Abstract
Background and Aims
Prostaglandin (PG) D2 activates two receptors, DP and CRTH2. Antagonism of CRTH2 has been shown to promote anti-allergic and anti-inflammatory effects. We investigated whether CRTH2 may play a role in Crohn’s disease (CD) focusing on eosinophils, which are largely present in the inflamed mucosa of CD patients and express both receptors.
Methods
Using the 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis model, involvement of CRTH2 in colitis was investigated by pharmacological antagonism, immunohistochemistry, Western blotting, immunoassay and leukocyte recruitment. Chemotactic assays were performed with isolated human eosinophils. Biopsies and serum samples of CD patients were examined for presence of CRTH2 and ligands, respectively.
Results
High amounts of CRTH2-positive cells including eosinophils are present in the colonic mucosa of mice with TNBS colitis and human CD. The CRTH2 antagonist OC-459, but not the DP antagonist MK0524, reduced inflammation scores and decreased TNF-α, IL-1β, and IL-6 as compared to control mice. OC-459 inhibited recruitment of eosinophils into the colon and also inhibited CRTH2-induced chemotaxis of human eosinophils in vitro. Eosinophil-depleted ΔdblGATA knockout mice were less sensitive to TNBS-induced colitis while IL-5 transgenic mice with lifelong eosinophilia were more severely affected than wild-types. In addition, we show that serum levels of PGD2 and Δ12-PGJ2 were increased in CD patients as compared to control individuals.
Conclusion
CRTH2 plays a pro-inflammatory role in TNBS-induced colitis. Eosinophils contribute to the severity of the inflammation, which is improved by a selective CRTH2 antagonist. CRTH2 may, therefore, represent an important target in the pharmacotherapy of CD.
Keywords: CRTH2, eosinophils, Crohn’s disease
Introduction
Prostaglandin (PG) D2 acts via two distinct G protein-coupled receptors, the D-type prostanoid receptor (DP or DP1), and the chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2 or DP2). PGD2 is an important mediator in inflammatory reactions with either pro- or anti-inflammatory effects. Which of the effects occur depends on the type, activation, interaction, tissue and cellular presence of the PGD2 receptors involved 1,2. Of the two PGD2 receptors, CRTH2 is the one linked to allergic responses and inflammation, representing a potential drug target for asthma and rhinitis 2-4. The receptor is present on human eosinophils, basophils, Th2 cells, and monocytes/macrophages (unpublished data) whereas in rodents, neutrophils may also express CRTH2 5-8. DP expression is found on many types of leukocytes including dendritic cells, lymphocytes, neutrophils and eosinophils 2,9 and seems to convey anti-inflammatory actions in colitis models 10,11. During inflammation, CRTH2 was demonstrated as a strong mediator of chemotaxis 7,12. Antagonism of CRTH2 has been shown to prevent nasal 13 and lower airway inflammation induced by Aspergillus fumigatus 14, and croton oil-induced dermatitis in the late-phase 8. The mechanisms involved in the anti-asthmatic and anti-inflammatory effects of CRTH2 antagonists thus include inhibition of eosinophil and neutrophil recruitment into inflamed tissue 8,14. A recent study shows CRTH2 expression also on innate lymphoid cells and a likely involvement of these cells in pulmonary inflammation 15. In contrast, CRTH2−/− knockout mice displayed more severe arthritic manifestations and a higher amount of macrophages than the wild type mice in the inflamed paw of complete Freund’s adjuvant (CFA)-induced joint inflammation 16 suggesting that CRTH2-mediated influx of leukocytes into inflamed tissue is dependent on the type and location of the inflammation. Previously, we could demonstrate that DP and CRTH2 exert different activities in the pathogenesis of ulcerative colitis, one form of inflammatory bowel disease (IBD) 11. We showed that blockade of CRTH2, but not of DP, during dextran sulfate sodium (DSS)-induced colitis improved disease severity and inhibited neutrophil and lymphocyte influx, indicating that CRTH2 drives intestinal inflammation at the level of leukocyte infiltration into the colon 11.
In the present study, we assessed whether CRTH2 plays a role in Crohn’s disease (CD), another form of IBD with a pathogenesis distinct to ulcerative colitis. As a model of experimental CD, we used the 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis model, which has important characteristics of CD 17. Contrary to the DSS-induced colitis, the chemical agent TNBS acts as a hapten that initiates an immune response and results in transmural inflammation, edema and cytokine secretion, strongly reminiscent of CD 17,18. Like CD, TNBS-induced inflammation is dominated by the presence of Th1 cells and macrophages, and a surge of eosinophils into the colon 17-19. Although eosinophils are thought to exert a pro-inflammatory role in IBD 20, a recent work in eosinophil-depleted PHIL knockout mice demonstrated that eosinophils could exert protective effects in acute experimental colitis 21, highlighting that the role of eosinophils in the pathogenesis of IBD is still unclear. Since eosinophils highly express CRTH2 and DP 6,9,12, we focused on the role of eosinophils in the development of TNBS colitis and whether the CRTH2 receptor may play a role therein. We used human eosinophils for in vitro studies of chemotaxis, and eosinophil-depleted ΔdblGATA knockout 22 and IL-5 transgenic mice (with a >10fold higher capacity to produce eosinophils than wild-type littermates 23) in the TNBS inflammation model.
Materials and methods
Patients
For colonic tissue biopsies, CD patients (n=5, mean age ± SD: 29.2 ± 14.0) and healthy (control) subjects (n=6, mean age: 46 ± 16.4) (for detailed patients’ characteristics see Supplementary material) were recruited by the Department of Internal Medicine, Medical University of Graz and biopsies were collected as previously described 11. In CD patients, colonoscopy was done as part of the clinical workup. Control biopsies were obtained from patients undergoing colonoscopy as part of the colon cancer screening program and from patients with diagnostic workup of occult or overt gastrointestinal bleeding, with a normal colonoscopy. Significant comorbidities, infections, pregnancy, taking nonsteroidal anti-inflammatory drugs (including acetylsalicylic acid) were all criteria for exclusion. Biopsies were taken from inflamed segments of the colon and were immediately frozen for Western blot experiments. Histological sections from CD patients with colonic inflammation were used for immunohistochemical staining. For the measurement of prostaglandins, blood was collected from CD patients (n=31; mean age ± SD: 34.8 ± 9.9; 15 females, 16 males) and healthy subjects (n=15; mean age ± SD: 38.9 ± 18.5; 8 females, 7 males) in Vacuette ® serum tubes (Greiner-Bio-One, Kremsmünster, Austria), and frozen at −80°C until use. The study was approved by the Ethics Committee of the Medical University of Graz (protocol numbers: 24-281 ex 11/12), and all participants provided written, informed consent.
