Abstract
The treatment of patients with chronic myelomonocytic leukemia (CMML) with transplant has not been optimized. We retrospectively reviewed the data for 83 consecutive patients with CMML (47 with CMML-1/2 and 36 with CMML progressed to acute myeloid leukemia) who received an allogeneic stem cell transplant at our institution between April 1991 and December 2013 to identify factors associated with improved survival and determine whether treatment with hypomethylating agents before transplant improves progression-free survival. The median age of the cohort was 57 years. Seventy-eight patients received induction treatment before transplant, with 37 receiving hypomethylating agents and 41 receiving cytotoxic chemotherapy. Patients treated with a hypomethylating agent had a significantly lower cumulative incidence of relapse at 3 years post-transplant (22%) than those treated with other agents (35%; p=0.03), whereas TRM at 1 year post-transplant did not significantly differ between the groups (27% and 30%, respectively; p=0.84). The lower relapse rate resulted in a significantly higher 3-year PFS rate in patients treated with a hypomethylating agent (43%) than in those treated with other agents (27%; p=0.04). Our data support the use of hypomethylating agents before allogeneic stem cell transplantation for patients with CMML to achieve morphologic remission and improve progression-free survival of these patients. Future studies are needed to confirm these findings.
Keywords: chronic myelomonocytic leukemia, myeloproliferative neoplasms, secondary acute myeloid leukemia, allogeneic stem cell transplantation, hypomethylating agents
INTRODUCTION
Chronic myelomonocytic leukemia (CMML) is a clonal hematopoietic stem cell disorder characterized by peripheral blood monocytosis and features of both a myeloproliferative neoplasm and a myelodysplastic syndrome. According to the 2008 World Health Organization (WHO) classification, CMML belongs to a category of mixed myeloproliferative/myelodysplastic neoplasms and has two subtypes, CMML-1 and CMML-2, depending on the number of blasts and promonocytes present in the bone marrow and peripheral blood.(1) To date, there is no consensus on the optimal therapy for CMML owing to the heterogeneity of the disease. Treatment modalities for CMML include supportive care, hypomethylating agents, cytotoxic chemotherapy, and allogeneic hematopoietic stem cell transplant (allo-SCT), which is the only curative treatment modality for patients with CMML.(2–9) However, allo-SCT for this disease has been associated with higher treatment-related mortality (TRM) and relapse rates, and, in general, worse outcomes than for other myeloproliferative neoplasms.(10) Data regarding allo-SCT outcomes in patients with CMML are currently limited to small retrospective series, and no prospective studies have been performed for CMML patients because of the relatively low number of patients with CMML treated with allogeneic transplantation. Moreover, timing of allo-SCT and benefit of induction therapy, in particular treatment with a hypomethylating agent before transplant, has not been studied. We therefore performed a retrospective analysis in a larger number of CMML patients who underwent allogeneic transplantation to identify factors associated with improved outcomes and determine whether treatment with hypomethylating agents before transplantation improves survival in these patients.
PATIENTS AND METHODS
All 83 consecutive patients 18 years of age or older with a diagnosis of CMML confirmed at The University of Texas MD Anderson Cancer Center (UTMDACC) who underwent allo-SCT between April 1991 and December 2013 were identified through review of the institution’s medical records and included in this analysis. Histologic subtypes at the time of diagnosis were classified according to the 2008 WHO definitions.(1) Forty-seven patients had CMML-1 or CMML-2 (CMML-1/2) (n=40 CMML-1 and n=7 CMML-2), and 36 of the patients had CMML that had progressed to secondary acute myeloid leukemia (CMML/AML). CMML-specific cytogenetic risk levels were determined at diagnosis according to the classification system described by Such et al.(11) All patients provided written informed consent for transplant in accordance with the Declaration of Helsinki. The Institutional Review Board of UTMDACC approved the treatment protocols and this retrospective study.
Treatment before transplantation and transplant procedures
We assessed the use of pre-transplant treatments and the agents used for those treatments on the basis of data extracted from the medical records. Pre-transplant induction therapies were various, mostly either 1–2 courses of conventional chemotherapy (idarubicin plus cytarabine; 7+3 regimen(12) or idarubicin plus clofarabine plus cytarabine; CIA regimen)(13) or at least 3 courses of hypomethylating agents (5-azacytidine or decitabine). The choice and dose of the pre-transplant treatments were based on the treating physician’s decision, disease status at diagnosis, and patient’s performance status. Patients who received hydroxyurea, supportive cares alone, or less than 3 cycles of hypomethylating agents before transplant were considered no induction therapy.
