Abstract
Glycosylation chemistry of 3-deoxy-D-manno-oct-2-ulosonic acid units has been considerably developed within the last decade. This review covers major achievements with respect to improved yields and anomeric selectivity as well as suppression of the elimination side reaction via selection of dedicated protecting groups and appropriate activation of the anomeric center.
Keywords: Kdo, Oligosaccharide, Synthesis, Glycal, Glycosyl donor
Introduction
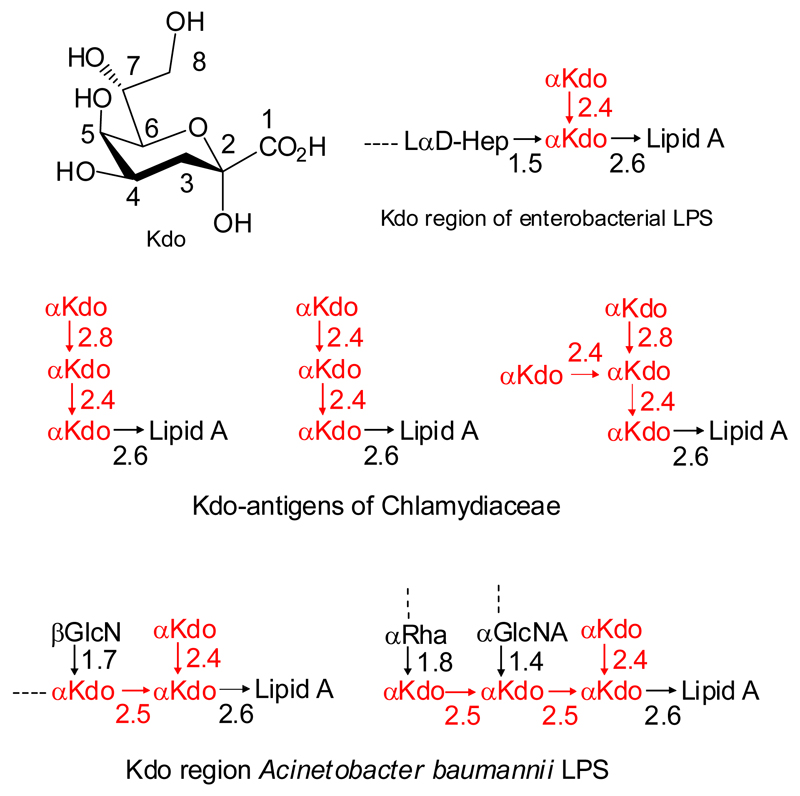
3-Deoxy-D-manno-oct-2-ulosonic acid (Kdo) is a non-mammalian higher-carbon saccharide which occurs in the cell wall glycans of Gram-negative bacteria, but has also been found in plants and algae.1–3 Kdo residues are of particular biomedical importance as constituents of bacterial lipopolysaccharides (LPS) as well as capsular polysaccharides.4 Due to their non-mammalian origin, Kdo units are recognized by components of the native and adaptive immune system as demonstrated by binding studies and crystal structures of liganded Kdo units in the binding sites of germ-line encoded antibodies and in complex with Toll-like receptor 4/MD-2.5 In LPS, Kdo is part of the structurally conserved inner core region, which interlinks the lipid A region to the outer core sugars and the O-antigenic polysaccharide. Figure 1 illustrates the most relevant Kdo-structures in the inner core of Gram-negative bacterial LPS.4 The synthesis of these oligosaccharide ligands and neoglycoconjugates derived therefrom is thus regarded as a highly relevant task for development of glycoarrays, immunoreagents and vaccines.
Figure 1.
Numbering scheme for Kdo and structures of selected Kdo oligosaccharides from pathogenic bacteria.
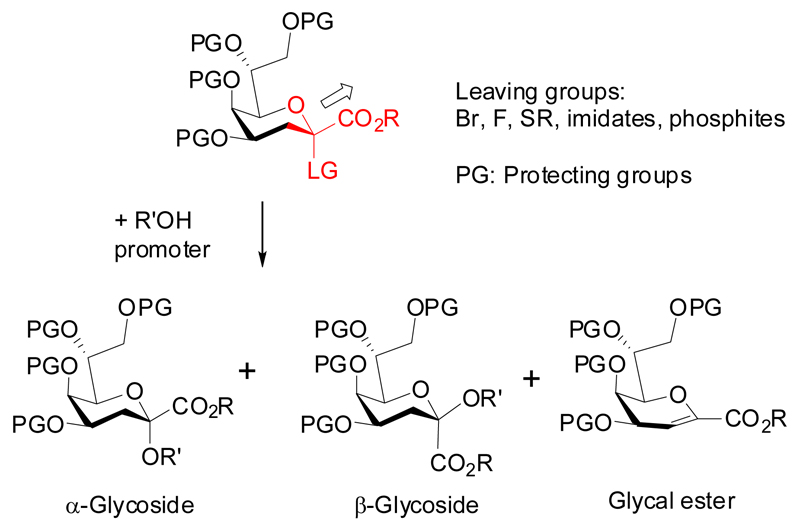
The synthesis of glycosides of Kdo shares several features with the structurally related 5-N-acetyl neuraminic acid (Neu5Ac), albeit with the difference that Kdo glycosides have been found in both anomeric configurations. Most of the synthetic work on Kdo chemistry in the past had been directed to the preparation of α-glycosides. Glycosylation reactions of Kdo donors are impaired by facile formation of a 2,3-dehydro product (glycal ester), the strong deactivation of the anomeric position by the adjacent carboxylic group and the absence of a stereodirecting group next to the anomeric center which limits stereochemical control of product formation. In addition, the increased steric load being present across a ketosidic linkage and the instability of the ketoside under acidic conditions has to be considered (Fig 2). Glycosylation reactions of Kdo monosaccharide derivatives with primary alcohols and reactive glycosyl acceptors, however, are usually doable in good yields and anomeric selectivities.
Figure 2.
Glycosylation products of Kdo donors.
The unfavorable properties, however, are much more demanding in the assembly of Kdo oligomers such as those depicted in Figure 1, resulting in many cases in poor to modest yields only and requiring substantial efforts in product purification. A number of reviews covering the synthesis of Kdo and important Kdo-containing bacterial oligosaccharides have been published previously and the reader should consult these for further information.6 The present review summarizes and highlights recent accomplishments, which have been reported in the past decade in the field of Kdo-oligosaccharide synthesis with a focus on α-ketosides, but will also cover selected examples for β-Kdo glycosides. The synthesis of the Kdo monosaccharide is beyond the scope of this review, but the reader is referred to a past review and two reports for multigram-preparation of Kdo.7–9
Kdo halide donors
Kdo bromide donors have mostly been used in the 1980ies and 1990ies under Koenigs-Knorr and Helferich conditions with varying success in the synthesis of Kdo oligosaccharides. The donors are easily available by reaction of the anomeric acetate in HBr/AcOH or TiBr4 in CH2Cl2 and are fairly stable when stored at low temperature. The use of toxic heavy-metal based promoters, the modest stereoselectivities and facile elimination side reaction leading to glycal ester byproducts, however, limits the use of Kdo bromide donors, in particular for preparation of oligosaccharides in a larger scale.
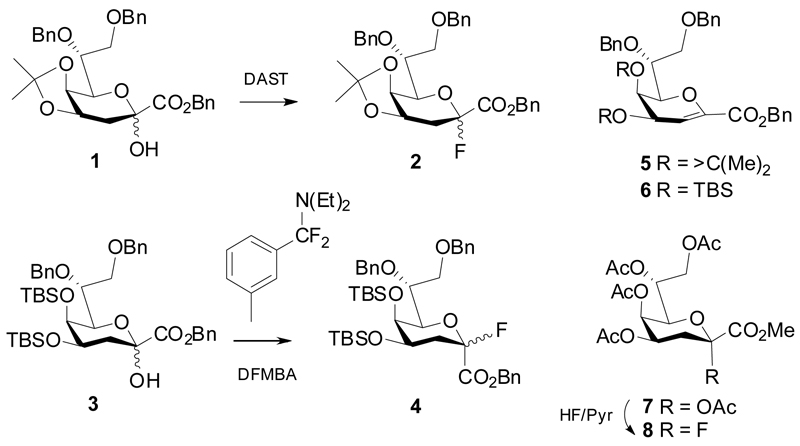
Kdo fluoride donors are accessible from the respective hemiketal precursors such as 1 and 3 by reaction with DAST, which leads to preferential formation of the α-anomeric fluorides, while reaction of 3 with N,N-diethyl-α,α-difluoro-(m-methylbenzyl)amine (DFMBA) mainly gives the β-anomeric product 4.10 Both reactions proceed with concomitant formation of glycal ester byproducts 5 and 6. Another option is the conversion of the anomeric acetate 7 with HF in pyridine to yield the α-fluoride 8 (Scheme 1).11
Scheme 1.
