Abstract
Introduction:
Alzheimer’s disease (AD) is one of the most common neurodegenerative disorders, which has much benefited from animal models to find the basics of its pathophysiology. In our previous work (Haghani, Shabani, Javan, Motamedi, & Janahmadi, 2012), a non-transgenic rat model of AD was used in electrophysiological studies. However, we did not investigate the histological aspects in the mentioned study.
Methods:
An AD model was developed through bilateral injection of amyloid-β peptides (Aβ) into the frontal cortices. Behavioral and histological methods were used to assess alterations in the memory and (ultra)structures. Furthermore, melatonin has been administered to assess its efficacy on this AD model.
Results:
Passive avoidance showed a progressive decline in the memory following Aβ injection. Furthermore, Nissl staining showed that Aβ neurotoxicity caused shrinkage of the CA1 pyramidal neurons. Neurodegeneration was clearly evident from Fluoro-jade labeled neurons in Aβ treated rats. Moreover, higher NF-κB immunoreactive CA1 pyramidal neurons were remarkably observed in Aβ treated rats. Ultrastructural analysis using electron microscopy also showed the evidence of subcellular abnormalities. Melatonin treatment in this model of AD prevented Aβ-induced increased NF-κB from immunoreaction and neurodegeneration.
Discussion:
This study suggests that injection of Aβ into the frontal cortices results in the memory decline and histochemical disturbances in CA1 pyramidal neurons. Furthermore, melatonin can prevent several histological changes induced by Aβ.
Keywords: Amyloid-β, Frontal cortex, Hippocampus, Memory, Melatonin, Animal model
1. Introduction
Alzheimer’s disease (AD) is one of the most prevalent neurodegenerative disorders. One of the main hypotheses about the pathology of AD is amyloid-β (Aβ) aggregations, which is known to be a basis for the majority of structural and functional changes in the vulnerable loci of the brain, including frontal cortex areas, entorhinal cortex, and the hippocampus (Huang & Muck, 2012).
The pathological signs of AD in human include the appearance of Aβ aggregation firstly in frontal cortex areas and over time its spread to the subcortical structures in a frontotemporal fashion (Arriagada, Growdon, Hedley-Whyte, & Hyman, 1992; Harris et al., 2010; Nath et al, 2012). We exploited this point of view in a rat model of AD to study electrophysiological properties of CA1 pyramidal neurons and the cellular basis of Aβ-induced changes in the neuronal intrinsic excitability (Haghani, Shabani, Javan, Motamedi, & Janahmadi, 2012; Haghani, Janahmadi, & Shabani, 2012; Esmaeili Tazangi, Moosavi, Shabani, & Haghani, 2015). To develop this model, Aβ (1–42) peptides were deeply injected into the frontal cortices bilaterally. However, histological aspects of this model were not investigated.
The usage of melatonin has a long history in the prevention of age- and AD-dependent cognitive declines. Data from clinical trials indicated that melatonin supplementation improves sleep, ameliorates sundowning and slows down the progression of cognitive impairment in patients with AD (Wang & Wang, 2006; Rosales-Corral et al., 2012). Furthermore, although it has long been shown that melatonin efficiently protects the neuronal cells from Aβ-mediated toxicity via antioxidant and anti-amyloid properties (Cheng, Feng, Zhang, & Zhang, 2006), therapeutic effects of melatonin are still under intense research (Jun et al., 2013; Liu et al., 2013; Rudnitskaya et al., 2015; Stefanova et al., 2015). In this regard, injection of Aβ peptides into frontal cortex was used as a less clarified cause in the early onset and in vivo model of AD. Also, we were prompted to further investigate the effectiveness of melatonin treatment on the potential behavioral changes and histological alterations in the hippocampus.
Present study was done to address some of the histological consequences of toxicity in the hippocampus induced by frontal cortex injection of Aβ peptides and to further examine the effectiveness of melatonin treatment against these potentially Aβ-induced cognitive and histologic alterations. We showed that remotely injected Aβ peptides did exert memory decline and had detrimental histochemical effects in the most vulnerable cells of the hippocampus, i.e. CA1 pyramidal neurons. Additionally, melatonin treatment prevented some of the Aβ-induced histopathologies.
2. Methods
2.1. Animals
Male Wistar rats (7–8 weeks old) weighting 100–120 g were kept in a 12:12 h light-dark cycle (lights on at 6 a.m.) and humidity-controlled animal house with water and food ad libitum. The experiments were conducted in accordance with the animal care and guidelines approved by the Institutional Ethic Committees at Iran University of Medical Sciences and Shefa Neuroscience Research Center. Rats were divided into four groups including, control, sham (in which normal saline 3μL was injected into the frontal cortex bilaterally), Aβ injected, and Aβ injected plus melatonin treatment. The number of animals used in each experiment will be appropriately mentioned in the results.
2.2. Animal surgery and melatonin treatment
Aβ (1–42, Sigma, UK) was dissolved in the normal saline at a concentration of 10 ng/μL, aliquoted and stored at −80°C until use. For animal surgery, rats were anesthetized intraperitoneally (i.p.) with a mixture of ketamine (80 mg/kg) and xylazine (20 mg/kg). 3μL of prepared Aβ was bilaterally injected into the frontal cortex using a 10-μL Hamilton syringe and stereotaxic apparatus (Bregma coordinates: 3.2 mm anteroposterior, 2 mm mediolateral, and 3mm depth) according to the literature (Haghani, Shabani, Javan, Motamedi, & Janahmadi, 2012; Altobellia, Cimini, Espositoc, Iuvonec, & Ciminia, 2015). Sham-vehicle rats underwent the same surgical procedure, except that normal saline (as a vehicle of Aβ peptide) was bilaterally injected. A separate group of Aβ treated rats received daily i.p. injection of melatonin 30 mg/kg for 10 days starting from the day of surgery. The histochemical experiments were conducted 10 days after Aβ injection.
