Abstract
The intestinal microbiota undergoes active remodeling in the first 6 to 18 months of life, during which time the characteristics of the adult microbiota are developed. This process is strongly influenced by the early diet and enteric pathogens. Enteric infections and malnutrition early in life may favor microbiota dysbiosis and small intestinal bacterial overgrowth, resulting in intestinal barrier dysfunction and translocation of intestinal bacterial products, ultimately leading to low-grade, chronic, subclinical systemic inflammation. The leaky gut–derived low-grade systemic inflammation may have profound consequences on the gut–liver–brain axis, compromising normal growth, metabolism, and cognitive development. This review examines recent data suggesting that early-life enteric infections that lead to intestinal barrier disruption may shift the intestinal microbiota toward chronic systemic inflammation and subsequent impaired cognitive development.
Keywords: cognition, enteric infections, environmental enteropathy, intestinal microbiome, malnutrition
INTRODUCTION
Despite technological advances of the 21st century, diarrheal diseases still account for a staggering death toll worldwide in children under 5 years of age, largely owing to poverty combined with deplorable yet potentially remediable poor sanitation and hygiene. Most deaths occur in developing countries, with the largest numbers recorded in sub-Saharan Africa and Asia, where poverty is the greatest. Over the past 2 decades, mortality in children under 5 years of age has decreased substantially, but reductions have been variable in resource-limited regions.1,2 Improvements in global health initiatives, such as oral rehydration therapy, have contributed to declines in mortality due to diarrheal disease, yet 1.3 million people still die every year across all ages.3 In 2011, 700 000 children under 5 years of age died because of sequelae of diarrheal illness, with a high proportion of deaths occurring in the first 2 years of life.2 However, a considerable proportion of the enteric infection burden is subclinical and may be present in a chronic or prolonged state. Since this type of infection does not necessarily lead to hospitalization, it is a silent and neglected health issue that is difficult to track and likely to be largely unrecognized by official health statistics.
Recently, much effort has gone into understanding how prolonged or repeated enteric infections (with or without diarrhea) are associated with an unhealthy gut environment that may lead to metabolic and even epigenetic consequences, with lasting negative effects on growth, cognition, and educational achievement that can reduce the likelihood of an individual reaching his or her full potential.4–6 Prolonged or repeated enteric infections have long-lasting consequences, since the effects may extend throughout a lifetime and may even be multigenerational, leading to an unresolved vicious cycle of poverty, low education, and costly poor health and well-being of individuals and society.7
Although chronic low-grade intestinal inflammation has been increasingly recognized as a factor contributing to poor intestinal absorption of nutrients,8 the underlying mechanisms remain poorly understood. Recently, it has been shown that genetically engineered mice with toll-like receptor 5 deficiency only in enterocytes demonstrate low-grade inflammation and develop metabolic syndrome. These effects were associated with changes in the intestinal microbiota and levels of bacterial lipopolysaccharide (LPS) and flagellin, since antibiotic treatment reduced these effects.9
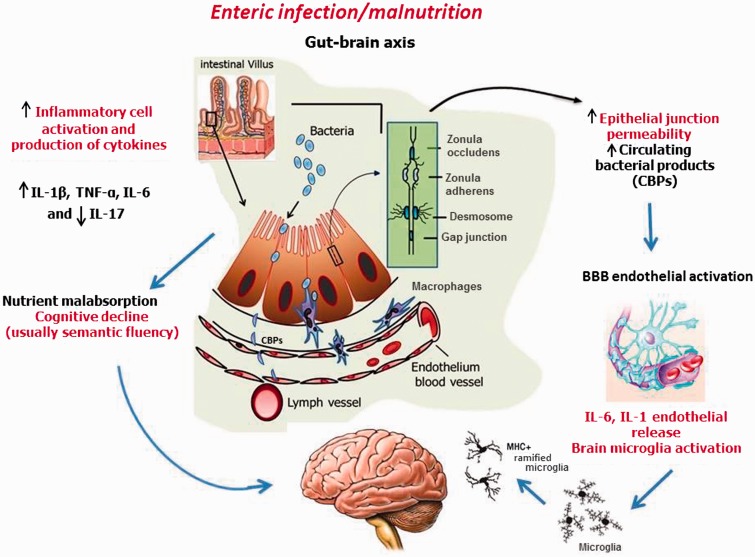
In this review, novel findings pointing to increasing evidence of the often-hidden costs of the “impoverished gut” are examined, shedding light on how enteric pathogens (often coupled with inadequate diets) can shift the balance of the intestinal microbiota toward inflammation, malabsorption, and potential low-grade liver and brain inflammation and have significant consequences for metabolism and cognitive development in children afflicted by enteric diseases early in life. Figure 1 shows a proposed model of how prolonged (even if subclinical) intestinal barrier dysfunction can lead to a status of chronic local and systemic inflammation, with profound effects on brain development.
Figure 1.
Proposed model of how a prolonged state of enteric infection/malnutrition causes systemic inflammation, thereby affecting the gut–brain axis. Malnutrition and repeated enteric infection in the first 2 years of life may cause intestinal barrier leakage. Intestinal dysbiosis may facilitate luminal-to-blood pathogenic bacterial translocation and, consequently, low-grade systemic inflammation. Circulating bacterial products may activate the endothelial cells, which form the blood–brain barrier, to release proinflammatory cytokines to prime and activate microglial cells. An early-in-life subclinical neuroinflammatory state may affect cognitive development in children. Abbreviations and symbols: ↑, increased; ↓, decreased; BBB, blood–brain barrier; CBPs, circulating blood products; IL, interleukin; MHC, major histocompatibility complex; TNF-α, tumor necrosis factor-α.
Environmental enteropathy and the intestinal microbiota
Intestinal microbiota.
The intestinal microbiota, approximately 100 trillion microbial cells, is composed of a remarkable range of bacterial (and other microbial) taxa that outnumber human body cells. The most abundant bacterial species are members of the phyla Firmicutes and Bacteroidetes, with smaller numbers being representative of the phyla Proteobacteria, Fusobacteria, Cyanobacteria, Verrucomicrobia, and Actinobacteria, among others.10,11 The gut microbiota is composed mainly of anaerobes, which outnumber facultative anaerobes and aerobic bacteria by approximately 2 to 3 orders of magnitude.12 The intestinal microbiota exists in a symbiotic relationship with the host that is reflective or a result of interactions with an intact intestinal mucosal barrier and an effective mucosal immune system.13 Changes in both bacterial abundance and species diversity within the gastrointestinal tract are locale dependent; specifically, a dynamic, diet-luminal-driven microbiota, which may alter intestinal absorptive capacity, is present, especially in the distal small bowel.14
Dietary factors, intestinal immunity, and the microbiota.
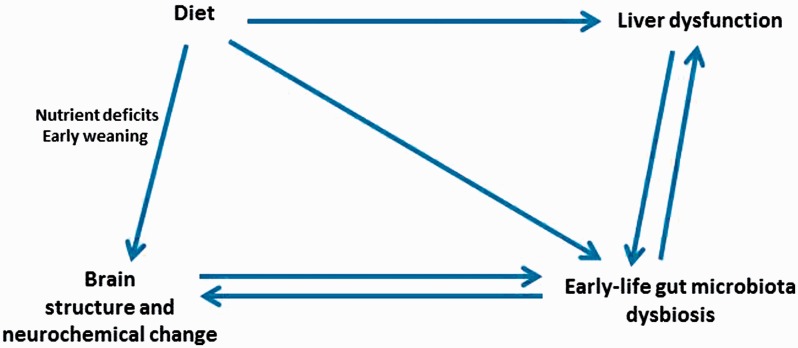
The intestinal microbiota is in a state of flux during the first 3 years of life before stabilizing15 and is affected by enteric infections and changes in diet that shape the mucosal immune system and influence nutrient absorption, thus having potential long-term consequences.16 Dietary factors (such as nutrient composition and change in diets) are key determinants of the composition of the microbiota and may influence the numbers of intestinal bacteria.17 Figure 2 shows the dynamic interactions between dietary factors and the intestinal microbiota that may profoundly affect liver and brain functions early in life. These interactions have resurged as an intense area of research.18–21 Potentially harmful bacteria proliferate under certain conditions in the intestinal lumen and may show increased translocation patterns, disrupting the balance of homeostatic translocation of commensal bacteria needed to induce mucosal production of immunoglobulin A.22 Proliferation of pathogenic bacteria may be amplified when intestinal motility is inadequate. If this inadequate motility is chronic and low grade, it may not trigger a diarrheal response, which might otherwise lead to washout of luminal bacterial overgrowth. There is a close relationship between the intestinal microbiota and mucosal immunity. Intestinal bacteria have a role in human intestinal B-cell function23 and the formation and activity of intestinal TH17 effector T cells.24 They also suppress production of regulatory T cells. Commensal bacteria are important to control regulatory T cell populations in the small intestine.25 Certain components of the gut microbiota are critical to the induction of regulatory T cells.26,27 In a recent study, mice receiving orally administered microbiota enriched with regulatory T-cell–inducing species showed improved outcomes in models of allergic diarrhea and experimental colitis after 3 weeks of treatment.28
Figure 2.