Animal model
Male CD1 mice were obtained from Charles River (Sulzfeld, Germany). ΔdblGATA knockout and IL-5 transgenic mice (both BALB/C background) were originally obtained from Dr. Helene Rosenberg (National Institute of Health, Bethesda, MD, USA) and were bred in our own animal facilities. After matching the animals by sex, age and body weight, TNBS colitis was induced as previously described 24 (see Supplementary material). Subsequently, colon samples were frozen immediately in liquid N2 or fixed in 10% neutral-buffered formalin and later processed for cytokine measurement, Western blot and histopathology. For the colitis experiments, we used control (CTRL) or vehicle-treated (VEH) groups, and groups receiving the CRTH2 antagonist OC-459 (abbreviated as OC) (Cayman Chemicals, Ann Arbor, MI, USA.) and the DP antagonist MK0524 (abbreviated as MK) (Cayman) alone or in combination (OC+MK). The antagonists OC-459 and MK0524 were injected subcutaneously (s.c.) 1 × as a pretreatment on the day before the TNBS application, followed by 2 × daily s.c. for 3 days. Experimental procedures in mice were approved by the Austrian Federal Ministry of Science and Research (protocol number 66.010/0018/-WF/V/3b/2015) and performed in accordance with the ARRIVE guidelines for reporting experiments involving animals 25.
Cytokine measurement
The colon samples were placed into extraction buffer (20 mg/50 μl), mechanically homogenized and sonicated. After normalization of protein concentrations, cytokine concentrations were measured from the extract. For the detection of TNF-α, IL-1β and IL-6, we employed mouse Readyset&go ELISA kits (eBioscience Inc., San Diego, CA, USA), according to the manufacturer’s protocol.
Western blot
Western blots were performed as described previously 11. For separation and detection of protein, SDS-PAGE (Life Technologies, Invitrogen, Vienna, Austria) was performed and gels were blotted onto polyvinylidene difluoride (PVDF) membranes (Merck Millipore, Billerica, MA, USA). Membranes were blocked in TBS-tween buffer containing 5% milk powder and subsequently incubated with rabbit anti-CRTH2 antibody (1:1000; Acris Antibodies, Herford, Germany), and mouse anti-β-actin antibodies (Sigma, St. Louis, MO, USA) overnight at 4°C. Membranes were washed and incubated with HRP-conjugated anti-rabbit antibodies (1:7500; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h at room temperature. Protein expression of CRTH2 was normalized to the respective actin level.
Liquid chromatography–mass spectrometry
For the quantification of PGD2, Δ12-PGJ2, PGE2, PGF2α, Thromboxane (TX) B2 and 11-dehydro (dh)-TXB2 in human sera, liquid chromatography/mass spectrometry (LC/MS/MS) was performed as previously outlined 11 (for detailed description see Supplementary material).
Immunohistochemistry/histochemical staining of colon tissue
Paraffin-embedded sections of human colon from CD patients and controls were cut (5 μm) and deparaffinized. For immunohistochemistry, sections were microwaved for 2 × 5-min cycles in 10 mM citrate buffer, and processed by ABC method according to the manufacturer’s protocol (Vectastain ABC kit; Vector Laboratories, Burlingname, CA, USA). Sections were incubated with rabbit anti-CRTH2 (1:200; Acris Antibodies, Herford, Germany) 11, visualized with 3-3′-diaminobenzidine (DAB) and counterstained with hematoxylin. CD4+ T cells were stained with a monoclonal mouse anti-human CD4 (clone 4B12; dilution 1:20; Labvision, Fremont, USA) as recommended by the suppliers. Images were taken with a high resolution digital camera (Olympus DP 50) and analyzed by Cell^A imaging software (Olympus, Vienna, Austria). Only contrast and brightness of images were adjusted. Sirius Red (Direct Red 80®, Sigma) was used to stain eosinophils in deparaffinized sections.
Eosinophil chemotaxis assay
Migration of eosinophils was studied in microBoyden chemotaxis chambers, as described before 11. 50 μl of purified human eosinophils (2×106 cells/ml), pretreated with antagonist (1 μM OC-459) were transferred into the top wells of the chamber separated from the bottom wells by a 5 μm pore-size polyvinylpyrrolidone-free polycarbonate filter. Assay buffer or agonists (PGD2 or 13,14-dihydro-15-keto (DK)-PGD2 [DK-PGD2]) at 30 nM) were loaded into the bottom wells. Baseline migration was determined with assay buffer. The chamber was incubated in a humidified incubator (37 °C for 1 h). After removing the membrane, cells that had migrated to the bottom wells cells were enumerated by flow cytometry (FACSCalibur, Becton-Dickinson, Mountain View, CA, USA).
Isolation and flow cytometric analysis of lamina propria leukocytes
As described previously 11, colon was removed, rinsed in Hank’s Buffered Salt Solution (HBSS), weighed, cut into small pieces, and transferred into HBSS containing 4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES) and penicillin/streptomycin (PS). Samples were incubated at 37°C and washed 4 × for 10 min with the HBSS/HEPES/PS buffer. After washing, samples were rinsed in complete RPMI 1640 medium (5 min) and then incubated with 100 U/ml collagenase type 2 (Life Technologies) (1 h at 37°C). Afterwards, the cell suspension was passed through a 40-μm cell strainer and centrifuged (400 × g; 7 min). Samples were washed twice in PBS, fixative solution was added, and samples were kept on ice until analysis on a FACSCalibur flow cytometer. Data were normalized to colon weight and expressed as percentage of total cells.
