Abstract
Context: It is unclear how in utero vitamin D deficiency affects the extraskeletal health of children, despite the known risks for adverse pregnancy/birth outcomes. Objective: This systematic review seeks to assess the effect of in utero vitamin D exposure on childhood allergy and infection outcomes using the PRISMA guidelines. Data Sources: MEDLINE, Cochrane Library, and Web of Science databases were searched. Study Selection: Literature published through April 2015 was searched for studies reporting on the association between maternal pregnancy or cord blood vitamin D status and childhood allergy and infection. Data Extraction: Of 4175 articles identified, 43 studies met the inclusion criteria. They examined a wide variety of outcomes, using many different vitamin D cutoff values in their analyses. Data Synthesis: For most outcomes, results were inconsistent, although there appeared to be a protective effect between higher in utero vitamin D status and childhood lower respiratory tract infection (5 of 10 studies). Conclusions: More research is needed on childhood allergy and infection outcomes, and future studies should standardize outcome reporting, especially with regard to cutoff values for vitamin D concentrations. Evidence of a protective association between in utero vitamin D exposure and lower respiratory tract infection was found, while the other outcomes were either understudied or showed inconsistent results.
PROSPERO registration no. CRD42013006156.
Keywords: allergy, epidemiology, infection, pediatrics, pregnancy, vitamin D
INTRODUCTION
Vitamin D deficiency is a widespread, global health concern, especially in industrialized countries and in people with darker skin pigmentation.1–3 Deficiency exists in nearly all populations, including children and pregnant women.3,4 This prevalent deficiency is concerning because vitamin D is a hormone with wide-ranging and only partially understood effects in the human body. Substantial evidence supports the importance of vitamin D for calcium homeostasis and bone health, and a growing body of evidence is accumulating for the extraskeletal effects of vitamin D on the outcomes of infection, allergy, autoimmune disease, cardiovascular disease, and cancer.5–9
Vitamin D is a group of fat-soluble secosteroids that exist in a number of forms. Initially, a form of calciferol (either cholecalciferol [vitamin D3] or ergocalciferol [vitamin D2]), which is an inactive provitamin, is produced in the skin upon exposure to ultraviolet B radiation (D3) or is ingested through foods and supplements (D3 or D2). Calciferol is then converted in the liver to the major circulating metabolite, the prehormone calcifediol [25(OH)D], before it is hydroxylated, primarily in the kidneys, to the active hormone, calcitriol [1,25(OH)2D]. Parathyroid hormone regulates 1,25(OH)2D production in response to serum calcium levels. Because of this, 1,25(OH)2D levels can be normal in vitamin D–deficient states, as parathyroid hormone levels increase in an effort to maintain calcium concentrations. Physicians and researchers most commonly use 25(OH)D as a marker of vitamin D status because it is outside of the direct influence of parathyroid hormone and has a long half-life of 2 to 3 weeks, accurately reflecting vitamin D status in the body.10
There are no universally agreed upon cutoff values for defining vitamin D deficiency in any population because data about the extraskeletal effects of deficiency are controversial and incomplete. A 25(OH)D level of 50 nmol/L is currently deemed adequate for skeletal health, but many experts advocate for 75 nmol/L 25(OH)D as the threshold for positively affecting extraskeletal health.8,11,12 Some research even points to levels greater than 100 nmol/L as being optimal.13 Correspondingly, current supplementation guidelines are constantly changing as new information emerges about the effects and safety profile of vitamin D.
Basic science research has revealed a variety of ways that vitamin D affects the immune system. For instance, macrophages are able to synthesize their own 1,25(OH)2D and use it to increase the synthesis of cathelicidin, a potent antimicrobial peptide.14 Evidence from animal and human studies has shown that, besides affecting the innate immune response, vitamin D also helps shift the adaptive immune system away from a proinflammatory TH1 phenotype to a more balanced phenotype with increased T regulatory cells – intriguing data for allergy and autoimmune disease.15–18 Vitamin D, therefore, may have important effects on the developing immune system, potentially influencing the health of young children throughout their lives.
Many groups now research the effects of in utero vitamin D exposure on maternal and childhood outcomes; however, there is still no consensus on the effects of vitamin D on extraskeletal health in any age group. A recent systematic review and meta-analysis showed an association between lower maternal 25(OH)D levels during pregnancy and increased risk of poor obstetric and birth outcomes such as gestational diabetes, preeclampsia, bacterial vaginosis, and small-for-gestational-age births.19 As no such expansive systematic review exists for in utero vitamin D exposure and childhood infection and allergy outcomes, this review aims to shed light on these topics. To assess the effects of in utero vitamin D exposure on subsequent childhood immune function, randomized controlled supplementation trials as well as observational studies that assessed vitamin D status in either maternal serum or cord blood were reviewed in relation to a variety of childhood infectious and allergic diseases.
METHODS
The standards set by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement were used in executing and writing this review (see Table S1 in the Supporting Information online for the completed checklist).20 The review protocol was registered with the PROSPERO database before screening commenced (no. CRD42013006156). The PICOS (Participants, Intervention, Comparisons, Outcomes, Study Design) criteria used to design this review are shown in Table 1.
Table 1.
PICOS criteria employed to the define research question
| Criteria | Description |
|---|---|
| Participants | Pregnant women and their children up to 18 y of age |
| Intervention/exposure | In utero vitamin D status in either maternal blood or cord blood |
| Comparisons | Sufficient vs insufficient vitamin D status or analysis of vitamin D status by quartile |
| Outcomes |
|
| Study design | Randomized controlled trials, nonrandomized controlled trials, cohort, and case–control studies |
Abbreviations: HIV, human immunodeficiency virus; IgE, immunoglobulin E.
Data sources and search strategy
The following databases were searched on April 28, 2015: MEDLINE (PubMed, 1946 to date), Cochrane Library (Wiley, 2015 issue 4), and Web of Science (1900 to date). The search included indexed terms and text words to capture concepts associated with vitamin D and pregnancy/in utero/fetus and children in articles published from database inception to the date of the search. There were no language or study design restrictions. The search strategy was adjusted for the syntax appropriate for each database (see Table S2 in the Supporting Information online for the full MEDLINE search strategy).
Selection criteria
The aim of the search strategy was to identify studies that reported on the association between maternal pregnancy or cord blood vitamin D status and the childhood health outcomes of infection, allergy, and autoimmune disease. Randomized controlled trials, nonrandomized controlled trials, cohort, and case–control studies were included, but not ecological studies. Any study that did not report a blood-based vitamin D concentration was excluded, such as studies based on nutritional intake or vitamin D supplementation but without corresponding blood 25(OH)D levels. This was done because pregnant women have shown heterogeneous responses to vitamin D supplementation,21,22 and a more direct measurement of exposure was sought for this review. The only restriction placed on study participants was that the outcomes must have been determined in children up to the age of 18 years. Animal and cell culture studies were also excluded.
The primary outcomes of interest encompassed respiratory infection, gastrointestinal tract infection, atopic dermatitis, allergic rhinitis, asthma, and wheezing. Secondary outcomes included other allergic disease, all other infections besides maternal–fetal HIV transmission, markers of allergy (e.g., total and specific immunoglobulin E [IgE] levels), autoimmune diseases, lung function, and other immunological outcomes related to atopy (e.g., TH1:TH2 ratio, cytokine production).
Study selection
After the search was performed and all records were identified, 2 reviewers performed an independent eligibility assessment. For initial screening, the record titles and abstracts were used. Further screening involved reading full-text publications and supplemental information from studies that initially satisfied the selection criteria. Disagreements between reviewers were discussed and resolved, involving a third reviewer when necessary. All references of the included articles and previous reviews were scanned in a similar fashion to ensure no articles were missed outside of the original search. The review of multiple reports of the same study was avoided by comparing author name, study location, sample size, and outcomes.
Data extraction
In order to collect and organize data from the eligible studies, a web-based electronic data extraction form was created prior to the screening process using Research Electronic Data Capture, hosted by Dartmouth Hitchcock Medical Center.23 The form was thoroughly tested before a single reviewer performed the complete data extraction. The following information was extracted: study design, characteristics of study participants, study inclusion and exclusion criteria, type of intervention and/or exposure, recruitment and fallout rates, and all pertinent outcome measures. When necessary, authors of studies were contacted for further explanation of their results.
