Abstract
Context: Evidence from previous reviews is supportive of the hypothesis that whole grains may protect against various cancers. However, the reviews did not report risk estimates for both whole grains and cereal fiber and only case–control studies were evaluated. It is unclear whether longitudinal studies support this conclusion. Objective: To evaluate associations between whole grains and cereal fiber in relation to risk of lifestyle-related cancers data from longitudinal studies was evaluated. Data Sources: The following 3 databases were systematically searched: PubMed, EMBASE, and Cochrane CENTRAL. Study Selection: A total of 43 longitudinal studies conducted in Europe and North America that reported multivariable-adjusted risk estimates for whole grains (n = 14), cereal fiber (n = 23), or both (n = 6) in relation to lifestyle-related cancers were included. Data Extraction: Information on study location, cohort name, follow-up duration, sample characteristics, dietary assessment method, risk estimates, and confounders was extracted. Data Synthesis: Of 20 studies examining whole grains and cancer, 6 studies reported a statistically significant 6%–47% reduction in risk, but 14 studies showed no association. Of 29 studies examining cereal fiber intake in relation to cancer, 8 showed a statistically significant 6%–49% reduction in risk, whereas 21 studies reported no association. Conclusions: This systematic review concludes that most studies were suggestive of a null association. Whole grains and cereal fiber may protect against gastrointestinal cancers, but these findings require confirmation in additional studies.
Keywords: cancer risk, cereal fiber, longitudinal studies, systematic review, whole grains.
INTRODUCTION
Whole grains are widely recommended as an integral part of a healthy diet,1 so their significance in chronic disease prevention is of strong interest. Whole grains represent the intact, ground, cracked, or flaked kernel of grains for which the starchy endosperm, germ, and bran are found in the same relative portions as they exist in the intact grain even after separation and reconstitution of the grain.1 Whole grains provide both nutrients and non-nutrients, which confer numerous health benefits.2,3 Whole grains can be consumed as a single food, such as ready-to-eat breakfast cereals, brown rice, popcorn, buckwheat, bulgur, millet, oatmeal, quinoa, grain barley, whole rye, and whole wheat.1 Alternatively, whole grains can be consumed as an ingredient in foods, such as whole-wheat flour incorporated into crackers or breads. Refined grains have been processed to remove the outer bran and inner germ, retaining only the endosperm. This results in a substantial reduction in content of dietary fiber, B vitamins, iron, magnesium, vitamin E, and other components; many refined grain products may be subsequently enriched with these vitamins and minerals, but not with fiber.1
The 2015–2020 Dietary Guidelines for Americans recommend consuming at least half of the daily grain servings as whole grains to reduce chronic disease risk.1 Similarly, the World Cancer Research Fund and American Institute for Cancer Research cancer prevention guidelines recommend eating relatively unprocessed cereals with every meal and limiting the intake of refined starchy foods.4 Nevertheless, whole grain intakes fall short of dietary guidance. Data from a nationally representative sample suggest that less than 10% of American adults consume the recommended 3 or more whole-grain servings per day,5 with no significant increases in whole-grain intakes over the past 10 years.6
A review of case–control evidence suggests that whole grains protect against various cancers.7,8 Cancer is a major public health burden in the United States, with high prevalence and incidence rates.9 The World Cancer Research Fund and American Institute for Cancer Research claim that altering diet may play a pivotal role in reducing cancer incidence.4 A protective impact of whole grains on cancer risk is biologically plausible.10–12 Whole grains are a rich source of antioxidants, vitamins, trace minerals, phytate, phenolic acids, lignans, and phytoestrogens with anticarcinogenic properties. Whole grains are also a major source of fiber. Due to the hypothesized association between dietary fiber and cancer,13,14 it is important to consider whether cereal fiber alone or whole grains, as a collective package of measured and unmeasured dietary constituents, are associated with cancer risk.
Evidence on whole grains and cereal fiber has been previously reviewed in relation to obesity, type 2 diabetes, and cardiovascular disease.2 A recent review showed that high intakes of cereal fiber and whole grains had a protective impact on the risk of these chronic diseases.2 However, for cancer, previous reviews on the association between whole grains and risk of first incident cancer have been limited to evidence from case–control studies and do not report risk estimates for cereal fiber.7,8 Moreover, only one review, which focused on colorectal cancer, summarized the evidence from prospective cohorts and nested case–controls on the impact of whole grains and total dietary fiber but not fiber from grains.14 Therefore, the purpose of the present systematic review is to summarize the evidence from longitudinal epidemiological studies evaluating the impact of both whole grain and cereal fiber consumption on the risk of any cancer. The insights from this literature review provide important information that helps identify gaps in the current literature and the methodological inconsistencies in assessing dietary consumption of whole grains; the findings may also be useful for guiding clinical practice and policy initiatives for preventing cancer.
POTENTIAL MECHANISMS LINKING WHOLE GRAINS TO CANCER RISK
Whole grains may impact cancer risk through a variety of mechanisms. First, whole grains promote increased chewing and higher satiation, by virtue of their fiber content, structural integrity, and particle size, leading to lower body adiposity.15 Whole-grain foods are also characterized by lower energy density, resulting in reduced caloric intake without a concomitant increase in hunger.15 Whole grains have also been shown to influence body composition and measures of central adiposity.16,17 In particular, higher consumption of whole grains is inversely associated with percentage body fat, trunk fat mass, waist circumference, and waist-to-hip ratio.16,17 In addition to their impact on weight gain, whole grains are associated with lower risk of type 2 diabetes.2 High body mass index (BMI) and diabetes are, in turn, associated with higher cancer risk due to metabolic dysregulation observed among obese and diabetic individuals.18,19 Therefore, improved glycemic response and reduced insulin resistance may be a potential mechanism by which they are protective against cancer.20,21
Whole grains may also mediate cancer risk through their impact on sex hormones.10 Whole-grain foods are a source of phytoestrogens, such as lignans and isoflavones, which influence endogenous sex hormone production, metabolism, and biological activity. These substances reduce circulating estrogen levels, inhibit tumor initiation and growth, and decrease early markers of risk for mammary and colon carcinogenesis (reviewed in Slavin10). Based on these proposed mechanisms, whole grains may be particularly beneficial in reducing the risk of hormone-related cancers.10 Whole-grain foods are also a rich source of antioxidants, including vitamins (e.g., vitamin E), trace minerals (e.g., selenium), phenolic acids, lignans, and phytoestrogens, which collectively reduce oxidative damage and stress, thereby reducing cancer risk.22 Whole grains are an important source of magnesium, which has been associated with reduced risk of colorectal cancer.23
The protective role of whole grains in cancer risk, specifically colorectal cancer, may be attributed to the health benefits of their dietary fiber content.22 Dietary fiber, particularly from cereal sources, increases fecal bulk and reduces intestinal transit time, thereby diluting carcinogens and reducing their absorption by the intestinal epithelium. Cereal fiber, in addition to the resistant starch and oligosaccharides in whole grains, is fermented in the colon into short-chain fatty acids including butyrate.15 Butyrate is the preferred fuel for mucosal cells and has proapoptotic and antineoplastic potential, thus decreasing tumor growth. They also lower intestinal pH, thereby reducing the solubility of free bile acids and diminishing their availability for carcinogenic activity.15 Finally, cereal fiber has been associated with lower body adiposity2 and lower serum estrogen levels (reviewed in Aune et al.13), thereby potentially reducing risk for adiposity-related cancers.
METHODS
Approach and methodology
For this systematic review, a comprehensive search of 3 databases—PubMed, EMBASE, and Cochrane CENTRAL—for articles in the English language published from January 1, 1990, through October 17, 2014, was conducted. The Population, Intervention, Comparison, Outcomes, and Study Design criteria24 (Table 1) were used to formulate and narrow the focus of the following research question: Among adults, is consumption of whole grains and cereal fiber in the highest vs lowest category of intake associated with reduced risk of first-incident cancer in longitudinal studies? The Preferred Reporting Items for Systematic Reviews and Meta-Analyses method24 was used, as applicable for the topic of this review, to track and report progress on the systematic search for cohort and experimental studies examining the association between whole grains and cereal fiber in relation to cancer risk among adults.
Table 1.
PICOS criteria for inclusion and exclusion of studies
| Parameter | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population |
|
|
| Intervention/Exposure | Highest category of whole grain and cereal fiber intake (quintiles, quartiles, servings/week, servings/day, grams/day) | |
| Comparison | Lowest category of whole grain and cereal fiber intake (quintiles, quartiles, servings/wk, servings/d, g/d) | |
| Outcomes |
|
|
| Study design |
|
|
The following search terms were used, as appropriate, for each of the 3 databases to capture articles that report risk estimates on whole grain and cereal fiber consumption in relation to cancer risk: (“whole-grain” OR “whole grains” OR “wholegrains” OR “whole meal” OR “wholemeal” OR “cereal” OR “cereals” OR “cereal fiber” OR “cereal fibers” OR “grain fiber” OR “grain fibers” OR “grain fibre” OR “bran” OR “brans”) AND (“cancer” OR “cancers” OR “neoplasm” OR “neoplasms”).