Statistical analysis
Data were analyzed either by Student’s t-test or one-way ANOVA, followed by Tukey’s post hoc test, using GraphPad Prism® (GraphPad Software Inc., La Jolla, CA, USA). P values of <0.05 were considered significant.
Additional information on Methods and Materials has been added to the Supplementary materials.
Results
Increased levels of CRTH2 in colon biopsies of CD patients
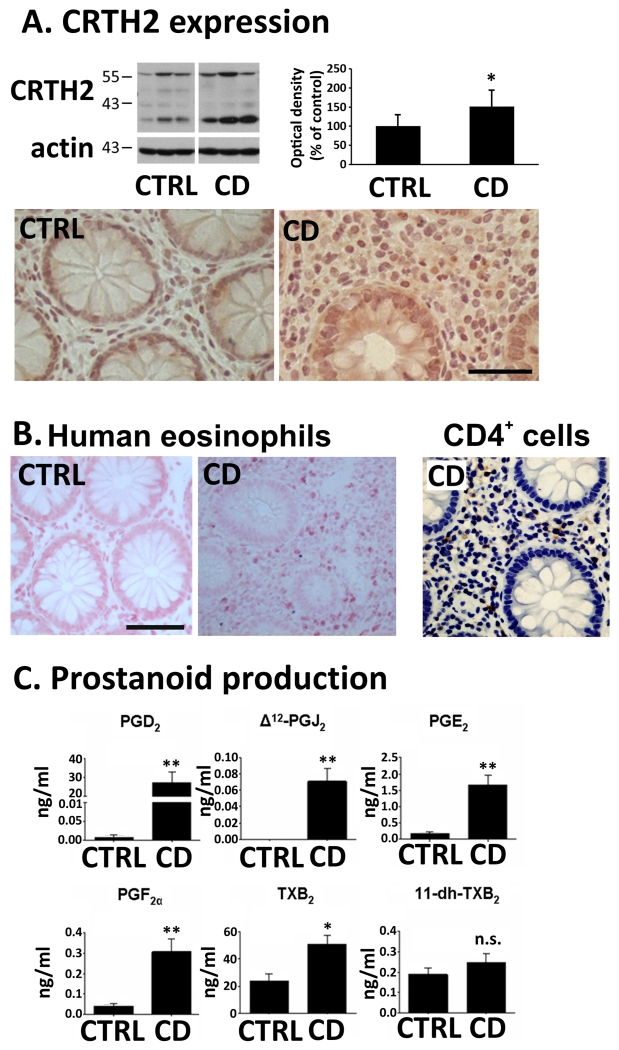
Western blots of colonic biopsies from CD patients revealed increased CRTH2 content (of ~40%) as compared to biopsies obtained from healthy individuals (Fig. 1). Bands were distributed between ~35-55 kDa, which, according to Nagata et al.26, represent the different N-glycosylation states of the CRTH2 protein. Immunohistochemistry confirmed the presence of CRTH2 in the lamina propria and epithelial cells of CD patients (Fig. 1A). We also employed Sirius Red staining in colonic sections of biopsies from CD patients to demonstrate the presence of eosinophils 27. Confirming previous reports 28, biopsies of colon mucosa from CD patients showed high levels of eosinophils. Representative images of Sirius Red-stained colonic sections from CD patients and healthy subjects (control; abbreviated as CRTL in graphs) are shown in Fig.1B. Since CD4+ T cells can also express CRTH2, CD4+ immunostaining of a biopsy of the colon mucosa from a CD patient is shown. Similar amounts of CD4+ T cells (24.4±10.3/visual field) and eosinophils (18.1±5.0/visual field) were counted in the sections of the colonic mucosa from CD patients (data are means±SD from 6-10 visual fields/section; 4 CD patients evaluated).
Fig. 1. CRTH2 and eosinophils in human colonic biopsies. Serum prostanoid content in CD patients.
(A) CRTH2 Western blots and immunohistochemistry (representative blots and images from n=5-7). (B) Sirius Red-stained eosinophils in sections of colonic biopsies (representative images from n=3) Calibration bars: 50μm. Image on the right shows CD4+ T cell staining in a section of a colonic biopsy from a Crohn’s disease (CD) patient. (C) Serum prostanoid levels of CD and healthy subjects (CTRL) (15 CTRL, 31 CD). Means±SD; *p<0.05; **p<0.01; Student’s t-test.
Increased production of endogenous CRTH2 ligands in CD patients
After investigating the presence of CRTH2 receptors in human colonic biopsies, we measured the endogenous ligands of CRTH2, i.e. of PGD2 and its metabolites (and also of other prostanoids), in sera of CD patients and healthy subjects (control; CTRL). We found increased levels of PGD2 and of its metabolite Δ12-PGJ2 29 in the CD group in comparison to the healthy group (Fig.1C). Production of 11-dh-TXB2, which has been described as a full agonist of CRTH2 30, was slightly but not significantly increased (Fig.1C). However, its parent molecule, TXB2, which is a stable product of TXA2 and which is rapidly metabolized to 11-dh-TXB2 31, was markedly increased in CD patients (Fig.1.C). Other prostaglandins, i.e. PGE2 and PGF2α (Fig.1.C), were also elevated in CD patients.
