Abstract
Introduction:
Depression is a mental disorder that highly associated with immune system. Therefore, this study compares the serum concentrations of IL-21, IL-17, and transforming growth factor β (TGF-β) between patients with major depressive disorder and healthy controls.
Methods:
Blood samples were collected from 41 patients with major depressive disorder and 40 healthy age-matched controls with no history of malignancies or autoimmune disorders. The subjects were interviewed face to face according to DSM-IV diagnostic criteria. Depression score was measured using completed Beck Depression Inventory in both groups. The serum concentrations of IL-21, IL-17, and TGF-β were assessed using ELISA.
Results:
The mean score of Beck Depression score in the patient and control groups was 35.4±5.5 and 11.1±2.3. IL-17 serum concentrations in the patients and the control group were 10.03±0.6 and 7.6±0.6 pg/mL, respectively (P=0.0002). TGF-β level in the patients group was significantly higher than compare to the control group; 336.7±20.19 vs. 174.8±27.20 pg/mL, (P<0.0001). However, the level of IL-21 was not statistically different between the two groups 84.30±4.57 vs. 84.12±4.15 pg/mL (P>0.05).
Conclusion:
Considering pro-inflammatory cytokines, current results support the association of inflammatory response and depressive disorder. So, it seems that pro-inflammatory factors profile can be used as indicator in following of depression progress and its treatment impacts.
Keywords: Major depressive disorder, Interleukin-17, interleukin-21, Transforming growth factor-beta, Inflammatory response
1. Introduction
There is robust evidence suggests that dysregulation of immune system, particularly the cytokine system, is associated with the pathophysiology of major depressive disorder (MDD) (Dubas-Slemp, Marmurowska-Michałowska, Szuster-Ciesielska, Kamińska, & Kandefer-Szerszeń, 2002; Schiepers, Wichers, & Maes, 2005; Irwin & Miller, 2007). Although the central nervous system affects the immune system via the autonomic nervous system and neuroendocrine system, the immune system inversely affects the central nervous system, via a cytokine network secreted by immune cells, to control behaviors and emotions (Muñoz-Fernández & Fresno, 1998). Cytokines are both immunoregulators and modulators of neural functions. Pro-inflammatory cytokines including interleukin 1 (IL-1), IL-6, tumor necrosis factor α (TNF-α), and interferon γ (IFN-γ) play important roles in the central nervous system, such as controlling neuronal and glial activation, proliferation, differentiation, as well as affecting neuronal plasticity, synaptogenesis, and tissue repair (Muñoz-Fernández & Fresno, 1998).
Two decades ago, Mossman and Coffman proposed that CD4+T cells differentiate into two subsets with reciprocal functions and patterns of cytokine secretion, termed T-helper 1 (Th1) and Th2 (Murphy & Reiner, 2002). Th1 cells are characterized by production of IFN-γ and induce cell-mediated immunity against intracellular pathogens, while Th2 cells produce IL-4 and stimulate humoral immunity against parasitic helminthes. This paradigm was maintained until 2005, when a third T-cell subset, known as Th17, was identified (Harrington et al., 2005). IL-17 was reported with its major signature of releasing IL-17 (Xu & Cao, 2010).
TGF-β plays an essential role in differentiation of CD4+T cells toward regulatory T cells (Tregs) or Th17 cells. The combination of TGF-β and IL-6 promotes the differentiation of Th17 cells and inhibits Treg cells differentiation in mice (Nam et al., 2008; Passos et al., 2010), whereas TGF-β plus retinoic acid inhibits Th17 cells differentiation and promotes the Treg cells (Mucida & Cheroutre, 2007; Mucida et al., 2007).
In a pro-inflammatory context, IL-1, IL-21 and IL-23 are effective in Th17 differentiation. Considering the increased level of TGF-β and the role of TGF-β for production of IL-17 (Duvallet et al., 2011), it is expected that the level of IL-17 be higher than the controls; however, a recently published study reported that the serum concentrations of IL-17 in patients with major depressive disorder was not different from the controls (Kim et al., 2013). Moreover, desipramine, an antidepressant, decreases CD4+IL-17+Th17 cells (Zhang et al., 2013). Furthermore, escitalopram also decrease IL-17 levels in patients with depression (Munzer et al., 2013).
