Abstract
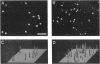
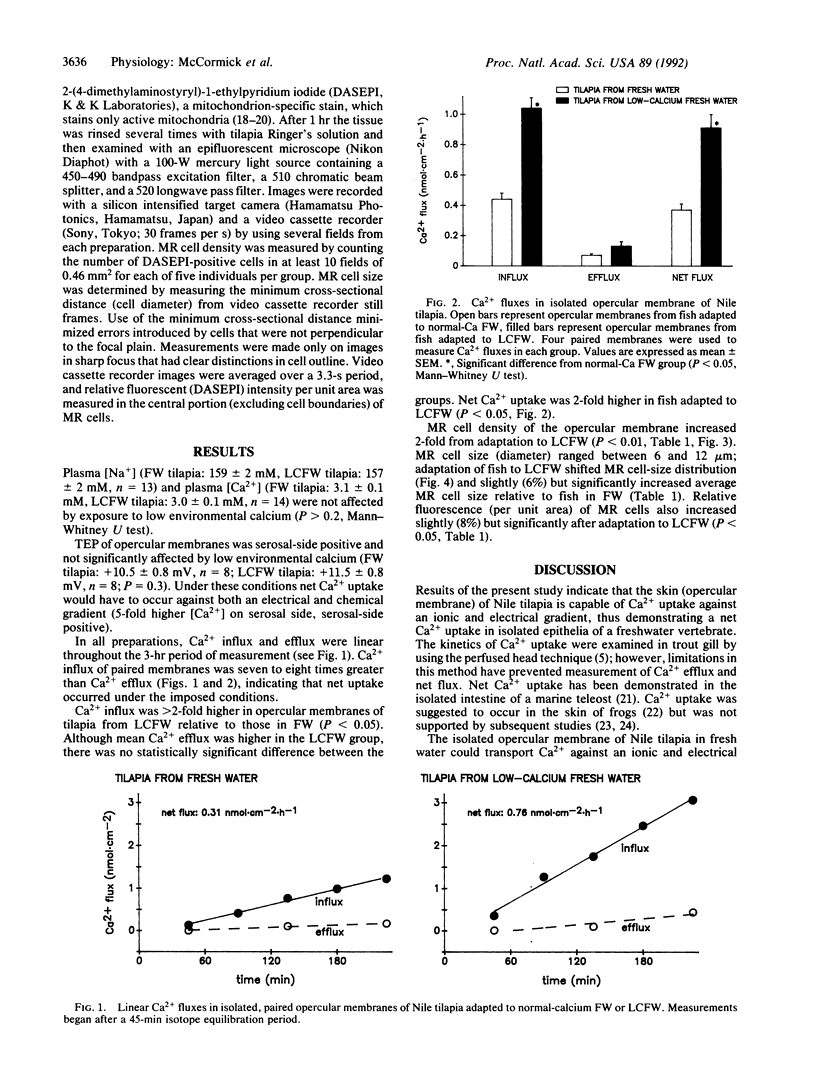
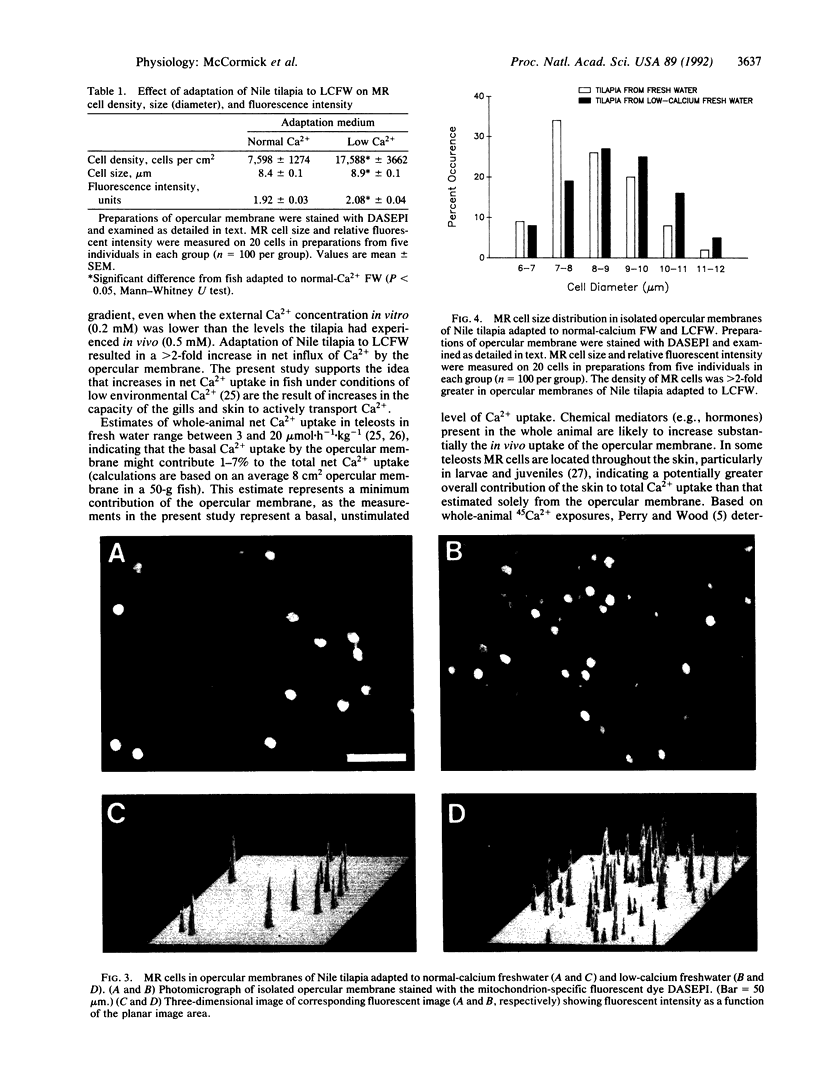
The skin, particularly the opercular membrane of some teleosts, contains mitochondrion-rich "chloride" cells and has been widely used as a model to study branchial salt-extrusion mechanisms in seawater fish. Skin isolated from the operculum of the freshwater Nile tilapia (Oreochromis niloticus) can transport Ca2+ against an ionic and electrical gradient. Adaptation of Nile tilapia to a low-Ca2+ environment increased the capacity of the opercular membrane to transport Ca2+. The density of mitochondrion-rich cells increased in parallel with Ca2+ transport capacity. The results demonstrate net Ca2+ uptake by vertebrate skin and strongly implicate mitochondrion-rich cells as the site of Ca2+ uptake in fresh water.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS J., COPELAND D. E. Chloride excretion in the head region of fundulus heteroclitus. Biol Bull. 1950 Dec;99(3):381–385. doi: 10.2307/1538468. [DOI] [PubMed] [Google Scholar]
- Bereiter-Hahn J. Dimethylaminostyrylmethylpyridiniumiodine (daspmi) as a fluorescent probe for mitochondria in situ. Biochim Biophys Acta. 1976 Jan 15;423(1):1–14. doi: 10.1016/0005-2728(76)90096-7. [DOI] [PubMed] [Google Scholar]
- Dharmamba M., Bornancin M., Maetz J. Environmental salinity and sodium and chloride exchanges across the gill of Tilapia mossambica. J Physiol (Paris) 1975 Dec;70(5):627–635. [PubMed] [Google Scholar]
- Flik G., Fenwick J. C., Kolar Z., Mayer-Gostan N., Wendelaar Bonga S. E. Whole-body calcium flux rates in cichlid teleost fish Oreochromis mossambicus adapted to freshwater. Am J Physiol. 1985 Oct;249(4 Pt 2):R432–R437. doi: 10.1152/ajpregu.1985.249.4.R432. [DOI] [PubMed] [Google Scholar]