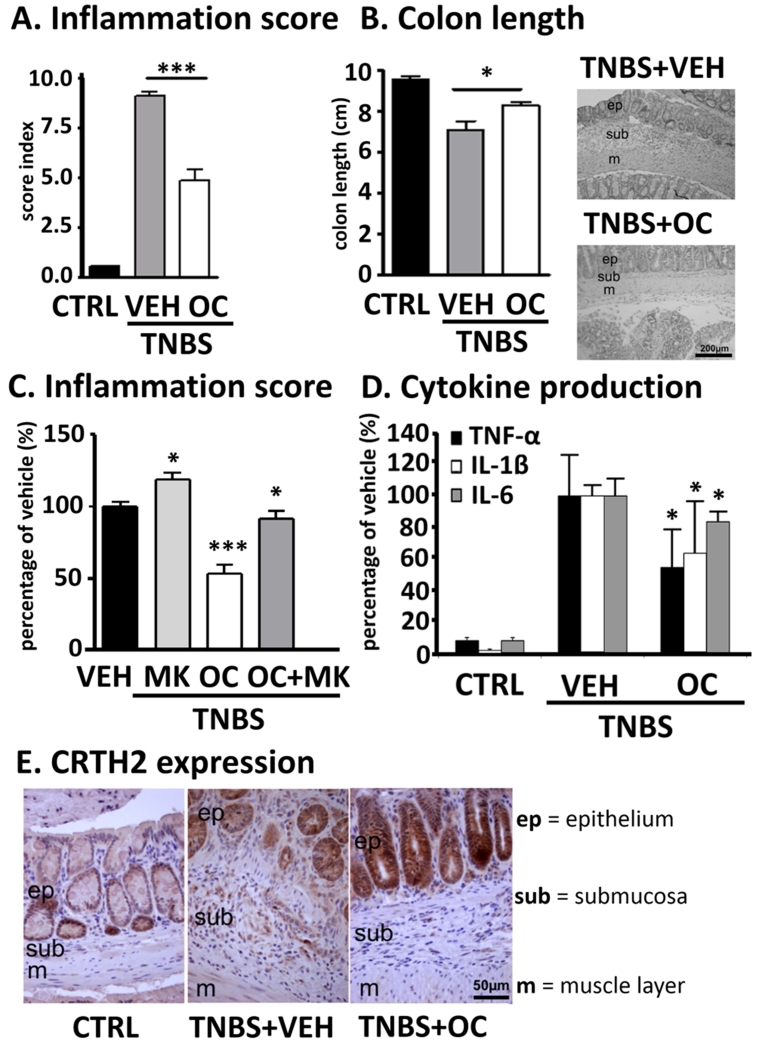
CRTH2 antagonist OC-459 improves, whereas DP antagonist MK0524 exacerbates TNBS-induced colitis in mice
To evaluate the effect of a selective CRTH2 antagonist in TNBS colitis, OC-459 was used at a dose, at which it caused reduction in blood eosinophilia after systemic treatment with DK-PGD2 32. Twice daily treatment with OC-459 (0.1 mg/kg, s.c.) decreased the inflammation scores in mice by almost 50% (Fig. 2A), preventing colon shortening and histological damage of the mucosa (Fig. 2B). Levels of the pro-inflammatory cytokines TNF-α, IL-1β and IL-6 were decreased by ~30-50% (Fig. 2D). We also investigated the effect of MK0524, a selective antagonist and inverse agonist at DP 33,34. As previously also observed in a DSS model 11, MK0524 (1 mg/kg) worsened inflammation scores in TNBS colitic mice by ~20%. Co-application with OC-459 diminished the protective effects of the CRTH2 antagonist (Fig. 2C). CRTH2 was identified by immunohistochemistry in mouse colon revealing similar staining patterns to human colonic biopsies (i.e. CRTH2 immunoreactivity in epithelial and lamina propria cells) (Fig. 2E).
Fig. 2. CRTH2 antagonist OC-459 improves while DP antagonist MK0524 worsens TNBS-induced colitis.
(A) OC-459 (OC), but not MK0524 (MK) (C), decreased inflammation scores, and prevented (B) colon shortening and (D) cytokine production vs. vehicle (VEH)-treated mice. (E) CRTH2-immunoreactivity in the lamina propria and epithelial cells in sections of mouse colon. Means±SD; *p<0.05; ***p<0.001; one-way ANOVA, n=6-10. Controls (CTRL, no TNBS).
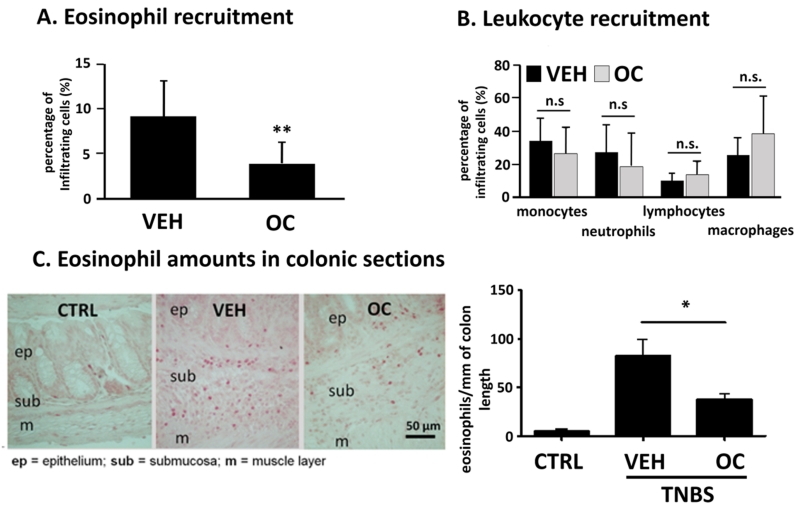
OC-459 inhibits eosinophil recruitment into the colon during TNBS colitis
Because of the prominent role of CRTH2 in cell migration, we measured the recruitment of leukocytes into the colonic lamina propria during TNBS colitis by flow cytometry. Twice daily treatment with OC-459 inhibited the infiltration of eosinophils into the colon of mice (Fig.3A) but failed to significantly inhibit neutrophil and lymphocyte infiltration (Fig.3B). Inhibition of eosinophilic infiltration to the colon was additionally evaluated by counting Sirius Red-stained eosinophils in sections of whole colon (whole colons were prepared as “Swiss rolls” 35). Similar to our flow cytometric results, a ~50% reduction of eosinophils into the colonic lamina propria was observed in OC-459-treated vs. vehicle-treated animals (Fig.3C).
Fig. 3. OC-459 inhibits eosinophil recruitment during TNBS colitis.
(A) Colonic infiltration of eosinophils but not of other leukocytes (B) was reduced in OC-459-treated (OC) vs. vehicle (VEH)-treated mice (n=9). (C) Presence of eosinophils were counted by Sirius Red staining in whole colon sections (n=5-13). Means±SD; *p<0.05; **p<0.01; one-way ANOVA and Student’s t-test. Controls (CTRL, no TNBS).