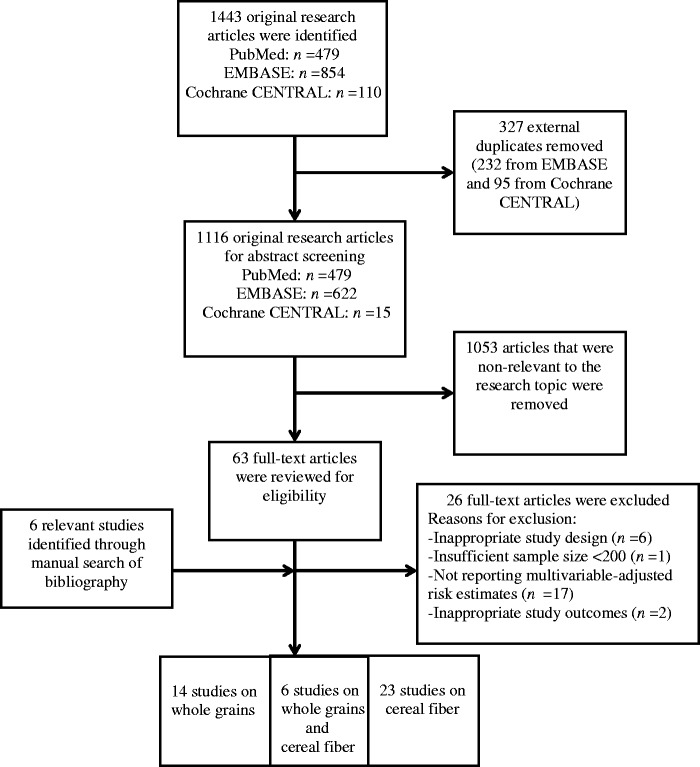
The literature search process is outlined in Figure 1. A total of 1443 research articles resulted from our search: 479 from PubMed, 854 from EMBASE, and 110 from Cochrane CENTRAL. After discarding 327 duplicates (95 from Cochrane CENTRAL and 232 from EMBASE), a total of 1116 research articles were identified for screening by 2 researchers who independently reviewed all manuscripts. Additionally, bibliographies were manually searched to supplement the online search process and ensure that all relevant studies were captured. After removing 1053 articles that were not relevant to the research topic based on abstract screening and identifying 6 additional studies manually, 69 full-text manuscripts were reviewed. There were no relevant randomized controlled trials.
Figure 1.
Flow diagram of the literature search process. A total of 43 studies were included in the final analysis after applying inclusion and exclusion criteria. Fourteen studies reported risk estimates on whole grains, 23 studies reported risk estimates on cereal fiber, and 6 studies reported risk estimates on both whole grains and cereal fiber in relation to cancer, respectively.
A total of 43 longitudinal studies were selected for inclusion in this systematic review. Fourteen studies reported risk estimates for whole grains, 23 reported risk estimates for cereal fiber, and 6 studies reported risk estimates for both, resulting in a total of 20 studies on whole grains and 29 on cereal fiber in relation to cancer, respectively. These studies evaluated whole grains and cereal fiber in relation to certain lifestyle-related cancers, which include female cancers (breast, ovarian, and endometrial), genitourinary cancers (renal, bladder, and prostate cancers), gastroinstestinal cancers (esophageal, gastric, liver, pancreatic, small intestinal, and colorectal cancers), head and neck cancers (oral cavity, pharyngeal, laryngeal, and overlapping regions cancers), and cancers of the reticuloendothelial system (lymphoma, myeloma, and leukemia). There was 1 study on lymphoma, 15 studies on female cancers, 20 studies on gastrointestinal cancers, 6 studies on genitourinary cancers, and 1 study on head and neck cancers.
Inclusion and exclusion criteria
Original research manuscripts were included if they 1) were intervention studies and observational studies, 2) reported estimates for risk of any cancer, 3) had a total sample size of at least 200 subjects, 4) presented hazard ratios or rate ratios, 5) conducted follow-up in cancer cases, and 6) presented multivariate analyses (not univariate analyses). Editorials, reviews, meta-analyses, cross-sectional studies, and case–control studies were excluded. Studies published in a language other than English were also excluded. Research articles that reported risk estimates for whole-grain food sources and total, soluble, and insoluble fiber with no data on total intake of whole grains and cereal fiber were also excluded from this review.
Data extraction
From each original research article, the following data were extracted: the lead author’s last name, year of publication, study location, cohort name, mean or median duration of follow-up, sample size, sex, age, number of cancer cases, method of dietary assessment (food frequency questionnaires [FFQs], diet records, or 24-h recalls), exposure (whole grains and/or cereal fiber), contrast (highest vs lowest category of intake), risk estimates (relative risks, hazard ratios, odds ratios), 95% confidence intervals (CIs), and confounders adjusted for in the final reported models.
Two investigators conducted the search. Both investigators reviewed all abstracts and, subsequently, full-text articles to identify relevant manuscripts for this review based on the inclusion and exclusion criteria. Both authors extracted the data from the relevant research articles and subsequently checked the extracted data to include study setting, year, design, sample, risk estimates, CIs, and covariates for accuracy. Both persons were required to independently assess the articles and then come to an agreement about any articles in question based on meeting the inclusion criteria. The objective parameters, including the sample size, study design, reporting of multivariable-adjusted risk estimates for whole grains and cereal fiber, and appropriate study outcome (first incidence of cancer), were considered to determine if the study’s quality merited its inclusion in this review. Investigators agreed that all included studies were deemed of good quality; however, an individual quality rating was not provided for each study.
RESULTS
Whole grains and cancer risk
A total of 20 longitudinal studies25–44 evaluated the associations between whole-grain intake and cancer risk, reporting risk estimates from tertile, quartile, and quintile analysis and/or regression analysis (Table 2; Figure 2). Fourteen studies25–38 reported a null association between whole-grain intake and cancer risk. Four studies reported a borderline significant reduction in cancer risk,31,36–38 whereas 1 study observed a nonsignificant increase in risk with higher levels of whole-grain intake.27 Six studies reported a 6%–47% statistically significant reduction in cancer risk among participants in the highest vs lowest category of whole-grain intake.39–44 These studies focused on colorectal,42–44 upper digestive tract,41 renal,40 and head and neck cancers39 as outcomes. The results from all studies are summarized by cancer site in the sections below, as the impact of whole grains on cancer may vary by cancer site.
Table 2.