Responses to induction therapy were evaluated according to the International Working Group response criteria before transplant.(14) All donors and recipients had high-resolution molecular typing of human leukocyte antigen class I and II antigens. Donor types were defined according to previously described criteria.(15) Conditioning regimens varied; most patients received either fludarabine in combination with busulfan or fludarabine combined with melphalan. The impact of conditioning regimens on outcomes was analyzed by their dose intensity using the Center for International Blood and Marrow Transplant Research criteria for myeloablative (MAC) and reduced-intensity conditioning (RIC) regimens.(16) Graft-versus-host disease (GVHD) prophylaxis consisted of tacrolimus 0.015 to 0.03 mg/kg (starting on day –2) and methotrexate of 5 mg/m2 on day +1, +3, and +6. Patients who received transplantations from matched unrelated or mismatched donors received an additional dose of methotrexate of 5 mg/m2 on day +11 and 1 mg/kg of rabbit antithymocyte globulin IV on day –2 and –1 before allo-SCT. Acute GVHD (aGVHD) and chronic GVHD (cGVHD) were graded according to consensus criteria that were reported previously.(17, 18)
Endpoint definitions and statistical analyses
We analyzed the impact of disease and transplant characteristics on the outcomes of transplant, including the characteristics of age, Karnofsky performance status, the 2008 WHO histologic subtype, bone marrow blast count immediately before transplant, cytogenetics at diagnosis, the use of hypomethylating agents before transplant, remission status before transplant, year of transplant, conditioning intensity, donor type, development of aGVHD and cGVHD. The primary endpoint was progression-free survival (PFS). The secondary endpoints were overall survival (OS), TRM, relapse incidence through last follow-up and incidences of aGVHD and cGVHD. All of these outcomes were measured from the time of allo-SCT.
PFS was defined as the time until disease relapse or death from any cause; data for patients who were alive without relapse were censored at the date of last contact. OS was defined as the time until death from any cause; surviving patients were censored at the date of last contact. Relapse was defined as the recurrence of disease according to the 2008 WHO criteria.(1) TRM was defined as death related to allo-SCT during continuous CR. OS and PFS were calculated using the Kaplan-Meier method. Univariate comparisons of all endpoints were done using the log-rank test. The cumulative incidence function with the competing risks method was used to estimate the endpoints of relapse, TRM, aGVHD, and cGVHD. A Cox proportional hazards model (19) or the Fine and Gray method(20) for competing hazards was used for multivariate regression. Variables were included in the multivariate model if they were conceptually important [i.e. if they approached (p<0.1)] or attained statistical significance in the univariate regression model. A P value of less than 0.05 was considered for statistical significance. Analyses were performed using the Stata statistics program (version 13).
RESULTS
Patient and transplant characteristics
The median age was 57 years (range 18–78 years). Thirty-three patients (39.7%) were older than 60 years. CMML-specific cytogenetic risk levels at diagnosis according to the classification system as described by Such et al.(11) were low, intermediate, and high risk in 46 (55.4%), 19 (22.9%), and 18 (21.6%) patients, respectively. There were no significant differences in characteristics between patients with CMML1/2 and those with CMML/AML as shown in Table 1. Fourteen patients (30%) and 10 patients (28%) in CMML-1/2 and CMML/AML achieved a complete remission before transplant (p=0.87).
Table 1.
Patient and transplant characteristics by the CMML category.
| All patients | CMML-1/2 | CMML/AML | P value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N=83 | %, Range | N=47 | %, Range | N=36 | %, Range | ||
| Median age (year) | 57 | 18–78 | 58 | 18–74 | 56 | 18–78 | 0.75 |
|
| |||||||
| Age>60 years | 33 | 39.8 | 21 | 46.6 | 12 | 33.3 | 0.52 |
|
| |||||||
| Median blast percentage at transplant | 3 | 0–83 | 3 | 0–17 | 4 | 0–83 | 0.43 |
|
| |||||||
| Gender:Male | 58 | 70 | 34 | 72 | 24 | 66 | 0.84 |
|
| |||||||
| Complex cytogenetics (≥3 abnormalities) | 8 | 9.6 | 3 | 6.3 | 5 | 13.9 | 0.21 |
|
| |||||||
| CMML-specific cytogenetics | 0.12 | ||||||
| Low | 46 | 55.4 | 30 | 63.8 | 16 | 44.4 | |
| Intermediate | 19 | 22.9 | 9 | 19 | 10 | 27.8 | |
| High | 18 | 21.7 | 8 | 17 | 10 | 27.8 | |
|
| |||||||