There is a lot of controversy regarding TGF-β level in major depressive disorder. In an animal model of depression, an increased TGF-β level was found (Hong et al., 2013). In addition, this increased level of TGF-β causes an imbalance between Th17 and Treg cells (Hong et al., 2013). It is suggested that TGF-β has an important role on MDD (Lee & Kim, 2010). However, other reported that the level of TGF-β was decreased in patients with depression than the healthy controls (Musil et al., 2011; Sutcigil et al., 2007). In contrary, higher TGF-β production in major depressive disorder is reported in other studies (Kim et al., 2008; Kim et al., 2007). Nevertheless, another study reported that TGF-β levels of patients with MDD were not different from normal controls (Lee & Kim, 2006).
It is shown that IL-21 is another cytokine highly expressed by mouse Th17 cells. IL-21 is induced by IL-6 in activated T cells, a process that is dependent on STAT3 but not ROR-gamma. IL-21 potently induces Th17 differentiation and suppresses Foxp3 expression, which requires STAT3 and ROR-gamma, which is encoded by Rorc. IL-21 deficiency impairs the generation of Th17 cells and results in protection against experimental autoimmune diseases (Passos et al., 2010).
IL-21 induces the function of CD4+ and CD8+T lymphocytes leading to the differentiation of B cells into memory cells. In addition, it increases secretion of some inflammatory molecules. Therefore, it is suggested that suppression of IL-21 could be beneficial in treatment of chronic inflammatory disease (Sarra, Cupi, Pallone, & Monteleone, 2012). IL-21 is supposed as a target for treating inflammatory diseases (Sarra, Franze, Pallone, & Monteleone, 2011). In addition, an opposing role for IL-21 and TGF-β in a chronic inflammatory disease is reported (MacDonald, Bell, & Monteleone, 2011). It seems IL-17 and IL-21 are pro-inflammatory cytokines that their roles in recruiting inflammatory cells and potentiating of this event should be considered.
Due to evidences suggest that major depression is associated with signs of immunological activation and also cytokine hypersecretion may be involved in the aetiology of depressive disorders, the present study focuses IL-17, TGF-β and IL-21 serum concentration in patient with major depressive disorder.
2. Materials & Methods
2.1. Subjects
Participants were 41 patients with major depressive disorder (11 males and 30 females) of mean age, 35.6±12.3 years. The patients were a convenient sample of patients recruited from a clinic of psychiatry. They were interviewed face to face according to Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV; 1994) diagnostic criteria. All of interviews were performed in same method by one psychiatrist. The Beck Depression Inventory Second Edition (BDI-II) is a 21-item self-report instrument intended to assess the existence and severity of symptoms of depression as listed in the American Psychiatric Association’s DSM-IV. Each of the 21 items corresponding to a symptom of depression is summed to give a single score for the BDI-II. There is a four-point scale for each item ranging from 0 to 3. Total score of 0–13 is considered minimal range, 14–19 is mild, 20–28 is moderate, and 29–63 is severe. Patients with coexisting autoimmune, chronic, and acute inflammatory diseases were excluded from the study.
The control group included 40 subjects matched for age and sex (16 males and 24 females) with mean age, 36.3±6.6 years selected randomly from healthy individuals undergoing checkup. During sample collection, it was ensured that subjects had neither history of major depression, congenital anomaly, history of autoimmune disease, and cancer nor any infection, acute or chronic disease. Individuals in both groups completed Beck Depression Inventory to measure depression score. Demographic features for both of groups is summarized in table 1.
Table 1.
Demographic features in patients with MDD and healthy controls.
| Characteristics | Patients (n=41) | Controls (n=40) |
|---|---|---|
| Age (years) | 12.3±35.6 | 6.6±36.3 |
| Male | 11 | 16 |
| Female | 30 | 24 |
| BMIa (kg/m2) | 10.23±24.30 | 2.74±22.86 |
| Beck Depression score | 5.5±35.4 | 2.3±11.1 |
BMI: body mass index.
The protocol for the present study was approved by the Ethics Committee of the Jahrom University of Medical Sciences (Jahrom, Iran). Informed consent was obtained from all subjects who participated in this study. Peripheral venous blood samples (5 ml) were collected by venipuncture before any clinical intervention. After serum separation, the serum samples were stored at −70°C until cytokine measurement.