Assessment of risk of bias in individual studies
The data extractor assessed potential bias and validity in all included studies. The Newcastle-Ottawa Scale was used to assess the risk of bias in observational studies and the Cochrane Collaboration’s tool to assess risk of bias in randomized trials.24,25 Each tool evaluates multiple sources of bias and places an objective risk-of-bias value on each study. A specific cutoff value for assessing high- vs low-quality studies with either tool was not used because no such values have been rigorously determined; instead, the scales were used as qualitative comparators.
Risk of bias across studies
To assess bias across studies, published articles were read carefully for missing outcomes (measured, but not reported), and trial registries and other published protocols were examined, when possible, to compare with the final product.
Data synthesis and analysis
Relative risks and odds ratios (ORs) of infectious and allergic disease in children were considered the primary measures of treatment and exposure effect. Differences between means and other calculations of correlation coefficients were also used. Only adjusted summary statistics were reported, and when multiple adjustments were made, the most adjusted values were reported.
A meta-analysis could not be performed because of the heterogeneity of outcomes and the differences in the measurement of exposure between studies. Therefore, a summarized, qualitative review of the findings is presented, with similar results placed next to each other in the tables and text.
RESULTS
Study identification and selection
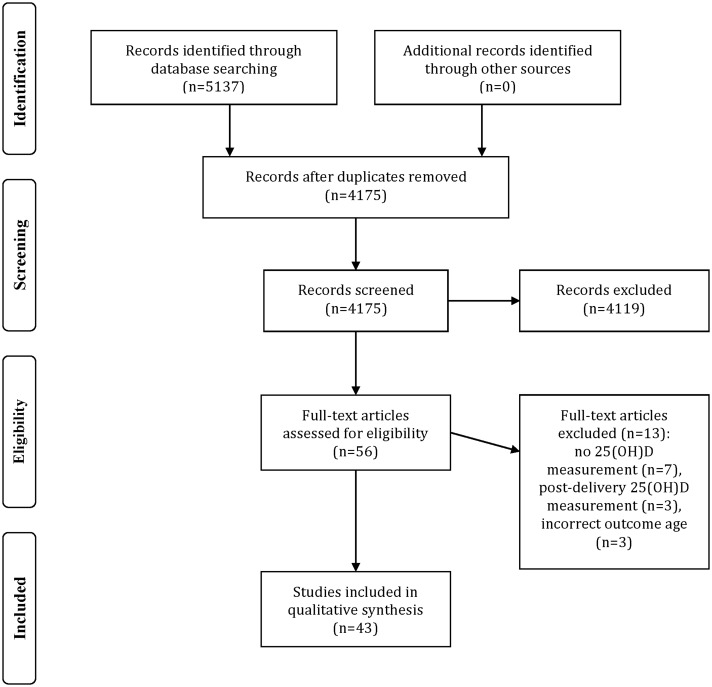
The search resulted in 4175 articles, with no additional articles found during the reference list search. After the initial title and abstract screening, 56 studies remained. Reasons for most of the initial exclusions were as follows: nonhuman subjects, lack of maternal or cord blood 25(OH)D data, or inappropriate outcomes (e.g., calcium, parathyroid hormone, and rickets). The 56 articles were reduced to 43 after review of the full-length papers. Seven were removed because of a lack of circulating vitamin D biomarker data, another 3 because they measured vitamin D status after delivery, and another 3 because they reported adult rather than pediatric outcomes (Figure 1)
Figure 1.
Flow diagram for identification and selection of studies
Of the 43 studies found, only 2 were randomized controlled trials. Though there are more published trials that examine the outcomes of interest, there were none that also measured cord or maternal 25(OH)D levels. The other 41 articles were observational, consisting of 6 case–control and 35 cohort studies. For 5 cohorts, there were 2 separate published papers that examined different variables within different subsets of the cohort.
Study characteristics
The studies varied in sites of origin, latitude, and ethnicity. Most cohorts excluded women with prior serious medical issues, problems during pregnancy, congenital defects, or premature delivery, except as follows: 2 studies (same cohort) included women with prior HIV infection,26,27 4 studies preferentially recruited parents with a history of allergic disease,28–31 and 3 studies included all expectant mothers who presented to the study sites, regardless of health status.32–34
Risk of bias
When assessed by the Cochrane Risk of Bias Assessment Tool,25 the randomized control trial by Goldring et al.35 included in this review scored low risk for all bias measures except performance bias, which scored higher because of lack of participant blinding (data not shown). Study personnel, though, were adequately blinded during outcome assessment and data analysis. Overall, the trial scored low risk for bias. The other randomized controlled trial, by Raqib et al.,36 scored low risk for all bias measures.
When the Newcastle-Ottawa Scale24 was used, the observational study scores ranged from 5 to 9 out of a maximum score of 9, lower scores indicating an increased risk of bias (see Table S3 in the Supporting Information online). Four studies scored 5, seven studies scored 6, and the rest scored 7 or higher. Seven studies had 2 different scores due to certain outcomes that involved a higher risk of bias than others.
Vitamin D status
Sixteen of the studies analyzed maternal serum 25(OH)D, 23 analyzed cord blood 25(OH)D, 2 analyzed both, 1 analyzed both maternal and neonatal blood within 72 hours of delivery, and 1 analyzed neonatal dried blood spots. For those that analyzed maternal serum, the timing of collection varied: 1 study collected from the first trimester, 4 from the second trimester, 10 from the third trimester, 1 from the first or second trimester, 1 from the second or third trimester, and 1 from any trimester.
Vitamin D status was also reported in multiple ways: means and medians (with interquartile ranges and/or standard errors/deviations), quartiles, and quintiles. The quartiles and quintiles were either natural or preset. Many studies used a variety of cutoff values to denote deficiency (<25, <27.5, <30, 30–50, <50 nmol/L), insufficiency (27.5–50, 25–50, 25–74.9, 30–50, 50–75 nmol/L), and sufficiency (>27.5, >30, >50, 50–75, >75, >80 nmol/L) or used other terms like severe deficiency (<30 nmol/L), low vitamin D (<80 nmol/L), and optimal vitamin D (>75 nmol/L). Some studies only set a deficiency cutoff value, and nearly half of the studies did not set any cutoffs, assessing outcomes only by quartiles/quintiles, 10–20 nmol/L increments, mean differences, and/or continuous 25(OH)D status.
The average 25(OH)D level in each study ranged between 43.5 and 73.7 nmol/L for maternal serum and between 17 and 82 nmol/L for cord blood.
Allergic disease
Of the 21 studies that assessed the association between vitamin D status and allergic disease, 12 measured 25(OH)D in cord blood, 9 in maternal serum (with 1 study assessing both), and 1 in dried blood spots taken within the first 3 days of life. The results are shown in Table 2.
Table 2.
Studies included in the analysis that examined allergic disease
| Reference | Study location | Study design, participants | Exposure and summary statistics for main outcome measures | Main outcome measures |
|---|---|---|---|---|
| Goldring et al. (2013)35 | London, United Kingdom |
|
|
|
| Baiz et al. (2014)44 | Nancy and Poitiers, France |
|
|
|
| Camargo et al. (2011)32 | Wellington and Christchurch, New Zealand |
|
|
|
| Chawes et al. (2014)30 | Copenhagen, Denmark |
|
|
|
| Chiu et al. (2014)43 | Keelung, Taiwan |
|
|
|
| Chiu et al. (2015)48 | Keelung, Taiwan |
|
|
|
| Gale et al. (2008)47 | Southampton, United Kingdom |
|
|
|
| Jones et al. (2012)38 | Perth, Australia |
|
|
|
| Jones et al. (2015)31 | Perth, Australia |
|
|
|
| Mommers et al. (2009)39 | The Netherlands |
|
|
|
| Morales et al. (2012)40 | Menorca Island, Valencia, Sabadell, and Gipuzkoa, Spain |
|
|
|
| Pike et al. (2012)42 | Southampton, United Kingdom |
|
|
|
| Rothers et al. (2011)29 | Tucson, Arizona, USA |
|
|
|
| Stelmach et al. (2015)45 | Poland |
|
|
|
| Weisse et al. (2013)50 | Leipzig, Germany |
|
|
|
| Wills et al. (2013)41 | United Kingdom |
|
|
|
| Zosky et al. (2014)46 | Western Australia |
|
|
|
| Magnus et al. (2013)33 | Norway |
|
|
|
| Mullins et al. (2012)52 | Canberra, Australia |
|
|
|
Abbreviations: IgE, immunoglobulin E; HR, hazard ratio; OR, odds ratio; RR, relative risk; CI, confidence interval; IQR, interquartile range; SD, standard deviation; NS, not significant; SPT, skin prick test.