| MDAPS | 0.08 | ||||||
| Low | 5 | 5 | 4 | 8.5 | 1 | 2.8 | |
| Intermediate-1 | 11 | 13.3 | 6 | 12.7 | 5 | 13.9 | |
| Intermediate-2 | 7 | 8.4 | 4 | 8.5 | 3 | 8.3 | |
| High | 3 | 3.6 | 2 | 4.2 | 1 | 2.8 | |
|
| |||||||
| Prior Allo-SCT | 5 | 6 | 3 | 6.3 | 2 | 5.6 | 0.97 |
|
| |||||||
| Prior ASCT | 4 | 4.8 | 3 | 6.3 | 1 | 2.8 | 0.55 |
|
| |||||||
| Hypomethylating treatment | 37 | 44.6 | 23 | 48.9 | 14 | 38.9 | 0.25 |
|
| |||||||
| Response before transplant | 0.08 | ||||||
| CR/mCR | 24 | 28.9 | 18 | 38.3 | 6 | 16.7 | |
|
| |||||||
| Karnofsky ≥90 | 51 | 70.8 | 29 | 61.7 | 22 | 66.7 | 0.36 |
|
| |||||||
| Conditioning | 0.13 | ||||||
| MAC | 64 | 77 | 36 | 76.5 | 28 | 77.7 | |
|
| |||||||
| Stem cell source | 0.44 | ||||||
| PB | 48 | 57.8 | 27 | 57.4 | 21 | 58.3 | |
| BM | 35 | 42.2 | 20 | 42.5 | 15 | 41.7 | |
|
| |||||||
| Donor | 0.74 | ||||||
| MRD | 30 | 36.1 | 17 | 36.1 | 13 | 36.1 | |
| MUD | 47 | 56.6 | 28 | 59.5 | 19 | 52.8 | |
| MMD | 6 | 7.2 | 2 | 4.2 | 4 | 11.1 | |
|
| |||||||
| Engraftment | 75 | 90.4 | 42 | 89.3 | 33 | 91.7 | 0.69 |
|
| |||||||
| Median time to ANC engraftment (day) | 13 | 8–46 | 13 | 9–30 | 13 | 8–46 | 1.0 |
|
| |||||||
| Median time to platelet engraftment (day) | 15 | 8–90 | 16 | 8–90 | 14 | 8–78 | 0.83 |
|
| |||||||
| Chimerism | 0.23 | ||||||
| Donor | 44 | 53 | 26 | 55.3 | 18 | 50 | |
| Mixed | 29 | 34.9 | 16 | 34 | 13 | 36.1 | |
| Autologous | 1 | 1.2 | 0 | 0 | 1 | 2.8 | |
|
| |||||||
| Final response | 0.88 | ||||||
| CCR/CR | 74 | 89.2 | 33 | 82.5 | 34 | 94.5 | |
Abbreviations: MDAPS - The MD Anderson Prognostic Scoring System; AML - acute myeloid leukemia; CMML - chronic myelomonocytic leukemia; Allo-SCT - allogeneic stem cell transplantation; ASCT - autologous stem cell transplantation; CR/mCR - complete remission/marrow complete remission; MAC - myeloablative conditioning; PB - peripheral blood; BM - bone marrow; MRD - matched related donor; MUD - matched unrelated donor; MMD - mismatched related and unrelated donor; ANC - absolute neutrophil count; CCR - complete cytogenetic remission
Seventy-eight patients (94%) received induction treatment before transplant - 37 patients (44.6%) with a hypomethylating agent (either 5-azacytidine or decitabine) for at least 3 courses (median 6 courses) and 41 patients (49.3%) with acute myeloid leukemia–type induction chemotherapy. The other 5 patients received standard supportive care and/or hydroxyurea before transplant. Patient and transplant characteristics did not significantly differ between the patients treated with hypomethylating agents and the patients treated with conventional chemotherapy (without hypomethylating agents) or given supportive care alone except more patients who did not receive hypomethylating agents underwent allo-SCT before the year 2005 (67% versus 0%; p<0.001) (Table 2). A complete remission (CR) or marrow complete remission (mCR) before transplant was seen in 41% of patients (N=15) treated with hypomethylating agents and 20% of patients (N=9) treated with other agents (p=0.12).
Table 2.
Patient and transplant characteristics by treatment with hypomethylating agent.
| Hypomethylating Treatment | P value | ||||
|---|---|---|---|---|---|
|
| |||||
| Yes | No | ||||
|
| |||||
| N=37 | %, Range | N=46 | %, Range | ||
| Median age (years) | 60 | 18–78 | 55.5 | 17–75 | 0.43 |
|
| |||||
| Age>60 | 18 | 48.6 | 15 | 32.6 | 0.17 |
|
| |||||
| Median ANC engraftment (day) | 12 | 11–30 | 13 | 8–56 | 0.54 |
|
| |||||
| Median platelet engraftment (day) | 14 | 9–44 | 16 | 8–90 | 0.63 |
|
| |||||
| Median percentage of blast at the time of transplant | 3 | 0–83 | 4 | 0–53 | 0.49 |
|
| |||||
| Gender: - Male | 26 | 68 | 32 | 70 | 1.00 |
|
| |||||
| Histology at diagnosis | 0.60 | ||||
| CMML-1/2 | 23 | 62 | 24 | 52 | |
| CMML/AML | 14 | 37.8 | 22 | 47.8 | |
|
| |||||
| CR/mCR before transplant | 15 | 40.5 | 9 | 19.5 | 0.12 |
|
| |||||
| Complex cytogenetics | 3 | 8.1 | 5 | 10.9 | 0.72 |
|
| |||||
| CMML specific cytogenetics | 0.72 | ||||
| Low | 21 | 56.8 | 25 | 54.3 | |
| Intermediate | 7 | 18.9 | 12 | 26.1 | |
| High | 9 | 24.3 | 9 | 19.6 | |
|
| |||||
| Prior AlloSCT | 2 | 5.4 | 3 | 6.5 | 1.00 |
|
| |||||
| Prior ASCT | 1 | 2.7 | 3 | 6.5 | 0.62 |
|
| |||||
| Karnofsky >90 | 23 | 67.6 | 28 | 73.7 | 0.61 |
|
| |||||
| Transplant before 2005 | 0 | 0 | 30 | 67 | 0.001 |
|
| |||||
| Conditioning | |||||
| MAC | 30 | 81 | 34 | 73.9 | 0.60 |
|
| |||||
| Stem cell source | 0.09 | ||||
| PB | 24 | 64.9 | 24 | 52.2 | |
| Marrow | 35 | 35.2 | 22 | 47.8 | |
|
| |||||
| Donor | 0.94 | ||||
| MRD | 14 | 37.8 | 16 | 34.8 | |
| MUD | 20 | 54.1 | 27 | 58.7 | |