Cohort studies evaluating whole-grain consumption in relation to cancer riska
| Reference | Location | Sample (n) | Outcome | Contrast | RR/HR (95% CI) | Covariates |
|---|---|---|---|---|---|---|
| Female cancers | ||||||
| Kasum et al. (2001)25 | USA; Iowa Women’s Health Study |
|
|
|
0.89 (0.61–1.29); Ptrend = .24 | Age, energy, education, BMI, smoking, vitamin use, fruits and vegetables, red meat, refined/whole grain, total fat, saturated fat, age at menarche, age at menopause, number of live births, and HRT |
| Nicodemus et al. (2001)27 | USA; Iowa Women’s Health Study |
|
|
|
1.21 (0.96–1.5); Ptrend = .02 | Age, energy, HRT, history of benign breast disease, family history of breast cancer, mammography status, age at first live birth, number of live births, weight, waist-to-hip ratio, vitamin use, education, vitamin A, and refined/whole grain |
| Fung et al. (2005)28 | USA; Nurses Health Study |
|
|
|
|
Age, smoking status, BMI, multivitamin use, energy, physical activity, family history of breast cancer, history of benign breast disease, duration of menopause, age at menopause, HRT, age at menarche, parity, age at first birth, BMI at age 18, weight change since age 18, adult height, and alcohol |
| Egeberg et al. (2009)30 | Denmark; Diet, Cancer and Health Cohort Study |
|
|
|
|
Parity, age at first birth, education, HRT, duration of HRT use, alcohol, and BMI |
| Aarestrup et al. (2012)26 | Denmark; Diet, Cancer and Health Cohort |
|
|
|
|
Menopausal status, HRT, HRT type, parity, age at first pregnancy, BMI, smoking, and energy |
| Genitourinary cancers | ||||||
| Egeberg et al. (2011)29 | Denmark; Diet, Cancer and Health Cohort Study |
|
Prostate cancer1081 cases |
|
|
Height, weight, education, red and processed meat, dairy products, and smoking |
| Nimptsch et al. (2011)33 | USA; Health Professionals Follow-up Study |
|
|
|
1.13 (1.03–1.24); Ptrend = .001 | BMI, height, history of diabetes, family history of prostate cancer, race/ethnicity, smoking, vigorous physical activity, energy, alcohol, calcium, alpha-linolenic acid, and tomato sauce |
| Drake et al. (2012)34 | Sweden; Malmo Diet and Cancer Cohort |
|
|
|
1.00 (0.78–1.28); Ptrend = .803 | Age, year of study entry, season of data collection, energy, height, waist, physical activity, smoking, educational, birth in Sweden, alcohol, calcium, selenium, competing risk by death from all causes except prostate cancer |
| Daniel et al. (2013)40 | USA; NIH–AARP Diet and Health Study |
|
|
|
0.84 (0.73–0.98); Ptrend = .05 | Age, sex, education, race, marital status, family history of any cancer, BMI, smoking, hypertension, diabetes, alcohol, red meat, energy, fruits, vegetables, legumes |
| Gastrointestinal cancers | ||||||
| Kasum et al. (2002)41 | USA; Iowa Women’s Health Study |
|
|
|
Age, smoking, alcohol, and energy | |
| McCullough et al. (2003)35 | USA; Cancer Prevention Study II Nutrition Cohort |
|
|
|
|
Age, physical activity, aspirin, smoking, family history of colorectal cancer, BMI, education, energy, multivitamin use, total calcium, red meat, and HRT |
| Wu et al. (2004)36 | USA; Health Professionals Follow-up Study |
|
|
|
0.75 (0.57–1.00); Ptrend = N/A | Age, family history of colorectal cancer, history of endoscopy, physical activity, smoking before age 30, race, aspirin use, and energy |
| Larsson et al. (2005)37 | Sweden; Swedish Mammography Cohort |
|
|
|
|
Age, BMI, education, energy, saturated fat, calcium, red meat, fruits, and vegetables |
| Schatzkin et al. (2007)42 | USA; NIH–AARP Diet and Health Study |
|
|
|
0.79 (0.70–0.89); Ptrend < .001 | Sex, physical activity, smoking, menopausal status, HRT, red meat, dietary calcium, dietary folate, and energy |
| Schatzkin et al. (2008)38 | USA; NIH–AARP Diet and Health Study |
|
|
|
0.59 (0.33–1.05); Ptrend = .06 | Age, sex, education, family history of cancer, smoking, BMI, physical activity, HRT, red meat, total fat, energy, and fiber |
| Egeberg et al. (2010)44 | Denmark; Diet, Cancer and Health Cohort Study |
|
|
|
|
BMI, alcohol, education, red and processed meat, HRT, and leisure time physical activity |
| Fung et al. (2010)43 |
|
|
|
|
|
Age, BMI, alcohol, family history of colorectal cancer, physical activity, aspirin, colonoscopy, history of polyps, smoking, energy, and multivitamin use |
| Kyro et al. (2013)31 | Scandinavia (Denmark, Sweden, Norway) |
|
|
|
|
Alcohol. smoking, education, HRT, red and processed meat, BMI, energy, and other grain products |
| Head and neck cancers | ||||||
| Kasum et al. (2002)41 | USA; Iowa Women’s Health Study |
|
|
|
Age, smoking, alcohol, and energy | |
| Lam et al. (2011)39 | USA; NIH–AARP Diet and Health Study |
|
|
|
|
Energy, education, BMI, physical activity, alcohol, smoking dose, red meat, and fiber |
| Hematologic cancers | ||||||
| Thompson et al. (2010)32 | USA; Iowa Women’s Health Study |
|
|
Whole-grain intake: >17 vs <4.6 servings/wk | 0.88 (0.66–1.17) | Age and energy |
a Dietary intakes of whole grains were measured using a food frequency questionnaire in all studies except the study by Drake et al. (2012)34, where a 7-day menu book and 1-hour dietary interview were also used.
b These hazard ratios do not have an associated confidence interval due to the small number of site-specific cancer cases, which limited statistical testing.
Abbreviations: BMI, body mass index; CI, confidence interval; ER, Estrogen Receptor; HR, hazard ratio; HRT, hormone replacement therapy; N/A, Not Applicable; NIH–AARP, National Institutes of Health–American Association of Retired Persons; PR, Progesterone Receptor; RR, relative risk.
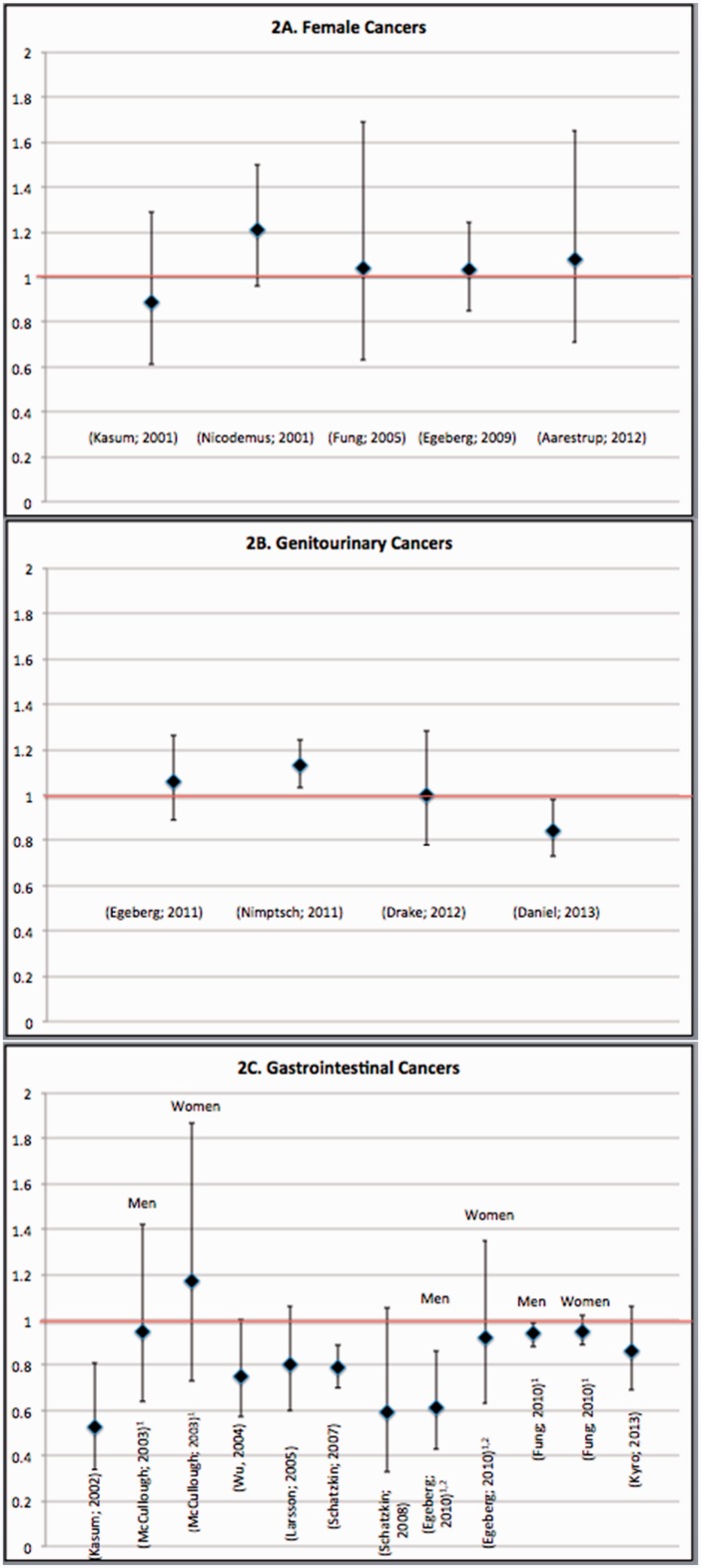
Figure 2.
Hazard ratios/relative risk ratios (95% confidence intervals) for associations between whole-grain intake and risk of (A) female cancers, (B) genitourinary cancers, and (C) gastrointestinal cancers. For studies of gastrointestinal cancers, in which risk estimates were not available for the sample as a whole, risk estimates for men and women are presented. For studies on colorectal cancers that provided risk estimates for colorectal, colon, and rectal cancers, results for colorectal cancer are presented, with the exception of the study by Egeberg et al. (2010),44 for which risk estimates for colon cancer are shown.
Female cancers.