- Flik G., Perry S. F. Cortisol stimulates whole body calcium uptake and the branchial calcium pump in freshwater rainbow trout. J Endocrinol. 1989 Jan;120(1):75–82. doi: 10.1677/joe.0.1200075. [DOI] [PubMed] [Google Scholar]
- Flik G., Wendelaar Bonga S. E., Fenwick J. C. Active Ca2+ transport in plasma membranes of branchial epithelium of the North-American eel, Anguilla rostrata LeSueur. Biol Cell. 1985;55(3):265–272. doi: 10.1111/j.1768-322x.1985.tb00436.x. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Machen T. E., Bern H. A. Chloride secretion and conductance of teleost opercular membrane: effects of prolactin. Am J Physiol. 1982 Mar;242(3):R380–R389. doi: 10.1152/ajpregu.1982.242.3.R380. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Scheffey C. The chloride cell: definitive identification as the salt-secretory cell in teleosts. Science. 1982 Jan 8;215(4529):164–166. doi: 10.1126/science.7053566. [DOI] [PubMed] [Google Scholar]
- Ichii T., Mugiya Y. Effects of a dietary deficiency in calcium on growth and calcium uptake from the aquatic environment in the goldfish, Carassius auratus. Comp Biochem Physiol A Comp Physiol. 1983;74(2):259–262. doi: 10.1016/0300-9629(83)90597-2. [DOI] [PubMed] [Google Scholar]
- Karnaky K. J., Jr, Degnan K. J., Garretson L. T., Zadunaisky J. A. Identification and quantification of mitochondria-rich cells in transporting epithelia. Am J Physiol. 1984 May;246(5 Pt 2):R770–R775. doi: 10.1152/ajpregu.1984.246.5.R770. [DOI] [PubMed] [Google Scholar]
- Karnaky K. J., Jr, Degnan K. J., Zadunaisky J. A. Chloride transport across isolated opercular epithelium of killifish: a membrane rich in chloride cells. Science. 1977 Jan 14;195(4274):203–205. doi: 10.1126/science.831273. [DOI] [PubMed] [Google Scholar]
- Keys A., Willmer E. N. "Chloride secreting cells" in the gills of fishes, with special reference to the common eel. J Physiol. 1932 Nov 5;76(3):368–378.2. doi: 10.1113/jphysiol.1932.sp002932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S. D. Cortisol directly stimulates differentiation of chloride cells in tilapia opercular membrane. Am J Physiol. 1990 Oct;259(4 Pt 2):R857–R863. doi: 10.1152/ajpregu.1990.259.4.R857. [DOI] [PubMed] [Google Scholar]
