Abstract
Coconut oil is being heavily promoted as a healthy oil, with benefits that include support of heart health. To assess the merits of this claim, the literature on the effect of coconut consumption on cardiovascular risk factors and outcomes in humans was reviewed. Twenty-one research papers were identified for inclusion in the review: 8 clinical trials and 13 observational studies. The majority examined the effect of coconut oil or coconut products on serum lipid profiles. Coconut oil generally raised total and low-density lipoprotein cholesterol to a greater extent than cis unsaturated plant oils, but to a lesser extent than butter. The effect of coconut consumption on the ratio of total cholesterol to high-density lipoprotein cholesterol was often not examined. Observational evidence suggests that consumption of coconut flesh or squeezed coconut in the context of traditional dietary patterns does not lead to adverse cardiovascular outcomes. However, due to large differences in dietary and lifestyle patterns, these findings cannot be applied to a typical Western diet. Overall, the weight of the evidence from intervention studies to date suggests that replacing coconut oil with cis unsaturated fats would alter blood lipid profiles in a manner consistent with a reduction in risk factors for cardiovascular disease.
Keywords: cardiovascular disease, cholesterol, coconut, lauric acid, medium-chain triglycerides
INTRODUCTION
Coconut oil has been an important edible oil for the food industry for many years and is normally termed or classified as a lauric oil, a tropical oil, or a confectionery fat.1 The usual commercial product is either refined, bleached, and deodorized coconut oil or, more recently, virgin (unrefined) coconut oil.2 The production of coconut oil has been increasing worldwide. In 2010, 3.5 million metric tons were produced, with the major producers being the Philippines (1.7 million metric tons), Indonesia (0.7 million metric tons), and India (0.5 million metric tons).3 Of this total, around 2.0 million metric tons were exported, with the major exporter being the Philippines. Consumption in the United States in 2010 was reported as 0.4 million metric tons, with an average consumption of 1.28 kg per capita per annum. Consumption in the European Union was reported as 0.6 million metric tons, with a similar average consumption of 1.3 kg per capita per annum.
One of the advantages of coconut oil is its resistance to oxidation and polymerization, which makes it a stable oil for cooking. For example, it is suitable for single-use shallow frying, although it is not recommended for continuous deep-fat frying because of its low smoke point, which may lead to the production of potentially carcinogenic substances upon overheating.4
Because of its high content of saturated fatty acids (92%), coconut oil has always been classified, along with butter, palm oil, and animal fats, as a source of saturated fat to be consumed at low levels in the diet (Table 1).5–7 In recent years, numerous claims on websites and in the commercial literature have likened coconut oil to medium-chain triglycerides, asserting that it behaves atypically compared with other foods high in saturated fat and is beneficial for human health.2 Research on manufactured medium-chain triglycerides in the literature cannot be applied to coconut oil because the triglycerides predominant in coconut oil are different in their structure, absorption, and metabolism.
Table 1.
Composition of coconut products
| Coconut product | Component (per 100 g of product) |
||||||
|---|---|---|---|---|---|---|---|
| Water | Energy | Protein | Fat | SFA | CHO | Fiber | |
| (g) | (kJ) | (g) | (g) | (g) | (g) | (g) | |
| Raw coconut flesha | 45 | 1470 | 3.2 | 36 | 33 | 3.6 | 7.7 |
| Coconut, desiccateda | 2 | 2530 | 5.6 | 62 | 58 | 6.1 | 19.2 |
| Coconut cream, canneda | 71 | 858 | 1 | 20 | 19 | 3.7 | 0.6 |
| Coconut oil (EV or RBD)b | 0 | 3700 | 0 | 100 | 92 | 0 | 0 |
| Hydrogenated coconut oil (CF92)b | 0 | 3700 | 0 | 100 | 100 | 0 | 0 |
| Coconut waterc | 95 | 80 | 0.7 | 0.2 | 0 | 3.7 | 1.1 |
A comparison of the fatty acid composition of coconut oil, medium-chain triglycerides (derived from coconut oil or palm kernel oil), and butterfat is shown in Table 2.8–10 Medium-chain triglyceride oils are made predominantly of C8:0 (caprylic) and C10:0 (capric) fatty acids. Research on medium-chain triglyceride oils has been focused on these synthesized esters of C:8 and C:10 fatty acids.8 Both are classified as medium-chain fatty acids. The main fatty acid in coconut oil is lauric acid (C12:0). Lauric acid can be classified as either a medium-chain or a long-chain fatty acid. In terms of digestion and metabolism, however, it behaves more as a long-chain fatty acid because the majority of it (70%–75%) is absorbed with chylomicrons.11 In comparison, 95% of medium-chain fatty acids are absorbed directly into the portal vein.12
Table 2.
Comparison of the properties of coconut oil, medium-chain triglycerides, and butterfat
| Property | Coconut oila | Hydrogenated coconut oilb | MCTs derived from coconut oil or palm kernel oilc | Butterfatb |
|---|---|---|---|---|
| Butyric acid 4:0 (percentage of TFAs) | 0 | 0 | 0 | 4.3 |
| Caproic acid 6:0 (percentage of TFAs) | 1 | <1 | <2 | 2.3 |
| Caprylic acid 8:0 (percentage of TFAs) | 9 | 5.4 | 50–80 | 1.4 |
| Capric acid 10:0 (percentage of TFAs) | 7 | 5.8 | 20–50 | 2.8 |
| Lauric acid 12:0 (percentage of TFAs) | 47 | 48.3 | <3 | 3.1 |
| Myristic acid 14:0 (percentage of TFAs) | 16.5 | 18.8 | <1 | 9 |
| Palmitic acid16:0 (percentage of TFAs) | 7.5 | 9.8 | 0 | 22 |
| Stearic acid 18:0 (percentage of TFAs) | 3 | 11.7 | 0 | 15 |
| Oleic acid 18:1 cis (percentage of TFAs) | 6.4 | 0.2 | 0 | 26 |
| Elaidic acid 18:1 trans (percentage of TFAs) | 0 | 0 | 0 | 5 |
| Linoleic acid 18:2 (percentage of TFAs) | 1.5 | 0 | 0 | 1.9 |
| Total SFAs (percentage of TFAs) | 92 | 99.8 | 100 | 60 |
| Triglycerides, carbon number range | C28–C52 | C28–C52 | C24–C32 | C28–C54 |
| C24–C30 content | <4% | <4% | 95% | <1% |
| Mean MW of triglycerides | 638 | 638 | 512 | 690 |
| Physical characteristics | Solid at ambient temperature | Melts at 36°C | Liquid at all temperatures | Solid at ambient temperature |
Medium-chain fatty acids are more water soluble than long-chain fatty acids and are solubilized in the aqueous phase of the intestinal contents without forming micelles, thereby undergoing faster absorption.13 Medium-chain fatty acids are also weak electrolytes and are highly ionized at neutral pH, which further increases their solubility.14 This marked difference in solubility occurs at chain lengths of C:10 and less, which therefore excludes lauric acid.
