Abstract
Introduction:
Prenatal stress has deleterious effects on the development of the brain and is associated with behavioral and psychosocial problems in childhood and adulthood. This study aimed to determine the protective effect of L-arginine on fetal brain under maternal stress.
Methods:
Twenty pregnant Wistar rats (weighting 200–230 g) were randomly divided into 4 groups (n=5 for each group). The first nonstress and stress groups received 2 mL of normal saline and the other nonstress and stress two groups received L-arginine (200 mg/kg, IP) from their 5th to 20th days of pregnancy. The pregnant rats were killed on 20th day and the brain fetuses removed and prefrontal cortical thickness, total neurons in the prefrontal cortex and in the areas of CA1, CA2, and CA3 of the hippocampus were measured and counted. Nitrite levels in the brain were measured as an indicator for nitric oxide (NO) level.
Results:
There was a significant decrease of mean number of pyramidal cells in the CA1 in prenatal stress group compared to nonstress and nonstress plus arginine groups. The NO level in brain tissue increased significantly in the stress plus arginine (3.8±0.4 nmol/mg) and in nonstress rats (2.9±0.3 nmol/mg) compared to the stress group (1.8±0.1 nmol/mg). Prefrontal cortical thickness decreased significantly in stress rats (1.2±0.09 mm) compared to the nonstress plus arginine (1.7±0.15 mm) and nonstress (1.6±0.13 mm) groups.
Discussion:
Results indicated that prenatal stress could lead to neurodegeneration of hippocampus and prefrontal cortex of rat fetuses. L-arginine as a precursor of NO synthesis had neuroprotective effect during prenatal stress and could be used an effective treatment for stress.
Keywords: Fetuses, Hippocampal formation, L-arginine, Pregnancy, Rats, Stress
1. Introduction
Stress in the mother during pregnancy is associated with several effects on the fetal brain development (Charil, Laplanteb, Vaillancourtc, & Kinga, 2010). Prenatal stress, especially during critical periods of organogenesis can be associated with serious health problems such as schizophrenia and autism in childhood and adulthood ( Van & Selten, 1998; Beversdorf et al,. 2005). The maternal and fetal Hypothalamic-Pituitary-Adrenal (HPA axis) are involved in stress. Therefore, in stressful conditions, the high level of maternal corticosterone is released and reaches to the fetus (Vaughan et al., 2015). In most cases the high level of cortisol was contributed in the pathologies of the embryonic period and led to behavioral and physical abnormalities in the human body (Buss et al., 2012). The maternal HPA axis affects placental Corticotropin-Releasing Hormone (CRH) activity, and the high level of placental CRH is associated with the intrauterine growth and spontaneous preterm birth (Glynn et al., 2001; Wadhwa, Porto, Chicz-DeMet, & Sandman, 1998). As previously described, during prenatal stress the CRH increased and transferred through the blood brain barrier to fetus brain, and has side effects on hippocampal neurogenesis by activating CRH receptors (Seress, Abraham, Tornoczky & Kosztolanyi, 2001).
Immobilization and mental stress induce the release of free radicals such as superoxide, hydroxyl, nitric oxide (and since there are no comprehensive defense in the brain), which may lead to neuronal death (Rather & Saravanan, 2013). The effect of prenatal stress on physical, reproductive, and behavioral outcomes is probably due to the maternal stress on fetal brain (Charil, Laplanteb, Vaillancourtc, & Kinga, 2010). Anti-anxiety drugs like benzodiazepines is effective in treating symptoms of stress for a short time, but if treatment stops, drowsiness, shakiness, sleeplessness, and loss of appetite will be side effects of this medication (Anthierens, 2010). However, some patients need other treatments for a long term. L-arginine acts as a free radical scavenger and improves the endothelial function and reduces the oxidative stress (Tripathi & Pandeym, 2013; Lassala et al., 2010). Nitric oxide (NO) is an important paracrine substance that is generated in the presence of L-arginine and causes blood vessels to dilate and subsequently increases blood flow (Tripathi & Pandeym, 2013).