- McCormick S. D. Fluorescent labelling of Na+, K(+)-ATPase in intact cells by use of a fluorescent derivative of ouabain: salinity and teleost chloride cells. Cell Tissue Res. 1990 May;260(3):529–533. doi: 10.1007/BF00297233. [DOI] [PubMed] [Google Scholar]
- Naon R., Mayer-Gostan N. Ca2+-stimulated ATPase activities in the gill of the eel: interactions of Mg2+ ions. Am J Physiol. 1989 Feb;256(2 Pt 2):R313–R322. doi: 10.1152/ajpregu.1989.256.2.R313. [DOI] [PubMed] [Google Scholar]
- Payan P., Mayer-Gostan N., Pang P. K. Site of calcium uptake in the fresh water trout gill. J Exp Zool. 1981 May;216(2):345–347. doi: 10.1002/jez.1402160219. [DOI] [PubMed] [Google Scholar]
- Perry S. F., Flik G. Characterization of branchial transepithelial calcium fluxes in freshwater trout, Salmo gairdneri. Am J Physiol. 1988 Mar;254(3 Pt 2):R491–R498. doi: 10.1152/ajpregu.1988.254.3.R491. [DOI] [PubMed] [Google Scholar]
- Watlington C. O., Burke P. K., Estep H. L. Calcium flux in isolated frog skin; the effect of parathyroid substances. Proc Soc Exp Biol Med. 1968 Jul;128(3):853–856. doi: 10.3181/00379727-128-33141. [DOI] [PubMed] [Google Scholar]
- Young P. S., McCormick S. D., Demarest J. R., Lin R. J., Nishioka R. S., Bern H. A. Effects of salinity, hypophysectomy, and prolactin on whole-animal transepithelial potential in the tilapia Oreochromis mossambicus. Gen Comp Endocrinol. 1988 Sep;71(3):389–397. doi: 10.1016/0016-6480(88)90267-5. [DOI] [PubMed] [Google Scholar]
- Zadunaisky J. A., Lande M. A. Calcium content and exchange in amphibian skin and its isolated epithelium. Am J Physiol. 1972 May;222(5):1309–1315. doi: 10.1152/ajplegacy.1972.222.5.1309. [DOI] [PubMed] [Google Scholar]