Abstract
Background
We aimed to define normative values for novel pressure topography metrics for high-resolution pharyngeal-esophageal manofluorography. The effects of age, gender and bolus properties were examined.
Methods
Concurrent high-resolution manometry (HRM) and videofluoroscopy data were collected from 22 younger (aged 21–40) and 22 older (aged 60–80) healthy subjects. Pressure topography was analyzed by correlating pressure domains with videofluoroscopic events. Nine pressure topography metrics of the pharyngeal and proximal esophageal swallow were extracted; four of these were also compared with previously obtained esophageal HRM studies to assess the effects of catheter diameter.
Key Results
Older individuals exhibited more vigorous contractility in the pharynx than did younger subjects with all bolus types, but the greatest values for both groups were with effortful swallow and on that measure the age groups were similar. Upper esophageal sphincter (UES) intrabolus pressure during sphincter opening was also greater in the older subjects. Some gender differences were observed, particularly related to proximal esophageal contractile vigor. Bolus consistency had no consistent effect. Studies using the larger catheter diameter resulted in significantly greater contractile vigor in the UES and proximal esophagus.
Conclusions and Inferences
Older adults exhibited more vigorous pharyngeal contractions than young adults, albeit within a similar range of capacity, perhaps reflecting a compensatory response to other age-related physiological changes. Greater UES intrabolus pressures observed during bolus transit in the older group likely reflect reduced UES compliance with age. Normative data on novel HRM metrics collected in this study can serve as a reference for future clinical studies.
Keywords: dysphagia, high-resolution manometry, Manofluorography, pharyngeal swallow, upper esophageal sphincter
Manofluorography, the concurrent use of manometry and videofluorography for the evaluation of pharyngeal swallowing disorders, can provide complementary diagnostic information, not available from the videofluoroscopic swallow study (VFSS) alone. Technological advances in recent years have led to substantial improvements in manometric devices and the development of high-resolution pharyngeal-esophageal manometry with pressure topography (HRPEPT) that can now overcome many previous shortcomings. Furthermore, recently designed computer software (Manoscan360 Given Imaging Sierra Scientific Instruments, Los Angeles, CA) now enables the simultaneous recording of manometry and VFSS, producing a synchronized data file that facilitates correlating pressure topography data with fluoroscopic images.
High-resolution manometry (HRM) and esophageal pressure topography, have improved the accuracy of diagnosis of esophageal disorders, facilitating improved management (1–4). Similarly, studies using HRM to evaluate pharyngeal swallowing disorders have recently emerged, demonstrating its added value (5–12). However, for HRPEPT to be used as a clinical tool, normative data are required to serve as a reference for studies in clinical populations. The main goal of this study was to develop novel pressure topography metrics of HRPEPT studies suitable for application in clinical studies and to establish normative values for these metrics in asymptomatic young and old adult subjects.
Previous studies have examined age-related changes in pharyngeal swallow pressures using conventional manometry systems with equivocal results (13–18). The advantages of HRPEPT should allow for the clarification of age-related changes in pharyngeal deglutitive pressures reflecting possible changes in muscular function with aging. Decline in pharyngeal muscular function or reduced muscular reserve may predispose older individuals to developing dysphagia and pneumonia in states of illness (19,20). Therefore, an additional aim of this study was to examine possible age and gender related differences in pharyngeal swallow pressure topography.
Methods
Subjects
Subjects were recruited by fliers and via the Aging Research Registry of Northwestern University’s Buehler Center. Fifty-one subjects were recruited to the study. Following exclusion or withdrawal of 5 subjects, and removal of 2 damaged data files,, subject groups consisted of 22 healthy individuals between the ages of 21–40 (median age 27 years) and 22 healthy individuals aged 60–80 (median age 74 years). The latter age range was chosen because studies have shown that age-related effects in the normal swallow typically begin around age 60 (20–24). The participants had no history of previous head and neck surgery or neurologic, otolaryngologic or upper gastrointestinal disorder potentially affecting swallowing. In order to screen for swallow-related symptoms, subjects completed the Individual Perception of Function Questionnaire (see appendix). This questionnaire is a modified version of the Patient’s Perception of Swallow Function Questionnaire, which is similar to that used in previous studies with head and neck cancer patients (25–27). Modifications included the omission of reference to pre-treatment versus post-treatment swallow function. Any subject responding positively to questions in the questionnaire (which indicate difficulty or symptoms) was excluded, with the exception of those reporting occasional mild heartburn or occasional hoarseness following prolonged periods of speech. Informed consent was obtained from each subject. The protocol was approved by the Northwestern University Institutional Review Board.
