Abstract
β-Cryptoxanthin, a carotenoid found in fruits and vegetables such as tangerines, red peppers, and pumpkin, has several functions important for human health. Most evidence from observational, in vitro, animal model, and human studies suggests that β-cryptoxanthin has relatively high bioavailability from its common food sources, to the extent that some β-cryptoxanthin–rich foods might be equivalent to β-carotene–rich foods as sources of retinol. β-Cryptoxanthin is an antioxidant in vitro and appears to be associated with decreased risk of some cancers and degenerative diseases. In addition, many in vitro, animal model, and human studies suggest that β-cryptoxanthin–rich foods may have an anabolic effect on bone and, thus, may help delay osteoporosis.
Keywords: antioxidant, β-cryptoxanthin, bone, cancer, vitamin A.
INTRODUCTION
β-Cryptoxanthin is an oxygenated carotenoid with a chemical structure similar to, but more polar than, β-carotene. Although β-carotene is present in large amounts in numerous fruits and vegetables, β-cryptoxanthin is found at high concentrations in only a small number of foods. Table 1 lists the foods with the highest concentrations of β-cryptoxanthin.1–3
Table 1.
Common foods rich in β-cryptoxanthina
| Food | β-Cryptoxanthin (µg/100 g of food) |
|---|---|
| Butternut squash | 3471 |
| Persimmons | 1447 |
| Hubbard squash | 1119 |
| Hot chili peppers | 1103 |
| Tangerines (canned; raw) | 775; 407 |
| Papaya | 589 |
| Sweet red peppers | 490 |
| Rose hips | 483 |
| Sweet pickles | 271 |
| Carrots | 199 |
| Kumquats | 191 |
| Orange juice | 169 |
| Sweet corn | 161 |
| Oranges | 116 |
aData from the USDA/ARS National Nutrient Database For Standard Reference, Release 27 (2014).1
As with other carotenoids, the amount of β-cryptoxanthin in fruits and vegetables seems to depend on cultivar, stage of maturity, growing conditions, storage methods, and season.4–9 Many of the best sources of β-cryptoxanthin are citrus fruits. Not surprisingly, concentrations of β-cryptoxanthin in citrus fruits and in human plasma are highest during the ripening season, in late fall and winter.10,11
Many β-cryptoxanthin–rich foods (such as tangerines and peaches) are eaten raw or juiced, but others (such as pumpkin and butternut squash) are baked or added to mixed dishes. Food processing decreases β-cryptoxanthin concentrations in foods. However, food processing and cooking may increase or decrease the bioaccessibility (i.e., the amount of the nutrient that could be absorbed from the food by a human or other organism) of β-cryptoxanthin in the food. Milder, shorter cooking methods generally improve bioaccessibility because they soften and disrupt cell walls and denature proteins that can bind β-cryptoxanthin.4,5,12–14 However, harsh or prolonged processing, such as refining, drying, or prolonged boiling, can isomerize or destroy carotenoids.4,5,12–14
Despite being found in a limited number of foods that are not staples of the diet, β-cryptoxanthin is a common carotenoid in human blood. In the United States, it is generally the fourth most abundant carotenoid.3,4,15,16
ABSORPTION OF β-CRYPTOXANTHIN
Mechanism
To be absorbed, β-cryptoxanthin must be freed from its food matrix, emulsified into oil droplets, and taken up by cells of the intestinal lining. Few studies have focused on the absorption and metabolism of β-cryptoxanthin. However, like other carotenoids, β-cryptoxanthin seems to be absorbed into the intestine by 2 mechanisms. At low physiological concentrations, it is absorbed mainly by facilitative transport, assisted by enzymes such as scavenger receptor class B type 1 (SR-B1), an epithelia transporter also involved in cholesterol and lipid uptake,17,18 and cluster determinant 36.16 At high pharmacological doses, this active transport mechanism is supplemented by passive diffusion.19–21
Most in vitro, animal model, and human studies suggest that β-cryptoxanthin is better absorbed from its major food sources than are other common carotenoids. For example, a comparison of the apparent bioavailability (the fraction of the nutrient that becomes absorbed and available for use or storage) of retinoid-forming carotenoids (β-cryptoxanthin, α-carotene, and β-carotene) showed that β-cryptoxanthin was move bioavailable in every population studied.22 This is supported by other studies that found β-cryptoxanthin from orange fruits to be more bioavailable than β-carotene–rich foods.23,24
There are several reasons why the absorption of β-cryptoxanthin from dietary sources might be greater than that of most other common carotenoids. SR-B1 preferentially facilitates the absorption of xanthophylls (such as β-cryptoxanthin and lutein) over carotenes (such as α- and β-carotene).25 In addition, the position of a carotenoid incorporated into a mixed micelle depends on its hydrophobicity: the less polar the carotenoid, the more likely it is to be located in the interior of the micelle, where it is less available for absorption. β-Cryptoxanthin is more hydrophilic than other important carotenoids such as lycopene, β-carotene, and α-carotene3,4 and is thus believed to have relatively higher absorbability due to its presence on the outer surface of micelles and its higher solubility in the aqueous environment of the intestine. Indeed, the percentage of β-cryptoxanthin incorporated into micelles during in vitro digestion is 3 times greater than that of β-carotene under similar conditions.26
Carotenoids are crystalline in form and are dissolved within oil droplets in the chromoplasts of yellow and orange fruits such as papaya, tangerines, and sweet potatoes.27,28 Considerable evidence shows they are extracted and digested more easily than carotenoids bound to pigment–protein complexes within the chloroplasts of leafy green vegetables.4,23,24,27,29 The addition of oil or fat increases the bioaccessibility of most carotenoids in foods,3,4,30,31 though there is limited information available on this for β-cryptoxanthin.
Absorbed β-cryptoxanthin can either be converted to retinal in the enterocytes or packaged into chylomicrons and excreted into lymph.20,32 These chylomicrons can then be secreted into the bloodstream, where they can be transported into cells. During fasting states, approximately 75% of plasma β-carotene and lycopene is associated with low-density lipoprotein and about 25% with high-density lipoprotein Oxygenated xanthophylls, such as β-cryptoxanthin, are more evenly distributed between low-density lipoprotein and high-density lipoprotein.20,32,33 Chylomicron remnants are transported to the liver, where some β-cryptoxanthin is converted into retinal and, subsequently, into retinol and retinyl esters.20,33
Bioaccessibility and bioavailability
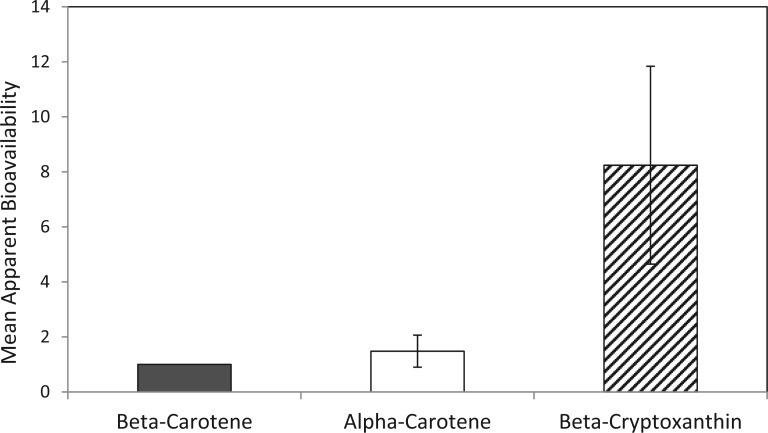
β-Cryptoxanthin appears to be much better absorbed than other carotenoids.22–24 A comparison of apparent bioavailability between different provitamin A carotenoids showed that β-cryptoxanthin–rich foods had 725% greater bioavailability than β-carotene–rich foods (Figure 1).22
Figure 1.
Apparent bioavailability of alpha-carotene and beta-cryptoxanthin from typical diets relative to the bioavailability of beta-carotene from the same diet (±SEM). Data from 9 food frequency questionnaires of 5824 individuals and 4 food record studies of 113 individuals; Burri et al. (2011).22
The absorption of β-carotene from food varies greatly, for reasons that include the food matrix, the presence or absence of fat, and human factors such as health status or genetic differences.34–38 The absorption of β-cryptoxanthin is probably influenced by many of the same factors that influence the absorption and bioavailability of other carotenoids: it appears to depend on the nutritional status, genetics, and digestive health of the subject, the amount and type of carotenoids present, the food matrix, and the presence of fat and other nutrients in the diet.3,4,10,20,32,33 Within foods, many carotenoids are bound to proteins that must be denatured by bile acids.3,4 Very low-fat diets can decrease carotenoid digestion and absorption, but the effect of fat in the diet is inconsistent and appears to be small.38–40 β-Cryptoxanthin may be less affected by these conditions than other carotenoids. In a study comparing carotenoid plasma levels in chronic cholestatic patients with those in age-matched control subjects, β-cryptoxanthin was the only carotenoid not significantly lower in the control group,41 which may suggest that β-cryptoxanthin is more effectively absorbed and transported despite fat malabsorption or compromised liver function.
The bioaccessibility of free and esterified β-cryptoxanthin seems to be identical.42,43 This suggests that humans are capable of efficiently cleaving various β-cryptoxanthin esters in the body, resulting in the existence of only free-form β-cryptoxanthin in the body.
A human study that directly compared the bioavailability of carotenoids from carrot, tomato, and papaya in 16 nonsmoking men and women showed that β-carotene bioavailability from papaya was 3 times greater than carotenoid bioavailability from tomatoes or carrots, and lycopene bioavailability from papaya was 2.6 times greater than that from carrots or tomatoes, suggesting that food matrix impacts bioavailability, with carotenoids in fruit being more bioavailable.44 Furthermore, β-cryptoxanthin from papaya was 2.9 times more bioavailable than β-carotene from papaya, potentially indicating that the β-cryptoxanthin molecule itself was more bioavailable than β-carotene. Thus, observational, in vitro, and human intervention studies suggest that β-cryptoxanthin has greater bioaccessibility and bioavailability than other common carotenoids such as β-carotene, α-carotene, and lycopene.
TISSUE STORAGE AND EXCRETION OF β-CRYPTOXANTHIN
Few studies have investigated the tissue storage and excretion of β-cryptoxanthin, and there is essentially no information on this from human studies,3,4,20 mostly because the metabolism of carotenoids in humans is atypical. Unlike most animals, humans absorb some carotenoids whole from the food matrix, with the rest being hydrolyzed by pancreatic enzymes.3,4,20 Animals that metabolize carotenoids similarly to humans are rare.45,46 The best small animal models of carotenoid metabolism appear to be the Mongolian gerbil and the ferret.