A total of 5 prospective cohort studies (Figure 2A) evaluated whole-grain intake in relation to risk of breast27,28,30 and endometrial cancer.25,26 There was no significant association between whole-grain intakes and risk of these female cancers in any study. However, 1 analysis using the Iowa Women’s Health Study cohort was suggestive of a nonstatistically significant 21% higher risk of postmenopausal breast cancer among participants in the highest vs lowest quintile of whole-grain intake, and a statistically significant linear trend across the categories of intake was observed (relative risk, 1.21; 95% CI, 0.96–1.50; Ptrend = .02).27
Genitourinary cancers.
Four prospective cohort studies (2 conducted in Scandinavia29,34 and 2 in the United States33,40; Figure 2B) evaluated whole grains in relation to cancers of the genitourinary organs—namely, prostate cancer29,33,34 and renal cell carcinoma.40 Of 3 studies examining whole grains and prostate cancer risk,29,33,34 2 reported a null association,29,34 whereas 1 study was suggestive of a 13% increased risk of prostate cancer with higher consumption of whole grains (P = .001).33 Contrary to these findings, the 1 US prospective study on renal cell carcinoma found a statistically significant 16% reduction in the risk of this cancer among participants in the highest vs lowest quintile of whole grain consumption (hazard ratio [HR], 0.84; 95% CI, 0.73–0.98; Ptrend = .05).40
Gastrointestinal cancers.
Whole grain consumption and risk of gastrointestinal cancers has been evaluated in 9 studies (3 conducted in Europe31,37,44 and 6 in the United States35,36,38,41–43 (Figure 2C), primarily in relation to colorectal cancer31,35–37,42–44 but also in relation to small intestinal38 and upper aerodigestive tract cancers, which included esophageal and gastric cancers.41 Findings from approximately half of these studies were suggestive of whole grains protecting against gastrointestinal cancers.41–44 Four studies reported a statistically significant 6%–53% reduction in cancer risk with higher whole grain consumption.41–44 Among these studies, 1 study of a US cohort43 and 1 study of a Danish cohort44 found a protective impact among men only; however, in the Danish cohort, this was only observed in relation to colon but not rectal cancer.44 In contrast, there was no statistically significant association between whole grains and gastrointestinal cancers in 5 studies.31,35–38 Nevertheless, in 4 of these studies, evidence was suggestive of a borderline significant 14%–41% reduction in risk when comparing participants in the highest vs lowest category of whole-grain intake.31,36–38
Head and neck cancers.
The impact of whole grain consumption on risk of head and neck cancers was evaluated in 2 prospective cohort studies,39,41 which were suggestive of a protective impact of whole grains. In the National Institutes of Health–American Association of Retired Persons (NIH-AARP) cohort, higher whole-grain intakes were associated with a statistically significant 29% reduction in the risk of these cancers among women (HR, 0.71; 95% CI, 0.51–0.97), and a statistically significant linear trend was observed across the increasing categories of whole-grain intake (Ptrend = .005). However, only a non-significant 15% reduction in risk was observed among men (HR, 0.85; 95% CI, 0.72–1.01; Ptrend = .139).39 Similarly, in the Iowa Women’s Health Study, higher consumption of whole grains was associated with a 39%–66% lower risk of oral/pharyngeal, nasopharyngeal/salivary, and laryngeal cancers.41
Hematologic cancers.
One prospective cohort study evaluated whole grains in relation to hematologic cancers.32 This study examined whole-grain intake in relation to non-Hodgkin’s lymphoma using data from the Iowa Women’s Health Study cohort. The results were indicative of a null association. There were no studies on whole grains and lymphoma in men.
Cereal fiber and cancer risk
A total of 29 prospective cohort studies26,38–42,45–67 in US, Canadian, and European populations evaluated cereal fiber consumption in relation to cancer risk (Table 3; Figure 3). Eight studies reported a statistically significant 6%–49% reduction in risk of gastrointestinal and head and neck cancers.38,39,41,42,45–48 In contrast, 21 studies26,40,49–61 reported a null association, primarily in relation to female and genitourinary cancers. However, 2 studies were suggestive of reduced risk of breast52 and colorectal cancer61 with higher cereal fiber intake, but these associations were not statistically significant. These results are discussed separately in the sections below for female, genitourinary, gastrointestinal, and head and neck cancers.
Table 3.
Cohort studies evaluating cereal fiber consumption in relation to cancer riska
| Reference | Location | Sample (n) | Outcome | Contrast | RR/HR (95% CI) | Covariates |
|---|---|---|---|---|---|---|
| Female cancers | ||||||
| Terry et al. (2002)49 | Canada; Canadian National Breast Screening Study |
|
|
|
0.90 (0.78–1.04); Ptrend = .13 | Study center, treatment allocations, age, BMI, smoking, education, vigorous physical activity, oral contraceptives, HRT, parity, history of benign breast disease, history of breast self-exam, family history of breast cancer, menopausal status, energy, alcohol, dietary calcium, vitamin C, vitamin E, folic acid, and saturated fat |
| Cho et al. (2003)50 | USA; Nurses Health Study II |
|
|
|
0.91 (0.69–1.21); Ptrend = .21 | Smoking, height, parity, age at first birth, BMI, age at menarche, family history of breast cancer, history of benign breast disease, oral contraceptives, menopausal status, alcohol, energy, and animal fat |
| Giles et al. (2006)51 | Australia; Melbourne Collaborative Cohort Study |
|
|
|
1.08 (0.95–1.23); Ptrend = .33 | Age at attendance, country of birth, energy, and HRT |
| Cade et al. (2007)52 | UK; UK Women’s Cohort Study |
|
|
|
|
Age, BMI, physical activity, smoking, oral contraceptives, HRT, number of children, alcohol, and energy |
| Silvera et al. (2007)57 | Canada; Canadian National Breast-Feeding Study |
|
|
|
|
|
| Suzuki et al. (2008)53 | Sweden; Swedish Mammography Cohort |
|
|
|
|
Age, height, BMI education, parity, age at first birth, age at menarche, age at menopause, type of menopause, oral contraceptives, HRT, family history of breast cancer among first-degree relatives, history of benign breast disease, energy, energy-adjusted total fat, fruits and vegetables, alcohol, fruit fiber, vegetable fiber, and other fibers |
| Park et al. (2009)54 |
|
|
|
|
