Abstract
Brassica vegetables are common components of the diet and have beneficial as well as potentially adverse health effects. Following enzymatic breakdown, some glucosinolates in brassica vegetables produce sulforaphane, phenethyl, and indolylic isothiocyanates that possess anticarcinogenic activity. In contrast, progoitrin and indolylic glucosinolates degrade to goitrin and thiocyanate, respectively, and may decrease thyroid hormone production. Radioiodine uptake to the thyroid is inhibited by 194 μmol of goitrin, but not by 77 μmol of goitrin. Collards, Brussels sprouts, and some Russian kale (Brassica napus) contain sufficient goitrin to potentially decrease iodine uptake by the thyroid. However, turnip tops, commercial broccoli, broccoli rabe, and kale belonging to Brassica oleracae contain less than 10 μmol of goitrin per 100-g serving and can be considered of minimal risk. Using sulforaphane plasma levels following glucoraphanin ingestion as a surrogate for thiocyanate plasma concentrations after indole glucosinolate ingestion, the maximum thiocyanate contribution from indole glucosinolate degradation is estimated to be 10 μM, which is significantly lower than background plasma thiocyanate concentrations (40–69 μM). Thiocyanate generated from consumption of indole glucosinolate can be assumed to have minimal adverse risks for thyroid health.
Keywords: broccoli, Chinese cabbage, glucosinolate, glucoraphanin, indole, kale, phase II enzymes
INTRODUCTION
Dietary intake of vegetables from the genus Brassica, which includes broccoli, broccoli rabe, kale, turnip, Brussels sprouts, Chinese cabbage, and cauliflower, has been associated with various health-promoting effects1 that are attributed to the more than 100 diverse β-thioglucoside-N-hydroxysulfates, termed glucosinolates.2,3 When these glucosinolates are acted upon by the enzyme myrosinase from adjacent plant cells, typically after mastication, they form isothiocyanates, some of which immediately break down to release a permanently charged thiocyanate ion. Extensive reviews of enzymatic products of glucosinolate breakdown by myrosinase and epithiospecifier protein and of nonenzymatic breakdown of glucosinolates into the major classes of isothiocyanates, thiocyanates, nitriles, indole-3-carbinol, various diindolylmethane dimers, etc., have been conducted previously.2–4
Through their pioneering work in the early 1990s, the research groups of Talalay and Zhang5,6 identified broccoli as having significant anticarcinogenic activity and found the compound responsible for this activity to be glucoraphanin, a glucosinolate.6 Using cell lines and animal studies, they demonstrated that the enzymatic degradation product of glucoraphanin, sulforaphane, has significant anticarcinogenic activity.2 Sulforaphane has been described as “the most potent inducer of phase II enzymes identified to date”7 and has shown benefits in alleviating multiple chronic conditions, including allergic respiratory inflammation from oxidant stimuli in the upper airway caused by asthma or air pollutants.7 It has also been associated with decreased risk of various cardiovascular diseases,8 lung cancer,9 prostate cancer,10 urinary cancer,11 and colon cancer.12 In addition to sulforaphane, other products of myrosinase-induced degradation of glucosinolate have been demonstrated to have significant anticarcinogenic benefits. Such compounds include low-molecular-weight aliphatic isothiocyanates,13 phenethyl isothiocyanate,14,15 and two products of indole glucosinolate degradation, indole-3-carbinol and diindolylmethane.16,17
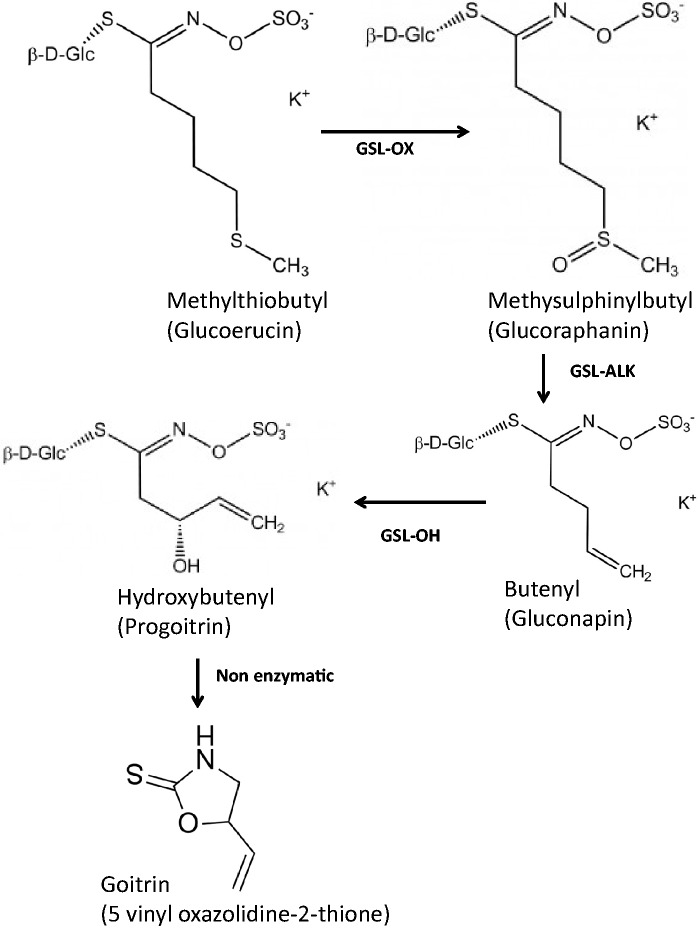
In contrast to these beneficial effects of glucosinolates, there is evidence (primarily from animal studies) of adverse effects on the thyroid caused by the glucosinolate progoitrin, from which the product of myrosinase-induced degradation is goitrin (Figure 1).18–21 Iodine is a dietary micronutrient required for the production of thyroid hormone, and the potential adverse effects of goitrin and thiocyanates are based on their ability to inhibit iodine utilization by the thyroid. Despite the development of high-performance liquid chromatography−mass spectrometry methods for measuring goitrin and other products of glucosinolate hydrolysis in plasma,22 there are no data on concentrations of goitrin in human plasma following the ingestion of brassica vegetables, which could be helpful in establishing nutritional safety guidelines. One study assessed the change in radioactive iodine uptake by the thyroid glands of human subjects following the administration of recrystallized goitrin.23 The authors reported that 25 mg (194 μmol) was the minimal amount of goitrin required to decrease the uptake of radioiodine; in contrast, a smaller ingested amount, 10 mg (70 μmol), caused no inhibition of uptake.
Figure 1.