Experimental Setup
A 2.75 mm outer diameter solid-state manometric assembly with 36 circumferential sensors spaced at 7.5 mm intervals was used (Sierra Scientific Instruments, Los Angeles, CA). Each of the 36 sensors contained 12 circumferentially distributed sensors. Before recording, the transducers were calibrated at 0 and 300 mmHg using externally applied pressure. The data acquisition frequency was 35 Hz for each sensor. Video was recorded by Manoscan360™ at a rate of 30 frames/second and analyzed by Manoview™ ESO 3.0, Sierra Scientific Instruments, Los Angeles CA). A PanasonicAJ-D455digitalvideocasetterecorder was also used to record the VFSS study at a rate of 30 frames per second.
Study Procedures
Participants were screened prior to the study by phone to assess eligibility. Subjects were instructed to fast for at least 4 hours prior to the procedure. Following consent, subjects were interviewed to obtain medical history and then filled out the Individual Perception of Swallow Questionnaire. At the start of the procedure, subjects were seated in an upright position. Following the administration of topical anesthesia to one nostril, the manometric catheter was inserted transnasally. The VFSS was conducted in the lateral view, apart from the last 3 swallows, which were conducted in the anterior-posterior view. A penny was taped behind the right ear of the subjects to serve as a “ruler” to correct for magnification. Subjects were asked to keep their head straight throughout the study. The swallow protocol consisted of three trials each of: 1 ml thin liquid barium, 5 ml thin liquid barium, 10 ml thin liquid barium, 3 ml pudding barium, ¼ Lorna Doone cookie with 3ml barium paste, 5ml thin liquid barium in anterior-posterior view. In nineteen of the studies, three additional swallows of 3ml pudding, in which subjects were instructed to “swallow with effort, with all the muscles in your throat” (effortful swallows), were added to the protocol.
In the majority of studies (66%), an additional step was taken to aid in the temporal synchronization of the manometry and video outputs within the Manoview™ software following the removal of the manometry catheter. The manometry catheter was held in front of the fluoroscope and one sensor was tapped or lightly pinched while the fluoroscope was on.
Data Analysis
Data analysis began with the temporal synchronization of the videofluoroscopic and manometry output. This was done by identifying the moment of UES opening on both the VFSS and pressure topography and adjusting the timing of the Manoview™ video output so that they were temporally matched. In 30 studies, timing synchrony was also verified by matching the moment of release of sensor pinch on fluoroscopy with the corresponding pressure signal on the topography. Following synchronization, physiological events in the pharyngeal swallow (e.g. tongue base retraction) were carefully matched with the corresponding events on the pressure topography. The UES was located for each individual on 1ml swallows by noting the sensor number located in the center of the UES high pressure band on topography at baseline, locating this sensor on videofluorography, and noting the position relative to the subject’s vocal folds, which are always clearly seen on fluoroscopy and move in unison with the UES. This individualized UES location relative to vocal folds was then used to track UES movement during the swallow and guide measurements relating to the UES.
The following parameters were then obtained from the manometric output: pharyngeal contractile integral (PhCI), maximal pressure of pharyngeal contraction (P-Max), velopharyngeal closure integral (VPCI), tongue base/pharyngeal wall integral (TBI), hypopharyngeal contractile integral (HPCI), UES integrated relaxation pressure (UES-IRP), Post-deglutitive UES contractile integral (PD-UESCI), maximum post-deglutitive UES contraction (UES-Max), and proximal esophageal contractile integral (PCI). Description of these measures and how they were obtained is detailed in Table 1.
Table 1.
