in mice. J Agric Food Chem. 2012;60:11942–11951. [DOI] [PubMed] [Google Scholar]
- 153.Bhattacharyya S, Dudeja PK, Tobacman JK. Carrageenan-induced NFκB activation depends on distinct pathways mediated by reactive oxygen species and Hsp27 or by Bcl10. Biochim Biophys Acta. 2008;1780:973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bhattacharyya S, Dudeja PK, Tobacman JK. ROS, Hsp27, and IKKβ mediate dextran sodium sulfate (DSS) activation of IκBα, NFκB, and IL-8. Inflamm Bowel Dis. 2009;15:673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ohkawara T, Nishihira J, Nagashima R, et al. Polaprezinc protects human colon cells from oxidative injury induced by hydrogen peroxide: relevant to cytoprotective heat shock proteins. World J Gastroenterol. 2006;12:6178–6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ohkawara T, Takeda H, Kato K, et al. Polaprezinc (N-(3-aminopropionyl)-L-histidinato zinc) ameliorates dextran sulfate sodium-induced colitis in mice. Scand J Gastroenterol. 2005;40:1321–1327. [DOI] [PubMed] [Google Scholar]
- 157.Van Molle W, Van Roy M, Van Bogaert T, et al. Protection of zinc against tumor necrosis factor induced lethal inflammation depends on heat shock protein 70 and allows safe antitumor therapy. Cancer Res. 2007;67:7301–7307. [DOI] [PubMed] [Google Scholar]
- 158.Sargeant HR, Miller HM, Shaw MA. Inflammatory response of porcine epithelial IPEC J2 cells to enterotoxigenic E . coli infection is modulated by zinc supplementation. Mol Immunol. 2011;48:2113–2121. [DOI] [PubMed] [Google Scholar]
- 159.Lodemann U, Einspanier R, Scharfen F, et al. Effects of zinc on epithelial barrier properties and viability in a human and a porcine intestinal cell culture model. Toxicol in vitro. 2013;27:834–843. [DOI] [PubMed] [Google Scholar]
- 160.Hu CH, Song ZH, Xiao K, et al. Zinc oxide influences intestinal integrity, the expressions of genes associated with inflammation and TLR4-myeloid differentiation factor 88 signaling pathways in weanling pigs. Innate Immun. 2013;20:478–486. [DOI] [PubMed] [Google Scholar]
- 161.Guilloteau P, Zabielski R, David JC, et al. Sodium-butyrate as a growth promoter in milk replacer formula for young calves. J Dairy Sci. 2009;92:1038–1049. [DOI] [PubMed] [Google Scholar]
- 162.Renis M, Calandra L, Scifo C, et al. Response of cell cycle/stress-related protein expression and DNA damage upon treatment of CaCo2 cells with anthocyanins. Br J Nutr. 2008;100:27–35. [DOI] [PubMed] [Google Scholar]
- 163.Noda S, Tanabe S, Suzuki T. Naringenin enhances intestinal barrier function through the expression and cytoskeletal association of tight junction proteins in Caco-2 cells. Mol Nutr Food Res. 2013;57:2019–2028. [DOI] [PubMed] [Google Scholar]
- 164.Hosokawa N, Hirayoshi K, Nakai A, et al. Flavonoids inhibit the expression of heat shock proteins. Cell Struct Funct. 1990;15:393–401. [DOI] [PubMed] [Google Scholar]
- 165.Hosokawa N, Hirayoshi K, Kudo H, et al. Inhibition of the activation of heat shock factor in vivo and in vitro by flavonoids. Mol Cell Biol. 1992;12: 3490–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Koishi M, Hosokawa N, Sato M, et al. Quercetin, an inhibitor of heat shock protein synthesis, inhibits the acquisition of thermotolerance in a human colon carcinoma cell line. Jpn J Cancer Res. 1992;83:1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li X, Yang Y, Liu S, et al. Grape seed extract supplementation attenuates the heat stress-induced responses of jejunum epithelial cells in Simmental × Qinchuan steers. Br J Nutr. 2014;112:347–357. [DOI] [PubMed] [Google Scholar]
- 168.Pinton P, Oswald IP. Effect of deoxynivalenol and other type B trichothecenes on the intestine: a review. Toxins (Basel). 2014;6:1615–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.El Golli-Bennour E, Bacha H. Hsp70 expression as biomarkers of oxidative stress: mycotoxins' exploration. Toxicology. 2011;287:1–7. [DOI] [PubMed] [Google Scholar]
- 170.Bensassi F, El Golli-Bennour E, Abid-Essefi S, et al. Pathway of deoxynivalenol- induced apoptosis in human colon carcinoma cells. Toxicology. 2009;264: 104–109. [DOI] [PubMed] [Google Scholar]
- 171.Lallès JP, Lessard M, Oswald IP, et al. Consumption of fumonisin B1 for 9 days inducesstress proteins along the gastrointestinal tract of pigs. Toxicon. 2010; 55:244–249. [DOI] [PubMed] [Google Scholar]