Pathway for conversion of methylthiobutyl glucosinolate to goitrin. The enzymes glucosinolate oxidase (NCBI accession no. J3760740) (GSL-OX), glucosinolate alkenylation (NCBI accession no. EF611253) (GSL-ALK), and glucosinolate hydroxylation (NCBI accession no. FJ376074) (GSL-OH) and the nonenzymatic reaction are indicated below or to the side of the arrows.
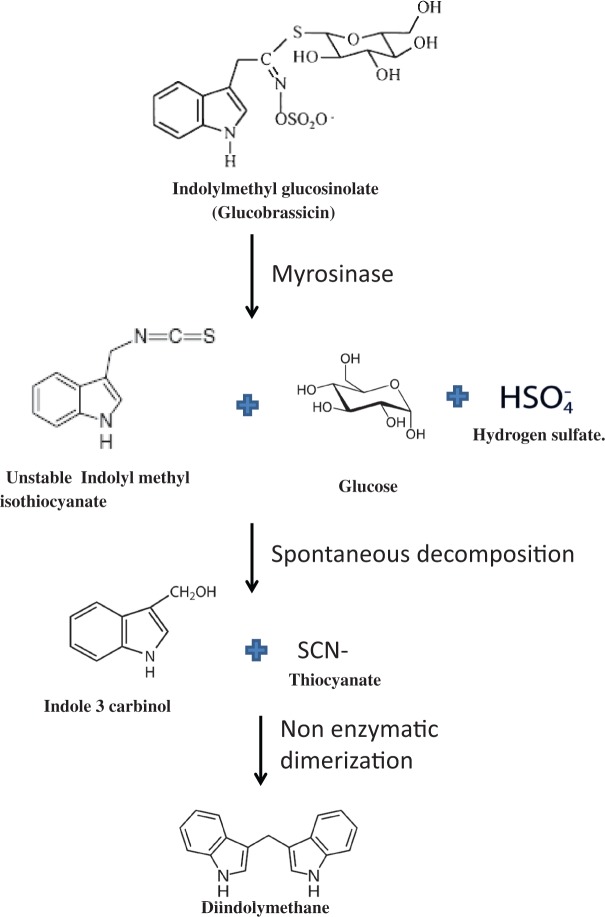
Additional adverse effects on the thyroid have been suggested to result from the thiocyanate ion that is produced by myrosinase-induced degradation of indole glucosinolates (Figure 2).4 The thiocyanate ion is a competitive inhibitor of the sodium/iodide symporter located on the basolateral membrane of the thyroid follicular cell.24,25 Thus, exposure to thiocyanate can reduce iodide uptake by the thyroid gland26 and has the potential to result in decreased synthesis of thyroid hormone (hypothyroidism). The thiocyanate ion also occurs naturally in humans, in whom its oxidation by peroxidase in saliva constitutes part of the innate defense in the oral cavity.27 Hurst28 has reviewed the chemistry of thiocyanate oxidation by myeloperoxidase to enhance the bactericidal effect of phagocytic killing by neutrophils. One study examined the addition of thiocyanate to raw cow’s milk, since stimulation of oxidation of exogenous thiocyanate to the short-lived hypothiocyanite anion (OSCN−) by lactoperoxidase from milk has bactericidal effects, assisting in milk preservation.29 To test whether these thiocyanate concentrations could have an effect on thyroid function in humans, Dahlberg et al.29 added 8 mg of thiocyanate each day to the milk consumed by human volunteers and reported no effect on serum thyroxine, triiodothyronine, or thyrotropin concentrations after 12 weeks. Serum thiocyanate concentrations were 69 μM and 121 μM before and after milk ingestion, respectively, among nonsmokers after the 12-week study period.30 These data confirm other reports demonstrating serum thiocyanate concentrations among nonsmokers,30,31 as thiocyanate is also produced from cigarette smoke as a metabolite of cyanide.
Figure 2.
Pathway for the enzymatic and nonenzymatic degradation of indole glucosinolates, which results in production of thiocyanate and the anticarcinogenic compounds indole-3-carbinol and diindolylmethane. Abbreviations: , hydrogen sulfate; SCN−, thiocyanate.
From a plant-breeding perspective, it is important to understand if it would be useful to breed for enhanced levels of indole glucosinolates in brassica vegetables to maximize the anticarcinogenic benefits described above. Alternatively if the thiocyanate concentrations resulting from myrosinase degradation of indoles are detrimental to thyroid function, plant breeders should seek to decrease indole glucosinolate concentrations in brassica vegetables. There is extensive literature on the effects of high goitrin concentrations in Brassica napus seed meal fed to animals (as a byproduct of oil production) and the breeding of low-goitrin lines for use in animal feed.19 Since synthesis of the glucosinolate responsible for goitrin is only a 2-step enzymatic process from synthesis of the highly beneficial glucoraphanin that yields sulforaphane,32 it is important to identify the health risks and benefits of glucosinolate ingestion. Accordingly, it would be desirable to have brassica vegetables with high glucoraphanin concentrations and the lowest possible progoitrin concentrations.
The genetics of the synthesis of glucosinolates in general33 and, specifically, of the enzymes responsible for synthesis of the positive and negative health attributes described above (i.e., indole glucosinolates, glucoraphanin, and progoitrin)32,34,35 have been well studied. Natural variation in these synthetic enzymes in the major commercial hybridizing Brassica species (i.e., Brassica oleracae, B. napus, and Brassica rapa) has resulted in considerable variation in progoitrin and indole glucosinolate concentrations in common brassica vegetables, suggesting that breeding studies could produce more healthful cultivars. However, Agerbirk et al.4 have suggested that, despite the benefits of the indole glucosinolates indole-3-carbinol and diindolylmethane, high concentrations of indole glucosinolates might have adverse effects.
In contrast to considerable research on broccoli (B. oleracae) and glucoraphanin, much less work has been done on B. rapa, which is widely consumed in China as bok choy and Chinese cabbage, in Italy as Cima di rapa, and in the United States as broccoli rabe. Chinese studies show that bok choy (B. rapa) is the most widely consumed brassica vegetable in China and that brassica vegetable consumption, as measured by urinary isothiocyanate concentrations, is associated with a significant reduction in breast cancer risk among Chinese women.36,37
This review aims to summarize the concentrations of goitrin found in various brassica vegetables, to assess human exposure to dietary goitrogens as measured by plasma goitrin and thiocyanate concentrations, and to outline the potential health benefits and adverse effects of brassica vegetable ingestion.