HRPEPT metrics
| Measure | Description |
|---|---|
| Pharyngeal Contractile Integral (PhCI) |
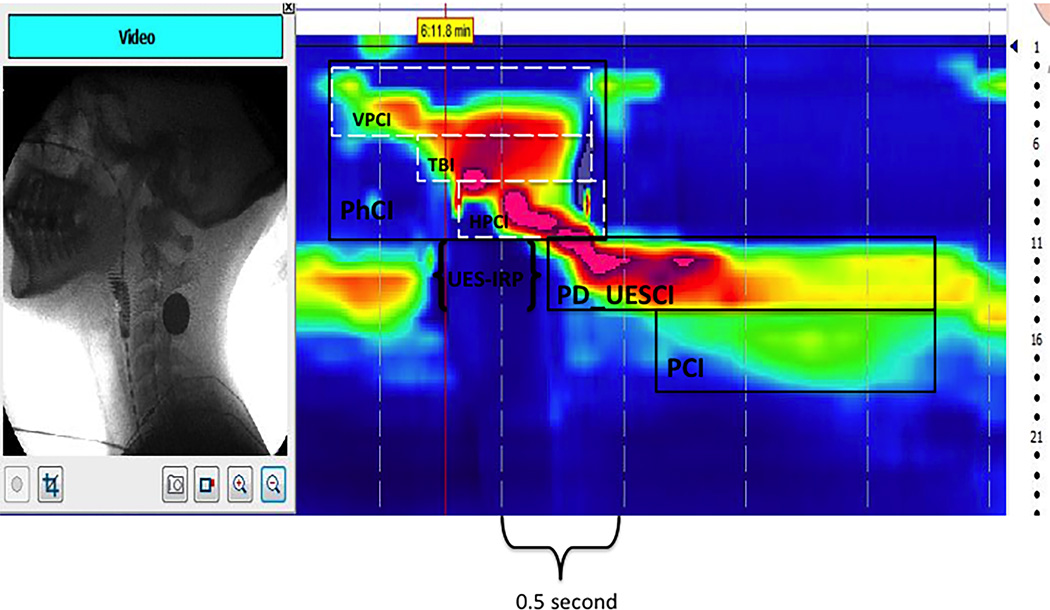
A global measure of pharyngeal contractile vigor and persistence within a space-time box on the pressure topography plot spanning from the velopharynx superiorly to the upper margin of the UES at the moment of closure inferiorly as viewed on VFSS (see Figure 1). The PCI is the mean pressure within this domain multiplied by duration (s) and span (cm) in units of mmHg•s•cm. |
| Maximal pharyngeal contraction pressure (P-Max) |
Maximal pressure within the area of pharyngeal space-time box (see PhCI, above) calculated automatically with Manoview software in units of mmHg. |
| Velopharyngeal Closure Integral (VPCI) |
A metric reflecting the vigor of velopharyngeal closure during the pharyngeal swallow measured within a space-time box on the pressure topography plot corresponding to velopharyngeal closure as viewed on VFSS (see Figure 1). VPCI is the mean pressure multiplied by the duration (s) and span (cm) within this box in units of mmHg•s•cm. |
| Tongue base/pharyngeal wall Integral (TBI) |
A metric reflecting the vigor of tongue base to pharyngeal wall opposition during the swallow measured within a space-time box on the pressure topography plot corresponding to the base of the tongue as viewed on VFSS (see Figure 1). TBI is the mean pressure multiplied by the duration (s) and span (cm) within this box in units of mmHg•s•cm. |
| Hypopharyngeal Contractile Integral (HPCI) |
A metric reflecting the robustness of the pharyngeal contraction highly reflective of pressure changes within the UES during the pharyngeal swallow measured within a space-time box on the pressure topography plot spanning from the tongue base superiorly to the inferior margin of the UES inferiorly as viewed on VFSS (see Figure 1). HPCI is the mean pressure multiplied by the duration (s) and span (cm) within this box in units of mmHg•s•cm. |
| UES integrated relaxation pressure (UES-IRP) |
A metric reflective of deglutitive intra-sphincteric intrabolus pressure, indicative of the completeness of UES relaxation and sphincter compliance. UES-IRP is the mean pressure during the 0.25 s of maximal deglutitive relaxation. The Manoview software automatically calculates this in units of mmHg once the UES area is manually demarcated (see Figure 1). Since the software references this to gastric pressure, the average gastric pressure needs to be added to the Manoview value to convert to an atmospheric pressure reference. |
| Post-deglutitive UES Contractile Integral (PD-UESCI) |
A metric reflective the post-deglutitive contractile response of the UES measured within the space-time box capturing UES high-pressure band. The box terminates at the location on the pressure topography plot that matches the end of the proximal esophageal contraction (or the beginning of the transition zone) (see Figure 1). PD-UESCI is the mean pressure multiplied by the duration (s) and span (cm) within this box in units of mmHg•s•cm. |
| Maximum post- deglutitive UES pressure (UES-Max) |
Maximal pressure within the PD-UESCI space/time box, calculated automatically by the Manoview software in units of mmHg. |
| Proximal Esophageal Contractile Integral (PCI) |
A metric reflective the vigor of the deglutitive contractile response of the proximal esophagus measured within the space-time box spanning from the inferior margin of the UES to the sharp drop in pressure (change of color from green to blue) marking the beginning of the transition zone (see Figure1). PCI is the mean pressure multiplied by the duration (s) and span (cm) within this box in units of mmHg•s•cm. |
Comparison of studies with different catheter diameter
To assess the effects of catheter diameter on the HRPEPT parameters, measures relating to the UES and proximal esophagus were extracted from HRM studies of asymptomatic adults (ages 19–48 yrs.) previously obtained at Northwestern Memorial Hospital using a 4.2mm diameter catheter. Since these studies were conducted to assess esophageal function, the most proximal sensor was often too distally located to assess velopharyngeal and pharyngeal contractility. Hence, UES and proximal esophageal measures from 22 of these studies were compared to the same metrics extracted from 22 subjects from the younger group of the current study to investigate possible effects of catheter diameter. All swallows compared were 5ml liquid conducted in the sitting position.