Synthesis of Kdo fluoride donors.
The groups of Kusumoto and Fukase have to be credited for developing these Kdo fluoride donors into a methodology, which is suitable for efficient coupling to protected lipid A derivatives. As a highlight, the total synthesis of Re-LPS from Escherichia coli is to be mentioned, wherein the Kdo fluorides served for constructing the α-(2→6)-linkage to the glucosamine disaccharide backbone as well as introduction of the lateral Kdo unit in an α-(2→4)-linkage.12 The introduction of bulky silyl substituents, or alternatively, the locking of the pyranose ring in a skew/boat conformation by an isopropylidene group at O-4 and O-5, allowed for significant improvement of the α-selectivity in the glycosylation reaction, presumably by sterically shielding the β-face.13 In general, however, an excess of donor and promoter has to be employed and significant amounts of glycal ester byproducts 5 and 6 were formed. A useful feature of the fluoride donors is the option of a glycodesilylation approach induced by liberation of HF from the BF3·Et2O promoter. Two recent examples are illustrated in schemes 2 and 3.
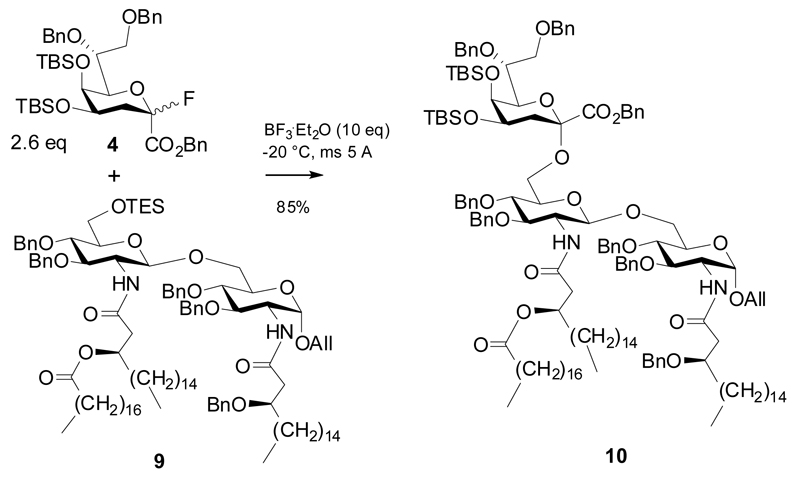
Scheme 2.
Glycosylation of a 6-O-TES-protected diglucosamine acceptor.
Scheme 3.
Glycosylation of a diglucosamine acceptor en route to Kdo-lipid A of Neisseria meningitidis.
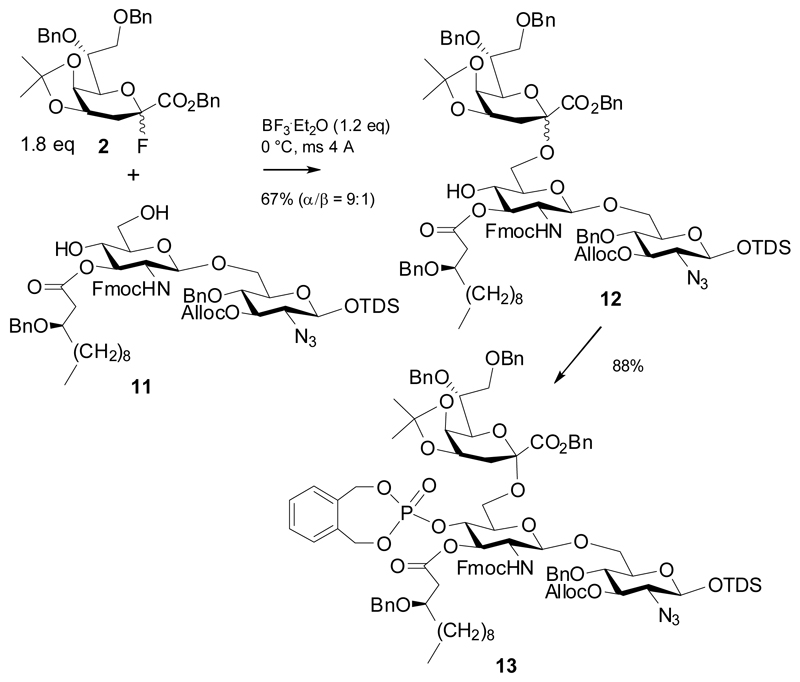
In 2007, Fukase et al succeeded in synthesizing an LPS fragment from Helicobacter pylori.10 Coupling of the 6-O-triethylsilyl protected disaccharide acceptor 9 was performed using 10 equivalents of promoter and 2.6 equivalents of fluoride donor 4 to give a high yield of the α-(2→6)-linked trisaccharide 10. Molecular sieves 5Å had been added to scavenge HF, which was formed in the reaction (Scheme 2). In 2008, the group of Boons synthesized a trisaccharide corresponding to a Kdo-lipid A fragment of Neisseria meningitidis LPS along similar lines (Scheme 3).14
Coupling of donor 2 to 3’-O-acylated disaccharide acceptor 11 was achieved with only a slight excess of promoter (relative to 11) and produced a 9:1 α/β mixture of trisaccharide 12. Separation of the anomeric mixture was achieved after 4’-O-phosphotriester formation with Watanabe reagent, which gave 13 in 88% yield.
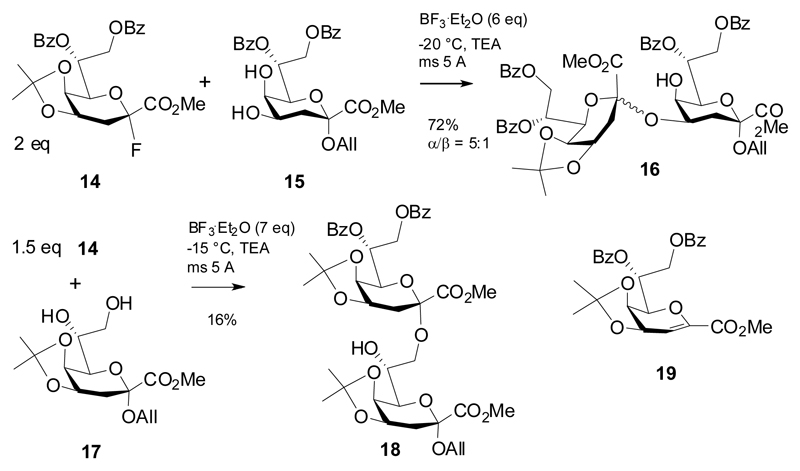
The reactivity of these Kdo donors, however, also depends on their protecting group patterns. “Disarming” substituents introduced at the side chain lead to decreased yields in glycosylation reactions. In 2011, Ichiyanagi et al employed the 7,8-di-O-benzoyl protected donor 14 for coupling reactions to 4-OH and 8-OH Kdo acceptors (Scheme 4).15 Again, a large excess of promoter was employed and triethylamine was added to capture released HF and to increase the product yield. While coupling to the 4-OH group of diol acceptor 15 produced a good yield of the disaccharide 16 and in fair anomeric selectivity, the α-(2→8)-linked disaccharide 18 was obtained in a rather poor yield only. The authors did not disclose the amount of elimination product 19 formed.
Scheme 4.