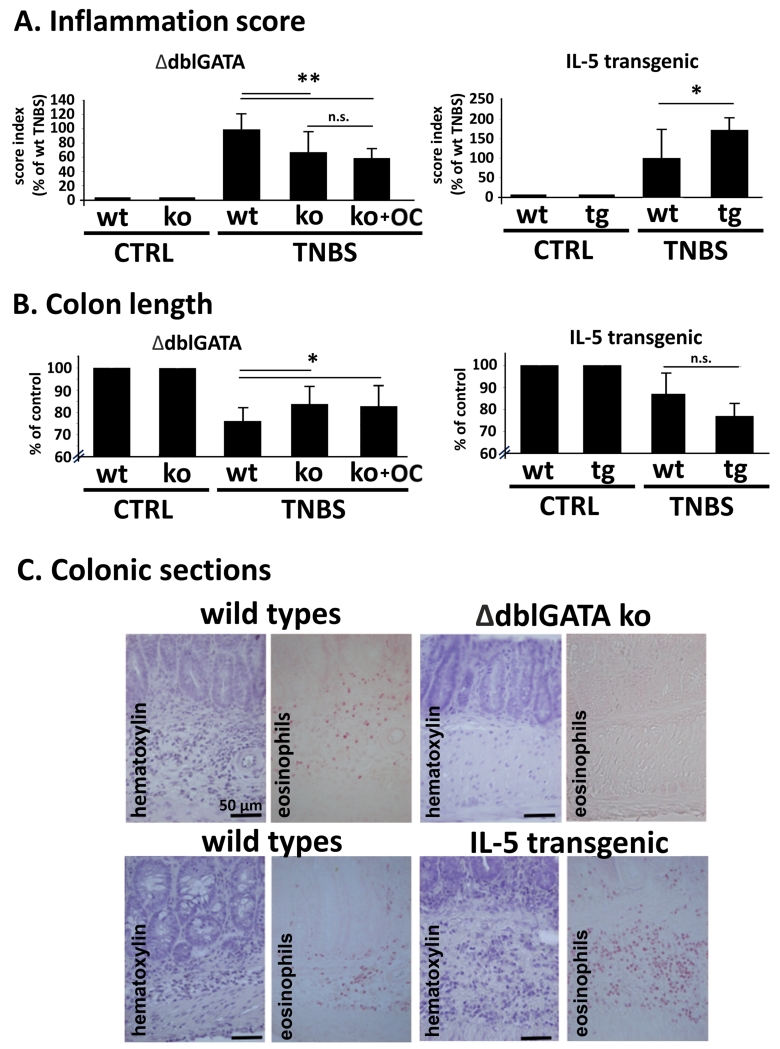
Eosinophils play a pro-inflammatory role in TNBS colitis
A pro-inflammatory role of eosinophils has been mainly described in DSS colitis models 36-38. To test whether eosinophils may also exert a pro-inflammatory role in our TNBS model, we used two genetically modified mice strains, namely ΔdblGATA knockout mice, that completely lack presence of eosinophils 37, and IL-5 transgenic mice, which have an enhanced eosinophil production. We found that ΔdblGATA knockout mice were less sensitive to TNBS-induced colitis as compared to wild-types (Fig. 4A) exhibiting reduced inflammation scores, colon shortening (Fig.4B) and an improved histopathology of the colonic mucosa (Fig. 4C). Inflammation score and colon length of ΔdblGATA knockout mice treated with OC-459 (0.1 mg/kg s.c.; Fig. 4A and B) did not differ from vehicle-treated ΔdblGATA knockout mice indicating that eosinophils are indeed affected by the blockade with OC-459. In contrast to their wild-type littermates, IL-5 transgenic mice showed severe macroscopic signs of colitis (Fig. 4A) and pronounced tissue damages (Fig. 4C).
Fig. 4. TNBS colitis in ΔdblGATA knockout and IL-5 transgenic mice.
(A, B) Inflammation was reduced in ΔdblGATA knockouts (ko), but enhanced in IL-5 transgenic (tg) mice vs. wild-types (wt). Treatment of ΔdblGATA knockouts (ko) with 0.1 mg/kg OC-459 (OC) had no further effect on inflammation score and colon length. (C) Images show lack (ΔdblGATA ko) or high presence (IL-5 tg) of eosinophils in sections of inflamed colon of TNBS-induced colitis (n=7-12). Means±SD; *p<0.05; **p<0.01; one-way ANOVA. Controls (CTRL, no TNBS);
Inhibition of eosinophil chemotaxis by OC-459
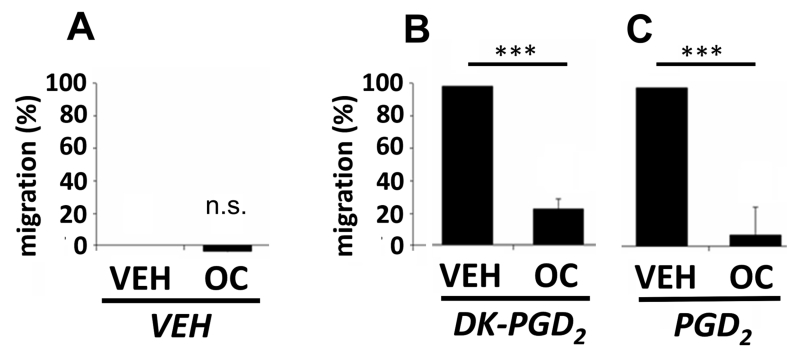
As a result of the TNBS experiments with the new CRTH2 antagonist and its prominent effect on eosinophils in the recruitment assay, CRTH2 was investigated in receptor-induced chemotaxis of human eosinophils using an in-vitro chemotaxis assay. To selectively activate CRTH2, we used DK-PGD2 11, whereas for the simultaneous activation of DP and CRTH2 receptors, PGD2 was used. First, the basal receptor activity was assessed without the addition of any agonists (only vehicle added). Under these conditions, OC-459 slightly but not significantly reduced basal migration, which was set at zero (hence the negative values in the graph basal migration) (Fig. 5A). Further, we induced chemotaxis by DK-PGD2, and we detected high amounts of cells migrating towards the DK-PGD2 gradient (Fig. 5B). OC-459 markedly decreased this effect. When using PGD2 as a chemoattractant, migration of eosinophils was similarly blocked by OC-459 (Fig. 5C).
