Abstract
Context: Studies suggest that appropriate nutritional modifications can improve the natural conception rate of infertile couples. Objectives: The purpose of this study was to review the human trials that investigated the relation between nutrition and male infertility. Data Sources: A comprehensive systematic review of published human studies was carried out by searching scientific databases. Article selection was carried out in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses. The American Dietetic Association Research Design and Implementation Checklist was also used for quality assessment. Data Extraction: A total of 502 articles were identified, of which 23 studies met the inclusion criteria. Data Synthesis: Results indicated that a healthy diet improves at least one measure of semen quality, while diets high in lipophilic foods, soy isoflavones, and sweets lower semen quality. Conclusion: The role of daily nutrient exposure and dietary quality needs to be highlighted in male infertility. Mechanistic studies addressing the responsible underlying mechanisms of action of dietary modifications are highly warranted. Systematic Review Registration: PROSPERO 2013: CRD42013005953. Available at: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013005953.
Keywords: diet, food compounds, male infertility, nutrition, sperm quality
INTRODUCTION
Infertility is a global medical concern affecting 60–80 million couples worldwide,1 and studies report that male factor infertility contributes to more than 40% of infertility cases globally.1,2 Several different disorders can cause male infertility, including idiopathic oligospermia (low sperm counts, for which a specific treatment remains unknown),3–6 complete asthenozoospermia (absence of sperm motility; implicated in 19% of infertile cases),7 and isolated asthenozoospermia (low sperm motility, caused by sperm dysfunction, varicocele, infection, or genetic factors; implicated in 24% of infertile cases).8–10
In the 1970s, several purported causes of male factor infertility received considerable attention. As a consequence, the World Health Organization published both new standardized diagnostic approaches toward male factor infertility11 and standardized techniques of semen analysis.12–14 Furthermore, the introduction of assisted reproductive techniques, such as in vitro fertilization and intracytoplasmic sperm injection,15 revolutionized reproductive medicine, revealing the role of the male factor in couple infertility.16 However, in the last decade, increasing concerns about the ethics, economics, and side effects of assisted reproductive techniques have been raised worldwide.17,18 Different studies reported that in vitro fertilization and intracytoplasmic sperm injection may be associated with major congenital malformations, risk of retinoblastoma, and impaired development of offspring.19–22 Although assisted reproductive techniques are able to mechanically rectify some types of male infertility disorders, the fundamental reasons behind male infertility remain untouched.23,24
Recent studies illustrate that nutrition and lifestyle factors play a critical role in the normal function of the reproductive system.25,26 Apart from demographic and certain known lifestyle factors (such as age, smoking, and alcohol intake), there is increasing evidence indicating the crucial role of nutrition in decreased sperm quality.27–30 Recent evidence from both animal and human studies indicates that male obesity and high-fat diets result in impaired reproductivity, affecting the molecular and physical structure of sperm as well as the health of the developing fetus and subsequent offspring.27,31–39 In a recent animal study, Rato et al.39 reported that the testicular physiology is sensitive to alterations of whole-body metabolism and that the testicular metabolism can be disturbed by high-energy diets. Disruption of testicular metabolism is associated with decreased sperm quality.27 Some components of high-energy diets, such as trans fatty acids and saturated fats, have the potential to adversely affect the testicular lipid metabolism and impair sperm production. In another animal study, Bakos et al.40 reported that paternal-diet-induced obesity decreased both the motility and the fertilizing ability of sperm in male mice. Morgan et al.34 also reported that elevated levels of plasma cholesterol induced by a cholesterol-rich diet negatively affected spermatogenesis in rabbits. To date, many human studies have investigated semen quality and male infertility, but most of these focus on isolated micronutrients (such as different antioxidants, zinc, or folate), while studies of a specific diet or food compound are scarce.
Males of reproductive age have been affected by the spread of unhealthy eating behaviors2,41 and the rapid negative changes in dietary patterns, such as high intakes of saturated fatty acids, trans fats, and sodium and low intakes of fruits and vegetables (rich in antioxidants and flavonoids). Excessive amounts of reactive oxygen species have a serious deleterious effect on sperm DNA, leading to formation of 8-oxodeoxyguanosine, the major oxidative product of sperm DNA (which causes fragmentation and mutagens). It has therefore been tempting to reduce the effect of reactive oxygen species on sperm by administering antioxidant supplements.42–45 Additionally, overconsumption of high-energy diets rich in saturated fatty acids and trans fats may result in the accumulation of fatty acids and fat-soluble toxicants within the testicular environment, leading to impaired spermatogenesis and low testosterone synthesis by Leydig cells.27
Since available findings introduce nutrition as one of the major environmental factors influencing the health of the reproductive system,2,29,46 it seems crucial to include diet and nutrition counseling in the treatment protocols for infertile men. It is also important to pinpoint the number of studies investigating the relationship between diet and male infertility, as the number of publications on this topic has increased in recent years. Although the importance of diet is clear for obese or underweight infertile patients, there are many infertile patients with normal body weight who have degrees of dietary imbalances. Furthermore, at the hypothalamic level, the effects of dietary composition override the effects of body weight that directly and indirectly impact fertility and optimum responsiveness to infertility treatments. In addition, many recent studies report that inappropriate dietary habits (such as meal skipping or false dieting), low intake of antioxidants, and nutrient deficiencies have been observed in male factor infertility.25,28,47–50
The aim of this study was to systematically review published studies that investigated the relationship between nutrition, diet, or food compounds and male factor infertility. As far as can be determined, this is the first systematic review of studies that examined the relation between nutrition or dietary compounds and male infertility. Although reviews that investigated the association between diet or nutrition and male factor infertility are available, they were either nonsystematic or focused on one specific food compound or micronutrient.
METHODS
Literature search
A comprehensive review of human studies published in English over the last 2 decades was carried out by searching PubMed, Google Scholar, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Embase in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).51 Two reviewers, in collaboration with senior nutrition and infertility specialists, developed the search strategy. Two primary investigators independently completed the literature search, identification of studies, data abstraction, and tabulation; discordance was resolved by discussions with the lead researcher. The search used a combination of terms as both Medical Subject Headings (MeSH) and keywords or free-text words. Male infertility–related keywords (“male infertility” OR “sperm dysfunction” OR “sperm DNA damage” OR “varicocele” OR “idiopathic oligozoospermia” OR “asthenozoospermia” OR “oligoasthenoteratozoospermia”) were used in combination with words relating to food, diet or nutrients (“diet” OR “nutrition” OR “nutriceutical” OR “nutrient” OR “food” OR “vitamin E” OR “vitamin C” OR “vitamin A” OR “zinc” OR “l-carnitine” OR “N-acetyl cysteine” OR “antioxidant” OR “glutathione” OR “coenzyme Q10” OR “selenium”). In addition, a manual search of bibliographies of original research and previous reviews was performed to identify additional relevant articles.
Study selection and eligibility
Analytical studies that investigated the effect of food intake, dietary pattern, or micronutrient supplementation on male infertility were included in the study. A PICOS (Patient, Intervention, Comparator, Outcome, Study) design structure2,52 was used to develop the study questions and the inclusion/exclusion criteria (Table 1).
Table 1.