| MMD | 3 | 8.1 | 3 | 6.5 | |
|
| |||||
| Engraftment | 33 | 89.2 | 42 | 91.3 | 0.13 |
|
| |||||
| Chimerism | 0.11 | ||||
| Donor | 16 | 43.2 | 28 | 60.9 | |
| Mixed | 17 | 45.9 | 12 | 26.1 | |
| Autologous | 0 | 0 | 1 | 2.2 | |
|
| |||||
| Final response | 0.21 | ||||
| CCR/CR | 30 | 81.1 | 44 | 95.6 | |
Abbreviations: MDAPS - The MD Anderson Prognostic Scoring System; Allo-SCT - allogeneic stem cell transplantation; ASCT - autologous stem cell transplantation; MAC - myeloablative conditioning; CR/mCR - complete remission/marrow complete remission; PB: peripheral blood; MRD - matched related donor; MUD - matched unrelated donor; MMD - mismatched related and unrelated donor; ANC - absolute neutrophil count; CCR - complete cytogenetic remission
The median time from diagnosis to allo-SCT was 8 months (range 3–86 months). There was no difference in median time to transplant in patients who received induction therapy with hypomethylating agents and those who received conventional chemotherapy or supportive care (6 months versus 9 months; p=0.32). However, patients with CMML/AML had shorter duration from diagnosis to transplant compared with CMML-1/2 (4 months versus 11 months; p=0.02). Thirty, 47 and 6 patients received transplants from matched related donors (MRD), matched unrelated donors (MUD), and mismatched related or unrelated donors, respectively. The sources of hematopoietic stem cells were peripheral blood for 48 patients (57.8%) and bone marrow for 35 patients (42.2%). Sixty-four patients (77.1%) received MAC and 19 patients (22.9%) RIC regimens.
Transplant outcomes by CMML category
At the last follow up, 29 patients were alive (18 of these patients received induction treatment with hypomethylating agents) with the median follow-up duration of 48 months. The transplant outcomes are summarized in Table 3. The cumulative incidence of engraftment at day 30 post-transplant for the entire group was 98%. The median time to neutrophil and platelet engraftment was 13 days and 15 days, respectively.
Table 3.
Transplant outcomes by CMML category and treatment with hypomethylating agent.
| Outcome | Total (%) | CMML1/2 (%) | CMML/A ML (%) | P value | Hypomet hylating treatment Yes (%) | Hypomet hylating treatment No (%) | P value |
|---|---|---|---|---|---|---|---|
| aGVHD all grades | 35.9 | 33.2 | 38.1 | 0.74 | 28.2 | 35.8 | 0.05 |
| aGVHD grade 2–4 | 11.7 | 13.1 | 8.3 | 0.37 | 12.8 | 11.3 | 0.72 |
| aGVHD grade 3–4 | 5.8 | 9.2 | 4.7 | 0.65 | 8.4 | 3.7 | 0.36 |
| cGVHD | 37.5 | 42.2 | 35 | 0.02 | 35 | 38.2 | 0.64 |
| Chronic extensive GVHD | 23.9 | 30.6 | 15.6 | 0.04 | 26.7 | 19.2 | 0.36 |
| 100-day TRM | 24.7 | 20.8 | 25.2 | 0.78 | 18.1 | 21.2 | 0.55 |
| 1-year TRM | 31.1 | 29 | 35.3 | 0.76 | 27.4 | 29.8 | 0.84 |
| 3-year CI of relapse | 33.3 | 35.4 | 26.7 | 0.39 | 22.4 | 34.9 | 0.03 |
| 3-year PFS | 33.9 | 35 | 27.4 | 0.31 | 43.2 | 27.4 | 0.04 |
| 3-year OS | 35.2 | 36.1 | 32.2 | 0.62 | 45.2 | 38.7 | 0.22 |
Abbreviations: aGVHD - acute graft-versus-host disease, cGVHD - chronic graft-versus-host disease, TRM - treatment-related mortality, CI - cumulative incidence, PFS - progression free survival, OS - overall survival
The cumulative incidences of TRM at day 100 and at 1 year post-transplant for the entire cohort were 25% and 31%, respectively. Causes of early death within 100 days were infection 64%, organ failure 24% and severe acute GVHD 12%. TRM rates did not differ significantly between the CMML subtypes; patients with CMML-1/2 and those with CMML/AML had 1-year TRM rates of 29% and 35%, respectively (p=0.76).
The cumulative incidence of aGVHD (all grades) at 100 days post-transplant was 36%, whereas the grade 2–4 aGVHD was only 12%. Patients with CMML-1/2 and those with CMML/AML developed grade 2–4 aGVHD at 100 days post-transplant at rates of 13% and 8%, respectively (p=0.37). The overall cumulative incidence of cGVHD at 1 year after transplant was 38%, higher in patients with CMML-1/2 than in those with CMML/AML (43% vs. 35%; p=0.02). The cumulative incidences of extensive cGVHD at 1 year post-transplant was 24% overall, 31% for patients with CMML-1/2 and 16% for CMML/AML (p=0.04).
The cumulative incidence of disease relapse at 3 years post-transplant for the entire cohort was 33%, with no differences between the two groups - 35% and 27% for CMML-1/2 and CMML/AML, respectively (p=0.39), while the 3-year PFS rate was 34% for the entire cohort, 35% for patients with CMML-1/2 and 27% for patients with CMML/AML (p=0.31; Figure 1A). The 3-year OS rates for the CMML-1/2 and CMML/AML groups were 36% and 32%, respectively (p=0.62).