0.93 (0.85–1.02); Ptrend = .27 | Race, education, BMI, age at first birth, parity, family history of breast cancer, age at menopause, physical activity, smoking, HRT, breast biopsy, gynecologic surgery, alcohol, fruits and vegetables, total fat, and energy |
| Hedelin et al. (2011)58 | Sweden |
|
|
|
1.17 (0.74–1.87); Ptrend = N/A | Age, oral contraceptives, age at menarche, parity, HRT, energy, alcohol, saturated fat, meat, and fish |
| Aarestrup et al. (2012)26 | Denmark; Diet, Cancer and Health Cohort |
|
|
|
|
|
| Deschasaux et al. (2013)55 | France; Supplementation en Vitamines et Mineraux Antioxydants (SU.VLMAX) study |
|
|
|
1.43 (0.81–2.53); Ptrend = .1 | Age, intervention group, smoking, education, physical activity, height, BMI, number of dietary records, without-alcohol energy, alcohol, total fat, overall healthy dietary pattern, family history of breast cancer, menopausal status, HRT, and number of children |
| Ferrari et al. (2013)56 | Europe; European Prospective Investigation into Cancer and Nutrition cohort |
|
|
|
1.01 (0.95–1.08); Ptrend = .85 | Menopausal status, weight, interaction between weight and menopausal status, height, smoking, education, physical activity, age at menarche, age at first full-term birth, contraceptives, HRT, age at menopause, energy, and alcohol |
| Genitourinary cancers | ||||||
| Suzuki et al. (2009)59 | Europe; European Prospective Investigation into Cancer and Nutrition cohort |
|
|
|
1.03 (0.89–1.18); Ptrend = .85 | Age, energy, height, weight smoking, education, and marital status |
| Daniel et al. (213)40 | USA; NIH–AARP Diet and Health Study |
|
|
|
0.99 (0.84–1.15); Ptrend = .59 | Age, sex, education, race, marital status, family history of any cancer, BMI, smoking, hypertension, diabetes, alcohol, red meat, energy, fruits, vegetables, and legumes |
| Deschasaux et al. (2014)60 | France; Supplementation en Vitamines et Mineraux Antioxydants (SU.VLMAX) study |
|
|
|
1.05 (0.83–1.75); Ptrend = .7 | Age, energy without alcohol, intervention group, number of 24-h dietary records, smoking, education, physical activity, height, BMI, alcohol, family history of prostate cancer, prostate-specific antigen, dietary calcium, processed meat, tomato product, vitamin E, and blood selenium |
| Gastrointestinal cancers | ||||||
| Giovannucci et al. (1994)62 | USA; Health Professionals Follow-up Study |
|
|
|
1.28 (0.78–2.09); Ptrend = .16 | Age, energy, previous polyps, previous endoscopic screening, parental history of colorectal cancer, smoking, aspirin use, red meat, methionine, and alcohol |
| Terry et al. (2001)63 | Sweden; Swedish Mammography Cohort Study |
|
|
|
0.91 (0.69–1.20); Ptrend = .82 | Age, red meat, dairy products, and energy |
| Kasum et al. (2002)41 | USA; Iowa Women’s Health Study |
|
|
|
Age, smoking, alcohol, and energy | |
| Bingham et al. (2003)45 | Europe; European Prospective Investigation into Cancer and Nutrition cohort |
|
|
|
0·78 (0.62–0.98); Ptrend = .06 | Age, weight, height, sex, nonfat energy, and energy from fat |
| Mai et al. (2003)65 | USA; Breast Cancer Detection Demonstration Project |
|
|
|
1.02 (0.76–1.37); Ptrend = N/A | Age, nonsteroidal anti-inflammatory drugs, smoking, alcohol, calcium, vitamin D, red meat, height, BMI, and education |
| Bingham et al. (2005)64 | Europe; European Prospective Investigation into Cancer and Nutrition cohort |
|
|
|
0.93 (0.76–1.15); Ptrend = .44 |
|
| Lin et al. (2005)66 | USA; Women’s Health Study |
|
|
|
0.97 (0.66–1.42); Ptrend = .69 | Age, BMI, treatment assignment, family history of colorectal cancer, colon polyps, physical activity, smoking, aspirin, red meat, alcohol, energy, menopausal status, and HRT |
| Michels et al. (2005)61 | USA; Nurses Health Study and Health Professionals Follow-up Study |
|
|
|
|
|
| Mendez et al. (2007)48 | Europe; European Prospective Investigation into Cancer and Nutrition cohort |
|
|
|
0.69 (0.48–0.99); Ptrend = .01 | Sex, height, weight, education, smoking, other types of fiber, and stratification by age and center |
| Nomura et al. (2007)67 | USA; Multiethnic Cohort Study |
|
|
|
|
Age, ethnicity, time since cohort entry, family history of colorectal cancer, history of colorectal polyps, smoking, BMI, physical activity, aspirin use, multivitamin use, HRT, alcohol, red meat, folate, vitamin D, calcium, and energy |
| Schatzkin et al. (2007)42 | USA; NIH–AARP Diet and Health Study |
|
|
|
0.86 (0.76–0.98); Ptrend = .01 | Sex, physical activity, smoking, menopausal status, HRT, red meat, dietary calcium, dietary folate, and energy |
| Schatzkin et al. (2008)38 | USA; NIH–AARP Diet and Health Study |
|
|
|
0.51 (0.29–0.89); Ptrend = .01 | Age, sex, education, family history of cancer, smoking, BMI, physical activity, HRT, red meat, total fat, energy, and whole grain |
| Hansen et al. (2012)46 | Scandinavia; HELGA cohort |
|
|
|
|
BMI, smoking, education, HRT, alcohol, red, and processed meat |
| Murphy et al. (2012)47 | Europe; European Prospective Investigation into Cancer and Nutrition cohort |
|
|
|
|
|
| Head and neck cancers | ||||||
| Kasum et al. (2002)41 | USA; Iowa Women’s Health Study |
|
|
|
Age, smoking, alcohol, and energy | |
| Lam et al. (2011)39 | USA; NIH–AARP Diet and Health Study |
|
|
|
|
Energy, education, BMI, physical activity, alcohol, smoking dose, red meat, and grains |
a Cereal fiber intake was measured using a food frequency questionnaire in all studies, except the study by Drake et al. (2012),34 for which a 7-day menu book and a 1-hour dietary interview were also used, and the studies by Deschasaux et al. (2013),55 Suzuki et al. (2009)59 and Deschasaux et al. (2014),60 for which 24-hour recalls were used.
b These hazard ratios do not have an associated confidence interval due to the small number of site-specific cancer cases, which limited statistical testing.
Abbreviations: BMI, body mass index; CI, confidence interval; ER, Estrogen Receptor; HR, hazard ratio; HRT, hormone replacement therapy; N/A, Not Applicable; NIH–AARP, National Institutes of Health–American Association of Retired Persons; PR, Progesterone Receptor; RR, relative risk.
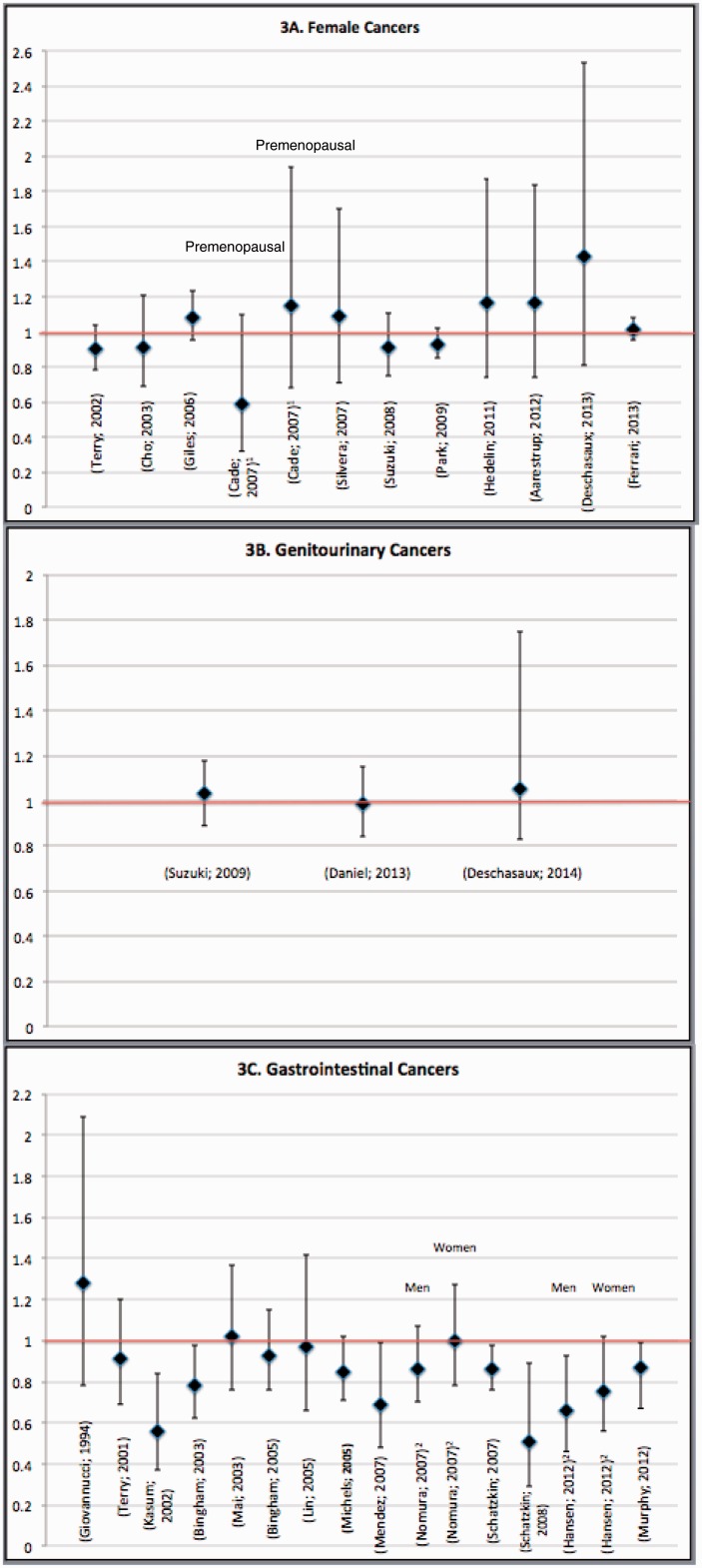
Figure 3.
Hazard ratios/relative risk ratios (95% confidence intervals) are shown for associations between cereal fiber intake in relation to risk of (A) female cancers, (B) genitourinary cancers, and (C) gastrointestinal cancers. For female cancers, risk estimates are presented for the whole sample, with the exception of one study by Cade et al. (2007),52 for which risk estimates were only reported separately for pre- and postmenopausal women. For gastrointestinal cancers, risk estimates are reported for the whole sample unless risk estimates were only reported separately for men and women.
Female cancers.