L-arginine is a basic amino acid and essential for the synthesis of substances such as protein, creatinine, and nitric oxide, which is needed during stress or illness (Böger, 2007). It has been documented that low level of L-arginine in premature infants is accompanied with intestinal and cardiovascular disease (Tripathi & Pandeym, 2013). In the presence of the stress and infections the catabolism of the protein increases and intestinal absorption of the L-arginine impairs and it is lost through the urinary tract (Böger, 2007). This finding is supported by study that demonstrated the L-arginine deficiency in the pregnancy period causes intrauterine growth retardation and impaired cardiovascular and neurological development (Tripathi & Pandeym 2013, Lassala et al., 2010). In the neuroendocrine system, NO could modulate the stress-induced activation of the HPA axis and affect CRH, growth hormone, and luteinizing hormone (Kishimoto, Tsuchiya, Emson, & Nakayama, 1996). L-arginine is a substrate of nitric oxide synthesis and there is a little knowledge about the relation between stress and NO in the embryonic period. Therefore, this study aimed to assess the effect of L-arginine on prefrontal cortex, hippocampus of the fetal brain following immobilization stress under maternal stress.
2. Methods
2.1. Animal groups and prenatal stress procedure
The study protocol was approved by the Ethics Committee for Animal Experiment at Yasuj University of Medical Sciences, School of Medicine in Yasuj, Iran. Twenty Wistar rats weighing 200–230g were housed. After 10 days, one male and one female rat were put together in one cage at 8 p.m. and the observation of vaginal plug was considered as the zero day of the pregnancy. For immobilization stress, iron fenestrate box was designed (7cm×5.5cm) and animals were immobilized for 6 h daily from their 5th to 20th days of the pregnancy. This device immobilized the rats without their being compressed, pinched, or screaming. During the immobilization process, animals were completely deprived of food and water (Zaidi et al., 2014). Animals were randomly allocated to four groups (five rats in each group): The nonstress (nonst) and stress (st) groups received 2 mL (IP) of normal saline daily from the 5th until 20th days of pregnancy. Other groups of nonstress (nonst+arg) and stress (st+arg) received L-arginine (200 mg/kg, IP) daily from the 5th until 20th days of pregnancy. The rats had free access to food and water, except when they were under stress.
2.2. Tissue processing
Pregnant rats were sacrificed (on the 20th day of their pregnancy) under deep anesthesia with an overdose of ketamine (200 mg/kg) and xylazine (20 mg/kg), then their fetuses were removed by laparotomy and expose of the uterine. Twenty-five prenatal (P20 days) brains (5 male fetuses from each group) were chosen for morphometric analysis. The brain specimens were acquired by opening the calvaria and then stored in formalin solution 10%. Next, serial coronal sections, 10 μm thick, were made out of each block on a freezing microtome (Leica cryostat, CM 3000). Every fifth section from the serial section was mounted onto gelatin coated adhesive slides, stained with Trichrome Masson or Hematoxylin and eosin (H&E), then dehydrated and cover slipped. As previously described, with using of the frame (25μm×25μm) with Motic microscope by an eyepiece of 100×objective lens (West, Slomianka, & Gundersen, 1991), total neurons in the prefrontal cortex and in the areas of CA1, CA2, and CA3 of left hippocampus were counted. The thickness of the prefrontal cortex was measured anterior to bregma as described (Dorph-Petersen, Nyengaard, & Gundersen, 2001). The image of the sections were taken and analyzed using the computer-assisted morphometric software (Olysia; Olympus, Japan), and low-power magnification (×4) images with the aid of a digital camera (DP 11) attached to the microscope (Olympus A×70).
2.3. NO measurements
Twenty-five male fetal brains (5 fetuses from each group) were chosen for measuring nitrite levels in the brain as an indicator for NO. Folch solution was prepared by mixing chloroform and methanol in a ratio of 2:1 (vol/vol). The fetal brain samples were then homogenized with the Folch solution and preserved at −20°C. After 90 minutes, the substrate extract was centrifuged at 11000 rpm and its NO content was assayed according to the Griess reaction (Borgerding & Murphy, 1995) using a spectrophotometer. Results were converted into a standard curve, which showed the NO absorption level, using 1, 2, 3, and 4 mol nitrite sodium solution. For measure of NO the nitrits concentration were expressed in nmol per gr of protein.
2.4. Statistical analysis
Data were expressed as a mean±SD; 1-way ANOVA followed by post-hoc Tukey test were used to determine statistical differences in each group. A statistically significant difference was accepted at P<0.05.