PICOS criteria for inclusion and exclusion of studies
| Parameter | Inclusion/exclusion criteria | Data extraction |
|---|---|---|
| Patient | Infertile men (men with the following disorders: sperm dysfunction, sperm DNA damage, varicocele, or idiopathic oligozoospermia) | Location, age, patient groups (trials only), type of infertility |
| Intervention | Oral administration of micronutrients (reports in which this was given in addition to other compounds were also eligible), assessment of food intake or dietary pattern | Type of micronutrient or assessment, dosage, duration of follow-up, any additional compound given |
| Comparators | Placebo, no micronutrient intake, different diets or food intakes | Numbers in the intervention and comparator groups, when relevant (trials only) |
| Outcome | Pregnancy rate, semen parameters, and semen quality (sperm concentration, motility, and morphology) | Details of sperm parameters, method of identification, treatment, and outcome. Effect of the intervention on clinical endpoints |
| Study design | Experimental studies (randomized trials), longitudinal studies (prospective and retrospective cohorts), case–control studies, and cross-sectional studies | Type of study design, and numbers in the intervention and comparator groups (trials only) |
Potentially appropriate studies were independently assessed by evaluating the title and abstract or, if no abstract was available, the full text of the article. The full text of all potentially eligible references was obtained for further application of inclusion criteria and quality assessments.
No limit was applied with respect to the duration and dosage of food or micronutrient consumption, and the references were imported into bibliographic database software to automatically exclude duplicates (Endnote, version 4). Furthermore, the latest publication was used when multiple articles for a single study were present. Review articles and commentaries were excluded after hand searching reference lists. Gray literature (conference abstracts and unpublished studies) was not included.
Quality assessment
The American Dietetic Association Research Design and Implementation Checklist for primary research was used for quality assessments.53 This checklist, which includes 10 questions, covers the following domains: research question, subject selection, comparable groups, description of withdrawal handling, blinding, protocol description, outcomes definition, statistical analysis, conclusions, and sponsorship/funding bias. Four key questions about subject selection, comparable groups, protocol description, and outcome definitions received the most consideration: (1) Was the selection of study subjects/patients free from bias? (2) Were study groups comparable? (3) Were intervention/therapeutic regimens/exposure factor or procedure and any comparison(s) described in detail? Were intervening factors described? and (4) Were outcomes clearly defined and the measurements valid and reliable? Each study was considered eligible for inclusion if the answers to all 4 key questions plus 1 additional question were “yes.” A negative response to any of the 4 key questions resulted in the exclusion of study under assessment. In addition, reviewers were blinded to the authors, institutions/affiliations, and journal names during quality assessment. A third party carried out the blinding process.
Data extraction
Data were extracted by using a predefined data extraction sheet. Data on lead author, year of publication, study design, population, study inclusion criteria, type of disorder, intervention/assessment, duration of follow-up, major outcome(s), and study conclusion were extracted. After extraction, the lead researcher checked the extracted data to minimize the possibility of errors. Studies were then categorized into 2 groups: studies that investigated the effect of micronutrients on male infertility, and studies that investigated the effect of food intake or dietary pattern on male infertility.
RESULTS
Overview of identified studies
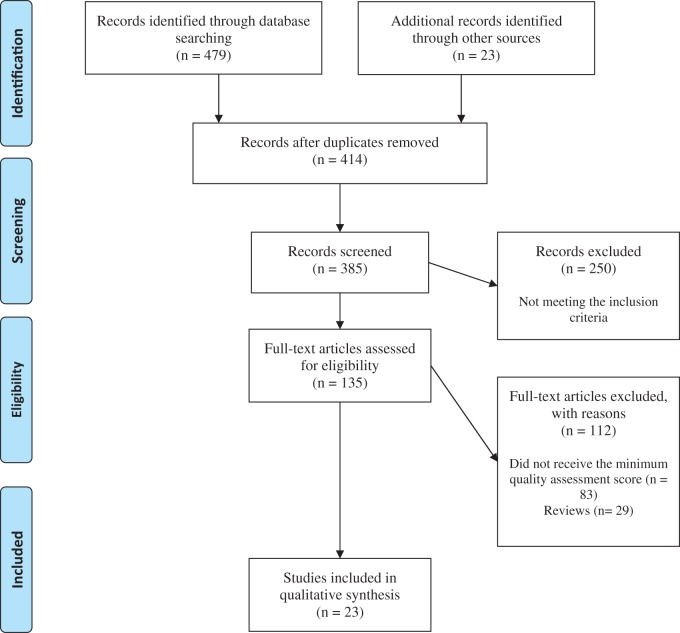
A total of 502 potentially relevant citations were identified through the search of electronic databases and other reference sources or information provided by relevant experts (Figure 1). A total of 135 articles were collected as full texts for quality assessment and potential inclusion. Of these, 23 articles (n = 2794 included patients) received the minimum required quality assessment score and met the inclusion criteria. Of the 23 included studies, 7 (n = 1084 patients) evaluated the effect of dietary habits and food intake on semen parameters and quality,2,25,28,48 4 (n = 232 patients) evaluated the effect of carnitine consumption on sperm parameters and quality,54–57 3 (n = 148 patients) investigated the effect of selenium on sperm parameters,58–60 2 (n = 481 patients) evaluated the effect of coenzyme Q10 on sperm quality and parameters,61,62 and each of the remaining articles investigated the effect of saffron, soy-based foods, vitamin C, clomiphene citrate, n-3 polyunsaturated fatty acids, beta carotene, and ubiquinol on sperm parameters.63–69 The 23 articles included patients from 9 different countries: Iran (6 articles),2,61–63,67,69 the United States (7 articles),28,54,58,64,70–72 Italy (3 articles),55–57 Egypt (2 articles),66,68 the United Kingdom (1 article),60 Germany (1 article),65 Poland (1 article),59 Spain (1 article),48 and Brazil (1 article).25
Figure 1.
PRISMA flow diagram of the literature search and selection process
Study hypotheses
Sixteen (70%) of the 23 studies hypothesized that the designated supplementation would result in improved sperm quality and parameters. The remaining 7 studies that investigated dietary habits hypothesized that diets rich in fruits or vegetables may maintain or improve semen quality.
Study designs
The included studies comprised 12 randomized controlled trials,54,55,57–60,62,63,65–69 3 open-label studies,56,61,68 4 observational studies,25,48,70,71 3 cross-sectional surveys,28,64,72 and 1 case–control study.2 The largest part of the data pool came from randomized control trials, with sample sizes ranging from small (n = 21) to large (n = 230).
Interventions or assessments
The majority of the included studies (n = 14, 60%) investigated the effect of a specific supplementation on sperm parameters, whereas 7 studies (30%) assessed the effect of dietary intakes and 2 studies (10%) examined the effect of specific food compounds (saffron and soy foods).63,64 Furthermore, the included articles varied considerably in the nature, dosage, and duration of interventions. Apart from the 7 studies that assessed the effect of dietary intake, 6 studies58,59,61–63,69 investigated the effect of a single agent, while the remaining articles considered a combination of different agents. The single agents were saffron,63 coenzyme Q10,61,62,69 and selenium.58,59 The average duration of the studies was 24 weeks.
Outcomes of interest
All of the included studies in this review reported the outcome in terms of semen variables, pregnancy incident, or both. Taken together, more than half of the included studies reported improvement in at least one of these outcome measures in the treatment group. Seven studies that investigated the effect of food intake and dietary habits reported a positive association between higher intake of processed meat and sweets and the risk of low sperm quality (Table 2).2,25,28,48,63,64,70–72 The remaining studies, which investigated the role of specific micronutrient(s) on indicators of semen quality, reported conflicting results (Table 3).17,54–62,65–67,69
Table 2.
