Abstract
Glutamine, reviewed extensively in the last century, is a key substrate for the splanchnic bed in the whole body and is a nutrient of particular interest in gastrointestinal research. A marked decrease in the plasma glutamine concentration has recently been observed in neonates and adults during acute illness and stress. Although some studies in newborns have shown parenteral and enteral supplementation with glutamine to be of benefit (by decreasing proteolysis and activating the immune system), clinical trials have not demonstrated prolonged advantages such as reductions in mortality or risk of infections in adults. In addition, glutamine is not able to combat the muscle wasting associated with disease or age-related sarcopenia. Oral glutamine supplementation initiated before advanced age in rats increases gut mass and improves the villus height of mucosa, thereby preventing the gut atrophy encountered in advanced age. Enterocytes from very old rats continuously metabolize glutamine into citrulline, which allowed, for the first time, the use of citrulline as a noninvasive marker of intestinal atrophy induced by advanced age.
Keywords: glutaminase, glutamine synthetase, longevity, metabolism, sarcopenia
INTRODUCTION
Glutamine is a nonessential amino acid but becomes a conditionally essential amino acid in catabolic states because of the body’s inability to synthesize sufficient amounts of glutamine during stress.1–4 In other words, normal plasma levels of glutamine are insufficient to meet increased demands under stress. Low concentrations of glutamine in plasma reflect reduced stores in muscle, and this reduced availability of glutamine in the catabolic state seems to correlate with increased morbidity and mortality. Aging, which is characterized by reduced physical activity resulting mainly from the inevitable age-related loss of muscle mass or to skeletal muscle atrophy, a condition referred to as sarcopenia,5–15 may be related to a “physiological” catabolic state. Consequently, it is important to discuss the role of glutamine in sarcopenia because glutamine synthesis can be depressed in skeletal muscle as a result of the muscle loss that occurs with aging.
The aim of this review is to present current data on the regulation of glutamine metabolism, with particular reference to animal data, in order to gain a better understanding of the cell mechanisms involved and, specifically, to determine what is currently known about the role of glutamine in nutrition (needs and utilization). Animal data are extended to elderly people, bearing in mind that, as reported by Welle,16 the metabolism rate is faster in rats than in humans. The potential role of glutamine in nutrition during aging, especially the relevance of glutamine supply for well-being and longevity, is highlighted.
PHYSIOLOGICAL IMPORTANCE AND BIOCHEMISTRY OF GLUTAMINE
This review covers only the enzymes responsible for the synthesis and degradation of glutamine in the cell. The data presented essentially concern skeletal muscle, liver, gut, and other rapidly dividing cells.
Two principal enzymes regulate intracellular metabolism of glutamine.17 The enzyme glutaminase catalyzes the hydrolysis of glutamine to glutamate, while glutamine synthetase catalyzes the synthesis of glutamine from glutamate and ammonia. Replicating cells, such as enterocytes, lymphocytes, endothelial cells, and tumor cells, tend to be avid consumers of glutamine and, in general, contain far greater amounts of glutaminase than glutamine synthetase. Skeletal muscle and lung, which synthesize and release net amounts of glutamine into the bloodstream, contain substantial amounts of glutamine synthetase. The liver contains both enzymes because it can switch from net glutamine utilization to net production, depending on physiological and nutritional conditions.
OVERVIEW OF MOLECULAR AND CELLULAR ASPECTS OF GLUTAMINE METABOLISM IN MAMMALS AND HUMANS
The major metabolic roles of glutamine (molecular and cellular mechanisms) in mammals and humans are presented in the first section of Table 1.3,4,17–24 The characteristics of glutamine, as well as the role of glutamine in metabolic pathways in the cell, are described in this part of the table. In the second part of Table 1, the regulatory roles of glutamine in several cell-specific processes are listed.25–52 The concept of the classical “ideal protein,” including glutamine as a nonessential amino acid, was defined according to Wu et al.51 in 2013.
Table 1.
Overview of the metabolic roles of glutamine
| References | |
|---|---|
| (1)Characteristics of glutamine and its role in metabolic pathways in the cell | |
| The most abundant free amino acid in the body. Classified among the NEAAs, which include glutamate, proline, glycine, and arginine | Boelens et al. (2001),18 Buchman (2001),19 Bulus et al. (1989),20 Chiba et al. (2009),21 Coster et al. (2004),22 Epler et al. (2003),23 Lacey & Wilmore (1990),3 Smith (1990),4 Souba (1991),17 Welbourne & Joshi (1994)24 |
| In catabolic states, reclassified as a conditionally essential amino acid; consequently, it plays a role in nutrition and health | |
| Skeletal muscle is the largest tissue reservoir of glutamine and is quantitatively the most important site of glutamine synthesis. Cytosolic glutamine synthetase, which is a longevity-related gene, is responsible for this synthesis | |
| Small intestine and kidneys are the major sites of glutamine utilization under normal or acidotic conditions | |
| Intracellular glutamine concentration generally decreases in catabolic states. In humans, this change can be prevented or attenuated by glutamine supplementation (enteral or parenteral) | |
| The liver can synthesize or degrade glutamine, depending on the nutritional conditions | |
| (2)Regulatory roles of glutamine in cell-specific processes | Boukhettala et al. (2012),25 Brasse-Lagnel et al. (2009),26 Yi et al. (2015)27, Brasse-Lagnel (2010),28 Curi et al. (2005)29 (2007),30 Deniel et al. (2007),31 Larson et al. (2007),35 Le Bacquer et al. (2001),36 Häussinger et al. (2001),32 Kadowaki & Kanazawa (2003),33 Krause et al. (2002),34 Matés et al. (2006),37 Nakajo et al. (2005),38 Naomoto et al. (2005),39 Nishikawa et al. (2007),40 Papaconstantinou (2000),41 Rhoads & Wu (2009),42 Roth et al. (2002),45 Roth (2007),43 (2008),44 Rutten et al. (2006),46 van de Poll et al. (2007),47,52 Watford (1993),48 Xi et al. (2011),49 Ziegler et al. (2003)50 |
| Nitrogen shuttle to form urea (via glutaminase) | |
| Major transport form of nitrogen and carbon within the body | |
| Oxidative fuel for rapidly dividing cells (enterocytes, fibroblasts, malignant cells, immune cells) | |
| Gluconeogenic precursor (liver and kidneys) | |
| Lipogenic precursor (adipocytes) | |
| Cell integrity (apoptosisa, cell proliferation) | |
| Cell swelling (notably in the liver and brain) | |
| Protein synthesis (in particular, contractile protein synthesis) and degradation (resulting from 3 major systems: cathepsin, calpain, and ATP-ubiquitin-dependent proteolysis) | |
| Redox potential (glutathione synthesis from glutamine) | |
| Respiratory burst (glutamine increases superoxide anion production) | |
| Insulin resistance or insulin secretion | |
| Extracellular matrix synthesis | |
| Many genes related to metabolism | |
| Activation of several proteins | |
| Enhancement of transcription factors (sp1, C/EBP, FXR/RXR, NF-κB) | |
| Intracellular signaling pathways (e.g., glutamine is a key regulator for cell growth through the mammalian target of rapamycin pathway) | |
| Signal transduction | |
| Cell defense and repair (e.g., glutamine is a potent enhancer of heat-shock proteins) | |
| Neurotransmission | |
| Immune function (cytokine production) | |
| (3)The classical “ideal protein” concept should include both EAAs and NEAAs (including glutamine) to improve protein accretion, efficiency of food utilization, growth, and health in animals and humans | Wu et al. (2013)51 |
Abbreviations: ATP, adenosine triphosphate; EAAs, essential amino acids; NEAAs, nonessential amino acids; NF-κB, nuclear factor κB.
aApoptotic signaling mechanisms activated in response to glutamine deprivation are cell-type specific. New findings indicate that glutamine availability is strongly related to the induction of apoptosis. Glutamine works both as a nutrient and as a signaling molecule and acts directly or indirectly on the pathways leading to programmed cell death (e.g., glutamine deprivation occurs as a protective mechanism to limit apoptosis associated with cellular stress in the intestine).