3. Results
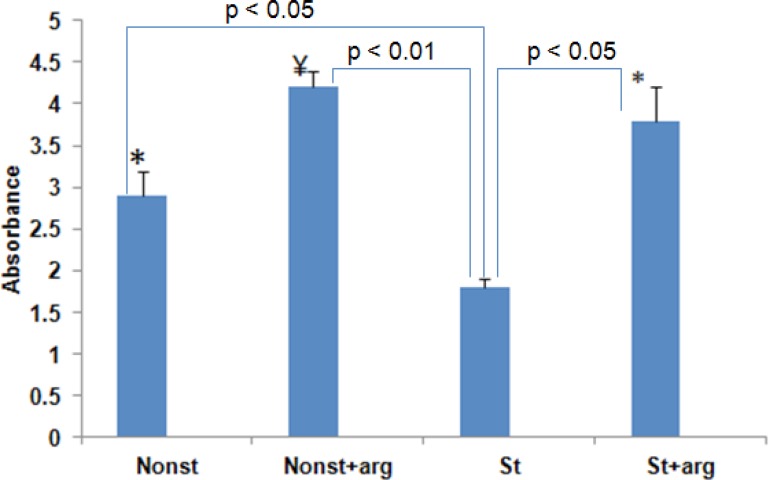
We examined that whether neurogenesis and NO level in the brain was affected by immobilization stress or L-arginine (200mg/kg) injection in the prenatal period. There was a significant increase in NO level (mean±SD) in the st+arg and nonst+arg groups with 3.8±0.4 nmol/mg and 4.2±0.2 nmol/mg, respectively compared to the stress group, 1.8±0.1 nmol/mg (P<0.01) rats (Figure 1). Also, the mean of NO level in nonstress rats 2.9±0.3 nmol/mg elevated significantly compared to the stress group (P<0.05).
Figure 1.
Nitric oxide levels in in the 20-day-old embryo of stressed and nonstress groups following treatment with L-arginine. The bars show mean values±SD, *P<0.05; compared to the st (stress) groups. ¥P<0.01, compared to the st (stress) group.
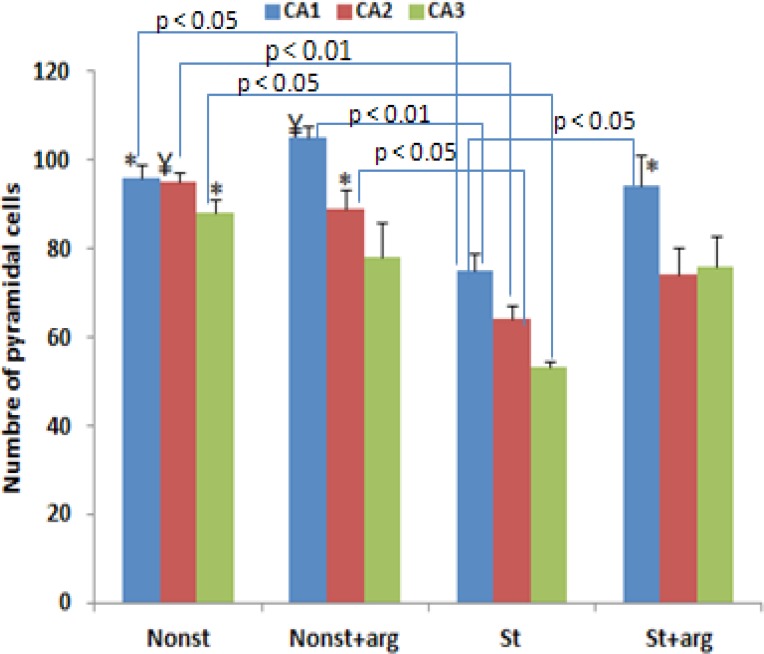
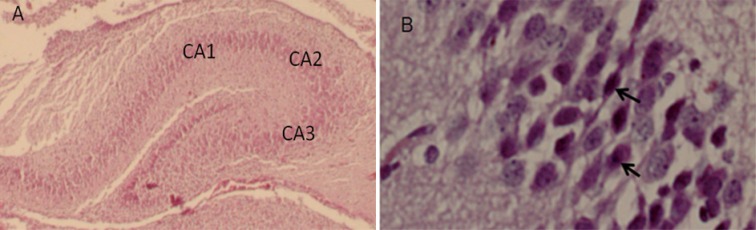
Although the mean number of pyramidal neurons in the CA2 region was not different from other groups, there was a significant decrease in the mean number of pyramidal cell in the CA1 in prenatal stress group compared to nonstress and nonst+arg groups (Figure 2). The mean number of pyramidal neurons in the CA2 increased in prenatal stress rats with L-arginine compared to that of the stress rats (Figure 2). Histological study showed that the perikaryon of pyramidal neurons in the stress rats were irregular-shaped associated with heterochromatin clumps (Figure 3).