2.2. Enzyme linked immunosorbent assay (ELISA)
The amounts of IL-17, TGF-β and IL-21 in the patients and controls sera were measured at the same time by the same technician, using ELISA-kits (eBiosciences, San Diego, CA, USA). ELISA procedure was performed in one run by the one operator. Briefly, premixed standards were reconstituted in PBS (PH 7.2), generating a stock concentration of 500, 1000, 4000 pg/mL for IL-17, TGF-β and IL-21, respectively. Sensitivity for IL-17 was 4 pg/mL and minimal cross reactivity IL-17 to the recombinant human IL-17AF heterodimer is observed at 0.4%. Sensitivity for IL-21 and TGF-β were 8 and 8 pg/ml, respectively. The standard stocks were serially diluted in Reagent Diluent to generate 7 points for the standard curves. Diluted Capture Antibody was added to a 96-well, flat-bottomed, polystyrene microtiter plate, at final volume of 100 μl. Plates were sealed and incubated overnight at room temperature, then washed with Wash Buffer. Premixed standards or samples (100 μl) were added to each well, covered with an adhesive strip and incubated for overnight at 4°C. After incubation and washing, 100 μl of the premixed Detection Antibody was added to each well and the plate was covered with a new adhesive strip and incubated for 2 hours at room temperature. After incubation and washing, Streptavidin-HRP was added to each well (100 μl). The incubation was terminated after 20 min at room temperature and the plates were kept away from direct light. Then, 50 μl of Stop Solution was added to each well, and the optical density of each well was immediately determined using a microplate reader set to 450 nm. The results were expressed in pg/ml.
2.3. Statistical analysis
The serum concentrations of IL-17, IL-21 and TGF-β in the peripheral blood was evaluated to the corresponding values from control samples using nonparametric Mann-Whitney and ANCOVA test using SPSS software v. 11.5 (SPSS, Chicago, IL,173 USA). The variable levels were evaluated by means of Prism 4 software (Inc; San Diego CA, USA, 2003). P<0.05 was regarded as significant in all statistical analysis.
3. Results
The mean of Beck Depression score in the patient and control groups was 35.4±5.5 and 11.1±2.3, respectively. This score is significantly different between the two groups (t=25.3, df=79, P<0.001).
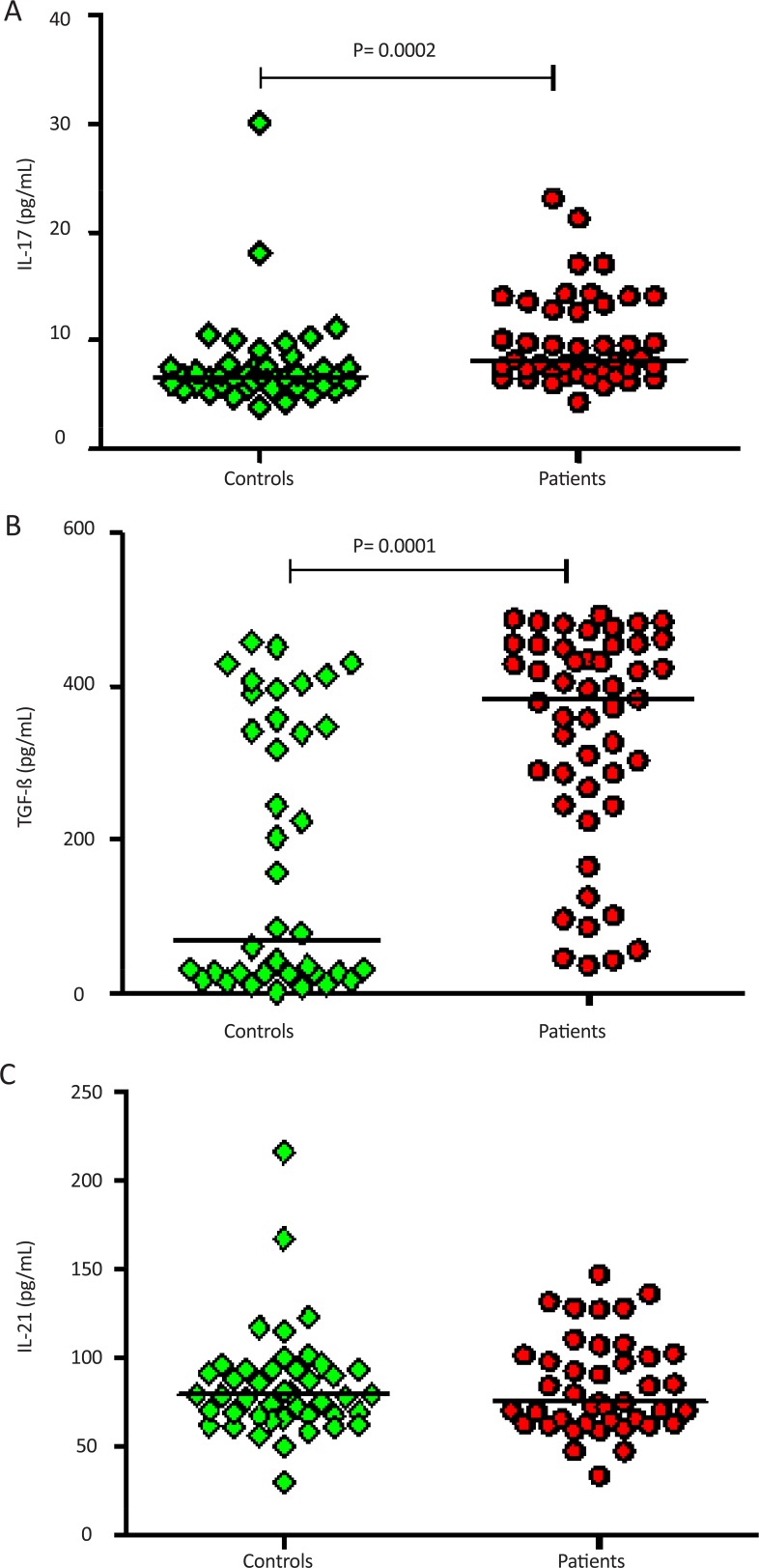
The mean±SEM of the IL-17 in the patients and the control group was 10.03±0.6 and 7.6±0.6 pg/ml, respectively. This level was significantly different between the two groups (P=0.0002); however, there was no significant difference in serum concentrations of IL-17 between healthy controls compared to male and female patients 7.6±0.6 vs. 10.84±1.41 and 9.43±0.63 pg/mL, respectively (P>0.05) (Figure 1A). In addition, the level of TGF-β was statistically different between the patients and controls. Its level in the patients group was significantly higher than that of the control group 336.7±20.19 vs. 174.8±27.20 pg/mL, (P<0.0001) (Figure 1B). However, the level of IL-21 was not statistically different between groups 84.30±4.57 vs. 84.12±4.15 pg/ml) (P>0.5) (Figure 1C). Like IL-17 and TGF-β, serum level of IL-21 between male and females had no significant difference (data not shown).