Effect of glutamine starvation on cell survival and apoptosis
The consequences of glutamine deprivation on cellular survival and gene expression have led to the construction of a new paradigm for this amino acid, namely that limited extracellular glutamine supplies modulate stress and apoptotic responses. Under these conditions, plasma glutamine levels decline and, as a result, the cells suffer from glutamine starvation. Apoptotic signaling mechanisms involved in the response to glutamine deprivation are cell-type specific. New findings indicate that glutamine availability is strongly related to the induction of apoptosis and that glutamine works both as a nutrient and as a signaling molecule, acting directly or indirectly on the pathways leading to programmed cell death.53 In addition, glutamine-starved cells show reduced expression of the 70 000 Mr heat-shock protein, which is an important factor for cell survival.54 Consequently, glutamine-utilizing cells possess molecular mechanisms to detect the availability of glutamine and to respond specifically to changes in extracellular glutamine concentrations.54,55
Regulative potential of glutamine: relation to glutathione metabolism
In animal studies, Roth44 demonstrated that administration of glutamine increases tissue concentrations of reduced glutathione. Glutamine (via glutamate), cysteine, and glycine are the precursor amino acids for glutathione, which is present within the cell in a reduced form and an oxidized form. The ratio of reduced glutathione to oxidized glutathione is the most important regulator of the cellular redox potential. Thus, glutamine in either its free or dipeptide form influences this potential by enhancing, even more so in catabolic states,56,57 the formation of glutathione, the major endogenous antioxidant in mammalian cells that protects against oxidative injury and cell death.58
Glutamine signaling in the intestine
Glutamine is an essential nutrient for small intestine function. It functions as a signal to enhance cell survival in the intestine, it inhibits apoptosis in intestinal cells, it is necessary for tight junction stabilization, and it has anti-inflammatory effects in the intestine (e.g., it decreases production of proinflammatory interleukins 6 and 8, enhances production of anti-inflammatory interleukin 10, and reduces expression of NF-κB protein).42 Hence, glutamine plays a key role in the metabolism of rapidly dividing cells, including enterocytes and lymphocytes, which may contribute to its beneficial clinical effects. Gut mucosal homeostasis is achieved through a balance between cell proliferation and apoptosis. Whereas glutamine upregulates anti-apoptotic proteins and downregulates pro-apoptotic proteins in T cells, it prevents apoptosis in rat epithelial cell lines derived from gut mucosa; moreover, glutamine starvation induces apoptosis through caspase activation.31,35 In brief, glutamine may play a role in the gut-protective effect by inhibiting apoptosis via downregulation of the transcription factor Sp3, by contributing to cell survival during physiological stress by induction of autophagy, by modulating intestinal barrier function under basal and inflammatory conditions, or by its anti-inflammatory effect via induction of nuclear degradation of the NF-κB p65 subunit.59,60
Glutamine is a key regulator of amino acid–controlled cell growth through the mammalian target of rapamycin (mTOR) signaling pathway in rat intestinal epithelial cells.27,28,31,39,42,61,62 Amino acids are important signaling regulators, especially for p70 S6 kinase and eIF-4E binding protein 1 via mTOR, which have 2 structurally and functionally distinct complexes termed mTORC1 and mTORC2. mTORC1 is activated by nutrients (amino acids), growth factors, and cellular energy, while mTORC2 is activated by growth factors alone. The mTOR pathway that regulates major cellular functions (growth and proliferation) plays a role in health and disease as well as in aging.43,63–65 Glutamine inhibits the activation of p70 S6 kinase and the phosphorylation of eIF-4E binding protein 1 induced by arginine and leucine in rat intestinal epithelial cells. In contrast, in healthy subjects in the fed state, enteral proteins, but not glutamine, increased protein synthesis via an mTOR-independent pathway in humans.66 Nakajo et al.38 have proposed a new concept for the biological role of phosphorylation of mTOR and intestinal cell growth: the signal induced by glutamine may stimulate cellular proliferation and increase cell number, whereas leucine or arginine induces the signal for cell growth (increase in cell size) in rat intestinal epithelial cells. Glutamine suppresses only mTOR signaling for cell growth, and therefore it is considered an essential amino acid for cell culture.38 The intracellular signaling pathways involved in controlling intestinal glutamine transport during acidosis have been studied in Caco-2 cells, a model of human enterocytes. Metabolic acidosis stimulates glutamine transport via signaling pathways that lead to transcription of the glutamine transporter gene and to intestinal glutamine absorption.23
Glutamine signaling in the liver
As in the intestine, glutamine has a direct regulatory potential in autophagic proteolysis in the liver caused by a lysosomotropic toxicity of ammonia derived from glutamine degradation. Indeed, in most visceral tissues, the autophagic pathway is responsible for the bulk of proteolysis and is most sensitive to amino acid regulation.67 Glutamine, like leucine, tyrosine, phenylalanine, proline, methionine, tryptophane, and histidine, may have a direct regulatory role in the liver, possibly via amino acid receptors or sensors that enable recognition of these amino acids at the plasma membrane, leading subsequently to their involvement in intracellular signaling.33 Moreover, glutamine stimulates a number of metabolic pathways of intermediary metabolism in the liver. For example, glutamine activates phosphoenolpyruvate carboxykinase (PEPCK) in the liver and increases the phosphorylation state of p70 S6 kinase, a key enzyme in liver protein synthesis. Glutamine is known to induce cell swelling and to mediate the inhibition of autophagic proteolysis; glutamine’s antiproteolytic effect may be mediated through osmotic swelling and the p38MAPK pathway.68
IMPACT OF AGING ON REGULATION OF GLUTAMINE METABOLISM
Aging is defined as the progressive changes that occur after maturity in various organs, leading to a decrease in their functional ability and possible alterations in metabolic pathways.69–72 Because protein ingestion is crucial for the maintenance of a variety of body functions, the requirements of protein in the elderly are a major factor in maintaining skeletal muscle mass; the amount of protein ingested that induces maximal muscle protein synthesis must be higher in elderly than in young individuals in order to combat anabolic resistance in the elderly.5,73–77
Dysregulation of autophagy78–80 contributes notably to aging. Autophagy, a lysosomal process involved in the maintenance of cellular homeostasis, is inhibited by the insulin-amino acid-mTOR signaling pathway that controls both protein synthesis and longevity (see next paragraph). During aging, autophagy declines and insulin resistance can develop.81 Thus, autophagy can provide protection against aging and cell death. Indeed, the best way to increase autophagy in vivo is by restricting calorie intake, which may promote longevity during aging.82–84 Moreover, glutamine inhibits autophagy and regulates cell growth.85 The role of glutamine metabolism in autophagy is related to the activation of mTOR by leucine, which is an activator of glutaminolysis.86
Aging is also characterized by protein wasting (see next paragraph), and glutamine, which is the most abundant amino acid in the blood, may be a hallmark of catabolic states. Indeed, a low concentration of glutamine in plasma reflects reduced stores in muscle, and this reduced availability of glutamine in the catabolic state seems to correlate with increased morbidity and mortality.18 Moreover, in catabolic states, glutamine may be replenished by supplementation of branched-chain amino acids. Branched-chain amino acids, especially leucine, stimulate protein synthesis, inhibit proteolysis (in cell culture and animals), and promote glutamine synthesis.87,88 Depletion of plasma glutamine may result in loss of branched-chain amino acids and increased protein wasting.89 When the “glutamine trap” (e.g., phenylbutyrate) is used to deplete plasma glutamine,90 skeletal muscle glutamine is not depleted, regardless of the age of rats, and muscle glutamine synthetase is not increased.91 Despite this, there are few documented data in the literature on the role of glutamine in aging.
Glutamine: the potential cornerstone of protein turnover in skeletal muscle during aging
The potential regulatory effect of glutamine in aging has implications for the development and treatment of age-related muscle loss and strength loss (sarcopenia). There is a progressive decrease in lean body mass with aging and, consequently, in total body protein, due largely to a loss of skeletal muscle protein.77,92 Sarcopenia is a highly significant public health problem. These changes in skeletal muscle are attributed largely to various molecular mediators that affect fiber size, mitochondrial homeostasis, and apoptosis and highlight the mTORC1 pathway as a key therapeutic target to prevent sarcopenia.93–97 Indeed, in skeletal muscle, the activation of mTORC1 is involved in the regulation of protein synthesis and controls skeletal muscle mass.98 This progressive loss of skeletal muscle with aging is attributed to a disruption in the regulation of protein turnover in skeletal muscle99 and may be considered as a “physiological” catabolic state. For this reason, protein and amino acids, notably glutamine, as dietary supplementation may have potential usefulness in fighting against muscle atrophy during aging. Thus far, however, dietary supplementation with proteins or amino acids seems inefficient in limiting the atrophy processes, and neither leucine100–102 (or essential amino acids103,104) nor glutamine105 fights against the loss of skeletal muscle. Protein supplementation increased muscle mass gain only during resistance-type exercise training in elderly people.106,107 Essential amino acids combined with resistance exercise also increased protein synthesis via mTORC1 signaling.108 During a 5-week exercise program, cysteine-rich whey protein increased lean body mass and decreased fat mass in comparison with a control diet, but the authors made no mention of the effect on skeletal muscle mass per se.109 Thus, neither glutamine nor essential amino acids such as leucine are able to fight against sarcopenia; the increase in protein synthesis is not sufficient to increase muscle mass (Table 2).