Summary of the studies that investigated dietary patterns or intakes of food compounds
| Reference | Intervention or assessment | Inclusion criteria | Size of population; type of study | Outcome(s) | Conclusion |
|---|---|---|---|---|---|
| Afeiche et al. (2014)70 | Dietary assessment | Men aged 18–55 y, from couples using their own gametes for IUI or assisted reproductive technologies, without a history of vasectomy | n = 155; observational | Total sperm count, sperm concentration, progressive motility, sperm morphology, and semen volume | Low-fat dairy intake, particularly low-fat milk, is related to higher sperm concentration and progressive motility |
| Afeiche et al. (2014)71 | Dietary assessment | Men aged 18–55 y, from couples using their own gametes for IUI or assisted reproductive technologies, without a history of vasectomy | n = 155; observational | Semen quality parameters | Processed meat intake was negatively associated with sperm morphology, whereas fish intake was positively associated with total sperm count and sperm morphology |
| Afeiche et al. (2014)72 | Dietary assessment | Men aged 18–22 y, able to read and speak English, and able to contact their mother and ask her to complete a questionnaire | n = 189; cross-sectional | Semen quality parameters | Processed red meat intake was inversely related to total sperm count and total progressive motile sperm count |
| Gaskins et al. (2012)28 | Dietary assessment | Men aged 18–22 y, able to read and speak English, and able to contact their mother and ask her to complete a questionnaire | n = 188; observational | Semen quality parameters | A diet rich in fruits, vegetables, fish, chicken, and whole grains may improve at least 1 measure of semen quality |
| Mendiola et al. (2009)48 | Assessment of recorded dietary habits and food consumption | Men with severe or moderate oligozoospermia and severe teratozoospermia | n = 60 (30 T and 30 C); observational | Semen parameters, hormone levels, Y microdeletions, and karyotypes | Frequent intake of lipophilic foods such as meat products or milk may negatively affect semen quality, whereas intake of some fruits or vegetables may maintain or improve semen quality |
| Braga et al. (2012)25 | Assessment of recorded dietary and social habits | Male patients undergoing ICSI cycles | n = 250; observational | Semen parameters and ICSI outcomes | Intakes of fruits, cereals, and vegetables were positively related to sperm quality and implantation rate. Meat intake, as well as food deprivation or high BMI, was negatively correlated with ICSI success |
| Eslamian et al. (2012)2 | Assessment of usual dietary intakes | Men with asthenozoospermia | n = 241 (72 T and 169 C); observational | Semen volume and sperm concentration, motility, and morphology | A higher intake of processed meat and sweets was positively associated with the risk of asthenozoospermia, whereas a high intake of fruits, vegetables, poultry, skim milk, and seafood was associated with a lower risk |
| Chavarro et al. (2008)64 | Assessment of intake of 15 soy-based foods in the previous 3 mo | Male partners in subfertile couples | n = 99; observational | Semen quality | Higher intake of soy foods and soy isoflavones is associated with lower sperm concentration |
| Safarinejad et al. (2011)63 | Two saffron capsules, twice daily (total of 60 mg) for 26 wk | Age <45 y, unable to conceive a child for >24 mo before the study with the same partner, total testicular volume >12 mL, and a normal fertile female partner | n = 230 (114 T and 116 C); RCTs | Semen parameters and total seminal plasma antioxidant capacity | Saffron does not statistically improve semen parameters in infertile men with idiopathic OAT |
Abbreviations: BMI, body mass index; C, control group; ICSI, intracytoplasmic sperm injection; IUI, intrauterine insemination; OAT, oligoasthenoteratozoospermia; RCT, randomized controlled trial; T, treatment group.
Table 3.
Summary of studies that investigated intake of micronutrients
| Reference | Intervention or assessment | Inclusion criteria | Size of population; type of study | Outcome(s) | Conclusion |
|---|---|---|---|---|---|
| Sigman et al. (2006)54 | LC 2000 mg/d and LAC 1000 mg/d for 24 wk | Infertile men with sperm motility of 10%–50% | n = 21 (12 T and 9 C); RCT | Sperm motility and total motile sperm counts | Carnitine supplementation demonstrated no clinically or statistically significant effect on sperm motility or total motile sperm counts |
| Balercia et al. (2005)55 | LC 3 g/d or LAC 3 g/d or combined LC 2 g/d and LAC 1 g/d for 24 wk | Sperm concentration >20 × 106/mL, sperm forward motility <50%, and normal sperm morphology >30% | n = 59 (44 T and 15 C); RCT | Variations in semen parameters and variations in total oxyradical scavenging capacity of the seminal fluid | Administration of LC and LAC is effective in increasing kinetic features of sperm in patients with idiopathic asthenozoospermia and improves the total oxyradical scavenging capacity of the seminal fluid in the same population |
| Vicari & Calogero (2001)57 | Carnitines (LC 1 g and LAC 0.5 g twice daily) for 12 wk | Chronic abacterial PVE | n = 54; RCT | Sperm parameters | Treatment with carnitines is effective only in patients with abacterial PVE, elevated ROS production, and normal seminal WBC concentration |
| Vicari et al. (2002)56 | Carnitines for 16 wk | Chronic abacterial PVE, oligozoospermia, asthenozoospermia with forward progression, or teratozoospermia | n = 98; open prospective | Semen variables, production of ROS, and pregnancy outcome | Antioxidant treatment with carnitines is effective in patients with abacterial PVE and increased seminal leukocyte concentrations, provided these patients have been pretreated with nonsteroidal anti-inflammatory drugs |
| Safarinejad et al. (2011)61 | CoQ10 300 mg orally twice daily for 48 wk | Sperm count of <5 × 106/mL, >2 y of failed attempts at conception, and no female factors | n = 287; open prospective | Semen parameters and pregnancy rates | CoQ10 supplementation improves semen quality, with beneficial effect on pregnancy rate |
| Safarinejad (2009)62 | CoQ10 300 mg daily for 26 wk | Unwilling childlessness at least 24 mo in duration with a female partner, no known medical condition that could account for infertility, total testicular volume ≥12 mL on ultrasound, and a normal fertile female partner | n = 194 (98 T and 96 C); RCT | Semen analyses | Significant improvement in certain semen parameters |
| Hawkes et al. (2009)58 | Se (300 mg/d) for 48 wk | Self-reported absence of disease (hypertension, diabetes, sexually transmitted disease, cancer, etc.) as well as clinically normal blood count, blood chemistries, thyrotropin, and semen analysis | n = 42 (22 high Se and 20 low Se); RCT | Semen quality | No significant effect of Se supplementation on any parameter of sperm motility |
| Iwanier & Zachara (1995)59 | Se 200 pg/d | Infertile men | n = 42 (33 patients + 9 fertile men); RCT | Sperm count, sperm motility, sperm morphology | Se supplementation did not improve sperm count, sperm motility, or sperm morphology |
| Scott et al. (1998)60 | Se (100 µg/d), Se + Vit A (1 mg/d), Vit C (10 mg) and Vit E (15 mg) for 12 wk | Men with reduced sperm motility | n = 64; RCT | Blood Se concentration, sperm motility, sperm count | Se supplementation in subfertile men with low Se status can improve sperm motility and the chance of successful conception |
| Rolf et al. (1999)65 | Vit C (1000 mg), Vit E (800 mg) or identical placebo capsule for 8 wk | Men with infertility persisting longer than 1 y, asthenozoospermia | n = 31 (16 C and 15 T); RCT | Volume, PH, and color of ejaculate, sperm parameters | No changes in semen parameters observed during treatment, and no pregnancies initiated during treatment. High-dose antioxidative treatment combined with vitamins C and E did not improve conventional semen parameters or 24 h sperm survival rates |
| Ghanem et al. (2010)66 | Clomiphene citrate (25 mg/d) and Vit E (400 mg/d) for 24 wk | Men with unexplained infertility, sperm concentration of <20 × 106, sperm total motility of <50%, and normal sperm morphology >30% | n = 60 (30 T and 30 C); RCT | Pregnancy incidence and variations in semen parameters | Treatment significantly increased the pregnancy rate and improved sperm count and progressive sperm motility |
| Safarinejad et al. (2011)67 | EPA and DHA, 1.84 g/d for 32 wk | Unwanted childlessness of at least 24 mo with same female partner; no known medical condition that could account for infertility, and total testicular volume >12 mL | n = 211 (106 T and 105 C); RCT | Changes in semen parameters and composition of n-3 fatty acids; seminal plasma antioxidant status | Significant positive association between long-chain n-3 PUFAs (EPA and DHA) and poor semen quality among infertile men with idiopathic OAT, and positive association between long-chain n-3 PUFAs and seminal plasma antioxidant status |
| Comhaire et al. (2000)68 | Beta carotene (30 mg daily), alpha tocopherol (180 mg), essential fatty acids (0.25 g) and arachidonic acid (0.10 g) daily for 24 wk | Male factor infertility | n = 27; open prospective | Sperm parameters | Treatment did not improve sperm motility or morphology or decrease the concentration of round cells and WBCs in semen. Sperm concentration increased in oligozoospermic men. Treatment significantly reduced ROS |
| Safarinejad et al. (2012)69 | Ubiquinol 200 mg/d for 26 wk | Less than 14% normal forms, oligozoospermia by a sperm concentration of <20 × 106/ml, and asthenozoospermia by <50% of motile spermatozoa with forward progression | n = 191 (96 T and 95 C); RCT | Sperm density, sperm motility, and sperm strict morphology | Significantly effective in improving sperm density, sperm motility, and sperm morphology in men with unexplained OAT |
Abbreviations: CoQ10, coenzyme Q10; C, control group; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid;. ICSI, intracytoplasmic sperm injection; LAC, l-acetyl-carnitine; LC, l-carnitine; Se, selenium; OAT, oligoasthenoteratozoospermia; PUFA, polyunsaturated fatty acid; PVE, prosthetic valve endocarditis; RCT, randomized control trial; ROS, reactive oxygen species; T, treatment group; Vit, vitamin; WBC, white blood cells.