Eleven prospective cohort studies conducted in Europe and North America (Figure 3A) examined cereal fiber consumption in relation to the risk of breast,49–56 ovarian,57,58 and endometrial cancers.26 There was no statistically significant association in any study, although a borderline significant 41% reduction in breast cancer risk with higher cereal fiber consumption was observed among premenopausal women in 1 study performed in the United Kingdom (HR, 0.59; 95% CI, 0.32–1.10; Ptrend = .05).52
Genitourinary cancers.
Three prospective cohort studies (Figure 3B) evaluated cereal fiber in relation to genitourinary cancers.40,59,60 A null association was found in both studies on prostate cancer59,60 and in the study on renal cell carcinoma.40
Gastrointestinal cancers.
The majority of studies on cereal fiber and cancer focused on gastrointestinal cancers. Of 14 prospective cohort studies (Figure 3C), 11 studies examined cereal fiber in relation to colorectal cancers.42,45–47,61–67 However, there was only 1 study per cancer type for small intestinal cancer,38 upper aerodigestive tract cancers,41 and gastric adenocarcinoma.48 Similar to the studies on whole grains, approximately half of the evaluated studies were indicative of a protective impact of cereal fiber on gastrointestinal cancer risk.42,45–47 Seven studies were indicative of a null association between cereal fiber and colorectal cancer.61–67 However, 1 analysis of the Nurses Health Study and Health Professional Follow-up Study showed borderline significant 15% and 21% reductions in colorectal cancer risk in the pooled sample (HR, 0.85; 95% CI, 0.71–1.02) and among men (HR, 0.79; 95% CI, 0.60–1.05), respectively.61 In contrast, 7 studies in American and European cohorts indicated a statistically significant 6%–65% reduction in risk of colorectal cancer,42,45–47 small intestinal cancer,38 upper aerodigestive tract cancers, including esophageal and gastric cancers,41 and gastric adenoma.48 A statistically significant linear trend was observed across the categories of cereal fiber intake.38,41,42,48
Head and neck cancers.
Two prospective cohort studies that evaluated cereal fiber consumption in relation to head and neck cancers were suggestive of a protective association.39,41 In the NIH-AARP cohort, results indicated a statistically significant 44% reduction in head and neck cancer risk among women (HR, 0.66; 95% CI, 0.47–0.91), and a statistically significant linear trend was observed across the categories of cereal fiber intake (Ptrend = .003). However, there was no association among men. Similarly, in the Iowa Women’s Heath Study, higher cereal fiber consumption was associated with approximately 48% lower risk of oral/pharyngeal and nasopharyngeal/salivary cancers.41
DISCUSSION
This systematic review integrates epidemiological evidence from longitudinal observational studies on whole grains and cereal fiber in relation to cancer risk. Taken together, the majority of these studies were indicative of a null association. However, most of the studies that reported lack of an association evaluated whole grains and cereal fiber in relation to lymphoma, female, and prostate cancers as an outcome. In contrast, approximately half of the studies evaluating gastrointestinal cancers and the 2 studies on renal cell carcinoma and head and neck cancers were suggestive of a protective impact of whole grains and cereal fiber on cancer risk, but evidence was limited to draw firm conclusions. It is notable that among studies reporting risk estimates for both whole grains and cereal fiber, results were consistent for both exposures with the exception of one study. In that study,40 an inverse association was observed between whole-grain intake and renal cell carcinoma risk, but no association was observed for cereal fiber, suggesting that consuming whole grains may confer an additional protective impact independent of their cereal fiber content. However, this finding is unusual given the high correlation between whole-grain and cereal fiber intake, estimated at 0.7 in a recent report from the Iowa Women’s Health Study.68
Among the reviewed studies, it is notable that whole grains and cereal fiber have been studied mostly in relation to colorectal cancer. Hence, this review highlights a knowledge gap that remains to be addressed to better understand these relationships for other types of cancer. Furthermore, there were no studies on whole grains in relation to ovarian cancer. A single study evaluated whole grains in relation to lymphoma and was indicative of a null result. Similarly, there was only 1 study per cancer site for renal cell adenoma, gastric adenoma, small intestinal cancers, and aerodigestive tract cancers, and evidence was limited for prostate and endometrial cancers. Therefore, additional research is needed to clarify associations for these cancer sites.
Despite the biological plausibility of the hypothesized associations between whole grains and cereal fiber in relation to cancer risk, several methodological issues should be considered when examining the epidemiological evidence. Importantly, the inability to accurately and consistently measure whole-grain intake represents a confounding impediment to the interpretation of the existing evidence. First, studies may have varied in the definition of whole grains. Initially, whole-grain foods were defined as foods with 25% or more whole grains and bran by weight, including high-fiber bran cereals.69 Recently, high-fiber bran cereals have been excluded from this definition.70 Moreover, this definition contradicts the Food and Drug Administration definition that a whole-grain food is one that contains 51% or more whole-grain ingredients by weight per reference amount that is typically consumed.70 Most of the studies reviewed herein did not explicitly state their definition of whole-grain foods, which may have accounted for, at least in part, the differences in results.
Moreover, some studies in the literature report risk estimates for one or multiple whole-grain food sources in relation to cancer without reporting associations for total whole-grain intake. Although a food source may capture the majority of whole-grain intake in some countries (e.g., rye bread in Finland), this is not the case in other countries such as the United States. In contrast, other studies that evaluate total whole-grain intake do not report risk estimates for the various food sources, which is important due to a potential differential physiologic role for different types of grains. Hence, future studies should report risk estimates for both total whole-grain consumption and whole-grain foods in relation to cancer.
The reviewed studies were published over a time period of more than 2 decades during which the methodology used to measure cereal fiber—namely, the AOAC (Association of Official Analytical Chemists) method—has been refined.71 This may have resulted in varying estimates of the fiber content of cereal products over time, which may account, at least in part, for differences in study findings. Studies also varied in the measurement tools used to assess intakes of whole grains and cereal fiber. Although most studies used food frequency questionnaires, some studies also used diet records and 24-hour recalls, which may lead to various degrees of measurement error, making study results difficult to compare. Moreover, most food frequency questionnaires were not designed to measure consumption of whole grains and, therefore, include limited relevant food sources. This, coupled with the generally low consumption of whole-grain foods in the general population, may have made the range of intake too narrow to detect an association.
Another methodological issue and potential explanation for inconsistency in results across the studies is lack of adjustment for important cancer risk factors in some studies. Studies did not consistently adjust for BMI, family history of cancer, cancer screening habits, education or socioeconomic status, physical activity, red and processed meat intake, fat intake, antioxidant intake, and other factors. This is important because the impact of diet on cancer risk may vary by tumor pathology in addition to clinical, demographic, and other lifestyle characteristics. Moreover, not all studies evaluated these associations separately in men and women. In some of the studies in this review, a protective impact of whole grains on cancer risk was observed in men only,43,44 indicating that interactions with sex should be evaluated because associations between diet and cancer may be different for men and women. Additionally, not all studies on whole grains in relation to cancer adjusted for fiber and vice versa; this may have led to significant associations due to unaddressed confounding and difficulties in separating their independent roles.
Another important consideration when interpreting these results is study design. Although some short-term randomized controlled trials have evaluated whole grains and cereal fiber in relation to other chronic diseases, no such studies were located for cancer. Randomized controlled trials with sufficient sample size and long duration of follow-up that comprehensively adjust for relevant dietary, medical, and lifestyle factors may help clarify these associations and help assess the impact of confounding as an explanation for findings thus far. However, intervention studies may not be feasible due to cost and duration considerations. Therefore, well-designed observational studies that use reliable dietary measures, consistent definitions of whole grains, and sufficient follow-up and that comprehensively control for all appropriate confounders may be able to clarify these associations, especially for gastrointestinal cancers, for which the presently available evidence is most promising.
Finally, the literature on racial differences in nutritional factors and cancer risk is generally limited. All observational studies in this systematic review were conducted in European and North American cohorts. The available evidence is largely for Caucasian populations, although some studies enrolled racially diverse cohorts.28,35,38–40,42,43 Those studies did not stratify by race, however, possibly due to limited sample sizes. Additional research is warranted among other racial and ethnic groups which may have a unique cancer risk profile due to health disparities and for whom associations between diet and cancer may vary. Moreover, these groups may have limited access to healthful foods such as whole-grain products and, consequently, different levels of whole-grain intake. Therefore, health initiatives targeting whole-grain consumption may need to be tailored to the needs of these specific population groups.