Table 2.
Effect of glutamine and other amino acids on protein turnover in skeletal muscle with aging
| Supplementation | Physical activity | Whole-body improvement or preservation of muscle functionality | Change in fat body mass | Changed in lean body mass | Skeletal muscle atrophy | References |
|---|---|---|---|---|---|---|
| Leucine-rich whey protein or leucine alone or essential amino acids | Bed rest or no physical activity | Yes | Decreased or maintained | Increased or maintained | No effective change | Koopman et al. (2008),101 Dillon et al. (2009),103 Ferrando et al. (2010),104 Walker et al. (2011),108 Casperson et al. (2012),100 Magne et al. (2013)102 |
| Glutamine | None | Yes | NR | NR | No effective change | Mignon et al. (2007)105 |
| Protein (2×15 g) | 24 wk of progressive resistance exercise | Yes | NR | Increased | No effective change | Tieland et al. (2012),107 Evans et al. (2013)106 |
| Cysteine-rich whey protein | 5 wk of exercise program | Yes | Decreased | Increased | NR | Dröge (2005)109 |
Abbreviation: NR, not reported.
Glutamine deficiency, which stimulates cell apoptosis, may stimulate sarcopenia, thereby triggering a low-level inflammatory process.43 Moreover, aging is known to induce a dysregulation of immune and inflammation functions that may affect protein synthesis rates in lymphoid tissues and plasma proteins.110 Aging has been also described as a condition characterized by anabolic resistance to nutrients, especially amino acids, which impairs muscle protein synthesis and contributes to muscle wasting. Table 2 provides an overview of the effect of amino acids on protein turnover in skeletal muscle. Under inflammatory conditions, branched-chain amino acids, notably leucine, can be transaminated to glutamate in order to increase the synthesis of glutamine, which is a substrate highly consumed by inflammatory cells such macrophages.111 But in old rats, both the production and the release of glutamine in muscle are reduced in relation to the increased splanchnic sequestration of leucine and the reduced renal and intestinal glutamine uptake in order to maintain whole-body glutamine homeostasis.112 Thus, glutamine depletion has a role in the sarcopenia and low-grade inflammation observed with aging.
Effect of aging on whole-body glutamine metabolism
A link can be made between aging and physical inactivity (experimental bed rest in healthy volunteers) since, as previously reported, inactivity contributes to sarcopenia.113 Physical activity generally decreases with age. Physical inactivityreduces whole-body glutamine availability due to downregulated de novo synthesis and, more generally, to anabolic resistance.114,115 This alteration also results in decreased cytosolic ratios of glutamine to branched-chain amino acids (leucine, valine, isoleucine) and glutamine to aromatic amino acids (tyrosine, phenylalanine). These changes in the profiles of free amino acids may be caused by sedentary lifestyle, nutrition, and immobilization, as commonly observed in the aging process.116 However, in very old rats aged 25 months (female rats, whose mean life expectancy is ≈27 months, with a maximum life span of 32 months), aging does not induce changes either in plasma or muscle glutamine concentrations (0.59±0.13 vs 0.55±0.15 mM in plasma and 3.21±0.60 vs 3.18±0.85 µmol/g in muscle).117 Advanced age in rats corresponds to an age of about 75–80 years in humans. Glutamine deficiency in bed rest in humans also alters monocyte or macrophage activity, decreases the formation of heat-shock proteins, stimulates cell apoptosis, and shifts the cellular redox potential by altering glutathione synthesis in lymphocytes. However, glutamine is able to preserve hepatic glutathione after hepatic injury,44 as measured in the liver of very old female rats.59
Impact of aging on glutamine synthesis in skeletal muscle: in vivo study using 13C magnetic resonance spectroscopy
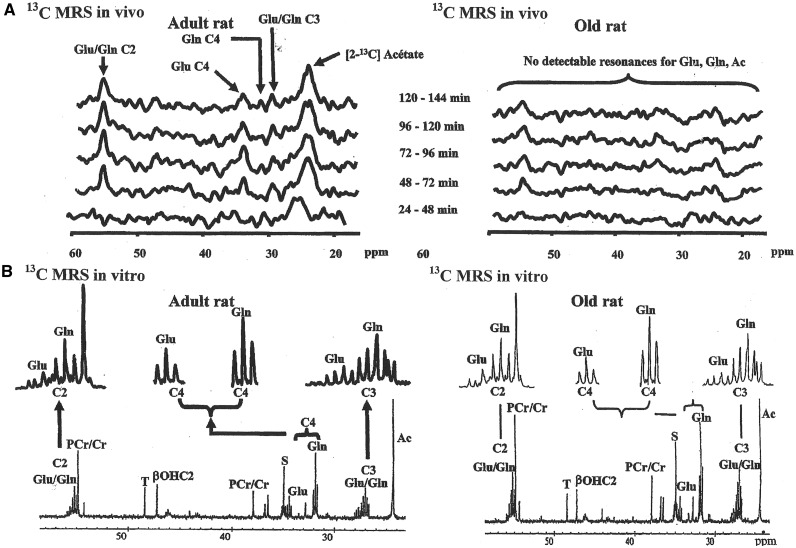
Skeletal muscle is the principal organ of the synthesis of glutamine, which is exported to other tissues such as the liver, gut, and immune cells according to the requirements of the cell. These requirements are dependent on age, as evidenced by the increase in glutamine synthetase activity with aging, reported below. Under normal healthy conditions, glutamine synthetase activity measured in skeletal muscle is higher in female and male rats of advanced age (about 25–27 months) than in 8- to 22-month-old rats. In contrast, very old age (29–32 months, which corresponds to >85-year-old humans, including centenarians) is associated with significant muscle wasting, resulting in 2 subpopulations of low or high glutamine synthetase activity. The low glutamine synthetase activity may reflect a state of lesser stress, as if these survivors may be physiologically younger than their age would indicate. High levels, such as those reported in the 25- to 27-month-old rats, may be considered an indicator of stress and frailty and, thus, the need for more glutamine.117 Moreover, glutamine synthetase in muscle is a longevity-related gene and increases with caloric restriction in mice.21 Because glutamine synthetase activity increases with fasting, whatever the age of animals,118 glutamine synthesis was compared directly in skeletal muscle from 2-[13C] acetate by magnetic resonance spectroscopy in fasted 25-month-old female Wistar rats and adult rats (8 months) (V. Mezzarobba, D. Meynial-Denis, and J.P. Renou, unpublished data, 2000). In old rats, glutamine and glutamate were undetectable in muscle in vivo but were detected in muscle extract at the end of the experiment (Figure 1). Consequently, flows of glutamine and glutamate through skeletal muscle were too quick to be detected by magnetic resonance spectroscopy in vivo. A metabolic schema is shown in Figure 2. This method was well described and assessed by Hwang and Choi119.
Figure 1.
13C magnetic resonance spectrometry study of skeletal muscle from adult (8 mo) and aged (25 mo) rats: (A) Kinetics of in vivo spectra between 24 and 144 min of [2-13C] acetate perfusion, and (B) in vitro spectrum obtained at the end of [2-13C] acetate perfusion (V. Mezzarobba, D. Meynial-Denis, and J.P. Renou, unpublished data, 2000). Abbreviations: Ac, acetate; βOHC2, β-hydroxybutyrate; Glu, glutamate; Gln, glutamine; PCr/Cr, ratio of phosphocreatine to creatine; ppm, parts per million (MRS unit); S, unknown resonance; MRS, magnetic resonance spectroscopy; T, unknown resonance.
Figure 2.
Hypothetical fluxes of both glutamine and glutamate through the skeletal muscle to the plasma. Abbreviations: αKG, α-ketoglutarate; Gln, glutamine; Glu, glutamate; GS, glutamine synthetase; TCA, tricarboxylic acid.