Figure 2.
Total pyramidal neuron number of CA1, CA2, and CA3 subdivisions of the left hippocampus in different groups of 20-day-old embryo. The bars show mean values±SD.*P<0.05; ¥P<0.01, compared to the st (stress) groups.
Figure 3.
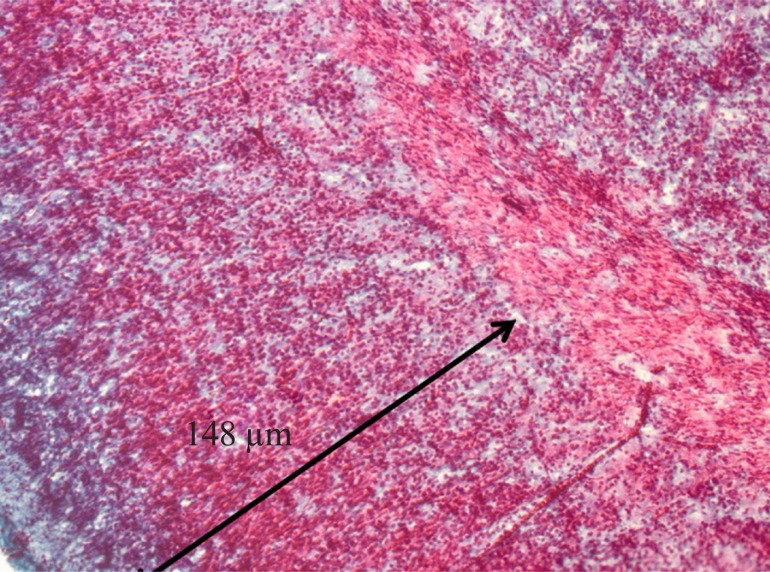
A light micrograph shows a section through the rat hippocampus (of 20-day-old embryo). The CA1, CA2, and CA3 are Cornu Ammonis (A). B photomicrograph (arrow) showing of pyramidal cells of CA1 at high magnification. Scale bars=200 μm in A, 20 μm in B.
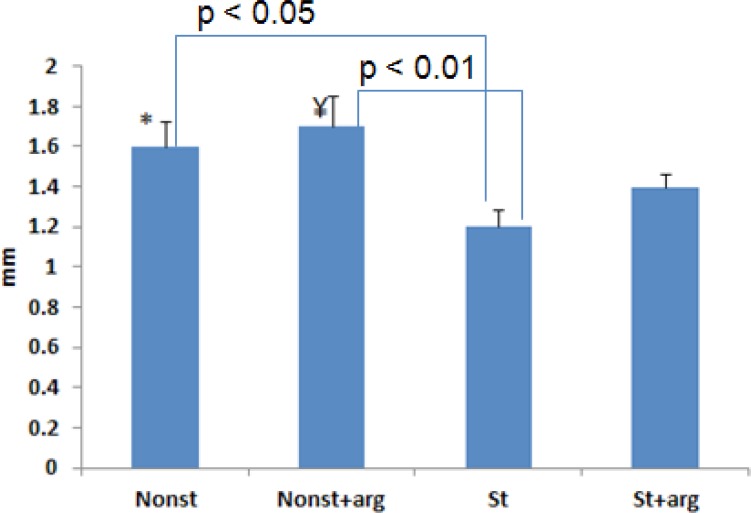
Prefrontal cortical thickness decreased significantly in st rats (1.2±0.09 mm) compared to the nonst+arg (1.7±0.15 mm) and nonst (1.6±0.13 mm) groups (Figure 4, 5). Although prefrontal cortical thickness increased in the st+arg compared to the st group, this increase was not significant.
Figure 4.
Prefrontal cortical thickness in different groups of 20-day-old embryos. There was no significant difference between the st group and st+arg rats. The bars show mean values±SD.*P<0.05; ¥P<0.01, compared to the st (stress) groups.
Figure 5.
Coronal section through the brain to illustrate the prefrontal cortex of 20-day-old embryo with H&E staining, scale bar: 100μm.