Dynamic interactions between diet, intestinal microbiota, brain, and liver.
In the intestinal luminal environment, bacterial symbionts such as Bacteroides may control the population of harmful enteric pathogens such as Salmonella and Shigella species, which have a more limited capacity to produce saccharolytic enzymes.29 Consequently, these pathogens are poorly adapted to compete with symbionts for nutrients from the host diet, restricting their luminal colonization.30 In addition, symbiotic bacteria may reduce the virulence of enteric pathogens by stimulating epithelial Toll-like receptors. This stimulation triggers the expression of a variety of antimicrobial proteins that might play a role in limiting the penetration of enteric pathogens into the epithelial barrier.31
As enteric pathogens colonize the gut, they may induce intestinal inflammation and excess free radicals. Infection with Salmonella typhimurium induces inflammation and production of reactive oxygen species via NADPH (nicotinamide adenine dinucleotide phosphate) oxidase activity during respiratory burst of phagocytes.32 Reactive oxygen species oxidize thiosulfate (which alone may be relatively innocuous), which is produced by the epithelium, resulting in the production of tetrathionate. This compound facilitates respiration by Salmonella, providing a growth advantage to this pathogen.33 In addition, overt inflammation in the intestinal milieu may cause a state of disequilibrium in the microbiome that favors overgrowth of some pathogenic species, including certain Escherichia coli pathotypes.34
Helminths and intestinal microbiota.
A small number of studies have investigated the impact of helminthic infections on the intestinal microbiota and inflammation.35,36 A recent study evaluated microbial communities after nematode infection and showed significantly higher levels of cecal γ-Proteobacteria/Enterobacteriaceae and Bacteroides/Prevotella organisms than in uninfected controls, which may constitute an anti-inflammatory factor in inflammatory bowel disease.37 Interestingly, Cantacessi et al35. did not find significant changes in the intestinal microbiota communities after Necator americanus infection in healthy volunteers, but they did not evaluate subjects living in areas where enteric infections were endemic. One recent study assessing juvenile rhesus macaques with idiopathic chronic diarrhea did find amelioration of the intestinal barrier function and reductions in TH1 inflammatory gene expression (and activation of mucosal TH2 response) following infection with whipworm Trichuris trichiura. These alterations in intestinal inflammation were accompanied by changes in the microbial communities associated with the intestinal mucosa.38 Nevertheless, it is not known how different classes of helminths and helminthic burden interact with other intestinal comorbid conditions to influence intestinal inflammatory responses.
Small intestinal bacterial overgrowth.
Small intestinal bacterial overgrowth is considered a result of intestinal dilation and stasis, which together promote excess bacterial proliferation and inflammation.39,40 It may occur when the small intestinal microbiota is altered41 and is likely affected by diet (e.g., high-carbohydrate diets with resultant luminal distension caused by impairment of digestion and absorption and changes to the composition of the luminal contents). Small intestinal bacterial overgrowth may lead to more inflammation and impaired nutrient absorption. In addition, it may lead to malabsorption of dietary fat, with important health consequences for children with enteric infections, when energy intake is important for catch-up growth.42,43 Small intestinal bacterial overgrowth (detected by increased intestinal hydrogen and methane concentrations in breath) has been identified in Brazilian children with poor nutritional status living in slums.44,45
Small intestinal barrier dysfunction may lead to small intestinal bacterial overgrowth,45 thereby causing dysbiosis in the proximal intestine. Small intestinal bacterial overgrowth has been correlated with liver steatosis (nonalcoholic fatty liver disease),46 an escalating health condition worldwide. In addition, reduced liver function leads to lower levels of potentially bacteriostatic bile acids (as well as small intestinal bacterial overgrowth by inducing bile acid deconjugation and fatty acid diarrhea47) that reach the small intestine through the enterohepatic circulation. Corroborating data demonstrate that administration of conjugated bile acid to rats with cirrhosis improves bile secretion and reduces intestinal bacterial overgrowth and translocation.48 There is little information available regarding bile acid and parasite growth; however, some findings suggest that bile acids may regulate Cryptosporidium parvum49 and Giardia lamblia50 excystation and invasiveness.
Commensal intestinal bacteria are considered key to maintain the intestinal epithelial barrier function. Studies using probiotics have documented improved expression of the zonula occludens-1 protein in a model of dextran sodium sulfate-induced colitis in BALB/c mice.51,52 The dysbiosis or disruption of resident microbiota induced by enteric pathogens may affect the regulatory benefits of commensal bacterial to the intestinal epithelial barrier.
Environmental enteropathy and liver function
Intestinal bacterial translocation and liver clearance of LPS.
Environmental enteropathy increases the intestinal proinflammatory state during chronic intestinal epithelial breakdown, which in turn is associated with overgrowth and translocation of small intestinal bacteria. Key proinflammatory cytokines can trigger the secretion of acute-phase liver proteins and enhance both the production of B-cell antibodies and the proliferation of T cells. If malnutrition occurs simultaneously, the latter effect is more likely compromised.53 In addition, the cytokine-mediated inflammatory response can further impair the intestinal barrier function, causing even more bacterial translocation. In addition to causing low-grade systemic inflammation, translocated bacterial products can enter the liver from the small and large bowel via the portal circulation and reach the space of Disse near the hepatocytes. Kupffer cells are the main liver cells responsible for phagocytosis of bacteria and therefore respond to any liver insult. Any impairment in liver function may compromise the clearance of LPS. Increased intestinal interferon-γ levels have been associated with impaired intestinal cell monolayers in vitro, along with depletion of tight-junction proteins.54 Interestingly, in patients with Crohn’s disease who show subclinical intestinal inflammation, a marked positive correlation between fecal lactoferrin, serum C-reactive protein (a liver acute-phase response), and interleukin 6 was identified55 following bowel resection. Unpublished data (A.A.M. Lima, M.D. DeBoer, and R.L. Guerrant, et al.) support this association between intestinal inflammatory markers and systemic inflammation, showing a positive correlation between serum C-reactive protein and fecal myeloperoxidase (a marker of intestinal inflammation) in Brazilian children attending a nutrition clinic in an area of endemic malnutrition and enteric diseases.
Environmental enteropathy–driven systemic inflammation, disrupted iron metabolism, and brain myelination.
Another important aspect of the leaky gut–induced systemic inflammation is related to iron metabolism. It is noteworthy that systemic inflammation may directly affect intestinal absorption of iron by sequestrating iron within intestinal enterocytes.56 One mechanism is through an increase in the hepatic hormone hepcidin, which binds to ferroportin, causing it to be internalized and, hence, preventing the efflux of iron from the enterocyte.38 Inflammation can also upregulate hepcidin synthesis by various brain cells (astrocytes, microglia, and neurons), which results in accumulation of intracellular iron and lack of iron availability for important developmental processes.57 These changes in iron metabolism may be detrimental to cognitive development, as oligodendrocytes require iron for myelin synthesis, a process accomplished by the release of iron from neuronal iron stores during myelination. Iron is also required for monoamine synthesis, neuronal metabolic activity, and the proper development of neuronal morphology.58,59 All of these processes are compromised when increased hepcidin levels reduce neuronal ferroportin activity.60 This is problematic, especially during the first 2 years of life, when myelination and dendritic arborization are occurring rapidly. In addition, parasitism early in life (especially due to ancylostomiasis) would potentially amplify this deleterious effect by exploiting the host.61 In mouse studies, the intestinal microbiota and intestinal inflammation were able to modulate liver hepcidin expression.62 Monocytes and macrophages also express hepcidin, which is regulated primarily by inflammatory mediators and infectious agents.63
Given the importance of iron for proper brain development, iron supplementation may be prudent in certain populations, especially in those with a high prevalence of deficiency. However, areas of the world where prevalence of iron deficiency is high are often areas where susceptibility to enteric infections is also high, leading to an inflammatory state within affected individuals. As noted above, this inflammation is likely to upregulate the production of hepcidin, which prevents the release of iron from enterocytes, thereby greatly reducing the efficacy of iron supplementation. In addition, there is concern that iron supplementation in such individuals may trigger a more proinflammatory state in the intestinal tract, as iron supplementation may favor bacterial proliferation.43 Iron supplementation may not be adequate if the causal mechanism is intestinal bacterial dysbiosis, since iron supplementation may even favor bacterial proliferation and therefore sustain a more proinflammatory state in the intestinal tract.64
Chronic systemic inflammation (even if low grade) may alter brain development, which may result in lasting neurological deficits without leading to conspicuous brain lesions. In addition, neonatal systemic inflammation disrupts white-matter programming, leading to impairments in oligodendrocyte and axon maturation, likely more pronounced during a time of increased vulnerability.65 In addition, gut microbiota may influence the deleterious effects of chronic exposure to heavy metals on the brain.66
Iron supplementation and cognition
Multiple studies have assessed the relation of iron supplementation with cognitive development in children under 5 years of age.67 Studies conducted in infants have consistently reported findings of lower mental development test scores,68 lower motor test scores,68,69 and differences in social-emotional behavior70 in those who are iron-deficient anemic vs those who are iron sufficient. However, these same studies report persistent cognitive differences in the groups, even after iron treatment. These findings are generally interpreted as the iron deficiency having occurred during a critical period of development, such that the negative effects are not reversible with supplementation during this age. When examining the studies of iron supplementation and cognitive development in toddlers and preschool-aged children, there are consistent findings of lower scores on learning tasks and language and motor development in children who are iron-deficient anemic vs those who are iron sufficient. These individual studies show remarkable agreement about supplementation reversing these negative effects. However, meta-analyses of such studies have not been consistent in finding support for supplementation.71 Although many of these studies conducted in children under 5 years of age were carried out in populations susceptible to repeated enteric infections, none of them examined the relation between enteric infection, the intestinal microbiota, the iron status of the individual, and cognitive development.