Impact of aging on glutamine synthetase in skeletal muscle: in vitro biochemistry study
Glutamine synthetase, a gluco/corticoid-induced enzyme that plays a key role in glutamine synthesis, is preserved in skeletal muscle during aging but not in self-contracted muscle such as the heart.120 In contrast, other steroids, such as sex steroids (progesterone, estradiol), do not affect glutamine synthetase activity in either the muscle or the heart, whatever the age of animals. The activity of glutamine synthetase in the heart is not regulated by glucocorticoids but is dependent on mineralocorticoid hormones (aldosterone).121 In brief, glutamine synthetase is regulated by glucocorticoids in skeletal muscle but not in the heart. An acute depletion of plasma glutamine does not modify the upregulation of muscle glutamine synthetase activity in response to fasting in either adult or aged rats.91 In old rats, there is increased glutamine synthetase activity (in 25- to 27-month-old female and 24- to 27-month-old male rats, which are very old animals, since mortality at this age is about 60%), suggesting a greater need for glutamine. In some very old female rats, low glutamine synthetase activity may be associated with longevity or may reflect a limitation of glutamine production due to extremely advanced age per se.117
Impact of aging on glutamine synthesis in the liver
The liver is an important organ in glutamine homeostasis. So, it has a particular role in nitrogen salvage, and urea production, and glutamine metabolism.122–126 It has the ability both to degrade glutamine and to synthesize glutamine because the enzymes responsible, which are located in different hepatocyte populations, are active simultaneously. In the liver acinus (functional unit of the liver), ureagenesis and glutaminase (L-glutamine amidohydrolase; EC 3.5.1.2) activity are localized predominantly in the periportal area, whereas glutamine synthetase (L-glutamate:ammonia ligase [ADP-forming]; EC 6.3.1.2) activity is found exclusively in a small perivenous hepatocyte population (≈7% of all hepatocytes of an acinus). The pericentral expression pattern of glutamine synthetase in the liver is due to the upstream region of this enzyme.127 Because ammonium ions at low concentration are effectively removed by glutamine synthetase, but not by urea synthesis, both pathways contribute to ammonia detoxification in the liver acinus. The liver may switch from net glutamine utilization to net production, depending on physiological and nutritional conditions. For this reason, it is interesting to study the regulation of glutamine metabolism during aging. Although a large proportion of proteases (to which glutamine synthetase belongs) are known to be oxidatively modified with aging in the liver and neutral or alkaline proteolytic activity is maintained during aging, native glutamine synthetase remains active. Indeed, Sahakian128 demonstrated that hepatocyte glutamine synthetase decreased by 40%–50% between 3 and 26 months of age, irrespective of gender. By contrast, it has been reported that, in fed male Wistar rats, liver glutamine synthetase activity remained constant, whatever the age of animals (2–24 months) (Figure 3). Moreover, glutaminase slightly and continuously increases with age (2–24 months) to become significantly different at 24 months (Figure 3).129 In contrast to these findings, Spindler130 demonstrated that glutaminase, which is a key enzyme in liver nitrogen disposal, increased in caloric-restricted aged mice. This author also reported that glutamine synthetase decreased by about 40% in these conditions, which is in good agreement with the findings of Pinel et al.117 It is noteworthy that glutaminase was shown to be significantly increased only at 24 months in male Wistar rats, but at this age, 50% of them died.117
Figure 3.
Effect of advanced age on interorgan glutamine flow between muscle, blood, and liver in Wistar rats. Glutamine orientation toward the gut and immune cells was not studied. Young rats were aged 2 months and very old rats 27 months. Muscle is the principal organ of glutamine synthesis. Glutamine is released from muscle into the blood. The liver uptakes glutamine and metabolizes it in urea, which is released into the blood. Adapted from Mouchard et al.,129 Pinel et al.,117 Meynial-Denis et al.,91 and Mezzarobba et al.118 Abbreviations: Gln, glutamine; Glu, glutamate; NH4+, ammonium; TCA, tricarboxylic acid.
Impact of aging on glutamine synthesis in the gut
Glutamine may have gut-protective effects. Since the expression of Schlafen 3, a negative regulator of cellular proliferation, decreases by 8- to 10-fold in the colonic mucosa of aged rats,131 it would be interesting to study the effect of glutamine on the expression of the Schlafen 3 gene. Glutamine also contributes to the suppression of the tricarboxylic acid cycle as an oxidative and synthetic pathway with aging in jejunal epithelial cells.132
In healthy humans, approximately 10%–15% of glutamine taken up by the intestines is converted to citrulline.133,134 Quantitatively, glutamine is considered a major precursor of intestinal citrulline release.47,52,135,136 The intestines consume glutamine at a rate that is dependent on glutamine supply. Because glutamine breakdown in the gut produces citrulline, there is a good relation between the amount of metabolically active gut tissue and the production of citrulline in this tissue.46,137,138
To investigate whether the regulation of glutamine metabolism remains the same throughout aging, the production of citrulline from glutamine in the gut of old female rats was studied. Glutamine was used as long-term intermittent supplementation to evaluate the state of the gastrointestinal tract with advanced age (see section below, Long-term supplementation in rats).59
De novo glutamine synthesis plays a major role in the maintenance of intracellular glutamine pools in Caco-2 cells. In this model, glutamine availability affects the rates of protein synthesis. Caco-2 cells represent a model of human intestine enterocytes because the cell line is of human origin and originally derives from a colon carcinoma; these cells undergo enterocytic differentiation in vitro and share many characteristics with normal human enterocytes. Glutamine deprivation in Caco-2 cells is achieved either by maintaining the cell in glutamine-free medium or by using methionine sulfoximine, an inhibitor of glutamine synthetase.36
In brief, recent studies highlighted a critical role for glutamine in enterocytes, namely the activation of the mTOR signaling pathway. In catabolic states, glutamine has been reported to enhance intestinal and whole-body growth, to promote enterocyte proliferation and survival, and to regulate intestinal barrier function.139 Thus, glutamine holds great promise in protecting the gut from atrophy and injury in mammals and in humans during aging.
Impact of aging on glutamine metabolism at the cellular level
Senescence-associated changes in the metabolic phenotype of human endothelial cells are related to glutaminolysis, which is an important target for in vitro induction of senescence. Indeed, a prerequisite for glutaminolysis is the overexpression of glutaminase, the first enzyme within the glutaminolytic pathway. Cell proliferation was found to correlate with glutaminase overexpression. Thus, premature senescence of human endothelial cells is induced by the inhibition of glutaminase (demonstrated by the use of a glutaminase inhibitor as 6-diaso-5-oxo-L-norleucine).140 Senescence and apoptosis act as parallel pathways by which severely damaged cells are eliminated from the body by the innate immune system.141 Programmed cell death pathways are promising targets for interventions in aging and aging-related diseases. But to inhibit them, muscle atrophy that occurs with aging must be prevented.142
EFFECT OF GLUTAMINE SUPPLEMENTATION ON AGING
Can glutamine supplementation have an effect on age-related muscle wasting and immunity, consequently playing a role in intestinal activity? In other words, can glutamine contribute to the well-being or the longevity of the elderly?
Short-term supplementation in rats.
Glutamine was added to drinking water for 7 consecutive days each month (20% of dietary protein) after 5-day fasting to determine whether glutamine synthetase enhancement induced by fasting disappeared with glutamine supply irrespective of animal age. This process was associated with re-feeding. The effect of glutamine supplementation was compared with the effect of alanine and glycine supplementation. Only glutamine supplementation was able to significantly decrease glutamine synthetase activity in the skeletal muscle of very old rats (27 months), whereas supplementation with other amino acids decreased upregulated glutamine synthetase activity in adult rats.143
Five-month supplementation in rats.
Supplementation with glutamine was the same as that given previously but was an intermittent oral supplementation given during the last 5 months of life (7 consecutive days each month). Long-term treatment with glutamine (started before advanced age was reached) had several effects on very old rats (in good agreement with Neu et al.144): (1) it prevented the loss of body weight; (2) it did not prevent the inevitable sarcopenia, regardless of nutritional state (inefficient in modifying the rates of protein turnover); and (3) it decreased upregulated glutamine synthetase activity only in the fed state. Whole-body glutamine requirements in the rat may be satisfied in the fed state but would not be met during catabolic states (fasting) with advanced age. Glutamine may also have an essentially beneficial role for the gut, maintaining both intestinal integrity and intestinal immune function.104
Long-term supplementation in rats
Muscle.