When fatty acids are combined into triglycerides, the triglycerides themselves can also be termed medium-chain or long-chain. Medium-chain triglycerides have a total carbon number of C24:0 to C30:0. Only around 4% of the triglycerides in coconut oil are of this length. Triglycerides containing lauric acid have a higher molecular weight and are metabolized differently than the lower-molecular-weight triglycerides, which contain only C8 and C10 chains (medium-chain triglycerides). The mean molecular weight of triglycerides in coconut oil is 638, whereas that of medium-chain triglyceride oils is 512. The lower molecular weight of medium-chain triglycerides facilitates the action of pancreatic lipase. Consequently, medium-chain triglycerides are hydrolyzed faster and more completely than longer-chain triglycerides.8 It is therefore inaccurate to consider coconut oil to contain either predominantly medium-chain fatty acids or predominantly medium-chain triglycerides. Thus, the evidence on medium-chain triglycerides cannot be extrapolated to coconut oil.
Epidemiological evidence from populations who consume substantial amounts of coconut is frequently cited as evidence that coconut oil does not have negative effects on cardiovascular health. It is important to note, however, that most indigenous populations have consumed either coconut flesh or squeezed coconut cream.15 The extraction and use of coconut oil in edible applications is a relatively recent phenomenon. Furthermore, when earlier data were collected, coconut products were consumed as part of a traditional diet, which was characterized by a low intake of processed foods. Subsequent to this, a large shift toward the Western diet has occurred among many indigenous populations, as evidenced by imports of unhealthy foods such as corned beef, fast food, and processed ingredients, leading to huge increases in obesity and poor health.16
The purpose of this narrative review, therefore, is to systematically assess the research available on the consumption of coconut oil, coconut milk, or coconut cream in humans, along with the effect of these products on cardiovascular disease (CVD) or related risk factors.
Search strategy
A search was conducted of the MEDLINE and Scopus databases to the end of 2013 for English-language research articles or reviews of studies performed in humans. The search terms used were “coconut” or “cocos nucifera” in the title, abstract, or keywords. To ensure all relevant articles were identified, search terms related to CVD or risk factors were not included in the search.
Studies were included if they were conducted in humans and met the following criteria: the study was related to consumption of edible coconut, coconut oil, squeezed coconut, coconut milk, or coconut cream; coconut was the main intervention or focus of analysis; and outcomes were relevant to CVD.
Studies were excluded if they were published as letters, conference abstracts, opinion pieces, nonsystematic reviews, or books. Articles were further excluded if the research was related to medium-chain triglycerides (rationale provided above); if they reported animal or ex vivo studies; if the study was related to the history of coconut production or use; if coconut was part of a mixed intervention and it was not possible to determine its individual impact; if the study was conducted in a special clinical population, e.g., patients with liver cirrhosis or formula-fed infants; if study outcomes such as lipid profiles were a minor part of the experimental results and were not a major objective of the study; if the study was also a drug trial; if the study investigated the effect of lauric acid rather than coconut; or if the study lacked a control group.
All articles were extracted into an EndNote library and duplicate studies removed. Exclusion criteria were applied by first screening titles and then screening abstracts, after which full papers were reviewed as necessary. Additional articles were obtained by manually searching bibliographies and coconut-related websites.
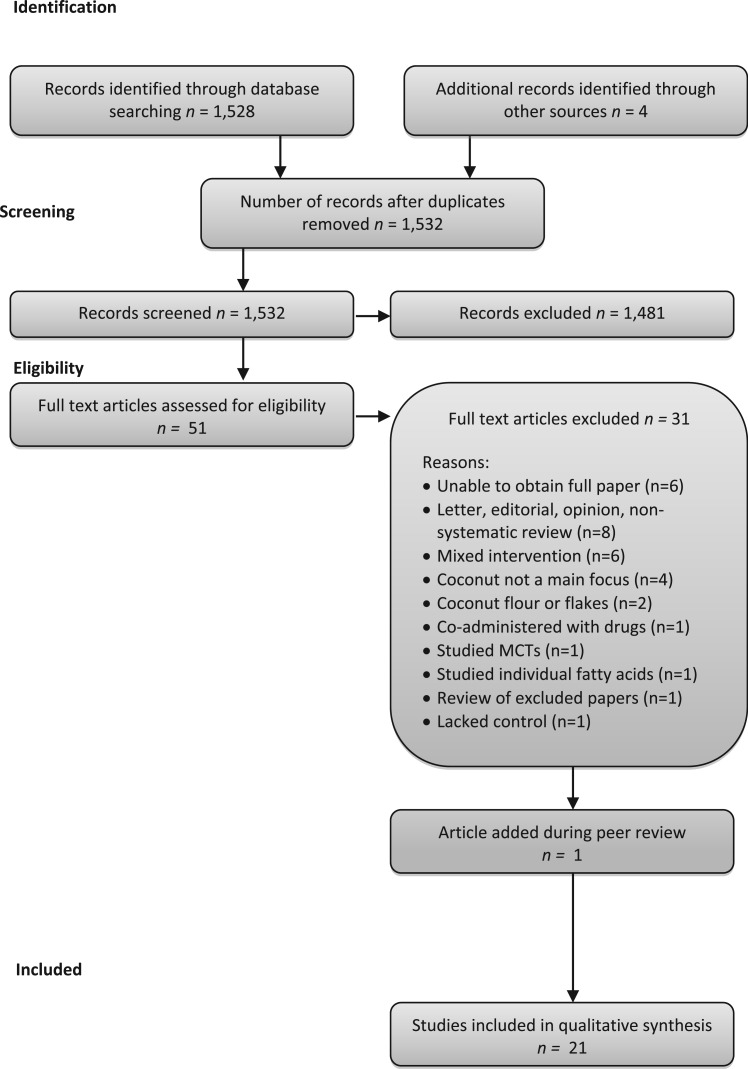
The database search identified 1528 unique articles (Figure 1). A further 4 papers were identified from bibliography and Web searches, yielding a total of 1532 to be examined. Screening by title and abstract review reduced the number to 51. The first 2 authors reviewed full texts of the remaining articles against the exclusion criteria and made consensus decisions about inclusion and exclusion. One paper was identified during peer review and was added. A total of 21 papers were identified for inclusion in this review.
Figure 1.