All statistics are after maximal adjustment. Values significant at the P = 0.05 level in bold.
Thirteen studies assessed wheeze in a variety of ways, including persistent, early, late, or any wheeze (with regard to age of presentation). One study did not report the wheeze outcomes,37 and 8 showed no significant associations.31,35–43 The remaining 4 studies reported a negative association between 25(OH)D (3 cord, 1 maternal) and wheezing.32,44–46 Baiz et al.44 analyzed cord blood 25(OH)D as a continuous variable and found an association for early, transient wheezing (OR 0.67; 95%CI 0.54–0.81) but found none for late-onset or persistent wheezing. Camargo et al.32 found a negative association between cord blood 25(OH)D and wheezing from birth to age 5 years: <25 nmol/L (reference) vs 25–75 nmol/L (OR 0.61; 95%CI 0.44–0.86) and ≥75 nmol/L (OR 0.47; 95%CI 0.30–0.72), while Stelmach et al.45 showed a negative association between cord blood 25(OH)D and wheezing through age 2 years. This was strongest and most consistent with wheezing associated with viral infection (via multiple 25(OH)D comparisons). Finally, Zosky et al.46 found a negative association between maternal 25(OH)D and wheezing at age 6 years (≥50 vs <50 nmol/L) (OR 0.51; 95%CI 0.28–0.94), but not at age 14 years.
Twelve studies examined asthma, with all but 3 showing no association. All 3 studies with significant results used different cutoffs to examine maternal 25(OH)D. One study showed a positive association (OR 5.40; 95%CI 1.09–26.65), while the other 2 found negative associations (OR 0.67; 95%CI 0.48–0.95 and OR 0.22; 95%CI 0.06–0.92.)33,47,48 The 9 studies with nonsignificant results used a variety of 25(OH)D thresholds.29,30,32,40–44,46
None of the 6 studies looking at the association between 25(OH)D and allergic rhinitis reported significant associations.29,30,41,43,44,48 One study mentioned collecting data but did not report on the outcome.35
The 12 studies that examined atopic dermatitis (eczema) found conflicting results. Four studies revealed negative associations with cord blood 25(OH)D (3 analyzed it continuously, the other via a set of 3 cutoff values),31,38,44,48 while 2 others showed higher odds of atopic dermatitis in children born to mothers in the highest quartile of maternal 25(OH)D (>75 nmol/L in 1 and an unnamed nmol/L value in the other) compared with lower quartiles (<30 nmol/L and an unnamed cutoff for the third quartile).39,47 One of the latter 2 studies was published only as an abstract,39 and the other, by Gale et al.,47 looked at multiple childhood eczema outcomes: visible eczema at the 9-month visit, UK Working Party diagnosis at 9 months of age,49 and parental report at 9 years of age. Only the visible eczema outcome was significant. The remaining 6 studies showed no association between maternal or cord blood 25(OH)D and atopic dermatitis.30,35,41,43,45,50 One study did not report on the outcome.37
While 7 studies listed clinician-diagnosed food allergies as one of their measured outcomes, 2 did not report data on this outcome,35,37 and another was unable to assess allergies because of a small sample size.51 Of the remaining 4 studies, 2 found no association.38,45 Mullins et al.,52 though, found a decrease in peanut allergy in the third quartile (75–99.9 nmol/L) compared with the reference second quartile (50–74.9 nmol/L) but found no significant differences between the first or fourth quartiles compared with the reference quartile. The study by Weisse et al.50 showed a positive association between cord blood 25(OH)D, analyzed ordinally by quartile, and food allergy during the second year of life (OR 4.65; 95%CI 1.50–14.48) but no association with food allergy during the first year of life or with total incidence throughout the 2-year period. The same study also found a positive association between maternal 25(OH)D and food allergy during the second year of life as well as total 2-year incidence (OR 3.66; 95%CI 1.36–9.87 and OR 1.91; 95%CI 1.09–3.37, respectively), but not during the first year of life.
Five studies looked at general atopic sensitization using either total eosinophil counts35 or total IgE.29,30,35,41,50 Of these studies, only 1 revealed a significant protective association with 25(OH)D: Rothers et al.29 showed a 0.27 (95%CI 0.08–0.47) increase in log(total IgE) when comparing the group with cord blood 25(OH)D <50 nmol/L with the reference group (50–74.9 nmol/L). Other studies investigated specific allergic sensitization via the skin prick test or specific IgE levels in response to certain aeroallergens and food allergens. The 8 studies that used the skin prick outcome found no associations with either maternal or cord blood 25(OH)D.29,31,35,38,41,42,46 All but one30 of the studies looking at specific IgE levels found significant associations between maternal or cord blood 25(OH)D levels and allergic sensitization (detectable IgE levels). Two studies of the same cohort (1 analyzing cord blood and 1 analyzing maternal serum) found negative associations.43,48 Two other studies found a positive association between maternal 25(OH)D levels and detectable specific IgE,39,50 while the Rothers et al.29 study found a U-shaped association, with higher odds of having detectable specific IgE levels in infants with both lower and higher cord blood 25(OH)D levels compared with the reference quartile (50–74.9 nmol/L).
Finally, as reported in Table 3, Liu et al.51 showed that higher cord blood 25(OH)D was associated with lower levels of detectable specific IgE levels at 2 to 3 years in a subgroup of participants who had the interleukin-4 genotype rs2243250 CC/CT genotype. Interestingly, participants with the rs2243250 TT genotype showed an opposite association, with higher levels of 25(OH)D related to higher specific IgE levels (P for interaction = 0.003). Liu et al.37 also found other genotype-specific protective and harmful associations between cord blood 25(OH)D and detectable specific IgE in 1- to 3-year-olds, but the genotype–vitamin D interactions were not significant after adjusting for multiple testing.
Table 3.
Studies included in the analysis that examined allergic disease by genotype
| Reference | Study location | Study design, participants | Exposure and summary statistics for main outcome measures | Main outcome measuresa |
|---|---|---|---|---|
| Liu et al. (2011)37 | Boston, USA |
|
|
|
| Liu et al. (2013)51 | Boston, USA |
|
|
|
Abbreviations: CI, confidence interval; IgE, immunoglobulin E; kUA/L, international unit for IgE; OR, odds ratio; NS, not significant; SD, standard deviation; SNP, single nucleotide polymorphism.
aAll statistics are after maximal adjustment. Values significant at the P = 0.05 level are shown in bold type.
Infectious disease
Eighteen studies that examined the effect of blood vitamin D concentration on infectious disease outcomes in children were found, of which 10 measured cord blood, 7 measured maternal serum, and 1 measured both maternal and neonatal 25(OH)D levels after less than 3 days of life. Their results are shown in Table 4.
Table 4.
Studies included in the analysis that examined infectious disease
| Reference | Study location | Study design, participants | Exposure and summary statistics | Main outcome measuresa |
|---|---|---|---|---|
| Belderbos et al. (2011)53 | Utrecht, The Netherlands |
|
|
RSV LRTI (reported + RSV swab) (0–1 y): 0.16 (0.04 to 0.63) |
| Camargo et al. (2011)32 | Wellington and Christchurch, New Zealand |
|
|
|
| Cetinkaya et al. (2015)59 | Istanbul, Turkey |
|
|
|
| Chawes et al. (2014)30 | Copenhagen, Denmark |
|
|
|
| Cizmeci et al. (2014)60 | Istanbul, Turkey |
|
Cord blood 25(OH)D: median difference | Early-onset sepsis vs control (0–3 d): 31.5 vs 52.4 nmol/L, P = 0.038 |
| Finkelstein et al. (2012)26 | Dar es Salaam, Tanzania |
|
|
|
| Finkelstein et al. (2010)27 | Dar es Salaam, Tanzania |
|
|
|
| Gad et al. (2015)61 | Cairo, Egypt |
|
Cord blood 25(OH)D: unpaired t test of mean difference (SD) | Congenital pneumonia vs control (0–3 d): 86.94 (45.33) vs 232.63 (90.16) nmol/L, P = 0.0001 |
| Gale et al. (2008)47 | Southampton, United Kingdom |
|
|
|
| Goldring et al. (2013)35 | London, United Kingdom |
|
|
|
| de Jongh et al. (2014)56 | Southampton, United Kingdom |
|
|
|
| Luczynska et al. (2014)55 | Ulm, Germany |
|
|
|
| Magnus et al. (2013)33 | Norway |
|
|
|
| Mohamed et al. (2013)54 | Abha, Saudi Arabia |
|
|
|
| Morales et al. (2012)40 | Menorca Island, Valencia, Sabadell, and Gipuzkoa, Spain |
|
|
|
| Shin et al. (2013)57 | South Korea |
|
|
|
| Skowronsk a-Jozwiak et al. (2014)58 | Lodz, Poland |
|
|
|
| Stelmach et al. (2015)45 | Poland |
|
|
|
Abbreviations: CI, confidence interval; IQR, interquartile range; LRTI, lower respiratory tract infection; NS, not significant; OR, odds ratio; RR, relative risk; RSV, respiratory syncytial virus; SD, standard deviation; SE, standard error; URTI, upper respiratory tract infection.
aAll statistics are after maximal adjustment. Values significant at the P = 0.05 level are shown in bold type.