Figure 1.
PFS by CMML category (A) and treatment with hypomethylating agent before transplant (B)
Transplant outcomes by treatment with hypomethylating agents
Successful engraftment was seen in 33 patients treated with hypomethylating agents (89%) and 42 patients treated with other agents (91%; p=0.13). At day 100 post-transplant, 30 patients treated with hypomethylating agents (81%) and 44 patients treated with other treatments (96%) achieved complete remission or complete cytogenetic remission (p=0.21).
Patients treated with a hypomethylating agent had a significantly lower cumulative incidence of relapse at 3 years post-transplant (22%) than those treated with other agents (35%; p=0.03), whereas TRM at 1 year post-transplant did not significantly differ between the groups (27% and 30%, respectively; p=0.84). The lower relapse rate resulted in a significantly higher 3-year PFS rate in patients treated with a hypomethylating agent (43%) than in those who received other treatment (27%; p=0.04) (Figure 1B). The benefits of hypomethylating agent treatment on relapse and PFS were seen only in patients who achieved a complete remission before transplant whereas patients who were not in remission had similar relapse rate and survival. However, therapy with hypomethylating agents before transplant did not significantly influence the 3-year OS rate (45% in those treated with hypomethylating agents and 39% in those treated with other agents; p=0.22).
Factors predicting transplant outcomes
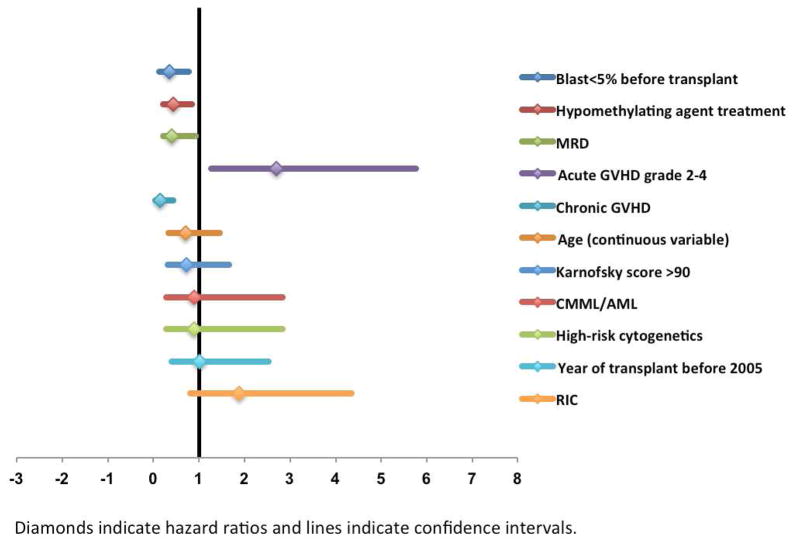
In the univariate analysis for PFS, factors associated with longer PFS were less than 5% bone marrow blasts before transplant (p=0.02), treatment with a hypomethylating agent (p=0.04), a transplant from an MRD (p=0.002), and the development of cGVHD (p<0.001). Conversely, the development of grade 2–4 aGVHD was associated with shorter PFS (p=0.02). All these factors remained significant in multivariate regression analysis. The independent prognostic factors for PFS were a blast count of less than 5% immediately before transplant (hazard ratio [HR] 0.36, 95% CI 0.14–0.78, p=0.04), treatment with a hypomethylating agent (HR 0.44, 95% CI 0.23–0.86, p=0.03), a transplant from an MRD (HR 0.41, 95% CI 0.22–0.94, p=0.03), development of grade 2–4 aGVHD (HR 2.7, 95% CI 1.27–5.77, p=0.01), and development of cGVHD (HR 0.15, 95% CI 0.05–0.45, p=0.001) (Figure 2). Also, in the multivariate analysis using remission status before transplant together with hypomethylating agent treatment, we found that both factors were an independent predictor for longer PFS.
Figure 2.
Forest plot representation of factors included in the multivariate analysis for progression-free survival.
Abbreviations: MRD - matched-related donor; GVHD - graft-versus-host disease; CMML/AML - CMML that had progressed to secondary acute myeloid leukemia; RIC - reduced-intensity conditioning
In the univariate analysis for disease relapse, a blast percentage of less than 5% (p=0.03), treatment with a hypomethylating agent (p=0.03), transplant from an MRD (p=0.02), and development of cGVHD (p=0.02) were each associated with a lower relapse incidence. All these factors also were independent predictors of lower relapse incidence in multivariate analysis, with HRs of 0.28 (95% CI 0.11–0.49, p=0.02) for less than 5% bone marrow blasts at the time of transplant, 0.67 (95% CI 0.43–0.88, p=0.03) for the use of hypomethylating agents, 0.87 (95% CI 0.66–0.95, p=0.04) for using an MRD, and 0.22 (95% CI 0.13–0.39, p=0.02) for cGVHD.
In univariate analysis for TRM, the development of grade 2–4 aGVHD (p=0.005) and age more 60 years (p=0.04) each predicted higher TRM. However, only grade 2–4 aGVHD was a significant predictor of higher TRM in multivariate analysis (HR 3.8, 95% CI 2.13–6.22, p=0.03). Age, CMML cytogenetic risk category, conditioning intensity and year of transplant did not predict transplant outcomes.