Flow diagram of the literature search
Epidemiological evidence
Indigenous populations who consume significant amounts of coconut products include those of India, Sri Lanka, the Philippines, Polynesia, and Melanesia.17–21 Their health statistics are often quoted as evidence that consuming coconut oil poses no risk of CVD.22 However, observational studies in these population groups cannot show causation and are prone to confounding because many different factors can simultaneously affect a specific health outcome or indicator. Furthermore, cross-sectional studies cannot show a temporal sequence because measures are taken at a single point in time. They are highly prone to recall bias and reverse causation. Observational studies also have inherent limitations when used for dietary assessment because they have a strong bias toward underestimation of habitual energy intake. Of the observational studies identified for inclusion in this review, the 8 key papers are discussed below.
The Pukapuka and Tokelau Island study by Prior et al.20 found a low incidence of CVD in the populations of these two islands, despite a large portion of energy intake (34% among Pukapukans and 63% among Tokelauans) and dietary fat intake being from coconut flesh. It has been reported that the diets of these two populations were low in sugar and high in fiber-rich foods, resulting in low cholesterol levels (4.5 mmol/L and 4.6 mmol/L in Pukapukans and Tokelauans, respectively). The higher saturated fat intake among the Tokelauans was associated with significantly higher total cholesterol (0.87–1.00 mmol/L) among the different age groups compared with the Pukapukans (all P < 0.05).
Forty years ago, a study of Tokelauans who migrated to and had lived in New Zealand for approximately 7 years was conducted.15 Lipid profiles and diets for 1200 residents of New Zealand were compared with those of 800 people still living in Tokelau. For the male migrants, whose diet and lifestyle had changed, plasma total cholesterol was higher by approximately 0.4 mmol/L (4.7–5.1 mmol/L), low-density lipoprotein cholesterol (LDL-C) was higher by 0.4 mmol/L (3.0–3.4 mmol/L), and high-density lipoprotein cholesterol (HDL-C) was lower by 0.06 mmol/L (1.22–1.16 mmol/L).15 For the people of Tokelau, 50% of their energy intake was from fat, predominantly coconut, either as grated coconut flesh or as coconut cream. Their diet consisted primarily of coconut, breadfruit, and fish. Coconut oil was not consumed per se. For the migrants who moved to New Zealand, their lifestyle and entire diet changed to include less fish and considerably more dairy products, meat, and sugar. Thus, the differences cannot be attributed to the presence or absence of coconut oil.
The Kitava studies examined cross-sectional age relations of cardiovascular risk factors in 203 Melanesian people between the ages of 20 and 86 years in Papua New Guinea.19,23,24 Coconut is a staple food of the Kitavans, who have a very low incidence of CVD. Fat intake (mainly from coconut) among Kitavans as a percentage of energy was low, at 21%. The Kitavan diet consisted of whole coconut, tubers, fish, and fruit. The average total cholesterol was reported as 4.7 mmol/L, with LDL-C being 3.1 mmol/L. Life expectancy in this study was most highly correlated with body mass index, the mean of which for those studied was 20 kg/m2.
A cross-sectional study of 1508 Samoans living in American and Western Samoa compared people consuming a traditional diet, which included coconut products, with those consuming a Western-style diet.17 The Western diet was associated with an increased incidence of metabolic syndrome and, therefore, an increased risk of developing CVD. These authors did not measure total cholesterol or LDL-C but focused on components of the metabolic syndrome: abdominal obesity, hypertension, hypertriglyceridemia, low HDL-C, and elevated fasting glucose. They concluded that the results of this study provide evidence of the potential protective effect of a neotraditional eating pattern in American Samoa and Samoa. However, they state that, given the cross-sectional nature of this study, the results should be confirmed in a prospective study and in clinical trials to separate the effects of specific nutrients from the influence of the surrounding dietary pattern. The neotraditional dietary profile was characterized by a high intake of coconut products and seafood and a low intake of processed foods, including potato chips, rice, and soft drinks.
Coconut consumption is associated with higher levels of serum HDL-C in epidemiological studies, and claims of coconut being beneficial to heart health have been attributed to this effect.22 An analysis of the results of a longitudinal cohort study of 1839 Filipino women aged 35–69 years by Feranil et al.25 reveals that, while HDL-C levels did indeed rise with an increase in coconut oil intake (1.01–1.09 mmol/L, P = 0.001), total cholesterol (4.63–5.02 mmol/L, P = 0.001), and LDL-C (2.97–3.23 mmol/L, P = 0.001) also rose. The serum triglyceride levels of the participants rose with increasing coconut oil intake (1.43–1.51 mmol/L, P = 0.001). The ratio of total cholesterol to HDL-C was unaffected by coconut intake (P = 0.81). When viewed as a whole, these results do not indicate either a beneficial or a detrimental effect on serum lipid profiles.
Sabitha et al.26 conducted a cross-sectional study in 140 middle-aged Indian men with and without type 2 diabetes who self-reported typically consuming coconut oil or sunflower oil as 13% to 20% of their total energy intake over the past 6 years. Lipid profiles and oxidative stress parameters were compared between the groups and did not show any statistically significant differences between coconut oil and sunflower oil. While the authors concluded that type of dietary fat may not be a major contributory factor to oxidative stress in this population, the observational nature of the study design does not allow any firm conclusions to be drawn.
Population studies have been performed in several other countries: 2 studies from Tanzania looked at obesity and dyslipidemia,27,28 1 each from Indonesia and India looked at coronary heart disease,18,29 and 1 from India examined hypertension.30 Confounding factors between study groups, such as smoking and sugar consumption, make it unclear whether coconut in the diet has any positive or negative effect on CVD and its risk factors.
Clinical trials and intervention studies
Eight intervention studies met the inclusion criteria (Table 3).31–38 Four were crossover trials,31,33,37,38 3 were sequential feeding studies,32,34 and 1 was a randomized parallel community-based trial.35 Two studies were conducted in New Zealand,31,32 2 in the United States,33,37 2 in Sri Lanka,34,35 and 2 in Malaysia.36,38 The primary outcomes were related to serum lipids (n = 8)31–38 and markers of inflammation or oxidative stress (n = 1).38
Table 3.