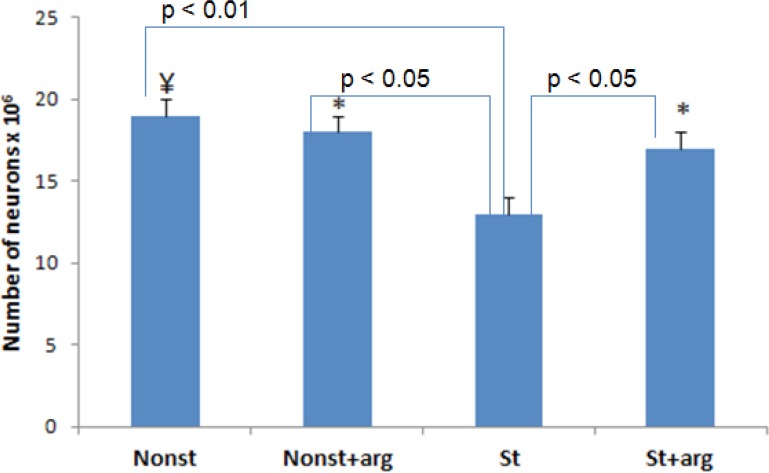
Furthermore, the mean number of prefrontal cortical neurons in prenatal stress rats decreased to 13.2×106±2.1, compared to the nonst number: 19.4×106±1.1, nonst+arg number: 18.1×106±0.9, and st+arg number: 17.3×106±1.9, respectively (Figure 6).
Figure 6.
Prefrontal cortical neuron number in different groups of 20-day-old embryos. The bars show mean values±SD. *P<0.05; ¥P<0.01, compared to the st (stress) groups.
4. Discussion
Prenatal stress has deleterious effects on the brain neurogenesis during the early development and impairs the memory process (Lemaire, Koehl, Le Moal, & Abrou, 2000). Result of this study showed that prenatal restrained rats had a lower number of hippocampal pyramidal cells, cortical neurons, and cortical thickness compared to their non-prenatally stressed counterparts. The reason for difference in frontal cortical thickness between the stress and nonstress rats is probably due to the immobilization stress that can change activity of neurotransmitters and neurohormonal in brain region, which is linked to the HPA axis (Ahmad, Rasheed, Chand, Maury, & Banum, 2012).
Immobilization stress contributes to the physical and psychological stress, which increase corticosterone, epinephrine, and norepinephrine hormone release, and eventually exacerbation of neurodegeneration (Calza, Giardino, & Ceccatelli, 1993). Prenatal stress could disrupt the function of the HPA axis and may result in increase the corticosterone secretion that inhibits cell proliferation (Lajud, & Torner, 2015). This result is supported by experimental evidence indicating that the high level of cortisol could deteriorate the normal development of the brain and reduce hippocampal sizes (Lajud, & Torner, 2015; Mandyam et al., 2008). The high level of cortisol in the male fetuses could worsen the placental inflammation and the uteroplacental abnormality is associated with neurodegenerative effect (Charil, Laplanteb, Vaillancourtc, & Kinga 1010, Mandyam et al 2008). Moreover, the increase of stress hormone during the fetal brain development in fetus rats is associated with a reduction in the number of neurons in the prefrontal and hippocampal formation (Charil, Laplanteb, Vaillancourtc, & Kinga, 1010). Prolonged immobilization stress leads to the stimulation of hypothalamus to release Corticotropin-Releasing Hormone (CRH) that act as a mediator of stress to suppress food intake and loss of body weight (Alario, Gamello, Beats, & Trancho, 1987), whereas L-arginine could increase food intake in rats through hypothalamus administration (Malfatti, 2015).
In this study, L-arginine induced the considerable production of nitric oxide in brain tissue of animals treated with L-arginine compared to the stress group. Nitric oxide is a reactive molecule that participates in the brain response to stress (Garry, Ezra, Rowland, Westbrook, & Pattinson, 2015). The delayed restraint stress changes neuronal Nitric Oxide Synthase (NOS) expression in the neuronal region of the limbic system and affects the cell proliferation from postnatal until aging in hippocampus (De Oliveira et al. 2000; Echeverry, Guimaraes, & Delbel, 2004). The expression of neuronal NOS is related to some part of brain such as hippocampus, pituitary, prefrontal cortex, and medial amygdaloid nucleus that participated in response to stress stimuli (Graeff, 1990). Prefrontal cortex could react to the Inducible nitric oxide synthase (iNOS) after 3h of restrained stress and cause the induction of NO production (Gdek-Michalska et al., 2012). In this regard, delay stress creates molecular changes and down-regulated glucocorticoid receptor mRNA in the hippocampus (McEwen & Magarinos, 1997).