LITERATURE SEARCH
In this narrative review, a systematic search strategy of the Web of Science and Google Scholar databases for articles published from 1950 to 2014 was employed, using combinations of the search terms “indole glucosinolate,” “progoitrin,” “goitrin,” “plasma thiocyanate,” and “thyroid,” to include all studies that measured goitrin concentrations. Units were converted to micromoles per 100 grams of fresh weight to compare measured values from the published papers. If dry matter percentages were provided in the papers, they were used to convert dry weights to fresh weights, and if no moisture content was available, a dry matter content of 13.6% was used, since this was found to be typical of the ratio of fresh to lyophilized brassica vegetables (P.F. and R.B, unpublished data, 2012).
In many cases, the maturity of the plant tissues was not provided. Yang and Quiros32 only analyzed leaves from 6-week-old greenhouse plants. The leaves and floral heads from the D’Arrigo Bros. Co. were obtained from 4- to 6-week-old field-grown plants that were probably larger because of greater soil volume.
In this review, the species designation of the vegetables is as reported in the original articles. It should be noted that the Latin binomial Brassica campestris, a diploid with 2n=20, has been replaced by B. rapa. B. oleracae is also a diploid, with 2n=18, but B. napus is an amphidipoid, with 4n=38, probably arising from an ancient combination of the B. oleracae and B. rapa genomes.38
Glucosinolate concentrations in brassica vegetables
Some of the glucosinolates examined here were measured from plants that were seeded in D’Arrigo Bros. fields, harvested, stored for no more than several days under refrigeration as is done for commercial crops, and analyzed using large sample sizes of fresh material (2–4 g). The glucosinolate analyses were performed using an in-house proprietary method based on the intact glucosinolate method of Wade et al.,39 which utilizes a hydrophilic interaction liquid chromatography column. These modifications were made for several reasons: to shorten the processing time and avoid possible indole degradation, to eliminate interfering phenolic compounds by anion exchange pretreatment, to obtain separation of 13 peaks, and to increase throughput. As the B. rapa samples did not contain sinigrin, this glucosinolate was used as an internal standard after measuring the concentration using the molar extinction coefficient of twice-crystallized sinigrin40 and after calibrating the spectrophotometer with the alkaline dichromate solution of Haupt.41 The relative absorption coefficient of sinigrin at 229 was taken from Agerbirk et al.42 Using the fragmentation patterns described by Clarke,3 the identity of the peaks was confirmed using high-performance liquid chromatography–mass spectrometry at the University of California Davis Genome Center and at the Stanford University Vincent Coates Foundation Mass Spectrometry Laboratory.
Estimation of human plasma thiocyanate concentrations derived from brassica vegetables
Population data from the United States have shown urinary thiocyanate concentrations resulting from low-level environmental exposure43 but have not been systemically evaluated on a similar scale for thiocyanate levels in plasma. As thiocyanate concentrations in human plasma derived from the action of myrosinase on indole glucosinolates have not been reported in the literature, the ratio of the glucoraphanin ingested to the sulforaphane plasma concentration was used as a surrogate, taking into account that 1 μmol of indole glucosinolate can produce no more than 1 μmol of thiocyanate.42 As further support for the use of the glucoraphanin to sulforaphane ratio as a surrogate for indole glucosinolate to thiocyanate ratios, the demonstration by Cramer and Jeffery44 that 74% of the ingested sulforaphane could be accounted for in the urine in 24 hours as N-acetyl-cysteine, (which results from the conjugation of sulforaphane with glutathione), implies that, while the thiocyanate to indole glucosinolate ratio could be lower than the sulforaphane to glucoraphanin ratio, it could not be more than 26% higher.
Indole glucosinolate concentrations in brassica vegetables that produce the thiocyanate ion upon mastication
There are 4 indole glucosinolates that could produce thiocyanates by the action of myrosinase: (1) the unsubstituted indole glucosinolate known as glucobrassicin; (2) an indole glucosinolate with a methoxy group on the N in the heterocycle, known as neoglucobrassicin; (3) an indole glucosinolate with a methoxy group on the 4 position of the 6-membered ring, known as 4-methoxy-glucobrassicin; and (4) an indole glucosinolate with a hydroxyl group on the 4 position of the 6-membered ring, known as 4-hydroxyglucobrassicin. Table 1 22,32,45–52 summarizes the data available for these indole glucosinolates.
Table 1.
Glucosinolate concentrations in brassica vegetables
| Species | Common name | Tissue type | No. tested | Mean concentration (range) in µmol/100 g of fresh weight |
Reference/source | |||
|---|---|---|---|---|---|---|---|---|
| Glucoraphanin (may provide anticancer benefits) | Thiocyanate-producing indole glucosinolates (may have adverse thyroidal effects) | Progoitrin (may have adverse thyroidal effects) | Total glucosinolates | |||||
| Brassica rapa | Napo (turnip) | Flower buds and leaves | 20 | 0 | 17 (5–32) | 1.49 (0–5) | 489 (63–1157) | Rosa (1997)47 |
| B. rapa ssp rapa | Turnip tops | Leaves | 45 | 0 | 9 (1–26) | 9.96 (1–72) | 400 (133–644) | Cartea et al (2012)48 |
| B. rapa | Turnip | Young leaves | 18 | 0 | 5 | 0 | 533 | Yang & Quiros (2010)32 |
| Brassica rapa L. ssp pekinensis | Chinese cabbage | Leaves | 62 | 0 | 28 (7–72) | 20.44 (8–78) | 194 (39–662) | Lee et al. (2014)49 |
| B. rapa | Chinese cabbage | Young leaves | 39 | 0 | 27 (5–139) | 2.24 (0−20) | 42 (6−254) | Yang & Quiros (2010)32 |