Summary of intervention studies investigating coconut oil
| Reference | Study design (country) | Participants | Intervention | Comparator | Outcome (mmol/L, except where specified) |
|---|---|---|---|---|---|
| Cox et al. (1995)31 | Randomized crossover trial (New Zealand) | n = 28 (13 M, 15 F) adults, 29–67 y, TC 5.5–7.9 mmol/L, TG <3 mmol/L |
|
|
|
| Cox et al. (1998)32 | Sequential feeding trial (New Zealand) | n = 41 (24 M, 17 F) healthy Pacific Islanders living in New Zealand | 39 g coconut oil to supply 17 g lauric acid per day for 6 wk. Total fat supplied 36% energy (84 g) and carbohydrate 47% energy | Butter (39 g) and safflower oil (24 g) to provide 17 g palmitic acid and 17 g linoleic acid for 6 wk each |
|
| Mendis et al. (2001)35 | Randomized feeding trial (Sri Lanka) | n = 54 (42 M, 12 F) Sri Lankans, half of whom were hypercholesterolemic |
|
Phase 2: Same fat intake as Diet A, with the addition of 3.3% energy from soyabean and sesame oils to a total of 24% total energy from fat for 52 wk (PUFA:SFA ratio = 1.1) (Diet B) | In both phases, fat was replaced with carbohydrate, and energy intakes decreased. |
| Phase 1: | |||||
| |||||
| Mendis & Kumarasunderam (1990)34 | Sequential feeding trial (Sri Lanka) | n = 25 healthy adult male prison inmates (20–26 y), normal BMI | 70% of fat from coconut fat for 8 wk (PUFA:SFA ratio = 0.25). Total fat provided ≈30% of energy | 70% of fat from soyabean oil for 8 wk (PUFA:SFA ratio = 4.0). Total fat provided ≈30% of energy |
|
| Ng et al. (1991)36 | Sequential feeding trial (Malaysia) | n = 83 (61 M, 22 F), healthy, normolipidemic adults | Approximately 75% of energy was from fat from coconut oil. Participants were randomized to 1 of 3 dietary sequences: | Approximately 75% of energy was from fat from palm olein or corn oil |
|
| |||||
| Reiser et al. (1985)37 | Randomized crossover trial (USA) | n = 19 normolipidemic male medical students (12 completed all 3 diets) | 60% of fat from coconut oil, beef fat, or safflower oil, each for 5 wk. 35% of total energy from fat | Habitual diet at baseline and during washout periods |
|
| Fisher et al. (1983)33 | Crossover trial (USA) | n = 9 normolipidemic males (18–37 y) | Mostly formula diet containing 31% fat as coconut oil (with and without added cholesterol) for 9 d | Mostly formula diet containing 31% fat as corn oil (with and without added cholesterol) for 9 d | Coconut significantly increased TC, VLDL, IDL + LDL, and HDL cholesterol as well as TG when compared with corn oil. |
| In comparison with ad libitum (baseline) diets: | |||||
| |||||
| Coconut oil shifted ApoE toward lower-density lipoproteins (VLDL, IDL, and LDL). Subgroup analysis showed no effect of ApoE phenotype on variables measured | |||||
| Voon et al. (2011)38 | Randomized crossover trial (Malaysia) | n = 45 (36 F, 9 M) normal weight and overweight healthy adults (average age 30 y) | Meals for 5 wk provided 30% energy from fat, two-thirds of which was from coconut oil (20% total energy) | Meals for 5 wk provided 30% energy from fat, two-thirds of which was from palm oil or extra virgin olive oil |
|
Abbreviations: ApoA-1, apolipoprotein A-1; ApoA-II, apolipoprotein A-II; ApoB, apolipoprotein B; ApoE, apolipoprotein E; BMI, body mass index; CETA, cholesteryl ester transfer activity; F, females; HDL, high-density lipoprotein cholesterol; IDL, intermediate-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; Lp(a), lipoprotein a; M, males; N/S, not statistically significant; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; TC, total cholesterol; TG, triglycerides; tHcy, total homocysteine; VLDL, very low-density lipoprotein cholesterol.
Coconut and blood lipids and lipoproteins
Cox et al.31 conducted a randomized crossover trial to assess the effects of coconut oil, butter, and safflower oil on lipids and lipoproteins in moderately hypercholesterolemic individuals. Twenty-eight participants (13 men, 15 women) followed three 6-week experimental diets of similar macronutrient distribution with the 3 different test fats providing 50% of total dietary fat. Fat as a percentage of energy ranged from 35% to 37%. After both the butter and the coconut oil interventions, total cholesterol (6.8 mmol/L and 6.4 mmol/L, respectively) and LDL-C (4.5 mmol/L and 4.2 mmol/L, respectively) were significantly higher than after the safflower oil intervention (total cholesterol, 6.1 mmol/L; LDL-C, 3.9 mmol/L), and butter raised both outcome measurements significantly more than coconut oil (P < 0.001). There was no statistically significant difference in HDL-C levels between interventions. Concentrations of triglycerides were significantly lower after the coconut oil (P = 0.01) and safflower oil (P = 0.02) interventions compared with the butter intervention, with no difference between coconut oil and safflower oil (P = 0.48).
In a larger trial, Cox et al.32 carried out a sequential, nonrandomized feeding experiment in 41 healthy people of Pacific ethnicity to assess the effects of coconut oil, butter, and safflower oil on blood lipids and lipoproteins. The participants consumed each of the 3 test diets for 6 weeks. Fat was consumed as 36% total energy, with test fats providing 46% of dietary fat intake. The authors confirmed their earlier findings,31 in that total cholesterol and LDL-C levels were highest with the butter diet (5.61 mmol/L and 4.08 mmol/L, respectively) and lowest with the safflower oil diet (5.1 mmol/L and 3.5 mmol/L, respectively), with coconut oil in between the two (5.47 mmol/L and 3.79 mmol/L, respectively). Total cholesterol and LDL-C levels were significantly higher in both the butter and the coconut oil interventions compared with the safflower oil intervention (P < 0.01) but did not differ significantly between butter and coconut oil. Both butter (1.16 mmol/L) and coconut oil (1.21 mmol/L) significantly raised HDL-C compared with safflower oil (1.06 mmol/L) (P < 0.01), with no significant difference between coconut oil and butter. Plasma triglyceride levels were reduced from 1.98 mmol/L in all 3 test groups, with the coconut oil group showing the lowest concentration (1.61 mmol/L) compared with the safflower oil group (1.77 mmol/L) and the butter group (1.86 mmol/L). However, this effect was not statistically significant between treatments (P = 0.18). The results of both these studies by Cox et al.31,32 suggest that coconut consumption has a detrimental effect on blood lipids and lipoproteins compared with safflower oil, a cis unsaturated fat. The results are less clear when comparing coconut with butter. One study reported significantly lower total cholesterol and LDL-C following coconut oil consumption in comparison with butter consumption,31 whereas the other study reported no difference.32
The randomized crossover study by Voon et al.,38 conducted in 45 healthy young Malaysian adults, examined a range of biomarkers that included homocysteine, C-reactive protein, and fasting and postprandial lipid levels after consumption of meals with different fat compositions over a 5-week period. The diets were high in protein (20% of energy), with the test fats (palm olein, coconut oil, and virgin olive oil) providing 67% of total fat, which in turn was 30% of energy. The levels of homocysteine and other inflammatory biomarkers such as C-reactive protein were not significantly different between the 3 treatments. Lipid profiles showed that the coconut oil group had significantly higher total cholesterol (4.95 mmol/L vs 4.65 mmol/L) and LDL-C (3.30 mmol/L vs 3.06 mmol/L) levels compared with the olive oil group (all P < 0.05). Although HDL-C concentrations were significantly higher in the coconut oil group compared with olive oil group (1.37 mmol/L vs 1.28 mmol/L, P < 0.05), ratios of total cholesterol to HDL-C were not significantly different between the 3 test diets. Other outcomes did not differ significantly between the palm olein treatment and the other 2 treatments. Similar to the studies above, coconut oil consumption was associated with a more atherogenic profile in terms of total and LDL cholesterol. It is important to note, however, that there was no difference in the ratio of total cholesterol to HDL-C, which is a strong predictor of CVD risk.