Statistical Analysis
The mean, standard deviation and ranges on each trial type were calculated for each pressure topography metric. A three-way factorial MANOVA (2*2*6) was conducted to test the effects of age, gender and swallow type on the metric. Two-way factorial MANOVAs were also separately conducted for each swallow type to assess age and gender differences within each swallow type. Additionally, a one-way MANOVA was done to compare the effects of catheter diameter on the metrics.
Results
Patient tolerance, clinical findings and technical issues
Catheter placement was well tolerated in all but two participants. In one case, a 26-year-old female could not tolerate catheter insertion and elected to end the study. In the second case, a 31-year-old male fainted immediately prior to catheter insertion. One other study was excluded from analysis due to abnormal findings (recurrent aspiration), despite no complaints relating to the swallow. Two additional studies were excluded due to a medical history that could potentially affect the swallow.
All included subjects had normal swallow studies. Occasional penetration of thin liquids into the vestibule was noted in 12 subjects (4 younger, 8 older). In two of these (one younger, one older), a single occurrence of aspiration of small amounts of thin liquid was also noted. Three participants from the older group showed mild vallecular residue after the swallow. Cervical osteophytes were noted in three participants and a cricopharyngeal (CP) bar in three subjects, none of which impeded bolus flow. Manometric data could not be analyzed from two studies due to technical issues (one damaged file and one study displaying distorted topography).
Normative Values of HRPEPT Pharyngeal Metrics
Mean, standard deviation and the observed ranges for each pharyngeal pressure topography metric are displayed in Tables 2 and 3. The mean values are based on the average of all swallows of all subjects combined, for each specified group and swallow type.
Table 2.
Statistical summary of pharyngeal HREPT measurements for 44 normal subjects; 22 young and 22 elderly
| Liquid | Pudding (3ml) | Cookie | ||||
|---|---|---|---|---|---|---|
| 1 ml | 5 ml | 10 ml | Effortful | |||
| PhCI (mmHg•s•cm) | 308 ± 110 (169 –504) |
308 ± 111 (162 – 504) |
320 ± 106 (176 – 541) |
305 ± 94 (161 –480) |
495 ± 252 (235 – 1060) |
296 ± 99 (148 –500) |
| (young) | 259 ± 58* (169 – 374) |
256 ± 84* (154 – 433) |
276 ± 97* (164 – 502) |
272 ± 87* (159 –475) |
494 ± 270 (229 – 1081) |
259 ± 92* (138 –483) |
| (old) | 355 ± 128* (180 – 708) |
363 ± 110* (216 – 588) |
364 ± 97* (191 – 548) |
339 ± 89* (170 –487) |
497 ± 238 (231 – 1094) |
333 ± 94* (173 –501) |
| P-Max (mmHg) | 228 ± 60 (148 – 327) |
229 ± 62 (142 – 338) |
222 ± 51 (153 – 317) |
233 ± 61 (157 –364) |
247 ± 69 (146 – 353) |
218 ± 45 (154 –294) |
| (young) | 207 ± 61* (138 – 379) |
211 ± 64* (130 – 342) |
202 ± 47* (134 – 286) |
216 ± 59* (149 –345) |
232 ± 56 (143 – 330) |
200 ± 37* (148 –268) |
| (old) | 248 ± 52* (163 – 328) |
249 ± 54* (173 – 340) |
241 ± 47* (173 – 334) |
249 ± 58* (164 –365) |
262 ± 77 (174 – 475) |
237 ± 44* (168 –324) |
| VPCI (mmHg•s•cm) | 122 ± 64 (43 – 276) |
129 ± 63 (51 – 291) |
147 ± 69 (63 – 319) |
116 ± 54 (37 – 243) |
199 ± 103 (65 – 429) |
109 ± 49 (31 – 204) |
| (young) | 103 ± 39* (0 – 172) |
106 ± 52* (18 – 211) |
125 ± 61* (54 – 292) |
107 ± 57* (9 – 231) |
215 ± 130 (114 – 463) |
94 ± 40* (29 – 184) |
| (old) | 140 ± 76* (60 – 333) |
152 ± 65* (70 – 308) |
167 ± 70* (72 – 323) |
125 ± 50* (41 – 248) |
184 ± 69 (63 – 334) |
122 ± 53* (33 – 230) |
| TBI (mmHg•s•cm) | 103 ± 38 (52 – 172) |
99 ± 39 (43 – 166) |
94 ± 38 (43 – 160) |
103 ± 34 (49 –170) |
164 ± 93 (61 – 383) |
99 ± 36 (47 – 171) |
| (young) | 93 ± 26* (52 – 158) |
88 ± 33* (42 – 160) |
86 ± 37* (44 – 166) |
94 ± 32* (45 –175) |
178 ± 111 (58 – 413) |
92 ± 39 (39 – 197) |
| (old) | 112 ± 44* (50 – 215) |
110 ± 41* (49 – 177) |
102 ± 38* (38 – 159) |
111 ± 34* (60 – 169) |
151 ± 71 (69 – 340) |
106 ± 32 (59 – 172) |
| HPCI (mmHg•s•cm) | 82 ± 40 (37 – 155) |
73 ± 36 (31 – 142) |
71 ± 32 (28 – 134) |
81 ± 36 (35 – 154) |
127 ± 101 (46 – 337) |
80 ± 35 (34 – 156) |
| (young) | 59 ± 21* (31 – 107) |
55 ± 21* (25 – 93) |
55 ± 24* (20 – 105) |
63 ± 25* (31 – 109) |
99 ± 56 (44 – 236) |
66 ± 32* (30 – 157) |
| (old) | 103 ± 42* (49 – 166) |
92 ± 39* (45 – 162) |
86 ± 33* (46 – 142) |
98 ± 37* (53 – 171) |
154 ± 125 (65 – 531) |
94 ± 32* (51 – 163) |
Values are mean ± sd (5–95th percentile)
Asterisks denote statistical significance (p<0.05) for comparison between the two age groups
Table 3.