Fig. 5. OC-459 inhibits human eosinophil migration in vitro.
(A) OC-459 (OC) had no effect on basal activity (B) but inhibited DK-PGD2- and (C) PGD2-induced migration of human eosinophils (VEH set at 100%). (n=7) Means±SD; ***p<0.001; Student’s t-test; n. s., not significant; VEH = vehicle.
Discussion
TNBS-induced experimental colitis models are widely used to study CD as they share many clinical features and histopathological changes with the human condition 17,18. We were specifically interested in the role of eosinophils in this model because eosinophils express both PGD2 receptors, with CRTH2 being a key regulator of eosinophil migration 9,12. This fact prompted us to investigate a selective and potent antagonist for CRTH2, OC-459 32, which has already shown beneficial effects in adult patients with active, corticosteroid-dependent or corticosteroid-refractory eosinophilic esophagitis 39 and in patients with airway inflammation 40. We can show that OC-459 leads to an improvement of TNBS colitis, reducing eosinophil influx to the colon. In vitro, the antagonist can directly inhibit the migration of human eosinophils.
The first important finding in our study was that the recruitment of eosinophils to the colon during TNBS colitis was significantly decreased in OC-459-treated mice. This was accompanied by an amelioration of the inflammatory response, and a reduction in the levels of TNF-α, IL-1β and IL-6. Similar to ulcerative colitis 11, colonic biopsies of CD patients in our study showed an increased content of CRTH2 as compared to healthy individuals. In addition, endogenous ligands of CRTH2, such as PGD2 and Δ12-PGJ2 were found to be increased in sera of Crohn’s patients as compared to healthy subjects, indicating an active ligand-CRTH2 axis in CD.
Both human CD and ulcerative colitis display extensive infiltration of eosinophils into inflamed areas of the colon 28,41. Higher amounts of eosinophils in CD than in ulcerative colitis that correlated with disease severity were also recorded 28, suggesting a prominent pro-inflammatory activity of eosinophils in CD. In a previous study, we demonstrated that the CRTH2 antagonist Cay10595 provided protection in DSS colitis by inhibiting the accumulation of neutrophils and lymphocytes in the colon while alterations in the number of infiltrated eosinophils were not detected 11. However, in the current “CD-like” TNBS-induced colitis model, we measured a much higher influx of eosinophils into the colon (5-fold higher than in DSS colitis). In addition, the amount of monocytes was also higher in the TNBS than the DSS model (~30 vs. ~15%), indicating that different populations of infiltrated leukocytes are involved in the pathogenesis of these models. This inhibitory effect of OC-459 on eosinophil migration may have occurred at the eosinophil itself, as demonstrated by our in vitro migration assays. Of particular interest, we did not observe significant inhibitory effects of OC-459 on the recruitment of other leukocytes in our study. However, in accordance with previous studies 32, we observed that the migration of eosinophils towards DK-PGD2, a CRTH2 ligand, and towards PGD2, a ligand for CRTH2 and DP, was inhibited by the CRTH2 antagonist in vitro in a similar manner. It should be mentioned that eosinophils from CD patients are activated and have shown increased spontaneous and ligand–induced migration in vitro as compared to those from healthy individuals 42. Studying the PGD2 metabolites (many of which are ligands of CRTH2), over the course of CD may, therefore, shed new light on the role of the two PGD2 receptors in eosinophil migration during inflammation. Since we were interested to uncover whether the decreased eosinophil infiltration into the colon by OC-459 treatment was pivotal to the improvement of TNBS colitis, we carried out the TNBS model in eosinophil-depleted ΔdblGATA knockout mice, which showed a strong reduction in disease severity. Our results are in accordance with others who used ΔdblGATA knockout mice in the DSS model 37, but are in contrast with recent findings that suggested protective effects of eosinophils in colitis models of eosinophil-deficient PHIL knockout mice 21. The discrepancy between these results may lie in the use of different knockout mouse strains because by use of our TNBS model, we could clearly confirm the findings of the DSS models 36,37. In addition, IL-5 transgenic mice that produce a large amount of eosinophils 23 were more affected by TNBS colitis than the wild type mice, supporting the notion that eosinophils aggravate disease severity in acute mouse colitis. Although immunohistochemical images show CRTH2 in colonic epithelial cells we do not suspect it to be part of the beneficial effects by OC-459. Wound healing and proliferation experiments in Caco-2 cells using CRTH2 antagonists failed to show an effect (data not shown). It should be noted that the reduction TNF-α, IL-1β and IL-6 levels could be a direct effect of the compound on cytokine release from T cells and macrophages beside inhibition of migration in these cells. These cell types are known to produce cytokines during colitis and both Th2 cells and macrophages have shown release of cytokines on activation with the CRTH2 agonist DK-PGD2 43,44.
To summarize, the CRTH2 antagonist OC-459 ameliorated colon inflammation and reduced the influx of eosinophils into the colonic lamina propria. Since CRTH2 is expressed also in other leukocytes and epithelial cells, eosinophil-unrelated beneficial effects of the antagonist may likely contribute. However, eosinophil-depleted ΔdblGATA knockout mice were clearly less affected by the colitis model than the wild types while IL-5 transgenic mice displayed severe colitis. In addition, OC-495 did not improve inflammation in the ΔdblGATA knockout mice suggesting that the effect of the compound on the inflammation indeed is brought about via blockade of eosinophils. CRTH2 is also known to be expressed on CD4+ T-cells, which infiltrate the inflamed colon and contribute to the disease. However, in a recent study we could show that in the blood of ulcerative colitis patients, CRTH2 immunofluorescence was much higher on eosinophils as compared to CD3+/CD4+ T cells 11 suggesting a prominent role for CRTH2 on eosinophils maybe also in Crohn’s disease.
Taken together, the results strongly point at a pro-inflammatory role of eosinophils during TNBS colitis that can be improved by treatment with a selective CRTH2 antagonist. Our data, therefore, suggest that CRTH2 antagonism in Crohn’s disease may be a valuable option for pharmacotherapy.
Supplementary Material
Acknowledgments
We would like to thank Veronika Pommer for excellent technical assistance.