Supplementation was the same as above but was given for 50% of the rats’ lifetime. Glutamine synthetase activity increased in skeletal muscle with aging in very old fed and fasted animals. The enhancement of glutamine synthetase activity (1.5- to 2-fold) in 25- to 27-month-old rats may be a consequence of aging-induced stress104 and will occur if glutamine supplementation is not interrupted before the study begins. Long-term treatment with glutamine before advanced age but discontinued 15 days before rat sacrifice is effective in increasing plasma glutamine to the same levels as those in adult rats and in maintaining plasma glutamine in very old rats, but it has no long-lasting effect on the glutamine synthetase activity of skeletal muscle with advanced age.145
Intestine.
Glutamine was added to the drinking water of very old (27 months) female rats for 10 months of their life span for 7 consecutive days a month (20% of dietary protein; average of the 10 glutamine treatments, 0.8±0.1 g/rat/d).59
Long-term treatment with glutamine initiated before advanced age maintains rat body weight and has a beneficial effect on enterocytes by increasing gut mass and improving the villus height of mucosa, thereby preventing the gut atrophy encountered in advanced age. The mucosal enzyme activities required for citrulline synthesis in the gut are preserved in the gut of very old rats, as reported by Crenn et al.137 in humans. Therefore, citrulline can be used, for the first time, as a noninvasive marker of intestinal atrophy induced by advanced age.
Further investigations are warranted to explore the effect of very old age on this glutamine–citrulline interrelation in the gut in vivo in humans. Intestinal atrophy with advanced age (reduction in the jejunal surface area), which has been widely documented, may contribute to the frailty syndrome and, consequently, have implications for public health.59
Supplementation in humans
Although the beneficial effects of glutamine supplementation were demonstrated as early as 1990, few data from healthy humans were reported.4,146 Healthy elderly subjects account for a very small part of the general population and are of interest primarily to nutritional researchers rather than medical doctors. Medical researchers are more interested in glutamine supplementation as a means of improving patient health. They tend to focus on the illness in humans, whereas nutritional scientists place greater emphasis on prevention. The aging of the population will become an important societal question as the number of very old individuals increases worldwide.
The interest in glutamine has been altered in the last century because this amino acid does not combat muscle-wasting during disease or age-related sarcopenia. For this reason, few data on the healthy elderly are reported in the literature. Nevertheless, glutamine is of continuing interest in medical research because of its potential role in improving the well-being of ill humans (see the reviews of Boelens et al.,18 and Neu et al.144). Gut function in both healthy and ill old humans is incompletely understood and needs to be more fully investigated. As far as is known, there is only 1 published report about collagen synthesis after supplementation with glutamine.147
CONCLUSION
Preventive nutrition should be developed in France and other countries to maintain the well-being and good health of humans as long as possible, particularly as aging progresses. Glutamine added to classical amino acid nutritional supplementation may contribute to the preservation of the gut by decreasing villus atrophy and maintaining gut function. Glutamine may therefore constitute an essential factor in the well-being and good health of very old individuals. In short, this review demonstrates that the function of glutamine goes beyond that of a simple metabolic fuel or protein precursor, as previously assumed, but instead is both a nutrient and a signaling molecule.
Acknowledgments
The author extends special thanks to Dennis Bier and Morey Haymond for their advice and encouragement.
Declaration of interest. The authors have no relevant interests to declare.
References
- 1.Biolo G, Zorat F, Antonione R, et al. Muscle glutamine depletion in the intensive care unit. Int J Biochem Cell Biol. 2005;37:2169–2179. [DOI] [PubMed] [Google Scholar]
- 2.Bode BP. Recent molecular advances in mammalian glutamine transport. J Nutr. 2001;131(9 suppl):2475S–2485S; discussion 2486S–2487S. [DOI] [PubMed] [Google Scholar]
- 3.Lacey JM, Wilmore DW. Is glutamine a conditionally essential amino acid? Nutr Rev. 1990;48:297–309. [DOI] [PubMed] [Google Scholar]
- 4.Smith RJ. Glutamine metabolism and its physiologic importance. JPEN J Parenter Enteral Nutr. 1990;14(4 suppl):40S–44S. [DOI] [PubMed] [Google Scholar]
- 5.Boirie Y, Morio B, Caumon E, et al. Nutrition and protein energy homeostasis in elderly. Mech. Ageing Dev. 2014;136–137:76–84. [DOI] [PubMed] [Google Scholar]
- 6.Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech. 2013;6:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43:748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giresi PG, Stevenson EJ, Theilhaber J, et al. Identification of a molecular signature of sarcopenia. Physiol Genomics. 2005;21:253–263. [DOI] [PubMed] [Google Scholar]
- 9.Johnson MA, Dwyer JT, Jensen GL, et al. Challenges and new opportunities for clinical nutrition interventions in the aged. J Nutr. 2011;141:535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paddon-Jones D, Campbell WW, Jacques PF, et al. Protein and healthy aging. Am J Clin Nutr. 2015;101(suppl):1339S–1345S. [DOI] [PubMed] [Google Scholar]
- 11.Rosca MG, Lemieux H, Hoppel CL. Mitochondria in the elderly: is acetylcarnitine a rejuvenator? Adv Drug Del Rev. 2009;61:1332–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solerte SB, Gazzaruso C, Bonacasa R, et al. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am J Cardiol. 2008;101(suppl):69E–77E. [DOI] [PubMed] [Google Scholar]
- 13.Wall BT, van Loon LJ. Nutritional strategies to attenuate muscle disuse atrophy. Nutr Rev. 2013;71:195–208. [DOI] [PubMed] [Google Scholar]
- 14.Wall BT, Gorissen SH, Pennings B, et al. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS One. 2015;10:e0140903 doi:10.1371/journal.pone.0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deutz NE, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welle S. Human Protein Metabolism. Vol 9 New York: Springer Verlag; 1999. [Google Scholar]
- 17.Souba WW. Glutamine: a key substrate for the splanchnic bed. Annu Rev Nutr. 1991;11:285–308. [DOI] [PubMed] [Google Scholar]
- 18.Boelens PG, Nijveldt RJ, Houdijk AP, et al. Glutamine alimentation in catabolic state. J Nutr. 2001;131(9 suppl):2569S–2577S; discussion 2590S. [DOI] [PubMed] [Google Scholar]
- 19.Buchman AL. Glutamine: commercially essential or conditionally essential? A critical appraisal of the human data. Am J Clin Nutr. 2001;74:25–32. [DOI] [PubMed] [Google Scholar]
- 20.Bulus N, Cerosimo E, Ghishan F, et al. Physiologic importance of glutamine. Metabolism. 1989;38:1–5. [DOI] [PubMed] [Google Scholar]
- 21.Chiba T, Kamei Y, Shimizu T, et al. Overexpression of FOXO1 in skeletal muscle does not alter longevity in mice. Mech Ageing Dev. 2009;130:420–428. [DOI] [PubMed] [Google Scholar]
- 22.Coster J, McCauley R, Hall J. Glutamine: metabolism and application in nutrition support. Asia Pac J Clin Nutr. 2004;13:25–31. [PubMed] [Google Scholar]
- 23.Epler MJ, Souba WW, Meng Q, et al. Metabolic acidosis stimulates intestinal glutamine absorption. J Gastrointest Surg. 2003;7:1045–1052. [DOI] [PubMed] [Google Scholar]
- 24.Welbourne TC, Joshi S. Enteral glutamine spares endogenous glutamine in chronic acidosis. JPEN J Parenter Enteral Nutr. 1994;18:243–247. [DOI] [PubMed] [Google Scholar]