Lower respiratory tract infection (LRTI) was the most common infectious disease outcome studied, although the definition of LRTI varied, ranging from only including pneumonia or bronchiolitis to broadly including bronchiolitis, pneumonia, croup, bronchitis, and/or otherwise unspecified chest infections. Four of the 10 studies that measured LRTI used maternal 25(OH)D as an exposure, while the other 6 used cord blood 25(OH)D. Five studies found significant protective associations between 25(OH)D (2 maternal and 3 cord blood) and LRTI using different 25(OH)D comparison groups.33,40,53–55 These studies assessed 25(OH)D as a continuous variable or by comparing high vs low 25(OH)D: (>50 vs <25 nmol/L, ≥75 vs <50 nmol/L, ≥75 vs <75 nmol/L, or fourth quartile [>92.4 nmol/L] vs reference quartile [<54.6 nmol/L]). Moreover, 1 of these aforementioned studies also found a relation between lower cord blood 25(OH)D and greater severity of LRTI.54 In contrast, 2 studies found increased odds of LRTI in children born to mothers with higher 25(OH)D levels (>75 vs <30 and <25 nmol/L).47,56 Three studies found no association between cord blood 25(OH)D and LRTI.30,35,57
Studies also examined other respiratory infection outcomes. In addition to LRTI, Gale et al.47 also looked at other respiratory infections but found no association between maternal 25(OH)D levels and overall chest infections, bronchitis, or other respiratory infections (such as otitis media, upper respiratory tract infections, etc.). Stelmach et al.45 also found no association between 25(OH)D and the occurrence of more than 1 respiratory infection in the first 2 years of life. Shin et al.,57 on the other hand, found increased odds of any respiratory infection and nasopharyngitis in children with lower cord 25(OH)D but found no association with otitis media. Skowronska-Jozwiak et al.58 found more recurrent respiratory infections in children born to vitamin D–deficient mothers. Camargo et al.32 found decreased odds of any upper or lower respiratory tract infection as well as any other infections (e.g., urinary tract, skin, and common viral infections) in children with cord blood 25(OH)D ≥75 nmol/L compared with those with <25 nmol/L (OR 0.49; 95%CI 0.27–0.89 and OR 0.42; 95%CI 0.21–0.86, respectively). de Jongh et al.56 also looked at otitis media and found no association with 25(OH)D but did find decreased odds of prolonged cough symptoms in children born to vitamin D–sufficient mothers in the first 6 months of life (this association not seen after 6 months up to age 2 years).
A few studies examined general symptoms of infection as outcomes. Two studies by Finkelstein et al.26,27 of HIV-positive mothers and their children also looked at cough symptoms and found a decreased risk in children with mothers whose 25(OH)D level was sufficient (≥80 vs <80 nmol/L). One of those 2 studies also measured childhood tuberculosis infections and showed a similar negative association between maternal 25(OH)D levels and infection rates.27 Finally, the Gale et al.47 study found increased odds of diarrheal illness in children born to mothers with 25(OH)D >75 nmol/L vs <30 nmol/L. The de Jongh56 and Finkelstein et al.26,27 studies also looked at diarrhea symptoms but found no association with 25(OH)D.
Two studies found lower cord and maternal 25(OH)D levels in case subjects with neonatal sepsis compared with healthy controls.59,60 One of these studies only included patients with culture-proven sepsis, while the other did not find significant associations in the subset of cases with culture-proven sepsis. The Gad et al.61 study similarly found lower cord 25(OH)D levels in subjects with neonatal pneumonia compared with healthy controls.
Cord blood cellular and chemical immunological outcomes
Table S4 in the Supporting Information online shows the results of the 9 studies that looked at immunological outcomes in cord blood rather than direct clinical outcomes. This included cytokine release by stimulated mononuclear cells, regulatory T cell percentage of leukocytes, innate immunity gene expression, antimicrobial peptide concentration, and cytokine concentration in cord blood. Of the 5 studies that looked at regulatory T cells in cord blood, 3 found no associations.28,62,63 Vijayendra Chary et al.64 found a positive relationship between cord blood 25(OH)D concentrations and regulatory T cell percentages, while Weisse et al.50 found a negative relationship with cord blood 25(OH)D, but not with maternal 25(OH)D as an exposure. Guven et al.62 and Romero et al.63 looked at a variety of other data points related to cord blood lymphocytes, focusing on T-helper cells, and found no correlation with cord blood 25(OH)D.
Chi et al.28 found positive associations between cord blood 25(OH)D concentrations and interferon (IFN)-α and IFN-γ release by stimulated cord blood mononuclear cells. This effect was found in cells stimulated by either lipopolysaccharide or peptidoglycan (innate immunity stimuli) but not by phytohemagglutinin (nonspecific stimuli, indicator of overall T-cell function). Vijayendra Chary et al.64 and Zittermann et al.65 found a positive correlation between cord blood 25(OH)D levels and cord blood interleukin-10 concentrations. Vijayendra Chary et al.64 also found a positive correlation for transforming growth factor β concentrations.
The Raqib et al.36 study showed a negative relationship between cord blood 25(OH)D level and the concentration of LL37, the active antimicrobial C-terminal peptide of cathelicidin, in both cord blood serum and the cytoplasm of cord blood mononuclear cells. This did not equate to a change in bacterial killing capacity, though. The Mandic Havelka et al.66 study showed no association between cord blood 25(OH)D level and the concentration of LL37.
Walker et al.67 examined whether adult monocytes cultured in either vitamin D–sufficient or –deficient cord blood plasma would differ in their gene expression upon the addition of either innate or adaptive immune system stimuli. They found that cells cultured in vitamin D–deficient plasma (<30 nmol/L) had lower expression of the cathelicidin gene and other genes involved in vitamin D metabolism, which was reversible by the addition of exogenous 25(OH)D to the cultures.
Lung function and inflammation
Six studies that examined lung function and inflammation outcomes (forced vital capacity, forced expiratory volume 1, forced expiratory volume 25–75, bronchial hyper-responsiveness, and exhaled nitric oxide) were found, the results of which are shown in Table S5 in the Supporting Information online.30,35,41,42,46,69 Only 1 study found any significant association: Chawes et al.30 described a positive relationship between maternal 25(OH)D level and forced vital capacity at 6 years, but not at 14 years (children born to vitamin D–sufficient mothers could expel larger lung volumes at maximum effort). None of the lung function results were compared with actual clinical diagnoses of asthma or atopy.
Autoimmune disease
The search revealed 2 case–control studies in this category, both of which studied the association between maternal vitamin D status during pregnancy and type 1 diabetes in offspring in Scandinavian countries (results not shown). One of the studies, by Miettinen et al.,34 used first-trimester blood samples and found no significant differences in maternal 25(OH)D between the cases and controls. The other, though, by Sørensen et al.,69 found decreased odds of type 1 diabetes in the offspring of mothers in the highest quartile of 25(OH)D concentration (>89 nmol/L) compared with the lowest quartile (≤54 nmol/L) (OR 0.42; 95%CI 0.20–0.89).
DISCUSSION
This systematic review found that the current evidence linking direct measurement of in utero vitamin D exposure to childhood allergic outcomes is largely nonexistent or equivocal, although the weight of evidence is beginning to point toward a lack of association for wheeze, asthma, and allergic rhinitis. There is stronger and more consistent evidence, however, that higher in utero vitamin D exposure lowers the risk of LRTI in young children. The evidence for an association of in utero vitamin D with other pediatric infectious disease outcomes is lacking, as the few studies that examined these outcomes found equivocal results.
Allergic disease
Table 5 shows the estimated the strength of evidence for each major outcome.
Table 5.