A study in the Mongolian gerbil (Meriones unguiculatus),showed that most β-cryptoxanthin was stored in the liver,47 with adipose tissue and blood being the other important storage sites. High concentrations of carotenoids per gram of tissue are also found in the adrenal gland. These results are similar to findings for β-carotene3,4,20,33,48–51 and other common carotenoids such as lycopene and phytoene.52,53 Another similarity is the primary route of excretion, the feces.3,4,20,33,48–53 The tissue distribution of β-cryptoxanthin was similar to that in male rats, except that the rat may have higher concentrations in the spleen and brain.54 The results suggested that β-cryptoxanthin concentrations in gerbil tissues are saturable, because concentrations did not differ between the groups given the highest dosages of β-cryptoxanthin, except in the pancreas and in the intestines, where they are excreted. In this, too, β-cryptoxanthin appears to be similar to β-carotene.3,4,33,37
The only long-lived metabolites of β-cryptoxanthin in the gerbils were retinoids (retinol and retinyl esters). Other possible metabolites, such as β-apo-10′-carotenal, β-ionone, 3-hydroxy-β-apo-10′-carotenal, 3-hydroxyretinol, and 3,4-didehydroretinol, were not found in any tissue. More studies on the absorption, metabolism, and excretion of β-cryptoxanthin are needed. The available studies suggest that, once absorbed, β-cryptoxanthin is stored in similar tissues as β-carotene and does not seem to form detectable amounts of potentially bioactive metabolites such as apocarotenals, 3-hydroxyretinol, or 3,4-didehydroretinol.
CONCENTRATIONS OF β-CRYPTOXANTHIN IN BLOOD, BREAST MILK, AND TISSUES
β-Cryptoxanthin concentrations in humans are not tightly regulated. Instead, they vary proportionally with dietary intake.3,4,20,33,55–57 No large-scale national or international studies have focused on determining the dietary intake of β-cryptoxanthin. However, intake of β-cryptoxanthin has been measured along with intakes of other common carotenoids in the diet or blood in a variety of populations worldwide. Most dietary surveys report that oranges, orange juice, and tangerines are the major dietary sources of β-cryptoxanthin.1,3,15,20,55–57 β-Cryptoxanthin consumption varies widely by country, with unusually high consumptions in Spain and Japan,10,58,59 where tangerines are common in the diet, and very low consumption in most developing countries, including Bangladesh.60
Plasma β-cryptoxanthin concentrations correlate with total intake of fruits and vegetables, as is typical for carotenoids.3,4,22,61–63 Studies show that correlations between dietary intakes and plasma concentrations of β-cryptoxanthin are similar to those for most carotenoids, in the range of r = 0.2–0.5.61–63
Concentrations of carotenoids in human breast milk depend mainly on diet and lactation stage,64–66 with colostrum (days 4–6 postpartum) containing higher concentrations than more mature milk. Concentrations of β-cryptoxanthin in breast milk vary widely between countries, ranging from 0.012 to 0.080 µmol/L (0.26–2.3 nmol/g lipid).64 Interestingly, concentrations of retinol in breast milk were correlated with β-cryptoxanthin concentrations in Australia, Chile, Japan, and the United Kingdom, but not in the other countries studied (including the United States and Canada). There are few studies of β-cryptoxanthin concentrations in human tissues, but β-cryptoxanthin appears to accumulate in adipose tissue in humans,67 as it does in gerbils and rats.47,54 It has also been detected in human brain.68
β-CRYPTOXANTHIN AS A SOURCE OF RETINOL
Mechanisms of retinol formation
It was established many years ago that β-cryptoxanthin forms retinol (vitamin A).69–72 Despite this, research on the mechanism of retinol formation from carotenoids has focused almost exclusively on β-carotene. In fact, no recent research has focused on the mechanisms by which β-cryptoxanthin forms retinol. However, some studies of β-carotene metabolism include data on β-cryptoxanthin.73–76 These studies suggest that the same enzymes involved in β-carotene cleavage to retinal can also cleave β-cryptoxanthin. Generally, the kinetics of these reactions differ, suggesting that β-carotene is the preferred target of these enzymes. However, because the same enzymes that cleave β-carotene to retinal also cleave β-cryptoxanthin with relatively good efficiencies, it is probable that β-cryptoxanthin forms retinol by the same mechanisms used for β-carotene.
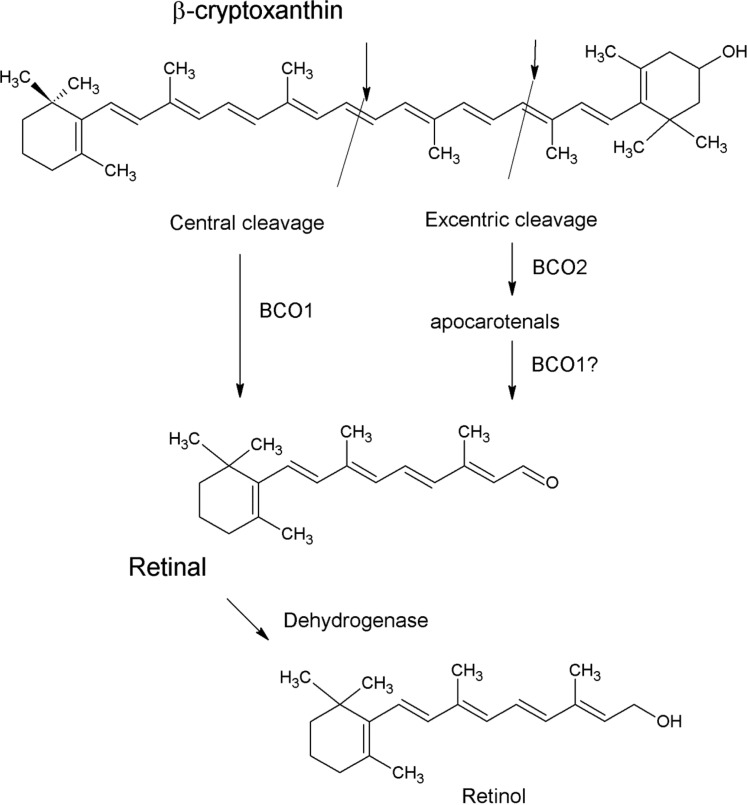
There are a few mechanisms by which β-cryptoxanthin might form retinol. For example, β-cryptoxanthin is known to be centrally cleaved by β-carotene 15,15′ oxygenase 1 (BCO1) into retinal, which is hydrolyzed to retinol. BCO1 catalyzes the cleavage of all retinoid-forming carotenoids, including β-cryptoxanthin, into retinal.25,73,74,76 This reaction occurs primarily in the enterocytes but can also occur in other tissues, such as the liver.33,73,74,76 BCO1 has a slightly alkaline pH optimum and is dependent upon ferrous iron.33,73,74 It is found in mucosal and glandular cells of the stomach, small intestine, colon, liver cells, exocrine pancreas, prostate, endometrium, mammary tissues, kidney tubular cells, skin epithelium, and skeletal muscle. BCO1 appears to be the most important enzyme cleaving β-carotene to retinol. It also cleaves β-cryptoxanthin to form retinal and 3-hydroxyretinal .33,73,74 This potential mechanism is shown in Figure 2.
Figure 2.
Schematic of probable mechanisms of retinol formation from β-cryptoxanthin.
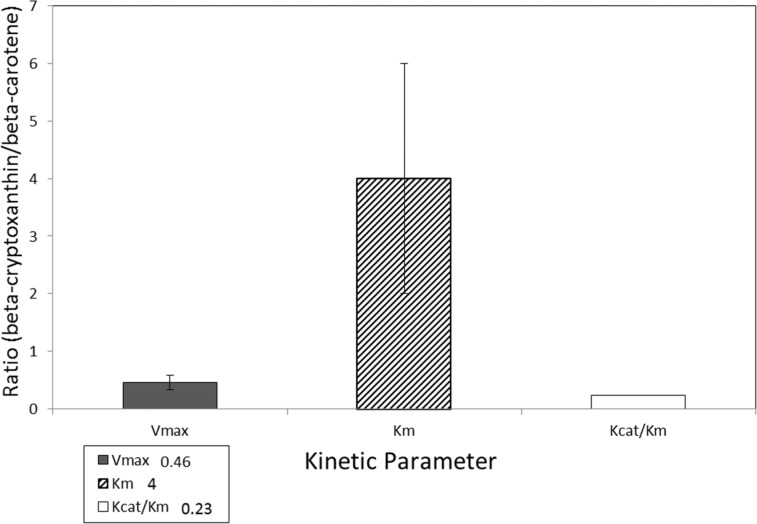
A second mechanism involves the enzyme β-carotene-9′,10′-oxygenase 2 (BCO2). This enzyme cleaves many carotenoids asymmetrically into retinal and β-10′carotenals, which can be metabolized into short-chain carbonyl compounds or retinoic acid.33,74,75,77,78 BCO2 cleaves a wide variety of carotenoids, including β-carotene, β-cryptoxanthin, lutein, and lycopene, eccentrically to an apocarotenal that can be cleaved to retinal.33,74,75,77,78 BCO2 (CMO2) cleaves β-cryptoxanthin in the ferret, but its cleavage activity toward β-cryptoxanthin is lower than that toward zeaxanthin or lutein (Figure 2).75 A third possible mechanism involves BCO2 cleaving β-cryptoxanthin into an apocarotenal, which is then cleaved to retinal by BCO1.33,75,77 So far, all mechanistic studies have shown that β-cryptoxanthin is a somewhat poorer substrate for BCO1 than is β-carotene.25,33,73,75,76 A typical comparison of the reaction kinetics for β-carotene and β-cryptoxanthin is shown in Figure 3, which shows that the kinetics of BCO1 is substantially faster when β-carotene is the substrate.
Figure 3.
Ratio of β-carotene 15,15′ oxygenase 1 (BCO1) kinetic parameters (Vmax, Km, Kcat/Km) for β-cryptoxanthin/β-carotene. Data from an in vitro study by Dela Sena et al. (2013).76
The mechanistic results summarized in this section suggest that, once absorbed, β-cryptoxanthin is a poorer substrate for carotenoid cleavage enzymes than is β-carotene. However, the amount of retinol that can be provided by a provitamin A carotenoid depends on several things, such as the structure of the carotenoid, the food matrix in which it occurs, and the health and physiological status of the person involved.79
In vitro, epidemiological, animal model, and human studies suggest that β-cryptoxanthin is much better absorbed from at least some of its major food sources than is β-carotene. Thus, both the mechanisms by which β-cryptoxanthin is absorbed from food and the mechanisms by which β-cryptoxanthin is cleaved to form retinol are likely to be important.
Factors influencing the formation of retinol from β-cryptoxanthin
Few studies have focused on determining the factors that influence the formation of retinol from β-cryptoxanthin. However, research on β-carotene should provide some insight into these factors. The amount of retinol formed from carotenoids varies greatly from person to person, with 30%–50% of people converting very little β-carotene to retinal.80–82 The reasons why some people (called “low-” or “non-responders”) absorb and convert less carotenoid to retinol are complex and multifactorial, and are related to characteristics of the carotenoid, diet, person, and environment.79 However, recent evidence suggests that an important reason for this variability is genetic polymorphisms, especially of the genes for BCO1 or SR-B1.