| Brassica campestris L. ssp chinensis var tai-tsai Hort. | Tai tsai | Leaves | 1 | 0 | 65 | 31.17 | 175 | He et al. (2003)50 |
| Brassica campestris L. ssp chinensis var communis Tsen et Lee | Pak choi | Leaves | 1 | 0 | 37 | 2.18 | 85 | He et al. (2003)50 |
| B. rapa | Broccoletto | Young leaves | 2 | 0 | 16 (15–17) | 0.90 (0.80–1.0) | 151 (145–157) | Yang & Quiros (2010)32 |
| B. rapa | Japanese green | Young leaves | 1 | 0 | 19 | 2 | 68 | Yang & Quiros (2010)32 |
| B. rapa | Oriental green | Young leaves | 1 | 0 | 2 | 0.09 | 19 | Yang & Quiros (2010)32 |
| B. rapa | Pak choi | Young leaves | 3 | 16 | 21 (9–6.77) | 8 (0–4) | 56 (43–77) | Yang & Quiros (2010)32 |
| B. rapa | Chiifu | Young leaves | 1 | 0 | 68 | 37 | 135 | D’Arrigo Bros. |
| B. rapa | Chiifu | Floral heads | 1 | 5 | 99 | 68 | 256 | D’Arrigo Bros. |
| B. rapa | Tsoi-sim | Young leaves | 1 | 1 | 72 | 17.22 | 177 | D’Arrigo Bros. |
| B. rapa | Tsoi-sim | Floral heads | 1 | 2 | 134 | 23.78 | 328 | D’Arrigo Bros. |
| B. rapa | Choho | Young leaves | 1 | 1 | 149 | 2.36 | 209 | D’Arrigo Bros. |
| B. rapa | Broccoli rabe (commercial varieties) | Young leaves | 10 | 1 (0–6) | 83 (53–118) | 3.27 (0–7) | 601 (853–317) | D’Arrigo Bros. |
| B. rapa | Broccoli rabe (commercial varieties) | Floral heads | 10 | 13 (2–42) | 187 (115–208) | 4.55 (2–10) | 809 (649–1003) | D’Arrigo Bros. |
| B. rapa | European (Cima de rapa varieties) | Young leaves | 11 | 0 (0–1) | 79 (40–136) | 1.45 (0–3) | 526 (303–820) | D’Arrigo Bros. |
| B. rapa | European (Cima de rapa varieties) | Floral heads | 11 | 3 (0–9) | 122 (63–181) | 1.39 (0–5) | 674 (480–888) | D’Arrigo Bros. |
| Brassica oleracea | Broccoli | Heads | 6 | 64 (30–88) | 59 (42–72) | 0.87 (0–4) | 188 (102–263) | Carlson et al. (1987)51 |
| B. oleracea | Broccoli | Heads | 50 | 96 (11–296) | 26 (5–85) | 13.68 (0–108) | 178 (35–486) | Kushad et al. (1999)52 |
| B. oleracea | Broccoli (Bravado cultivar) | Heads | 1 | 97 | 61 | 0 | 159 | D’Arrigo Bros. |
| B. oleracea | Broccoli | Heads | 105 | 0.09 | Tian et al. (2005)53 | |||
| B. oleracea | Broccoli | Heads | 30 | 3.90 | Song et al. (2005)22 | |||
| Brassica alboglabra Bailey | Chinese kale | Leaves | 1 | 127 | 28.63 | 119 | 421 | He et al. (2003)50 |
| Brassica oleracea L. convar acephala var sabellica | Kale | Leaves | 1 | 270 | 6.77 | 2 | 429 | He et al. (2003)50 |
| B. oleracea var acephala | Galega kale | Flower buds and leaves | 20 | 117 (49–211) | 16.11 (2–60) | 0 | 468 (353–672) | Rosa (1997)47 |
| Kale | Leaves | 4 | 55 (44–70) | 5.90 (0–23) | 4 (0–8) | 220 (64–307) | Carlson et al. (1987)51 | |
| B. oleracea L. var acephala | Kale | Leaves | 1 | 274 | 7 | 5 | 584 | Ku et al. (2014)54 |
| B. oleracea | Kale | Leaves | 5 | 42 (15–62) | 840 (446–1172) | 0 | 884 (478–884) | D’Arrigo Bros. |
| Kale (Siberian) | Leaves | 1 | 29 | 102 | 158.1 | 694 | Carlson et al. (1987)51 | |
| Brassica napus ssp pabularia | Kale (Russian) | Leaves | 1 | 53 | 202 | 365.9 | 667 | Ku et al. (2014)54 |
| B. napus | Kale | Leaves | 3 | 55 (3–118) | 465 (216–803) | 176.6 (29–368) | 828 (330–1241) | D’Arrigo Bros. |
| B. oleracea convar acephala var medullosa Thell | Collards | Leaves | 1 | 3 | 198 | 314.16 | 731 | He et al. (2003)50 |
| B. oleracea | Collards | Leaves | 5 | 5 (0–13) | 108 (67–165) | 70.08 (17–130) | 439 (316–600) | Carlson et al. (1987)51 |
| B. oleracea | Brussels sprouts | Button | 6 | 8 (0–23) | 392 (328–469) | 8.33 (1–25) | 553 (466–601) | Carlson et al. (1987)51 |
| B. oleracea | Brussels sprouts | Heads | 4 | 13 (5–19) | 84 (63–108) | 42.66 (15–100) | 329 (227–504) | Kushad et al. (1999)52 |
| B. oleracea | Brussels sprouts | Heads | 0.6 | 2.40 | Song et al. (2005)22 | |||
| B. oleracea | Brussels sprouts | Heads | 15 | 133.0 | Tian et al. (2005)53 | |||
The brassica vegetables with the highest total indole glucosinolate concentrations are kale (B. oleracae leaves; 840 μmol/100 g), kale (B. napus leaves; 465 μmol/100 g), and Brussels sprouts (392 μmol/100 g). Those with intermediate concentrations are B. rapa tsoi-sim heads (134 μmol/100 g), choho (149 μmol/100 g), broccoli rabe commercial varieties (187 μmol/100 g), and broccoli heads (188 μmol/100 g). The brassica vegetables with the lowest values are Chinese cabbages, turnips, pak choi, and Chinese greens, which contain glucosinolate concentrations that range from 5 to 99 μmol/100 g of fresh weight.
The two species of kale sold commercially (i.e., kale of the species B. oleracae and Russian/Siberian kale of the species B. napus) contain markedly different glucosinolate profiles. The brassica with the highest total indole glucosinolate concentration was B. oleracae kale (840 μmol/100 g), which did not contain any progoitrin, but the B. napus kale (465 μmol/100 g) had a progoitrin concentration of 176 μmol/100 g, putting it into the category of potential risk for thyroid damage.23
Progoitrin concentrations in brassica vegetables that produce goitrin upon mastication
Based on the measurements by Langer et al.,23 who investigated goitrin doses that impaired thyroidal iodine uptake (i.e., 70 μmol was associated with no inhibition, whereas 194 μmol inhibited iodine uptake), the brassica vegetables were categorized into 3 classes: (1) those with progoitrin concentrations over 100 μmol/100 g of fresh weight [i.e., the Siberian (Russian) kales belonging to the species B. napus, one collard (B. oleracae), and one Brussels sprout (B. oleracae)]; (2) those with goitrin concentrations between 10 and 100 μmol/100 g of fresh weight [i.e., Chinese cabbages (B. rapa), some collards, and Brussels sprouts]; and (3) those with goitrin concentrations less than 10 μmol/100 g of fresh weight [i.e., turnip tops, some Chinese and Japanese greens, commercial broccoli rabe (B. rapa), commercial broccoli (B. oleracae) varieties, and the kales belonging to the species B. oleracae].