In stress situations such as depression and anxiety, oxygen consumption increases in the brain tissue, which in turn leads to an increase in the production of free radicals in mice (Kumar, Garg, Gaur, & Kumar, 2009). L-arginine with increase of NO could improve the endothelium-mediated vascular functions such as enhanced vasodilation, which had neuroprotective effects during pregnancy (Böger, 2007). The present result confirms the result of other studies that NOS inhibitors such as NG-nitro-L-arginine methyl ester could impair locomotor functions in rat (Del Bel et al., 2002) and this effect was prevented by pretreatment with L-arginine (Moncada, Palmer, & Higgs, 1991). L-arginine could induce vasodilation with increase in NO synthesis, which improves nutritive brain blood flow. Further, there is a report that showed the growth hormone level increased in plasma after intravenous injection of L-arginine (Bode-Böger et al. 1999).
A regulatory function of NO has been proposed for the stress susceptibility and adaptation in rats (Gulati, Ray, Masood, & Vijayan, 2006). Restrain stress induces Elevated Plus Maze (EPM) in rats, but L-arginine could improve the EPM activity with increase in NO (Ahmed Khan & Ghosh, 2011). Suppression of NO synthase during prenatal development could cause the anxiolytic effect, whereas L-arginine abolishes the anxiolytic-like effect of withaferin in rats (Ahmed Khan & Ghosh, 2011). Furthermore, L-arginine stimulates the secretion of different hormones and insulin in mammals, especially in premature infants (Reitano, Grasso, Distefano, & Messina, 1971). Result of this experiment is supported by our previous study in that stress decreases the level of NO and has a significant impact in the reduction of the number and thickness of the prefrontal cortex in mature rats (Ebadi et al., 2010). Previous studies reported that prenatal stress has effect on cell proliferation, decreased synaptic density in the CA3 area, and density of NO producing in hippocampal formation in mature rats (Lemaire, Koehl, Le Moal, & Abrou, 2000).
Our results indicate that prolong immobilization prenatal stress has profound deleterious effects on embryonic neurogenesis in the prefrontal cortex and hippocampal formation of fetus rats (20-day-old). The other major finding of this study demonstrated that L-arginine as a precursor of nitric oxide (NO) synthesis has neuroprotective effect during prenatal stress and could be used as an effective treatment for stress. Future studies should be performed to clear the influence of L-arginine on molecular neurobiology following prenatal stress.
Acknowledgments
The authors are grateful to Yasuj University of Medical Sciences for providing grants and supporting this study.
References
- Ahmad A., Rasheed N., Chand K., Maurya R., Banum N., Palit G. (2012). Restraint stress-induced central monoaminergic & oxidative changes in rats & their prevention by novel Ocimum sanctum compounds. Indian Journal of Medical Research, 135 (4), 548– 554. [PMC free article] [PubMed] [Google Scholar]
- Ahmed Khan Z., Ghosh A. R. (2011). L-Arginine abolishes the anxiolytic-like effect of withaferin A in the elevated plus-maze test in rats. African Journal of Pharmacy and Pharmacology, 5 (2), 234– 237. [Google Scholar]
- Alario P., Gamello A., Beats M. J., Trancho G. (1987). Body weight gain, food intake and adrenal development in chronic noise stressed rats. Physiology & Behavior, 40 (1), 29– 32. [DOI] [PubMed] [Google Scholar]
- Anthierens S. Pasteels I. Habraken H. Steinberg P. Declercq T. Christiaens T. (2010) Barriers to nonpharmacologic treatments for stress, anxiety, and insomnia: family physicians attitudes toward benzodiazepine prescribing. Canadian Family Physician, 56 (11), 398– 406. [PMC free article] [PubMed] [Google Scholar]
- Beversdorf D. Q., Manning S. E., Hillier A., Anderson S. L., Nordgren R. E., Walters S. E., Nagaraja H. N., Cooley W. C., Gaelic S. E., Bauman M. L. (2005). Timing of prenatal stressors and autism. Journal of Autism and Developmental Disorders, 35 (4), 471– 478. [DOI] [PubMed] [Google Scholar]