Glycosylation of 17 with a peracetylated Kdo bromide donor was also low-yielding according to a previous report.16
3-Iodo-Kdo fluoride donors
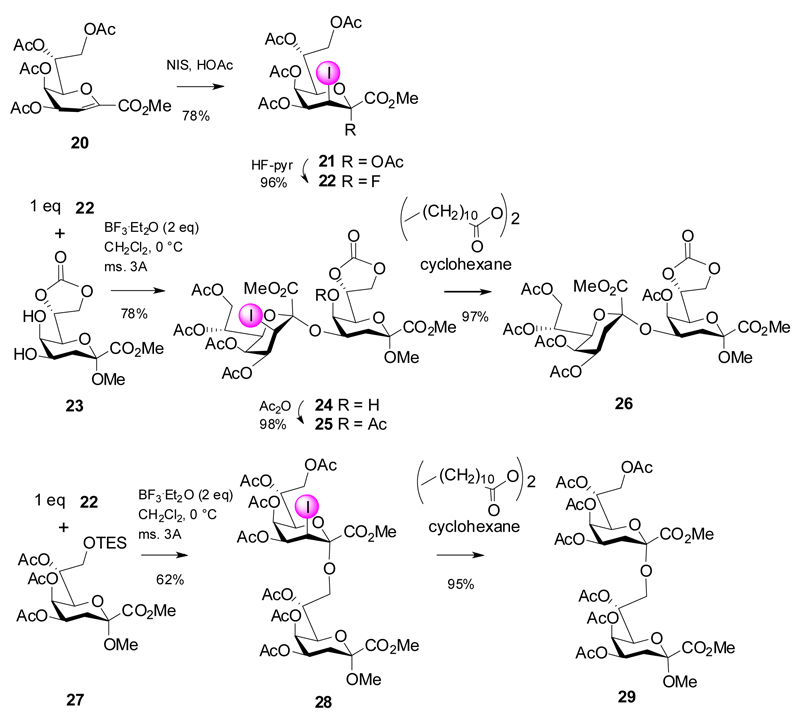
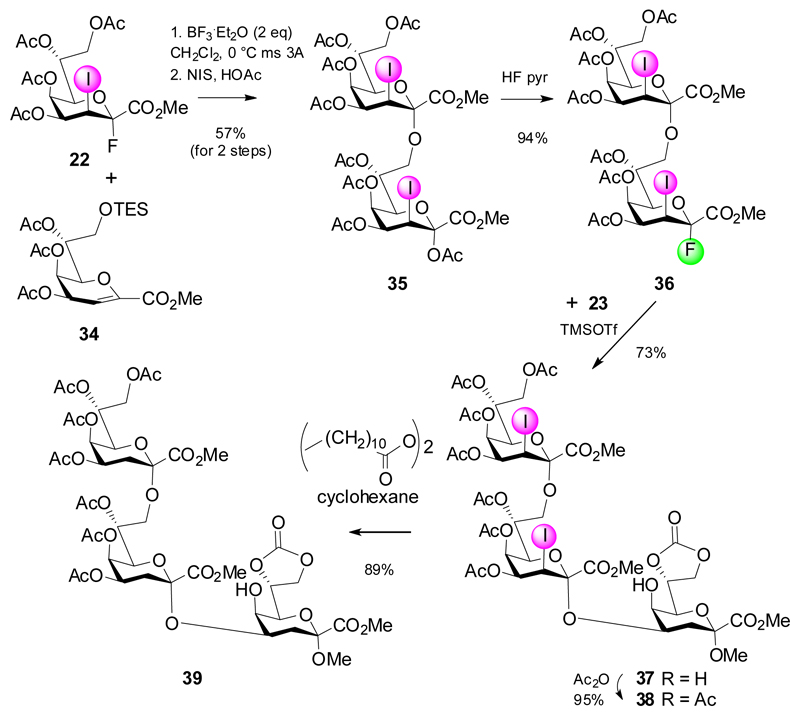
In order to work around the lack of stereochemical control, auxiliary stereodirecting groups at C-3 such as 3-iodo, 3-phenylselenyl and 3-thio groups had previously been used for Kdo-glycoside formation, but also in the field of neuraminic acid chemistry.17,18 Recently the favorable stereodirecting effect of a 3-iodo substituent has been combined with a fluoride leaving group. In contrast to fluoride donors 2, 4 and 14, 3-iodo-Kdo donors do not require bulky or acetal type protecting groups at O-4 and O-5, which tend to increase the formation of the elimination products. Furthermore, these donors are easily accessible in good overall yields from Kdo glycal esters via an acetoxyiodination reaction to give mainly the 2,3-trans-diaxial products followed by treatment with HF-pyridine (Scheme 5).19,20 This way, fluoride 22 was obtained as single anomer and was bench-stable at room temperature for several weeks.
Scheme 5.
Synthesis of Kdo disaccharides.
The favorable glycosylation properties were demonstrated then in the assembly of the α-(2→4)- and α-(2→8)-linked Kdo disaccharides 26 and 29, respectively.21 Reaction of an equimolar amount of fluoride 22 with diol 23 afforded 24 as single anomer with only minor formation of glycal ester byproduct (4%). The glycodesilylation approach was equally effective using the 8-O-TES derivative 27 to yield 28 in 62% yield. Both 3-iodo disaccharides were reduced in excellent yields to the corresponding Kdo-derivatives 26 and 29, respectively, using atom transfer hydrogenation with lauroyl peroxide in refluxing cyclohexane, thereby avoiding the use of toxic tin reagents.22
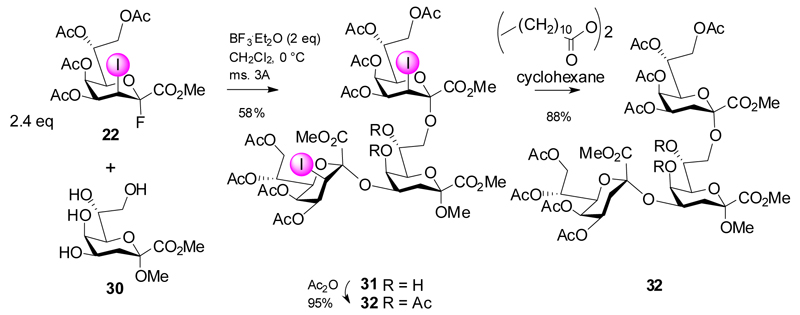
Eventually donor 22 was applied to prepare the Chlamydia and Chlamydophila specific Kdo trisaccharides (Scheme 6). Indeed, glycosylation of the tetraol 30 could be directed in a regioselective fashion to provide the 4,8-branched trisaccharide. The use of 2.4 equivalents of donor 22 was sufficient to give trisaccharide 31 in 58% yield accompanied by isolation of the α-(2→7)-linked regioisomeric trisaccharide (7%) and elimination product 20 (11%). Subsequent O-acetylation and deiodination was straightforward to give the branched Chl. psittaci trisaccharide 31 in high yield and in a minimum number of steps. The strategy was then further elaborated to include a disaccharide fluoride donor derived from the respective disaccharide glycal and again capitalizing on a glycodesilylation approach (Scheme 7). Reaction of the 8-O-TES glycal ester 34 with donor 22 afforded disaccharide glycal ester intermediate, which was directly subjected to acetoxyiodination giving 35 in 57% yield. Conversion into fluoride 36 followed by TMSOTf-promoted coupling to diol 23 proceeded smoothly (→ 37) as well as the subsequent steps (→ 38, 39).
Scheme 6.
Synthesis of a 4,8-dibranched Kdo trisaccharide.
Scheme 7.
Synthesis of the linear Chlamydia-specific Kdo trisaccharide 39.