Dietary habits and food intake.
All 7 included studies that investigated the role of diet and food intake on sperm variables reported that diet plays a key role in the improvement of sperm parameters. Although Gaskins et al.28 reported no effect of a Western dietary pattern on semen quality, they found that a prudent dietary pattern (high intake of fruits and vegetables, low intake of meat/fat/processed food) was associated with increased sperm motility. Furthermore, Braga et al.,25 Eslamian et al.,2 and Mendiola et al.48 reported that fat-rich foods (e.g., processed meat), soy isoflavones, and sweets decrease semen quality. In a recent study, Afeiche et al.72 demonstrated that consumption of processed red meat has an inverse effect on sperm concentration and total progressive motile count. Two separate 2014 studies also reported the positive effects of fish71 and low-fat dairy intake (particularly low-fat milk)70 on indicators of semen quality.
Saffron.
In a 2011 study by Safarinejad et al.,63 the effect of saffron consumption as an herbal remedy to improve semen parameters was evaluated. Oral intake of 2 saffron capsules (60 mg/d) for 26 weeks did not significantly improve semen parameters in infertile men with idiopathic oligoasthenoteratozoospermia. Furthermore, saffron administration did not improve total seminal plasma antioxidant capacity.
Carnitine.
l-carnitine and l-acetylcarnitine were used in combination in 4 of the studies in this review.54–57 Although Balercia et al.,55 Vicari et al.,56 and Vicari and Calogero57 reported that carnitine administration had a positive effect on sperm variables and total oxyradical scavenging capacity of the seminal fluid, Sigman et al.54 found no significant effect on sperm motility or total motile sperm counts in their 2006 trial.
Coenzyme Q10.
In 2 different large trials, Safarinejad61,62 investigated the effect of coenzyme Q10 over periods of 26 and 48 weeks. In the first trial, from 2009, 300 mg of daily consumption of coenzyme Q10 for 26 weeks resulted in significant improvement in semen parameters. In the second trial, from 2011, in which the effect of coenzyme Q10 on semen quality parameters and pregnancy rate was investigated, consumption of the same supplement (300 mg twice daily for 48 weeks) was found to have a positive effect on both the sperm parameters and the pregnancy rate.
Selenium.
Two of the randomized control trials, by Iwanier and Zachara59 and Hawkes et al.,58 investigated the effect of selenium consumption on sperm parameters, but neither reported a positive result. Selenium intake, neither 200 mg/d for 12 weeks59 nor 300 mg/d for 48 weeks,58 resulted in no significant improvement in sperm parameters.
In a 1998 study by Scott et al.,60 however, the combined use of selenium (100 µg) and vitamin A (1 mg) showed that selenium supplementation in subfertile men with low selenium status can improve sperm motility and the chance of successful conception.
DISCUSSION
This systematic review, which investigated the effect of nutrition and diet on male infertility, highlights the paucity of studies on this topic. There were only 23 articles that met the inclusion criteria, and within these articles, a notable disparity was observed in study design, trial quality, and final results. The inherent variability prevented any quantitative meta-analysis of the reported results, but this review has shown that evidence of the association between diet or nutrition and male infertility is currently scarce. This study, which systematically assessed all identified human studies on this topic, supports the results of earlier systematic and nonsystematic reviews by demonstrating that future research requires stronger study designs.1,47,73 The key methodological component of study design was the implementation of the American Dietetic Association checklist for identification of articles for inclusion. Not surprisingly, the variation in the results of included articles makes it difficult to draw a robust conclusion.
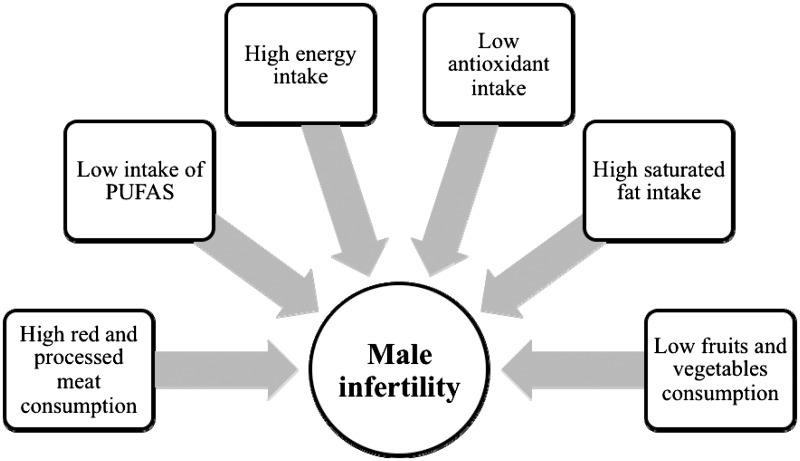
The most commonly reported assessments or interventions in the included studies were dietary patterns and intake of food compounds. However, most of the articles that investigated dietary patterns had an observational design, which limits the ability to determine causality between dietary effects and parameters of semen quality. These studies reported that higher consumption of fruits, green vegetables, fish, chicken, whole grains, and low-fat dairy products and decreased intake of meat, processed foods, sweets, and high-fat products have the potential to positively affect the quality of semen (Figure 2).
Figure 2.
Common nutrition-related factors that may result in male factor infertility. Abbreviation: PUFA, polyunsaturated fatty acids.
Data suggests that low-fat milk intake may be associated with increased levels of circulating insulin growth factor 1 (IGF-1) and insulin.70 Results from animal studies indicate that insulin has the potential to rescue spermatogenesis in type 1 diabetic mice74 as well as increase sperm motility and sperm concentration in rats.9 Furthermore, it has been demonstrated that IGF-1 protects equine Leydig cells from undergoing apoptosis in vitro.75 Spermatogenesis is an active cell division process that requires insulin and IGF-1. IGF-1 could bind to Leydig cell insulin receptors and activate them.76 Therefore, it is possible that the suggested positive effects of low-fat dairy consumption on parameters of semen quality represent a biological effect in humans. Nevertheless, further research is required to shed light on the true biological effect and proposed mechanisms.