SR-B1 has a role in transporting carotenoids in and out of cells.17 Single-nucleotide polymorphisms of genes regulating SR-B1 (such as SCARB1) are associated with altered plasma carotenoid levels.17,18,33 Expression of SCARB1 and BCO1 genes in intestinal cells has been shown to be affected by intestinal transcription factor ISX, which in turn is influenced by retinoic acid through the binding of retinoic acid receptors (RARs). ISX is a retinoic acid–sensitive gatekeeper that controls carotenoids. Expression of ISX increases when retinoic acid is present, which in turn decreases SCARBI/BCO1 expression.33 Single-nucleotide polymorphisms in SCARB1 were related to β-cryptoxanthin plasma concentrations. For example, concentrations were 40% higher in male carriers of allele A in SCARB1 exon 1 than in men with the GG genotype and were 28% lower in women possessing a T allele at SCARB1 exon 8 than in female CC carriers.83
Single-nucleotide polymorphisms appear to be common in the BCO1 gene.33,83–88 Most studies on genetic modifications have focused on β-carotene, but several have examined β-cryptoxanthin as well as other carotenoids. These studies have found that some, but perhaps not all, of the genetic polymorphisms that influence β-carotene metabolism also influence β-cryptoxanthin metabolism.84,86,87 The genetic polymorphisms identified so far do not appear to influence β-cryptoxanthin concentrations as much as they influence β-carotene concentrations.85,87 These results suggest that the amount of retinol formed from β-cryptoxanthin will vary from person to person, in part because of differences in genetics; however, these differences in genetics may not be as important for β-cryptoxanthin as they are for β-carotene.
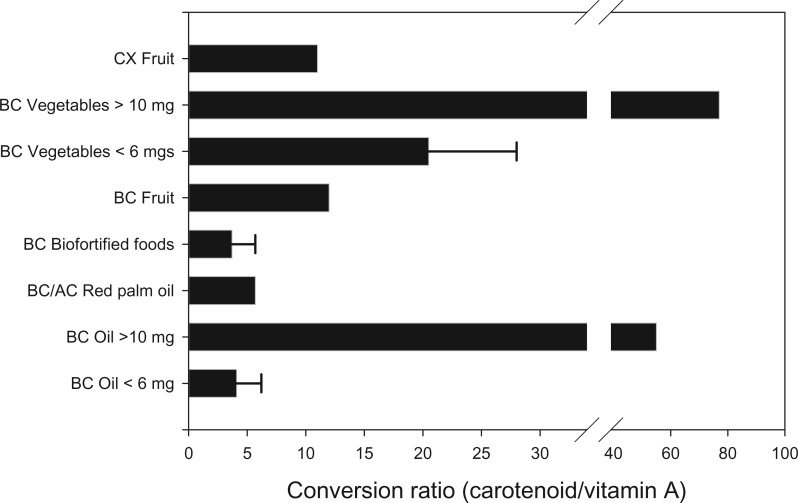
Single-nucleotide polymorphisms also influence the BCO2 gene, though the importance of this gene to β-cryptoxanthin cleavage is not known and is likely to be small.77,78 A second important influence on the amount of retinol formed from carotenoids is the food matrix, which exerts its influence primarily at the carotenoid absorption step.22–24 Recently, scientists have begun to study the effects of the food matrix on the amount of retinol formed from β-carotene–rich foods. These studies are summarized in Figure 4, which shows the conversion ratios determined for β-carotene–rich foods.89,90 The conversion ratio for β-cryptoxanthin was calculated from data from a study of β-cryptoxanthin in humans, described below.60 When available, the range of the conversion estimates is shown, with the thin black bar denoting the range between the low and the high estimates.
Figure 4.
Effects of the food matrix on the conversion ratio of carotenoid to vitamin A. Bars represent the mean conversion ratio of carotenoid from the food indicated. Abbreviations: AC, α-carotene; BC, β-carotene; CX, β-cryptoxanthin. Data from literature reviews by Haskell (2012)89 and Van Loo-Bouman et al. (2014).90
Essentially, β-carotene in oil is a better source of retinol than β-carotene in fruit, and β-carotene from fruit or from biofortified foods tends to be a better source of retinol than β-carotene from most vegetables.91 Furthermore, studies suggest that physiological concentrations (below 6 mg) form more retinol per milligram than do supraphysiological concentrations. Although information on β-cryptoxanthin is very limited, research shows that the absorption of β-cryptoxanthin is likely influenced similarly to that of β-carotene, i.e., by the food matrix and dosage.44,47,92
Animal studies comparing the bioefficacy of carotenoids for maintaining retinol status
Mongolian gerbils (Meriones unguiculatus) are accepted as the best small animal model for human carotenoid digestion.45,46 In gerbils, β-cryptoxanthin–rich maize was found to be as good as or better than β-carotene–rich maize in maintaining retinol status.92–94 Furthermore, gerbils fed 7 maize genotypes (3 with high concentrations of β-carotene and 4 with high concentrations of β-cryptoxanthin) that were equalized for retinol activity (assuming a 1:1 retinol activity equivalency between β-carotene and β-cryptoxanthin) maintained retinol status. The β-cryptoxanthin–rich maize maintained retinol status equally as well as the β-carotene–rich maize.92
In another study, gerbils were fed a low-retinoid, low-carotenoid diet supplemented with freeze-dried fruit (banana, mango, orange, tangerine, papaya) or a vitamin A supplement. Gerbils received a theoretical retinol intake of 32–37 nmol/d from fruit compared with 17 nmol/d from the vitamin A supplement.95 All fruit treatments except banana maintained retinoid concentrations in blood and liver, irrespective of whether the most abundant carotenoid was β-carotene or β-cryptoxanthin. These results suggest that β-cryptoxanthin–rich foods are comparable to β-carotene–rich foods in their ability to maintain vitamin A status in the gerbil.
Human study of β-cryptoxanthin conversion to retinol
Only one study in humans has attempted to compare the conversion of β-carotene and β-cryptoxanthin to retinol.60 In this single-site, parallel, randomized, placebo-controlled study that evaluated the absorption of β-carotene from orange sweet potato and the absorption of β-cryptoxanthin from tangerines, the effects of these foods on retinoids and carotenoids in plasma and breast milk were compared.60 Lactating women with marginal vitamin A status were recruited from Mirpur, Bangladesh, an area with poor socioeconomic status. Participants received a food and either a vitamin A (retinyl acetate) or a placebo corn oil capsule twice a day, 6 days a week, for 3 weeks. The amount of β-carotene supplied by sweet potatoes (12 mg) was more than twice that of the β-cryptoxanthin supplied by tangerines (5.3 mg), while the positive control group consumed 0.5 mg of vitamin A.
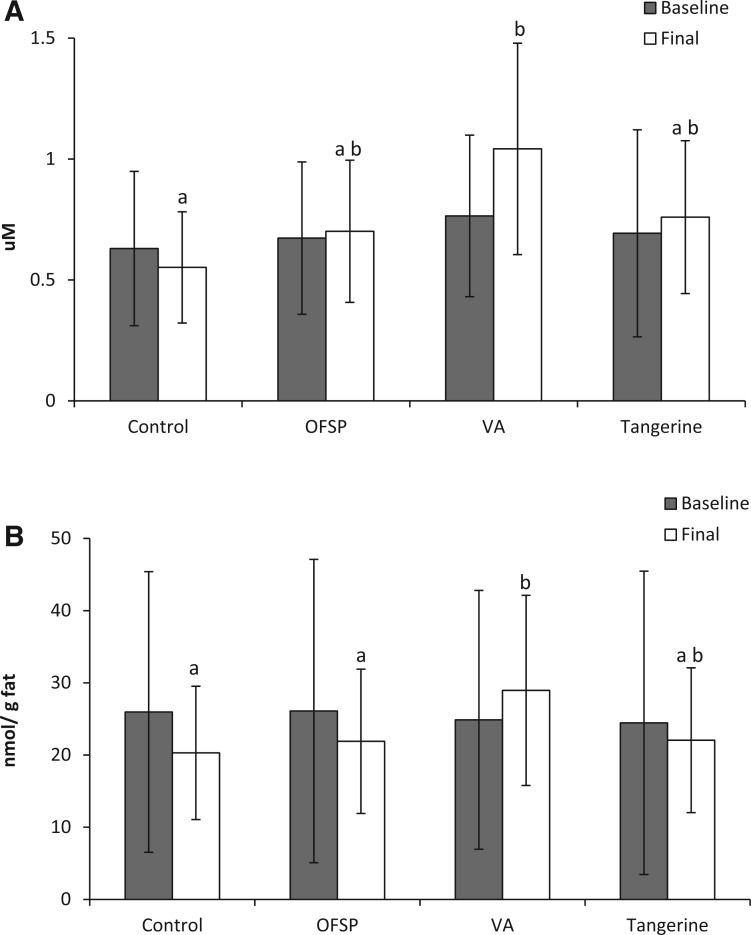
Plasma β-carotene increased 250% in the sweet potato group, while plasma β-cryptoxanthin increased 830% in the tangerine group. Thus, the relative absorption of β-cryptoxanthin was 4 times greater than that of β-carotene. Breast milk retinol increased significantly only in the vitamin A supplement group: 36%, or, when correcting for fat, 16%. When breast milk fat was taken into account, retinol levels in all but the vitamin A supplement group tended to decrease. Changes in breast milk retinoid concentrations (nmol/g fat) in the carotenoid treatment groups were not significantly different from those in the control group. Interestingly, though, the final breast milk retinoid concentrations in the tangerine group were also not significantly different from those in the vitamin A supplement group (P = 0.12). β-Carotene increased significantly in the sweet potato group, while β-cryptoxanthin in the tangerine group showed a large significant increase of 447%. Little is known about how well infants can convert carotenoids to retinol, but large increases in β-cryptoxanthin concentrations in breast milk might be beneficial to the infant.
Although sweet potato interventions providing similar concentrations of β-carotene have resulted in increased retinol status in some populations,89,90 this study found no significant change in status. The sweet potato group showed a nonsignificant increase in breast milk retinol concentrations, as did the tangerine group. The placebo control group had a nonsignificant decrease of 14% from baseline (Figures 5A and B).
Figure 5.