- 25.Boukhettala N, Claeyssens S, Bensifi M, et al. Effects of essential amino acids or glutamine deprivation on intestinal permeability and protein synthesis in HCT-8 cells: involvement of GCN2 and mTOR pathways. Amino Acids. 2012;42:375–383. [DOI] [PubMed] [Google Scholar]
- 26.Brasse-Lagnel C, Lavoinne A, Husson A. Control of mammalian gene expression by amino acids, especially glutamine. FEBS J. 2009;276:1826–1844. [DOI] [PubMed] [Google Scholar]
- 27.Yi D, Hou Y, Wang L, et al. L-Glutamine enhances enterocyte growth via activation of the mTOR signaling pathway independently of AMPK. Amino Acids. 2015;47:65–78. [DOI] [PubMed] [Google Scholar]
- 28.Brasse-Lagnel CG, Lavoinne AM, Husson AS. Amino acid regulation of mammalian gene expression in the intestine. Biochimie. 2010;92:729–735. [DOI] [PubMed] [Google Scholar]
- 29.Curi R, Lagranha CJ, Doi SQ, et al. Molecular mechanisms of glutamine action. J Cell Physiol. 2005;204:392–401. [DOI] [PubMed] [Google Scholar]
- 30.Curi R, Newsholme P, Procopio J, et al. Glutamine, gene expression, and cell function. Front Biosci. 2007;12:344–357. [DOI] [PubMed] [Google Scholar]
- 31.Deniel N, Marion-Letellier R, Charlionet R, et al. Glutamine regulates the human epithelial intestinal HCT-8 cell proteome under apoptotic conditions. Mol Cell Proteomics. 2007;6:1671–1679. [DOI] [PubMed] [Google Scholar]
- 32.Haussinger D, Graf D, Weiergraber OH. Glutamine and cell signaling in liver. J Nutr. 2001;131(9 suppl):2509S–2514S; discussion 2523S–2524S. [DOI] [PubMed] [Google Scholar]
- 33.Kadowaki M, Kanazawa T. Amino acids as regulators of proteolysis. J Nutr. 2003;133(6 suppl 1):2052S–2056S. [DOI] [PubMed] [Google Scholar]
- 34.Krause U, Bertrand L, Maisin L, et al. Signalling pathways and combinatory effects of insulin and amino acids in isolated rat hepatocytes. Eur J Biochem. 2002;269:3742–3750. [DOI] [PubMed] [Google Scholar]
- 35.Larson SD, Li J, Chung DH, et al. Molecular mechanisms contributing to glutamine-mediated intestinal cell survival . Am J Physiol Gastrointest Liver Physiol. 2007;293:G1262–G1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Bacquer O, Nazih H, Blottiere H, et al. Effects of glutamine deprivation on protein synthesis in a model of human enterocytes in culture. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1340–G1347. [DOI] [PubMed] [Google Scholar]
- 37.Matés JM, Segura JA, Alonso FJ, et al. Pathways from glutamine to apoptosis. Front Biosci. 2006;11:3164–3180. [DOI] [PubMed] [Google Scholar]
- 38.Nakajo T, Yamatsuji T, Ban H, et al. Glutamine is a key regulator for amino acid-controlled cell growth through the mTOR signaling pathway in rat intestinal epithelial cells. Biochem Biophys Res Commun. 2005;326:174–180. [DOI] [PubMed] [Google Scholar]
- 39.Naomoto Y, Yamatsuji T, Shigemitsu K, et al. Rational role of amino acids in intestinal epithelial cells (review). Int J Mol Med. 2005;16:201–204. [PubMed] [Google Scholar]
- 40.Nishikawa T, Tomiya T, Ohtomo N, et al. Stimulation by glutamine and proline of HGF production in hepatic stellate cells. Biochem Biophys Res Commun. 2007;363:978–982. [DOI] [PubMed] [Google Scholar]
- 41.Papaconstantinou HT, Chung DH, Zhang W, et al. Prevention of mucosal atrophy: role of glutamine and caspases in apoptosis in intestinal epithelial cells. J Gastrointest Surg. 2000;4:416–423. [DOI] [PubMed] [Google Scholar]
- 42.Rhoads M, Wu G. Glutamine, arginine, and leucine signaling in the intestine. Amino Acids. 2009;37:111–122. [DOI] [PubMed] [Google Scholar]
- 43.Roth E. Immune and cell modulation by amino acids. Clin Nutr. 2007;26:535–544. [DOI] [PubMed] [Google Scholar]
- 44.Roth E. Nonnutritive effects of glutamine. J Nutr. 2008;138:2025S–2031S. [DOI] [PubMed] [Google Scholar]
- 45.Roth E, Oehler R, Manhart N, et al. Regulative potential of glutamine – relation to glutathione metabolism. Nutrition. 2002;18:217–221. [DOI] [PubMed] [Google Scholar]
- 46.Rutten EP, Engelen MP, Wouters EF, et al. Metabolic effects of glutamine and glutamate ingestion in healthy subjects and in persons with chronic obstructive pulmonary disease. Am J Clin Nutr. 2006;83:115–123. [DOI] [PubMed] [Google Scholar]
- 47.van de Poll MC, Ligthart-Melis GC, Boelens PG, et al. Intestinal and hepatic metabolism of glutamine and citrulline in humans. J Physiol. 2007;581(pt 2):819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watford M. Hepatic glutaminase expression – relationship to kidney-type glutaminase and to the urea cycle. FASEB J. 1993;7:1468–1474. [DOI] [PubMed] [Google Scholar]
- 49.Xi P, Jiang Z, Zheng C, et al. Regulation of protein metabolism by glutamine: implications for nutrition and health. Front Biosci. 2011;16:578–597. [DOI] [PubMed] [Google Scholar]
- 50.Ziegler TR, Evans ME, Fernandez-Estivariz C, et al. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr. 2003;23:229–261. [DOI] [PubMed] [Google Scholar]
- 51.Wu G, Wu Z, Dai Z, et al. Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids. 2013;44:1107–1113. [DOI] [PubMed] [Google Scholar]
- 52.van de Poll MCG, Siroen MPC, Van Leeuwen PAM, et al. Interorgan amino acid exchange in humans: consequences for arginine and citrulline metabolism. Am J Clin Nutr. 2007;85:167–172. [DOI] [PubMed] [Google Scholar]
- 53.Fuchs BC, Bode BP. Stressing out over survival: glutamine as an apoptotic modulator. J Surg Res. 2006;131:26–40. [DOI] [PubMed] [Google Scholar]
- 54.Oehler R, Roth E. Regulative capacity of glutamine. Curr Opin Clin Nutr Metab Care. 2003;6:277–282. [DOI] [PubMed] [Google Scholar]
- 55.Meijer AJ, Lorin S, Blommaart EF, et al. Regulation of autophagy by amino acids and MTOR-dependent signal transduction. Amino Acids. 2015;47:2037–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Obled C, Papet I, Breuillé D. Metabolic bases of amino acid requirements in acute diseases. Curr Opin Clin Nutr Metab Care. 2002;5:189–197. [DOI] [PubMed] [Google Scholar]
- 57.Petry ER, Cruzat VF, Heck TG, et al. L-Glutamine supplementations enhance liver glutamine-glutathione axis and heat shock factor-1 expression in endurance-exercise trained rats. Int J Sport Nutr Exerc Metab. 2015;25:188–197. [DOI] [PubMed] [Google Scholar]
- 58.Mates JM, Segura JA, Alonso FJ, et al. Intracellular redox status and oxidative stress: implications for cell proliferation, apoptosis, and carcinogenesis. Arch Toxicol. 2008;82:273–299. [DOI] [PubMed] [Google Scholar]
- 59.Beaufrère AM, Neveux N, Patureau Mirand P, et al. Long-term intermittent glutamine supplementation repairs intestinal damage (structure and functional mass) with advanced age: assessment with plasma citrulline in a rodent model. J Nutr Heath Aging. 2014;18:814–819. [DOI] [PubMed] [Google Scholar]
- 60.Sakiyama T, Musch MW, Ropeleski MJ, et al. Glutamine increases autophagy under Basal and stressed conditions in intestinal epithelial cells. Gastroenterology. 2009;136:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xi P, Jiang Z, Dai Z, et al. Regulation of protein turnover by L-glutamine in porcine intestinal epithelial cells. J Nutr Biochem. 2012;23:1012–1017. [DOI] [PubMed] [Google Scholar]
- 62.Yao K, Yin Y, Li X, et al. Alpha-ketoglutarate inhibits glutamine degradation and enhances protein synthesis in intestinal porcine epithelial cells. Amino Acids. 2012;42:2491–2500. [DOI] [PubMed] [Google Scholar]
- 63.Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011;23:744–755. [DOI] [PubMed] [Google Scholar]
- 64.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mizunuma M, Neumann-Haefelin E, Moroz N, et al. mTORC2-SGK-1 acts in two environmentally responsive pathways with opposing effects on longevity. Aging Cell. 2014;13:869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coeffier M, Claeyssens S, Bole-Feysot C, et al. Enteral delivery of proteins stimulates protein synthesis in human duodenal mucosa in the fed state through a mammalian target of rapamycin-independent pathway. Am J Clin Nutr. 2013;97:286–294. [DOI] [PubMed] [Google Scholar]