Glucoraphanin concentrations that produce beneficial effects
Glucoraphanin was reported in large concentrations only in the B. oleracae species or in B. napus that includes the B. oleracae genome. None of the B. rapa varieties have large quantities of glucoraphanin; B. rapa Chiifu and some B. rapa broccoli rabe varieties have small levels of glucoraphanin, concentrated only in the head.
The anticarcinogenic benefits of sulforaphane, phenethyl isothiocyanate, and myrosinase-induced degradation products of indole glucosinolates can be maximized by raw consumption of the vegetables mentioned above or by the addition of functional myrosinase to denatured myrosinase (which results from cooking or blanching of frozen vegetables).53
Comparison between isothiocyanate plasma concentrations from brassica vegetable ingestion and endogenous thiocyanate plasma concentrations
In Table 2,29–31,54–57 plasma concentrations of thiocyanate in smokers and nonsmokers are compared with plasma concentrations of the isothiocyanate sulforaphane for use in estimating the potential thiocyanate plasma concentration resulting from ingestion of indole glucosinolate. After consuming as much as 200 g of raw broccoli, the plasma concentration of sulforaphane (i.e., the glucoraphanin degradation product) was 0.114 μM,22 while other reports demonstrated mean plasma levels of 0.04–0.07, 0.3, 0.4, and 1.9 μM.54–56 Broccoli that was fed raw had a higher sulforaphane concentration, as would be expected.57 The maximum plasma thiocyanate value reported for smokers was 121 μM.29 Choi et al.58 measured the concentrations of thiocyanate, cyanide, and organic isothiocyanates in the serum of rats after administration of a single dose of 50 μmol of various glucosinolates. As shown in Table 1, a dose of 50 μmol is comparable to the amount of glucoraphanin present in a 100-g serving of broccoli for a human, and thus the maximum value of 50 μM of sinigrin-derived thiocyanate they measured in rat plasma is an overestimation of what could be expected in humans.
Table 2.
Studies reporting human plasma concentrations of thiocyanate and sulforaphane
| Compound | No. of subjects | Condition | Reference |
|---|---|---|---|
| Thiocyanate ion (µM) | |||
| 69 | 37 | Nonsmokers before consuming 8 mg of thiocyanate daily | Dahlberg et al. (1984)29 |
| 121 | 37 | Nonsmokers after consuming 8 mg of thiocyanate daily for 12 wk | Dahlberg et al. (1984)29 |
| Mean (SD) 40±24 | 16 | Nonsmokers | Morgan et al. (2011),30 Nedoboy et al. (2014)31 |
| Mean (SD) 47±26 | 74 | Nonsmokers | Nedoboy et al. (2014)31 |
| Sulforaphane (µM) | |||
| 1.9 (mean); 0.37–7.3 (range) | 14 | After consumption of fresh, steamed, and microwaved broccoli and Brussels sprouts | Angelino & Jeffrey (2014)54 |
| 0.4 | 12 | After consumption of fresh broccoli in crossover study | Conaway et al. (2000)55 |
| 0.3 | 18 | After consumption of raw broccoli | Saha et al. (2012)57 |
| 0.114 | 5 | After consumption of 200 g of raw broccoli | Song et al. (2005)22 |
| 0.07 (raw); 0.04 (cooked) | 1 | After consumption of 200 g of raw or microwaved broccoli | Vermeulen et al. (2008)57 |
If 60–120 μmol of glucoraphanin per serving results in a sulforaphane concentration of less than 5 μM, an indole glucosinolate amount of 120 μmol might also result in no more than an approximately 5 μM increase in thiocyanate ion. This may be on the high side, since Agerbirk et al.4 reported that the myrosinase-catalyzed indole glucosinolate degradation pathway was very complex and likely leads to a lower stoichiometric conversion of indole glucosinolate to thiocyanate than glucoraphanin to sulforaphane. Another complicated issue in the conversion of glucosinolates to isothiocyanates is the genetic variation in myrosinase specificity for glucosinolate epimers and the variation in the epithiospecifier protein that results in nitrile, rather than thiocyanate, formation.59 Since the indole concentrations in many brassica vegetables are 200 μmol/100 g of fresh weight, this suggests the maximum thiocyanate increase would be on the order of 10 μM. Since this concentration is much less than 40–121 μM, the naturally occurring “control” thiocyanate concentration reported in various studies (Table 2), it is unlikely that thiocyanate arising from indole glucosinolates from normal serving sizes of common brassica vegetables would have significant adverse effects on thyroid function. However, it would be useful to confirm this with a study in which plasma thiocyanate levels are measured before and after consumption of a meal rich in indole glucosinolates.
Progoitrin concentrations that result in decreased thyroid hormone production in animals
It is useful to compare progoitrin concentrations in brassica vegetables for human consumption with the progoitrin concentrations in defatted rapeseed meal that have caused thyroid or other metabolic dysfunction when fed to livestock. Kelley and Bjeldanes60 found that, when a continuous diet of 154 μmol of progoitrin per 100 g was fed to rats, a hypothyroid state was produced, negatively affecting the glutathione S-transferase activities that are responsible for the conjugation and subsequent detoxification of many potentially toxic, electrophilic substances. In a comparison of two progoitrin-containing diets (71 μmol/100 g and 226 μmol/100 g) in lambs, progoitrin concentrations increased significantly in the lung and thyroid.61 Thomke et al.62 fed two diets containing goitrin concentrations of 383 μmol/100 g and 755 μmol/100 g to pigs and found that only the higher concentration diet significantly suppressed live weight gain and increased liver and thyroid masses.
From the previous data of Langer et al.,23 who reported that 77 μmol of goitrin did not decrease thyroidal radioiodine uptake,23 it is reasonable to infer that a single serving of brassica vegetables with less than 70 μmol of progoitrin would be unlikely to result in decreased thyroid hormone production in humans. Specifically, ingestion of commercial broccoli and broccoli rabe, which each contain less than 10 μmol progoitrin per 100-g serving, would appear to pose minimal risk of thyroidal toxicity. Excess consumption should be avoided, as an elderly woman in New York presented with myxedema coma (the most severe and a life-threatening form of hypothyroidism) after ingesting up to 1.5 kg of raw bok choy daily for several months.63
Animal feeding trials investigating the safety of rapeseed meal19 typically do not distinguish the concentration of glucosinolate progoitrin, whose degradation product isothiocyanate goitrin has known antithyroid effects, from the total glucosinolate concentration of the meal. Since the glucosinolates glucoraphanin and phenethyl glucosinolate have well-established anticarcinogenic properties, this broad selection against all glucosinolates might be counterproductive. Similarly, the work of Tang et al.,64 who used the benzene dithiol reagent to measure the sum of all isothiocyanates, cannot discriminate between profiles with or without high concentrations of goitrin or sulforaphane.