Studies investigating meat intake and parameters of semen quality are scarce but report a consistent negative effect of meat consumption, especially consumption of processed meat, on indicators of semen quality. High levels of saturated fatty acids or the presence of preservative agents or hormonal residues in processed meat could be among the major causes of such negative effects.29,77 It has been reported that processed red meats have higher concentrations of hormone residues than other meats.78 In addition, red meat includes small amounts of trans fatty acids that can negatively affect sperm quality. However, an increased intake of antioxidants (antioxidant support) with a higher intake of trans fatty acids may ameliorate the negative effects on sperm quality.71,79 More clinical trials with sufficient sample sizes are needed to further elucidate the cause-and-effect relationship between red or processed meat consumption and male reproductive potential.
A positive association of fish intake with sperm concentration and morphology could be mediated through increased intake of long-chain n-3 fatty acids. Sperm and testes have a higher concentration of long-chain polyunsaturated fatty acids (PUFAs), especially docosahexaenoic acid (DHA), compared with other cells or tissues in the human body.80 The structural integrity of the spermatozoa cell membrane plays a pivotal role in successful fertilization. This is because both the acrosome reaction and sperm–oocyte fusion are associated with the membrane’s fatty acid profile.81 The increase in the DHA level in the sperm membrane during sperm maturation suggests that testes have a very active fatty acid metabolism that results in the preferential accumulation of long-chain PUFAs and the more efficient metabolization of PUFAs into long-chain metabolites.71 Moreover, marine products are characterized by a high proportion of fat-soluble vitamins that play a crucial role in fertilization.81 Therefore, it is plausible that increased fish intake or fish oil supplementation may result in improved parameters of semen quality.
A dietary pattern that includes fruits and vegetables is rich in antioxidants such as vitamin E, vitamin C, and beta carotene. Numerous health benefits have been ascribed to antioxidants, mainly because they protect against generation of reactive oxygen species.42,44 The production of reactive oxygen species has the potential to negatively affect sperm motility and the capacity for sperm–oocyte fusion.82 Moreover, antioxidants are thought to have the ability to protect human spermatozoa against endogenous oxidative damage by neutralizing hydroxyl, superoxide, and hydrogen peroxide radicals and preventing sperm agglutination.12,13,71,79,83,84
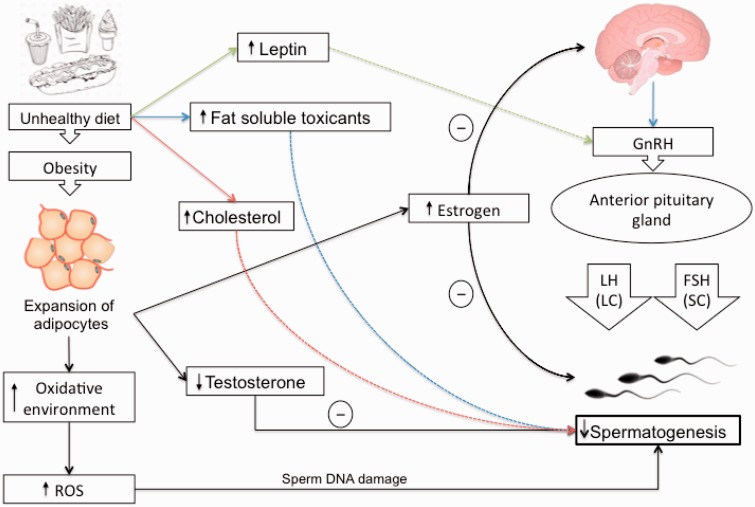
Deleterious effects of dietary factors can impair spermatogenesis, reduce sperm concentration and motility, and increase sperm DNA damage, especially in infertile obese men with diabetes, dyslipidemia, or metabolic syndrome, who are at increased risk of oxidative stress in the testicular microenvironment or the excurrent ductal system.47 Obesity, a consequence of high caloric load induced by a high-energy diet, is associated with increased number and size of adipocytes, and thus many proposed mechanisms for the role of obesity in male infertility focus on the abnormal level of adipose-derived hormones and adipokines.85 It has been reported that obese men seeking infertility treatments show a decreased ratio of testosterone to estrogen.86 This dysregulation is explained by the increased activity of aromatase cytochrome P450 enzyme, which is highly expressed in white adipose tissue.85,87 This enzyme plays a key role in the biosynthesis of estrogen, and obese males have a high rate of conversion of androgens into estrogen due to a high bioavailability of aromatase enzymes.87 Animal models have shown that high estrogen levels have a direct deleterious impact on spermatogenesis.88
Moreover, estrogen negatively regulates the secretion of gonadotropin-releasing hormone, luteinizing hormone, and follicle-stimulating hormone by the anterior pituitary gland. Luteinizing hormone and follicle-stimulating hormone are the functional links between brain and testicles, targeting, respectively, Sertoli cells (follicle-stimulating hormone) and Leydig cells (luteinizing hormone).27 The process of male reproduction is controlled by hypothalamic–pituitary–testicular (HPT) axis, and the signals derived from this neurohormonal network are closely related to the metabolism of testicular cells.89 According to current understanding, this complex system is highly sensitive to disturbances in energy homeostasis, and its dysregulation contributes to abnormalities in semen quality.27,90 Leptin, a hormone whose production is proportional to body fat mass, has the potential to act on multiple levels of the HPT axis. Studies showed that leptin can directly interact with its receptors on Leydig cells and regulates the synthesis of steroids within the testicles.91,92 Leptin can modulate the firing rate of gonadotropins as well.27 High amounts of leptin have been associated with impaired spermatogenesis and infertility, probably as a result of insufficient gonadotropin support, which leads to lower sperm quality.27,39 Moreover, increased intake of a high-energy diet can lead to leptin resistance, which can contribute to impairment of Leydig cells and dysfunction of the HPT axis, resulting in androgen deficiency.39
Additionally, paternal obesity resulting from consumption of a high-energy diet may affect the reproductive health of the offspring and play a significant role in the amplification of subfertility.93 Obesity is also negatively associated with the level of sex hormone–binding globulin.94,95 In other words, fat mass is a negative determinant of sex hormone–binding globulin levels because of reduced hepatic globulin synthesis caused by excessive levels of insulin.96 Cholesterol-rich diets have the potential to contribute to male infertility and to decrease semen quality via disruption of the blood–testes barrier.34 The blood–testes barrier, a structure consisting of tight junctions between Sertoli cells, limits the entry of toxins into the seminiferous tubules.97 During spermatogenesis, the blood–testes barrier undergoes rapid assembly and disassembly of tight junctions due to regulation of protein trafficking.98,99 Since uptake of extracellular cholesterol in Sertoli cells occurs via endocytosis, elevated plasma cholesterol is thought to promote endocytic accumulation of tight-junction proteins in Sertoli cells and disrupt the integrity of the blood–testes barrier, thereby contributing to impaired spermatogenesis34 (Figure 3).
Figure 3.
Overall effect of diet and nutrition on spermatogenesis. Increased intake of unhealthy diet may lead to impaired spermatogenesis due to elevated cholesterol, leptin, reactive oxygen species, and fat-soluble toxicants. Abbreviations: FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LC, Leydig cells; LH, luteinizing hormone; ROS, reactive oxygen species; SC, Sertoli cells.