- 67.Kalhan SC, Bier DM. Protein and amino acid metabolism in the human newborn. Annu Rev Nutr. 2008;28:389–410. [DOI] [PubMed] [Google Scholar]
- 68.Haussinger D, Schliess F. Glutamine metabolism and signaling in the liver. Front Biosci. 2007;12:371–391. [DOI] [PubMed] [Google Scholar]
- 69.Danner DB, Holbrook NJ. Alterations in gene expression with aging In: Schneider EL, Rowe JW, eds. Handbook of the Biology of Aging. 3rd ed London, UK: Academic Press; 1990:97–115. [Google Scholar]
- 70.Dice JF. Cellular and molecular mechanisms of aging. Physiol Rev. 1993;73:149–159. [DOI] [PubMed] [Google Scholar]
- 71.Evans WJ. Exercise and muscle metabolism in the elderly. In: Hutchinson ML, Munro HN, eds. Nutrition and Aging: London, UK: Academic Press; 1986:179–192. [Google Scholar]
- 72.Evans WJ. What is sarcopenia? J Gerontol A Bio Sci Med Sci. 1995;50A(special issue):5–8. [DOI] [PubMed] [Google Scholar]
- 73.Attaix D, Mosoni L, Dardevet D, et al. Altered responses in skeletal muscle protein turnover during aging in anabolic and catabolic periods. Int J Biochem Cell Biol. 2005;37:1962–1973. [DOI] [PubMed] [Google Scholar]
- 74.Dideriksen K, Reitelseder S, Holm L. Influence of amino acids, dietary protein, and physical activity on muscle mass development in humans. Nutrients. 2013;5:852–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S–1127S. [DOI] [PubMed] [Google Scholar]
- 76.Pedersen AN, Cederholm T. Health effects of protein intake in healthy elderly populations: a systematic literature review. Food Nutr Res. 2014;58 doi:10.3402/fnr.v58.23364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Young VR. Amino acids and proteins in relation to the nutrition of elderly people. Age Ageing. 1990;19:S10–S24. [DOI] [PubMed] [Google Scholar]
- 78.Bergamini E, Cavallini G, Donati A, et al. The role of macroautophagy in the ageing process, anti-ageing intervention and age-associated diseases. Int J Biochem Cell Biol. 2004;36:2392–2404. [DOI] [PubMed] [Google Scholar]
- 79.Codogno P, Meijer AJ. Autophagy: a potential link between obesity and insulin resistance. Cell Metab. 2010;11:449–451. [DOI] [PubMed] [Google Scholar]
- 80.Salminen A, Kaarniranta K. Regulation of the aging process by autophagy. Trends Mol Med. 2009;15:217–224. [DOI] [PubMed] [Google Scholar]
- 81.Fujita S, Glynn EL, Timmerman KL, et al. Supraphysiological hyperinsulinaemia is necessary to stimulate skeletal muscle protein anabolism in older adults: evidence of a true age-related insulin resistance of muscle protein metabolism. Diabetologia. 2009;52:1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cavallini G, Donati A, Bergamini E. Antiaging therapy: a novel target for antilipolytic drugs. Mini Rev Med Chem. 2014;14:551–556. [DOI] [PubMed] [Google Scholar]
- 83.Kitada M, Koya D. The use of calorie restriction mimetics to study aging. Methods Mol Biol. 2013;1048:95–107. [DOI] [PubMed] [Google Scholar]
- 84.Marino G, Pietrocola F, Madeo F, et al. Caloric restriction mimetics: natural/physiological pharmacological autophagy inducers. Autophagy. 2014;10:1879–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duran RV, Hall MN. Glutaminolysis feeds mTORC1. Cell Cycle. 2012;11:4107–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duran RV, Oppliger W, Robitaille AM, et al. Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell. 2012;47:349–358. [DOI] [PubMed] [Google Scholar]
- 87.Choudry HA, Pan M, Karinch AM, et al. Branched-chain amino acid-enriched nutritional support in surgical and cancer patients. J Nutr. 2006;136(1 suppl):314S–318S. [DOI] [PubMed] [Google Scholar]
- 88.Kimball SR, Jefferson LS. Control of protein synthesis by amino acid availability. Curr Opin Clin Nutr Metab Care. 2002;5:63–67. [DOI] [PubMed] [Google Scholar]
- 89.Le Bacquer O, Mauras N, Welch S, et al. Acute depletion of plasma glutamine increases leucine oxidation in prednisone-treated humans. Clin Nutr. 2007;26:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Darmaun D, Welch S, Rini A, et al. Phenylbutyrate-induced glutamine depletion in humans: effect on leucine metabolism. Am J Physiol Endocrinol Metabol. 1998;274(5 pt 1):E801–E807. [DOI] [PubMed] [Google Scholar]
- 91.Meynial-Denis D, Verdier L, Mignon M, et al. Does acute glutamine depletion enhance the response of glutamine synthesis to fasting in muscle in adult and old rats? Clin Nutr. 2005;24:398–406. [DOI] [PubMed] [Google Scholar]
- 92.Munro HN. Adaptation of body protein metabolism in adult and aging man. Clin Nutr. 1982;1:95–108. [DOI] [PubMed] [Google Scholar]
- 93.Dillon EL. Nutritionally essential amino acids and metabolic signaling in aging. Amino Acids. 2013;45:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fry CS, Drummond MJ, Glynn EL, et al. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle. 2011;1:11 doi:10.1186/2044-5040-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sakuma K, Aoi W, Yamaguchi A. The intriguing regulators of muscle mass in sarcopenia and muscular dystrophy. Front Aging Neurosci. 2014;6:230 doi:10.3389/fnagi.2014.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sandri M, Barberi L, Bijlsma AY, et al. Signalling pathways regulating muscle mass in ageing skeletal muscle: the role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology. 2013;14:303–323. [DOI] [PubMed] [Google Scholar]
- 97.Schiaffino S, Dyar KA, Ciciliot S, et al. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280:4294–4314. [DOI] [PubMed] [Google Scholar]
- 98.Sakuma K, Aoi W, Yamaguchi A. Current understanding of sarcopenia: possible candidates modulating muscle mass. Pflugers Arch. 2015;467:213–229. [DOI] [PubMed] [Google Scholar]
- 99.Mosoni L, Patureau Mirand P, Houlier ML, et al. Age-related changes in protein synthesis measured in vivo in rat liver and gastrocnemius muscle. Mech Ageing Dev. 1993;68:209–220. [DOI] [PubMed] [Google Scholar]
- 100.Casperson SL, Sheffield-Moore M, Hewlings SJ, et al. Leucine supplementation chronically improves muscle protein synthesis in older adults consuming the RDA for protein. Clin Nutr. 2012;31:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koopman R, Verdijk LB, Beelen M, et al. Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Br J Nutr. 2008;99:571–580. [DOI] [PubMed] [Google Scholar]
- 102.Magne H, Savary-Auzeloux I, Remond D, et al. Nutritional strategies to counteract muscle atrophy caused by disuse and to improve recovery. Nutr Res Rev. 2013;26:149–165. [DOI] [PubMed] [Google Scholar]
- 103.Dillon EL, Sheffield-Moore M, Paddon-Jones D, et al. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J Clin Endocrinol Metab. 2009;94:1630–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ferrando AA, Paddon-Jones D, Hays NP, et al. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr. 2010;29:18–23. [DOI] [PubMed] [Google Scholar]
- 105.Mignon M, Beaufrere AM, Combaret L, et al. Does long-term intermittent treatment with glutamine improve the well-being of fed and fasted very old rats? JPEN J Parenter Enteral Nutr. 2007;31:456–462. [DOI] [PubMed] [Google Scholar]
- 106.Evans WJ, Boccardi V, Paolisso G. Perspective: dietary protein needs of elderly people: protein supplementation as an effective strategy to counteract sarcopenia. J Am Med Dir Assoc. 2013;14:67–69. [DOI] [PubMed] [Google Scholar]
- 107.Tieland M, Dirks ML, van der Zwaluw N, et al. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13:713–719. [DOI] [PubMed] [Google Scholar]