Funding:
This work was supported by the Austrian Science Fund FWF [P25633 to RS, P22976 to GM, P22521 to AH]. JK was funded by the Austrian Science Fund FWF through the Doctoral College ‘Molecular Fundamentals of Inflammation’ (MOLIN). The funding source only provided financial support and was not involved in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- ABC
avidin biotin complex
- CD
Crohn’s disease
- CRTH2
chemoattractant receptor-homologous molecule expressed on Th2 cells receptor
- DSS
dextran sulfate sodium
- DK-PGD2
13,14-dihydro-15-keto-PGD2
- DP
D-type prostanoid receptor
- IBD
inflammatory bowel disease
- LC/MS/MS
liquid chromatography-mass spectrometry
- MK
MK0524
- OC
OC-495
- PGD2
prostaglandin D2
- PGE2
prostaglandin E2
- PGF2α
prostaglandin F2-alpha
- Δ12-PGJ2
prostaglandin J2
- PS
penicillin/streptomycin
- TNBS
2,4,6-trinitrobenzenesulfonic acid
- TXB2
thromboxane B2
- 11-dh-TXB2
11-dehydro-thromboxane B2
- VEH
vehicle
Footnotes
Conflicts of interest
None
Conference presentation:
48th Annual Meeting & 26th Postgraduate course of the Austrian Society of Gastroenterology & Hepatology (ÖGGH), Salzburg, Austria, 2015.
References
- 1.Kostenis E, Ulven T. Emerging roles of DP and CRTH2 in allergic inflammation. Trends Mol Med. 2006;12:148–158. doi: 10.1016/j.molmed.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Schuligoi R, Sturm E, Luschnig P, et al. CRTH2 and D-type prostanoid receptor antagonists as novel therapeutic agents for inflammatory diseases. Pharmacology. 2010;85:372–382. doi: 10.1159/000313836. [DOI] [PubMed] [Google Scholar]
- 3.Pettipher R. The roles of the prostaglandin D(2) receptors DP(1) and CRTH2 in promoting allergic responses. Br J Pharmacol. 2008;153(Suppl 1):S191–9. doi: 10.1038/sj.bjp.0707488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horak F, Zieglmayer P, Zieglmayer R, et al. The CRTH2 antagonist OC000459 reduces nasal and ocular symptoms in allergic subjects exposed to grass pollen, a randomised, placebo-controlled, double-blind trial. Allergy. 2012;67:1572–1579. doi: 10.1111/all.12042. [DOI] [PubMed] [Google Scholar]
- 5.Spik I, Brenuchon C, Angeli V, et al. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J Immunol. 2005;174:3703–3708. doi: 10.4049/jimmunol.174.6.3703. [DOI] [PubMed] [Google Scholar]
- 6.Monneret G, Gravel S, Diamond M, et al. Prostaglandin D2 is a potent chemoattractant for human eosinophils that acts via a novel DP receptor. Blood. 2001;98:1942–1948. doi: 10.1182/blood.v98.6.1942. [DOI] [PubMed] [Google Scholar]
- 7.Gervais FG, Cruz RP, Chateauneuf A, et al. Selective modulation of chemokinesis, degranulation, and apoptosis in eosinophils through the PGD2 receptors CRTH2 and DP. J Allergy Clin Immunol. 2001;108:982–988. doi: 10.1067/mai.2001.119919. [DOI] [PubMed] [Google Scholar]
- 8.Sarashina H, Tsubosaka Y, Omori K, et al. Opposing immunomodulatory roles of prostaglandin D2 during the progression of skin inflammation. J Immunol. 2014;192:459–465. doi: 10.4049/jimmunol.1302080. [DOI] [PubMed] [Google Scholar]
- 9.Schratl P, Royer JF, Kostenis E, et al. The role of the prostaglandin D2 receptor, DP, in eosinophil trafficking. J Immunol. 2007;179:4792–4799. doi: 10.4049/jimmunol.179.7.4792. [DOI] [PubMed] [Google Scholar]
- 10.Ajuebor MN, Singh A, Wallace JL. Cyclooxygenase-2-derived prostaglandin D(2) is an early anti-inflammatory signal in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G238–44. doi: 10.1152/ajpgi.2000.279.1.G238. [DOI] [PubMed] [Google Scholar]
- 11.Sturm EM, Radnai B, Jandl K, et al. Opposing roles of prostaglandin D2 receptors in ulcerative colitis. J Immunol. 2014;193:827–839. doi: 10.4049/jimmunol.1303484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Royer JF, Schratl P, Lorenz S, et al. A novel antagonist of CRTH2 blocks eosinophil release from bone marrow, chemotaxis and respiratory burst. Allergy. 2007;62:1401–1409. doi: 10.1111/j.1398-9995.2007.01452.x. [DOI] [PubMed] [Google Scholar]
- 13.Shiraishi Y, Takeda K, Domenico J, et al. Role of prostaglandin D2 and CRTH2 blockade in early- and late-phase nasal responses. Clin Exp Allergy. 2014;44:1076–1082. doi: 10.1111/cea.12280. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Zheng M, Qiao J, et al. Role of prostaglandin D2 /CRTH2 pathway on asthma exacerbation induced by Aspergillus fumigatus. Immunology. 2014;142:78–88. doi: 10.1111/imm.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tait Wojno ED, Monticelli LA, Tran SV, et al. The prostaglandin D receptor CRTH2 regulates accumulation of group 2 innate lymphoid cells in the inflamed lung. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsubosaka Y, Nakamura T, Hirai H, et al. A deficiency in the prostaglandin D2 receptor CRTH2 exacerbates adjuvant-induced joint inflammation. J Immunol. 2014;193:5835–5840. doi: 10.4049/jimmunol.1303478. [DOI] [PubMed] [Google Scholar]
- 17.Scheiffele F, Fuss IJ. Induction of TNBS colitis in mice. Curr Protoc Immunol. 2002 doi: 10.1002/0471142735.im1519s49. Chapter 15: Unit 15.19. [DOI] [PubMed] [Google Scholar]
- 18.Alex P, Zachos NC, Nguyen T, et al. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341–352. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pontell L, Castelucci P, Bagyanszki M, et al. Structural changes in the epithelium of the small intestine and immune cell infiltration of enteric ganglia following acute mucosal damage and local inflammation. Virchows Arch. 2009;455:55–65. doi: 10.1007/s00428-009-0795-x. [DOI] [PubMed] [Google Scholar]