Statistical summary of UES and proximal esophageal HREPT measurements for 46 normal subjects; 22 young and 22 elderly
| Liquid | Pudding (3ml) | Cookie | ||||
|---|---|---|---|---|---|---|
| 1 ml | 5 ml | 10 ml | Effortful | |||
| UES-IRP (mmHg) | 1 ± 8 (−11 – 18) |
0 ± 6 (−8 – 13) |
0 ± 7 (−9 – 12) |
3 ± 8 (−9 – 18) |
6 ± 13 (−10 – 31) |
6 ± 8 (−6 – 20) |
| (young) | −4 ± 5* (−12 – 2) |
−3 ± 4* (−9 – 3) |
−3 ± 4* (−9 – 3) |
−1 ± 6* (−9 – 7) |
−3 ± 6* (−18 – 6) |
3 ± 7* (−7 – 14) |
| (old) | 5 ± 8* (−6 – 21) |
4 ± 6* (−3 – 14) |
3 ± 7* (−5 – 20) |
8 ± 8* (−3 – 25) |
15 ± 13* (−1 – 42) |
9 ± 8* (−4 – 24) |
| PD-UESCI (mmHg•s•cm) |
407 ± 185 (149 – 817) |
407 ± 170 (195 – 758) |
390 ± 181 (134 – 747) |
485 ± 206 (196 – 892) |
488 ± 291 (167 – 1075) |
557 ± 293 (142 – 1186) |
| (young) | 425 ± 186 (145 – 863) |
408 ± 170 (180 – 716) |
394 ± 179 (158 – 748) |
476 ± 209 (138 – 899) |
463 ± 346 (130 – 1414) |
559 ± 310 (151 – 1317) |
| (old) | 391 ± 184 (188 – 857) |
405 ± 170 (196 – 778) |
387 ± 184 (76 – 748) |
494 ± 204 (200 – 902) |
512 ± 233 (204 – 1077) |
555 ± 276 (129 – 1060) |
| UES-Max (mmHg) | 201 ± 59 (116 – 298) |
209 ± 60 (132 – 303) |
212 ± 56 (122 – 301) |
219 ± 83 (130 – 306) |
235 ± 67 (148 – 345) |
218 ± 51 (133 – 317) |
| (young) | 205 ± 51 (107 – 295) |
205 ± 46 (118 – 285) |
204 ± 54 (108 – 289) |
206 ± 48* (109 – 274) |
218 ± 47 (146 – 325) |
215 ± 45 (126 – 294) |
| (old) | 198 ± 65 (123 – 310) |
214 ± 72 (141 – 322) |
219 ± 57 (150 – 311) |
232 ± 106* (138 – 342) |
250 ± 79 (147 – 439) |
222 ± 57 (130 – 327) |
| PCI (mmHg•s•cm) | 244 ± 214 (0 – 572) |
266 ± 218 (2 – 648) |
268 ± 244 (0 – 672) |
340 ± 254 (0 – 702) |
402 ± 360 (0 – 1422) |
452 ± 324 (0 – 1231) |
| (young) | 271 ± 150 (64 – 583) |
291 ± 180* (58 – 675) |
291 ± 196 (0 – 599) |
362 ± 214 (28 – 695) |
413 ± 338 (0 – 1212) |
473 ± 308 (0 – 1114) |
| (old) | 220 ± 257 (0 – 884) |
241 ± 250* (0 – 750) |
245 ± 286 (0 – 1106) |
318 ± 290 (0 – 1048) |
391 ± 386 (0 – 1526) |
432 ± 338 (93 – 1332) |
Values are mean ± sd (5–95th percentile)
Asterisks denote statistical significance (p<0.05) for comparison between the two age groups.