While the electron-deficient double bond in glycal ester 34 was fully compatible with the glycosylation conditions, electron rich alkenes compete via an iodonium ion transfer reaction which leads to significant consumption of donor due to formation of the elimination product 20. This feature excludes allyl protecting groups on 3-iodo-Kdo glycosyl donors and glycosyl acceptors.23
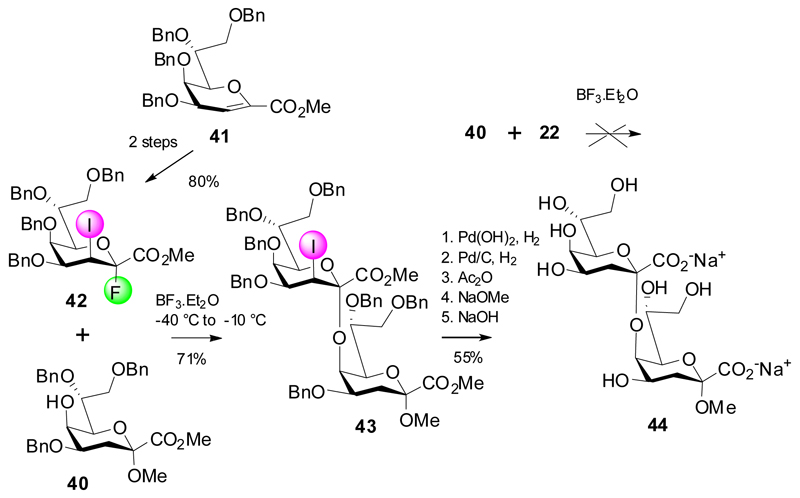
As seen from the previous examples, glycosylation of Kdo 4,5-diol acceptors never gave any (2→5)-linked products, due to the steric crowding at the acceptor site as well as expected for a ketosidic linkage being close to an ester group at the anomeric center of the donor. In fact, donor 22 also failed in forming a 5-O-substituted product when subjecting the “armed” 4,7,8-tri-O-benzyl Kdo acceptor 40 to the aforementioned glycosylation conditions. Hence, the “armed” glycosyl donor 42 was elaborated, and under careful temperature control the first successful 5-O-glycosylation with a Kdo donor derivative to produce 43 was accomplished in 71% yield, again with no β-product detected and only very minor formation of glycal ester 41.24 The ensuing sequence of hydrogenation/reacetylation and global deprotection gave the α-(2→5)-linked Kdo disaccharide 44 (Scheme 8). This approach opens future perspectives for the assembly of larger fragments of the Acinetobacter baumannii LPS core region.
Scheme 8.
Synthesis of the α-(2→5)-linked Kdo disaccharide 44.
Kdo glycal donors
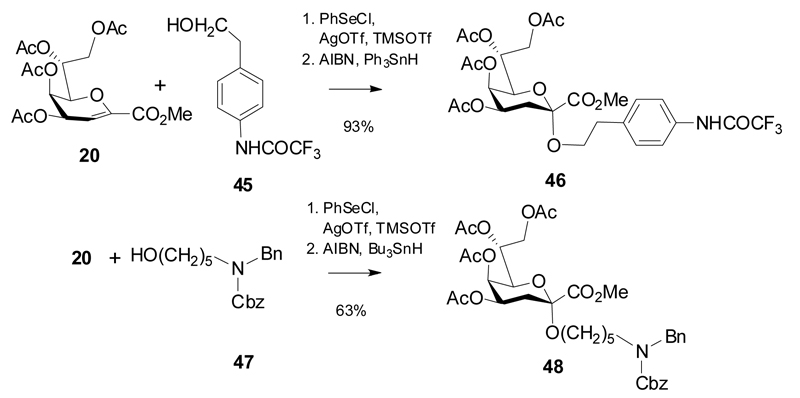
Kdo glycal ester derivatives can also directly serve as glycosylating agents of reactive glycosyl acceptor derivatives upon suitable activation, mostly using triflic acid as promoter. The merit of this approach is seen in a straightforward introduction of spacer groups on Kdo monosaccharide units allowing for subsequent application in neoglycoconjugate synthesis and generation of ligands for glycoarray preparation. As examples, following the first reports by Achiwa et al,25 reaction of glycal ester 20 with primary alcohols 45 and 47, respectively, promoted with AgOTf/TMSOTf and exploiting the stereochemical control of a temporary phenylselenyl auxiliary group were high-yielding and α-selective and furnished the protected spacer glycosides 46 and 48.26,27
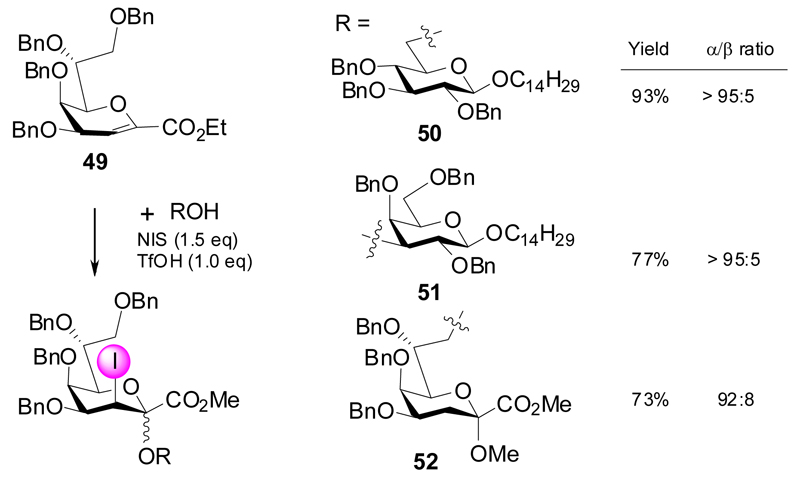
In 2006, the group of Takahashi developed a high-yielding and α-selective methodology on the basis of the electron-rich glycal ester 49, which -in the presence of triflic acid as promoter- could directly be activated leading to 3-iodo-Kdo glycosides when reacted with “armed” glycosyl acceptors (Scheme 10).28 This way, the 3-iodo-Kdo-(2→6)-, (2→3)- and (2→8)-linked gluco-, galacto- and Kdo-disaccharides 50-52 were isolated in very good yields and α-selectivities.
Scheme 10.
Activation of benzyl glycal ester with NIS/TfOH.
A similar α-glycoside formation of 3-iodo-Kdo had previously been observed by Oscarson in a TfOH-promoted reaction of glycal ester 20 with a spacer alcohol nucleophile.26
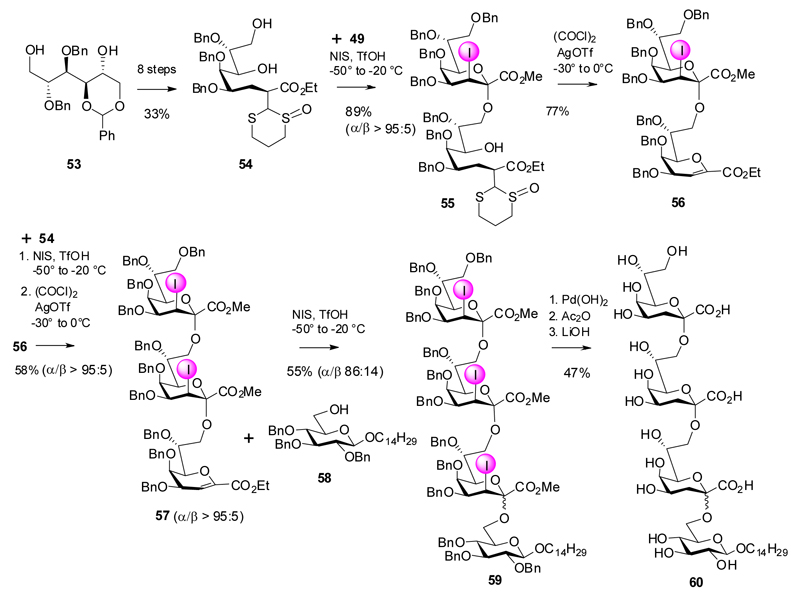
An iterative sequence was then elaborated starting from 2,3-di-O-benzyl-4,5-O-benzylidene mannitol 53, which was converted into the open-chain Kdo precursor 54. NIS/TfOH promoted coupling with the perbenzyl Kdo donor 49 gave an excellent yield of the α-(2→8)-pseudodisaccharide 55 followed by ring-closure and elimination of the thio-appendix to regenerate the glycal ester moiety (56). Repetition of the sequence and coupling of trisaccharide glycal 57 to glucoside acceptor 58 was carried out with reduced yield and anomeric selectivity to give tetrasaccharide 59. Removal of the iodo and benzyl substituents and ester hydrolysis afforded 60. It might be noted that α-(2→8)-Kdo trimers have not yet been found in nature.