- Böger R. H. (2007). The Pharmacodynamics of L-Arginine. Journal of Nutrition, 137 (6), 1650– 1655. [DOI] [PubMed] [Google Scholar]
- Bode-Böger S. M., Böger R. H., Löffler M., Tsikas D., Brabant G., Frölich J. C. (1999). L-Arginine stimulates NO-dependent vasodilation in humans—effect of somatostatin pretreatment. Journal of Investigative Medicine, 47 (1), 43– 50. [PubMed] [Google Scholar]
- Borgerding R. A., Murphy S. (1995). Expression of inducible nitric oxide synthase in cerebral endothelial cells is regulated by cytokine-activated astrocytes. Journal of Neurochemistry, 65 (3), 1342– 1347. [DOI] [PubMed] [Google Scholar]
- Buss C., Davis E. P., Shahbaba B., Pruessner J. C., Head K., Sandman C. A. (2012). Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proceedings of the National Academy of Sciences, 109 (20), 1312– 1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza L., Giardino L., Ceccatelli S. (1993). NOS mRNA in the paraventricular nucleus of young and old rats after immobilization stress. NeuroReport, 4 (6), 627– 30. [DOI] [PubMed] [Google Scholar]
- Charil A., Laplanteb D. P., Vaillancourtc C., Kinga S. (2010). Prenatal stress and brain development. Brain Research Reviews, 65 (1), 56– 79. [DOI] [PubMed] [Google Scholar]
- Del Bel E. A., Souza A. S., Guimarães F. S., da-Silva C. A., Nuccida-Silva L. P. (2002). Motor effects of acute and chronic inhibition of nitric oxide synthesis in mice. Psychopharmacology (Berl), 161 (1), 32– 37. [DOI] [PubMed] [Google Scholar]
- De Oliveira R. M., Del Bel E. A., Mamede-Rosa M. L., Padovan C. M., Deakin J. F., Guimarães F. S. (2000). Expression of neuronal nitric oxide synthase mRNA in stress-related brain areas after restraint in rats. Neuroscience Letter, 289 (2), 123– 126. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen K. A., Nyengaard J. R., Gundersen H. J. (2001). Tissue shrinkage and unbiased stereological estimation of particle number and size. Journal of Microscopy, 204 (3), 232– 46. [DOI] [PubMed] [Google Scholar]
- Ebadi B. Mehdizadeh M. Nahavandi A. Shariati T. Soleimani S. Delaviz H. (2010) Effects of nitric oxide on the pre-frontal cortex in stressed rats. Neural Regeneration Research, 5 (14), 1096– 1099. [Google Scholar]
- Echeverry M. B., Guimaraes F. S., Delbel E. A. (2004). Acute and delayed restraint stress-induced changes in nitric oxide producing neurons in limbic regions. Neuroscience, 125 (4), 981– 993. [DOI] [PubMed] [Google Scholar]
- Garry P. S., Ezra M., Rowland M. J., Westbrook J., Pattinson K. T. S. (2015). The role of the nitric oxide pathway in brain injury and its treatment from bench to bedside. Experimental Neurology, 263, 235– 243. [DOI] [PubMed] [Google Scholar]
- Gdek-Michalska A., Tadeusz J., Rachwalska P., Spyrka J., Bugajsk J. (2012). Effect of repeated restraint on homotypic stress-induced nitric oxide syntheses expression in brain structures regulating HPA axis. Pharmacological Report, 64 (6), 1381– 1390. [DOI] [PubMed] [Google Scholar]
- Glynn L. M., Wadhwa P. D., Dunkel Schetter C., Chicz-Demet A., Sandman C. A. (2001). When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. American Journal of Obstetrics and Gynaecology, 184 (4), 637– 642. [DOI] [PubMed] [Google Scholar]
- Mcnaughton N. (1995). Brain mechanisms of anxiety. New Zealand Journal of Psychology, 24 (2), 11– 18. [Google Scholar]
- Gulati K., Ray A., Masood A., Vijayan V.K. (2006). Involvement of nitric oxide in the regulation of stress susceptibility and adaptation in rats. Indian Journal of Experimental Biology, 44 (10), 809– 815. [PubMed] [Google Scholar]
- Kishimoto J., Tsuchiya T., Emson P., Nakayama Y. (1996). Immobilization-induced stress activates neuronal nitric oxide synthase (nNOS) mRNA and protein in hypothalamic-pituitary-adrenal axis in rats. Brain Research, 720 (2), 159– I71. [DOI] [PubMed] [Google Scholar]