The major potential negative effects of brassica consumption on thyroid hormone production have been suggested to result from the goitrin and thiocyanate content of these vegetables, as described above. However, decreased thyroidal radioiodine uptake has been also demonstrated to occur when allyl and methyl isothiocyanates (from mustards) were incubated with thyroid tissue slices at concentrations of 10−3 and 10−4M.18 Whereas the concentrations of isothiocyanates in the tissue slices with diminished iodine uptake were much higher than the background levels found in human plasma (Table 2), intracellular isothiocyanate concentrations can be orders of magnitude higher (i.e., mM). These high increased intracellular concentrations are due to diffusion of the isothiocyanates across the cell membrane. Once inside the cell, the isothiocyanates spontaneously form adducts with the cysteine thiol of glutathione.65 Following secretion, these conjugates are unstable and readily disassociate to their parent compounds,65 and therefore, conjugate concentrations in the millimolar range would also expose the tissues to the same range of isothiocyanate concentrations that Ermans and Bourdoux18 demonstrated in thyroid slices and reported as evidence of decreased iodine uptake.
Genetics of glucoraphanin and progoitrin synthesis
Progoitrin is the terminal glucosinolate produced in a chain of glucosinolate syntheses that start with methionine (Figure 1).32,34 After removal of the carboxylic acid and amine group, the methionine side chain is elongated to produce a methyl thiobutyl group. The thioether in this group is first oxidized by the enzyme glucosinolate oxidase (NCBI [National Center for Biotechnology Information] accession no. FJ3760740) (GSL-OX) to produce the methylsulfinyl group (i.e., glucoraphanin) and is then oxidized – through the loss of the CH3CO – by the enzyme glucosinolate alkenylase (GSL-ALK) (NCBI accession no. EF611253) to produce the unsaturated butenyl (gluconapin). Lastly, it is oxidized by the enzyme glucosinolate hydroxylase (GSL-OH) (NCBI accession no. FJ376074) to produce hydroxybutenyl, which, after a nonenzymatic reaction, cyclizes to produce goitrin.
In broccoli, the GSL-ALK enzyme is nonfunctional due to a 2-bp deletion.66 This results in termination of the pathway and, consequently, high glucoraphanin concentrations. The majority of the B. rapa species have a functional GSL-ALK gene, and thus the glucosinolate present at the highest concentration by far is the butenyl glucosinolate (D’Arrigo Bros. Co., unpublished data and Yang and Quiros32). This highly functional GSL-ALK gene is also responsible for very low glucoraphanin concentrations in B. rapa, since the glucoraphanin is converted in the next step of the pathway (i.e., to butenyl glucosinolate).
Li et al.34 examined crosses of broccoli × cauliflower, collard × broccoli, and collard × cauliflower and found that the hydroxylating gene GSL-OH segregated with high progoitrin concentrations in the collard × broccoli progeny. This indicates that progoitrin production is single-gene controlled and, due to common ancestry among Brassica species, could occur in most commercial brassica vegetable species. Fortunately, it only occurs in high concentrations in B. napus kale and some collards.
Liu et al.66 have utilized knowledge of this pathway and RNA silencing techniques to downregulate expression of the GSL-ALK genes in the problematic B. napus species used for oilseed production. Not only was the progoitrin reduced by 65% in the seeds, but the glucoraphanin concentration was increased to a high concentration of 4226 μmol/100 g of seeds. It would be interesting to examine the glucoraphanin concentration in the sprouts of this transgenic plant.
CONCLUSION
Components of brassica vegetables demonstrate important anticarcinogenic properties that must be balanced against the potential adverse effects of progoitrin and thiocyanate on thyroid hormone production. The consumption of typical serving sizes of raw, commercial B. oleracae and B. rapa varieties (i.e., broccoli, Chinese cabbage, bok choy, broccoli rabe) correspond to progoitrin- and thiocyanate-generating indole glucosinolate exposures at concentrations far lower than those likely to impair thyroid function. In contrast, excessive consumption (e.g., >1 kg/d for several months) of raw Russian/Siberian kale of the species B. napus, some collards, and Brussels sprouts, all of which have high progoitrin concentrations and thus can decrease iodine uptake into the thyroid to affect the synthesis of thyroid hormone, should be avoided.
Acknowledgments
The excellent technical assistance in Mass Spectral assignment of glucosinolate compounds to HPLC retention time by Vladimir Tolstikov, Director, University of California Davis Genome Center and Theresa McLaughlin at Stanford University Vincent Coates Foundation Mass Spectrometry Laboratory is gratefully acknowledged.
Author contributions. P.F. and R.B. designed and conducted the research; P.F., R.B., and A.M.L. analyzed the data and wrote the paper. All authors read and approved the final manuscript. Neither the National Institutes of Health nor D’Arrigo Bros. Co. had any role in the design, execution, or approval of the manuscript (the views expressed are of P.F. and R.B. and not of the company).
Funding. The study was supported in part by National Institutes of Health K23HD068552 (A.M.L.).
Declaration of interest. P.F. and R.B. are employees of D'Arrigo Bros. Co., of California (www.Andyboy.com), which grows and ships brassica vegetables.