Concentrations of retinol in breast milk before and after tangerine and sweet potato interventions. (A) Concentrations (μM ± SEM). (B) Concentration changes normalized for changes in milk fat concentration. Abbreviations: OFSP, treatment group fed orange-fleshed sweet potatoes; VA, treatment group given vitamin A supplement capsules. Data from a study of 135 lactating Bangladeshi women; Turner et al. (2013).60
Another recent study using sweet potatoes in this same population also failed to demonstrate increased retinol status,96 for unknown reasons. Taken together, these studies might mean that the conversion of carotenoids to retinol is limited in this population. The women in this study seemed to have low but adequate levels of iron (as assessed by concentrations of transferrin receptor, ferritin, and hemoglobin), but they might have had inadequate levels of protein, which might have resulted in poor carotenoid conversion.3,4,33 Additionally, foods in that study location are prepared in unhygienic conditions, and both mothers and infants have high exposure to disease. Unhygienic environments could increase enteropathy and chronic inflammation, which could decrease the effectiveness of carotenoid interventions.3,4,20 It is also possible that genetic polymorphism in this population might be important, since several common polymorphisms of the BCO1 gene are associated with decreased conversion of β-carotene to retinol.84–88 Carotenoid-based interventions to improve retinoid status may be less effective in impoverished populations, where codeficiencies and comorbidities are often present together, especially in ethnically isolated population groups (as was the case in these studies in Bangladesh).60,96
Although neither treatment was effective, β-cryptoxanthin from tangerines and β-carotene from sweet potatoes provided similar amounts of retinol (even though the amount of β-cryptoxanthin consumed was less than half the amount of β-carotene consumed, 5.3 mg/d vs 12 mg/d, and supposedly provided less than one-fourth of the retinyl equivalents). The relative apparent bioavailability of β-cryptoxanthin from tangerines was 10 times greater than that of β-carotene from sweet potatoes, and if breast milk results were corrected for the difference in the amount of carotenoids provided, then tangerines provided 5 times as much retinoid as sweet potatoes.
Clearly, more animal model and human studies are needed to determine the bioefficacy of β-cryptoxanthin–rich foods as retinoid sources. More studies on the absorption, metabolism, and retinyl equivalency of β-cryptoxanthin should be done, preferably with longer supplementation times in larger populations. Research on the metabolism of β-cryptoxanthin may be important scientifically and might also have practical implications in some areas of the world, specifically in Japan, where the consumption of β-cryptoxanthin is especially high and the amount of preformed retinol eaten is relatively low. Determining accurate values of the conversion of β-cryptoxanthin–rich foods might improve the estimates of retinol intake in Japan as well as in other areas of the world where food sources of β-cryptoxanthin are commonly consumed.59
β-CRYPTOXANTHIN AS AN ANTIOXIDANT
β-Cryptoxanthin can accept energy from singlet oxygen (1O2).3,4,97 The evidence that β-cryptoxanthin is an antioxidant in vitro at most physiological concentrations is persuasive.3,4,98–100 However, a recent review suggested that there is a difference between nonvitamin A–forming carotenoids (such as lycopene) and vitamin A–forming carotenoids (such as β-cryptoxanthin).100 Nonvitamin A–forming carotenoids protected against DNA damage in almost all circumstances, but vitamin A–forming carotenoids protected against DNA damage in some cases and enhanced DNA damage in others, especially when they were present at high concentrations.
There have been no in vivo studies of β-cryptoxanthin as an antioxidant. To act as an antioxidant in vivo, a carotenoid would need to be incorporated into the tissues in the correct location and at a suitable concentration relative to the oxidizing agent and the molecule that is to be protected.97 The concentrations of β-cryptoxanthin in most mammalian tissues generally are low compared with those of other dietary antioxidants such as vitamins E and C20,33; therefore, it is unlikely that β-cryptoxanthin is an important antioxidant for most people. However, more studies of both the antioxidant and pro-oxidant activity of β-cryptoxanthin would be useful.
β-CRYPTOXANTHIN AS A CANCER PREVENTIVE
A review of the literature on the relationship between carotenoids and cancer is beyond the scope of this review. Suffice it to say there is very strong epidemiological evidence for an association between low carotenoid intakes or low blood and tissue concentrations of carotenoids and higher risks of several types of cancers.101–105 However, cancer trials in which high doses of pure β-carotene were administered orally to people at risk of lung cancer showed no preventive effect and sometimes were associated with an increased risk of cancer, perhaps because high-dose β-carotene acted as a pro-oxidant in smokers.106,107
Most cancer prevention studies have focused on β-carotene or lycopene.101–107 Only a few human studies have compared β-cryptoxanthin intakes or concentrations with cancer incidence. All were observational studies. Results were mixed108–111 but favored a protective role for β-cryptoxanthin in lung cancer. For example, a recent study linking dietary information from the National Health and Nutrition Examination Survey III (NHANES III) with the NHANES III Linked Mortality File compared 161 subjects who died of lung cancer with controls and found that higher baseline serum β-cryptoxanthin was associated with a lower risk of lung cancer death.111
Recently, several laboratories have focused on identifying the mechanisms by which β-cryptoxanthin could prevent cancer.112–118 β-Cryptoxanthin appears to affect genetic regulation, antioxidant, and inflammatory markers in vitro. One promising area of research suggests that β-cryptoxanthin can increase the mRNA levels of RARβ117,118 and that β-cryptoxanthin transactivates RAR-mediated transcription activity of the retinoic acid response element (RARE). When comparing the ability of several carotenoids as RAR agonists, β-cryptoxanthin outperformed all carotenoids in terms of dose-dependent increases in β-galactosidase activity, a marker of agonist activity against RARα and RARγ.119 β-Cryptoxanthin may also be able to stimulate the differentiation of lung cancer cells and modulate immune response by Th2 cells via an RAR. Subsequent cell culture and animal studies investigated the chemopreventive effects of β-cryptoxanthin supplementation on lung cancer prevention. One cell culture study found that β-cryptoxanthin both inhibited lung cancer cell growth while inducing mRNA concentrations of RARβ in BEAS-2B cells and transactivated RAR-mediated transcription activity of RARE.120 In another study, β-cryptoxanthin supplementation significantly decreased smoke-induced squamous metaplasia and inflammation in the lung and lowered tumor necrosis factor α concentrations in lung tissue cells and macrophages in ferrets.121 Lastly, a study in mice showed that β-cryptoxanthin supplementation reduced nicotine-promoted lung tumor multiplicity and volume, in addition to decreasing emphysema. Supplementation also restored nicotine-suppressed expression of lung Sirtuin 1 (SIRT1), p53, and RARβ and increased survival probablility.122
There is evidence β-cryptoxanthin may inhibit other types of cancer as well. β-Cryptoxanthin-supplemented ICR mice had a decreased incidence of preneoplastic and neoplastic lesions of the urinary bladder.123 Furthermore, in a study in which F344 rats were provided varying doses of β-cryptoxanthin, the high-dose (25 ppm) group showed a decreased incidence of colon cancer.124
Thus, besides evidence that β-cryptoxanthin may be involved in functions associated with cancer prevention, there is also some evidence linking moderate intakes of β-cryptoxanthin with cancer-preventive effects. Therefore, it would be useful to obtain information on the amounts of β-cryptoxanthin in the diet that might be beneficial or harmful as well as information on the relationship between β-cryptoxanthin and smoking, alcohol consumption, or other factors that might contribute to the risk of cancer. Further research to gather this basic information is needed before the importance of β-cryptoxanthin in cancer prevention can be established.
β-CRYPTOXANTHIN AND BONE HEALTH
Bone is a dynamic tissue, constantly forming and reforming. Good nutrition is essential for bone homeostasis, with vitamin D and calcium playing essential roles.125 A series of cell culture and rodent studies suggest that β-cryptoxanthin may also be involved in bone health and homeostasis by promoting osteoclast formation and inhibiting osteoblast actions.126–141 Most of these studies were summarized in 2012.142 Collectively, they suggest that β-cryptoxanthin has an effect on bone health that is not duplicated by other carotenoids.
Moderate (probably physiological) concentrations of β-cryptoxanthin appeared to increase calcium content, protein content, and alkaline phosphatase activity in bone in vitro, changes that are inhibited by inhibitors of RNA polymerase II or protein synthesis, such as cycloheximide. Both calcium and alkaline phosphatase participate in the mineralization of bone. β-Cryptoxanthin also appeared to stimulate gene expression for proteins involved in bone formation and mineralization in osteoblasts, such as insulin-like growth factor 1 and transforming growth factor β1, an effect possibly mediated by protein kinase C or mitogen-activated protein kinase (MAPK).137 Transforming growth factor β1 is involved in the differentiation of pre-osteoblasts to osteoblasts. Furthermore, β-cryptoxanthin stimulated runt-related transcription factor 2 (Runx2, also known as core-binding factor α1 or CBFα1), a key transcription factor associated with osteoblast differentiation.130 Although retinoic acid, the active form of vitamin A, can also mediate bone formation, it did not always duplicate the actions of β-cryptoxanthin on gene expression in these studies.132
In vitro, β-cryptoxanthin inhibited bone resorption induced by parathyroid hormone or prostaglandin E2 by preventing osteoclast cell formation by receptor activator of nuclear factor κB ligand (RANKL).127 β-Cryptoxanthin decreased the number of mature osteoclasts in culture, an action that was inhibited by inhibitors of capase-3, suggesting that β-cryptoxanthin was inducing apoptotic cell death.133 Zinc appeared to have synergistic effects with β-cryptoxanthin, stimulating bone formation and inhibiting bone resorption.129,135,136
Animal studies provided some corroboration of the cell culture results. Rats fed moderately high doses of β-cryptoxanthin (50–100 µg/kg body weight) in combination with zinc sulfate (zinc, 1–5 mg/kg body weight) for 1 week showed increases of alkaline phosphatase activity and calcium concentrations in diaphyseal tissues.135 Additionally, young male or older female rats fed high doses of β-cryptoxanthin (100–500 µg/kg body weight) for 1 week also showed increased alkaline phosphatase and calcium in diaphyseal and metaphyseal tissues.131,138 Furthermore, several laboratories found that feeding moderate doses of β-cryptoxanthin to ovarectimized rats inhibited bone loss134,139,141 and periodontal bone resorption.140
There have been few human studies on the effects of β-cryptoxanthin or β-cryptoxanthin–rich foods on osteoporosis, most of which have been small, and results have been inconclusive. No significant associations were found in a prospective cohort study of carotenoid intakes and risk of hip fracture or other indices of bone health in the Framingham Osteoporosis Study.143,144 In another study conducted in the United States, β-cryptoxanthin intakes were higher in postmenopausal women with osteoporosis than in postmenopausal women without osteoporosis.145 On the other hand, epidemiological studies in Japan showed that high β-cryptoxanthin intake or the intake of Satsuma mandarins was associated with high bone mineral density in postmenopausal women.146–148 Furthermore, comparison of the highest and lowest tertiles of dietary intakes of vitamin C and β-cryptoxanthin in menopausal female subjects from Mikkabi, Japan, showed that these antioxidants were associated with higher bone mineral density (odds ratio of 0.40; 95% confidence interval [CI], 0.17–0.92).149 In a 4-year follow-up of the Mikkabi study, 15 postmenopausal women developed osteoporosis. After adjustments for confounders, the odds ratio for osteoporosis in the highest tertiles of serum β-cryptoxanthin was 0.07 (95%CI, 0.01–0.88). Serum β-cryptoxanthin was also inversely associated with osteopenia/osteoporosis (P = 0.037).150 There are many differences between these studies that could explain the varying results, including study design, study population, and diets. One factor that may be important is that the Japanese have one of the highest intakes of β-cryptoxanthin, coupled with relatively low intakes of preformed vitamin A. β-Cryptoxanthin is not a large part of the American diet, so intakes may have been too low to have had an effect.