This review benefits from several strengths, including its systematic and exhaustive approach, its use of the American Dietetic Association’s quality checklist, and its methodological rigor. Nevertheless, several potential limitations should be noted. Although broad search terms were used, multiple relevant scientific databases were searched, and reference lists were hand searched, it is possible that not all relevant publications were identified. In addition, the exclusion of non-English literature may have introduced unwanted bias in the findings.
Taken together, the 23 included studies provide contradictory evidence of the role of different dietary compounds in male infertility. A major finding of this review is the limited nature of studies on this topic, in terms of both number and quality. It is important to note, however, that the number of studies is increasing, as 35% of the articles included in this review were published since 2010.
CONCLUSION
The results of this review reinforce previous indications that the role of diet in male factor infertility still needs further research. Furthermore, they support the suggestion that a healthy diet is a safe way to improve at least one measure of semen quality. Hence, it is suggested that couples seeking assisted reproduction treatments be advised of the effect of diet quality on treatment success. Future research should aim to add to current knowledge of the effects of diet and dietary compounds in both testicular metabolism and sperm production. Animal studies may be helpful in developing further investigations on the role of diet and/or dietary compounds in sperm production and testicular cell metabolism. Such studies would be helpful for forming an in-depth knowledge of the molecular mechanisms and pathways related to male factor infertility and diet, since a majority of these pathways remain unknown. Moreover, additional clinical studies are imperative to clarify the optimum dosage and duration of treatment and to elucidate whether and how dietary compounds affect male infertility. Although clinical studies can be problematic, mostly due to financial burdens and regulatory requirements, they need to be carefully designed, preferably as multicenter, crossover, double-blind, randomized studies, and to have strict inclusion criteria as well as a sufficient sample size.
Acknowledgments
Parts of the results reported here were presented at the American Society for Nutrition’s 3rd Middle East Congress, Dubai, United Arab Emirates, February 19–21, 2014.
The authors would like to thank Dr Howard Glauert for his assistance during manuscript editing and Dr Alireza Alizadeh for critically reviewing the manuscript for important intellectual content during the revision process.
Author contributions. L.G. initiated the idea of the review, designed the study, supervised the project, and contributed to the drafting and finalizing of the manuscript. S.M. collected the data, assessed the articles, and wrote the final manuscript. A.J. collected the data, assessed the articles, conducted data tabulation, and contributed to the drafting of the manuscript. M.R.S. critically reviewed the article for important intellectual content; all authors approved the final manuscript.
Funding. There was no external source of funding for this work.
Declaration of interest. The authors have no relevant interests to declare.
References
- 1.Ross C, Morriss A, Khairy M, et al. A systematic review of the effect of oral antioxidants on male infertility. Reprod Biomed Online. 2010;20:711–723. [DOI] [PubMed] [Google Scholar]
- 2.Eslamian G, Amirjannati N, Rashidkhani B, et al. Intake of food groups and idiopathic asthenozoospermia: a case–control study. Hum Reprod. 2012;27:3328–3336. [DOI] [PubMed] [Google Scholar]
- 3.Anthony H. ABC of subfertility: male subfertility. BMJ. 2003;327:669–672.14500443 [Google Scholar]
- 4.Ortega C, Verheyen G, Raick D, et al. Absolute asthenozoospermia and ICSI: what are the options? Hum Reprod Update. 2011;17:684–692. [DOI] [PubMed] [Google Scholar]
- 5.Gdoura R, Kchaou W, Chaari C, et al. Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis and Mycoplasma genitalium infections and semen quality of infertile men. BMC Infect Dis. 2007;7:129 doi:10.1186/1471-2334-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuccarello D, Ferlin A, Garolla A, et al. A possible association of a human tektin-t gene mutation (A229V) with isolated non-syndromic asthenozoospermia: case report. Hum Reprod. 2008;23:996–1001. [DOI] [PubMed] [Google Scholar]
- 7.Curi SM, Ariagno JI, Chenlo PH, et al. Asthenozoospermia: analysis of a large population. Syst Biol Reprod Med. 2003;49:343–349. [DOI] [PubMed] [Google Scholar]
- 8.Luconi M, Forti G, Baldi E. Pathophysiology of sperm motility. Front Biosci. 2006;11:1433–1447. [DOI] [PubMed] [Google Scholar]
- 9.Jaiswal D, Sah R, Agrawal NK, et al. Combined effect of GSTT1 and GSTM1 polymorphisms on human male infertility in North Indian population. Reprod Sci. 2012;19:312–316. [DOI] [PubMed] [Google Scholar]
- 10.Martini AC, Tissera A, Estofán D, et al. Overweight and seminal quality: a study of 794 patients. Fertil Steril. 2010;94:1739–1743. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Towards more objectivity in diagnosis and management of male infertility. Int J Androl. 1987;10(supp1 7):1–53. [Google Scholar]
- 12.Nadjarzadeh A, Shidfar F, Amirjannati N, et al. Effect of coenzyme Q10 supplementation on antioxidant enzymes activity and oxidative stress of seminal plasma: a double-blind randomised clinical trial. Andrologia. 2014;46:177–183. [DOI] [PubMed] [Google Scholar]
- 13.Nadjarzadeh A, Sadeghi MR, Amirjannati N, et al. Coenzyme Q10 improves seminal oxidative defense but does not affect on semen parameters in idiopathic oligoasthenoteratozoospermia: a randomized double-blind, placebo controlled trial. J Endocrinol Invest. 2011;34:e224–e228. [DOI] [PubMed] [Google Scholar]
- 14.Thum C, Cookson AL, Otter DE, et al. Can nutritional modulation of maternal intestinal microbiota influence the development of the infant gastrointestinal tract? J Nutr. 2012;142:1921–1928. [DOI] [PubMed] [Google Scholar]
- 15.Palermo G, Joris H, Devroey P, et al. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–18. [DOI] [PubMed] [Google Scholar]
- 16.Comhaire FH, Mahmoud A. The role of food supplements in the treatment of the infertile man. Reprod Biomed Online. 2003;7:385–391. [DOI] [PubMed] [Google Scholar]
- 17.Comhaire F. Clinical andrology: from evidence-base to ethics: the `E' quintet in clinical andrology. Hum Reprod. 2000;15:2067–2071. [DOI] [PubMed] [Google Scholar]
- 18.Katz P, Nachtigall R, Showstack J. The economic impact of the assisted reproductive technologies. Nat Cell Biol. 2002;4(suppl.):S29–S32. [DOI] [PubMed] [Google Scholar]
- 19.Moll AC, Imhof SM, Cruysberg JR, et al. Incidence of retinoblastoma in children born after in-vitro fertilisation. Lancet. 2003;361:309–310. [DOI] [PubMed] [Google Scholar]
- 20.Hansen M, Kurinczuk JJ, Bower C, et al. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. New Engl J Med. 2002;346:725–730. [DOI] [PubMed] [Google Scholar]
- 21.Strömberg B, Dahlquist G, Ericson A, et al. Neurological sequelae in children born after in-vitro fertilisation: a population-based study. Lancet. 2002;359:461–465. [DOI] [PubMed] [Google Scholar]
- 22.Wennerholm UB, Bergh C, Hamberger L, et al. Incidence of congenital malformations in children born after ICSI. Hum Reprod. 2000;15:944–948. [DOI] [PubMed] [Google Scholar]