DISCUSSION
In this report, which we believe is the largest retrospective single-institution analysis looking at the effects of pre-transplant therapies of adult CMML patients treated with allo-SCT performed to date, we also have identified important factors that influence transplant outcomes. We conclude that (1) disease burden appreciated by the percentage of bone marrow blasts at the time of transplant determines prognosis after transplant; (2) allo-SCT can overcome the poor prognosis associated with high-risk CMML-specific cytogenetics; and (3) treatment with a hypomethylating agent before transplant decreases relapse rate and improves progression-free survival.
The prognostic significance of bone marrow blast percentage in patients with CMML has been previously evaluated in several studies(21–23) and has been incorporated in various prognostic models of CMML.(24–26) Investigators from UTMDACC developed the MD Anderson Prognostic Scoring System for CMML based on survival analysis of 213 patients with CMML. This scoring system included 4 baseline clinical characteristics including the bone marrow blast count at diagnosis, and was validated in a cohort of 250 patients with CMML from the same institution.(24, 25) However, for patients with CMML undergoing allo-SCT, the disease burden at the time of transplant seems to have a greater prognostic impact than the disease burden at diagnosis. Krishnamurthy et al. reported 3-year disease-free survival rates of 47% for patients who had less than 5% bone marrow blasts at transplant versus 20% for those with more than or equal to 5% blasts at the time of transplant(4), while Kröger et al. found that the 2-year disease-free survival was 33% in patients with less than 10% marrow blasts compared with 12% for those with more than or equal to 10% marrow blasts at the time of transplant.(5) Even though these findings were not statistically significant in either study, probably because of the small numbers of patients, both studies suggested a prognostic significance of bone marrow blast percentage immediately before transplant. In the present study, we have clearly shown that patients with less than 5% bone marrow blasts at transplant had a lower risk of relapse and better PFS than those with higher bone marrow blast count. However, we did not identify a significant difference in transplant outcomes between patients with CMML-1/2 and those with CMML/AML, stratified on the basis of blast count at diagnosis. Taken together, these findings suggest that the disease burden appreciated by the bone marrow blast percentage at the time of transplant rather than at diagnosis is an important prognostic factor for transplant outcomes, and that patients with CMML should be treated with induction therapy to achieve at least morphologic remission prior to transplant in order to improve survival after transplant.
Another important finding was that the cytogenetic risk did not significantly affect transplant outcomes in patients with CMML, in contrasts with previous findings. Unlike for other myelodysplastic syndromes, in which chromosomal abnormalities strongly affect treatment outcomes, the prognostic significance of chromosomal abnormalities in CMML remains unclear. Moreover, no specific cytogenetic alterations have been associated with CMML, although recurring chromosomal abnormalities have been reported in this disease.(25, 27, 28) The Spanish Cooperative Group for myelodysplastic syndromes investigated the prognostic significance of chromosomal abnormalities in patients with CMML and found that patients with low-risk (normal karyotype or loss of Y chromosome as a single anomaly), intermediate-risk (all other abnormalities except those considered high risk), and high-risk (presence of trisomy 8, abnormalities of chromosome 7, or complex karyotype) had 5-year OS rates of 35%, 26%, and 4%, respectively (p=0.001). However, these results reflected the natural history of this disease, as none of the patients in this study was treated with hypomethylating agents, and patients undergoing allo-SCT (n=4) or intensive acute myeloid leukemia–type chemotherapy (n=23) were censored from the survival analysis at the time of transplant or at the start of chemotherapy, respectively.(11) Although transplant outcomes according to the International Prognostic Scoring System (IPSS) or the Revised IPSS could not be analyzed in the present study (owing to limitations in the data available) and although these prognostic scoring systems were not developed for CMML patients, a few studies have suggested that high cytogenetic risk according to the IPSS is associated with increased relapse rate and worse survival post-transplant in patients with CMML.(5, 9) In a study from the Fred Hutchinson Cancer Research Center (which included both pediatric and adult patients), high cytogenetic risk according to the IPSS was associated with increased mortality rate and reduced relapse-free survival in patients with CMML treated with allo-SCT.(9) Our study showed that patients with low-risk, intermediate-risk, and high-risk cytogenetics according to the risk classification system used by the Spanish myelodysplastic syndrome cooperative group(11) had similar transplant outcomes; therefore, these results suggest that transplantation can overcome the poor prognosis of CMML with high-risk cytogenetics.
Treatment with hypomethylating agents, such as decitabine and 5-azacytidine, have proven efficacy not only for patients with myelodysplastic syndromes, but also for those with CMML. Several recently completed phase II studies, investigating treatment with these agents specifically for patients with CMML, showed overall response rates ranging from 25% to 70%, and median OS times ranging from 12 to 37 months.(29–35) However, the benefit of treatment with a hypomethylating agent before transplant in patients with CMML has not been addressed. Our study is the first to associate the treatment with hypomethylating agents with lower relapse rates and superior PFS after transplant, when compared with conventional induction chemotherapy or supportive care alone. Treatment with hypomethylating agents remained an independent prognostic factor for lower relapse and better PFS in multivariate analysis. The mechanism by which these agents reduce relapse rate post-transplant remains unclear; however a better suppression of malignant clones and minimization of residual disease in the bone marrow prior to transplant is possible. Even though remission rate before transplant in 2 groups was not different (41% in hypomethylating group vs. 20% in other patients, p=0.12), this may be due to a small number of patients in both groups, which is the main limitation of this study. Randomized prospective studies are needed to clarify this issue.