2.3. Passive avoidance behavioral test
Behavioral assessment in rats was evaluated using a passive avoidance shuttle box (Haghani, Shabani, Javan, Motamedi, & Janahmadi, 2012). Briefly, on the third day after Aβ injection, rats were subjected to the adaptation and learning sessions. To do this, rats were individually put into the light chamber and allowed to circulate freely in the shuttle box. During 60 s of adaptation, if the rat did not enter into the dark chamber, it was excluded from the rest of experiments. Two hours after adaptation, the learning task was done in which the rats were again individually placed into the light chamber and after 10 s the door between the chambers was lifted. Once the rat entered into the dark chamber an electrical foot shock (1 mA, 50 Hz square wave, 1.5 s) was applied. After 20 s, rats were removed from the dark chamber. This learning task was repeated every 2 min until the rats learnt not to enter the dark chamber during a 300 s period. The first and second retention sessions were conducted one day and one week later, respectively. In the learning and retention trials the latency of rats to enter the dark chamber via open door was considered as Step Through Latency (STL) and the maximum cutoff time for the STL was 300 s.
2.4. Histological assessments
2.4.1. Nissl staining
After the second retention session, the rats were deeply anesthetized with ether and sacrificed. Brains have been fixed via transcardial perfusion of phosphate buffered saline (PBS, 0.1 M, pH 7.4) followed by 200–250 mL paraformaldehyde solution (1%) (Saffarzadeh et al., 2015). Brains were removed and postfixed by fixative for one week at 4°C. Then, brains were dehydrated using a series of alcohols and xylene.
Finally, the paraffin embedded blocks were prepared. The blocks were cut coronally on a microtome at a thickness of 8 μm and mounted onto the silan-coated slides. Before staining, the sections on the glass slides were deparaffinized and then hydrated using a series of alcohols and distilled water, and finally stained using Cresyl violet for general histology. The soma diameter (in μm) of pyramidal neurons was assessed from the photos taken from CA1 pyramidal layer under 40X light microscopy. To quantify the soma diameters, a pyramidal-like neuron was randomly selected from pyramidal layer of the CA1 region from each slice and an average was taken from each neuron’s long, short, and an intersected oblique axes defined on its soma using Image J software by an experimenter blind to the experimental groups.
2.4.2. Fluoro-jade staining
Degenerating neuronal soma and their processes were detected using Fluoro-Jade B (Abcam, UK). Briefly, brain sections were deparaffinized on a 60°C heater for 20 min and incubated in each of the following solutions for the time indicated: alcohol 100%, 3 min; alcohol 70%, 1 min; distilled water (H2O), 1 min; potassium permanganate 0.06%, 15 min; H2O, 1 min; Fluoro-Jade B 0.001% in acetic acid 0.09%, 30 min; H2O, 2×1 min. Stained sections were allowed to dry at room temperature protected from the light. Sections were coverslipped and kept at 4°C avoided from the light (Schmued, Albertson, & Slikker, 1997). Then, they were examined using an inverted microscope (BX71, Olympus, Japan) equipped with the fluorescence filter. The pictures were captured by a digital camera. The number of green fluorescently labeled neurons per mm2 in CA1 pyramidal layer was counted from 3–4 sections per brain using Image J software.
2.4.3. NF-κB immunohistochemistry
After deparaffinization and rehydration using alcohol and H2O, the endogenous peroxidase activity was quenched by hydrogen peroxide 0.3% diluted in PBS (0.1 M, pH 7.4) for 10 min. After washing in PBS, sections were brought to a boil in 10 mM sodium citrate buffer (pH 6) for 10 min. After cooling and washing in PBS, sections were blocked with normal goat serum diluted in PBS for 20 min. Active NF-kB was probed by using an anti-p65 antibody, Biotinylated Goat Anti-Rat IgG (BA-9400, Vector Lab, Canada) diluted 1:50 in PBS at 4°C overnight. After washing, sections were incubated with VECTASTAIN Elite ABC reagents (PK-6100, Vector Lab, Canada) for 40 min at room temperature to visualize the binding of the antibody to p65. The chromogen diaminobenzidine tetrahydrochloride (DAB, Sigma) was used followed by the counterstaining with Hematoxylin. The pictures were captured by a digital camera. The number of immunoreactive brownish neurons per mm2 in CA1 pyramidal layer was counted from 3–4 sections per brain using Image J software (Butler, Moynagh, & O’Connor, 2002).
2.4.4. Ultrastructural analysis
The deeply anesthetized rats were transcardially per-fused with 200 mL of normal saline followed by 200 mL of paraformaldehyde 4% in PBS (0.1 M, pH 7.4) at 4°C. The hippocampi were removed and immersed in glutaraldehyde 2.5%. The specimens were then washed in PBS and postfixed in OsO4 1% for 2 h at room temperature.
After dehydration in ascending ethanols, they were embedded in Epon 812 resin (TAAB, UK) and polymerized for 48 h at 55°C. Semi-thin sections (0.3 μm) were stained with toluidine blue to identify CA1 pyramidal layer. Subsequently, 70 nm sections were cut and stained with uranyl acetate and lead. The sections were examined with a LEO 906 electron microscope (Zeiss, Germany). Ultrathin sections were analyzed to find changes in neuronal ultrastructure consistent with apoptosis, including swelling and vacuolization of mitochondria, nuclear pyknosis, and margination of nucleoli (Kermanian et al., 2012).
2.5. Statistical analyses
Statistical analysis was performed with SPSS software (version 17). Analysis with one-sample Kolmogorov-Smirnov showed that the distribution of data was normal. Therefore, the parametric test, One-way ANOVA was used followed by Tukey post-hoc test for all statistical comparisons. The values are presented as mean±S.E.M. P<0.05 was statistically considered significant.