- 108.Walker DK, Dickinson JM, Timmerman KL, et al. Exercise, amino acids, and aging in the control of human muscle protein synthesis. Med Sci Sports Exerc. 2011;43:2249–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dröge W. Oxidative stress and ageing: is ageing a cysteine deficiency syndrome? Philos Trans R Soc Lond B Biol Sci. 2005;360:2355–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Papet I, Dardevet D, Sornet C, et al. Acute phase protein levels and thymus, spleen and plasma protein synthesis rates differ in adult and old rats. J Nutr. 2003;133:215–219. [DOI] [PubMed] [Google Scholar]
- 111.Nicastro H, da Luz CR, Chaves DF, et al. Does branched-chain amino acids supplementation modulate skeletal muscle remodeling through inflammation modulation? Possible mechanisms of action. J Nutr Metab. 2012;2012:136937 doi:10.1155/2012/136937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jourdan M, Deutz NE, Cynober L, et al. Consequences of age-related splanchnic sequestration of leucine on interorgan glutamine metabolism in old rats. J Appl Physiol. 2013;115:229–234. [DOI] [PubMed] [Google Scholar]
- 113.Biolo G. Protein metabolism and requirements. World Rev Nutr Diet. 2013;105:12–20. [DOI] [PubMed] [Google Scholar]
- 114.Tessari P. Changes in protein, carbohydrate, and fat metabolism with aging: possible role of insulin. Nutr Rev. 2000;58:11–19. [DOI] [PubMed] [Google Scholar]
- 115.Agostini F, Biolo G. Effect of physical activity on glutamine metabolism. Curr Opin Clin Nutr Metab Care. 2010;13:58–64. [DOI] [PubMed] [Google Scholar]
- 116.Stuerenburg HJ, Stangneth B, Schoser BG. Age related profiles of free amino acids in human skeletal muscle. Neuroendocrinol Lett. 2006;27:133–136. [PubMed] [Google Scholar]
- 117.Pinel C, Coxam V, Mignon M, et al. Alterations in glutamine synthetase activity in rat skeletal muscle are associated with advanced age. Nutrition. 2006;22:778–785. [DOI] [PubMed] [Google Scholar]
- 118.Mezzarobba V, Torrent A, Leydier I, et al. The role of adrenal hormones in the response of glutamine synthetase to fasting in adult and old rats. Clin Nutr. 2003;22:569–575. [DOI] [PubMed] [Google Scholar]
- 119.Hwang JH, Choi CS. Use of in vivo magnetic resonance spectroscopy for studying metabolic diseases. Exp Mol Med. 2015;47:e139 doi:10.1038/emm.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meynial-Denis D, Mignon M, Miri A, et al. Glutamine synthetase induction by glucocorticoids is preserved in skeletal muscle of aged rats. Am J Physiol Endocrinol Metabol. 1996;271:E1061–E1066. [DOI] [PubMed] [Google Scholar]
- 121.Verdier L, Boirie Y, Van Drieesche S, et al. Do sex steroids regulate glutamine synthesis with age? Am J Physiol Endocrinol Metabol. 2002;282:E215–E221. [DOI] [PubMed] [Google Scholar]
- 122.Brosnan JT, Ewart HS, Squires SA, et al. Hormonal and dietary control of hepatic glutamine catabolism. Contrib Nephrol. 1994:109–114. [DOI] [PubMed] [Google Scholar]
- 123.Curthoys NP, Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr. 1995;15:133–159. [DOI] [PubMed] [Google Scholar]
- 124.Gebhardt R, Baldysiak-Figiel A, Krugel V, et al. Hepatocellular expression of glutamine synthetase: an indicator of morphogen actions as master regulators of zonation in adult liver. Prog Histochem Cytochem. 2007;41:201–266. [DOI] [PubMed] [Google Scholar]
- 125.Lopez HW, Moundras C, Morand C, et al. Opposite fluxes of glutamine and alanine in the splanchnic area are an efficient mechanism for nitrogen sparing in rats. J Nutr. 1998;128:1487–1494. [DOI] [PubMed] [Google Scholar]
- 126.Watford M, Chellaraj V, Ismat A, et al. Hepatic glutamine metabolism. Nutrition. 2002;18:301–303. [DOI] [PubMed] [Google Scholar]
- 127.Lie-Venema H, Labruyère WT, Van Roon MA, et al. The spatio-temporal control of the expression of glutamine synthetase in the liver is mediated by its 5'-enhancer. J Biol Chem. 1995;270:28251–28256. [DOI] [PubMed] [Google Scholar]
- 128.Sahakian JA, Szweda LI, Friguet B, et al. Aging of the liver: proteolysis of oxidatively modified glutamine synthetase. Arch Biochem Biophys. 1995;318:411–417. [DOI] [PubMed] [Google Scholar]
- 129.Mouchard ML, Bes S, Mignon M, et al. Fasting up-regulates muscle glutamine synthetase while it down-regulates liver glutamine synthetase in male rats during aging [published online September 17, 2008]. e-SPEN. 2008;3:309–315. doi:10.1016/j.eclnm.2008.07.019. [Google Scholar]
- 130.Spindler SR. Calorie restriction enhances the expression of key metabolic enzymes associated with protein renewal during aging. Ann N Y Acad Sci. 2001;928:296–304. [DOI] [PubMed] [Google Scholar]
- 131.Patel BB, Yu Y, Du J, et al. Schlafen 3, a novel gene, regulates colonic mucosal growth during aging [published online Feburary 19, 2009]. Am J Physiol Gastrointest Liver Physiol. 2009;296:G955–G962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fleming SE, Kight CE. The TCA cycle as an oxidative and synthetic pathway is suppressed with aging in jejunal epithelial cells. Can J Physiol Pharmacol. 1994;72:266–274. [DOI] [PubMed] [Google Scholar]
- 133.Crenn P, Hanachi M, Neveux N, et al. La citrullinémie un biomarqueur de la fonctionnalité intestinale [Circulating citrulline levels: a biomarker for intestinal functionality assessment]. Annale de Biologie Clinique. 2011;69:513–521. [DOI] [PubMed] [Google Scholar]
- 134.Curis E, Nicolis I, Moinard C, et al. Almost all about citrulline in mammals. Amino Acids. 2005;29:177–205. [DOI] [PubMed] [Google Scholar]
- 135.Fujita T, Yanaga K. Association between glutamine extraction and release of citrulline and glycine by the human small intestine. Life Sci. 2007;80:1846–1850. [DOI] [PubMed] [Google Scholar]
- 136.Breuillard CL, Cynober L, Moinard C. Citrulline and nitrogen homeostasis: an overview. Amino Acids. 2015;47:685–691. [DOI] [PubMed] [Google Scholar]
- 137.Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008;27:328–339. [DOI] [PubMed] [Google Scholar]
- 138.Deutz NEP. The 2007 ESPEN Sir David Cuthbertson Lecture: amino acids between and within organs. The glutamate-glutamine-citrulline-arginine pathway. Clin Nutr. 2008;27:321–327. [DOI] [PubMed] [Google Scholar]
- 139.Wang B, Wu G, Zhou Z, et al. Glutamine and intestinal barrier function. Amino Acids. 2015;47:2143–2154. [DOI] [PubMed] [Google Scholar]
- 140.Unterluggauer H, Mazurek S, Lener B, et al. Premature senescence of human endothelial cells induced by inhibition of glutaminase. Biogerontology. 2008;9:247–259. [DOI] [PubMed] [Google Scholar]
- 141.Hornsby PJ. Senescence and life span. Pflugers Arch. 2010;459:291–299. [DOI] [PubMed] [Google Scholar]
- 142.Shen J, Tower J. Programmed cell death and apoptosis in aging and life span regulation. Discov Med. 2009;8:223–226. [PubMed] [Google Scholar]
- 143.Mignon M, Leveque L, Bonnel E, et al. Does glutamine supplementation decrease the response of muscle glutamine synthesis to fasting in muscle in adult and very old rats? JPEN J Parenter Enteral Nutr. 2007;31:26–31. [DOI] [PubMed] [Google Scholar]
- 144.Neu J, DeMarco V, Li N. Glutamine: clinical applications and mechanisms of action. Curr Opin Clin Nutr Metab Care. 2002;5:69–75. [DOI] [PubMed] [Google Scholar]
- 145.Meynial-Denis D, Bielicki G, Beaufrere AM, et al. Glutamate and CO2 production from glutamine in incubated enterocytes of adult and very old rats [published online August 14, 2012]. J Nutr Biochem. 2013;24:688–692. [DOI] [PubMed] [Google Scholar]
- 146.Lowe DK, Benfell K, Smith RJ, et al. Safety of glutamine-enriched parenteral nutrient solutions in humans. Am J Clin Nutr. 1990;52:1101–1106. [DOI] [PubMed] [Google Scholar]
- 147.Williams JZ, Abumrad N, Barbul A. Effect of a specialized amino acid mixture on human collagen deposition. Ann Surg. 2002;236:369–374; discussion 374–375. [DOI] [PMC free article] [PubMed] [Google Scholar]