Reiser et al.37 conducted a relatively small randomized crossover trial in medical students that assessed the effects of coconut oil, safflower oil, and beef fat on fasting plasma lipid and lipoproteins. Nineteen male participants consumed 2 or 3 of the diets for 5 weeks each, eating their normal diet in between the test diets. The test fat provided 60% of energy from fat, with total fats providing 35% of energy. In comparison with the safflower oil diet (total cholesterol, 3.65 mmol/L; HDL-C, 1.03 mmol/L; LDL-C, 2.33 mmol/L), the coconut oil diet resulted in significantly higher concentrations of total cholesterol (4.34 mmol/L), HDL-C (1.19 mmol/L), and LDL-C (2.84 mmol/L), with no significant difference in triglycerides (0.81 mmol/L vs 0.88 mmol/L). In comparison with the beef fat diet (total cholesterol, 4.01 mmol/L; HDL-C, 1.03 mmol/L; LDL-C, 0.99 mmol/L), the coconut oil diet resulted in significantly higher total cholesterol and HDL-C and lower triglycerides, with no significant difference in LDL-C (2.53 mmol/L vs 2.84 mmol/L). Although this study sample is relatively small, the results are consistent with other research in terms of reporting significant increases in total and LDL cholesterol among those consuming the coconut oil diet.
Ng et al.36 conducted a 3-part sequential feeding study in 83 normocholesterolemic Malaysian participants. Participants were randomized to 1 of 3 groups and received a sequence of (1) coconut oil/palm oil/coconut oil; (2) coconut oil/corn oil/coconut oil; or (3) coconut oil for all three 5-week dietary periods. Fat as a percentage of energy ranged from 27% to 31%, with the study oils suppling approximately 75% of total fat. Following coconut oil feeding, subsequent feeding of palm oil or corn oil resulted in reductions in total cholesterol (19% and 36%, respectively), LDL-C (20% and 42%, respectively), and HDL-C (20% and 26%, respectively), all P < 0.01. Neither the ratio of LDL-C to HDL-C nor triglyceride levels were significantly different between the coconut oil and palm oil diets, but both were significantly lower after the corn oil diet compared with the coconut oil diet (both P < 0.01). Compared with baseline, coconut oil increased total cholesterol concentrations by more than 10% in all groups. It should be noted that, although this study had 83 participants, each completed only 1 of 3 sequences, meaning the number in whom the different oils were compared ranged from 26 to 27 participants. In addition, the participants did not receive the different oils in random order. Overall, these results indicate a detrimental effect on both total and LDL cholesterol. In addition, although HDL-C was higher after the coconut oil diet, the proportionally greater increase in LDL-C resulted in a significantly higher ratio of LDL-C to HDL-C compared with the ratio in participants who consumed the corn oil diet. Collectively, these findings indicate that coconut oil consumption results in an atherogenic profile when compared with corn oil consumption. Further, coconut oil offered no advantage over palm oil.
A randomized community-based feeding experiment with 60 Sri Lankans over a 62-week period assessed the effects of lowering coconut and saturated fat intake compared with partial replacement of saturated fat from coconut with polyunsaturated fat from soyabean and sesame oil.35 Both intervention diets were low in fat (20% total energy vs 24% total energy). The use of field workers who made regular visits to the households facilitated the collection of detailed dietary information and supervision of the intervention phases. The results showed that reducing the saturated fat intake or replacing a portion of the saturated fat in the diet with unsaturated fat resulted in improved serum lipoprotein profiles compared with baseline. Both groups showed a 24% to 27% decrease in the ratio of total cholesterol to HDL-C at 12 months compared with baseline (both groups, P < 0.002). The difference between groups was not significant. In addition, there were significant reductions in LDL-C and increases in HDL-C in both groups, with a significantly greater reduction in LDL-C in the soyabean and sesame oil group. The only adverse effect was a small but significant increase (8.2%) in triglycerides in the group supplemented with soyabean and sesame oil, but in this group some of the total fat was replaced by carbohydrate. A limitation of this study is the lack of a control group whose intake of coconut oil was not modified.
An earlier sequential feeding study by Mendis and Kumarasunderam34 assessed the effects of coconut oil consumption vs soyabean oil consumption over two 8-week periods, with a 3-week washout period, on 25 healthy males. Thirty percent of total energy was consumed as fat, the test fats constituting 70% of fat intake. In contrast to the later findings of Mendis et al.,35 this study showed a beneficial effect of soyabean oil on triglyceride levels (1.42–1.06 mmol/L) compared with baseline (P < 0.01). Additionally, the soyabean intervention significantly reduced total cholesterol (4.46–3.68 mmol/L) and LDL-C (2.95–2.27 mmol/L) (both P < 0.01) and lowered HDL-C from 1.10 to 0.94 mmol/L (P < 0.05). The coconut oil diet resulted in a return to levels of serum lipids that were not significantly different from baseline levels. Only total cholesterol was reported as being statistically significantly different between the coconut oil and soyabean oil interventions. Interestingly, although LDL-C was lower with soyabean oil than with coconut oil (2.27 ± 0.36 vs 2.84 ± 0.3 mmol/L), this difference was not statistically significant, a finding unique to this study.
Fisher et al.33 compared corn oil with coconut oil in mixed diets in a small number (9 healthy males) of participants for 9 days. They measured changes in blood lipids and lipoproteins and examined the effects of individual variations in lipoprotein metabolism on the end results. Coconut oil was found to have adverse effects, shifting the distribution of apolipoprotein E toward very low-density lipoprotein (VLDL) cholesterol fractions. Compared with the corn oil diet, the coconut oil diet was associated with significant increases in total cholesterol, VLDL cholesterol, intermediate-density lipoprotein cholesterol + LDL-C, HDL-C, total triglycerides, and VLDL triglycerides as well as significant increases in the apolipoprotein E content in intermediate-density lipoprotein cholesterol + LDL-C (all P < 0.01). An important limitation of this study is the small sample size, which hampers interpretation of the results.