3. Results
3.1. Bilateral Aβ injection into the frontal cortex caused memory impairment
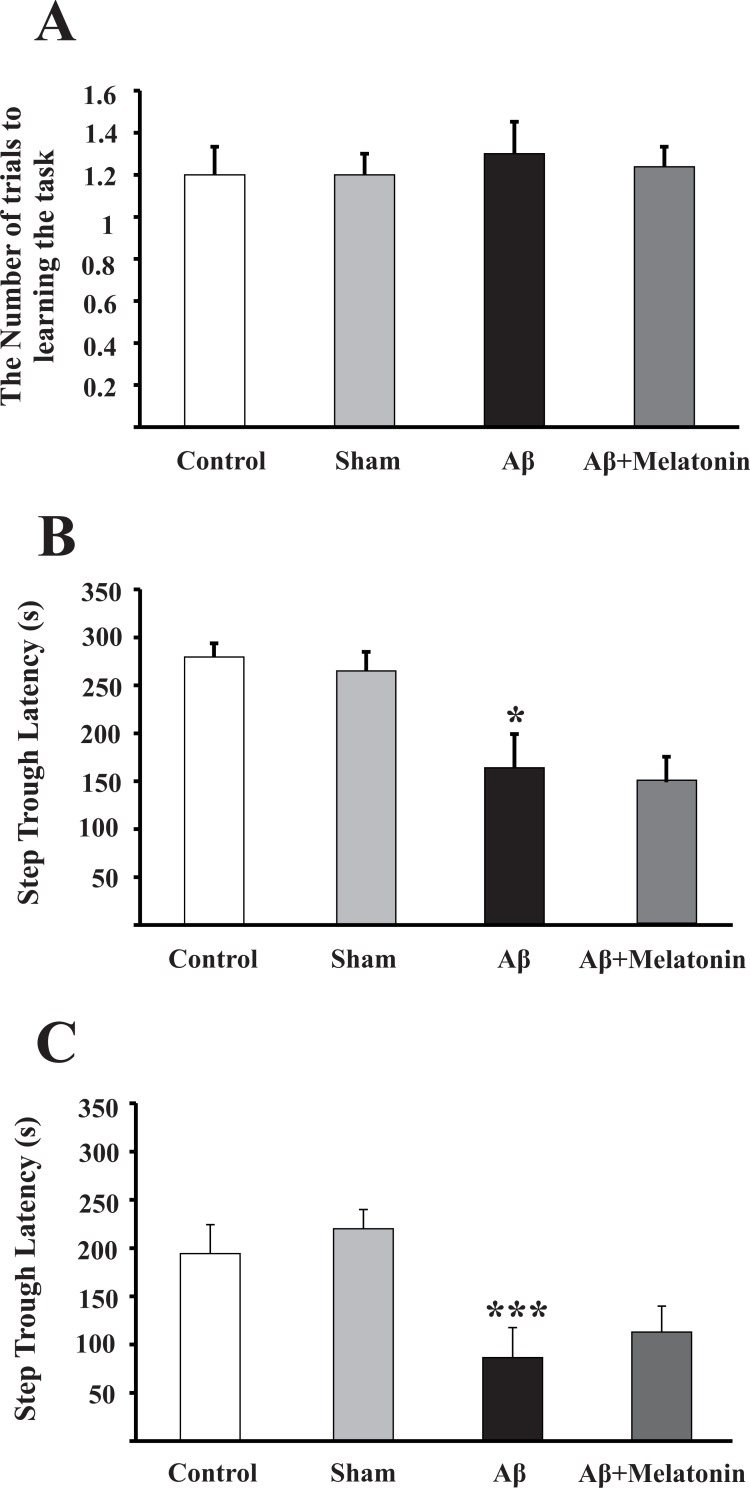
On the third day after Aβ injection, rats were subjected to the adaptation and learning sessions. During learning sessions, rats learnt not to enter dark chamber during 300 s after giving electrical foot shocks. The number of foot shocks was considered as a measure of learning ability (control 1.2±0.1, n=10; sham 1.2±0.1, n=7; Aβ-treated 1.3±0.15, n=10; Aβ+Melatonin treated 1.23±0.09, n=11; P=0.91 (Figure 1A).
Figure 1.
Injection of Aβ into the frontal cortices impaired memory. A) Leaning trial on the third day after Aβ injection as indicated by the number of shocks to learn not to enter the dark chamber during 300s period. B) The first retention trial, 24 h after learning trial and C) The second retention trial one week after the first retention trial. *P=0.019 and ***P=0.001 compared to control group. Analysis was done by one-way ANOVA with Turkey post-hoc test.
The first retention trial was assessed 1 day after leaning session, indicative of a short-term memory function, via measuring Step Through Latency (STL) to enter dark chamber. In intact rats, this latency was 279±14 s (n=10) and injection of 3 μL normal saline in each frontal cortex (sham rats) did not change this latency (265±20 s, n=7; P=0.52), while Aβ treatment significantly reduced the STL in the first retention trial compared to that of control rats (164±35 s, n=10, P=0.019; Figure 1B).
To have an estimate of a longer term memory function, probe test was measured 1 week after the first retention trial. At this time point, there was also no difference in the ability of rats in the passive avoidance task between control and sham groups (Control, 194±30 s, n=10; Sham, 220±20 s, n=7; P=0.76). However, STL was severely reduced in Aβ treated rats compared to control rats (86±31 s, n=10; P=0.001; Figure 1C).
Evaluating the effectiveness of melatonin therapy, however, showed that melatonin did not prevent Aβ-induced memory deficit neither in the first nor in the second retention trials (149±26 s, n=11, P=0.45 and 113±26 s, n=11, P=0.94, respectively; Figure 1B & C). These findings imply that injection of Aβ into deep frontal cortices can aggravate hippocampal dependent memory to an extent that even pharmacologically relevant dose of melatonin cannot prevent it.
3.2. Injection of Aβ peptide into the frontal cortex reduced the soma diameter of CA1 pyramidal neurons
Histological evaluation with Nissl staining 10 days after Aβ injections showed that the diameter of CA1 pyramidal soma did not change in sham operated rats (control 16.5±0.5 μm, n=12 slices from 4 rats; sham 17.1±0.4 μm, n=10 slices from 4 rats; P=0.91; Figure 2A–B, & E). However, after 10 days of Aβ peptide injection into the frontal cortex, the soma diameter of pyramidal neurons was significantly reduced compared to control rats (12.6±0.6 μm, n=14 slices from 5 rats; P=0.002; Figure 2C & E).
Figure 2.
Injection of Aβ into the frontal cortices reduced the soma size. Photomicrographs of Nissl staining of CA1 pyramidal neurons in control (A), sham (B), Aβ treated (C), and Aβ+melatonin treated rats (D). Shrunk and darkly stained pyramidal neurons are remarkable in C and D. Quantitative analysis of soma diameter between groups (E). **P=0.002 indicates comparison with control rats. Analysis was done by one-way ANOVA with Turkey post-hoc test. Scale bar shows 50 μm.
Moreover, melatonin treatment had no effect compared to Aβ treated rats (11.9±0.5 μm, n=11 slices from 3 rats, P=0.45; Figure 2D & E). These findings indicate that the injection of Aβ peptides into the frontal cortices reduces soma diameter of CA1 pyramidal neurons. However, melatonin treatment could not prevent this effect of Aβ peptides.