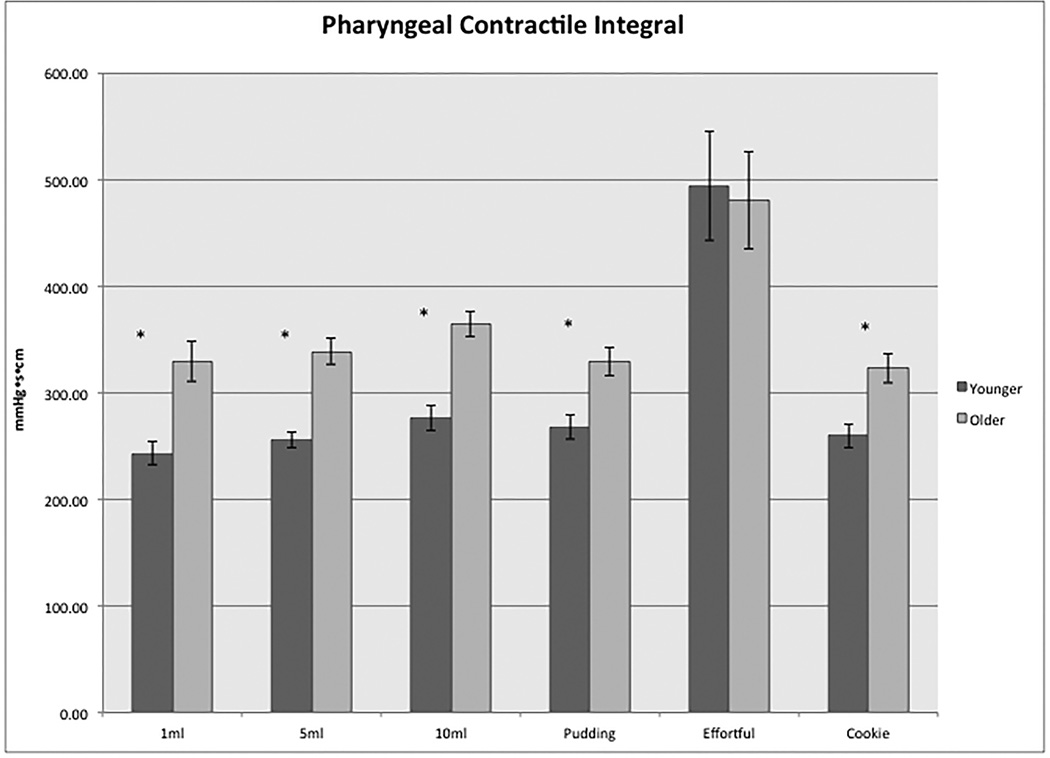
For all of the integrated topography pharyngeal metrics (PhCI, VPCI, TBI, and HPCI), values with effortful swallows were significantly greater than all other swallow types and values for older subjects were significantly greater than for the younger (p<0.05) for all swallow types with the exception of the effortful swallows (Figure 2). Effect size for the age group difference in PhCI was large on all swallow types, except for the effortful swallow (1ml liquid: d=1.0, 5ml liquid: d=1.1, 10ml liquid: d=0.9, pudding: d=0.8, effortful d=0, cookie: d=0.8).
Figure 2.
Differences in pharyngeal contractile integral (PhCI) between the younger and older subjects on each swallow type. Asterisks denote statistical significance (p<0.05) for comparison between the two age groups.
No consistent gender differences were seen. Less consistent differences and exceptions to this generalization among swallow types and between subject groups are noted below where applicable.
Maximal pharyngeal contraction pressure
Average values for the older subjects were significantly greater than for younger subjects for all swallow types except for effortful (p<0.05). The P-Max with effortful swallows was only significantly greater than with 10ml liquid and cookie swallows (p<0.05). Mean P-Max for the male group was significantly greater than for the female group on 5ml liquid, pudding and cookie trials (p<0.05).
Velopharyngeal Closure Integral
Significant differences were found in VPCI for 10ml liquid swallows versus all other trial types (p<0.05), but effortful values were still greatest. Mean values for the male group were significantly greater than the female group on trials of cookie swallows (p<0.05), while 10ml and effortful swallows were significantly greater for women versus male (p<0.05).
Tongue Base/Pharyngeal Wall Integral
Average values for the older subjects were significantly greater than for younger subjects for all swallow types except for cookie and effortful (p<0.05). The only significant gender difference observed was of greater values of effortful swallow in the female group compared to male (p<0.05).
Normative Values of HRPEPT UES and Proximal Esophageal Metrics
Mean, standard deviation and the observed ranges for each UES and proximal esophageal pressure topography metric are displayed in Table 3.