EMERGING AREAS OF RESEARCH
Similar to findings for other phytonutrients, new research suggests that β-cryptoxanthin might have other functions that can impact human health. For example, β-cryptoxanthin may influence some aspects of immune function (such as CD4+ lymphocytes and immunoglobulin [Ig] G, IgA, and IgM levels).151 β-Cryptoxanthin might also influence cholesterol homeostasis by inducing mitochondrial sterol 27-hydroxylase (CYP27A1).152 Finally, β-cryptoxanthin has been associated with decreased risk of insulin resistance and liver dysfunction, possibly helping to prevent nonalcoholic fatty liver disease.153 Further research in these areas is necessary to determine whether, when, and how β-cryptoxanthin could impact these functions.
Similarly, further research on the role of β-cryptoxanthin on eye health would be useful. There is considerable evidence suggesting that lutein and zeaxanthin have important roles in eye health.154–157 Lutein and zeaxanthin are xanthophylls with structures similar to that of β-cryptoxanthin, but no recent research has focused on β-cryptoxanthin in the eye, and little is known about β-cryptoxanthin transport in the eye or about potential interactions with lutein and zeaxanthin.
A few studies have assessed β-cryptoxanthin (as well as many other antioxidants) as a risk factor for ocular diseases, with mixed results. A cross-sectional study of the concentrations of antioxidant nutrients (including β-cryptoxanthin) in Indian patients with low antioxidant status showed an inverse correlation of cataract risk with β-cryptoxanthin (and other antioxidant) intake.158 A case–control study that measured β-cryptoxanthin concentrations in glaucoma found no significant correlation between risk and concentration.159 A few studies have measured β-cryptoxanthin (as well as other antioxidant nutrients) in patients with macular degeneration.160–163 An early study showed a trend toward greater risk with lower β-cryptoxanthin concentrations,160 but more recent studies showed no relationship between intake and risk.161–163 It should be noted, however, that β-cryptoxanthin intakes in these populations were generally much lower than intakes of β-carotene, lutein, vitamin E, or most other antioxidants measured. Thus, more research on the metabolism and potential effects of β-cryptoxanthin in the eye would be useful.
CONCLUSION
There is good evidence from in vitro, animal, and human studies that β-cryptoxanthin is well absorbed from its major food sources. Like other carotenoids, β-cryptoxanthin appears to be an antioxidant under most physiological conditions. Recent research indicates it may have anticarcinogenic effects, particularly with regard to lung cancer. There is also mounting evidence that β-cryptoxanthin–rich foods can provide substantial amounts of vitamin A. Due to its high bioavailability from foods, β-cryptoxanthin may have a greater potential to improve vitamin A status than previously assumed. Finally, there is intriguing evidence suggesting that β-cryptoxanthin might have an anabolic effect on bone, thereby offering some protection against osteoporosis.
Thus far, however, there are not enough data on the absorption, metabolism, or function of β-cryptoxanthin to accurately estimate its effects on health at this time. There are little data on (1) the dose-response and reaction kinetics of physiological concentrations of β-cryptoxanthin, (2) the normal variability of these reactions, or (3) the effects of genetic and host factors such as deficiencies of other nutrients or the nutrient interactions that might influence absorption, metabolism, or potential toxicity. There is also almost no information on the effects of the food matrix, cooking and food processing, or food storage on β-cryptoxanthin concentrations or bioavailability in foods. This is a promising area of research, but more work is necessary.
Acknowledgments
Thanks are extended to the following people who worked on the β-cryptoxanthin research performed by the vitamin A metabolism lab and made this review possible: Tami Turner, Xiao Qui, Jade Tso, and Maelle Rajaonary of the Western Human Nutrition Research Center for conducting the studies; Lacey Baldiviez of the UC Davis Department of Nutrition for her statistical support; and Sherri Goss of the Environmental Health and Safety service of the UC Davis campus for her veterinary assistance.
Declaration of interest: The authors have no relevant interests to declare.
REFERENCES
- 1.United States Department of Agriculture, Agricultural Research Service. National Nutrient Database for Standard Reference, Release 27. Beltsville, MD: USDA Nutrient Data Laboratory. http://www.nal.usda.gov/fnic/foodcomp/search. Accessed May 26, 2015.
- 2.SELFNutritionData. Foods highest In beta-cryptoxanthin. http://nutritiondata.self.com/foods-000136000000000000000.html. Updated January 2, 2014. Accessed September 2, 2014.
- 3.Maiani G, Castón MJ, Catasta G, et al. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res. 2009;53(suppl 2):S194–S218. [DOI] [PubMed] [Google Scholar]
- 4.Namitha KK, Negi PS. Chemistry and biotechnology of carotenoids. Crit Rev Food Sci Nutr. 2010;50:728–760. [DOI] [PubMed] [Google Scholar]
- 5.Boon CS, McClements DJ, Weiss J, et al. Factors influencing the chemical stability of carotenoids in foods. Crit Rev Food Sci Nutr. 2010;50:515–532. [DOI] [PubMed] [Google Scholar]
- 6.Mercadante AZ. Carotenoids in foods: sources and stability during processing and storage. In: Socaciu C, ed. Food Colorants: Chemical and Functional Properties. Boca Raton, FL: CRC Press; 2008:213–240. [Google Scholar]
- 7.de Faria AF, Hasegawa PN, Chagas EA, et al. Cultivar influence on carotenoid composition of loquats from Brazil. J Food Compos Anal. 2009;22:196–203. [Google Scholar]
- 8.Dhuique-Mayer C, Caris-Veyrat C, Ollitrault P, et al. Varietal and interspecific influence on micronutrient contents in citrus from the Mediterranean area. J Agri Food Chem. 2005;53:2140–2145. [DOI] [PubMed] [Google Scholar]
- 9.Kimura M, Rodriguez-Amaya DB, Yokoyama SM. Cultivar differences and geographic effects on the carotenoid composition and vitamin-A value of papaya. Lebens Wiss Technol. 1991;24:415–418. [Google Scholar]
- 10.Xiang J, Nagaya T, Huang XE, et al. Sex and seasonal variations of plasma retinol, α-tocopherol, and carotenoid concentrations in Japanese dietitians. Asian Pac J Cancer Prev. 2008;9:413–416. [PubMed] [Google Scholar]
- 11.Granado-Lorencio F, Olmedilla-Alonso B, Blanco-Navarro I, et al. Seasonal variation of serum α- and β-cryptoxanthin and 25-OH-vitamin D3 in women with osteoporosis. Osteoporos Int. 2008;19:717–720. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Amaya D, Kimura M. HarvestPlus Handbook For Carotenoid Analysis. Washington, DC: HarvestPlus; 2004. Technical Monograph Series 2. [Google Scholar]
- 13.Rodriguez-Amaya DB. Food carotenoids; analysis, composition and alterations during storage and processing of foods. Forum Nutr. 2003;56:35–37. [PubMed] [Google Scholar]
- 14.Pugliese A, O'Callaghan Y, Tundis R, et al. In vitro investigation of the bioaccessibility of carotenoids from raw, frozen and boiled red chili peppers (Capsicum annuum). Eur J Nutr. 2014;53:501–510. [DOI] [PubMed] [Google Scholar]
- 15.Ford ES, Gillespie C, Ballew C, et al. Serum carotenoid concentrations in US children and adolescents. Am J Clin Nutr. 2002;76:818–827. [DOI] [PubMed] [Google Scholar]
- 16.Olmedilla B, Granado F, Southon S, et al. Serum concentrations of carotenoids and vitamins A, E, and C in control subjects from five European countries. Br J Nutr. 2001;85:227–238. [DOI] [PubMed] [Google Scholar]
- 17.During A, Doraiswamy S, Harrison EH. Xanthophylls are preferentially taken up compared with β-carotene by retinal cells via a SRBI-dependent mechanism. J Lipid Res. 2008;49:1715–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borel P, Lietz G, Goncalves A, et al. CD36 and SR-B1 are involved in cellular uptake of provitamin A carotenoids by Caco-2 and HEK cells, and some of their genetic variants are associated with plasma concentrations of these micronutrients in humans. J Nutr. 2013;143:448–456. [DOI] [PubMed] [Google Scholar]
- 19.Hollander D, Ruble PE., Jr Beta-carotene intestinal absorption: bile, fatty acid, pH, and flow rate effects on transport. Am J Physiol. 1978;235:E686–E691. [DOI] [PubMed] [Google Scholar]
- 20.United States Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 21.Burri BJ, Clifford AJ. Carotenoid and retinoid metabolism: insights from isotope studies. Arch Biochem Biophys. 2004;430:110–119. [DOI] [PubMed] [Google Scholar]
- 22.Burri BJ, Chang JST, Neidlinger TR. β-Cryptoxanthin- and α-carotene-rich foods have greater apparent bioavailability than β-carotene-rich foods in Western diets. Br J Nutr. 2011;105:212–219. [DOI] [PubMed] [Google Scholar]
- 23.de Pee S, West C, Permaesih D, et al. Orange fruit is more effective than are dark-green, leafy vegetables in increasing serum concentrations of retinol and β-carotene in schoolchildren in Indonesia. Am J Clin Nutr. 1998;68:1058–1067. [DOI] [PubMed] [Google Scholar]
- 24.O’Connell OF, Ryan L, O’Brien NM. Xanthophyll carotenoids are more bioaccessible from fruits than dark green vegetables. Nutr Res. 2007;27:258–264. [Google Scholar]
- 25.Kim YS, Park CS, Oh DK. Hydrophobicity of residue 108 specifically affects the affinity of human β-carotene 15,15’-monooxygenase for substrates with two ionone rings. Biotechnol Lett. 2010;32:847–853. [DOI] [PubMed] [Google Scholar]
- 26.Dhuique-Mayer C, Amiot-Carlin M. β-Cryptoxanthin from citrus juices: bioaccessibility and uptake by Caco-2 cell culture model. Acta Horticult. 2009;841:129–134. [Google Scholar]
- 27.Schweiggert RM, Mezger D, Schimpf F, et al. Influence of chromoplast morphology on carotenoid bioaccessibility of carrot, mango, papaya, and tomato. Food Chem. 2012;135:2736–2742. [DOI] [PubMed] [Google Scholar]