3.3. Injection of Aβ peptides into the frontal cortex increased degeneration of CA1 pyramidal neurons
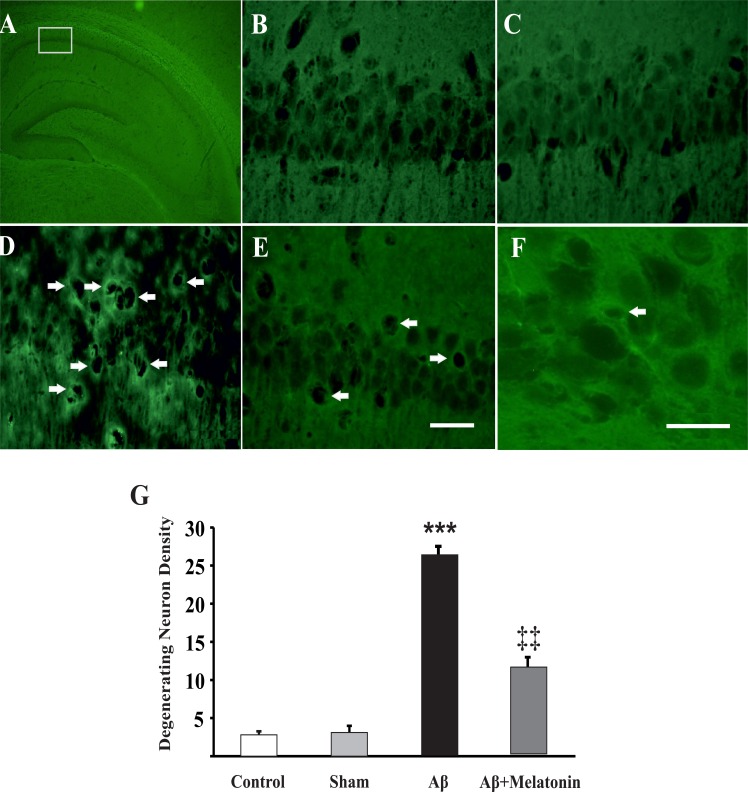
Cellular degeneration is one of the hallmarks of neurodegenerative disorders like AD. To address this issue in the present animal model of AD, neurodegeneration was assessed using a highly sensitive fluorescent dye, Fluoro-jade B. Analysis of the images from control and sham operated rats showed a few fluorescent neurons (per mm2) in the CA1 pyramidal layer (control 2.8±0.4 neurons, n=8 slices from 2 rats and sham 3.1±0.8 neurons, n=9 slices from 3 rats, P=0.95; Figure 3A–C). Interestingly, the tissues from Aβ treated rats compared to control rats showed more abundant fluorescent neurons in CA1 pyramidal layer (26.4±0.8 neurons, n=10 slices from 3 rats, P<0.001; Figure 3D & F). In spite of a significant reduction in the number of degenerating neurons by 10 days treatment with melatonin (12.9±1.2 neurons, n=12 slices from 3 rats; P=0.005, Figure 3E & G), this reduction did not reach the control levels.
Figure 3.
Injection of Aβ into frontal cortices induced neurodegeneration. Fluoro-jade photomicrographs of the whole hippocampus (A), CA1 pyramidal neurons of control (B), sham (C), Aβ treated (D), and Aβ+melatonin treated rats (E). Higher resolution image of fluorescent neurons from an Aβ treated rat (F). Arrows show degenerating fluorescent neurons. Scale bars show 50 μm for B-E and 20 μm for F. G) Quantitative analysis of the number of fluorescent neurons in different groups. ***P<0.001 and ‡‡P=0.005 indicate comparisons with control and Aβ groups, respectively. Analysis was done by one-way ANOVA with Turkey post-hoc test.
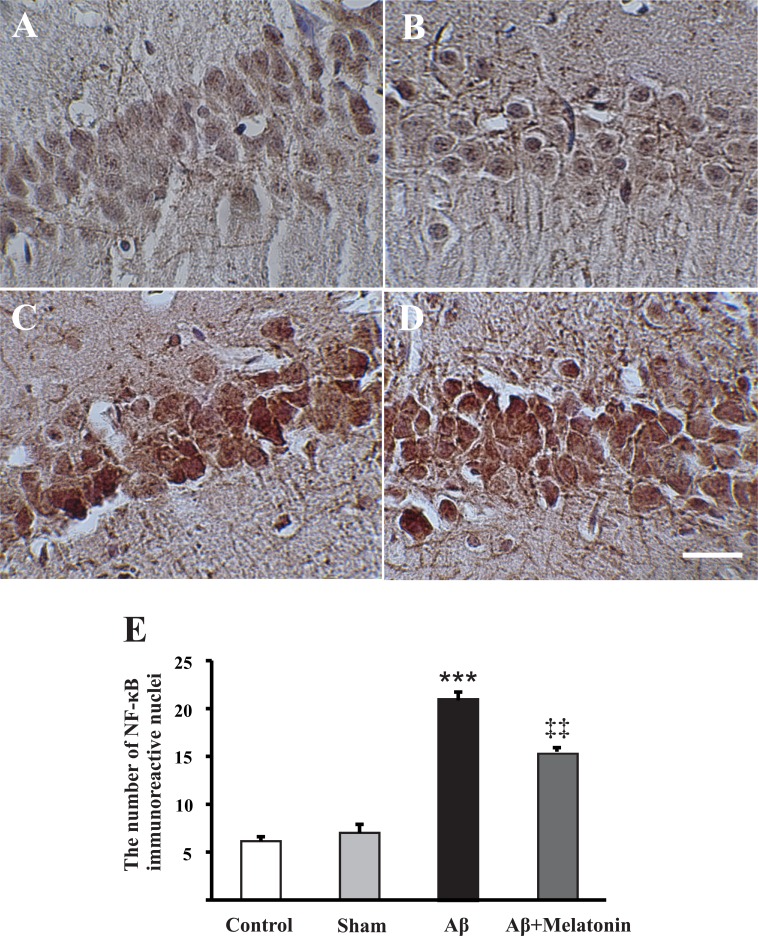
3.4. Injection of Aβ peptides into the frontal cortex increased NF-κB immunoreactivity in CA1 pyramidal neurons
NF-κB is one of the main signaling molecules activated and participated in the physiological and pathophysiological phenomena, including inflammation. Upon activation, this complex molecule translocates from the cytoplasm to the nucleus to alter gene transcription. In this regard, we measured the number of immunoreactive CA1 pyramidal neurons (per mm2) in our AD model. There were not differences between control and sham operated rats (6.14±0.4 neurons, n=10 slices from 3 rats; 7.1±0.8 neurons, n=12 slices from 3 rats, respectively; P=0.48; Figure 4A & B); however, in Aβ treated rats compared to controls, there was an evidence of enhanced NF-κB immuno-reaction in the CA1 pyramidal neurons (21±0.8 neurons, n=13 slices from 4 rats, P<0.001; Figure 4C). Ten days of melatonin treatment decreased Aβ-enhanced NF-κB immunoreactivity in these neurons (15.8±0.4 neurons, n=10 slices from 4 rats; P=0.003, Figure 4 & E).