- 20.Wedemeyer J, Vosskuhl K. Role of gastrointestinal eosinophils in inflammatory bowel disease and intestinal tumours. Best Pract Res Clin Gastroenterol. 2008;22:537–549. doi: 10.1016/j.bpg.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Masterson JC, McNamee EN, Fillon SA, et al. Eosinophil-mediated signalling attenuates inflammatory responses in experimental colitis. Gut. 2015;64:1236–1247. doi: 10.1136/gutjnl-2014-306998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu C, Cantor AB, Yang H, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dent LA, Strath M, Mellor AL, et al. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990;172:1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schicho R, Bashashati M, Bawa M, et al. The atypical cannabinoid O-1602 protects against experimental colitis and inhibits neutrophil recruitment. Inflamm Bowel Dis. 2011;17:1651–1664. doi: 10.1002/ibd.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGrath JC, Drummond GB, McLachlan EM, et al. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagata K, Tanaka K, Ogawa K, et al. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol. 1999;162:1278–1286. [PubMed] [Google Scholar]
- 27.Kim SW, Kim DW, Khalmuratova R, et al. Resveratrol prevents development of eosinophilic rhinosinusitis with nasal polyps in a mouse model. Allergy. 2013;68:862–869. doi: 10.1111/all.12132. [DOI] [PubMed] [Google Scholar]
- 28.Smyth CM, Akasheh N, Woods S, et al. Activated eosinophils in association with enteric nerves in inflammatory bowel disease. PLoS One. 2013;8:e64216. doi: 10.1371/journal.pone.0064216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzpatrick FA, Wynalda MA. Albumin-catalyzed metabolism of prostaglandin D2. Identification of products formed in vitro. J Biol Chem. 1983;258:11713–11718. [PubMed] [Google Scholar]
- 30.Bohm E, Sturm GJ, Weiglhofer I, et al. 11-Dehydro-thromboxane B2, a stable thromboxane metabolite, is a full agonist of chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2) in human eosinophils and basophils. J Biol Chem. 2004;279:7663–7670. doi: 10.1074/jbc.M310270200. [DOI] [PubMed] [Google Scholar]
- 31.Westlund P, Kumlin M, Nordenstrom A, et al. Circulating and urinary thromboxane B2 metabolites in the rabbit: 11-dehydro-thromboxane B2 as parameter of thromboxane production. Prostaglandins. 1986;31:413–443. doi: 10.1016/0090-6980(86)90106-1. [DOI] [PubMed] [Google Scholar]
- 32.Pettipher R, Vinall SL, Xue L, et al. Pharmacologic profile of OC000459, a potent, selective, and orally active D prostanoid receptor 2 antagonist that inhibits mast cell-dependent activation of T helper 2 lymphocytes and eosinophils. J Pharmacol Exp Ther. 2012;340:473–482. doi: 10.1124/jpet.111.187203. [DOI] [PubMed] [Google Scholar]
- 33.Labrecque P, Roy SJ, Frechette L, et al. Inverse agonist and pharmacochaperone properties of MK-0524 on the prostanoid DP1 receptor. PLoS One. 2013;8:e65767. doi: 10.1371/journal.pone.0065767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sturino CF, O’Neill G, Lachance N, et al. Discovery of a potent and selective prostaglandin D2 receptor antagonist, [(3R)-4-(4-chloro-benzyl)-7-fluoro-5-(methylsulfonyl)-1,2,3,4-tetrahydrocyclopent a[b]indol-3-yl]-acetic acid (MK-0524) J Med Chem. 2007;50:794–806. doi: 10.1021/jm0603668. [DOI] [PubMed] [Google Scholar]
- 35.Moolenbeek C, Ruitenberg EJ. The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Lab Anim. 1981;15:57–59. doi: 10.1258/002367781780958577. [DOI] [PubMed] [Google Scholar]
- 36.Ahrens R, Waddell A, Seidu L, et al. Intestinal macrophage/epithelial cell-derived CCL11/eotaxin-1 mediates eosinophil recruitment and function in pediatric ulcerative colitis. J Immunol. 2008;181:7390–7399. doi: 10.4049/jimmunol.181.10.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vieira AT, Fagundes CT, Alessandri AL, et al. Treatment with a novel chemokine-binding protein or eosinophil lineage-ablation protects mice from experimental colitis. Am J Pathol. 2009;175:2382–2391. doi: 10.2353/ajpath.2009.090093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Forbes E, Murase T, Yang M, et al. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J Immunol. 2004;172:5664–5675. doi: 10.4049/jimmunol.172.9.5664. [DOI] [PubMed] [Google Scholar]
- 39.Straumann A, Hoesli S, Bussmann C, et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy. 2013;68:375–385. doi: 10.1111/all.12096. [DOI] [PubMed] [Google Scholar]
- 40.Pettipher R, Hunter MG, Perkins CM, et al. Heightened response of eosinophilic asthmatic patients to the CRTH2 antagonist OC000459. Allergy. 2014;69:1223–1232. doi: 10.1111/all.12451. [DOI] [PubMed] [Google Scholar]
- 41.Lampinen M, Backman M, Winqvist O, et al. Different regulation of eosinophil activity in Crohn’s disease compared with ulcerative colitis. J Leukoc Biol. 2008;84:1392–1399. doi: 10.1189/jlb.0807513. [DOI] [PubMed] [Google Scholar]
- 42.Coppi LC, Thomazzi SM, de Ayrizono ML, et al. Comparative study of eosinophil chemotaxis, adhesion, and degranulation in vitro in ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2007;13:211–218. doi: 10.1002/ibd.20018. [DOI] [PubMed] [Google Scholar]
- 43.Xue L, Gyles SL, Wettey FR, et al. Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol. 2005;175:6531–6536. doi: 10.4049/jimmunol.175.10.6531. [DOI] [PubMed] [Google Scholar]
- 44.Jandl K, Stacher E, Balint Z, et al. Activated prostaglandin D receptors on macrophages enhance neutrophil recruitment into the lung. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.