Strength of evidence of the association between major outcomes and in utero vitamin D status
| Outcome | Type of finding (n = no. of studies) | Association with strongest evidence | Strength of evidencea |
|---|---|---|---|
| Allergic diseases | |||
| Wheeze | No association (n = 8), negative association (n = 4) | None | ++ |
| Asthma | No association (n = 9), positive association (n = 1), negative association (n = 2) | None | ++ |
| Atopic dermatitis/eczema | No association (n = 6), positive association (n = 2), negative association (n = 4) | None | + |
| Allergic rhinitis | No association (n = 6) | None | ++ |
| Food allergy measured via physician diagnosis | No association (n = 2), positive association (n = 1), negative association (n = 1) | None | + |
| Allergic sensitization without clinical corroboration | Specific sensitization measured via specific IgE: no association (n = 1), positive association (n = 2), negative association (n = 2), U-shaped association (n = 1), positive association in genetic subgroups only (n = 1), negative association in genetic subgroups only (n = 1) | Positive | + |
| Specific sensitization measured via skin prick tests: no association (n = 8) | None | +++ | |
| General sensitization measured via total IgE or eosinophil count: no association (n = 4), negative association (n = 1) | None | ++ | |
| Infectious diseases | |||
| Lower respiratory tract infection | No association (n = 3), positive association (n = 2), negative association (n = 5) | Negative | ++ |
| Upper respiratory tract infection | No association (n = 2), negative association (n = 1) | None | + |
| Tuberculosis | Negative association (n = 1) | Negative | + |
| Any respiratory tract infection | No association (n = 2), negative association (n = 3) | Negative | + |
| Any infection | Negative association (n = 1) | Negative | + |
| Cough | Negative association (n = 3) | Negative | + |
| Diarrhea | No association (n = 3), 1 positive association (n = 1) | None | + |
Abbreviation: IgE, immunoglobulin E.
aStrength of evidence supporting the associations was based on number and quality of studies and was defined as follows: strong (+++), moderate (++), and weak (+).
Infant and childhood wheezing is a frequently used outcome for measuring atopy, as it is a common asthma symptom and a risk factor for later development of asthma. The studies reviewed showed little evidence for an association between in utero vitamin D exposure and childhood wheezing. Despite its use, wheeze is not an optimal outcome because only a minority of children who wheeze will ever go on to develop asthma. The prevalence of childhood wheezing is much higher than that of asthma, and it is difficult to know which “wheezers” have predilection for asthma.70,71 Given the strong link between childhood wheezing and viral respiratory tract infection (due to smaller airway caliber and inflammation), wheezing could also act as a marker for increased risk of infection or for higher symptom severity when infected.72 Four studies looked at both of these outcomes: Camargo et al.32 found a negative association (similar in size as well) between cord blood 25(OH)D and both wheeze and respiratory infection. Goldring et al.35 found no association between cord blood 25(OH)D and either upper or lower respiratory tract infection. Stelmach et al.45 found a negative association between cord blood 25(OH)D and wheeze, but none for respiratory infections. Morales et al.,40 however, found no association between maternal 25(OH)D level and wheezing in the offspring but saw a reduced odds of LRTI with higher maternal 25(OH)D levels. To better elucidate this link, all future studies should measure both wheezing and respiratory infection in tandem.
Asthma is a difficult-to-measure outcome in birth cohorts because its age of presentation is later and more variable and its prevalence lower compared with most other outcomes of interest. For this reason, despite the predominant finding of no association, this outcome remains understudied, and larger sample sizes are needed in future cohorts. Although studies that looked at asthma had an average of about 1000 participants each, this number is skewed positively, with 6 studies having a sample size of less than 300 and 1 having a sample size over 5000. Additionally, 2 of the 3 studies that found a significant association used an asthma diagnosis by 3 or 4 years of age (via parental report). It is generally considered that asthma cannot be reliably diagnosed before age 5 years because of the difficulty in administering lung function tests in younger children.73 Thus, in the aforementioned studies, it is possible that parents misclassified wheezing associated with illness or reactive airway disease as asthma because it presents similarly to asthma and is often treated with the same medications. The studies did not capture childhood wheezing, which would help elucidate this issue.
Ten of the 12 studies reporting on asthma used parental report of physician diagnosis without corroborating chart review or pulmonary function tests. Current guidelines recommend performing lung function testing, specifically spirometry, as a cornerstone of asthma diagnosis.73 Four studies measured both parental reports and pulmonary function tests,32,41,42,44 of which only 1 compared the outcomes: Wills et al.41 found 44% of the positive parental reports also included evidence of bronchial hyper-responsiveness as demonstrated via objective testing, indicating a low concordance between current asthma and parental report of asthma. That study did not appear to have distinguished between the 2 groups (parental report only vs parental report plus a proven test) in the analysis. Misdiagnosis may therefore be a major limitation in studies that rely on parental report to measure asthma outcomes. Linking vitamin D status with both asthma-type lung function tests and a clinical diagnosis of asthma in the same patients would provide the strongest evidence of an association in future studies.
Allergic rhinitis is another outcome with a later age of presentation. The 6 studies reporting on this outcome did not find it to be associated with in utero 25(OH)D exposure. The global prevalence of allergic rhinitis in children aged 6 to 7 years and 13 to 14 years is estimated to be 8.5% and 14.6%, respectively, in the most comprehensive survey study to date.74 Four of the reviewed studies (Baiz et al.,44 Chiu et al.,43,48 Wills et al.41) found similar percentages, but those of Chawes et al.30 and Rothers et al.29 reported a prevalence of 12% at age 7 years and 19.8% by age 5 years, respectively (none followed children beyond age 4–7.5 y). This variability is likely due to the difficulty in diagnosing allergic rhinitis because it shares symptoms with common upper respiratory infections and rhinorrhea. The diagnosis relies mostly on a history of symptoms related to allergen exposure or seasonality, which is difficult to determine in young children, especially those under the age of 5 years. Therefore, neither parental report nor chart review may adequately measure the true prevalence. Given the existing evidence and the difficulty with diagnosis, it unlikely that future studies will find an association between in utero 25(OH)D and allergic rhinitis in children under the age of 5 years. That said, allergic rhinitis is directly linked to aeroallergen sensitization, and several studies found an association with the latter outcome. This indicates the potential for an effect of vitamin D on allergic rhinitis. Future studies should attempt to look beyond 5 years of age to better capture the diagnosis and to also try to simultaneously measure allergic sensitization. Measuring specific IgE levels is superior to the skin prick test in measuring allergic sensitization in young children because the skin prick test can be affected by eczema, antihistamine and anti-inflammatory medications, and measurement error. It would be good to correlate measurement of specific IgE levels with clinical diagnoses of allergic disease in future studies, as the results do not reveal anything about a child’s morbidity as stand-alone values.
The evidence for an association of in utero 25(OH)D with atopic dermatitis remains conflicting. The age-at-outcome ascertainment and method of measurement (parental report, UK Working Party diagnosis, and visible eczema on exam) differed between many of the studies, but no pattern exists between studies with and studies without associations. The study size (164–5513 participants) and participant characteristics also varied but do not appear to explain the difference in outcomes.
Food allergy requires more research, as only 4 studies reported associations for this outcome and they differed in the method of diagnosis employed (physician diagnosis, skin prick test, and parental report) and the type of allergy studied, likely explaining the heterogeneity of results.
Liu et al.’s37,51 finding of vitamin D’s protective effect on allergic sensitization in participants with certain genotypes is intriguing and may explain the U-shaped association found in the Rothers et al.29 study. If at-risk genotypes are rare or underrepresented within study cohorts, this could also explain the lack of or the equivocal associations for most of the allergic outcomes. Measuring genotypes in future studies, while expensive, may help define subgroups for which in utero vitamin D exposure holds the greatest intervention potential.
Infectious disease
Vitamin D’s association with pediatric infectious disease outcomes has been less frequently studied than its association with allergic disease outcomes. Tuberculosis, and “any infection” were included in 1 study each, “upper respiratory infection” in 3, and “any upper respiratory infection” in 2, which are too few to conclude any association with reasonable certainty. Of the 7 total studies that examined cough and diarrhea (3 and 4 studies, respectively) 2 of the studies for each came from the same cohort, which comprised a very specific population (pregnant, HIV-positive women in Africa), so no firm conclusions can be drawn about these outcomes, either. Cough and diarrhea are surrogate measures of disease and are therefore not the best outcomes to measure unless required because of the study population and setting.