Figure 1.
Serum concentrations of IL-17, TGF-β and IL-21 in the peripheral blood of MDD patients and blood of normal controls. Presented data were analyzed with the nonparametric two-tailed Mann-Whitney test and the horizontal lines are showing the median of the groups. Significant difference was found in the serum concentrations of IL-17 (A) and TGF-β (B) among patients compared to healthy controls. No Significant difference was found in the serum concentrations of IL-21 (C) among patients compared to healthy controls.
4. Discussion
In the current study, the levels of the IL-17 and TGF-β in the patients group were significantly higher than that of the healthy controls. Considering pro-inflammatory cytokines, current results support the association of inflammatory response and depressive disorder.
Current results show that the level of IL-17 in the patients group was higher than the control group. This is in line with our hypothesis. As mentioned above, naive CD4+T cells are transformed into Th17 cells by the TGF-β (Duvallet et al., 2011). Considering the increased level of TGF-β and the role of TGF-β for production of IL-17 (Duvallet et al., 2011), it can be justified the increased level of IL-17 in the patients with major depressive disorder. Beurel et al. showed Th17 cells are increased in the brain during depression-like states, promote depression-like behaviors in mice (Beurel, Harrington, & Jope, 2013).
Additionally, the level of TGF-β in the current study was higher than that of the controls. It is in similar line with many previous studies in animal models of depression and patients with major depression (Kim et al., 2008; Kim et al., 2007). In Sutcigil’s study, TGF-β showed lower value in patients with major depression than healthy controls, and plasma TGF-β levels were significantly increased after 8-week treatment with sertraline (Sutcigil et al., 2007). Since TGF-β has multiple suppressive actions on T cells, B cells, macrophages, and other cells, sertraline may change the proinflammatory/anti-inflammatory cytokine balance via increased TGF-β levels in major depression (Lee & Kim, 2006).
Current results showed that the level of IL-21 was not different between the patient with major depressive disorder and the control group. This finding supports our hypothesis that the level of IL-21 and TGF-β would not be in same direction. One explanation is that major depression disorder is not a chronic inflammatory process such as rheumatoid arteritis. Therefore, targeting IL-21 in major depressive disorder might not be a target for treatment while its suppression could be beneficial for treating chronic inflammatory disease (Sarra, et al., 2012). Further studies should consider the factors such as exercise, gender, smoking, coronary artery disease, obesity, and pain (Janssen, Caniato, Verster, & Baune, 2010).