CONCLUSION
Currently, the American Institute for Cancer Research and the American Cancer Society cancer prevention guidelines recommend choosing whole grains instead of refined grains as part of a comprehensive lifestyle approach to reduce cancer incidence.4,72 Based on the results of the present qualitative review, a potential protective impact of whole grains and cereal fiber is limited to certain types of cancer, including head and neck cancers, renal cell carcinoma, and gastrointestinal cancers, which collectively constitute approximately a quarter of incident cancers.9 Therefore, at an individual and population level, the consumption of whole-grain foods should be encouraged, primarily due to their protective impact on cardiovascular disease and diabetes, but also due to their potential role in reducing risk of these cancers, especially among high-risk individuals. However, government policy thus far has provided little regulation of whole-grain labeling73 and has eased the standards that require more whole-grain foods as part of the healthier school meals initiative.74 Moreover, whole-grain health claims sanctioned by the US Food and Drug Administration have been limited to their potential role in reducing the risk of heart disease.73 Thus, future policy and public health initiatives should focus on providing more rigorous regulation for the labeling of whole-grain products and promoting sufficient whole-grain consumption in various population groups as an integral component of a healthy diet that reduces the risk of chronic disease, including cancer.
Acknowledgments
Author contributions. N.P. and N.M. conceived this project. N.M. wrote the paper, developed the research plan, and assisted with conducting the search for relevant manuscripts. J.M.N. conducted the research for relevant manuscripts and assisted with writing the methods section. E.V.B. and N.M.M. provided insights toward reviewing and revising the manuscript for important intellectual content. N.P. reviewed the manuscript for important intellectual content, was responsible for overseeing the research process and had primary responsibility for the final content. All co-authors provided substantive comments and editorial review and approved the final version of the manuscript.
Funding. This research was supported by the American Cancer Society Research Scholar Grant (#RSG-12-005-01-CNE) awarded to N.P. The funder did not play any role in the design, execution, or approval of this review.
Declaration of interest. NMM has received funding for investigator-initiated grants from the General Mills Bell Institute of Health and Nutrition and from ILSI North America for Dietary Fiber Database and is a scientific advisor for the Whole Grains Council. The other authors have no relevant interests to declare.
References
- 1.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans. 8th Edition. December 2015. Available at http://health.gov/dietaryguidelines/2015/guidelines/. Accessed April 7, 2016. [Google Scholar]
- 2.Cho SS, Qi L, Fahey GC, Jr, et al. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am J Clin Nutr. 2013;98:594–619. [DOI] [PubMed] [Google Scholar]
- 3.Slavin J. Whole grains and human health. Nutr Res Rev. 2004;17:99–110. [DOI] [PubMed] [Google Scholar]
- 4.World Cancer Research Fund, American Institute for Cancer Research. Food, Nutrition, and Physical Activity and the Prevention of Cancer: a Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 5.Reicks M, Jonnalagadda S, Albertson AM, et al. Total dietary fiber intakes in the US population are related to whole grain consumption: results from the National Health and Nutrition Examination Survey 2009 to 2010. Nutr Res. 2014;34:226–234. [DOI] [PubMed] [Google Scholar]
- 6.McGill CR, Devareddy L. Ten-year trends in fiber and whole grain intakes and food sources for the United States Population: National Health and Nutrition Examination Survey 2001–2010. Nutrients. 2015;7:1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs DR, Jr, Slavin J, Marquart L. Whole grain intake and cancer: a review of the literature. Nutr Cancer. 1995;24:221–229. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs DR, Jr, Marquart L, Slavin J, et al. Whole-grain intake and cancer: an expanded review and meta-analysis. Nutr Cancer. 1998;30:85–96. [DOI] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: Cancer J Clin. 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 10.Slavin JL. Mechanisms for the impact of whole grain foods on cancer risk. J Am Coll Nutr. 2000;19(Suppl 3):300S–307S. [DOI] [PubMed] [Google Scholar]
- 11.Cotterchio M, Boucher BA, Manno M, et al. Dietary phytoestrogen intake is associated with reduced colorectal cancer risk. J Nutr. 2006;136:3046–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotterchio M, Boucher BA, Kreiger N, et al. Dietary phytoestrogen intake—lignans and isoflavones—and breast cancer risk (Canada). Cancer Cause Control. 2008;19:259–272. [DOI] [PubMed] [Google Scholar]
- 13.Aune D, Chan D, Greenwood D, et al. Dietary fiber and breast cancer risk: a systematic review and meta-analysis of prospective studies. Ann Oncol. 2012;23:1394–1402. [DOI] [PubMed] [Google Scholar]
- 14.Aune D, Chan DS, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. Brit Med J. 2011;343:521–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borneo R, León AE. Whole grain cereals: functional components and health benefits. Food Funct. 2012;3:110–119. [DOI] [PubMed] [Google Scholar]
- 16.Harland JI, Garton LE. Whole-grain intake as a marker of healthy body weight and adiposity. Public Health Nutr. 2008;11:554–563. [DOI] [PubMed] [Google Scholar]
- 17.McKeown NM, Yoshida M, Shea MK, et al. Whole-grain intake and cereal fiber are associated with lower abdominal adiposity in older adults. J Nutr. 2009;139:1950–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–316. [DOI] [PubMed] [Google Scholar]
- 19.Shikata K, Ninomiya T, Kiyohara Y. Diabetes mellitus and cancer risk: review of the epidemiological evidence. Cancer Sci. 2013;104:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H, Stote KS, Behall KM, et al. Glucose and insulin responses to whole grain breakfasts varying in soluble fiber, β-glucan. Eur J Nutr. 2009;48:170–175. [DOI] [PubMed] [Google Scholar]
- 21.Samra RA, Anderson GH. Insoluble cereal fiber reduces appetite and short-term food intake and glycemic response to food consumed 75 min later by healthy men. Am J Clin Nutr. 2007;86:972–979. [DOI] [PubMed] [Google Scholar]
- 22.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev. 2010;23:65–134. [DOI] [PubMed] [Google Scholar]
- 23.Larsson SC, Bergkvist L, Wolk A. Magnesium intake in relation to risk of colorectal cancer in women. J Am Med Assoc. 2005;293:86–89. [DOI] [PubMed] [Google Scholar]
- 24.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasum CM, Nicodemus K, Harnack LJ, et al. Whole grain intake and incident endometrial cancer: the Iowa Women’s Health Study. Nutr Cancer. 2001;39:180–186. [DOI] [PubMed] [Google Scholar]
- 26.Aarestrup J, Kyrø C, Christensen J, et al. Whole grain, dietary fiber, and incidence of endometrial cancer in a Danish cohort study. Nutr Cancer. 2012;64:1160–1168. [DOI] [PubMed] [Google Scholar]
- 27.Nicodemus KK, Jacobs DR, Jr, Folsom AR. Whole and refined grain intake and risk of incident postmenopausal breast cancer (United States). Cancer Cause Control. 2001;12:917–925. [DOI] [PubMed] [Google Scholar]
- 28.Fung TT, Hu FB, Holmes MD, et al. Dietary patterns and the risk of postmenopausal breast cancer. Int J Cancer. 2005;116:116–121. [DOI] [PubMed] [Google Scholar]
- 29.Egeberg R, Olsen A, Christensen J, et al. Intake of whole-grain products and risk of prostate cancer among men in the Danish Diet, Cancer and Health cohort study. Cancer Cause Control. 2010;22:1133–1139. [DOI] [PubMed] [Google Scholar]
- 30.Egeberg R, Olsen A, Loft S, et al. Intake of whole grain products and risk of breast cancer by hormone receptor status and histology among postmenopausal women. Int J Cancer. 2009;124:745–750. [DOI] [PubMed] [Google Scholar]
- 31.Kyrø C, Skeie G, Loft S, et al. Intake of whole grains from different cereal and food sources and incidence of colorectal cancer in the Scandinavian HELGA cohort. Cancer Cause Control. 2013;24:1363–1374. [DOI] [PubMed] [Google Scholar]
- 32.Thompson CA, Habermann TM, Wang AH, et al. Antioxidant intake from fruits, vegetables and other sources and risk of non-Hodgkin's lymphoma: the Iowa Women’s Health Study. Int J Cancer. 2010;126:992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nimptsch K, Kenfield S, Jensen M, et al. Dietary glycemic index, glycemic load, insulin index, fiber and whole-grain intake in relation to risk of prostate cancer. Cancer Cause Control. 2011;22:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drake I, Sonestedt E, Gullberg B, et al. Dietary intakes of carbohydrates in relation to prostate cancer risk: a prospective study in the Malmö Diet and Cancer cohort. Am J Clin Nutr. 2012;96:1409–1418. [DOI] [PubMed] [Google Scholar]