This review found the evidence linking poorer in utero vitamin D status to LRTI to be moderate to strong, as half of the studies pointed to a negative association, including those with the 2 largest sample sizes. Additionally, the finding of a dose–response effect in 1 study that found decreased 25(OH)D levels to be associated with increased illness severity strengthens support for this association. Further research on this topic should use a more standardized definition of LRTI, one that certainly includes bronchiolitis and pneumonia but separates out croup (which strongly affects the upper airway as well) and more generalized chest infections, which may lead to misclassification of non-LRTI illnesses. The most important change recommended for this outcome is that future studies always attempt to measure LRTI via chart review, and not parental report, as LRTIs are diagnosed in physician’s offices far more frequently than other minor illnesses, given their severity and distinct clinical pictures.
Cord blood cellular and chemical immunology
The studies that performed basic immunological tests on cord blood, while fascinating, are limited by their lack of association with later clinical outcomes; moreover, each outcome was examined in only a small number of studies. These are mostly exploratory studies with small sample sizes examining novel outcomes, and they rarely adjust for adequate potential confounders such as season of birth and socioeconomic variables. For the purpose of this review, it is not possible to reach any firm conclusion from them. To enhance the mechanistic understanding of this, future studies would do well to look at immunological outcomes and childhood illness in the same cohorts.
Autoimmune disease
The association between in utero vitamin D and autoimmune disease in pediatric populations is largely unstudied. It is difficult to research autoimmune diseases outside of case–control studies, which do not provide the highest level of observational evidence, because of the low prevalence (much less than 1 in 1000) and late age of presentation for type 1 diabetes and other autoimmune disorders. Further research on this possible association would be fascinating but understandably difficult outside of locations that widely collect maternal or cord blood for large-scale data banks, again because of the low prevalence of these outcomes.
Limitations of the reviewed studies
Studies measured the exposure of 25(OH)D in many different ways. This made it impossible to perform a meta-analysis and difficult to compare results qualitatively between studies. While it would be helpful to see similar cutoff values used in future studies, no specific values based upon the current body of evidence can be recommended. More robust observational data, along with evidence showing that raising vitamin D via supplementation above a certain level reduces disease risk, is needed to create firm cutoff values.
A number of studies have shown that vitamin D supplementation during pregnancy can increase maternal and cord blood 25(OH)D.21,75,76 This increase is highly variable, though, as highlighted in 1 study that found an average increase in maternal 25(OH)D of 35.2 nmol/L (SD 31.7 nmol/L) after supplementation of 2000–4000 IU/d.21 This is why measured 25(OH)D, rather than supplementation status, was used for the purposes of this review. More trials that measure in utero 25(OH)D in addition to clinical outcomes in children are needed.
Maternal serum 25(OH)D is a commonly used measure of in utero vitamin D status, but assessment of cord blood obtained during delivery offers the most direct measurement of a fetus’s vitamin D exposure. The developing fetus relies entirely on its mother for vitamin D, and 25(OH)D readily crosses the placenta. Maternal and cord blood levels are directly associated, although, as seen in this review, cord blood levels are generally lower than maternal levels. That said, maternal and cord blood 25(OH)D levels are not precisely correlated, for poorly understood reasons. This may make it difficult to compare studies that measure maternal 25(OH)D with those that measure cord blood 25(OH)D. No guideline for adequate cutoff values for cord blood 25(OH)D exists, even for skeletal outcomes, so it is not possible to define deficiency and sufficiency using this biomarker. Additionally, all of the studies measured vitamin D at just a single time point during pregnancy. It is theoretically possible that the fetus is most vulnerable to vitamin D status during specific developmental periods, and a single measurement, usually in the third trimester, may not fully capture the relevant exposure. If studies do not measure vitamin D during this period of vulnerability, the effect size is likely to be underestimated.
The included studies largely relied on parental reporting for outcome assessment, which introduces the potential for misclassification error and recall bias. It is not known how often vitamin D status was shared with the study parents, which could influence their recall of illness as well. Overall, for the outcomes that can be measured via both physician diagnosis and/or parental report (e.g., wheezing, infections, allergies), 13 of 28 papers utilized parental questionnaires only, 1 used chart review only, and 6 included both in the analysis.
Sample sizes varied greatly, with 16 studies (excluding cord blood immunological studies) having fewer than 300 participants. Many of the outcomes of interest have a relatively high prevalence, so they were not necessarily underpowered, but it is possible that the difference between groups is smaller than predicted, leading to type II error in the smaller studies. That said, in this analysis, sample size did not appear to correlate with the presence or lack of significant associations.
While all but 1 observational study adjusted for potential confounders, the covariates that were included differed between studies. Overall measurements of covariates were good and included a wide variety of demographic and lifestyle factors that may confound the relationship between vitamin D and susceptibility to allergic and infectious diseases, such as season of birth, tobacco smoke exposure, breastfeeding, prematurity, maternal body mass index, parental income and education level, etc. Despite this, some studies did not include one or more of the following variables: ethnicity, maternal smoking, or season of birth, all of which were common confounders in the studies that did measure them. In addition, few studies measured nutritional intake, although this is thought to be a minor contribution to overall vitamin D status.22
Selective reporting bias was very hard to discern from the studies because of a general lack of published protocols. A few studies did not report on pertinent secondary outcomes they stated to have collected. These were often either minor outcomes or ones not related to the published material (e.g., infectious outcomes in an allergic disease study).
Strengths and weaknesses of the search strategy
This review is based on a very thorough search that was not limited by inputting outcomes into the search fields. Initially, an attempt was made to limit the number of titles/abstracts to review by inputting outcome terms, but it was determined early on that this strategy missed articles of interest. The strategy used (i.e., not inputting outcomes) ensured that the search captured the appropriate intervention/exposures but did not miss any that might have been overlooked as a result of the complexities of the medical subject headings for the most pertinent outcomes.
Embase was not searched because of a lack of access and the expense required to obtain it. This opens the possibility that some studies may have been missed, especially abstracts and European-based studies not widely published elsewhere.
Results in context
Systematic reviews have detailed robust evidence linking in utero vitamin D to pregnancy and birth outcomes such as preeclampsia, cesarean section, gestational diabetes, and birth weight.19,77–79 Very few previous systematic reviews have addressed the topic of vitamin D’s extraskeletal effects in children, and this review is the first to specifically focus on blood biomarkers of in utero vitamin D status. The most recent systematic review examining vitamin D in pregnancy and extraskeletal health effects in children is that of Christesen et al.,80 published in 2012. That review included fewer studies, a number of which used food questionnaires as an indirect measure of vitamin D status. The authors found evidence of decreased risk of allergic rhinitis and eczema, conflicting results for respiratory infections, wheezing, and type 1 diabetes, and a U-shaped association with allergic sensitization (assessed via inhalant allergen-specific IgE). Thorne-Lyman and Fawzi79 examined childhood infections in addition to birth and pregnancy outcomes in their 2012 review. Looking at a smaller selection of the same studies included in the present review, they found conflicting results with regard to respiratory infection and diarrheal disease.
The present review suggests no association between in utero vitamin D and childhood wheezing, while the Christesen et al.80 review reports conflicting evidence of an association. The use of the 25(OH)D biomarker of vitamin D exposure, rather than nutritional intake, as well as the inclusion of 4 additional (twice as many) studies makes the result of the present review more robust. As noted earlier, vitamin D intake is a poor indicator of status because it generally represents a small contribution to overall 25(OH)D production, and the increase in serum 25(OH)D in those who do take high supplementatal doses varies greatly.21,22
The present review did not find the same protective effect of vitamin D concentration against allergic rhinitis or eczema as Christesen et al.,80 but it included more studies (an additional 2 for allergic rhinitis and 5 for eczema). Additionally, the 2 studies unique to the Christesen et al.80 review, which showed significant associations for either eczema or allergic rhinitis, involved estimated vitamin D status based on food and supplement intake, rather than the serum biomarker. The specific IgE results in the present review are also more robust than those in the previous review, given the inclusion of 8 studies as opposed to just 1. Of note, the newer studies analyzed vitamin D status as a continuous variable or by using a single cutoff value, which precluded the reporting of a U-shaped effect, so it is not known whether this effect is a solitary finding.
Rather than the mixed results for LRTI found in both the Christesen et al.80 and the Thorne-Lyman and Fawzi79 reviews, the results of the present review, which includes a number of newer studies, point toward an association between lower in utero vitamin D exposure and a higher risk of LRTI.