DISCUSSION
This review identified a limited amount of human research on which to assess the merits of coconut oil or coconut products in relation to cardiovascular health. Much of the research has important limitations that warrant caution when interpreting results, such as small sample size, biased samples, inadequate dietary assessment, and a strong likelihood of confounding.
There is no robust evidence on disease outcomes, and most of the evidence is related to lipid profiles. In comparison with other fat sources, coconut oil did not raise total or LDL cholesterol to the same extent as butter in one of the studies by Cox et al.,31 but it did increase both measures to a greater extent than did cis unsaturated vegetable oils.
In total, 7 intervention studies directly compared coconut oil with oils containing cis unsaturated fat.31–34,36–38 The coconut oil interventions resulted in significantly higher total cholesterol in all 7 trials, with significantly higher LDL-C in 6 trials31–33,36–38 and with no significant difference in 1 trial.34 High-density lipoprotein cholesterol was significantly higher after the coconut oil interventions in 5 of the trials,32,33,36–38 with no significant difference observed in the remaining 2 trials.31,34 Ratios of total cholesterol to HDL-C or of LDL-C to HDL-C were examined in only 2 of these studies. One study reported a significantly higher LDL-C:HDL-C ratio after a coconut oil diet compared with a corn oil diet,36 while one reported a significantly lower ratio of total cholesterol to HDL-C following a coconut oil diet compared with an olive oil diet.38 Five studies reported no significant difference in triglyceride concentrations,31,32,34,37,38 while 2 studies reported lower triglyceride concentrations following a corn oil diet compared with a coconut oil diet.33,36
While the inconsistent findings on the effects of coconut oil on HDL-C, on the ratio of total cholesterol to HDL-C, and on the ratio of LDL-C to HDL-C make it difficult to predict the effects of coconut oil on CVD risk, it should be noted that the significantly lower LDL-C concentrations observed among participants who received cis unsaturated fat treatments compared with participants who received coconut oil diets ranged from 0.24 mmol/L to 1.03 mmol/L. It has been reported that every 1-mmol/L reduction in LDL-C is associated with a corresponding average 22% reduction in CVD mortality and morbidity.39 In addition, it has been calculated that, in New Zealand, a reduction in the incidence of ischemic heart disease in the order of 10% among the population can be achieved by substituting 5% of the daily energy (10–14 g) from saturated fat with polyunsaturated fat.40 On the basis of these predictions, it appears that consuming cis unsaturated fat in place of coconut oil is likely to result in substantial reductions in the risk of CVD.
Not all saturated fatty acids produce the same cholesterol-raising effects. Differences in the effects have been attributed to a combination of variations in the structural shape, the melting point, and the water solubility of the fatty acids.11 Fatty acids may affect the metabolism of fat in the liver as well as subsequent levels of cholesterol and lipoproteins in several ways, depending on how the fatty acid is presented to the liver, i.e., via the portal vein or via chylomicrons,11 or on the stereospecific position of the fatty acid in the triglyceride molecule.41
Ninety-five percent of medium-chain triglycerides are absorbed through the portal vein. The subsequent rise in cholesterol was reported to be 50% of that from palmitic acid.42 In contrast, less than 5% of palmitic acid is absorbed via the portal vein. Studies have reported that 25% to 30% of lauric acid is absorbed through the portal vein.11 Lauric acid, because of its shorter chain length and lower melting point, may impart less rigidity to triglyceride and phospholipid molecules than does palmitic acid and thus may have different effects on hepatic cholesterol and/or lipid metabolism. If only 70% of the lauric acid is absorbed via chylomicrons, then this may explain why LDL-C concentrations were lower after coconut oil consumption than after butterfat consumption in one of the studies by Cox et al.31
However, when the data from the 5 trials that directly compared coconut oil with another saturated fat are examined collectively, the results are largely inconsistent.31,32,36–38 The comparators in these interventions included butter in 2 trials,31,32 beef fat in 1 trial,37 and palm oil in the remaining 2 trials.36,38 There was a significant reduction in total and LDL cholesterol in the coconut oil arm compared with the butter arm in one study,31 but not in another.32 One study found no difference between palm oil and coconut oil with regard to changes in total and LDL cholesterol,38 while the other reported significantly higher concentrations following the coconut oil diet.36 Compared with the beef fat arm, total cholesterol, but not LDL-C, was significantly higher in the coconut oil arm.37 Concentrations of HDL-C were not different between coconut oil and butter treatments31,32 but were significantly higher in the coconut oil arm of a study that used beef fat as the comparator.37 Conversely, HDL-C was higher in the coconut oil arm than in the palm oil in one study,36 but not the other.38 Triglyceride concentrations were significantly lower in one of the studies in which the comparison was butter,31 but not in the other.32 Triglycerides were also significantly lower when coconut oil was compared with beef fat,37 but they did not differ when coconut oil was compared with palm oil.36,38 Only one study36 measured the LDL-C:HDL-C ratio and reported a lower ratio following the palm oil diet compared with the coconut oil diet. The total cholesterol to HDL-C ratio was measured only by Voon et al.,38 who reported no difference between the coconut oil diet and the palm oil diet. Collectively, these results do not provide evidence that coconut oil acts consistently different from other saturated fats in terms of its effects on blood lipids and lipoproteins.
In summary, this review found no evidence that coconut oil should be viewed differently from other sources of dietary saturated fat with regard to dietary recommendations. This is in line with recommendations from the American Heart Association and the US Department of Agriculture’s Dietary Guidelines for Americans, 201043, which suggest that coconut oil is not preferable to other saturated fats. Guidelines from both agencies continue to recommend that dietary saturated fat be limited to 7% to 10% of calories because it can increase the risk for heart disease.
It has been hypothesized that the more favorable lipid profiles and lower mortality rates observed in coconut-consuming populations are due to the foods that constitute the rest of their traditional diets.17 The majority of the Pacific Island populations, such as those from the islands of Tokelau and Pukapuka, traditionally ate no processed foods and consumed a diet high in fruit and vegetables, with the main protein source being fish. The original participants of the Kitava study had an active lifestyle without major influences from a Western diet. This is in contrast to the consumption of coconut oil in a typical Western diet, which contains more processed foods, less fish, less fruit and vegetables, and more saturated fat than recommended in dietary guidelines.44
Studies suggest that the consumption of coconut products that contain fiber, such as coconut flesh and coconut flour,45 within a traditional dietary pattern that includes sufficient polyunsaturated fats (omega-3) in the absence of excessive calories from refined carbohydrates does not pose a risk for heart disease. In contrast, the excessive use of coconut oil as the major lipid in the typical Western diet produces effects similar to those of other saturated fats. Despite claims that coconut oil may reduce cardiovascular risk factors, this review found no evidence indicating that coconut oil is preferable to other unsaturated plant oils.