Environmental enteropathy and genetic predisposition
APOE4 as a possible protective factor.
Some of the immune system responses to enteric pathogens may be strongly influenced by the host’s genetic background.72,73 One interesting gene is the apolipoprotein E4 gene (APOE4), a gene related to cholesterol transport and metabolism and increasingly recognized to have important immune-inflammatory roles.74 Children from a Brazilian shantytown bearing APOE4 and raised in areas where diarrhea and enteric infections are endemic have been found to have improved cognitive development when compared with non-APOE4 neighbors75,76; however, this was observed only in children who experienced heavy diarrhea burdens. In addition, one study documented a beneficial effect of APOE4 in reducing circulating C-reactive proteins in individuals exposed to heavy infection loads in an indigenous Bolivian population.77 Interestingly, in a recent hospital-based case–control study, individuals who harbored APOE4 had less nonalcoholic fatty liver disease.78 Undernourished APOE4-targeted replacement mice show better adaptation against experimental Cryptosporidium parvum infection than controls.79 Cryptosporidiosis has been found to be a strong factor to modulate the intestinal microbiota in immunosuppressed mice.80 More studies are needed to appreciate the effects of APOE4 in children with environmental enteropathy and the relationship with gut and liver function. Although APOE4 is a compelling target gene for understanding causality, the multifactorial effects of environmental enteropathy should highlight an array of genes, especially since multiple genes are likely to have lasting effects on cognition in children. Metagenomics of the early intestinal microbiota in light of environment exposures and host genetics is key for deciphering novel determinants of environmental enteropathy, especially when coupled with the study of innate immune-related genes.81
Microbial ecology and brain development in the first 2 years of life
Timeline of intestinal colonization from 0 to 24 months.
The construction of the intestinal microbiota starts after birth82 and continues to be actively remodeled over the first 3 years of life, reaching a maturation status similar to that of an adult.83,84 A number of factors play a role in shaping the intestinal microbiota (and other host-associated microbial communities), including mode of delivery, environmental exposures, and diet. Vaginal or cesarean delivery of infants impacts colonization of the intestinal tract by representative species from vaginal or skin communities, respectively. Intestinal colonization in the postnatal period is further shaped by the diet and is reflective of the metabolic capacity,85,86 a trend that continues into childhood and beyond.87,88 A small portion of microbes from other sources (e.g., environment, animals, water) are classified as pathogens, which introduce additional variability to the intestinal microbiota.
Enteric pathogens: prevalence from 0 to 24 months and links to brain function.
Increased pathogen exposure during the first 2 years of life is coincident with exposure to different reservoirs that include food,89,90 hygiene-related behaviors,91–93 and contaminated water sources.94,95 Global studies designed to measure the burden of diarrhea around the world highlight the prevalence of numerous pathogens associated with pediatric diarrhea.96–98 Pathogens with the highest prevalence across study sites include Campylobacter spp, rotavirus, enterotoxigenic E. coli, Cryptosporidium spp, and Shigella spp; several other enteric pathogens were identified in the studies but were locale dependent.99,100 In addition to the pathogen burden during diarrhea, the intestinal microbiota is also affected by lower bacterial diversity and higher levels of facultative anaerobes.101–103
Unearthing the impact of enteric disease on brain development and function in children is an underappreciated area11; nevertheless, research efforts are under way to understand the breadth of the relationship between enteric infection and brain function. Among the studies of predominant diarrheal pathogens in children, at least two reported associations of Cryptosporidium or Giardia infection and diarrhea in early life with cognitive function in later life.104,105 Rotavirus infection can potentially affect the central nervous system in pediatric patients,106 although the long-term impacts of this infection are unknown. In rodent models, systemic infection or LPS exposure during the postnatal period affects brain development and function in adulthood,107,108 signifying both pathogens and commensal organisms as contributing factors.
Additional factors that likely play a role in enteric infections and brain development/function during the first 2 years of life include hydration status and diet. Childhood diarrhea inevitably alters host hydration status, resulting in lower diversity of the intestinal microbiota.103 Dehydration is known to affect cognitive function in children and young adults, although information about its impact in early childhood is limited.109
Diet during the first 2 years of life.
As previously mentioned, microbial shifts in the intestinal community are coincident with the diet110; furthermore, remodeling of the intestinal microbial community during the introduction of solid food appears to be dependent on whether infants were breastfed.86 The quality and type of foods within a given diet influence the composition of the microbiota and, ultimately, host health (reviewed by Mitsuoka111 and Subramanian et al112.). A recent study found that malnutrition is associated with intestinal microbiota immaturity, which was only partially ameliorated by nutritional intervention in Bangladeshi children. The intestinal microbiota immaturity was measured by two indices, named the “relative microbiota maturity index” and the “microbiota-for-age Z-score,” the latter calculated on the basis of healthy children of similar chronologic age.113 Future research would potentially address whether intestinal microbiota immaturity is closely related to early-life enteric pathogen burden and whether it impacts developmental cognitive function.
One aspect of host health already mentioned is brain function. In the absence of infection, protein-energy malnutrition has an adverse effect on behavior compared with nutrient sufficiency in rats.114,115 Additional studies show that behaviors of undernourished dams and their offspring are negatively impacted,115,116 linking diet and microbiota (by proxy) to brain function. Metabolism of food by the host (and perhaps by the mother) has the potential to affect host genetics.
Brain development, metabolites, and the microbiota from birth to age 2.
The number of articles establishing links between the intestinal microbiota, the gut, and the brain has increased exponentially over the last decade, beginning with topics in the area of microbial endocrinology.117–119 The majority of studies utilize germ-free mouse systems to examine the impact of microbial colonization on brain-related functions, but these models are typically carried out in mature mice. Nevertheless, data from human and mouse studies can be combined to start building hypotheses. For example, a recent study measured important brain metabolites from birth to adolescence,120 some of which are exclusively part of human metabolism (e.g., N-acetylaspartate) and others that are acquired through diet and/or, potentially, the intestinal microbiota (e.g., myo-inositol). In humans, levels of myo-inositol decrease over the first 5 years of life, coincident with colonization and stabilization of the intestinal microbiota.83,84,120 To extend this further, a recent study conducted transcriptional and metabolomics analyses during mouse conventionalization and found positive correlations between measured metabolites and related host metabolic genes. Furthermore, the data suggest that myo-inositol levels decrease upon conventionalization, suggesting a role for the microbiota in regulating these levels.121 As more large-scale datasets are made available to the scientific community, additional interactions between the microbiota and host can be unraveled.
Potential effects of early-life intestinal microbiota on the brain.