CONCLUSION
This review highlights the reasons behind the lack of consensus among experts on what constitutes an adequate vitamin D status for optimal health, namely the limited and unclear evidence of associated outcomes. Pregnancy is perhaps the most hotly debated time period during which to study the effects of vitamin D, as in utero vitamin D may influence clinically important, potentially lifelong outcomes in children. Pregnancy is a very desirable point of intervention: it is a relatively short, defined period, and it precedes the development of illness and morbidity caused by potentially reversible exposures.
There was moderate to strong evidence for a protective association between in utero vitamin D exposure and LRTI. Most of the outcomes remain understudied. Larger studies, especially randomized controlled trials that measure serum 25(OH)D, are needed to further elucidate these topics. Future studies should also link certain outcomes together in the same participants, such as wheeze and respiratory infection; lung function tests and asthma; specific IgE and allergic rhinitis; and basic cord blood immunological tests and later allergic or infectious disease outcomes. Such research will help to elucidate the mechanisms behind the many potential extraskeletal effects of vitamin D.
Acknowledgments
Funding/support. D.G.-D. and M.R.K. are supported by NIGMS (National Institute of General Medicine Sciences) P20GM104416 from the NIH (National Institutes of Health), P01 ES022832 from the National Institute of Environmental Health Sciences at the NIH, and grant RD83544201 from the Environmental Protection Agency. These agencies had no role in the design and conduct of the study.
Declaration of interest. The authors have no relevant interests to declare.
SUPPORTING INFORMATION
The following Supporting Information is available through the online version of this article at the publisher’s website.
Table S1 PRIMSA 2009 Checklist
Table S2 Search strategies for Pubmed, April, 2015
Table S3 Newcastle-Ottawa Score for judging risk of bias in observational studies
Table S4 Studies included in the analysis examining cord blood cellular and chemical immunologic outcomes
Table S5 Studies included in the analysis examining lung function
REFERENCES
- 1.Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008;88:558S–564S. [DOI] [PubMed] [Google Scholar]
- 2.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48–54. [DOI] [PubMed] [Google Scholar]
- 3.Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev. 2008;66(10 suppl 2):S153–S164. [DOI] [PubMed] [Google Scholar]
- 4.Kumar J, Muntner P, Kaskel FJ, et al. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics. 2009;124:e362–e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung M, Lee J, Terasawa T, et al. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Int Med. 2011;155:827–838. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff-Ferrari HA, Willett WC, Orav EJ, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. New Engl J Med. 2012;367:40–49. [DOI] [PubMed] [Google Scholar]
- 7.Rajakumar K. Vitamin D, cod-liver oil, sunlight, and rickets: a historical perspective. Pediatrics. 2003;112:e132–e135. [DOI] [PubMed] [Google Scholar]
- 8.Holick MF. Vitamin D: extraskeletal health. Endocrinol Metabol Clin North Am. 2010;39:381–400, table of contents. [DOI] [PubMed] [Google Scholar]
- 9.Theodoratou E, Tzoulaki I, Zgaga L, et al. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ (Clinical research ed.). 2014;348:g2035 doi:10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr. 2008;87:1087s–1091s. [DOI] [PubMed] [Google Scholar]
- 11.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metabol. 2011;96:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142–1152. [DOI] [PubMed] [Google Scholar]
- 13.Hollis BW, Wagner CL. Vitamin D and pregnancy: skeletal effects, nonskeletal effects, and birth outcomes. Calcif Tissue Int. 2013;92:128–139. [DOI] [PubMed] [Google Scholar]
- 14.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. [DOI] [PubMed] [Google Scholar]
- 15.Boonstra A, Barrat FJ, Crain C, et al. 1α,25-Dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–4980. [DOI] [PubMed] [Google Scholar]
- 16.Gregori S, Casorati M, Amuchastegui S, et al. Regulatory T cells induced by 1α,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:1945–1953. [DOI] [PubMed] [Google Scholar]
- 17.Prietl B, Pilz S, Wolf M, et al. Vitamin D supplementation and regulatory T cells in apparently healthy subjects: vitamin D treatment for autoimmune diseases? Isr Med Assoc J. 2010;12:136–139. [PubMed] [Google Scholar]
- 18.Smolders J, Thewissen M, Peelen E, et al. Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis. PLoS One. 2009;4:e6635 doi:10.1371/journal.pone.0006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aghajafari F, Nagulesapillai T, Ronksley PE, et al. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ (Clinical research ed.). 2013;346:f1169 doi:10.1136/bmj.f1169. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097 doi:10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner CL, McNeil R, Hamilton SA, et al. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. Am J Obstet Gynecol. 2013;208:137.e1–137.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 suppl):1678S–1688S. [DOI] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells GA, Shea B, Connell O, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital website. 2011; http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed April 14, 2014. [Google Scholar]
- 25.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed.). 2011;343:d5928 doi:10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finkelstein JL, Mehta S, Duggan C, et al. Maternal vitamin D status and child morbidity, anemia, and growth in human immunodeficiency virus-exposed children in Tanzania. Pediatr Infect Dis J. 2012;31:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkelstein JL, Mehta S, Manji KP, et al. Maternal vitamin D status and child tuberculosis, anemia, and morbidity in Tanzania [abstract]. FASEB J. 2010;24:227.3. http://www.fasebj.org/cgi/content/meeting_abstract/24/1_MeetingAbstracts/227.3. Accessed December 12, 2013. [Google Scholar]
- 28.Chi A, Wildfire J, McLoughlin R, et al. Umbilical cord plasma 25-hydroxyvitamin D concentration and immune function at birth: the Urban Environment and Childhood Asthma study. Clin Exp Allergy. 2011;41:842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothers J, Wright AL, Stern DA, et al. Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona. J Allergy Clin Immunol. 2011;128:1093.e5–1099.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chawes BL, Bønnelykke K, Jensen PF, et al. Cord blood 25(OH)-vitamin D deficiency and childhood asthma, allergy and eczema: the COPSAC2000 birth cohort study. PLoS One. 2014;9:e99856 doi:10.1371/journal.pone.0099856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones AP, D'Vaz N, Meldrum S, et al. 25-hydroxyvitamin D3 status is associated with developing adaptive and innate immune responses in the first 6 months of life. Clin Exp Allergy. 2015;45:220–231. [DOI] [PubMed] [Google Scholar]
- 32.Camargo CA, Jr, Ingham T, Wickens K, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127:e180–e187. [DOI] [PubMed] [Google Scholar]
- 33.Magnus MC, Stene LC, Haberg SE, et al. Prospective study of maternal mid-pregnancy 25-hydroxyvitamin D level and early childhood respiratory disorders. Paediatr Perinat Epidemiol. 2013;27:532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miettinen ME, Reinert L, Kinnunen L, et al. Serum 25-hydroxyvitamin D level during early pregnancy and type 1 diabetes risk in the offspring. Diabetologia. 2012;55:1291–1294. [DOI] [PubMed] [Google Scholar]
- 35.Goldring ST, Griffiths CJ, Martineau AR, et al. Prenatal vitamin D supplementation and child respiratory health: a randomised controlled trial. PLoS One. 2013;8:e66627 doi:10.1371/journal.pone.0066627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raqib R, Ly A, Akhtar E, et al. Prenatal vitamin D3 supplementation suppresses LL-37 peptide expression in ex vivo activated neonatal macrophages but not their killing capacity. Br J Nutr. 2014;112:908–915. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Wang G, Hong X, et al. Gene-vitamin D interactions on food sensitization: a prospective birth cohort study. Allergy. 2011;66:1442–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones AP, Palmer D, Zhang G, et al. Cord blood 25-hydroxyvitamin D3 and allergic disease during infancy. Pediatrics. 2012;130:e1128–e1135. [DOI] [PubMed] [Google Scholar]