This review included studies published up to the end of 2013. The search was subsequently updated to include studies published up to November 2015. Although several studies were published during this period, most suffer fundamental flaws, and thus only one study met the inclusion criteria.46 In a further analysis of their previously reported study, Voon et al.38 used a randomized crossover study to compare the effects of virgin olive oil, palm olein, and coconut oil on cell adhesion molecules and thrombogenicity indices in 45 healthy adults in Malaysia. As mentioned previously, the diets contained 30% of energy from fat, and the study oils supplied two-thirds of total fat. There were no significant differences between the 3 oils for thrombogenicity indices, but olive oil significantly lowered proinflammatory leukotriene B4 compared with both palm olein and coconut oil. In addition, the plasma antiaggregatory 6-keto-prostaglandin F1α was significantly lower after the olive oil treatment compared with the palm olein treatment.
This additional study also fails to provide any evidence that consumption of coconut oil results in benefits preferable to those seen with plant cis unsaturated oils, and thus the conclusion of this review remains unchanged.
CONCLUSION
In summary, although evidence of an association between coconut consumption and risk factors for heart disease is mostly of very poor quality, it suggests that coconut oil, when compared with cis unsaturated plant oils, raises total cholesterol, HDL-C, and LDL-C, although not as much as butter does. The impact of coconut oil consumption on the ratio of total cholesterol to HDL-C was often not reported. No convincing evidence that consumption of coconut oil, as opposed to consumption of unsaturated oils, led to improved lipid profiles and a decreased risk of CVD was discovered during the literature search. Overall, the weight of the evidence to date suggests that replacing coconut oil with cis unsaturated fats would reduce CVD risk. Therefore, this review does not support popular claims purporting that coconut oil is a healthy oil in terms of reducing the risk of CVD. There was no evidence that coconut oil acted consistently different from other saturated fats in terms of its effects on blood lipids and lipoproteins. Given the limited number of intervention studies in this area, along with the methodological flaws evident in existing studies, further well-designed randomized trials that include appropriate controls, are adequately powered, and examine a range of CVD risk factors are required.
Acknowledgments
Funding. Funding for this review was provided by the National Heart Foundation of New Zealand (NHFNZ). Nutritionists from the NHFNZ assisted with both the initial literature review and the production of an internal report for their website but played no role in the writing of this paper.
Declaration of interest. The authors have no relevant interests to declare.
References
- 1.Eyres L. Handbook of Australasian Edible Oils. Auckland: Oils and Fats Specialist Group of the New Zealand Institute of Chemistry; 2007. [Google Scholar]
- 2.Marina A, Che-Man Y, Nazimah S, et al. Chemical properties of virgin coconut oil. J Am Oil Chem Soc. 2009;86:301–307. [Google Scholar]
- 3.Gunstone F. Lauric oils. Lipid Technol. 2010;22:168 doi:10.1002/lite.201000035. [Google Scholar]
- 4.Srivastava S, Singh M, George J, et al. Genotoxic and carcinogenic risks associated with the dietary consumption of repeatedly heated coconut oil. Br J Nutr. 2010;104:1343–1352. [DOI] [PubMed] [Google Scholar]
- 5.Sivakumaran S, Huffman L. The Concise New Zealand Food Composition Tables. 11th ed. Palmerston North, New Zealand: New Zealand Institute for Plant and Food Research Limited and Ministry of Health New Zealand; 2014. [Google Scholar]
- 6.US Department of Agriculture, Agriculture Research Service. USDA National Nutrient Database for Standard Reference. Nutrient Data Laboratory website; 2012. http://www.nal.usda.gov/fnic/foodcomp/search/. Accessed March 23, 2012. [Google Scholar]
- 7.Yong JW, Ge L, Ng YF, et al. The chemical composition and biological properties of coconut (Cocos nucifera L.) water. Molecules. 2009;14:5144–5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bach A, Babayan V. Medium-chain triglycerides: an update. Am J Clin Nutr. 1982;36:950–962. [DOI] [PubMed] [Google Scholar]
- 9.Eyres L. Analysis of edible oils and fats. Chem New Zeal. 1979;43:237–239. [Google Scholar]
- 10.Williams M, Tamai K, Hincenbergs T, et al. Hydrogenated coconut oil and tissue fatty acids in EFA-depleted and EFA-supplemented rats. J Nutr. 1972;102:847–856. [DOI] [PubMed] [Google Scholar]
- 11.Denke MA, Grundy SM. Comparison of effects of lauric acid and palmitic acid on plasma lipids and lipoproteins. Am J Clin Nutr. 1992;56:895–898. [DOI] [PubMed] [Google Scholar]
- 12.Swift LL, Hill JO, Peters JC, et al. Medium-chain fatty acids: evidence for incorporation into chylomicron triglycerides in humans. Am J Clin Nutr. 1990;52:834–836. [DOI] [PubMed] [Google Scholar]
- 13.Marten B, Pfeuffer M, Schrezenmeir J. Medium-chain triglycerides. Int Dairy J. 2006;16:1374–1382. [Google Scholar]
- 14.Timmermann F. Oils and Fats in the Nineties. Lystrup, Denmark: International Food Science Centre; 1992. [Google Scholar]
- 15.Stanhope JM, Sampson VM, Prior IA. The Tokelau Island Migrant Study: serum lipid concentration in two environments. J Chronic Dis. 1981;34:45–55. [DOI] [PubMed] [Google Scholar]
- 16.Parry J. Pacific Islanders pay heavy price for abandoning traditional diet. Bull World Health Organ. 2010;88:484–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiBello JR, McGarvey ST, Kraft P, et al. Dietary patterns are associated with metabolic syndrome in adult Samoans. J Nutr. 2009;139:1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar PD. The role of coconut and coconut oil in coronary heart disease in Kerala, South India. Trop Doc. 1997;27:215–217. [DOI] [PubMed] [Google Scholar]