- 23.Varghese AC, Goldberg E, Agarwal A. Current and future perspectives on intracytoplasmic sperm injection: a critical commentary. Reprod Biomed Online. 2007;15:719–727. [DOI] [PubMed] [Google Scholar]
- 24.Tarlatzis BC, Bili H. Intracytoplasmic sperm injection survey of world results. Ann N Y Acad Sci. 2000;900:336–344. [DOI] [PubMed] [Google Scholar]
- 25.Braga DP, Halpern G, Figueira RC, et al. Food intake and social habits in male patients and its relationship to intracytoplasmic sperm injection outcomes. Fertil Steril. 2012;97:53–59. [DOI] [PubMed] [Google Scholar]
- 26.Chavarro JE, Rich-Edwards JW, Rosner BA, et al. Caffeinated and alcoholic beverage intake in relation to ovulatory disorder infertility. Epidemiology. 2009;20:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rato L, Alves MG, Cavaco JE, et al. High-energy diets: a threat for male fertility? Obes Rev. 2014;15:996–1007. [DOI] [PubMed] [Google Scholar]
- 28.Gaskins AJ, Colaci DS, Mendiola J, et al. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27:2899–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attaman JA, Toth TL, Furtado J, et al. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod. 2012;27:1466–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong WY, Thomas CM, Merkus JM, et al. Male factor subfertility: possible causes and the impact of nutritional factors. Fertil Steril. 2000;73:435–442. [DOI] [PubMed] [Google Scholar]
- 31.Ng SF, Lin RC, Laybutt DR, et al. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature. 2010;467:963–966. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell M, Bakos HW, Lane M. Paternal diet-induced obesity impairs embryo development and implantation in the mouse. Fertil Steril. 2011;95:1349–1353. [DOI] [PubMed] [Google Scholar]
- 33.Palmer NO, Bakos HW, Owens JA, et al. Diet and exercise in an obese mouse fed a high-fat diet improve metabolic health and reverse perturbed sperm function. Am J Physiol Endocrinol Metabol. 2012;302:E768–E780. [DOI] [PubMed] [Google Scholar]
- 34.Morgan DH, Ghribi O, Hui L, et al. Cholesterol-enriched diet disrupts the blood-testis barrier in rabbits. Am J Physiol Endocrinol Metabol. 2014;307:E1125–E1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bakos HW, Henshaw RC, Mitchell M, et al. Paternal body mass index is associated with decreased blastocyst development and reduced live birth rates following assisted reproductive technology. Fertil Steril. 2011;95:1700–1704. [DOI] [PubMed] [Google Scholar]
- 36.Tunc O, Bakos HW, Tremellen K. Impact of body mass index on seminal oxidative stress. Andrologia. 2011;43:121–128. [DOI] [PubMed] [Google Scholar]
- 37.Keltz J, Zapantis A, Jindal S, et al. Overweight men: clinical pregnancy after ART is decreased in IVF but not in ICSI cycles. J Assist Reprod Genet. 2010;27:539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kort HI, Massey JB, Elsner CW, et al. Impact of body mass index values on sperm quantity and quality. J Androl. 2006;27:450–452. [DOI] [PubMed] [Google Scholar]
- 39.Rato L, Alves MG, Dias TR, et al. High-energy diets may induce a pre-diabetic state altering testicular glycolytic metabolic profile and male reproductive parameters. Andrology. 2013;1:495–504. [DOI] [PubMed] [Google Scholar]
- 40.Bakos HW, Mitchell M, Setchell BP, et al. The effect of paternal diet-induced obesity on sperm function and fertilization in a mouse model. Int J Androl. 2011;34(5pt1):402–410. [DOI] [PubMed] [Google Scholar]
- 41.Lioret S, McNaughton SA, Crawford D, et al. Parents' dietary patterns are significantly correlated: findings from the Melbourne Infant Feeding Activity and Nutrition Trial Program. Brit J Nutr. 2012;108:518–526. [DOI] [PubMed] [Google Scholar]
- 42.Mora-Esteves C, Shin D. Nutrient supplementation: improving male fertility fourfold. Semin Reprod Med. 2013;31:293–300. [DOI] [PubMed] [Google Scholar]
- 43.Ménézo YJ, Hazout A, Panteix G, et al. Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod BioMed Online. 2007;14:418–421. [DOI] [PubMed] [Google Scholar]
- 44.Mostafa T, Anis T, Imam H, et al. Seminal reactive oxygen species-antioxidant relationship in fertile males with and without varicocele. Andrologia. 2009;41:125–129. [DOI] [PubMed] [Google Scholar]
- 45.Zini A, San Gabriel M, Baazeem A. Antioxidants and sperm DNA damage: a clinical perspective. J Assist Reprod Genet. 2009;26:427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vujkovic M, de Vries JH, Dohle GR, et al. Associations between dietary patterns and semen quality in men undergoing IVF/ICSI treatment. Hum Reprod. 2009;24:1304–1312. [DOI] [PubMed] [Google Scholar]
- 47.Polito M, Conti A, Tiroli M, et al. Diet and male infertility. J Androl Sci. 2011;18:60–63. [Google Scholar]
- 48.Mendiola J, Torres-Cantero AM, Moreno-Grau JM, et al. Food intake and its relationship with semen quality: a case-control study. Fertil Steril. 2009;91:812–818. [DOI] [PubMed] [Google Scholar]
- 49.Comhaire F. The role of food supplementation in the treatment of the infertile couple and for assisted reproduction. Andrologia. 2010;42:331–340. [DOI] [PubMed] [Google Scholar]
- 50.Kefer JC, Agarwal A, Sabanegh E. Role of antioxidants in the treatment of male infertility. Int J Urol. 2009;16:449–457. [DOI] [PubMed] [Google Scholar]
- 51.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100 doi:10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moher D, Tricco AC. Issues related to the conduct of systematic reviews: a focus on the nutrition field. Am J Clin Nutr. 2008;88:1191–1199. [DOI] [PubMed] [Google Scholar]
- 53.Academy of Nutrition and Dietetics. Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process. Chicago, IL: American Dietetic Association; 2012. [Google Scholar]
- 54.Sigman M, Glass S, Campagnone J, et al. Carnitine for the treatment of idiopathic asthenospermia: a randomized, double-blind, placebo-controlled trial. Fertil Steril. 2006;85:1409–1414. [DOI] [PubMed] [Google Scholar]
- 55.Balercia G, Regoli F, Armeni T, et al. Placebo-controlled double-blind randomized trial on the use of L-carnitine, L-acetylcarnitine, or combined L-carnitine and L-acetylcarnitine in men with idiopathic asthenozoospermia. Fertil Steril. 2005;84:662–671. [DOI] [PubMed] [Google Scholar]
- 56.Vicari E, La Vignera S, Calogero AE. Antioxidant treatment with carnitines is effective in infertile patients with prostatovesiculoepididymitis and elevated seminal leukocyte concentrations after treatment with nonsteroidal anti-inflammatory compounds. Fertil Steril. 2002;78:1203–1208. [DOI] [PubMed] [Google Scholar]
- 57.Vicari E, Calogero AE. Effects of treatment with carnitines in infertile patients with prostato-vesiculo-epididymitis. Hum Reprod. 2001;16:2338–2342. [DOI] [PubMed] [Google Scholar]
- 58.Hawkes WC, Alkan Z, Wong K. Selenium supplementation does not affect testicular selenium status or semen quality in North American men. J Androl. 2009;30:525–533. [DOI] [PubMed] [Google Scholar]
- 59.Iwanier K, Zachara BA. Selenium supplementation enhances the element concentration in blood and seminal fluid but does not change the spermatozoal quality characteristics in subfertile men. J Androl. 1995;16:441–447. [PubMed] [Google Scholar]
- 60.Scott R, Macpherson A, Yates RW, et al. The effect of oral selenium supplementation on human sperm motility. Brit J Urol. 1998;82:76–80. [DOI] [PubMed] [Google Scholar]
- 61.Safarinejad MR. The effect of coenzyme Q10 supplementation on partner pregnancy rate in infertile men with idiopathic oligoasthenoteratozoospermia: an open-label prospective study. Int Urol Nephrol. 2011;44:689–700. [DOI] [PubMed] [Google Scholar]