- 28.Dhuique-Mayer C, Borel P, Reboul E, et al. β-Cryptoxanthin from citrus juices: assessment of bioaccessibility using an in vitro digestion/Caco-2 cell culture model. Br J Nutr. 2007;97:883–890. [DOI] [PubMed] [Google Scholar]
- 29.Jeffery J, Holzenburg A, King S. Physical barriers to carotenoid bioaccessibility. Ultrastructure survey of chromoplast and cell wall morphology in nine carotenoid-containing fruits and vegetables. J Sci Food Agric. 2012;92:2594–2602. [DOI] [PubMed] [Google Scholar]
- 30.Huang C, Tang Y, Chen C, et al. The bioaccessibility of β-carotene in stir- or deep-fried vegetables in men determined by measuring the serum response to a single ingestion. J Nutr. 2000;130:534–540. [DOI] [PubMed] [Google Scholar]
- 31.Mills JP, Tumuhimbise GA, Jamil KM, et al. Sweet potato β-carotene bioefficacy is enhanced by dietary fat and not reduced by soluble fiber intake in Mongolian gerbils. J Nutr. 2009;139:44–50. [DOI] [PubMed] [Google Scholar]
- 32.Tyssandier V, Lyan B, Borel P. Main factors governing the transfer of carotenoids from emulsion lipid droplets to micelles. Bioch Biophys Acta. 2001;1533:285–292. [DOI] [PubMed] [Google Scholar]
- 33.von Lintig J. Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Ann Rev Nutr. 2010;30:35–56. [DOI] [PubMed] [Google Scholar]
- 34.Thakkar SK, Maziya-Dixon B, Dixon AGO, et al. β-Carotene micellarization during in vitro digestion and uptake by Caco-2 cells is directly proportional to β-carotene content in different genotypes of cassava. J Nutr. 2007;137:2229–2233. [DOI] [PubMed] [Google Scholar]
- 35.Roman MJ, Burri BJ, Singh RP. Release and bioaccessibility of β-carotene from fortified almond butter during in vitro digestion. J Agric Food Chem. 2012;60:9659–9666. [DOI] [PubMed] [Google Scholar]
- 36.Bengtsson A, Brackmann C, Enejder A, et al. Effects of thermal processing on the in vitro bioaccessibility and microstructure of β-carotene in orange-fleshed sweet potato. J Agric Food Chem. 2010;58:11090–11096. [DOI] [PubMed] [Google Scholar]
- 37.During A, Hussain MM, Morel DW, et al. Carotenoid uptake and secretion by CaCo-2 cells: β-carotene isomer selectivity and carotenoid interactions. J Lipid Res. 2002;43:1086–1095. [DOI] [PubMed] [Google Scholar]
- 38.Brown MJ, Ferruzzi MG, Nguyen ML, et al. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am J Clin Nutr. 2004;80:396–403. [DOI] [PubMed] [Google Scholar]
- 39.Ribaya-Mercado JD, Maramag CC, Tengco LW, et al. Carotene-rich plant foods ingested with minimal dietary fat enhance the total-body vitamin A pool size in Filipino schoolchildren as assessed by stable-isotope-dilution methodology. Am J Clin Nutr. 2007;85:1041–1049. [DOI] [PubMed] [Google Scholar]
- 40.Maramag CC, Ribaya-Mercado JD, Rayco-Solon P, et al. Influence of carotene-rich vegetable meals on the prevalence of anemia and iron deficiency in Filipino schoolchildren. Eur J Clim Nutr. 2010;64:468–474. [DOI] [PubMed] [Google Scholar]
- 41.Floreani A, Baragiotta A, Martines D, et al. Plasma antioxidant levels in chronic choleosatic liver diseases. Aliment Pharmacol Ther. 2000;14:353–358. [DOI] [PubMed] [Google Scholar]
- 42.Breithaupt DE, Weller P, Wolters M, et al. Plasma response to a single dose of dietary β-cryptoxanthin esters from papaya (Carica papaya L.) or non-esterified β-cryptoxanthin in adult human subjects: a comparative study. Br J Nutr. 2003;90:795–801. [DOI] [PubMed] [Google Scholar]
- 43.Wingerath T, Stahl W, Sies H. β-Cryptoxanthin selectively increases in human chylomicrons upon ingestion of tangerine concentrate rich in β-cryptoxanthin esters. Arch Biochem Biophys. 1995;324:385–390. [DOI] [PubMed] [Google Scholar]
- 44.Schweiggert RM, Kopen RE, Villalobos-Gutierrez MG, et al. Carotenoids are more bioavailable from papaya than from tomato and carrot in humans: a randomized cross-over study. Br J Nutr. 2014;111:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanumihardjo SA. Mammalian models for understanding the mechanisms of retinol and retinoid actions. In: WHO Technical Consultation on Vitamin A in Newborn Health: Mechanistic Studies. Geneva, Switzerland: World Health Organization; 2012:93–108. [Google Scholar]
- 46.Lee CM, Boileau AC, Boileau TW, et al. Review of animal models in carotenoid research. J Nutr. 1999;129:2271–2277. [DOI] [PubMed] [Google Scholar]
- 47.La Frano MR, Zhu C, Burri BJ. Assessment of tissue distribution and concentration of β-cryptoxanthin in response to varying amounts of dietary β-cryptoxanthin in the Mongolian gerbil. Br J Nutr. 2014;111:968–978. [DOI] [PubMed] [Google Scholar]
- 48.Bendich A, Olson JA. Biological actions of carotenoids. FASEB J. 1989;119:665–668. [PubMed] [Google Scholar]
- 49.Olson JA. Serum levels of vitamin A and carotenoids as reflectors of nutritional status. J Natl Cancer Inst. 1984;73:1439–1444. [PubMed] [Google Scholar]
- 50.Ribaya-Mercado JD, Holmgren SC, Fox JG, et al. Dietary β-carotene absorption and metabolism in ferrets and rats. J Nutr. 1989;119:665–668. [DOI] [PubMed] [Google Scholar]
- 51.Gugger ET, Bierer TL, Henze TM, et al. β-Carotene uptake and tissue distribution in ferrets (Mustela putorius furo). J Nutr. 1992;122:115–119. [DOI] [PubMed] [Google Scholar]
- 52.Conlon LE, King RD, Moran NE, et al. Coconut oil enhances tomato carotenoid tissue accumulation compared to safflower oil in the Mongolian gerbil (Meriones unguiculatus). J Am Food Chem. 2012;60:8366–8394. [DOI] [PubMed] [Google Scholar]
- 53.Moran NE, Clinton SK, Erdman JW., Jr Differential bioavailability, clearance, and tissue distribution of the acyclic tomato carotenoids, lycopene and phytoene, in Mongolian gerbils. J Nutr. 2013;143:1920–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sugiera M, Ogawa K, Yano M. Absorption, storage and distribution of β-cryptoxanthin in rat after chronic administration of Satsuma mandarin (Citrus unshiu Marc.). Biol Pharm Bull. 2013;36:147–151. [DOI] [PubMed] [Google Scholar]
- 55.Murphy MM, Barraj LM, Herman D, et al. Phytonutrient intake by adults in the United States in relation to fruit and vegetable consumption. J Acad Nutr Diet. 2012;112:222–229. [DOI] [PubMed] [Google Scholar]
- 56.Talegawkar SA, Johnson EJ, Carithers TC, et al. Carotenoid intakes, assessed by food-frequency questionnaires (FFQs), are associated with serum carotenoid concentrations in the Jackson Heart Study: validation of the Jackson Heart Study Delta NIRI Adult FFQs. Public Health Nutr. 2008;11:989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.World Health Organization. Food and Agriculture Organization of the United Nations. Vitamin and Mineral Requirements in Human Nutrition. 2nd ed Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 58.Granado F, Olmedilla B, Blanco I, et al. Major fruit and vegetable contributors to the main serum carotenoids in the Spanish diet. Eur J Clin Nutr. 1996;50:246–250. [PubMed] [Google Scholar]
- 59.Matsuda-Inoguchi N, Date C, Sakurai K, et al. Reduction in estimated vitamin A intake induced by new food composition tables in Japan, where vitamin A is taken mostly from plant foods. Internat J Food Sci Nutr. 2006;57:279–291. [DOI] [PubMed] [Google Scholar]
- 60.Turner T, Burri BJ, Jamil KM, et al. The effects of daily consumption of β-cryptoxanthin–rich tangerines and β-carotene–rich sweet potatoes on retinoid and carotenoid concentrations in plasma and breast milk of Bangladeshi women with low retinoid status in a randomized controlled trial. Am J Clin Nutr. 2013;98:1200–1208. [DOI] [PubMed] [Google Scholar]
- 61.Hodge AM, Simpson JA, Fridman M, et al. Evaluation of an FFQ for assessment of antioxidant intake using plasma biomarkers in an ethnically diverse population. Public Health Nutr. 2009;12:2438–2447. [DOI] [PubMed] [Google Scholar]
- 62.Al-Delaimy WK, Slimani N, Ferrari P, et al. Plasma carotenoids as biomarkers of intake of fruits and vegetables: ecological-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Clin Nutr. 2005;59:1397–1408. [DOI] [PubMed] [Google Scholar]
- 63.McNaughton SA, Marks GC, Gaffney P, et al. Validation of a food-frequency questionnaire assessment of carotenoid and vitamin E intake using weighed food records and plasma biomarkers: the method of triads model. Eur J Clin Nutr. 2005;59:211–218. [DOI] [PubMed] [Google Scholar]
- 64.Patton S, Canfield L, Huston G, et al. Carotenoids of human colostrum. Lipids. 1990;25:159–165. [DOI] [PubMed] [Google Scholar]
- 65.Canfield LM, Clandinin MT, Davies DP, et al. Multinational study of major breast milk carotenoids of healthy mothers. Eur J Nutr. 2003;42:133–141. [DOI] [PubMed] [Google Scholar]
- 66.Schweigert FJ, Bathe K, Chen F, et al. Effect of the stage of lactation in humans on carotenoid levels in milk, blood plasma and plasma lipoprotein fractions. Eur J Nutr. 2004;43:39–44. [DOI] [PubMed] [Google Scholar]
- 67.Chung H-Y, Ferreira ALA, Epstein S, et al. Site-specific concentrations of carotenoids in adipose tissue: relations with dietary and serum carotenoid concentrations in healthy adults. Am J Clin Nutr. 2009;90:533–539. [DOI] [PubMed] [Google Scholar]
- 68.Craft NE, Haitema TB, Garnett KM, et al. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J Nutr Health Aging. 2004;8:156–162. [PubMed] [Google Scholar]
- 69.Deuel HJ, Jr, Meserve ER, Johnston CH, et al. Reinvestigation of the relative provitamin A potencies of cryptoxanthin and β-carotene. Arch Biochem. 1945;7:447–450. [Google Scholar]
- 70.Johnson RM, Swick RW, Baumann CA. The vitamin A activity of certain carotenoids in the chick. Arch Biochem. 1949;22:122–131. [PubMed] [Google Scholar]
- 71.Greenberg SM, Chatterjee A, Calbert CE, et al. A comparison of the provitamin A activity of β-cryptoxanthin in the chick. Arch Biochem. 1950;25:61–65. [PubMed] [Google Scholar]
- 72.John J, Kishore GS, Subbarayan C, et al. Metabolism and biological potency of cryptoxanthin in rat. India J Biochem. 1970;7:222–225. [Google Scholar]