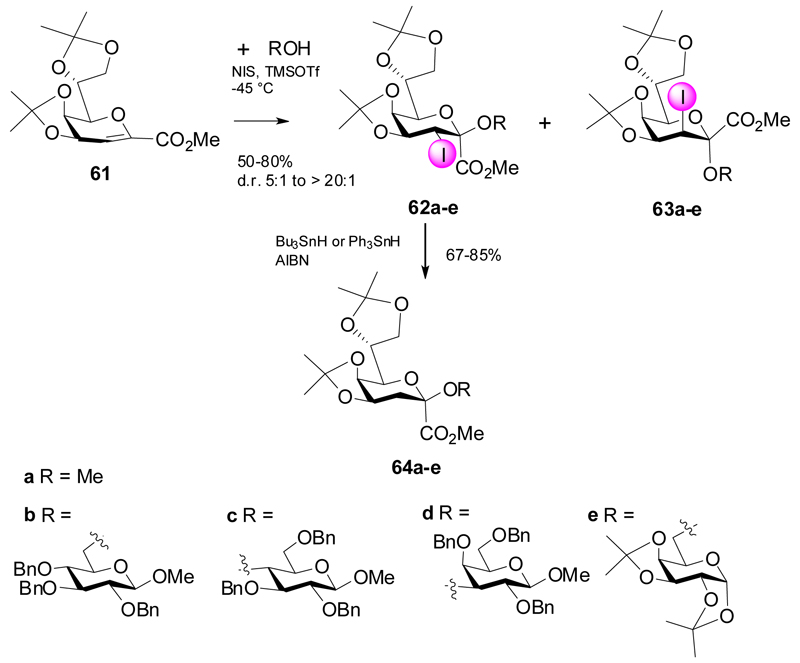
Recently, the group of Mong developed a strategy towards β-Kdo glycosides exploiting similar reactions of the bis-isopropylidene protected Kdo glycal ester 61.29 Reactions of 61 with various glycosyl aceptors was promoted by NIS/TMSOTf and led to preferential formation of β-glycosides 62a-e, due to the stereodirecting effect of the equatorial 3-iodo-group (Scheme 12). The predominant formation of the D-glycero-D-galacto configuration at C-3 can be rationalized on the basis of a steric collision of the incoming nucleophile with one of the methyl groups of the 4,5-O-isopropylidene group, which effectively shields the top position. The ratio of epimeric iodo products was also influenced by the choice of solvent and amount of NIS in the reaction. The presence of 2 equivalents of NIS and a MeCN-CH2Cl2 solvent mixture led to improved β-selectivity. Dehalogenation of 63a-e under radical conditions generated the corresponding Kdo-glycosides 64a-e.
Scheme 12.
Synthesis of β-Kdo derivatives.
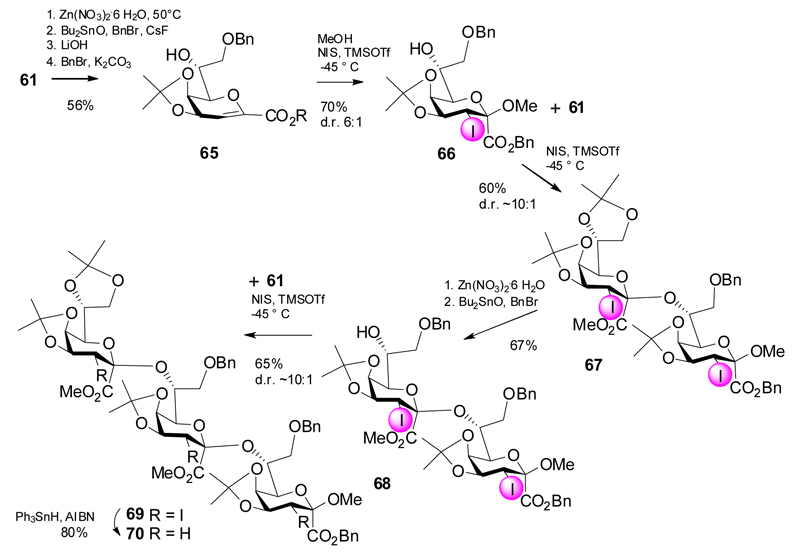
The methodology was further expanded to include β-(2→7)-linked Kdo di- and trimers, which correspond to fragments of capsular polysaccharides from E. coli, N. meningitidis and Sinorhizobium meliloti strains (Scheme 13).30,31 First, the glycal glycosyl acceptor 65 was derived from 61 via selective cleavage of the side-chain acetonide with aqueous Zn-nitrate followed by regioselective 8-O-benzylation and conversion into the methyl β-ketoside 66 in a 6:1 diastereomeric ratio. Reaction of 66 with glycal ester 61 afforded disaccharide 67 in 60% yield. The sequence of protecting group manipulation was repeated (→68) followed by a third glycosylation step and eventually provided the trimer 69. Simultaneous removal of three iodo-substituents under radical conditions gave trisaccharide 70, which, however, was not deprotected.
Scheme 13.
Synthesis of a β-(2→7)-linked Kdo trimer.
Kdo thioglycoside donors
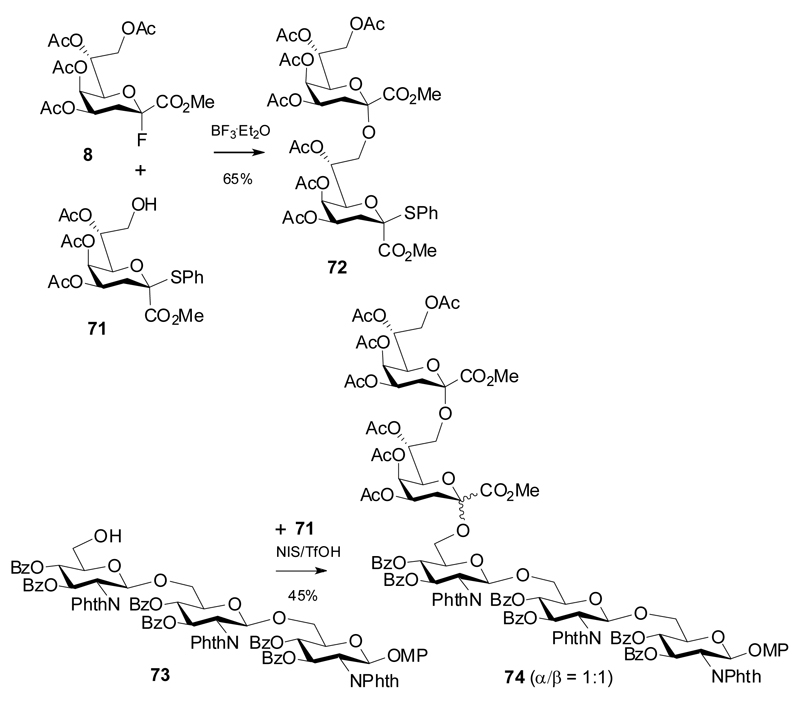
Kdo thioglycoside donors allow for various activation protocols using thiophilic promoters, but are susceptible to pronounced elimination reactions with less reactive glycosyl acceptors. These donors have mainly been applied for the preparation of spacer glycosides, in particular of those with a β-anomeric configuration.32,33 In 2007, Oscarson et al published a detailed and systematic model study with several Kdo thioglycoside donors equipped with acetyl, benzoyl and isopropylidene protecting groups in different solvents and with variations of reaction temperature and activating agents such as dimethyl(methylthio)sulfonium trifalte (DMTST), NIS/AgOTf, NIS and IBr.34 In short, this investigation showed preferential formation of β-ketosides at low temperatures, a shift towards higher α/β ratios in ether solvents (at the expense of reduced yields). Preferred formation of α-glycosides was again seen when using 4,5-O-isopropylidene protected donors. The moderate anomeric selectivities with thioglycoside donors are also evident in a pentasaccharide synthesis by Baasov, wherein the Kdo fluoride 8 was coupled to acceptor 71 to give the α-(2→8)-linked Kdo disaccharide thioglycoside 72 in 65% yield, but subsequent coupling to a triglucosamine acceptor 73 resulted in a 1:1 mixture of anomeric pentasaccharide 74 in moderate yield only (Scheme 14).35
Scheme 14.