The first 2 years of extrauterine life is a key time window for human brain development, with profound remodeling of synaptic circuitry, active processes of synaptogenesis and synaptic pruning, and myelination122 all occurring within the same time period that dietary transition from breastfeeding to solid foods takes place. Often, this transition is accompanied by the introduction of contaminants and pathogens in areas of poor environmental hygiene, facilitating the onset of early enteric infections.123,124
In addition, the maturation of the blood–brain barrier early in life has been recognized in mouse experiments to be regulated by the intestinal microbiota, which can affect blood–brain barrier tight-junction proteins, regardless of brain vascular density, having effects that last into adulthood.125 It is unclear how enteric infections early in life may affect the blood–brain barrier function by disrupting the normal intestinal microbiota in humans. Furthermore, early-life gut microbial populations may affect brain neurotransmission, increasing the risk for developmental neuropsychiatric disorders, such as autism and schizophrenia.126,127 It is noteworthy that luminal intestinal microbiota have been found to induce serotonin biosynthesis by stimulating enterochromaffin cells, which increase the supply of serotonin to circulating platelets.118
It is not known whether increased production of intestinal luminal serotonin is a mechanism that can potentially trigger later neuropsychiatric disorders. Recent studies have documented that intestinal microbiota produce different neurotransmitters,119,128,129 but it is unknown whether gut-derived neurotransmitters affect brain functions, especially during early brain development, and whether enteric pathogens could disturb these processes. Interestingly, Diaz Heijtz et al.,130 by conventionalizing germ-free mice early in life with the gut microbiota from specific-pathogen-free mice and testing anxiety and motor behaviors into adulthood, speculate that those behaviors may be programmed soon after birth, when the newborn mice are exposed to gut microbiota, since gut microbes may modulate levels of important neurotransmitters and their metabolites in the striatum in the adult mice.
An interesting study from Sudo et al.131 has documented that germ-free mice showed higher plasma levels of adrenocorticotropic hormone and corticosterone following restraint stress, with reduced expression of brain-derived neurotrophic factor in the cortex and hippocampus compared with levels in specific-pathogen-free mice. In addition, the response to stress increased following oral inoculation with enteropathogenic E. coli. The enhanced stress response was partially ameliorated with feces from specific-pathogen-free mice at an early stage, but not at a later stage, reinforcing that the establishment of normal commensal bacteria early in life is critical to support maturation of the hypothalamic–pituitary–adrenal axis.
Effects of leaky gut and intestinal and systemic inflammation on the brain
Convincing data are accumulating indicating that the brain (including the regions separated from the immune system by the blood–brain barrier) is significantly affected by sustained peripheral inflammation, which may activate microglia and perivascular macrophages, leading to a neuroinflammatory status.132
Of note, neonatal microglia are more active and more easily primed than adult microglia, owing to higher expression of major histocompatibility complex (MHC) II, CD86, and CD40, whereas perivascular macrophages (expressing mannose receptor CD163) with high MHC II expression are adapted to present antigens to peripheral T cells in the neonatal period.132–134 In addition, CD4+ T cells can traffic into the brain and may differentiate into TH1, TH17, and TH2 types, depending on microenvironment. T cells are recognized as having a critical role in the crosstalk between the innate and the acquired immune systems, influencing brain inflammation.135 It is also known that microglia can express mRNA for all Toll-like receptors except Toll-like receptor 10 and therefore can recognize pattern-recognition receptors, which can be especially important in the immature neonatal brain.136
In addition, inflammatory responses in the neonatal period may cause increased blood–brain barrier permeability,137 with white matter injury occurring even during subclinical inflammation,138 which could facilitate peripheral T-cell and monocyte/macrophage traffic to the brain. Neonatal systemic inflammation may have a lasting effect on behavior60,139 and cause neuropsychiatric disorders in adulthood.132
Role of LPS.
Following luminal bacterial translocation, LPS, found in the outer lipid bilayer in the cell wall of gram-negative bacteria, is a strong mediator of intestinal inflammation and activates the mucosal immune system. Lipopolysaccharide is released during bacterial proliferation and as a byproduct of bacterial lysis. Once LPS is released, the lipid A portion of the molecule is exposed and elicits biological responses.140 If LPS is not neutralized by the gut immune response, it may enter the bloodstream and circulate systemically. It is still unclear how peripheral LPS (or LPS-induced proinflammatory cytokines) leads to neuroinflammation. Cytokines may act directly on neuronal receptors, either following active transport across the blood–brain barrier141 or after passive diffusion in the circumventricular organs (not associated with the blood–brain barrier). Since LPS cannot easily enter the blood–brain barrier, systemic proinflammatory cytokines (like tumor necrosis factor-α) may mediate the inflammatory signaling effect to the brain, since these cytokines can cross the blood–brain barrier from the peripheral circulation.142 However blood–brain barrier endothelial cells may respond to LPS and cytokines by producing interleukin 6 and releasing it to the brain tissue.143 Furthermore, leptomeningeal cells may have a role in inducing proinflammatory responses in the brain after systemic inflammation.144
Recently, it was demonstrated that acute injection of a septic dose of LPS intraperitoneally could lead to breakdown of the blood–brain barrier and increased brain levels of proinflammatory cytokines with activated microglia in an experimental model.145 Systemic LPS (indirectly, by inducing blood–brain barrier epithelial cells or proinflammatory cytokine release) could activate microglia and cause neuroinflammation in the adult142 and aged brain.146,147 This may predispose to neurodegeneration,142 whereas aging reduces the responsiveness to the interleukin 4–induced M2 microglia phenotype.147 In addition, peripheral inflammation (induced by carrageenan injection into the paw of rats) can exacerbate the loss of dopaminergic neurons and the increase in brain inflammatory markers caused by intranigral injection of LPS.148 It has been suggested that disruption of the intestinal mucosa by increased translocation of gram-negative bacteria (including Hafnei alvei, Pseudomonas aeruginosa, Morganella morganii, Pseudomonas putida, Citrobacter koseri, and Klebsiella pneumoniae) is associated with major depression.149 However, it is still uncertain whether low doses of LPS can cause changes in the blood–brain barrier significant enough to cause microglial activation and neuroinflammation over extended periods of time.
As documented recently, low levels of systemic LPS do not seem to cause impairments to the blood–brain barrier or induce microglial activation and neuroinflammation in neonatal mice.150 In addition, microglial activation is not always associated with exacerbated brain injury. It may be neuroprotective and contribute to the regenerative remodeling following low levels of circulating LPS.151 Nevertheless, it is unclear whether enteric infections and undernutrition affect these protective responses. It is noteworthy that, in critical periods of brain development, microglia may be primed by a systemic inflammatory response early in life (or prenatally) and later become overactive during neurodegenerative processes that occur with aging, increasing the risk of neuronal death.152 Another intriguing possibility is that enteric pathogens could influence brain neuroinflammation by releasing factors carried by retrograde axonal transport via the enteric nerve plexus.153
Restoring balance in the gut–liver–brain axis (micronutrients and the intestinal microbiota)
The influence of early-life malnutrition and enteric infection on the intestinal microbiome (perhaps especially in the small intestine) is being increasingly recognized as an important factor modulating intestinal adaptive responses such as unbalanced innate (over-reactive) immune responses and impaired intestinal barrier function. If these deleterious effects occur during a time of profound changes in brain plasticity, such as actively ongoing myelination, neurogenesis, and synaptogenesis processes, they may negatively affect memory storage, executive function, and language circuitry maturation, ultimately leading to poor cognition.154,155 A compelling therapeutic strategy is to use nutritional supplementation that provides both preventive and curative benefits to children residing in areas where malnutrition and enteric diseases are endemic. One important rationale is to use key gut-trophic nutrients (e.g., zinc, glutamine, citrulline, arginine) to improve the intestinal barrier function, thereby reducing exposure of the intestinal lamina propria to pathogenic bacterial translocation. Apart from the nutritional effect per se, such nutrients/micronutrients may have an advantage in terms of interacting positively with the intestinal microbiota.
L-arginine and citrulline.
L-arginine has been shown to ameliorate intestinal epithelial barrier function by improving tight junctions156 and to diminish luminal intestinal bacterial translocation following intestinal barrier impairment.157–159 Recently, it has been shown that supplemental dietary L-arginine interacts with the intestinal microbiome to activate the immune system by enhancing intestinal Toll-like receptor signaling and the expression of secretory immunoglobulin A, mucins, and defensins.160 Arginine given as oral supplementation is converted to ornithine and urea by the liver, the former being a precursor of polyamines (a known cell proliferative factor161) by the activity of the ornithine decarboxylase enzyme. In addition, intestinal bacteria may convert dietary arginine to polyamines, which may aid in recovery of the intestinal mucosa following injury.162,163 Intestinal polyamines were found to be a procognition factor in elderly mice.162 However, one pitfall of oral arginine supplementation is that arginine may induce increased circulating levels of arginase and therefore lead to arginine breakdown and poor bioavailability in the target tissue.164,165 One way to overcome this problem is by oral citrulline supplementation. Citrulline, a precursor of arginine, is not known to induce excessive levels of arginase and is not subject to liver metabolism. In addition, citrulline supplementation was found to be beneficial in models of chemotherapy-induced intestinal mucositis and intestinal obstruction in mice.166,167
Glutamine, probiotics, and zinc.