- 73.Lindquist A, Andersson S. Biochemical properties of purified recombinant human β-carotene 15,15’ monooxygenase. J Biol Chem. 2002;277:23942–23948. [DOI] [PubMed] [Google Scholar]
- 74.Amengual J, Wadjaja-Adhi MAK, Rodriguez-Santiago S, et al. Two carotenoid oxygenases contribute to mammalian provitamin A metabolism. J Biol Chem. 2013;288:34081–34095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mein JR, Dolnikowski GG, Ernst H, et al. Enzymatic formation of apo-carotenoids from the xanthophyll carotenoids lutein, zeaxanthin and β-cryptoxanthin by ferret carotene-9',10'-monooxygenase. Arch Biochem Biophys. 2011;506:109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dela Sena C, Narayanasamy S, Riedl KM, et al. Substance specificity of purified recombinant human β-carotene 15, 15'-oxygenase (BCO1). J Biol Chem. 2013;288:37094–37103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lietz G, Oxley A, Boesch-Saadatmandi C, et al. Importance of β,β-carotene 15,15'-monooxygenase 1 (BCMO1) and β,β-carotene 9',10'-dioxygenase 2 (BCDO2) in nutrition and health. Mol Nutr Food Res. 2012;56:241–250. [DOI] [PubMed] [Google Scholar]
- 78.Tian R, Pitchford WS, Morris CA, et al. Genetic variation in the β, β-carotene-9', 10'-dioxygenase gene and association with fat colour in bovine adipose tissue and milk. Anim Genet. 2010;41:253–259. [DOI] [PubMed] [Google Scholar]
- 79.West CE, Castenmiller JJ. Quantification of the “SLAMENGHI” factors for carotenoid bioavailability and bioconversion. Int J Vitam Nutr Res. 1998;68:371–377. [PubMed] [Google Scholar]
- 80.Lin Y, Dueker SR, Burri BJ, et al. Variability of the conversion of β-carotene to vitamin A in women measured by using a double-tracer study design. Am J Clin Nutr. 2000;71:1545–1554. [DOI] [PubMed] [Google Scholar]
- 81.Hickenbottom SJ, Follett JR, Lin Y, et al. Variability in conversion of β-carotene to vitamin A in men as measured by using a double-tracer study design. Am J Clin Nutr. 2002;75:900–907. [DOI] [PubMed] [Google Scholar]
- 82.Ho CC, de Moura FF, Kim SH, et al. A minute dose of 14C-β-carotene is absorbed and converted to retinoids in humans. J Nutr. 2009;139:1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Borel P, Moussa M, Reboul E, et al. Human plasma levels of vitamin E and carotenoids are associated with genetic polymorphisms in genes involved in lipid metabolism. J Nutr. 2007;137:2653–2659. [DOI] [PubMed] [Google Scholar]
- 84.Ferrucci L, Perry JR, Matteini A, et al. Common variation in the β-carotene 15,15'-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet. 2009;84:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hendrickson SJ, Hazra A, Chen C, et al. β-Carotene 15,15'-monooxygenase 1 single nucleotide polymorphisms in relation to plasma carotenoid and retinol concentrations in women of European descent. Am J Clin Nutr. 2012;96:1379–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lietz G, Oxley A, Leung W, et al. Single nucleotide polymorphisms upstream from the β-carotene 15,15'-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J Nutr. 2012;142:161S–165S. [DOI] [PubMed] [Google Scholar]
- 87.Wang TT, Edwards AJ, Clevidence BA. Strong and weak plasma response to dietary carotenoids identified by cluster analysis and linked to β-carotene 15,15'-monooxygenase 1 single nucleotide polymorphisms. J Nutr Biochem. 2013;24:1538–1546. [DOI] [PubMed] [Google Scholar]
- 88.Hendrickson SJ, Lindstroem S, Eliassen AH, et al. Plasma carotenoid- and retinol-weighted multi-SNP scores and risk of breast cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. Cancer Epid Biomark Prev. 2013;22:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haskell MJ. The challenge to reach nutritional adequacy for vitamin A: β-carotene bioavailability and conversion – evidence in humans. Am J Clin Nutr. 2012;96:1193S–1203S. [DOI] [PubMed] [Google Scholar]
- 90.Van Loo-Bouwman CA, Naber THJ, Schaafsma G. A review of vitamin A equivalency of β-carotene in various food matrices for human consumption. Br J Nutr. 2014;111:2153–2166. [DOI] [PubMed] [Google Scholar]
- 91.La Frano MR, de Moura FF, Boy E, et al. Bioavailability of iron, zinc, and provitamin A carotenoids in biofortified staple crops. Nutr Rev. 2014;72:289–307. [DOI] [PubMed] [Google Scholar]
- 92.Schmaelzle S, Gannon B, Crawford S, et al. Maize genotype and food matrix affect the provitamin A carotenoid bioefficacy from staple and carrot-fortified feeds in Mongolian gerbils (Meriones unguiculatus). J Agric Food Chem. 2014;62:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davis C, Jing H, Howe JA, et al. β-Cryptoxanthin from supplements or carotenoid-enhanced maize maintains liver vitamin A in Mongolian gerbils (Meriones unguiculatus) better than or equal to β-carotene supplements. Br J Nutr. 2008;100:786–793. [DOI] [PubMed] [Google Scholar]
- 94.Davis CR, Howe JA, Rocheford TR, et al. The xanthophylls composition of biofortified maize (Zea mays Sp.) does not influence the bioefficacy of provitamin A carotenoids in Mongolian gerbils (Meriones unguiculatus). J Agric Food Chem. 2008;65:6745–6750. [DOI] [PubMed] [Google Scholar]
- 95.Arscott SA, Howe JA, Davis CR, et al. Carotenoid profiles in pro-vitamin A-containing fruits and vegetables affect the bioefficacy in Mongolian gerbils. Exp Biol Med (Maywood). 2010;235:839–848. [DOI] [PubMed] [Google Scholar]
- 96.Jamil KM, Brown KH, Jamil M, et al. Daily consumption of orange-fleshed sweet potato for 60 days increased plasma β-carotene concentrations but did not increase total body vitamin A pool size in Bangladeshi women. J Nutr. 2012;142:1896–1902. [DOI] [PubMed] [Google Scholar]
- 97.Britton G. Structure and properties of carotenoids in relation to function. FASEB J. 1995;9:1551–1558. [PubMed] [Google Scholar]
- 98.Stahl W, Nicolai S, Briviba K, et al. Biological activities of natural and synthetic carotenoids: induction of gap junctional communication and singlet oxygen quenching. Carcinogen. 1997;18:89–92. [DOI] [PubMed] [Google Scholar]
- 99.Chisté RC, Freitas M, Mercadante AZ, et al. Carotenoids inhibit lipid peroxidation and hemoglobin oxidation, but not the depletion of glutathione induced by ROS in human erythrocytes. Life Sci. 2014;99:52–60. [DOI] [PubMed] [Google Scholar]
- 100.Azqueta A, Collins AR. Carotenoids and DNA damage. Mutat Res. 2012;733:4–13. [DOI] [PubMed] [Google Scholar]
- 101.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 102.Gallicchio L, Boyd K, Matanoski G, et al. Carotenoids and the risk of developing lung cancer: a systematic review. Am J Clin Nutr. 2008;88:372–383. [DOI] [PubMed] [Google Scholar]
- 103.Nishino H, Murakosh M, Ii T, et al. Carotenoids in cancer chemoprevention. Cancer Metastasis Rev. 2009;21:257–264. [DOI] [PubMed] [Google Scholar]
- 104.Chatterjee M, Roy K, Janarthan M, et al. Biological activity of carotenoids: its implications in cancer risk and prevention. Curr Pharm Biotechnol. 2012;13:180–190. [DOI] [PubMed] [Google Scholar]
- 105.Wang L, Li B, Pan MX, et al. Specific carotenoid intake is inversely associated with the risk of breast cancer among Chinese women. Brit J Nutr. 2014;111:1686–1695. [DOI] [PubMed] [Google Scholar]
- 106.Holick CN, Michaud DS, Stolzenberg-Solomon R, et al. Dietary carotenoids, serum β-carotene, and retinol and risk of lung cancer in the Alpha-Tocopherol, Beta-Carotene Cohort Study. Am J Epidemiol. 2002;156:536–547. [DOI] [PubMed] [Google Scholar]
- 107.Albanes D, Heinonen OP, Taylor PR, et al. α-Tocopherol and β-carotene supplements and lung cancer incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88:1560–1570. [DOI] [PubMed] [Google Scholar]
- 108.Männistö S, Smith-Warner SA, Spiegelman D, et al. Dietary carotenoids and risk of lung cancer in a pooled analysis of seven cohort studies. Cancer Epidemiol Biomark Prev. 2004;13:40–48. [DOI] [PubMed] [Google Scholar]
- 109.Nishino H, Murakoshi M, Tokuda H, et al. Cancer prevention by carotenoids. Arch Biochem Biophys. 2009;483:165–168. [DOI] [PubMed] [Google Scholar]
- 110.Tanaka T, Shnimizu M, Moriwaki H. Cancer chemoprevention by carotenoids. Molecules. 2012;17:3202–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Min K, Min J. Serum carotenoid levels and risk of lung cancer death in US adults. Cancer Sci. 2014;105:736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu C, Han L, Riaz H, et al. The chemopreventive effect of β-cryptoxanthin from mandarin on human stomach cells (BGC-823). Food Chem. 2013;136:1122–1129. [DOI] [PubMed] [Google Scholar]
- 113.Kohno H, Taima M, Sumida T, et al. Inhibitory effect of mandarin juice rich in β-cryptoxanthin and hesperidin on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced pulmonary tumorigenesis in mice. Cancer Lett. 2001;174:141–150. [DOI] [PubMed] [Google Scholar]
- 114.Manabe Y, Hirata T, Sugawara T. Suppressive effects of carotenoids on the antigen-induced degranulation in RBL-2H3 rat basophilic leukemia cells. J Oleo Sci. 2014;63:291–294. [DOI] [PubMed] [Google Scholar]
- 115.Fu H, Wu C, Riaz H, et al. β-Cryptoxanthin uptake in THP-1 macrophages upregulates the CYP27A1 signaling pathway. Mol Nutr Food Res. 2014;58:425–436. [DOI] [PubMed] [Google Scholar]
- 116.San Millán C, Soldevilla B, Martín P, et al. β-Cryptoxanthin synergistically enhances the antitumoral activity of oxaliplatin through ΔNP73 negative regulation in colon cancer. Clin Cancer Res. 2015;21:4398–4409. [DOI] [PubMed] [Google Scholar]
- 117.Wishart DS, Jewison T, Guo AC, et al. β-Cryptoxanthin (HMDB33844). Human Metabolome Database 3.6. Edmonton, Alberta, Canada: University of Alberta and the Metabolomics Innovation Centre. http://www.hmdb.ca/metabolites/HMDB33844. Updated January 1, 2015. Accessed December 6, 2015. [Google Scholar]
- 118.Katsuura S, Imamura T, Bando N, et al. β-Carotene and β-cryptoxanthin but not lutein evoke redox and immune changes in RAW264 murine macrophages. Mol Nutr Food Res. 2009;53:1396–1405. [DOI] [PubMed] [Google Scholar]