Synthesis and glycosidation of a Kdo disaccharide donor
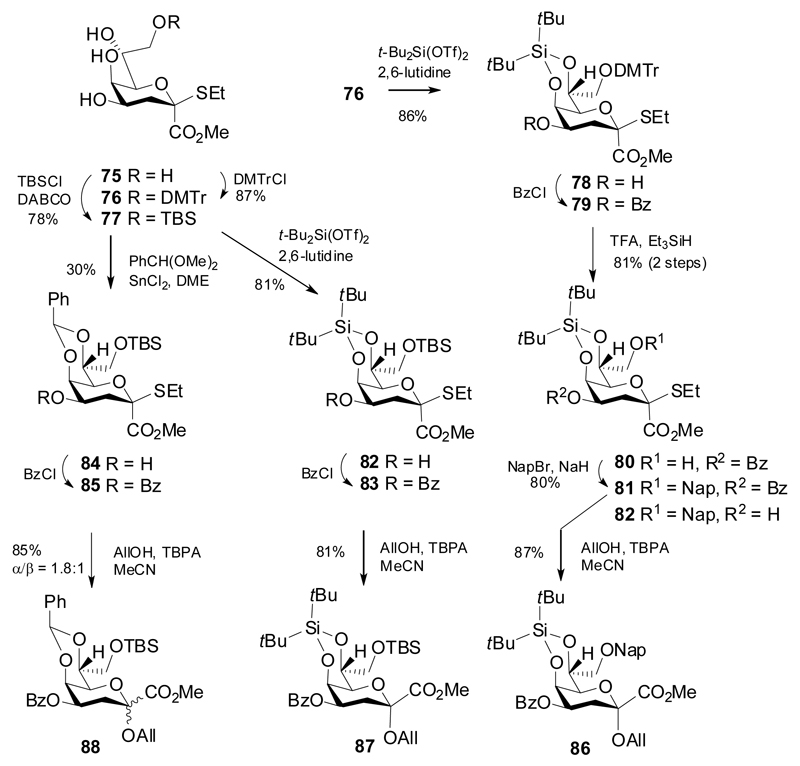
In 2015, however, a new type of conformational locking of Kdo was introduced by the Yang group, who elaborated a multistep synthesis of 5,7-O-tethered thioglycosyl donors.36 These donors exert excellent α-selectivity but also suffer from significant elimination, which require 2-4 equivalents of donor in order to achieve high yields. This was even the case with reactive glycosyl acceptors and primary alcohols and since the preparation of the donor is lengthy (Scheme 15), precious donor material is wasted. Nevertheless, this strategy is of significant value, as demonstrated in successful coupling of these donors to all acceptor positions of Kdo, including the unreactive 5-OH group.
Scheme 15.
Synthesis of 5,7-O-locked Kdo thioglycoside donor and acceptor derivatives.
Donor synthesis started from selective protection of position 8 of the ethyl 2-β-thio ketoside 75 by dimethoxytrityl (→76) and TBS (→77) groups, respectively, followed by introduction of a 5,7-O-DTBS or benzylidene tether to give the ethyl β-thioglycosides 78, 82 and 84 in high to modest yields. 4-O-Benzoylation gave 79, 83 and 85, respectively, and allowed for subsequent preparation of the 8-OH glycosyl acceptors via selective cleavage of the respective 8-O-protecting group (→80). Reprotection of 80 by a 2-naphthylmethyl group was followed by cleavage of the 4-O-benzoyl group to give the 4-OH acceptor 82. The allyl glycosides 86-88 were then prepared from donors 81, 83 and 85, respectively, using tris(4-bromophenyl)ammoniumyl hexachloroantimonate (TBPA) as promoter in MeCN. Notably, the 5,7-O-benzylidene protected donor 85 led to a poor anomeric ratio of the allyl glycoside 88, whereas the DTBS donors 81 and 83 affored only the α-products 86and 87.
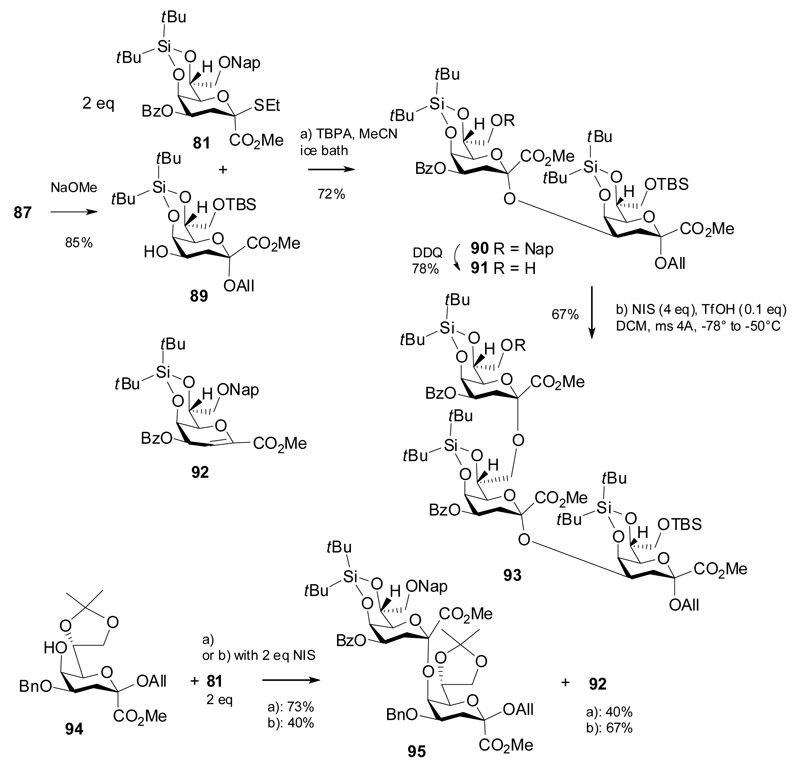
The Chlamydia-specific Kdo trisaccharide, disaccharide fragments derived therefrom, and the α-(2→5)-linked Kdo disaccharide were then prepared in a protected form (Scheme 16). Acceptor 89 was derived from allyl glycoside 87 by Zemplén transesterification and coupled with 2 equivalents of donor 81 in the presence of TBPA to give the α-(2→4)-linked disaccharide 90 in 72% yield (based on acceptor 89). DDQ-oxidation then afforded the alcohol 91, which was reacted with 81 under NIS/TfOH promotion to yield trisaccharide 93 in a respectable yield. The glycosylation of the β-allyl acceptor 94 was explored using both promoter systems. Again, the TBPA activation was superior in the glycosylation of the “armed” but still unreactive acceptor 94, as seen in the lower product yield of the NIS/TfOH promoted reaction and conversely, the higher amount of isolated elimination product 92. In view of the difficult and challenging formation of the α-(2→5)-linkage, this is an excellent result.
Scheme 16.
Synthesis of the Chlamydia-related Kdo trisaccharide and the α-(2→5)-linked Kdo disaccharide.
Eventually the authors also applied these new donors for the preparation of a protected trisaccharide corresponding to the inner core of Burkholderia and Proteus LPS (Scheme 17).
Scheme 17.
Synthesis of Burkholderia and Proteus-related LPS core structures.
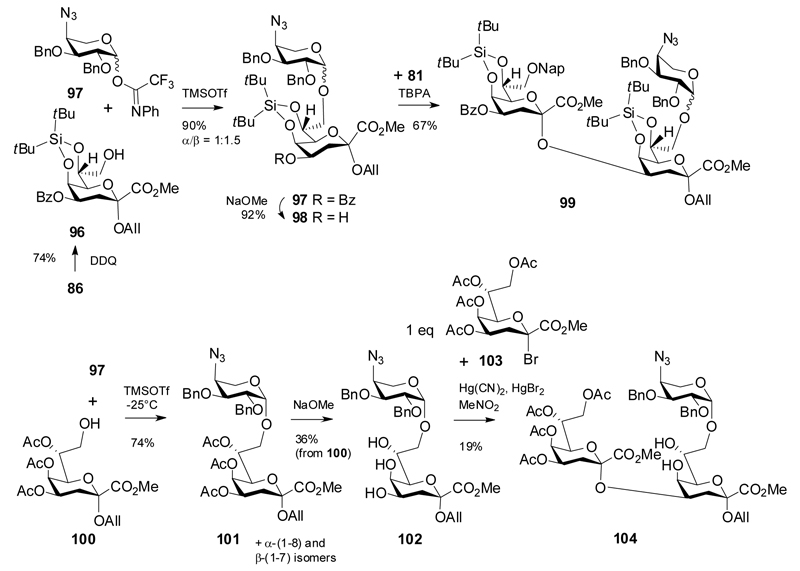
The primary alcohol 96 generated from 86 upon DDQ-oxidation was coupled with 4-amino-4-deoxy-L-arabinosyl donor 97 in very high yield but poor anomeric selectivity to give disaccharide 97.37,38 Subsequent removal of the 4-O-benzoyl group was followed by coupling of 98 with donor 81 in the presence of TBPA to give a good yield of the branched trisaccharide 99.