Glutamine has also been found to interact with the intestinal microbiome, improving host innate immune responses168 in addition to having well-known effects in protecting the intestinal barrier function and improving intestinal bacterial translocation in models of intestinal injury.169 Interestingly, dietary glutamine supplementation favored growth of Bacteroidetes instead of Firmicutes, improved Toll-like receptor 4 responses, and increased the level of antibacterial substances in the small intestine.168 Increased Firmicutes populations in the gut have been associated with obesity.170 In addition, probiotics such as Bifidobacterium, Saccharomyces, and Lactobacillus have been shown to protect the intestinal barrier function and stimulate host immunity early in life, which may have potentially long-term effects during the life span.171 Lactobacilli in the gut were found to be protective against Shigella diarrhea.101 Probiotics play a key role in regulating the intestinal microbiota and may have procognition effects.172 Although findings have been more contradictory, zinc supplementation has also been found to benefit the intestinal microbiota173,174 and to modulate the intestinal inflammatory responses,175,176 even reducing the virulence of pathogenic E. coli.177,178 Zinc supplementation is still the only nutritional therapy clearly recognized to protect children with diarrheal diseases in low-income countries.179 These findings highlight the benefits of certain nutrients in restoring or maintaining intestinal barrier function, but how these nutrients interact with the early-life intestinal microbiota when enteric pathogens are present is largely unknown. More studies are warranted to dissect how these interactions influence cognitive development in children and infants in settings of enteric infection and malnutrition.
CONCLUSION
It is increasingly recognized that a prolonged state of environmental enteropathy, an intestinal inflammatory condition that may profoundly limit an individual’s full potential and may go chronically unrecognized in children, in a setting of endemic enteric infections and malnutrition is a strong driving force toward poor development. As a malabsorptive condition, environmental enteropathy may also impair the transport of adequate key nutrients to the brain within an important time period of postnatal brain plasticity, especially in the first 2 years of life. This developmental window of time is the very same time when enteric infections and malnutrition prevail in children exposed to fecally contaminated environments, amplifying the vicious cycle. Although more studies have been published recently, there is still an important knowledge gap about how enteric pathogens interact with the early-life intestinal microbiota. These potential effects of environmental enteropathy on early cognitive development in children are still mostly unknown. Long-term cohort studies are key to dissect the determinants of these lasting effects. Any effective intervention to ameliorate environmental enteropathy should consider the interactions between the intestinal microbiota and the enteric pathogens that may contribute to poor cognition in children.
Acknowledgments
Funding. The Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) is carried out as a collaborative project supported by the Bill & Melinda Gates Foundation, the Foundation for the National Institutes of Health, and the Fogarty International Center, National Institutes of Health. R.J.S. is a recipient of Doris Duke Charitable Foundation Clinical Scientist Development award.
Declaration of interest. The authors have no relevant interests to declare.
REFERENCES
- 1.Bhutta ZA, Sommerfeld J, Lassi ZS, et al. Global burden, distribution, and interventions for infectious diseases of poverty. Infect Dis Poverty. 2014;3:21 doi:10.1186/2049-9957-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das JK, Salam RA, Bhutta ZA. Global burden of childhood diarrhea and interventions. Curr Opin Infect Dis. 2014;27:451–458. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray-Kolb LE, Rasmussen ZA, Scharf RJ, et al. The MAL-ED cohort study: methods and lessons learned when assessing early child development and caregiving mediators in infants and young children in 8 low- and middle-income countries. Clin Infect Dis. 2014;59(suppl 4):S261–S272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerrant RL, Oria RB, Moore SR, et al. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008;66:487–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBoer MD, Lima AA, Oria RB, et al. Early childhood growth failure and the developmental origins of adult disease: do enteric infections and malnutrition increase risk for the metabolic syndrome? Nutr Rev. 2012;70:642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keusch GT, Denno DM, Black RE, et al. Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clin Infect Dis. 2014;59(suppl 4):S207–S212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso C, Vicario M, Pigrau M, et al. Intestinal barrier function and the brain-gut axis. Adv Exp Med Biol. 2014;817:73–113. [DOI] [PubMed] [Google Scholar]
- 9.Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology. 2014;147:1363–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolling G, Wu M, Guerrant RL. Enteric pathogens through life stages. Front Cell Infect Microbiol. 2012;2:114 doi:10.3389/fcimb.2012.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sommer F, Backhed F. The gut microbiota – masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. [DOI] [PubMed] [Google Scholar]
- 13.Huang XZ, Zhu LB, Li ZR, et al. Bacterial colonization and intestinal mucosal barrier development. World J Clin Pediatr. 2013;2:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Aidy S, van den Bogert B, Kleerebezem M. The small intestine microbiota, nutritional modulation and relevance for health. Curr Opin Biotechnol. 2014;32C:14–20. [DOI] [PubMed] [Google Scholar]
- 15.Arrieta MC, Stiemsma LT, Amenyogbe N, et al. The intestinal microbiome in early life: health and disease. Front Immunol. 2014;5:427 doi:10.3389/fimmu.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang C, Zhu X, Liu N, et al. Lactoferrin up-regulates intestinal gene expression of brain-derived neurotrophic factors BDNF, UCHL1 and alkaline phosphatase activity to alleviate early weaning diarrhea in postnatal piglets. J Nutr Biochem. 2014;25:834–842. [DOI] [PubMed] [Google Scholar]
- 19.Clarke G, Grenham S, Scully P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–673. [DOI] [PubMed] [Google Scholar]
- 20.Barouei J, Moussavi M, Hodgson DM. Effect of maternal probiotic intervention on HPA axis, immunity and gut microbiota in a rat model of irritable bowel syndrome. PLoS One. 2012;7:e46051 doi:10.1371/journal.pone.0046051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohland CL, Kish L, Bell H, et al. Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology. 2013;38:1738–1747. [DOI] [PubMed] [Google Scholar]
- 22.Kinnebrew MA, Pamer EG. Innate immune signaling in defense against intestinal microbes. Immunol Rev. 2012;245:113–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He B, Xu W, Santini PA, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. [DOI] [PubMed] [Google Scholar]
- 24.Ivanov II, Frutos RL, Manel N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall JA, Bouladoux N, Sun CM, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. [DOI] [PubMed] [Google Scholar]
- 27.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. [DOI] [PubMed] [Google Scholar]
- 29.Xu J, Gordon JI. Honor thy symbionts. Proc Natl Acad Sci U S A. 2003;100:10452–10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stecher B, Robbiani R, Walker AW, et al. Salmonella enterica serovar Typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaishnava S, Behrendt CL, Hooper LV. Innate immune responses to commensal bacteria in the gut epithelium. J Pediatr Gastroenterol Nutr. 2008;46(suppl 1):E10–E11. [DOI] [PubMed] [Google Scholar]
- 32.Paiva CN, Bozza MT. Are reactive oxygen species always detrimental to pathogens? Antioxid Redox Signal. 2014;20:1000–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winter SE, Thiennimitr P, Winter MG, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duca F, Gerard P, Covasa M, et al. Metabolic interplay between gut bacteria and their host. Front Horm Res. 2014;42:73–82. [DOI] [PubMed] [Google Scholar]
- 35.Cantacessi C, Giacomin P, Croese J, et al. Impact of experimental hookworm infection on the human gut microbiota. J Infect Dis. 2014;210:1431–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra PK, Palma M, Bleich D, et al. Systemic impact of intestinal helminth infections. Mucosal Immunol. 2014;7:753–762. [DOI] [PubMed] [Google Scholar]