- 62.Safarinejad MR. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J Urol. 2009;182:237–248. [DOI] [PubMed] [Google Scholar]
- 63.Safarinejad MR, Shafiei N, Safarinejad S. A prospective double-blind randomized placebo-controlled study of the effect of saffron (Crocus sativus Linn.) on semen parameters and seminal plasma antioxidant capacity in infertile men with idiopathic oligoasthenoteratozoospermia. Phytotherapy Res. 2011;25:508–516. [DOI] [PubMed] [Google Scholar]
- 64.Chavarro JE, Toth TL, Sadio SM, et al. Soy food and isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Hum Reprod. 2008;23:2584–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rolf C, Cooper TG, Yeung CH, et al. Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high-dose vitamin C and vitamin E: a randomized, placebo-controlled, double-blind study. Hum Reprod. 1999;14:1028–1033. [DOI] [PubMed] [Google Scholar]
- 66.Ghanem H, Shaeer O, El-Segini A. Combination clomiphene citrate and antioxidant therapy for idiopathic male infertility: a randomized controlled trial. Fertil Steril. 2010;93:2232–2235. [DOI] [PubMed] [Google Scholar]
- 67.Safarinejad MR. Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: a double-blind, placebo-controlled, randomised study. Andrologia. 2011;43:38–47. [DOI] [PubMed] [Google Scholar]
- 68.Comhaire FH, Christophe AB, Zalata AA, et al. The effects of combined conventional treatment, oral antioxidants and essential fatty acids on sperm biology in subfertile men. Prostaglandins, Leukot Essent Fatty Acids. 2000;63:159–165. [DOI] [PubMed] [Google Scholar]
- 69.Safarinejad MR, Safarinejad S, Shafiei N, et al. Effects of the reduced form of coenzyme Q10 (ubiquinol) on semen parameters in men with idiopathic infertility: a double-blind, placebo controlled, randomized study. J Urol. 2012;188:526–531. [DOI] [PubMed] [Google Scholar]
- 70.Afeiche MC, Bridges ND, Williams PL, et al. Dairy intake and semen quality among men attending a fertility clinic [published online March 14, 2014]. Fertil Steril. 2014;101:1280.e2–1287.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Afeiche MC, Gaskins AJ, Williams PL, et al. Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a fertility clinic. J Nutr. 2014;144:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Afeiche MC, Williams PL, Gaskins AJ, et al. Meat intake and reproductive parameters among young men. Epidemiology. 2014;25:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Comhaire F, Ahmed Mahmoud A. An update on treatments and interventions for male infertility, and the role of nutriceutical food supplementation. J Pharm Nutr Sci. 2013;3:1–16. [Google Scholar]
- 74.Schoeller EL, Albanna G, Frolova AI, et al. Insulin rescues impaired spermatogenesis via the hypothalamic-pituitary-gonadal axis in Akita diabetic mice and restores male fertility. Diabetes. 2012;61:1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoon MJ, Roser JF. Insulin-like growth factor-I (IGF-I) protects cultured equine Leydig cells from undergoing apoptosis. Anim Reprod Sci. 2010;122:353–358. [DOI] [PubMed] [Google Scholar]
- 76.Holly JMP, Perks CM. Insulin-like growth factor physiology: what we have learned from human studies. Endocrinol Metabol Clin North Am. 2012;41:249–263. [DOI] [PubMed] [Google Scholar]
- 77.Willingham EJ. Environmental review: trenbolone and other cattle growth promoters: need for a new risk-assessment framework. Environ Pract. 2006;8:58–65. [Google Scholar]
- 78.Henricks DM, Gray SL, Owenby JJ, et al. Residues from anabolic preparations after good veterinary practice. APMIS. 2001;109:273–283. [DOI] [PubMed] [Google Scholar]
- 79.Valk E, Hornstra G. Relationship between vitamin E requirement and polyunsaturated fatty acid intake in man: a review. Int J Vitam Nutr Res. 2000;70:31–42. [DOI] [PubMed] [Google Scholar]
- 80.Jeong B-Y, Jeong W-G, Moon S-K, et al. Preferential accumulation of fatty acids in the testis and ovary of cultured and wild sweet smelt Plecoglossus altivelis. Comp Biochem Physiol B Biochem Mol Biol. 2002;131:251–259. [DOI] [PubMed] [Google Scholar]
- 81.Esmaeili V, Shahverdi AH, Moghadasian MH, et al. Dietary fatty acids affect semen quality: a review. Andrology. 2015;3:450–461. [DOI] [PubMed] [Google Scholar]
- 82.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–843. [DOI] [PubMed] [Google Scholar]
- 83.Agarwal A, Sekhon LH. The role of antioxidant therapy in the treatment of male infertility. Hum Fertil. 2010;13:217–225. [DOI] [PubMed] [Google Scholar]
- 84.Fraga CG, Motchnik PA, Shigenaga MK, et al. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci U S A. 1991;88:11003–11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Du Plessis SS, Cabler S, McAlister DA, et al. The effect of obesity on sperm disorders and male infertility. Nat Rev Urol. 2010;7:153–161. [DOI] [PubMed] [Google Scholar]
- 86.Tsai EC, Matsumoto AM, Fujimoto WY, et al. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care. 2004;27:861–868. [DOI] [PubMed] [Google Scholar]
- 87.Roth MY, Amory JK, Page ST. Treatment of male infertility secondary to morbid obesity. Nat Clin Pract End Met. 2008;4:415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hammoud AO, Gibson M, Peterson CM, et al. Obesity and male reproductive potential. J Andrology. 2006;27:619–626. [DOI] [PubMed] [Google Scholar]
- 89.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol. 2010;6:380–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alves M, Rato L, Carvalho R, et al. Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cell Mol Life Sci. 2013;70:777–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ishikawa T, Fujioka H, Ishimura T, et al. Expression of leptin and leptin receptor in the testis of fertile and infertile patients. Andrologia. 2007;39:22–27. [DOI] [PubMed] [Google Scholar]
- 92.Martins AD, Moreira AC, Sá R, et al. Leptin modulates human Sertoli cells acetate production and glycolytic profile: a novel mechanism of obesity-induced male infertility? Biochim Biophys Acta. 2015;1852:1824–1832. [DOI] [PubMed] [Google Scholar]
- 93.Fullston T, Palmer NO, Owens JA, et al. Diet-induced paternal obesity in the absence of diabetes diminishes the reproductive health of two subsequent generations of mice. Hum Reprod. 2012;27:1391–1400. [DOI] [PubMed] [Google Scholar]
- 94.MacDonald AA, Herbison GP, Showell M, et al. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16:293–311. [DOI] [PubMed] [Google Scholar]
- 95.Birkebæk NH, Lange A, Holland-Fischer P, et al. Effect of weight reduction on insulin sensitivity, sex hormone-binding globulin, sex hormones and gonadotrophins in obese children. Eur J Endocrinol. 2010;163:895–900. [DOI] [PubMed] [Google Scholar]
- 96.Vanbillemont G, Lapauw B, De Naeyer H, et al. Sex hormone–binding globulin at the crossroad of body composition, somatotropic axis and insulin/glucose homeostasis in young healthy men. Clin Endocrinol. 2012;76:111–118. [DOI] [PubMed] [Google Scholar]
- 97.Wong CH, Cheng CY. The blood-testis barrier: its biology, regulation, and physiological role in spermatogenesis. Curr Top Dev Biol. 2005;71:263–296. [DOI] [PubMed] [Google Scholar]
- 98.Xia W, Wong EWP, Mruk DD, et al. TGF-β3 and TNFα perturb blood–testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev Biol. 2009;327:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yan HHN, Mruk DD, Lee WM, et al. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]