- 39.Mommers M, Bronsveld A, van Ree R, et al. Vitamin D status during pregnancy and early life and atopic outcomes in childhood. Allergy. 2009;64(suppl 90):21. [Google Scholar]
- 40.Morales E, Romieu I, Guerra S, et al. Maternal vitamin D status in pregnancy and risk of lower respiratory tract infections, wheezing, and asthma in offspring. Epidemiology. 2012;23:64–71. [DOI] [PubMed] [Google Scholar]
- 41.Wills AK, Shaheen SO, Granell R, et al. Maternal 25-hydroxyvitamin D and its association with childhood atopic outcomes and lung function. Clin Exp Allergy. 2013;43:1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pike KC, Inskip HM, Robinson S, et al. Maternal late-pregnancy serum 25-hydroxyvitamin D in relation to childhood wheeze and atopic outcomes. Thorax. 2012;67:950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiu CY, Yao TC, Chen SH, et al. Low cord blood vitamin D levels are associated with increased milk sensitization in early childhood. Pediatr Allergy Immunol. 2014;25:767–772. [DOI] [PubMed] [Google Scholar]
- 44.Baiz N, Dargent-Molina P, Wark JD, et al. Cord serum 25-hydroxyvitamin D and risk of early childhood transient wheezing and atopic dermatitis. J Allergy Clin Immunol. 2014;133:147–153. [DOI] [PubMed] [Google Scholar]
- 45.Stelmach I, Majak P, Jerzynska J, et al. Cord serum 25-hydroxyvitamin D correlates with early childhood viral-induced wheezing. Respir Med. 2015;109:38–43. [DOI] [PubMed] [Google Scholar]
- 46.Zosky GR, Hart PH, Whitehouse AJ, et al. Vitamin D deficiency at 16 to 20 weeks' gestation is associated with impaired lung function and asthma at 6 years of age. Ann Am Thorac Soc. 2014;11:571–577. [DOI] [PubMed] [Google Scholar]
- 47.Gale CR, Robinson SM, Harvey NC, et al. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiu CY, Huang SY, Peng YC, et al. Maternal vitamin D levels are inversely related to allergic sensitization and atopic diseases in early childhood. Pediatr Allergy Immunol. 2015;26:337–343. [DOI] [PubMed] [Google Scholar]
- 49.Williams HC, Burney PG, Hay RJ, et al. The U.K. Working Party's diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol. 1994;131:383–396. [DOI] [PubMed] [Google Scholar]
- 50.Weisse K, Winkler S, Hirche F, et al. Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy. 2013;68:220–228. [DOI] [PubMed] [Google Scholar]
- 51.Liu X, Arguelles L, Zhou Y, et al. Longitudinal trajectory of vitamin D status from birth to early childhood in the development of food sensitization. Pediatr Res. 2013;74:321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mullins RJ, Clark S, Wiley V, et al. Neonatal vitamin D status and childhood peanut allergy: a pilot study. Ann Allergy Asthma Immunol. 2012;109:324–328. [DOI] [PubMed] [Google Scholar]
- 53.Belderbos ME, Houben ML, Wilbrink B, et al. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127:e1513–e1520. [DOI] [PubMed] [Google Scholar]
- 54.Mohamed WA, Al-Shehri MA. Cord blood 25-hydroxyvitamin D levels and the risk of acute lower respiratory tract infection in early childhood. J Trop Pediatr. 2013;59:29–35. [DOI] [PubMed] [Google Scholar]
- 55.Luczynska A, Logan C, Nieters A, et al. Cord blood 25(OH)D levels and the subsequent risk of lower respiratory tract infections in early childhood: the Ulm birth cohort. Eur J Epidemiol. 2014;29:585–594. [DOI] [PubMed] [Google Scholar]
- 56.de Jongh RT, Crozier SR, D'Angelo S, et al. Maternal 25-hydroxyvitamin D levels in relation to offspring respiratory symptoms and infections. Eur Respir J. 2014;43:1181–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin YH, Yu J, Kim KW, et al. Association between cord blood 25-hydroxyvitamin D concentrations and respiratory tract infections in the first 6 months of age in a Korean population: a birth cohort study (COCOA). Korean J Pediatr. 2013;56:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skowronska-Jozwiak E, Lebiedzinska K, Smyczynska J, et al. Effects of maternal vitamin D status on pregnancy outcomes, health of pregnant women and their offspring. Neuro Endocrinol Lett. 2014;35:367–372. [PubMed] [Google Scholar]
- 59.Cetinkaya M, Cekmez F, Buyukkale G, et al. Lower vitamin D levels are associated with increased risk of early-onset neonatal sepsis in term infants. J Perinatol. 2015;35:39–45. [DOI] [PubMed] [Google Scholar]
- 60.Cizmeci MN, Kanburoglu MK, Akelma AZ, et al. Cord-blood 25-hydroxyvitamin D levels and risk of early-onset neonatal sepsis: a case–control study from a tertiary care center in Turkey. Eur J Pediatrics. 2015;174:809–815. [DOI] [PubMed] [Google Scholar]
- 61.Gad GI, Abushady NM, Fathi MS, et al. Diagnostic value of anti-microbial peptide, cathelicidin in congenital pneumonia. J Matern Fetal Neonatal Med. 2015;28:2197–2200. [DOI] [PubMed] [Google Scholar]
- 62.Guven A, Ecevit A, Sozer O, et al. Correlation between the cord vitamin D levels and regulatory T cells in newborn. Eur J Pediatr. 2012;171:1161–1166. [DOI] [PubMed] [Google Scholar]
- 63.Romero V, Somers E, Williams J, et al. Cord blood vitamin D status and pro-allergic Th2 to Th1-associated chemokine ratios: is there a link [abstract]? Reprod Sci. 2014;21(3 suppl 1):366A. [Google Scholar]
- 64.Vijayendra Chary A, Hemalatha R, Seshacharyulu M, et al. Vitamin D deficiency in pregnant women impairs regulatory T cell function. J Steroid Biochem Mol Biol. 2015;147:48–55. [DOI] [PubMed] [Google Scholar]
- 65.Zittermann A, Dembinski J, Stehle P. Low vitamin D status is associated with low cord blood levels of the immunosuppressive cytokine interleukin-10. Pediatr Allergy Immunol. 2004;15:242–246. [DOI] [PubMed] [Google Scholar]
- 66.Mandic Havelka A, Yektaei-Karin E, Hultenby K, et al. Maternal plasma level of antimicrobial peptide LL37 is a major determinant factor of neonatal plasma LL37 level. Acta Paediatr. 2010;99:836–841. [DOI] [PubMed] [Google Scholar]
- 67.Walker VP, Zhang X, Rastegar I, et al. Cord blood vitamin D status impacts innate immune responses. J Clin Endocrinol Metabol. 2011;96:1835–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cremers E, Thijs C, Penders J, et al. Maternal and child's vitamin D supplement use and vitamin D level in relation to childhood lung function: the KOALA Birth Cohort Study. Thorax. 2011;66:474–480. [DOI] [PubMed] [Google Scholar]
- 69.Sørensen IM, Joner G, Jenum PA, et al. Maternal serum levels of 25-hydroxy-vitamin D during pregnancy and risk of type 1 diabetes in the offspring. Diabetes. 2012;61:175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cornish RP, Henderson J, Boyd AW, et al. Validating childhood asthma in an epidemiological study using linked electronic patient records. BMJ Open. 2014;4:e005345 doi:10.1136/bmjopen-2014-005345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cano-Garcinuno A, Mora-Gandarillas I. Wheezing phenotypes in young children: an historical cohort study. Prim Care Respir J. 2014;23:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jackson DJ, Lemanske RF., Jr The role of respiratory virus infections in childhood asthma inception. Immunol Allergy Clin North Am. 2010;30:513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.National Asthma Education and Prevention Program: Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma–Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94–S138. [DOI] [PubMed] [Google Scholar]
- 74.Mallol J, Crane J, von Mutius E, et al. The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three: a global synthesis. Allergol Immunopathol (Madr). 2013;41:73–85. [DOI] [PubMed] [Google Scholar]
- 75.Dawodu A, Saadi HF, Bekdache G, et al. Randomized controlled trial (RCT) of vitamin D supplementation in pregnancy in a population with endemic vitamin D deficiency. J Clin Endocrinol Metabol. 2013;98:2337–2346. [DOI] [PubMed] [Google Scholar]
- 76.Hollis BW, Johnson D, Hulsey TC, et al. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26:2341–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nassar N, Halligan GH, Roberts CL, et al. Systematic review of first-trimester vitamin D normative levels and outcomes of pregnancy. Am J Obstet Gynecol. 2011;205:208.e201–e207. [DOI] [PubMed] [Google Scholar]
- 78.Urrutia RP, Thorp JM. Vitamin D in pregnancy: current concepts. Curr Opin Obstet Gynecol. 2012;24:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thorne-Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2012;2 (suppl 1):75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Christesen HT, Elvander C, Lamont RF, et al. The impact of vitamin D in pregnancy on extraskeletal health in children: a systematic review. Acta Obstet Gynecol Scand. 2012;91:1368–1380. [DOI] [PubMed] [Google Scholar]