- Kumar A., Garg R., Gaur V., Kumar P. (2009) Nitric oxide mechanism in protective effect of imipramine and venlafaxine against acute immobilization stress-induced behavioral and biochemical alteration in mice. Neuroscience Letter, 467 (2), 72– 5. [DOI] [PubMed] [Google Scholar]
- Lajud N., Torner L. (2015). Early life stress and hippocampal neurogenesis in the neonate: sexual dimorphism, long term consequences and possible mediators. Frontiers in Molecular Neuroscience, 8 (3), 1– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassala A., Bazer F. W., Cudd T. A., Datta S., Keisler D. H., Satterfield M. C., Spencer T. E., Wu G. (2010). Parenteral administration of L-arginine prevents fetal growth restriction in undernourished ewes. Journal of Nutrition, 140 (7), 1242– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V., Koehl M., Le Moal M., Abrou D. N. (2000). Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proceedings of the National Academy of Sciences, 97 (20), 2011032– 11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfatti C. R., da Silva L. A., Pereira R. A., Michel R. G., Snak A. L., dos Santos F. S. (2015) Acute hypothalamic administration of L-arginine increases feed intake in rats. Revista de Nutrição, 28 (1), 19– 25. [Google Scholar]
- Mandyam C. D., Crawford E. F., Eisch A. J., Rivier C. L., Richardson H. N. (2008). Stress experienced in utero reduces sexual dichotomies in neurogenesis, microenvironment, and cell death in the adult rat hippocampus. Developmental Neurobiology, 68 (5), 575– 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S., Magarinos A. M. (1997). Stress effects on morphology and function of the hippocampus. Annals of the New York Academy of Sciences, 821 (21), 871– 884. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M. J., Higgs E. A. (1991). Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacological Reviews, 43 (2), 109– 142. [PubMed] [Google Scholar]
- Rather S. A., Saravanan N. (2013). Protective effect of gallic acid on immobilization induced stress in encephalon and myocardium of male albino Wistar rats. International Journal of Nutrition, Pharmacology, Neurological Disease, 3 (3), 269– 75. [Google Scholar]
- Reitano G., Grasso S., Distefano G., Messina A. (1971). The serum insulin and growth hormone response to arginine and to arginine with glucose in the premature infant. The Journal of Clinical Endocrinology and Metabolism, 33 (6), 924– 8. [DOI] [PubMed] [Google Scholar]
- Seress L., Abraham H., Tornoczky T., Kosztolanyi G. (2001). Cell formation in the human hippocampal formation from mid-gestation to the late postnatal period. Neuroscience, 105 (4), 831– 843. [DOI] [PubMed] [Google Scholar]
- Tripathi P., Pandeym S. (2013). L-arginine attenuates oxidative stress condition during cardiomyopathy. Indian Journal of Biochemistry & Biophysics, 50 (2), 99– 104. [PubMed] [Google Scholar]
- Van Os J., Selten J. P. (1998). Prenatal exposure to maternal stress and subsequent schizophrenia. The invasion of The Netherlands. British journal of Psychiatry, 172 (8), 324– 326. [DOI] [PubMed] [Google Scholar]
- Vaughan O. R., Fisher H. M., Dionelis K. N., Jefferies E. C., Higgins J. S., Musial B., et al. (2015). Corticosterone alters materno-fetal glucose partitioning and insulin signalling in pregnant mice. The Journal of Physiology, 593 (5), 1307– 1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa P. D., Porto M., Chicz-DeMet A., Sandman C.A. (1998). Maternal CRH levels in early third trimester predict length of gestation in human pregnancy. American Journal of Obstetrics & Gynaecology, 179 (4), 1079– 1085. [DOI] [PubMed] [Google Scholar]
- West M. J., Slomianka L., Gundersen H. J. G. (1991). Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. The Anatomical Record, 231 (4), 482– 7. [DOI] [PubMed] [Google Scholar]
- Zaidi S. K., Hoda N., Tabrez S., Ansari S. A., Jafri M. A., Khan M. S., et al. (2014). Protective effect of solanum nigrum leaves extract on immobilization stress induced changes in rat’s brain. Evidence-Based Complementary and Alternative Medicine, 1– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]