Both clinical and experimental studies indicate that stress and depression are associated with increased circulating concentrations of cytokines such as IL-1β, IL-6, IL-17 and IFN-γ and positive acute phase proteins, and hyperactivity of the hypothalamic–pituitary–adrenal (HPA)-axis (Connor & Leonard, 1998). The central action of cytokines may also account for the HPA axis hyperactivity that is frequently observed in depressive disorders, as proinflammatory cytokines may cause HPA axis hyperactivity by disturbing the negative feedback inhibition of circulating corticosteroids (CSs) on the HPA axis. Concerning the deficiency in serotonergic (5-HT) neurotransmission that is concomitant with major depression, cytokines may reduce 5-HT levels by lowering the availability of its precursor tryptophan (TRP) through activation of the TRP-metabolising enzyme indoleamine-2,3-dioxygenase (IDO) (Schiepers, Wichers, & Maes, 2005).
In conclusion, MDD is accompanied by immune dysregulation and activation of the inflammatory response system and can change pro-inflammatory cytokine profile according to current study with increased IL-17 and TGF-β serum concentrations. These findings indicate important role Th17 with IL-17 production in depression and it seems immune system can be influenced by disease state. Therefore, along with disease severity, increased Th17 activation-related inflammation in the patients with high score may result to disease deterioration. It seems Th17-associated cytokines like L-17 can promote depression, and specifically inhibiting the production or function of Th17 cells can suitable candidate to reduce vulnerability to depression behaviors, suggesting antidepressant effects may be attained by targeting Th17 cells. Meanwhile, current cytokine response clinically can be used as a predictor for following of depression progress and its treatment.
Acknowledgement
This work was financially supported by a grant from by Jahrom University of Medical Science.
Footnotes
Conflict of interest
The authors declare that they do not have any conflict of interest with respect to this manuscript.
Reference
- Beurel E., Harrington L. E., Jope R. S. ( 2013). Inflammatory T helper 17 cells promote depression-like behavior in mice. Biological Psychiatry, 73( 7), 622– 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor T. J., Leonard B. E. ( 1998). Depression, stress and immunological activation: the role of cytokines in depressive disorders. Life Sciences, 62( 7), 583– 606. [DOI] [PubMed] [Google Scholar]
- Dubas-Slemp H., Marmurowska-Michałowska H., Szuster-Ciesielska A., Kamińska T., Kandefer-Szerszeń M. ( 2002). The role of cytokines in depression. Psychiatria Polska, 37( 5), 787– 798. [PubMed] [Google Scholar]
- Duvallet E., Semerano L., Assier E., Falgarone G., Boissier M. C. ( 2011). Interleukin-23: a key cytokine in inflammatory diseases. Annual Medicine, 43( 7), 503– 511. [DOI] [PubMed] [Google Scholar]
- Harrington L. E., Hatton R. D., Mangan P. R., Turner H., Murphy T. L., Murphy K. M., et al. ( 2005). Interleukin 17–producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunology, 6( 11), 1123– 1132. [DOI] [PubMed] [Google Scholar]
- Hong M., Zheng J., Ding Z. Y., Chen J. H., Yu L., Niu Y., et al. ( 2013). Imbalance between Th17 and Treg cells may play an important role in the development of chronic unpredictable mild stress-induced depression in mice. Neuroimmunomodulation, 20( 1), 39– 50. [DOI] [PubMed] [Google Scholar]
- Irwin M. R., Miller A. H. ( 2007). Depressive disorders and immunity: 20 years of progress and discovery. Brain, Behavior, and Immunity, 21( 4), 374– 383. [DOI] [PubMed] [Google Scholar]
- Janssen D. G., Caniato R. N., Verster J. C., Baune B. T. ( 2010). A psychoneuroimmunological review on cytokines involved in antidepressant treatment response. Human Psychopharmacology, 25( 3), 201– 215. [DOI] [PubMed] [Google Scholar]
- Kim J. W., Kim Y. K., Hwang J. A., Yoon H. K., Ko Y. H., Han C., et al. ( 2013). Plasma levels of IL-23 and IL-17 before and after antidepressant treatment in patients with major depressive disorder. Psychiatry Investigation, 10( 3), 294– 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. K., Lee S. W., Kim S. H., Shim S. H., Han S. W., Choi S. H., et al. ( 2008). Differences in cytokines between non-suicidal patients and suicidal patients in major depression. Progress in Neuropsychopharmacological and Biological Psychiatry, 32( 2), 356– 361. [DOI] [PubMed] [Google Scholar]