- 37.Rausch S, Held J, Fischer A, et al. Small intestinal nematode infection of mice is associated with increased enterobacterial loads alongside the intestinal tract. PLoS One. 2013;8:e74026 doi:10.1371/journal.pone.0074026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broadhurst MJ, Ardeshir A, Kanwar B, et al. Therapeutic helminth infection of macaques with idiopathic chronic diarrhea alters the inflammatory signature and mucosal microbiota of the colon. PLoS Pathog. 2012;8:e1003000 doi:10.1371/journal.ppat.1003000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutierrez IM, Kang KH, Calvert CE, et al. Risk factors for small bowel bacterial overgrowth and diagnostic yield of duodenal aspirates in children with intestinal failure: a retrospective review. J Pediatr Surg. 2012;47:1150–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaufman SS, Loseke CA, Lupo JV, et al. Influence of bacterial overgrowth and intestinal inflammation on duration of parenteral nutrition in children with short bowel syndrome. J Pediatr. 1997;131:356–361. [DOI] [PubMed] [Google Scholar]
- 41.Duseja A, Chawla YK. Obesity and NAFLD: the role of bacteria and microbiota. Clin Liver Dis. 2014;18:59–71. [DOI] [PubMed] [Google Scholar]
- 42.Gibson PR, Barrett JS. The concept of small intestinal bacterial overgrowth in relation to functional gastrointestinal disorders. Nutrition. 2010;26:1038–1043. [DOI] [PubMed] [Google Scholar]
- 43.Jonas A, Avigad S, Diver-Haber A, et al. Disturbed fat absorption following infectious gastroenteritis in children. J Pediatr. 1979;95:366–372. [DOI] [PubMed] [Google Scholar]
- 44.Mello CS, Tahan S, Melli LC, et al. Methane production and small intestinal bacterial overgrowth in children living in a slum. World J Gastroenterol. 2012;18:5932–5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donowitz JR, Petri WA., Jr Pediatric small intestine bacterial overgrowth in low-income countries. Trends Mol Med. 2015;21:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. [DOI] [PubMed] [Google Scholar]
- 47.Mathias JR, Clench MH. Review: pathophysiology of diarrhea caused by bacterial overgrowth of the small intestine. Am J Med Sci. 1985;289:243–248. [DOI] [PubMed] [Google Scholar]
- 48.Lorenzo-Zuniga V, Bartoli R, Planas R, et al. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 2003;37:551–557. [DOI] [PubMed] [Google Scholar]
- 49.King BJ, Keegan AR, Phillips R, et al. Dissection of the hierarchy and synergism of the bile derived signal on Cryptosporidium parvum excystation and infectivity. Parasitology. 2012;139:1533–1546. [DOI] [PubMed] [Google Scholar]
- 50.Gillin FD, Boucher SE, Rossi SS, et al. Giardia lamblia: the roles of bile, lactic acid, and pH in the completion of the life cycle in vitro. Exp Parasitol. 1989;69:164–174. [DOI] [PubMed] [Google Scholar]
- 51.Miyauchi E, Morita H, Tanabe S. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. J Dairy Sci. 2009;92:2400–2408. [DOI] [PubMed] [Google Scholar]
- 52.Ukena SN, Singh A, Dringenberg U, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2:e1308 doi:10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rytter MJ, Kolte L, Briend A, et al. The immune system in children with malnutrition – a systematic review. PLoS One. 2014;9:e105017 doi:10.1371/journal.pone.0105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang S, Yu M, Sun L, et al. Interferon-γ-induced intestinal epithelial barrier dysfunction by NF-κB/HIF-1α pathway. J Interferon Cytokine Res. 2014;34:195–203. [DOI] [PubMed] [Google Scholar]
- 55.Ruffolo C, Scarpa M, Faggian D, et al. Subclinical intestinal inflammation in patients with Crohn's disease following bowel resection: a smoldering fire. J Gastrointest Surg. 2010;14:24–31. [DOI] [PubMed] [Google Scholar]
- 56.Tussing-Humphreys L, Pusatcioglu C, Nemeth E, et al. Rethinking iron regulation and assessment in iron deficiency, anemia of chronic disease, and obesity: introducing hepcidin. J Acad Nutr Diet. 2012;112:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Urrutia P, Aguirre P, Esparza A, et al. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem. 2013;126:541–549. [DOI] [PubMed] [Google Scholar]
- 58.Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69(suppl 1):S43–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murray-Kolb LE. Iron and brain functions. Curr Opin Clin Nutr Metab Care. 2013;16:703–707. [DOI] [PubMed] [Google Scholar]
- 60.Lieblein-Boff JC, McKim DB, Shea DT, et al. Neonatal E. coli infection causes neuro-behavioral deficits associated with hypomyelination and neuronal sequestration of iron. J Neurosci. 2013;33:16334–16345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loukas A, Constant SL, Bethony JM. Immunobiology of hookworm infection. FEMS Immunol Med Microbiol. 2005;43:115–124. [DOI] [PubMed] [Google Scholar]
- 62.Shanmugam NK, Trebicka E, Fu LL, et al. Intestinal inflammation modulates expression of the iron-regulating hormone hepcidin depending on erythropoietic activity and the commensal microbiota. J Immunol. 2014;193:1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang X, Rovin BH. Beyond anemia: hepcidin, monocytes and inflammation. Biol Chem. 2013;394:231–238. [DOI] [PubMed] [Google Scholar]
- 64.Jaeggi T, Kortman GA, Moretti D, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut. 2015;64:731–742. [DOI] [PubMed] [Google Scholar]
- 65.Favrais G, van de Looij Y, Fleiss B, et al. Systemic inflammation disrupts the developmental program of white matter. Ann Neurol. 2011;70:550–565. [DOI] [PubMed] [Google Scholar]
- 66.Breton J, Daniel C, Dewulf J, et al. Gut microbiota limits heavy metals burden caused by chronic oral exposure. Toxicol Lett. 2013;222:132–138. [DOI] [PubMed] [Google Scholar]
- 67.Hermoso M, Vucic V, Vollhardt C, et al. The effect of iron on cognitive development and function in infants, children and adolescents: a systematic review. Ann Nutr Metab. 2011;59:154–165. [DOI] [PubMed] [Google Scholar]
- 68.Akman M, Cebeci D, Okur V, et al. The effects of iron deficiency on infants' developmental test performance. Acta Paediatr. 2004;93:1391–1396. [PubMed] [Google Scholar]
- 69.Shafir T, Angulo-Barroso R, Calatroni A, et al. Effects of iron deficiency in infancy on patterns of motor development over time. Hum Mov Sci. 2006;25:821–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lozoff B, Castillo M, Clark KM, et al. Iron supplementation in infancy contributes to more adaptive behavior at 10 years of age. J Nutr. 2014;144:838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thompson J, Biggs BA, Pasricha SR. Effects of daily iron supplementation in 2- to 5-year-old children: systematic review and meta-analysis. Pediatrics. 2013;131:739–753. [DOI] [PubMed] [Google Scholar]
- 72.Mohamed JA, DuPont HL, Flores J, et al. Single nucleotide polymorphisms in the promoter of the gene encoding the lipopolysaccharide receptor CD14 are associated with bacterial diarrhea in US and Canadian travelers to Mexico. Clin Infect Dis. 2011;52:1332–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pinkerton RC, Oria RB, Kent JW, Jr, et al. Evidence for genetic susceptibility to developing early childhood diarrhea among shantytown children living in northeastern Brazil. Am J Trop Med Hyg. 2011;85:893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gale SC, Gao L, Mikacenic C, et al. APOε4 is associated with enhanced in vivo innate immune responses in human subjects. J Allergy Clin Immunol. 2014;134:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oria RB, Patrick PD, Zhang H, et al. APOE4 protects the cognitive development in children with heavy diarrhea burdens in Northeast Brazil. Pediatr Res. 2005;57:310–316. [DOI] [PubMed] [Google Scholar]
- 76.Oria RB, Patrick PD, Oria MO, et al. ApoE polymorphisms and diarrheal outcomes in Brazilian shanty town children. Braz J Med Biol Res. 2010;43:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vasunilashorn S, Finch CE, Crimmins EM, et al. Inflammatory gene variants in the Tsimane, an indigenous Bolivian population with a high infectious load. Biodemography Soc Biol. 2011;57:33–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Feo E, Cefalo C, Arzani D, et al. A case–control study on the effects of the apolipoprotein E genotypes in nonalcoholic fatty liver disease. Mol Biol Rep. 2012;39:7381–7388. [DOI] [PubMed] [Google Scholar]
- 79.Azevedo OG, Bolick DT, Roche JK, et al. Apolipoprotein E plays a key role against cryptosporidial infection in transgenic undernourished mice. PLoS One. 2014;9:e89562 doi:10.1371/journal.pone.0089562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ras R, Huynh K, Desoky E, et al. Perturbation of the intestinal microbiota of mice infected with Cryptosporidium parvum. Int J Parasitol. 2015;45:567–573. [DOI] [PubMed] [Google Scholar]
- 81.Sherman MP, Zaghouani H, Niklas V. Gut microbiota, the immune system, and diet influence the neonatal gut–brain axis. Pediatr Res. 2015;77:127–135. [DOI] [PubMed] [Google Scholar]
- 82.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ottman N, Smidt H, de Vos WM, et al. The function of our microbiota: who is out there and what do they do? Front Cell Infect Microbiol. 2012;2:104 doi:10.3389/fcimb.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69:1035S–1045S. [DOI] [PubMed] [Google Scholar]