- 19.Lindeberg S, Berntorp E, Nilsson-Ehle P, et al. Age relations of cardiovascular risk factors in a traditional Melanesian society: the Kitava Study. Am J Clin Nutr. 1997;66:845–852. [DOI] [PubMed] [Google Scholar]
- 20.Prior IA, Davidson F, Salmond CE, et al. Cholesterol, coconuts, and diet on Polynesian atolls: a natural experiment: the Pukapuka and Tokelau island studies. Am J Clin Nutr. 1981;34:1552–1561. [DOI] [PubMed] [Google Scholar]
- 21.Abeywardena M. Dietary fats, carbohydrates and vascular disease: Sri Lankan perspectives. Atherosclerosis. 2003;171:157–161. [DOI] [PubMed] [Google Scholar]
- 22.Dayrit C. Coconut oil: atherogenic or not? Philipp J Cardiol. 2003;31:97–104. [Google Scholar]
- 23.Lindeberg S, Lundh B. Apparent absence of stroke and ischaemic heart disease in a traditional Melanesian island: a clinical study in Kitava. J Intern Med. 1993;233:269–275. [DOI] [PubMed] [Google Scholar]
- 24.Lindeberg S, Nilsson-Ehle P, Vessby B. Lipoprotein composition and serum cholesterol ester fatty acids in nonwesternized Melanesians. Lipids. 1996;31:153–158. [DOI] [PubMed] [Google Scholar]
- 25.Feranil AB, Duazo PL, Kuzawa CW, et al. Coconut oil is associated with a beneficial lipid profile in pre-menopausal women in the Philippines. Asia Pac J Clin Nutr. 2011;20:190–195. [PMC free article] [PubMed] [Google Scholar]
- 26.Sabitha P, Vaidyanathan K, Vasudevan DM, et al. Comparison of lipid profile and antioxidant enzymes among south Indian men consuming coconut oil and sunflower oil. Indian J Clin Biochem. 2009;24:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Njelekela M, Kuga S, Nara Y, et al. Prevalence of obesity and dyslipidemia in middle-aged men and women in Tanzania, Africa: relationship with resting energy expenditure and dietary factors. J Nutr Sci Vitaminol. 2002;48:352–358. [DOI] [PubMed] [Google Scholar]
- 28.Njelekela M, Sato T, Nara Y, et al. Nutritional variation and cardiovascular risk factors in Tanzania – rural-urban difference. S Afr Med J. 2003;93:295–299. [PubMed] [Google Scholar]
- 29.Lipoeto NI, Agus Z, Oenzil F, et al. Dietary intake and the risk of coronary heart disease among the coconut-consuming Minangkabau in West Sumatra, Indonesia. Asia Pac J Clin Nutr. 2004;13:377–384. [PubMed] [Google Scholar]
- 30.Beegom R, Singh RB. Association of higher saturated fat intake with higher risk of hypertension in an urban population of Trivandrum in South India. Int J Cardiol. 1997;58:63–70. [DOI] [PubMed] [Google Scholar]
- 31.Cox C, Mann J, Sutherland W, et al. Effects of coconut oil, butter, and safflower oil on lipids and lipoproteins in persons with moderately elevated cholesterol levels. J Lipid Res. 1995;36:1787–1795. [PubMed] [Google Scholar]
- 32.Cox C, Sutherland W, Mann J, et al. Effects of dietary coconut oil, butter and safflower oil on plasma lipids, lipoproteins and lathosterol levels. Eur J Clin Nutr. 1998;52:650–654. [DOI] [PubMed] [Google Scholar]
- 33.Fisher EA, Blum CB, Zannis VI, et al. Independent effects of dietary saturated fat and cholesterol on plasma lipids, lipoproteins, and apolipoprotein E. J Lipid Res. 1983;24:1039–1048. [PubMed] [Google Scholar]
- 34.Mendis S, Kumarasunderam R. The effect of daily consumption of coconut fat and soya-bean fat on plasma lipids and lipoproteins of young normolipidaemic men. Br J Nutr. 1990;63:547–552. [DOI] [PubMed] [Google Scholar]
- 35.Mendis S, Samarajeewa U, Thattil RO. Coconut fat and serum lipoproteins: effects of partial replacement with unsaturated fats. Br J Nutr. 2001;85:583–589. [DOI] [PubMed] [Google Scholar]
- 36.Ng TK, Hassan K, Lim JB, et al. Nonhypercholesterolemic effects of a palm-oil diet in Malaysian volunteers. Am J Clin Nutr. 1991;53(4 suppl):1015S–1020S. [DOI] [PubMed] [Google Scholar]
- 37.Reiser R, Probstfield JL, Silvers A, et al. Plasma lipid and lipoprotein response of humans to beef fat, coconut oil and safflower oil. Am J Clin Nutr. 1985;42:190–197. [DOI] [PubMed] [Google Scholar]
- 38.Voon PT, Ng TK, Lee VK, et al. Diets high in palmitic acid (16:0), lauric and myristic acids (12:0 + 14:0), or oleic acid (18:1) do not alter postprandial or fasting plasma homocysteine and inflammatory markers in healthy Malaysian adults. Am J Clin Nutr. 2011;94:1451–1457. [DOI] [PubMed] [Google Scholar]
- 39.Cholesterol Treatment Trialists Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foster RH, Wilson N. Review of the evidence for the potential impact and feasibility of substituting saturated fat in the New Zealand diet. Aust N Z J Public Health. 2013;37:329–336. [DOI] [PubMed] [Google Scholar]
- 41.Decker EA. The role of stereospecific saturated fatty acid positions on lipid nutrition. Nutr Rev. 1996;54(4 pt 1):108–110. [DOI] [PubMed] [Google Scholar]
- 42.Cater NB, Heller HJ, Denke MA. Comparison of the effects of medium-chain triacylglycerols, palm oil, and high oleic acid sunflower oil on plasma triacylglycerol fatty acids and lipid and lipoprotein concentrations in humans. Am J Clin Nutr. 1997;65:41–45. [DOI] [PubMed] [Google Scholar]
- 43.US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. Washington, DC: US Government Printing Office; 2010. [Google Scholar]
- 44.University of Otago and Ministry of Health. A Focus on Nutrition: Key Findings of the 2008/09 New Zealand Adult Nutrition Survey. Wellington, New Zealand: Ministry of Health New Zealand; 2011. [Google Scholar]
- 45.Trinidad TP, Loyola AS, Mallillin AC, et al. The cholesterol-lowering effect of coconut flakes in humans with moderately raised serum cholesterol. J Med Food. 2004;7:136–140. [DOI] [PubMed] [Google Scholar]
- 46.Voon PT, Ng TKW, Lee VKM, et al. Virgin olive oil, palm olein and coconut oil diets do not raise cell adhesion molecules and thrombogenicity indices in healthy Malaysian adults. Eur J Clin Nutr. 2015;69:712–716. [DOI] [PubMed] [Google Scholar]