Figure 4.
Injection of Aβ into the frontal cortices increased NF-κB positive pyramidal neurons. Photomicrographs of NF-κB immunohistochemistry in CA1 pyramidal neurons in control (A), sham (B), Aβ treated (C), and Aβ+melatonin treated rats (D). ***P<0.001 and ‡‡P=0.003 comparisons with control and Aβ groups, respectively (E). Analysis was done by one-way ANOVA with Turkey post-hoc test. Scale bar shows 50 μm.
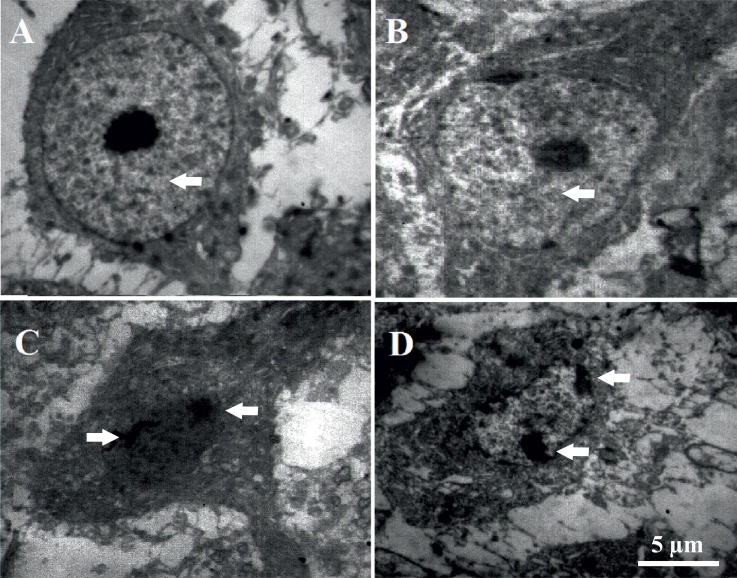
3.5. Injection of Aβ peptides into the frontal cortex induced ultra-structural changes in CA1 pyramidal neurons
Ultrastructural assessment using electron microscopy showed that in the control and sham operated rats, CA1 pyramidal neurons had oval nuclei with evenly dispersed chromatin and clear nucleoli in the center of nucleus (Figure 5A & B). Furthermore, both plasmalemma and nucleolemma were intact in neurons obtained from control and sham rats. In contrast, the CA1 pyramidal neurons from Aβ- and Aβ+mel atonin-treated rats showed the criteria similar to apoptotic neurons, in which degenerated neurons are evident in CA1 pyramidal layer, with nucleolemma invaginations implying the shrinkage of the nuclei and/or nuclear lysis, and chromatin aggregation (Figure 5C & D). Our results from electron microscopic study imply that injection of Aβ peptides into the frontal cortex can exert ultrastructural changes similar to the characteristics of apoptosis in the hippocampal neurons.
Figure 5.
Injection of Aβ into the frontal cortices induced ultra-structural changes in CA1 pyramidal neurons. Electron microscopic photomicrographs from CA1 pyramidal neurons of control (A) and sham rats (B), in which arrows show oval nuclei with evenly dispersed chromatin and clear nucleoli in the center of nucleus. Electron microscopic photomicrographs from CA1 pyramidal neurons of Aβ (C) and Aβ+melatonin (D) treated rats, respectively, in which arrows indicate nucleolemma invaginations and chromatin aggregation.
4. Discussion
In the present rat model of AD developed by injection of Aβ (1–42) peptide solution into the frontal cortical areas, behavioral changes and histochemical alterations were assessed in the most vulnerable hippocampal neurons in AD. Passive avoidance test showed a progressive decline in the memory, which is a behavior strongly dependent on the normal hippocampal function. Histological evaluations also verified evidence of alterations in the structure and biochemical properties of CA1 pyramidal neurons. Furthermore, daily treatment with melatonin was effective to prevent several histological disturbances, but not memory decline induced by Aβ injection into frontal cortices.
Postmortem studies show that Aβ aggregation in patients with AD firstly appears in the frontal cortex areas and then spreads out over time to the subcortical structures, including hippocampus (Arriagada, Growdon, Hedley-Whyte, & Hyman, 1992; Harris et al., 2010; Nath et al., 2012). Recently, we have benefited from this view in an in vivo rat model of AD to study electrophysiological properties and the cellular basis of excitability in CA1 pyramidal neurons (Haghani, Shabani, Javan, Motamedi, & Janahmadi, 2012; Haghani, Janahmadi, & Shabani, 2012). To develop this AD model, Aβ peptides have been injected deeply into the frontal cortices bilaterally. This model has advantages of rapidly developing and early onset of cognitive symptoms of AD (as we evaluated just 3 days after Aβ injection), including impairment in the passive avoidance memory. Genetic mice models of AD are mostly reported to show memory impairments after at least 4 months after birth (Kaczorowski, Sametsky, Shah, Vassar, & Disterhoft, 2011; Stevens & Brown, 2014; Cañete, Blázquez, Tobeña, Giménez-Llort, & Fernández-Teruel, 2014).