Our data suggest that treatment with hypomethylating agents should continue for at least three courses with the goal of achieving morphologic remission (<5% bone marrow blasts) before transplant. This amount of time would be enough for the transplant physician to identify a donor and prepare for transplant. Thus, we suggest that transplant be performed soon after the patient has achieved morphologic remission, as a longer period of treatment may increase the risk of disease progression and compromise transplant outcomes.
While this is the largest single-institution analysis in adult patients undergoing allogeneic transplantation for CMML and the first one to show that treatment with hypomethylating agents prior to transplant may impact survival after transplant, these data should be interpreted with caution as is from a non-randomized single center, retrospective in nature with a limited number of patients. Analysis or larger number of patients as well as controlled studies is needed to confirm these findings.
Highlights.
Disease burden appreciated by the percentage of bone marrow blasts at the time of transplant determines prognosis after transplant.
Allo-SCT can overcome the poor prognosis associated with high-risk CMML-specific cytogenetics.
Treatment with a hypomethylating agent before transplant decreases relapse rate and improves progression-free survival in patients with CMML
Acknowledgments
This work was supported in part by the National Institutes of Health through MD Anderson Cancer Center Support Grant P30CA16672.
The authors would like to thank Sarah Bronson for her critical editing of the manuscript.
Footnotes
Conflicts of interest: The authors have no potential conflicts of interest to declare.
Authorship
P.K. collected and analyzed data and wrote the manuscript; U.P. contributed with data collection, interpretation of the results and manuscript writing; A.J., S.G., R.E.F., G.R, J.C. contributed with data collection, reviewed and approved the manuscript; C.B.S. contributed with review of the pathology slides, reviewed and approved the manuscript; G.B., N.P., G.G.M., H.K., A.A., C.H., P.A., I.F.K., P.K., B.S.A., O.B., K.R., D.M., E.J.S., R.E.C contributed with treatment of patients, reviewed and approved the manuscript; S.O.C contributed with study design, data collection and interpretation, and manuscript writing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Swerdlow SHCE, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. France: Lyon, International Agency for Research on Cancer; 2008. [Google Scholar]
- 2.Elliott MA, Tefferi A, Hogan WJ, Letendre L, et al. Allogeneic stem cell transplantation and donor lymphocyte infusions for chronic myelomonocytic leukemia. Bone Marrow Transplant. 2006;37(11):1003–8. doi: 10.1038/sj.bmt.1705369. [DOI] [PubMed] [Google Scholar]
- 3.Kerbauy DM, Chyou F, Gooley T, Sorror ML, et al. Allogeneic hematopoietic cell transplantation for chronic myelomonocytic leukemia. Biol Blood Marrow Transplant. 2005;11(9):713–20. doi: 10.1016/j.bbmt.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Krishnamurthy P, Lim ZY, Nagi W, et al. Allogeneic haematopoietic SCT for chronic myelomonocytic leukaemia: a single-centre experience. Bone Marrow Transplant. 2010;45(10):1502–7. doi: 10.1038/bmt.2009.375. [DOI] [PubMed] [Google Scholar]
- 5.Kröger N, Zabelina T, Guardiola P, et al. Allogeneic stem cell transplantation of adult chronic myelomonocytic leukaemia. A report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Br J Haematol. 2002;118(1):67–73. doi: 10.1046/j.1365-2141.2002.03552.x. [DOI] [PubMed] [Google Scholar]
- 6.Laport GG, Sandmaier BM, Storer BE, et al. Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biol Blood Marrow Transplant. 2008;14(2):246–55. doi: 10.1016/j.bbmt.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittal P, Saliba RM, Giralt SA, et al. Allogeneic transplantation: a therapeutic option for myelofibrosis, chronic myelomonocytic leukemia and Philadelphia-negative/BCR-ABL-negative chronic myelogenous leukemia. Bone Marrow Transplant. 2004;33(10):1005–9. doi: 10.1038/sj.bmt.1704472. [DOI] [PubMed] [Google Scholar]
- 8.Ocheni S, Kroger N, Zabelina T, Zander AR, Bacher U. Outcome of allo-SCT for chronic myelomonocytic leukemia. Bone Marrow Transplant. 2009;43(8):659–61. doi: 10.1038/bmt.2008.366. [DOI] [PubMed] [Google Scholar]
- 9.Eissa H, Gooley TA, Sorror ML, et al. Allogeneic hematopoietic cell transplantation for chronic myelomonocytic leukemia: relapse-free survival is determined by karyotype and comorbidities. Biol Blood Marrow Transplant. 2011;17(6):908–15. doi: 10.1016/j.bbmt.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adekola K, Popat U, Ciurea SO. An update on allogeneic hematopoietic progenitor cell transplantation for myeloproliferative neoplasms in the era of tyrosine kinase inhibitors. Bone Marrow Transplant. 2014;49(11):1352–9. doi: 10.1038/bmt.2014.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Such E, Cervera J, Costa D, Sole F, et al. Cytogenetic risk stratification in chronic myelomonocytic leukemia. Haematologica. 2011;96(3):375–83. doi: 10.3324/haematol.2010.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berman E, Wiernik P, Vogler R, et al. Long-term follow-up of three randomized trials comparing idarubicin and daunorubicin as induction therapies for patients with untreated acute myeloid leukemia. Cancer. 1997;80(S11):2181–5. doi: 10.1002/(sici)1097-0142(19971201)80:11+<2181::aid-cncr3>3.3.co;2-q. [DOI] [PubMed] [Google Scholar]