- 85.Smith MI, Yatsunenko T, Manary MJ, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339:548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thompson AL, Monteagudo-Mera A, Cadenas MB, et al. Milk- and solid-feeding practices and daycare attendance are associated with differences in bacterial diversity, predominant communities, and metabolic and immune function of the infant gut microbiome. Front Cell Infect Microbiol. 2015;5:3 doi:10.3389/fcimb.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome . Nature. 2014;505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agustina R, Sari TP, Satroamidjojo S, et al. Association of food-hygiene practices and diarrhea prevalence among Indonesian young children from low socioeconomic urban areas. BMC Public Health. 2013;13:977 doi:10.1186/1471-2458-13-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kung'u JK, Boor KJ, Ame SM, et al. Bacterial populations in complementary foods and drinking-water in households with children aged 10-15 months in Zanzibar, Tanzania. J Health Popul Nutr. 2009;27:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saleh F, Ara F, Hoque MA, et al. Complementary feeding practices among mothers in selected slums of Dhaka city: a descriptive study. J Health Popul Nutr. 2014;32:89–96. [PMC free article] [PubMed] [Google Scholar]
- 92.Baker KK, Farzana FD, Ferdous F, et al. Association between moderate-to-severe diarrhea in young children in the Global Enteric Multicenter Study (GEMS) and types of handwashing materials used by caretakers in Mirzapur, Bangladesh. Am J Trop Med Hyg. 2014;91:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ngure FM, Humphrey JH, Mbuya MN, et al. Formative research on hygiene behaviors and geophagy among infants and young children and implications of exposure to fecal bacteria. Am J Trop Med Hyg. 2013;89:709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pedersen SH, Wilkinson AL, Andreasen A, et al. Cryptosporidium prevalence and risk factors among mothers and infants 0 to 6 months in rural and semi-rural northwest Tanzania: a prospective cohort study. PLoS Negl Trop Dis. 2014;8:e3072 doi:10.1371/journal.pntd.0003072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khalil K, Lindblom GB, Mazhar K, et al. Flies and water as reservoirs for bacterial enteropathogens in urban and rural areas in and around Lahore, Pakistan. Epidemiol Infect. 1994;113:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. [DOI] [PubMed] [Google Scholar]
- 97.Taniuchi M, Sobuz SU, Begum S, et al. Etiology of diarrhea in Bangladeshi infants in the first year of life analyzed using molecular methods. J Infect Dis. 2013;208:1794–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu J, Kabir F, Manneh J, et al. Development and assessment of molecular diagnostic tests for 15 enteropathogens causing childhood diarrhoea: a multicentre study. Lancet Infect Dis. 2014;14:716–724. [DOI] [PubMed] [Google Scholar]
- 99.Platts-Mills JA, McCormick BJ, Kosek M, et al. Methods of analysis of enteropathogen infection in the MAL-ED cohort study. Clin Infect Dis. 2014;59(suppl 4):S233–S238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Platts-Mills JA, Babji S, Bodhidatta L, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health. 2015;3:e564–e575. doi:10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lindsay B, Oundo J, Hossain MA, et al. Microbiota that affect risk for shigellosis in children in low-income countries. Emerg Infect Dis. 2015;21:242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pop M, Walker AW, Paulson J, et al. Diarrhea in young children from low-income countries leads to large-scale alterations in intestinal microbiota composition. Genome Biol. 2014;15:R76 doi:10.1186/gb-2014-15-6-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Monira S, Nakamura S, Gotoh K, et al. Metagenomic profile of gut microbiota in children during cholera and recovery. Gut Pathog. 2013;5:1 doi:10.1186/1757-4749-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berkman DS, Lescano AG, Gilman RH, et al. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359:564–571. [DOI] [PubMed] [Google Scholar]
- 105.Guerrant DI, Moore SR, Lima AA, et al. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four–seven years later in a poor urban community in northeast Brazil. Am J Trop Med Hyg. 1999;61:707–713. [DOI] [PubMed] [Google Scholar]
- 106.Kobayashi S, Negishi Y, Ando N, et al. Two patients with acute rotavirus encephalitis associated with cerebellar signs and symptoms. Eur J Pediatr. 2010;169:1287–1291. [DOI] [PubMed] [Google Scholar]
- 107.Bland ST, Beckley JT, Young S, et al. Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain Behav Immun. 2010;24:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dinel AL, Joffre C, Trifilieff P, et al. Inflammation early in life is a vulnerability factor for emotional behavior at adolescence and for lipopolysaccharide-induced spatial memory and neurogenesis alteration at adulthood. J Neuroinflammation. 2014;11:155 doi:10.1186/s12974-014-0155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.D'Anci KE, Constant F, Rosenberg IH. Hydration and cognitive function in children. Nutr Rev. 2006;64:457–464. [DOI] [PubMed] [Google Scholar]
- 110.Faith JJ, McNulty NP, Rey FE, et al. Predicting a human gut microbiota's response to diet in gnotobiotic mice. Science. 2011;333:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mitsuoka T. Development of functional foods. Biosci Microbiota Food Health. 2014;33:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Subramanian S, Blanton LV, Frese SA, et al. Cultivating healthy growth and nutrition through the gut microbiota. Cell. 2015;161:36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Subramanian S, Huq S, Yatsunenko T, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510:417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chaudhary R, Chugh M, Darokhan Z, et al. Physiological slowing and upregulation of inhibition in cortex are correlated with behavioral deficits in protein malnourished rats. PLoS One. 2013;8:e76556 doi:10.1371/journal.pone.0076556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Belluscio LM, Berardino BG, Ferroni NM, et al. Early protein malnutrition negatively impacts physical growth and neurological reflexes and evokes anxiety and depressive-like behaviors. Physiol Behav. 2014;129:237–254. [DOI] [PubMed] [Google Scholar]
- 116.Akitake Y, Katsuragi S, Hosokawa M, et al. Moderate maternal food restriction in mice impairs physical growth, behavior, and neurodevelopment of offspring. Nutr Res. 2015;35:76–87. [DOI] [PubMed] [Google Scholar]
- 117.Lyte M. Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013;9:e1003726 doi:10.1371/journal.ppat.1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barrett E, Ross RP, O'Toole PW, et al. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113:411–417. [DOI] [PubMed] [Google Scholar]
- 120.Blüml S, Wisnowski JL, Nelson MD, Jr, et al. Metabolic maturation of the human brain from birth through adolescence: insights from in vivo magnetic resonance spectroscopy. Cereb Cortex. 2013;23:2944–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.El Aidy S, Derrien M, Merrifield CA, et al. Gut bacteria–host metabolic interplay during conventionalisation of the mouse germfree colon. ISME J. 2013;7:743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev. 2014;72:267–284. [DOI] [PubMed] [Google Scholar]
- 123.Guerrant RL, DeBoer MD, Moore SR, et al. The impoverished gut – a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2013;10:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tshala-Katumbay D, Mwanza JC, Rohlman DS, et al. A global perspective on the influence of environmental exposures on the nervous system. Nature. 2015;527:S187–S192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158 doi:10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Finegold SM, Molitoris D, Song Y, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35(suppl 1):S6–S16. [DOI] [PubMed] [Google Scholar]
- 127.Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr Bull. 2008;34:1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sridharan GV, Choi K, Klemashevich C, et al. Prediction and quantification of bioactive microbiota metabolites in the mouse gut. Nat Commun. 2014;5:5492 doi:10.1038/ncomms6492. [DOI] [PubMed] [Google Scholar]
- 129.Asano Y, Hiramoto T, Nishino R, et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1288–G1295. [DOI] [PubMed] [Google Scholar]
- 130.Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol. 2004;558:263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol. 2012;71:444–457. [DOI] [PubMed] [Google Scholar]
- 133.Galea I, Palin K, Newman TA, et al. Mannose receptor expression specifically reveals perivascular macrophages in normal, injured, and diseased mouse brain. Glia. 2005;49:375–384. [DOI] [PubMed] [Google Scholar]
- 134.Fabriek BO, Van Haastert ES, Galea I, et al. CD163-positive perivascular macrophages in the human CNS express molecules for antigen recognition and presentation. Glia. 2005;51:297–305. [DOI] [PubMed] [Google Scholar]