Moreover, non-transgenic rat model of AD, used in the present study, has functional alterations at the cellular level as evidenced by altered intrinsic electrical properties in the hippocampal neurons (Haghani, Shabani, Javan, Motamedi, & Janahmadi, 2012; Haghani, Janahmadi, & Shabani, 2012) and impaired induction of plasticity via decreased probability of neurotransmitter release (Esmaeili Tazangi, Moosavi, Shabani, & Haghani, 2015). Therefore, structural and functional alterations seen in the hippocampal neurons undoubtedly could be attributed to the neurodegeneration induced by either a remote injury (due to the method of injection), and/or injected Aβ peptides. However, several questions have remained to address about histological properties and effectiveness of a conventional treatment, i.e. using melatonin in this model.
Impaired cognition and memory decline are the earliest symptoms of developing AD in humans. In this study, we assessed memory function of rats using passive avoidance test. The reliability and validity of this test have been demonstrated by numerous studies to assess the ability of cognitive function in rodents (Filali et al., 2012; Webster, Bachstetter, Nelson, Schmitt, & Van Eldik, 2014). Likewise, we also found a progressive deteriorating effect of Aβ on the memory function of rats.
Hippocampus, in the temporal lobe is one of the main targets of AD pathophysiology. Decreased hippocampal volume due to neuronal shrinkage and loss through an accelerated apoptosis in AD is detectable using imaging and pathological studies. In the present study, Nissl staining showed evidence of shrinkage in the CA1 pyramidal neurons, but not neuronal loss (data not shown). Shrinkage and neuronal loss have been reported in both animal models of AD (Bondolfi et al., 2002; Li et al., 2013; Zhang, Dong, Zhao, & Ma, 2014) and clinical studies (Simić, Kostović, Winblad, & Bogdanović, 1997; Zarow et al., 2005). It is worthy to note that the imaging studies implied that structural changes in the hippocampal CA1 area might be the underpinning of cognitive dysfunctions in AD (Lim et al., 2012).
Moreover, labeling with fluorescent dye, Fluoro-jade, approved activation of neurodegenerative processes in CA1 pyramidal neurons induced by Aβ injection into the frontal cortices, consistent with other studies showing increased number of degenerating neurons in AD animal models (Damjanac et al., 2007; Kemppainen, Hämäläinen, Miettinen, Koistinaho, & Tanila, 2014). Furthermore, consistent with findings using light microscopy, our study with electron microscopy showed ultra-structural evidence of apoptosis in CA1 pyramidal neurons of Aβ-treated rats. These findings are in line with other studies indicating detrimental effects of Aβ peptides on the subcellular structures in the hippocampal and cortical neurons (Li et al., 2012; Balietti et al., 2013). Our further investigation using staining with Congo red, to find amyloid plaques, however, did not indicate the formation of plaques in the CA1 region ten days after Aβ injection (data not shown).
Inflammation as one of the important features of AD in this study has been brought under scrutiny using histochemical measurement of NF-κB positive pyramidal neurons in CA1 pyramidal layer. NF-κB is a protein complex located in the vicinity of postsynaptic machinery and cytoplasm, which activates through physiological synaptic stimulations and during inflammatory processes (Butler, Moynagh, & O’Connor, 2002; Meffert, Chang, Wiltgen, Fanselow, & Baltimore, 2003). In this study, Aβ injection into the frontal cortices increased the number of NF-κB immunoreactive neurons, consistent with other studies in animal models of AD (Liu et al., 2007; Kim et al., 2009; He et al., 2011; Wu et al., 2011; Fakhfouri et al., 2012) and postmortem studies from patients with AD (Terai, Matsuo, & McGeer, 1996; Yoshiyama, Arai, & Hattori, 2001).
Since different etiologies have been attributed to AD, therapies aimed at completely preventing or curing AD remained immature and current therapeutics are only able to slow down its progression. Anti-oxidative and anti-amyloid properties of melatonin have been extensively studied (Cheng, Feng, Zhang, & Zhang, 2006; Rosales-Corral et al., 2012). A vast majority of studies used systemic treatment with melatonin, ranging 10–50 mg/kg/day, and demonstrated its neuroprotective effects in different animal models of AD (Wang, Ling, Cao, Zhu, & Wang, 2004; Feng et al., 2004; Yang et al., 2011). In the present study, treatment with melatonin (30 mg/kg/day) did not prevent or alleviate Aβ-induced memory decline, or decrease in soma diameter, and ultrastructural alterations. There are other evidence that melatonin treatment also fails to prevent amyloid burden and oxidative damage in Tg2576 model of AD in mice (Quinn et al., 2005).
However, we showed that melatonin prevented to a considerable extent an enhanced NF-κB immunoreactivity and neurodegeneration, indicating of its effectiveness to inhibit inflammatory pathways triggered by the intrafrontal cortex injection of Aβ.
In this study, we showed that the injection of Aβ into the frontal cortices impaired memory and resulted in the cellular features of AD in the hippocampus within 10 days. This experimental model of AD, besides its simplicity (to be developed in vivo), due to early onset of behavioral and histological manifestations of AD, may have an advantage for the electrophysiological and histological studies to assess Aβ induced neurotoxicity in a frontotemporal fashion, similar to that occurs naturally in patients with AD.
Acknowledgments
Financial support was provided by Iran University of Medical Sciences. The authors wish to thank Mr. Hassan Hosseini for his technical assistance and Drs. Fariba Karimzade and Marziyeh Darvish at Shefa Neuroscience Research Center for their comments during experiments.