A similar trisaccharide had previously been obtained with minimized protecting group manipulation via coupling of alcohol 100 with 97 to give an anomeric mixture of 101 and eventually an isolated overall yield of 36% for the 4,5,7-triol 102.38 Regioselective glycosylation of 102 was then achieved with 1 equivalent of the Kdo bromide donor 103 to give trisaccharide 104 in low yield.
Kdo trifluoroacetimidate donors
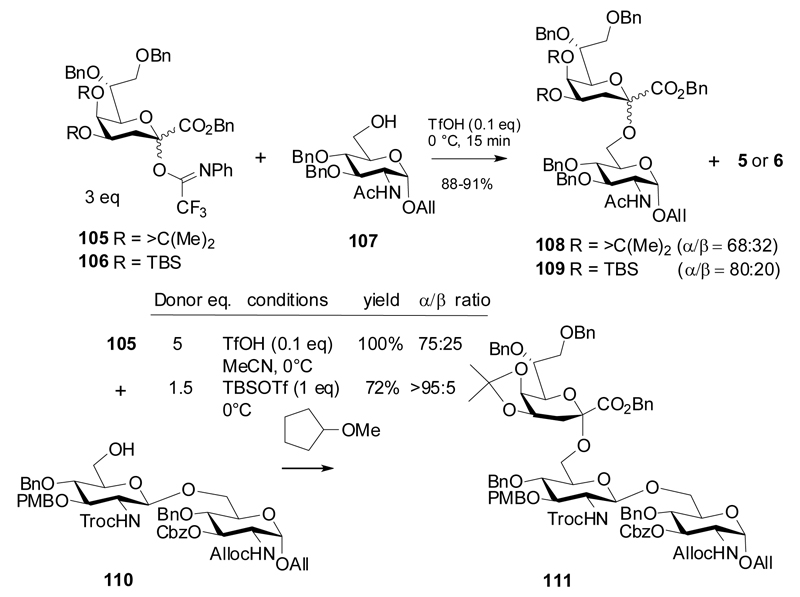
A few reports have been published describing the use of N-phenyltrifluoroacetimidate (NPTFA) donors of Kdo, which can be activated with TfOH or TBSOTf and TMSOTf as promoters. Fukase et al investigated the glycosylating properties of Kdo imidates 105 and 106, equipped with protecting groups identical to the previously used Kdo fluorides 2 and 4.39 Coupling of these donors to position 6 of mono- and disaccharide glucosamine acceptors 107 and 110 gave a fair anomeric selectivity but needed considerable excess of donor to achieve high yields of disaccharides 108, 109 and trisaccharide 111. Cyclopentyl methylether as well as acetonitrile as solvents led to an improved α-selectivity, and the use of microfluidic conditions reduced formation of the undesirable elimination product (Scheme 18). Trisaccharide 111 was then further transformed into a Helicobacter pylori LPS fragment.40
Scheme 18.
Glycosylation reactions with Kdo N-phenyl trifluoroacetimidates.
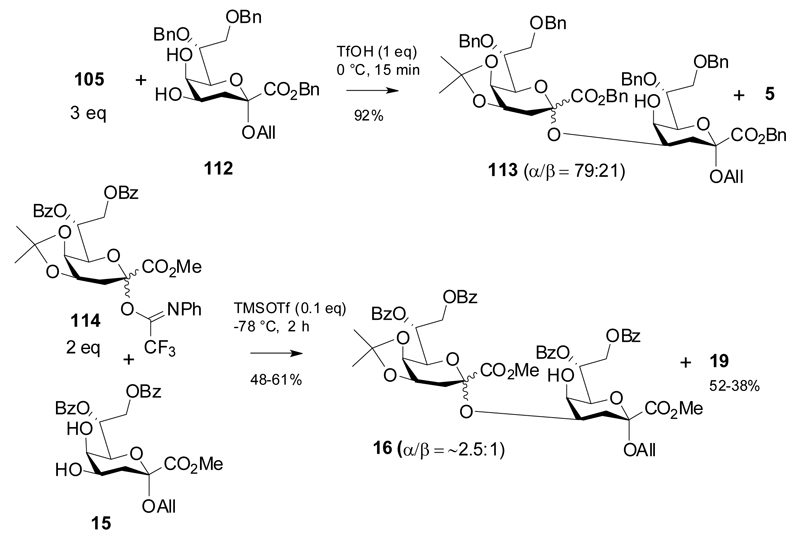
In addition, coupling of donor 105 to Kdo allyl glycoside 112 was performed under similar conditions to give Kdo disaccharide 113 in fair anomeric selectivity (Scheme 19).
Scheme 19.
Kdo disaccharide synthesis with Kdo N-phenyl trifluoroacetimidates.
A similar anomeric ratio was also observed by Ichiyanagi using the 7,8-di-O-benzoyl protected imidate 114 to give disaccharide 16.41 The authors also employed a related Kdo-phosphite donor, which, however, exhibited poor glycosylating properties.
Kdo 1-C-Arylglycal donors
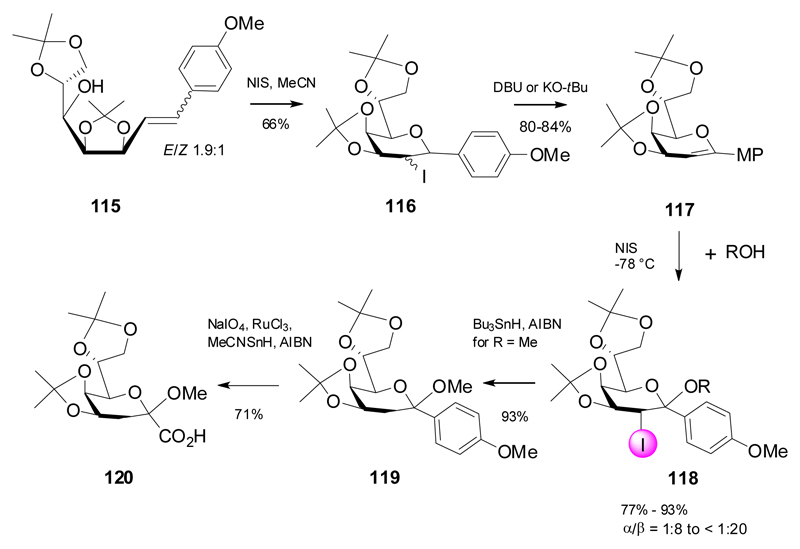
Recently, an indirect method toward β-linked Kdo glycosides was developed via formation of a glycal containing an anomeric carbon-linkage to an electron-rich aromatic group via ring-closure of olefin 115.42 Thus, NIS-treatment of 115 afforded an epimeric mixture of 2-iodo-2-deoxy-C-glycosides 116, which were converted into the corresponding p-methoxyphenyl glycal 117 under basic conditions (Scheme 20). Subsequent reaction with a series of alcohols in the presence of NIS proceeded via preferred formation of 2-iodo β-glycosides 118. The selective formation of the β-glycosides was rationalized on stereoelectronic grounds and on the skewed conformation enforced by the 4,5-O-isopropylidene group, thereby preventing the NIS attack from the top face and eventually leading to a pseudo trans-diaxial arrangement of the iodo-group and the anomeric OR group (see also Scheme 13). Radical deiodination of the methyl glycoside gave 119 and was followed by oxidative removal of the aromatic appendix to generate the carboxylic function leading to 120.
Scheme 20.
β-Kdo synthesis from 1-C-aryl glycal.
Conclusions and Outlook
As illustrated in this review, remarkable progress has been achieved in the past in the synthesis of α-glycosides of Kdo. In particular, locking of the pyranose ring conformation by 4,5-O-isopropylidene or 5,7-O-TDBS groups provides mainly the α-ketosides, albeit at the cost of a more pronounced elimination side reaction. The 3-iodo-Kdo fluoride donors have been proven to be versatile donors in α-Kdo oligomer assembly with options for block and regioselective synthesis, but have some limitations in selection of protecting groups. As a main very recent accomplishment the first syntheses of α-(2→5)-linked Kdo disaccharides should be mentioned. The stereodirecting temporary 3-iodo-group has also been of value in the construction of β-anomeric Kdo glycosides. Still, the chemistry of β-Kdo units needs to be further developed in the future and presently lags behind its α-anomeric counterparts.
Scheme 9.
Synthesis of spacer α-glycosides from glycal ester 20.
Scheme 11.
Synthesis of α-(2→8)-Kdo trimers.