- 135.Walsh JT, Watson N, Kipnis J. T cells in the central nervous system: messengers of destruction or purveyors of protection? Immunology. 2014;141:340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jack CS, Arbour N, Manusow J, et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–4330. [DOI] [PubMed] [Google Scholar]
- 137.Stolp HB, Dziegielewska KM, Ek CJ, et al. Breakdown of the blood–brain barrier to proteins in white matter of the developing brain following systemic inflammation. Cell Tissue Res. 2005;320:369–378. [DOI] [PubMed] [Google Scholar]
- 138.Wang X, Hellgren G, Lofqvist C, et al. White matter damage after chronic subclinical inflammation in newborn mice. J Child Neurol. 2009;24:1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stolp HB, Johansson PA, Habgood MD, et al. Effects of neonatal systemic inflammation on blood-brain barrier permeability and behaviour in juvenile and adult rats. Cardiovasc Psychiatry Neurol. 2011;2011:469046 doi:10.1155/2011/469046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rietschel ET, Kirikae T, Schade FU, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. [DOI] [PubMed] [Google Scholar]
- 141.Banks WA, Kastin AJ. Passage of peptides across the blood-brain barrier: pathophysiological perspectives. Life Sci. 1996;59:1923–1943. [DOI] [PubMed] [Google Scholar]
- 142.Qin L, Wu X, Block ML, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Verma S, Nakaoke R, Dohgu S, et al. Release of cytokines by brain endothelial cells: a polarized response to lipopolysaccharide. Brain Behav Immun. 2006;20:449–455. [DOI] [PubMed] [Google Scholar]
- 144.Wu Z, Zhang J, Nakanishi H. Leptomeningeal cells activate microglia and astrocytes to induce IL-10 production by releasing pro-inflammatory cytokines during systemic inflammation. J Neuroimmunol. 2005;167:90–98. [DOI] [PubMed] [Google Scholar]
- 145.Ghosh A, Birngruber T, Sattler W, et al. Assessment of blood-brain barrier function and the neuroinflammatory response in the rat brain by using cerebral open flow microperfusion (cOFM). PLoS One. 2014;9:e98143 doi:10.1371/journal.pone.0098143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sierra A, Gottfried-Blackmore AC, McEwen BS, et al. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–424. [DOI] [PubMed] [Google Scholar]
- 147.Fenn AM, Henry CJ, Huang Y, et al. Lipopolysaccharide-induced interleukin (IL)-4 receptor-α expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behav Immun. 2012;26:766–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hernandez-Romero MC, Delgado-Cortes MJ, Sarmiento M, et al. Peripheral inflammation increases the deleterious effect of CNS inflammation on the nigrostriatal dopaminergic system. Neurotoxicology. 2012;33:347–360. [DOI] [PubMed] [Google Scholar]
- 149.Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram-negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett. 2008;29:117–124. [PubMed] [Google Scholar]
- 150.Wang P, You SW, Yang YJ, et al. Systemic injection of low-dose lipopolysaccharide fails to break down the blood–brain barrier or activate the TLR4-MyD88 pathway in neonatal rat brain. Int J Mol Sci. 2014;15:10101–10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen Z, Jalabi W, Shpargel KB, et al. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J Neurosci. 2012;32:11706–11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ling Z, Zhu Y, Tong C, et al. Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp Neurol. 2006;199:499–512. [DOI] [PubMed] [Google Scholar]
- 153.Deretzi G, Kountouras J, Grigoriadis N, et al. From the “little brain” gastrointestinal infection to the “big brain” neuroinflammation: a proposed fast axonal transport pathway involved in multiple sclerosis. Med Hypotheses. 2009;73:781–787. [DOI] [PubMed] [Google Scholar]
- 154.Oria RB, Costa CM, Lima AA, et al. Semantic fluency: a sensitive marker for cognitive impairment in children with heavy diarrhea burdens? Med Hypotheses. 2009;73:682–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Patrick PD, Oria RB, Madhavan V, et al. Limitations in verbal fluency following heavy burdens of early childhood diarrhea in Brazilian shantytown children. Child Neuropsychol. 2005;11:233–244. [DOI] [PubMed] [Google Scholar]
- 156.Beutheu S, Ghouzali I, Galas L, et al. Glutamine and arginine improve permeability and tight junction protein expression in methotrexate-treated Caco-2 cells. Clin Nutr. 2013;32:863–869. [DOI] [PubMed] [Google Scholar]
- 157.Quirino IE, Cardoso VN, Santos R, et al. The role of L-arginine and inducible nitric oxide synthase in intestinal permeability and bacterial translocation. JPEN J Parenter Enteral Nutr. 2013;37:392–400. [DOI] [PubMed] [Google Scholar]
- 158.Costa KA, Soares AD, Wanner SP, et al. L-arginine supplementation prevents increases in intestinal permeability and bacterial translocation in male Swiss mice subjected to physical exercise under environmental heat stress. J Nutr. 2014;144:218–223. [DOI] [PubMed] [Google Scholar]
- 159.Castro IC, Oliveira BB, Slowikowski JJ, et al. Arginine decreases Cryptosporidium parvum infection in undernourished suckling mice involving nitric oxide synthase and arginase. Nutrition. 2012;28:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ren W, Chen S, Yin J, et al. Dietary arginine supplementation of mice alters the microbial population and activates intestinal innate immunity. J Nutr. 2014;144:988–995. [DOI] [PubMed] [Google Scholar]
- 161.Gao JH, Guo LJ, Huang ZY, et al. Roles of cellular polyamines in mucosal healing in the gastrointestinal tract. J Physiol Pharmacol. 2013;64:681–693. [PubMed] [Google Scholar]
- 162.Kibe R, Kurihara S, Sakai Y, et al. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci Rep. 2014;4:4548 doi:10.1038/srep04548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Blachier F, Davila AM, Benamouzig R, et al. Channelling of arginine in NO and polyamine pathways in colonocytes and consequences. Front Biosci (Landmark Ed). 2011;16:1331–1343. [DOI] [PubMed] [Google Scholar]
- 164.Curis E, Nicolis I, Moinard C, et al. Almost all about citrulline in mammals. Amino Acids. 2005;29:177–205. [DOI] [PubMed] [Google Scholar]
- 165.Abrahams VM, Kim YM, Straszewski SL, et al. Macrophages and apoptotic cell clearance during pregnancy. Am J Reprod Immunol. 2004;51:275–282. [DOI] [PubMed] [Google Scholar]
- 166.Antunes MM, Leocadio PC, Teixeira LG, et al. Pretreatment with L-citrulline positively affects the mucosal architecture and permeability of the small intestine in a murine mucositis model. JPEN J Parenter Enteral Nutr. 2016;40:279–286. [DOI] [PubMed] [Google Scholar]
- 167.Batista MA, Nicoli JR, Martins FS, et al. Pretreatment with citrulline improves gut barrier after intestinal obstruction in mice. JPEN J Parenter Enteral Nutr. 2012;36:69–76. [DOI] [PubMed] [Google Scholar]
- 168.Ren W, Duan J, Yin J, et al. Dietary L-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine. Amino Acids. 2014;46:2403–2413. [DOI] [PubMed] [Google Scholar]
- 169.Andrade ME, Araujo RS, de Barros PA, et al. The role of immunomodulators on intestinal barrier homeostasis in experimental models. Clin Nutr. 2015;34:1080–1087. [DOI] [PubMed] [Google Scholar]
- 170.Ley RE, Backhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thomas LV, Ockhuizen T, Suzuki K. Exploring the influence of the gut microbiota and probiotics on health: a symposium report. Br J Nutr. 2014;112(suppl 1):S1–S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Carabotti M, Scirocco A, Maselli MA, et al. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 173.Shen J, Chen Y, Wang Z, et al. Coated zinc oxide improves intestinal immunity function and regulates microbiota composition in weaned piglets. Br J Nutr. 2014;111:2123–2134. [DOI] [PubMed] [Google Scholar]
- 174.Broom LJ, Miller HM, Kerr KG, et al. Effects of zinc oxide and Enterococcus faecium SF68 dietary supplementation on the performance, intestinal microbiota and immune status of weaned piglets. Res Vet Sci. 2006;80:45–54. [DOI] [PubMed] [Google Scholar]
- 175.de Queiroz CA, Fonseca SG, Frota PB, et al. Zinc treatment ameliorates diarrhea and intestinal inflammation in undernourished rats. BMC Gastroenterol. 2014;14:136 doi:10.1186/1471-230X-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hu CH, Song ZH, Xiao K, et al. Zinc oxide influences intestinal integrity, the expressions of genes associated with inflammation and TLR4-myeloid differentiation factor 88 signaling pathways in weanling pigs . Innate Immun. 2013;20:478–486. [DOI] [PubMed] [Google Scholar]
- 177.Bolick DT, Kolling GL, Moore JH, et al. Zinc deficiency alters host response and pathogen virulence in a mouse model of enteroaggregative Escherichia coli-induced diarrhea. Gut Microbes. 2014;5:618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Medeiros P, Bolick DT, Roche JK, et al. The micronutrient zinc inhibits EAEC strain 042 adherence, biofilm formation, virulence gene expression, and epithelial cytokine responses benefiting the infected host. Virulence. 2013;4:624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Iannotti LL, Trehan I, Clitheroe KL, et al. Diagnosis and treatment of severely malnourished children with diarrhoea. J Paediatr Child Health. 2015;51:387–395. [DOI] [PubMed] [Google Scholar]