References
- Altobellia G. G., Cimini D., Espositoc G., Iuvonec T., Ciminia V. (2015). Analysis of calretin in early expression in the rat hippocampus after beta amyloid (1–42) peptide injection. Brain Research, 1610, 89–97. [DOI] [PubMed] [Google Scholar]
- Arriagada P. V., Growdon J. H., Hedley-Whyte E. T., Hyman B. T. (1992). Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology, 42(3), 631–639. [DOI] [PubMed] [Google Scholar]
- Balietti M., Giorgetti B., Casoli T., Solazzi M., Tamagnini F., Burattini C., et al. (2013). Early selective vulnerability of synapses and synaptic mitochondria in the hippocampal CA1 region of the Tg2576 mouse model of Alzheimer’s disease. Journal of Alzheimer’s Disease, 34(4), 887–896. [DOI] [PubMed] [Google Scholar]
- Bondolfi L., Calhoun M., Ermini F., Kuhn H. G., Wiederhold K. H., Walker L., et al. (2002). Amyloid-associated neuron loss and gliogenesis in the neocortex of amyloid precursor protein transgenic mice. Journal of Neuroscience, 22(2), 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M. P., Moynagh P. N., O’Connor J. J. (2002). Methods of detection of the transcription factor NF-kB in rat hippocampal slices. Journal of Neuroscience Methods, 119(2), 185–190. [DOI] [PubMed] [Google Scholar]
- Cañete T., Blázquez G., Tobeña A., Giménez-Llort L., Fernández-Teruel A. (2014). Cognitive and emotional alterations in young Alzheimer’s disease (3xTgAD) mice: Effects of neonatal handling stimulation and sexual dimorphism. Behavioral Brain Research, 281C, 156–171. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Feng Z., Zhang Q. Z., Zhang J. T. (2006). Beneficial effects of melatonin in experimental models of Alzheimer disease. Acta Pharmacologica Sinica, 27(2), 129–139. [DOI] [PubMed] [Google Scholar]
- Damjanac M., Rioux Bilan A., Barrier L., Pontcharraud R., Anne C., Hugon J., et al. (2007). Fluoro-Jade B staining as useful tool to identify activated microglia and astrocytes in a mouse transgenic model of Alzheimer’s disease. Brain Research, 1128, 40–49. [DOI] [PubMed] [Google Scholar]
- Esmaeili Tazangi P., Moosavi S. M., Shabani M., Haghani M. (2015). Erythropoetin improves synaptic plasticity deficits by decrease of the neurotransmitter release probability in the rat model of Alzheimer’s disease. Pharmacology and Biochemistry Behavior, 29, 15–21. [DOI] [PubMed] [Google Scholar]
- Fakhfouri G., Ahmadiani A., Rahimian R., Grolla A. A., Moradi F., Haeri A. (2012). WIN55212-2 attenuates amyloid-beta-induced neuroinflammation in rats through activation of cannabinoid receptors and PPAR-γ pathway. Neuropharmacology, 63(4), 653–666. [DOI] [PubMed] [Google Scholar]
- Feng Z., Chang Y., Cheng Y., Zhang B. L., Qu Z. W., Qin C., et al. (2004). Melatonin alleviates behavioral deficits associated with apoptosis and cholinergic system dysfunction in the APP 695 transgenic mouse model of Alzheimer’s disease. Journal of Pineal Research, 37(2), 129–136. [DOI] [PubMed] [Google Scholar]
- Filali M., Lalonde R., Theriault P., Julien C., Calon F., Planel E. (2012). Cognitive and non-cognitive behaviors in the triple transgenic mouse model of Alzheimer’s disease expressing mutated APP, PS1, and Mapt (3xTg-AD). Behavioral Brain Research, 234(2), 334–342. [DOI] [PubMed] [Google Scholar]
- Haghani M., Janahmadi M., Shabani M. (2012). Protective effect of cannabinoid CB1 receptor activation against altered intrinsic repetitive firing properties induced by Aβ neurotoxicity. Neuroscience Letters, 507(1), 33–37. [DOI] [PubMed] [Google Scholar]
- Haghani M., Shabani M., Javan M., Motamedi F., Janahmadi M. (2012). CB1 cannabinoid receptor activation rescues amyloid β-induced alterations in behavior and intrinsic electrophysiological properties of rat hippocampal CA1 pyramidal neurons. Cellular Physiology and Biochemistry, 29(3–4), 391–406. [DOI] [PubMed] [Google Scholar]
- Harris J. A., Devidze N., Verret L., Ho K., Halabisky B., Thwin M. T., et al. (2010). Transsynaptic progression of amyloid-β-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron, 68(3), 428–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F. Q., Qiu B. Y., Zhang X. H., Li T. K., Xie Q., Cui D. J., et al. (2011). Tetrandrine attenuates spatial memory impairment and hippocampal neuroinflammation via inhibiting NF-κB activation in a rat model of Alzheimer’s disease induced by amyloid-β(1–42). Brain Research, 89–96. [DOI] [PubMed] [Google Scholar]
- Huang Y., Mucke L. (2012). Alzheimer mechanisms and therapeutic strategies. Cell, 148(6), 1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun Z., Li Z., Fang W., Fengzhen Y., Puyuan W., Wenwen L., Zhi S., Bondy S. C. (2013). Melatonin decreases levels of S100β and NFΚB, increases levels of synaptophysin in a rat model of Alzheimer’s disease. Current Aging Science, 6(2), 142–9. [DOI] [PubMed] [Google Scholar]
- Kaczorowski C. C., Sametsky E., Shah S., Vassar R., Disterhoft J. F. (2011). Mechanisms underlying basal and learning-related intrinsic excitability in a mouse model of Alzheimer’s disease. Neurobiology of Aging, 32(8), 1452–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen S., Hämäläinen E., Miettinen P. O., Koistinaho J., Tanila H. (2014). Behavioral and neuropathological consequences of transient global ischemia in APP/PS1 Alzheimer model mice. Behavioral Brain Research, 275C, 15–26. [DOI] [PubMed] [Google Scholar]
- Kermanian F., Mehdizadeh M., Soleimani M., Ebrahimzadeh Bideskan A. R., Asadi-Shekaari M., Kheradmand H., et al. (2012). The role of adenosine receptor agonist and antagonist on Hippocampal MDMA detrimental effects; a structural and behavioral study. Metabolic Brain Diseases, 27(4), 459–469. [DOI] [PubMed] [Google Scholar]
- Kim T. I., Lee Y. K., Park S. G., Choi I. S., Ban J. O., Park H. K., et al. (2009). I-Theanine, an amino acid in green tea, attenuates beta-amyloid-induced cognitive dysfunction and neurotoxicity: reduction in oxidative damage and inactivation of ERK/p38 kinase and NF-kappaB pathways. Free Radical Biology and Medicine, 47(11), 1601–1610. [DOI] [PubMed] [Google Scholar]