- 119.Matsumoto A, Mizukami H, Mizuno S, et al. β-Cryptoxanthin, a novel natural RAR ligand, induces ATP-binding cassette transporters in macrophages. Biochem Pharmacol. 2007;74:256–264. [DOI] [PubMed] [Google Scholar]
- 120.Lian F, Hu KQ, Russell RM, et al. β-Cryptoxanthin suppresses the growth of immortalized human bronchial epithelial cells and non-small-cell lung cancer cells and up-regulates retinoic acid receptor β expression. Int J Cancer. 2006;119:2084–2089. [DOI] [PubMed] [Google Scholar]
- 121.Liu C, Bronson RT, Russell RM, et al. β-Cryptoxanthin supplementation prevents cigarette smoke-induced lung inflammation, oxidative damage, and squamous metaplasia in ferrets. Cancer Prev Res. 2011;4:1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Iskandar Liu C, Smith DE, et al. β-Cryptoxanthin restores nicotine-reduced lung SIRT1 to normal levels and inhibits nicotine-promoted lung tumorigenesis and emphysema in A/J mice. Cancer Prev Res. 2012;6:309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miyazawa K, Miyamoto S, Suzuki R, et al. Dietary β-cryptoxanthin inhibits N-butyl-N-(4-hydroxybutyl)nitrosamine-induced urinary bladder carcinogenesis in male ICR mice. Oncol Rep. 2007;17:297–304. [PubMed] [Google Scholar]
- 124.Narisawa T, Fukaura Y, Oshima S, et al. Chemoprevention by the oxygenated carotenoid β-cryptoxanthin of N-methynitrosourea-induced carcinogenesis in F344 rats. Jpn J Cancer Res. 1999;90:1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rondanelli M, Opizzi A, Perna S, et al. Update on nutrients involved in maintaining healthy bone. Endocrinol Nutr. 2013;60:197–210. [DOI] [PubMed] [Google Scholar]
- 126.Yamaguchi M, Uchimaya S. Effect of carotenoid on calcium content and alkaline phosphatase activity in rat femoral tissues in vitro: the unique anabolic effect of β-cryptoxanthin. Biol Pharm Bull. 2003;26:1188–1191. [DOI] [PubMed] [Google Scholar]
- 127.Uchiyama S, Yamaguchi M. Inhibitory effect of β-cryptoxanthin on osteoclast-like cell formation in mouse marrow cultures. Biochem Pharmacol. 2004;67:1297–1305. [DOI] [PubMed] [Google Scholar]
- 128.Yamaguchi M, Uchiyama S. β-Cryptoxanthin stimulates bone formation and inhibits bone resorption in tissue culture in vitro. Mol Cell Biochem. 2004;258:137–144. [DOI] [PubMed] [Google Scholar]
- 129.Uchiyama S, Ishiyama K, Hashimoto K, et al. Synergistic effect of β-cryptoxanthin and zinc sulfate on the bone component in rat femoral tissues in vitro: the unique anabolic effect with zinc. Biol Pharm Bull. 2005;28:2142–2145. [DOI] [PubMed] [Google Scholar]
- 130.Uchiyama S, Yamaguchi M. β-Cryptoxanthin stimulates cell differentiation and mineralization in osteoblastic MC3T3-E1 cells. J Cell Biochem. 2005;95:1224–1234. [DOI] [PubMed] [Google Scholar]
- 131.Uchiyama S, Yamaguchi M. Oral administration of β-cryptoxanthin prevents bone loss in streptozotocin-diabetic rats in vivo. Biol Pharm Bull. 2005;28:1766–1769. [DOI] [PubMed] [Google Scholar]
- 132.Uchiyama S, Yamaguchi M. β-Cryptoxanthin stimulates cell proliferation and transcriptional activity in osteoblastic MC3T3-E1 cells. Int J Mol Med. 2005;15:671–681. [PubMed] [Google Scholar]
- 133.Uchiyama S, Yamaguchi M. β-Cryptoxanthin stimulates apoptotic cell death and suppresses cell function in osteoclastic cells: change in their related gene expression. J Cell Biochem. 2006;98:1185–1195. [DOI] [PubMed] [Google Scholar]
- 134.Uchiyama S, Yamaguchi M. Oral administration of β-cryptoxanthin prevents bone loss in ovariectomized rats. Int J Mol Med. 2006;17:15–20. [PubMed] [Google Scholar]
- 135.Yamaguchi M, Uchiyama S, Ishiyama K, et al. Oral administration in combination with zinc enhances β-cryptoxanthin-induced anabolic effects on bone components in the femoral tissues of rats in vivo. Biol Pharm Bull. 2006;29:371–374. [DOI] [PubMed] [Google Scholar]
- 136.Yamaguchi M, Uchiyama S. Combination of β-cryptoxanthin and zinc has potent effects on apoptotic cell death and suppression of bone resorption-related gene expression in osteoclastic cells. Int J Mol Med. 2008;22:221–228. [PubMed] [Google Scholar]
- 137.Yamaguchi M, Weitzmann MN. The bone anabolic carotenoid β-cryptoxanthin enhances transforming growth factor-β1-induced SMAD activation in MC3T3 preosteoblasts. Int J Mol Med. 2009;24:671–675. [DOI] [PubMed] [Google Scholar]
- 138.Uchimaya S, Summida T, Yamaguchi M. Anabolic effect of β-cryptoxanthin on bone components in femoral tissues of aged rats in vivo and in vitro. J Health Sci. 2004;50:491–496. [Google Scholar]
- 139.Ikeda N, Sugiyama T, Suzuki T, et al. Effects of β-cryptoxanthin on bone metabolism in a rat model of osteoporosis. J Anim Vet Adv. 2012;11:30–35. [Google Scholar]
- 140.Matsumoto C, Ashida N, Yokoyama S, et al. The protective effects of β-cryptoxanthin on inflammatory bone resorption in a mouse experimental model of periodontitis. Biosci Biotech Biochem. 2013;77:860–862. [DOI] [PubMed] [Google Scholar]
- 141.Iino M, Kozai Y, Kawamata R, et al. Effects of β-cryptoxanthin on bone-formation parameters in the distal femoral epiphysis of ovariectomized mice. Oral Radiol. 2014;30:1–8. [Google Scholar]
- 142.Yamaguchi M. Role of carotenoid β-cryptoxanthin in bone homeostasis. J Biomed Sci. 2012;19:36 doi:10.1186/1423-0127-19-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sahni S, Hannan MT, Blumberg J, et al. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: a 17-year follow-up from the Framingham Osteoporosis Study. J Bone Miner Res. 2009;24:1086–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sahni S, Hannan MT, Blumberg J, et al. Inverse association of carotenoid intakes with 4-y change in bone mineral density in elderly men and women: the Framingham Osteoporosis Study. Am J Clin Nutr. 2009;89:416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang Z, Zhang Z, Penniston KL, et al. Serum carotenoid concentrations in postmenopausal women from the United States with and without osteoporosis. Int J Vitam Nutr Res. 2008;78:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sugiura M, Nakamura M, Ogawa K, et al. Bone mineral density in post menopausal female subjects is associated with serum antioxidant carotenoids. Osteoporos Internat. 2008;19:211–219. [DOI] [PubMed] [Google Scholar]
- 147.Sugiura M, Nakamura M, Ogawa K, et al. Dietary patterns of antioxidant vitamin and carotenoid intake associated with bone mineral density: findings from post-menopausal Japanese female subjects. Osteopos Internat. 2010;2010:1–10. [DOI] [PubMed] [Google Scholar]
- 148.Sugiura M. Nutritional epidemiologic survey of the relationship between satsuma mandarin intake and the risk for lifestyle-related diseases: Mikkabi Prospective Cohort Study. Jap Soc Food Sci Tech. 2014;61:373–381. [Google Scholar]
- 149.Sugiura M, Nakamura M, Ogawa K, et al. Dietary patterns of antioxidant vitamin and carotenoid intake associated with bone mineral density: findings from post-menopausal Japanese female subjects. Osteo Internat. 2011;22:143–152. [DOI] [PubMed] [Google Scholar]
- 150.Sugiura M, Nakamura M, Ogawa K, et al. High serum carotenoids associated with lower risk for bone loss and osteoporosis in post-menopausal Japanese female subjects: prospective cohort study. PLOS One. 2012;7:e52643 doi:10.1371/journal.pone.0052643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ghodratizadeh S, Kanbak G, Beyramzadeh M, et al. Effect of carotenoid β-cryptoxanthin on cellular and humoral response in rabbit. Vet Res Commun. 2014;38:59–62. [DOI] [PubMed] [Google Scholar]
- 152.Fu HF, Wu CJ, Riaz H, et al. β-Cryptoxanthin uptake in THP-1 macrophoges upregulates the CYP27A1 signalling pathway. Mol Nutr Food Res. 2014;58:425–436. [DOI] [PubMed] [Google Scholar]
- 153.Kobori M, Ni YH, Takahashi Y, et al. β-Cryptoxanthin alleviates diet-induced nonalcoholic steatohepatitis by suppressing inflammatory gene expression in mice. PLOS One. 2014;9:e98294 doi:10.1371/journal.pone.0098294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Johnson EJ. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr Rev. 2014;72:605–612. [DOI] [PubMed] [Google Scholar]
- 155.Aronow ME, Chew EY. Age-related Eye Disease Study 2: perspectives, recommendations, and unanswered questions. Curr Opin Ophthalmol. 2014;25:186–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chew EY. Nutrition effects on ocular diseases in the aging eye. Invest Ophthalmol Vis Sci. 2013;54:ORSF42–ORSF47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Koushan K, Rusovici R, Li W, et al. The role of lutein in eye-related disease. Nutrients. 2013;5:1823–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dherani M, Murthy GV, Gupta SK, et al. Blood levels of vitamin C, carotenoids and retinol are inversely associated with cataract in a North Indian population. Invest Ophthalmol Vis Sci. 2008;49:3328–3335. [DOI] [PubMed] [Google Scholar]
- 159.Kang JH, Pasquale LR, Willett W, et al. Antioxidant intake and primary open-angle glaucoma: a prospective study. Am J Epidemiol. 2003;158:337–346. [DOI] [PubMed] [Google Scholar]
- 160.Lyle BJ, Mares-Perlman JA, Klein BE, et al. Serum carotenoids and tocopherols and incidence of age-related nuclear cataract. Am J Clin Nutr. 1999;69:272–277. [DOI] [PubMed] [Google Scholar]
- 161.Zhou H, Zhao X, Johnson EJ, et al. Serum carotenoids and risk of age-related macular degeneration in a Chinese population sample. Invest Ophthalmol Vis Sci. 2011;52:4338–4344. [DOI] [PubMed] [Google Scholar]
- 162.Chong EW, Wong TY, Kreis AJ, et al. Dietary antioxidants and primary prevention of age-related macular degeneration: systematic review and meta-analysis. BMJ. 2007;335:755 doi:10.1136/bmj.39350.500428.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tan JS, Wang JJ, Flood V, et al. Dietary antioxidants and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology. 2008;115:334–341. [DOI] [PubMed] [Google Scholar]