Upper Esophageal Sphincter Integrated Relaxation Pressure
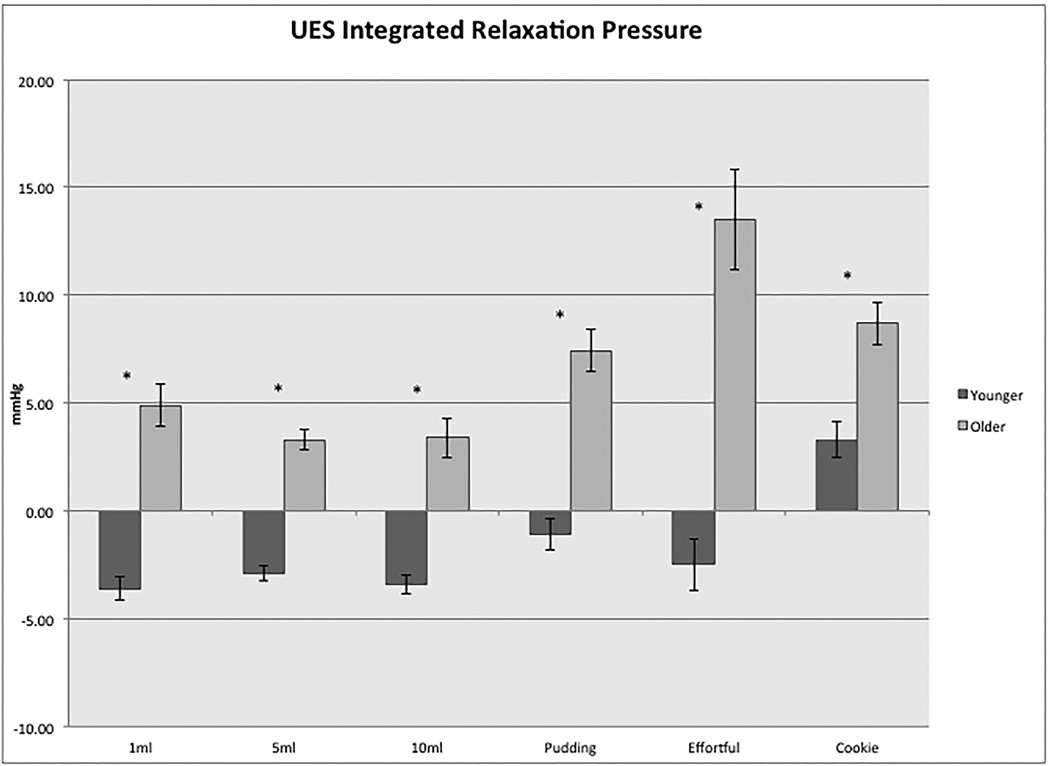
The UES-IRP was significantly greater with pudding, cookie and effortful swallows compared to all volumes of liquid swallow (p<0.05) and cookie swallows had significantly greater UES-IRP values than pudding swallows (p<0.05). Average UES-IRP for the older subjects were significantly greater (p<0.05) than for the young for all bolus trials (see Figure 3), with a large effect size for all (1ml liquid: d=1.4, 5ml liquid: d=1.3, 10ml liquid: d=1.2, pudding: d=1.3, effortful d=1.8, cookie: d=0.8). The three subjects with observed CP bar showed greater than average values of UES-IRP on most swallow types compared to the total group, and on some swallow types compared to the older group. All three differed more than one standard deviation from total group on pudding and cookie swallows.
Figure 3.
Differences in upper esophageal sphincter integrated relaxation pressure (UES-IRP) between the younger and older age groups with each swallow type. Asterisks denote statistical significance (p<0.05) for comparison between the two age groups.
Mean UES-IRP for the female group was significantly greater than the male group with cookie swallows (p<0.05) and values for male were greater than female for 5ml and 10ml swallows.
Post-deglutitive Upper Esophageal Sphincter Contractile Integra
The PD-UESCI was significantly greater for pudding and cookie swallows compared to all volumes of liquid swallow (p<0.05). Effortful swallows were significantly greater than 10ml liquid swallows (p<0.05). No significant differences were found between the age groups on this metric. Mean PD-UESCI for males was greater than for females with all swallow types, but reached significance only on 5ml and effortful swallows (p<0.05).
Maximal Post-Deglutitive Upper Esophageal Sphincter Pressure
Average UES-Max for effortful swallows was significantly greater than 1ml swallows (p<0.05). No significant difference was found for any other swallow types. No significant differences were found between the age groups in UES-Max, except for pudding swallows, in which older subjects had a slightly, yet statistically significantly, greater mean value. No gender differences were observed.