- Kim Y. K., Na K. S., Shin K. H., Jung H. Y., Choi S. H., Kim J. B. ( 2007). Cytokine imbalance in the pathophysiology of major depressive disorder. Progress in Neuropsychopharmacological and Biological Psychiatry, 31( 5), 1044– 1053. [DOI] [PubMed] [Google Scholar]
- Lee H. Y., Kim Y. K. ( 2010). Transforming growth factor-beta1 and major depressive disorder with and without attempted suicide: preliminary study. Psychiatry Research, 178( 1), 92– 96. [DOI] [PubMed] [Google Scholar]
- Lee K. M., Kim Y. K. ( 2006). The role of IL-12 and TGF-β1 in the pathophysiology of major depressive disorder. International Immunopharmacology, 6( 8), 1298– 1304. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T., Bell I., Monteleone G. ( 2011). The opposing roles of IL-21 and TGFbeta1 in chronic inflammatory bowel disease. Biochemical Society Transactions, 39( 4), 1061– 1066. [DOI] [PubMed] [Google Scholar]
- Mucida D., Cheroutre H. ( 2007). TGFβ and retinoic acid intersect in immune-regulation. Cell Adhesion & Migration, 1( 3), 142– 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D., Park Y., Kim G., Turovskaya O., Scott I., Kronenberg M., et al. ( 2007). Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science, 317( 5835), 256– 260. [DOI] [PubMed] [Google Scholar]
- Muñoz-Fernández M. A., Fresno M. ( 1998). The role of tumour necrosis factor, interleukin 6, interferon-γ and inducible nitric oxide synthase in the development and pathology of the nervous system. Progress in Neurobiology, 56( 3), 307– 340. [DOI] [PubMed] [Google Scholar]
- Munzer A., Sack U., Mergl R., Schonherr J., Petersein C., Bartsch S., et al. ( 2013). Impact of antidepressants on cytokine production of depressed patients in vitro. Toxins (Basel), 5( 11), 2227– 2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K. M., Reiner S. L. ( 2002). The lineage decisions of helper T cells. Nature Reviews Immunology, 2( 12), 933– 944. [DOI] [PubMed] [Google Scholar]
- Musil R., Schwarz M. J., Riedel M., Dehning S., Cerovecki A., Spellmann I., et al. ( 2011). Elevated macrophage migration inhibitory factor and decreased transforming growth factor-beta levels in major depression--no influence of celecoxib treatment. Journal of Affective Disorders, 134( 1–3), 217– 225. [DOI] [PubMed] [Google Scholar]
- Nam J. S., Terabe M., Kang M. J., Chae H., Voong N., Yang Y. A., et al. ( 2008). Transforming growth factor β subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer research, 68( 10), 3915– 3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos S. T., Silver J. S., O’Hara A. C., Sehy D., Stumhofer J. S., Hunter C. A. ( 2010). IL-6 promotes NK cell production of IL-17 during toxoplasmosis. Journal of Immunology, 184( 4), 1776– 1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarra M., Cupi M. L., Pallone F., Monteleone G. ( 2012). Interleukin-21 in immune and allergic diseases. Inflammation and Allergy Drug Targets, 11( 4), 313– 319. [DOI] [PubMed] [Google Scholar]
- Sarra M., Franze E., Pallone F., Monteleone G. ( 2011). Targeting interleukin-21 in inflammatory diseases. Expert Opinion on Therapeutic Targets, 15( 6), 695– 702. [DOI] [PubMed] [Google Scholar]
- Schiepers O. J., Wichers M. C., Maes M. ( 2005). Cytokines and major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 29( 2), 201– 217. [DOI] [PubMed] [Google Scholar]
- Sutcigil L., Oktenli C., Musabak U., Bozkurt A., Cansever A., Uzun O., et al. ( 2007). Pro-and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clinical and Developmental Immunology, 2007, 2007: 76396. doi: 10.1155/2007/76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Cao X. ( 2010). Interleukin-17 and its expanding biological functions. Cellular & Molecular Immunology, 7( 3), 164– 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhen H., Yao W., Bian F., Mao X., Yang X., et al. ( 2013). Antidepressant drug, desipramine, alleviates allergic rhinitis by regulating Treg and Th17 cells. International Journal of Immunopathol Pharmacology, 26( 1), 107– 115. [DOI] [PubMed] [Google Scholar]