- 35.McCullough ML, Robertson AS, Chao A, et al. A prospective study of whole grains, fruits, vegetables and colon cancer risk. Cancer Cause Control. 2003;14:959–970. [DOI] [PubMed] [Google Scholar]
- 36.Wu K, Hu FB, Fuchs C, et al. Dietary patterns and risk of colon cancer and adenoma in a cohort of men (United States). Cancer Cause Control. 2004;15:853–862. [DOI] [PubMed] [Google Scholar]
- 37.Larsson S, Giovannucci E, Bergkvist L, et al. Whole grain consumption and risk of colorectal cancer: a population-based cohort of 60000 women. Br J Cancer. 2005;92:1803–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schatzkin A, Park Y, Leitzmann MF, et al. Prospective study of dietary fiber, whole grain foods, and small intestinal cancer. Gastroenterology. 2008;135:1163–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam TK, Cross AJ, Freedman N, et al. Dietary fiber and grain consumption in relation to head and neck cancer in the NIH-AARP Diet and Health Study. Cancer Cause Control. 2011;22:1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniel CR, Park Y, Chow WH, et al. Intake of fiber and fiber-rich plant foods is associated with a lower risk of renal cell carcinoma in a large US cohort. Am J Clin Nutr. 2013;97:1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasum CM, Jacobs DR, Nicodemus K, et al. Dietary risk factors for upper aerodigestive tract cancers. Int J Ccancer. 2002;99:267–272. [DOI] [PubMed] [Google Scholar]
- 42.Schatzkin A, Mouw T, Park Y, et al. Dietary fiber and whole-grain consumption in relation to colorectal cancer in the NIH-AARP Diet and Health Study. Am J Clin Nutr. 2007;85:1353–1360. [DOI] [PubMed] [Google Scholar]
- 43.Fung TT, Hu FB, Wu K, et al. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. Am J Clin Nutr. 2010;92:1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Egeberg R, Olsen A, Loft S, et al. Intake of wholegrain products and risk of colorectal cancers in the Diet, Cancer and Health cohort study. Br J Cancer. 2010;103:730–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bingham SA, Day NE, Luben R, et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet. 2003;361:1496–1501. [DOI] [PubMed] [Google Scholar]
- 46.Hansen L, Skeie G, Landberg R, et al. Intake of dietary fiber, especially from cereal foods, is associated with lower incidence of colon cancer in the HELGA cohort. Int J Cancer. 2012;131:469–478. [DOI] [PubMed] [Google Scholar]
- 47.Murphy N, Norat T, Ferrari P, et al. Dietary fibre intake and risks of cancers of the colon and rectum in the European prospective investigation into cancer and nutrition (EPIC). PLoS One. 2012;7:e39361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mendez M, Pera G, Agudo A, et al. Cereal fiber intake may reduce risk of gastric adenocarcinomas: the EPIC-EURGAST study. Int J Cancer. 2007;121:1618–1623. [DOI] [PubMed] [Google Scholar]
- 49.Terry P, Jain M, Miller AB, et al. No association among total dietary fiber, fiber fractions, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:1507–1508. [PubMed] [Google Scholar]
- 50.Cho E, Spiegelman D, Hunter DJ, et al. Premenopausal dietary carbohydrate, glycemic index, glycemic load, and fiber in relation to risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2003;12(11 Pt 1):1153–1158. [PubMed] [Google Scholar]
- 51.Giles GG, Simpson JA, English DR, et al. Dietary carbohydrate, fibre, glycaemic index, glycaemic load and the risk of postmenopausal breast cancer. Int J Cancer. 2006;118:1843–1847. [DOI] [PubMed] [Google Scholar]
- 52.Cade JE, Burley VJ, Greenwood DC, UK Women’s Cohort Study Steering Group. Dietary fibre and risk of breast cancer in the UK Women’s Cohort Study. Int J Epidemiol. 2007;36:431–438. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki R, Rylander-Rudqvist T, Ye W, et al. Dietary fiber intake and risk of postmenopausal breast cancer defined by estrogen and progesterone receptor status—a prospective cohort study among Swedish women. Int J Cancer. 2008;122:403–412. [DOI] [PubMed] [Google Scholar]
- 54.Park Y, Brinton LA, Subar AF, et al. Dietary fiber intake and risk of breast cancer in postmenopausal women: the National Institutes of Health-AARP Diet and Health Study. Am J Clin Nutr. 2009;90:664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deschasaux M, Zelek L, Pouchieu C, et al. Prospective association between dietary fiber intake and breast cancer risk. PloS One. 2013;8:e79718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferrari P, Rinaldi S, Jenab M, et al. Dietary fiber intake and risk of hormonal receptor-defined breast cancer in the European Prospective Investigation into Cancer and Nutrition study. Am J Clin Nutr. 2013;97:344–353. [DOI] [PubMed] [Google Scholar]
- 57.Silvera SA, Jain M, Howe GR, et al. Dietary fiber intake and ovarian cancer risk: a prospective cohort study. Cancer Cause Control. 2007;18:335–341. [DOI] [PubMed] [Google Scholar]
- 58.Hedelin M, Lof M, Andersson TM, et al. Dietary phytoestrogens and the risk of ovarian cancer in the women’s lifestyle and health cohort study. Cancer Epidemiol Biomarkers Prev. 2011;20:308–317. [DOI] [PubMed] [Google Scholar]
- 59.Suzuki R, Allen NE, Key TJ, et al. A prospective analysis of the association between dietary fiber intake and prostate cancer risk in EPIC. Int J Cancer. 2009;124:245–249. [DOI] [PubMed] [Google Scholar]
- 60.Deschasaux M, Pouchieu C, His M, et al. Dietary total and insoluble fiber intakes are inversely associated with prostate cancer risk. J Nutr. 2014;144:504–510. [DOI] [PubMed] [Google Scholar]
- 61.Michels KB, Fuchs CS, Giovannucci E, et al. Fiber intake and incidence of colorectal cancer among 76,947 women and 47,279 men. Cancer Epidemiol Biomarkers Prev. 2005;14:842–849. [DOI] [PubMed] [Google Scholar]
- 62.Giovannucci E, Rimm EB, Stampfer MJ, et al. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994;54:2390–2397. [PubMed] [Google Scholar]
- 63.Terry P, Giovannucci E, Michels KB, et al. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst. 2001;93:525–533. [DOI] [PubMed] [Google Scholar]
- 64.Bingham SA, Norat T, Moskal A, et al. Is the association with fiber from foods in colorectal cancer confounded by folate intake? Cancer Epidemiol Biomarkers Prev. 2005;14:1552–1556. [DOI] [PubMed] [Google Scholar]
- 65.Mai V, Flood A, Peters U, et al. Dietary fibre and risk of colorectal cancer in the Breast Cancer Detection Demonstration Project (BCDDP) follow-up cohort. Int J Epidemiol. 2003;32:234–239. [DOI] [PubMed] [Google Scholar]
- 66.Lin J, Zhang SM, Cook NR, et al. Dietary intakes of fruit, vegetables, and fiber, and risk of colorectal cancer in a prospective cohort of women (United States). Cancer Cause Control. 2005;16:225–233. [DOI] [PubMed] [Google Scholar]
- 67.Nomura AM, Hankin JH, Henderson BE, et al. Dietary fiber and colorectal cancer risk: the multiethnic cohort study. Cancer Cause Control. 2007;18:753–764. [DOI] [PubMed] [Google Scholar]
- 68.Jacobs DR., Jr Nutrition: the whole cereal grain is more informative than cereal fibre. Nat Rev Endocrinol. 2015;11:389–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacobs D, Meyer KA, Kushi LH, et al. Whole-grain intake may reduce the risk of ischemic heart disease death in postmenopausal women: the Iowa Women’s Health Study. Am J Clin Nutr. 1998;68:248–257. [DOI] [PubMed] [Google Scholar]
- 70.US Food and Drug Administration. Guidance for Industry: A Food Labeling Guide, 2014. Washington, DC: US Food and Drug Administration; 2014. http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm064919.htm. Accessed September 2, 2015. [Google Scholar]
- 71.Hollmann J, Themeier H, Neese U, et al. Dietary fibre fractions in cereal foods measured by a new integrated AOAC method. Food Chem. 2013;140:586–589. [DOI] [PubMed] [Google Scholar]
- 72.Kushi LH, Doyle C, McCullough M, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention. CA: Cancer J Clin. 2012;62:30–67. [DOI] [PubMed] [Google Scholar]
- 73.Whole Grains Council. Government guidance, 2013. http://wholegrainscouncil.org/whole-grain-stamp/government-guidance. Accessed September 2, 2015.
- 74.United States Department of Agriculture. Whole Grain Resource for the National School Lunch and School Breakfast Programs: A Guide to Meeting the Whole Grain-rich Criteria, 2014. http://www.fns.usda.gov/sites/default/files/WholeGrainResource.pdf. Accessed September 2, 2015.