References
- 1.Wagner AE, Terschluesen AM, Rimbach G. Health promoting effects of brassica-derived phytochemicals: from chemopreventive and anti-inflammatory activities to epigenetic regulation. Oxid Med Cell Longev. 2013;2013:964539 doi:10.1155/2013/964539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. [DOI] [PubMed] [Google Scholar]
- 3.Clarke DB. Glucosinolates, structure and analysis in food. Anal Methods. 2010;2:310–325. [Google Scholar]
- 4.Agerbirk N, de Vos M, Kim JH, et al. Indole glucosinolate breakdown and its biological effects. Phytochem Rev. 2009;8:101–120. [Google Scholar]
- 5.Prochaska HJ, Santamaria AB, Talalay P. Rapid detection of inducers of enzymes that protect against carcinogens. Proc Natl Acad Sci U S A. 1992;89: 2394–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Talalay P, Cho CG, et al. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;89:2399–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riedl MA, Saxon A, Diaz-Sanchez D. Oral sulforaphane increases phase II antioxidant enzymes in the human upper airway. Clin Immunol. 2009;130:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angeloni C, Leoncini E, Malaguti M, et al. Modulation of phase II enzymes by sulforaphane: implications for its cardioprotective potential. J Agric Food Chem. 2009;57:5615–5622. [DOI] [PubMed] [Google Scholar]
- 9.Tan XL, Spivack SD. Dietary chemoprevention strategies for induction of phase II xenobiotic-metabolizing enzymes in lung carcinogenesis: a review. Lung Cancer. 2009;65:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers KF, Bacon JR, Kemsley EK, et al. Gene expression profile of primary prostate epithelial and stromal cells in response to sulforaphane or iberin exposure. Prostate. 2009;69:1411–1421. [DOI] [PubMed] [Google Scholar]
- 11.Munday R, Zhang Y, Fahey JW, et al. Evaluation of isothiocyanates as potent inducers of carcinogen-detoxifying enzymes in the urinary bladder: critical nature of in vivo bioassay. Nutr Cancer. 2006;54:223–231. [DOI] [PubMed] [Google Scholar]
- 12.Traka M, Gasper AV, Smith JA, et al. Transcriptome analysis of human colon Caco-2 cells exposed to sulforaphane. J Nutr. 2005;135:1865–1872. [DOI] [PubMed] [Google Scholar]
- 13.La Marca M, Beffy P, Della Croce C, et al. Structural influence of isothiocyanates on expression of cytochrome P450, phase II enzymes, and activation of Nrf2 in primary rat hepatocytes. Food Chem Toxicol. 2012;50:2822–2830. [DOI] [PubMed] [Google Scholar]
- 14.Gao N, Budhraja A, Cheng S, et al. Phenethyl isothiocyanate exhibits antileukemic activity in vitro and in vivo by inactivation of Akt and activation of JNK pathways. Cell Death Dis. 2011;2:e140 doi:10.1038/cddis.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li RW, Li C, Wang TT. Transcriptomic alterations in human prostate cancer cell LNCaP tumor xenograft modulated by dietary phenethyl isothiocyanate. Mol Carcinog. 2013;52:426–437. [DOI] [PubMed] [Google Scholar]
- 16.Bonnesen C, Stephensen PU, Andersen O, et al. Modulation of cytochrome P-450 and glutathione S-transferase isoform expression in vivo by intact and degraded indolyl glucosinolates. Nutr Cancer. 1999;33:178–187. [DOI] [PubMed] [Google Scholar]
- 17.Degner SC, Papoutsis AJ, Selmin O, et al. Targeting of aryl hydrocarbon receptor-mediated activation of cyclooxygenase-2 expression by the indole-3-carbinol metabolite 3,3'-diindolylmethane in breast cancer cells. J Nutr. 2009;139:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ermans AM, Bourdoux P. Antithyroid sulfurated compounds. In: Environmental Goitrogenesis. Boca Raton, FL: CRC Press; 1989:16–31. [Google Scholar]
- 19.Tripathi MK, Mishra AS. Glucosinolates in animal nutrition: a review. Animal Feed Sci Technol. 2007;132:1–27. [Google Scholar]
- 20.Latté KP, Appel KE, Lampen A. Health benefits and possible risks of broccoli – an overview. Food Chem Toxicol. 2011;49:3287–3309. [DOI] [PubMed] [Google Scholar]
- 21.Vanderpas J. Nutritional epidemiology and thyroid hormone metabolism. Annu Rev Nutr. 2006;26:293–322. [DOI] [PubMed] [Google Scholar]
- 22.Song L, Morrison JJ, Botting NP, et al. Analysis of glucosinolates, isothiocyanates, and amine degradation products in vegetable extracts and blood plasma by LC-MS/MS. Anal Biochem. 2005;347:234–243. [DOI] [PubMed] [Google Scholar]
- 23.Langer P, Michajlovskij N, Sedlák J, et al. Studies on the antithyroid activity of naturally occurring L-5-vinyl-2-thiooxazolidone in man. Endokrinologie. 1971;57:225–229. [PubMed] [Google Scholar]
- 24.Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458 doi:10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- 25.Tonacchera M, Pinchera A, Dimida A, et al. Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid. 2004;14:1012 doi:10.1089/thy.2004.14.1012. [DOI] [PubMed] [Google Scholar]
- 26.Di Bernardo J, Iosco C, Rhoden KJ. Intracellular anion fluorescence assay for sodium/iodide symporter substrates. Anal Biochem. 2011;415:32–38. [DOI] [PubMed] [Google Scholar]
- 27.Ihalin R, Loimaranta V, Tenovuo J. Origin, structure, and biological activities of peroxidases in human saliva. Arch Biochem Biophys. 2006;445:261–268. [DOI] [PubMed] [Google Scholar]
- 28.Hurst JK. What really happens in the neutrophil phagosome? Free Radic Biol Med. 2012;53:508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahlberg PA, Bergmark A, Björck L, et al. Intake of thiocyanate by way of milk and its possible effect on thyroid function. Am J Clin Nutr. 1984;39:416–420. [DOI] [PubMed] [Google Scholar]
- 30.Morgan PE, Pattison DI, Talib J, et al. High plasma thiocyanate levels in smokers are a key determinant of thiol oxidation induced by myeloperoxidase. Free Radic Biol Med. 2011;51:1815–1822. [DOI] [PubMed] [Google Scholar]
- 31.Nedoboy PE, Morgan PE, Mocatta TJ, et al. High plasma thiocyanate levels are associated with enhanced myeloperoxidase-induced thiol oxidation and long-term survival in subjects following a first myocardial infarction. Free Radic Res. 2014;48:1256–1266. [DOI] [PubMed] [Google Scholar]
- 32.Yang B, Quiros CF. Survey of glucosinolate variation in leaves of Brassica rapa crops. Genet Resour Crop Evol. 2010;57:1079–1089. [Google Scholar]
- 33.Sønderby IE, Geu-Flores F, Halkier BA. Biosynthesis of glucosinolates – gene discovery and beyond. Trends Plant Sci. 2010;15:283–290. [DOI] [PubMed] [Google Scholar]
- 34.Li G, Riaz A, Goyal A, et al. Inheritance of three major genes involved in the synthesis of aliphatic glucosinolates in Brassica oleracea. Amer Soc Hort Sci. 2001;126:426–431. [Google Scholar]