- Li G., Cheng H., Zhang X., Shang X., Xie H., Zhang X., et al. (2013). Hippocampal neuron loss is correlated with cognitive deficits in SAMP8 mice. Neurological Sciences, 34(6), 963–969. [DOI] [PubMed] [Google Scholar]
- Li W. Z., Li W. P., Huang D. K., Kan H. W., Wang X., Wu W. Y., et al. (2012). Dexamethasone and Aβ25–35 accelerate learning and memory impairments due to elevate amyloid precursor protein expression and neuronal apoptosis in 12-month male rats. Behavioral Brain Research, 227( 1), 142– 149. [DOI] [PubMed] [Google Scholar]
- Lim H. K., Jung W. S., Ahn K. J., Won W. Y., Hahn C., Lee S. Y., et al. (2012). Relationships between hippocampal shape and cognitive performances in drug-naïve patients with Alzheimer’s disease. Neuroscience Letters, 516(1), 124–129. [DOI] [PubMed] [Google Scholar]
- Liu Q., Zhang J., Zhu H., Qin C., Chen Q., Zhao B. (2007). Dissecting the signalling pathway of nicotine-mediated neuroprotection in a mouse Alzheimer’s disease model. FASEB Journal, 21(1), 61–73. [DOI] [PubMed] [Google Scholar]
- Liu X. J., Yuan L., Yang D., Han W. N., Li Q. S., Yang W., et al. (2013). Melatonin protects against amyloid-β-induced impairments of hippocampal LTP and spatial learning in rats. Synapse, 67(9), 626–36. [DOI] [PubMed] [Google Scholar]
- Meffert M. K., Chang J. M., Wiltgen B. J., Fanselow M. S., Baltimore D. (2003). NF-κB functions in synaptic signalling and behavior. Nature Neuroscience, 6(10), 1072–1078. [DOI] [PubMed] [Google Scholar]
- Nath S., Agholme L., Kurudenkandy F. R., Granseth B., Marcusson J., Hallbeck M. (2012). Spreading of neurodegenerative pathology via neuron-to-neuron transmission of β-amyloid. Journal of Neuroscience, 32(26), 8767–8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J., Kulhanek D., Nowlin J., Jones R., Praticò D., Rokach J., et al. (2005). Chronic melatonin therapy fails to alter amyloid burden or oxidative damage in old Tg2576 mice: implications for clinical trials. Brain Research, 1037(1), 208–213. [DOI] [PubMed] [Google Scholar]
- Rosales-Corral S. A., Acuña-Castroviejo D., Coto-Montes A., Boga J. A., Manchester L. C., Fuentes-Broto L., Korkmaz A., et al. (2012). Alzheimer’s disease: pathological mechanisms and the beneficial role of melatonin. Journal of Pineal Research, 52(2), 167–202. [DOI] [PubMed] [Google Scholar]
- Rudnitskaya E. A., Maksimova K. Y., Muraleva N. A., Logvinov S. V., Yanshole L. V., Kolosova N. G., Stefanova N. A. (2015). Beneficial effects of melatonin in a rat model of sporadic Alzheimer’s disease. Biogerontology, 16(3), 303–16. [DOI] [PubMed] [Google Scholar]
- Saffarzadeh F., Eslamizade M. J., Ghadiri T., Modarres Mousavi S. M., Hadjighassem M., Gorji A. (2015). Effects of TRPV1 on the hippocampal synaptic plasticity in the epileptic rat brain. Synapse, 69, 375– 83. [DOI] [PubMed] [Google Scholar]
- Schmued L. C., Albertson C., Slikker W. J. (1997). Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Research, 751 ( 1), 37– 46. [DOI] [PubMed] [Google Scholar]
- Simić G., Kostović I., Winblad B., Bogdanović N. (1997). Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer’s disease. Journal of Comparative Neurology, 379(4), 482– 494. [DOI] [PubMed] [Google Scholar]
- Stefanova N. A., Maksimova K. Y., Kiseleva E., Rudnitskaya E. A., Muraleva N. A., Kolosova N. G. (2015). Melatonin attenuates impairments of structural hippocampal neuroplasticity in OXYS rats during active progression of Alzheimer’s disease-like pathology. Journal of Pineal Research, 10.1111/jpi.12248. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Stevens L. M., Brown R. E. (2014). Reference and working memory deficits in the 3xTg-AD mouse between 2 and 15-months of age: A cross-sectional study. Behavioral Brain Research, 278C, 496–505. [DOI] [PubMed] [Google Scholar]
- Terai K., Matsuo A., McGeer P. L. (1996). Enhancement of immunoreactivity for NF-kappa B in the hippocampal formation and cerebral cortex of Alzheimer’s disease. Brain Research, 735(1), 159–168. [DOI] [PubMed] [Google Scholar]
- Wang D. L., Ling Z. Q., Cao F. Y., Zhu L. Q., Wang J. Z. (2004). Melatonin attenuates isoproterenol-induced protein kinase A overactivation and tau hyperphosphorylation in rat brain. Journal of Pineal Research, 37(1), 11–16. [DOI] [PubMed] [Google Scholar]
- Wang J. Z., Wang Z. F. (2006). Role of melatonin in Alzheimer-like neurodegeneration. Acta Pharmacologica Sinica, 27(1), 41–49. [DOI] [PubMed] [Google Scholar]
- Webster S. J., Bachstetter A. D., Nelson P. T., Schmitt F. A., Van Eldik L. J. (2014). Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Frontiers in Genetics, 5, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Wang A., Min Z., Xiong Y., Yan Q., Zhang J., et al. (2011). Lipoxin A4 inhibits the production of proinflammatory cytokines induced by β-amyloid in vitro and in vivo. Biochemistry and Biophysics Research Communications, 408(2), 382–387. [DOI] [PubMed] [Google Scholar]
- Yang X., Yang Y., Fu Z., Li Y., Feng J., Luo J., et al. (2011). Melatonin ameliorates Alzheimer-like pathological changes and spatial memory retention impairment induced by calyculin A. Journal of Psychopharmacology, 25(8), 1118–1125. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y., Arai K., Hattori T. (2001). Enhanced expression of I-kappaB with neurofibrillary pathology in Alzheimer’s disease. Neuroreport, 12(12), 2641–2645. [DOI] [PubMed] [Google Scholar]
- Zarow C., Vinters H. V., Ellis W. G., Weiner M. W., Mungas D., White L., et al. (2005). Correlates of hippocampal neuron number in Alzheimer’s disease and ischemic vascular dementia. Annals of Neurology, 57(6), 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Dong S., Zhao G., Ma Y. (2014). 7.0 T nuclear magnetic resonance evaluation of the amyloid beta (1–40) animal model of Alzheimer’s disease: comparison of cytology verification. Neural Regeneration Research, 9(4), 430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]