- 13.Nazha A, Kantarjian H, Ravandi F, et al. Clofarabine, idarubicin, and cytarabine (CIA) as frontline therapy for patients </=60 years with newly diagnosed acute myeloid leukemia. Am J Hematol. 2013;88(11):961–6. doi: 10.1002/ajh.23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–25. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 15.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14(7):748–58. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15(3):367–9. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 18.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Cox D. Regression models and life tables (with Discussion) Journal of the Royal Statistical Society. 1972;(34):187–200. [Google Scholar]
- 20.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 21.Worsley A, Oscier DG, Stevens J, et al. Prognostic features of chronic myelomonocytic leukaemia: a modified Bournemouth score gives the best prediction of survival. Br J Haematol. 1988;68(1):17–21. doi: 10.1111/j.1365-2141.1988.tb04173.x. [DOI] [PubMed] [Google Scholar]
- 22.del Cañizo MC, Sanz G, San Miguel JF, et al. Chronic myelomonocytic leukemia--clinicobiological characteristics: a multivariate analysis in a series of 70 cases. Eur J Haematol. 1989;42(5):466–73. doi: 10.1111/j.1600-0609.1989.tb01472.x. [DOI] [PubMed] [Google Scholar]
- 23.Fenaux P, Jouet JP, Zandecki M, et al. Chronic and subacute myelomonocytic leukaemia in the adult: a report of 60 cases with special reference to prognostic factors. Br J Haematol. 1987;65(1):101–6. doi: 10.1111/j.1365-2141.1987.tb06142.x. [DOI] [PubMed] [Google Scholar]
- 24.Beran M, Wen S, Shen Y, et al. Prognostic factors and risk assessment in chronic myelomonocytic leukemia: validation study of the M.D. Anderson Prognostic Scoring System. Leuk Lymphoma. 2007;48(6):1150–60. doi: 10.1080/10428190701216386. [DOI] [PubMed] [Google Scholar]
- 25.Onida F, Kantarjian HM, Smith TL, et al. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: a retrospective analysis of 213 patients. Blood. 2002;99(3):840–9. doi: 10.1182/blood.v99.3.840. [DOI] [PubMed] [Google Scholar]
- 26.Mufti GJ, Stevens JR, Oscier DG, et al. Myelodysplastic syndromes: a scoring system with prognostic significance. Br J Haematol. 1985;59(3):425–33. doi: 10.1111/j.1365-2141.1985.tb07329.x. [DOI] [PubMed] [Google Scholar]
- 27.Bacher U, Haferlach T, Kern W, et al. Conventional cytogenetics of myeloproliferative diseases other than CML contribute valid information. Ann Hematol. 2005;84(4):250–7. doi: 10.1007/s00277-004-0977-1. [DOI] [PubMed] [Google Scholar]
- 28.Haase D, Germing U, Schanz J, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110(13):4385–95. doi: 10.1182/blood-2007-03-082404. [DOI] [PubMed] [Google Scholar]
- 29.Adès L, Sekeres MA, Wolfromm A, et al. Predictive factors of response and survival among chronic myelomonocytic leukemia patients treated with azacitidine. Leuk Res. 2013;37(6):609–13. doi: 10.1016/j.leukres.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Aribi A, Borthakur G, Ravandi F, et al. Activity of decitabine, a hypomethylating agent, in chronic myelomonocytic leukemia. Cancer. 2007;109(4):713–7. doi: 10.1002/cncr.22457. [DOI] [PubMed] [Google Scholar]
- 31.Braun T, Itzykson R, Renneville A, et al. Molecular predictors of response to decitabine in advanced chronic myelomonocytic leukemia: a phase 2 trial. Blood. 2011;118(14):3824–31. doi: 10.1182/blood-2011-05-352039. [DOI] [PubMed] [Google Scholar]
- 32.Costa R, Abdulhaq H, Haq B, et al. Activity of azacitidine in chronic myelomonocytic leukemia. Cancer. 2011;117(12):2690–6. doi: 10.1002/cncr.25759. [DOI] [PubMed] [Google Scholar]
- 33.Fianchi L, Criscuolo M, Breccia M, et al. High rate of remissions in chronic myelomonocytic leukemia treated with 5-azacytidine: results of an Italian retrospective study. Leuk Lymphoma. 2013;54(3):658–61. doi: 10.3109/10428194.2012.719617. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Manero G, Gore SD, Cogle C, et al. Phase I study of oral azacitidine in myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J Clin Oncol. 2011;29(18):2521–7. doi: 10.1200/JCO.2010.34.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong E, Seymour JF, Kenealy M, et al. Treatment of chronic myelomonocytic leukemia with azacitidine. Leuk Lymphoma. 2013;54(4):878–80. doi: 10.3109/10428194.2012.730615. [DOI] [PubMed] [Google Scholar]