- 35.Chen G-J, Si Y, Cao B-H, et al. Analysis of combining ability and heredity parameters of glucosinolates in Chinese kale. Afr J Biotechnol. 2010;53:9026–9031. [Google Scholar]
- 36.Fowke JH, Shu XO, Dai Q, et al. Urinary isothiocyanate excretion, Brassica consumption, and gene polymorphisms among women living in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2003;12:1536–1539. [PubMed] [Google Scholar]
- 37.Fowke JH, Chung FL, Jin F, et al. Urinary isothiocyanate levels, Brassica, and human breast cancer. Cancer Res. 2003;63:3980–3986. [PubMed] [Google Scholar]
- 38.Wang X, Wang H, Wang J, et al. The genome of the mesopolyploid crop species Brassica rapa. Nat Genet. 2011;43:1035–1039. [DOI] [PubMed] [Google Scholar]
- 39.Wade KL, Garrard IJ, Fahey JW. Improved hydrophilic interaction chromatography method for the identification and quantification of glucosinolates. J Chromatogr A. 2007;1154:469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwimmer S. Spectral changes during the action of myrosinase on sinigrin. Acta Chemica Scandinavica. 1961;15:535–544. [Google Scholar]
- 41.Haupt GW. An alkaline solution of potassium chromate as a transmittancy standard in the ultraviolet. J Res Natl Bureau Standards. 1952;48:414–423. [Google Scholar]
- 42.Agerbirk N, Petersen BL, Olsen CE, et al. 1,4-Dimethoxyglucobrassicin in Barbarea and 4-hydroxyglucobrassicin in Arabidopsis and Brassica. J Agric Food Chem. 2001;49:1502–1507. [DOI] [PubMed] [Google Scholar]
- 43.Bruce GM, Corey LM, Mandel JH, et al. Urinary nitrate, thiocyanate, and perchlorate and serum thyroid endpoints based on NHANES 2001 to 2002. J Occup Environ Med. 2013;55:52–58. [DOI] [PubMed] [Google Scholar]
- 44.Cramer JM, Jeffery EH. Sulforaphane absorption and excretion following ingestion of a semi-purified broccoli powder rich in glucoraphanin and broccoli sprouts in healthy men. Nutr Cancer. 2011;63:196–201. [DOI] [PubMed] [Google Scholar]
- 45.Rosa EAS. Glucosinolates from flower buds of Portugese Brassica crops. Phytochemistry. 1997;44:1415–1419. [Google Scholar]
- 46.Cartea ME, de Haro A, Obregón S, et al. Glucosinolate variation in leaves of Brassica rapa crops. Plant Foods Hum Nutr. 2012;67:283–288. [DOI] [PubMed] [Google Scholar]
- 47.Lee M-K, Chun J-H, Byeon DH, et al. Variation of glucosinolates in 62 varieties of Chinese cabbage (Brassica rapa L. ssp. pekinensis) and their antioxidant activity. Food Sci Technol. 2014;58:93–101. [Google Scholar]
- 48.He H, Liu L, Song SH, et al. Evaluation of glucosinolate composition and contents in Chinese brassica vegetables. Acta Hort. 2003;620:85–92. [Google Scholar]
- 49.Carlson DG, Daxenbichler ME, VanEtten CH, et al. Glucosinolates in crucifer vegetables: broccoli, brussels sprouts, cauliflower, collards, kale, mustard greens and kohlrabi. J Amer Soc Hort Sci. 1987;112:173–178. [Google Scholar]
- 50.Kushad MM, Brown AF, Kurilich AC, et al. Variation of glucosinolates in vegetable crops of Brassica oleracea. J Agric Food Chem. 1999;47:1541–1548. [DOI] [PubMed] [Google Scholar]
- 51.Tian Q, Rosselot RA, Schwartz SJ. Quantitative determination of intact glucosinolates in broccoli, broccoli sprouts, Brussels sprouts, and cauliflower by high-performance liquid chromatography–electrospray ionization–tandem mass spectrometry. Anal Biochem. 2005;343:93–99. [DOI] [PubMed] [Google Scholar]
- 52.Ku KM, Jeffery EH, Juvik JA. Exogenous methyl jasmonate treatment increases glucosinolate biosynthesis and quinone reductase activity in kale leaf tissue. PLoS One. 2014;9:e103407 doi:10.1371/journal.pone.0103407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dosz EB, Jeffery EH. Modifying the processing and handling of frozen broccoli for increased sulforaphane formation. J Food Sci. 2013;78:H1459–H1463. [DOI] [PubMed] [Google Scholar]
- 54.Angelino D, Jeffery E. Glucosinolate hydrolysis and bioavailability of resulting isothiocyanates: focus on glucoraphanin. J Funct Foods. 2014;7:67–76. [Google Scholar]
- 55.Conaway CC, Getahun SM, Liebes LL, et al. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer. 2000;38:168–178. [DOI] [PubMed] [Google Scholar]
- 56.Saha S, Hollands W, Teucher B, et al. Isothiocyanate concentrations and interconversion of sulforaphane to erucin in human subjects after consumption of commercial frozen broccoli compared to fresh broccoli. Mol Nutr Food Res. 2012;56:1906–1916. [DOI] [PubMed] [Google Scholar]
- 57.Vermeulen M, Klöpping-Ketelaars IW, van den Berg R, et al. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J Agric Food Chem. 2008;56:10505–10509. [DOI] [PubMed] [Google Scholar]
- 58.Choi EJ, Zhang P, Kwon H. Determination of goitrogenic metabolites in the serum of male Wistar rat fed structurally different glucosinolates. Toxicol Res. 2014;30:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bernardi R, Finiguerra MG, Rossi AA, et al. Isolation and biochemical characterization of a basic myrosinase from ripe Crambe abyssinica seeds, highly specific for epi-progoitrin. J Agric Food Chem. 2003;51:2737–2744. [DOI] [PubMed] [Google Scholar]
- 60.Kelley MK, Bjeldanes LF. Modulation of glutathione S-transferase activity and isozyme pattern in liver and small intestine of rats fed goitrin- and T3-supplemented diets. Food Chem Toxicol. 1995;33:129–137. [DOI] [PubMed] [Google Scholar]
- 61.Mabon N, et al. Chemical changes and influences of rapeseed antinutritional factors on lamb physiology and performance. 3. Antinutritional factors in plasma and organs. Animal Feed Sci Technol. 2000;85:111–120. [Google Scholar]
- 62.Thomke S, Petterson H, Neil M, et al. Skeletal muscle goitrin concentration and organ weights in growing pigs fed diets containing rapeseed meal. Animal Feed Sci Technol. 1998;73:207–215. [Google Scholar]
- 63.Chu M, Seltzer TF. Myxedema coma induced by ingestion of raw bok choy. N Engl J Med. 2010;362:1945–1946. [DOI] [PubMed] [Google Scholar]
- 64.Tang L, Paonessa JD, Zhang Y, et al. Total isothiocyanate yield from raw cruciferous vegetables commonly consumed in the United States. J Funct Foods. 2013;5:1996–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y. The molecular basis that unifies the metabolism, cellular uptake and chemopreventive activities of dietary isothiocyanates. Carcinogenesis. 2012;33:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Z, Hirani AH, McVetty PBE, et al. Reducing progoitrin and enriching glucoraphanin in Brassica napus seeds through silencing of the GSL-ALK gene family. Plant Mol Biol. 2012;79:179–189. [DOI] [PubMed] [Google Scholar]