Acknowledgments
The author gratefully acknowledges financial support of work performed in the authors laboratory by the Austrian Science Fund FWF (grants P 22909, P 24921 and P 26919).
References
- 1.Raetz CRH, Whitfield C. Annu Rev Biochem. 2002;71:635. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.York WS, Darvill AG, McNeil M, Albersheim P. Carbohydr Res. 1985;138:109. [Google Scholar]
- 3.Becker B, Lommerse JPM, Melkonian M, Kamerling JP, Vliegenthart JFG. Carbohydr Res. 1995;267:313. [Google Scholar]
- 4.Holst O. FEMS Microbiol Lett. 2007;271:3. doi: 10.1111/j.1574-6968.2007.00708.x. [DOI] [PubMed] [Google Scholar]
- 5.(a) Gomery K, Müller-Loennies S, Brooks CL, Brade L, Kosma P, Di Padova F, Brade H, Evans SV. Proc Natl Acad Sci U S A. 2012;109:20877. doi: 10.1073/pnas.1209253109. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. Nature. 2009;458:1191. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 6.(a) Oscarson S, Hansson J. Curr Org Chem. 2000;4:535. [Google Scholar]; (b) Oscarson S. Carbohydr Chem. 2012;45:40. [Google Scholar]; (c) Kosma P. In: Microbial Glycobiology. Moran AP, Holst O, Brennan PJ, von Itzstein M, editors. Elsevier; Amsterdam: 2009. pp. 429–454. [Google Scholar]; (d) Cipolla L, Gabrielli L, Bini D, Russo LN, Shiakh N. Nat Prod Rep. 2010;27:161. doi: 10.1039/c004750n. [DOI] [PubMed] [Google Scholar]; (e) Kosma P, Zamyatina A. In: Bacterial Lipopolysaccharides. Knirel YA, Valvano MA, editors. Springer; Wien, New York: 2011. pp. 131–161. [Google Scholar]; (f) Pradhan TK, Mong TKKT. Isr J Chem. 2015;55:285. [Google Scholar]
- 7.Li LS, Wu Y-L. Curr Org Chem. 2003;7:447. [Google Scholar]
- 8.Mikula H, Blaukopf M, Sixta G, Stanetty C, Kosma P. In: Carbohydrate Chemistry-Proven Synthetic Methods. van derMarel G, Codee J, editors. Vol. 2. CRC Press; Boca Raton, London, New York: 2014. pp. 207–211. [Google Scholar]
- 9.Feng Y, Dong J, Xu F, Liu A, Wang L, Zhang Q, Chai Y. Org Lett. 2015;17:2388. doi: 10.1021/acs.orglett.5b00901. [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto Y, Iwata M, Imakita N, Shimoyama A, Suda Y, Kusumoto S, Fukase K. Tetrahedron Lett. 2007;48:6577. [Google Scholar]
- 11.Hashimoto S, Hayashi M, Nayori R. Tetrahedron Lett. 1984;25:1379. [Google Scholar]
- 12.Orbe M, Luthman K, Wåglund T, Claesson R, Csöregh I. Carbohydr Res. 1991;211:1. doi: 10.1016/0008-6215(90)80066-c. [DOI] [PubMed] [Google Scholar]
- 13.Yoshizaki H, Fukuda N, Sato K, Oikawa M, Fukase K, Suda Y, Kusumoto S. Angew Chem Int Ed. 2001;40:1475. doi: 10.1002/1521-3773(20010417)40:8<1475::AID-ANIE1475>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Gaekwad J, Wolfert MA, Boons G-J. Chem Eur J. 2008;14:558. doi: 10.1002/chem.200701165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichiyanagi T, Fukunaga M, Tagashira R, Hayashi S, Nanjo M, Yamasaki Tetrahedron. 2011;67:5964. [Google Scholar]
- 16.Kosma P, Hofinger A, Müller-Loennies S, Brade H. Carbohydr Res. 2010;345:704. doi: 10.1016/j.carres.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 17.(a) Ikeda K, Akamatsu S, Achiwa K. Chem Pharm Bull. 1990;38:279. doi: 10.1248/cpb.38.1766. [DOI] [PubMed] [Google Scholar]; (b) van der Klein PAM, Boons GJPGH, Veeneman GH, van der Marel GA, van Boom JH. Synlett. 1990:311. [Google Scholar]
- 18.Boons G-J, Demchenko AV. Chem Rev. 2000;100:4539. doi: 10.1021/cr990313g. [DOI] [PubMed] [Google Scholar]
- 19.Claesson A, Luthman K. Acta Chem Scand B. 1982;36:719. [Google Scholar]
- 20.Kosma P, Sekljic H, Balint G. J Carbohydr Chem. 1996;15:701. [Google Scholar]
- 21.Pokorny B, Kosma P. Chem Eur J. 2015;21:305. doi: 10.1002/chem.201405424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boivin J, Quiclet-Sire B, Ramos L, Zard SZ. Chem Commun. 1997:353. [Google Scholar]
- 23.Pokorny B, Kosma P. ChemOpen. 2015;4:722. doi: 10.1002/open.201500126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pokorny B, Kosma P. Org Lett. 2015;17:110. doi: 10.1021/ol5033128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda K, Akamatsu S, Achiwa K. Carbohydr Res. 1989;189:C1. [Google Scholar]
- 26.Ekelöf K, Oscarson S. Carbohydr Res. 1995;278:289. doi: 10.1016/0008-6215(95)00269-3. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Martin CE, Seeberger PH. Chem Sci. 2011;3:896. [Google Scholar]
- 28.Tanaka H, Takahashi D, Takahashi T. Angew Chem Int Ed. 2006;45:770. doi: 10.1002/anie.200503299. [DOI] [PubMed] [Google Scholar]
- 29.Pradhan TK, Lin CC, Mong K-KT. Org Lett. 2014;16:1474. doi: 10.1021/ol500275j. [DOI] [PubMed] [Google Scholar]
- 30.Willis LM, Whitfield C. Proc Natl Acad Sci USA. 2013;110:20753. doi: 10.1073/pnas.1312637110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraysse N, Lindner B, Kaczynski Z, Sharypova L, Holst O, Niehaus K, Poinsot V. Glycobiol. 2005;15:101. doi: 10.1093/glycob/cwh142. [DOI] [PubMed] [Google Scholar]
- 32.Boons JGPH, Delft FL, van der Klein PAM, van der Marel GA, van Boom JH. Tetrahedron. 1992;48:885. [Google Scholar]
- 33.Laroussarie A, Barycza B, Andriamboavonjy H, Kenfack MT, Blériot Y, Gauthier C. J Org Chem. 2015;80:10386. doi: 10.1021/acs.joc.5b01823. [DOI] [PubMed] [Google Scholar]
- 34.Mannerstedt K, Ekelöf K, Oscarson S. Carbohydr Res. 2007;342:631. doi: 10.1016/j.carres.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 35.Solomon D, Fridman M, Zhang J, Baasov T. Org Lett. 2001;3:4311. doi: 10.1021/ol010257h. [DOI] [PubMed] [Google Scholar]
- 36.Huang J-S, Huang W, Meng X, Wang X, Gao P-C, Yang J-S. Angew Chem Int Ed. 2015;54:10894. doi: 10.1002/anie.201505176. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Oishi S, Martin CE, Seeberger PH. J Am Chem Soc. 2013;135:6262. doi: 10.1021/ja401164s. [DOI] [PubMed] [Google Scholar]
- 38.Blaukopf M, Müller B, Hofinger A, Kosma P. Eur J Org Chem. 2012:119. doi: 10.1002/ejoc.201101171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimoyama A, Fujimoto Y, Fukase K. Synlett. 2011:2359. [Google Scholar]
- 40.Shimoyama A, Saeki A, Tanimura N, Tsutsui H, Miyake K, Suda Y, Fujimoto Y, Fukase K. Chem Eur J. 2011;17:14464. doi: 10.1002/chem.201003581. [DOI] [PubMed] [Google Scholar]
- 41.Yi R, Ogaki A, Fukunaga M, Nakajima H, Ichiyanagi T. Tetrahedron. 2014;70:3675. [Google Scholar]
- 42.Qian Y, Feng J, Parvez M, Ling C-C. J Org Chem. 2012;77:96. doi: 10.1021/jo201531j. [DOI] [PubMed] [Google Scholar]