Proximal Esophageal Contraction
Pudding swallow PCI values were greater than liquid swallows, but significantly greater only compared to 1ml and 10ml swallows (p<0.05). Cookie swallows were significantly greater than all other swallow types except for the effortful swallow (p<0.05). Effortful swallows had significantly greater PCI values than did all liquid swallows. Although PCI values were greater for the younger subjects on all swallows types, this reached significance only with 5ml swallows. PCI values were larger in men compared to women on all bolus types, reaching significance for all bolus types except 1ml liquid, which approximated significance (p=0.54). The effect size for this gender difference ranged from a low to medium for the different trial types (1ml liquid: d=0.3, 5ml liquid: d=0.3, 10ml liquid: d=0.4, pudding: d=0.3, effortful d=0.6, cookie: d=0.4). The gender differences for proximal esophageal contractions are likely driven mostly by gender differences in the older group, in which the average PCI in older male were substantially greater than female (359 mmHg•s•cm. versus 212 mmHg•s•cm.) and less by the younger group, in which gender differences were small (354mmHg•s•cm. in male versus 310mmHg•s•cm. in female). PCI values of asymptomatic subjects in this study showed high variability, particularly in the older subject group (Coefficient of variance in total of groups=85%). Absence of proximal esophageal contraction was noted on some swallows in 12 (26%) of subjects (3 younger, 9 older).
UES Location and Movement During the Swallow
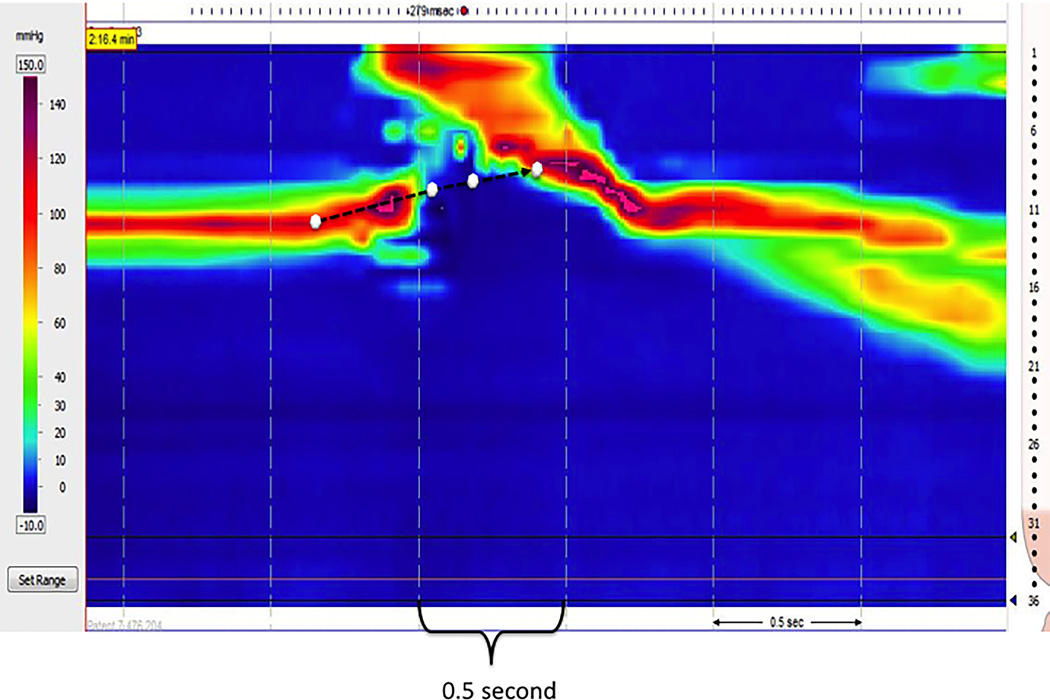
On average, the UES centered 13.8 mm distal to the underside of the vocal cords as derived from 1ml swallows of all participants. The mean excursion of the UES relative to the HRM catheter at key temporal transitions during the swallow are summarized in Table 4. Figure 4 illustrates an example of UES movement on the topography plot of a typical subject.
Table 4.
Elevation of the UES relative to the HRM catheter at key temporal landmarks of the swallow (mean, SD, 41 studies). At baseline the UES was centered an average of 13.8 mm distal to the under side of the vocal folds as derived from fluoroscopy. Because the UES elevates to a greater degree than the HMR catheter, it is centered above its baseline position to varying degrees throughout the swallow. The duration of UES opening was 0.49 ± 0.30 s.
| Time: | UES opening | Maximal laryngeal elevation |
UES closure |
|---|---|---|---|
| UES to HRM catheter relative elevation (sensors) |
2.0 (0.6) | 2.5 (0.5) | 2.3 (0.6) |
| UES to HRM catheter relative elevation (mm) |
14.3 (4.5) | 18.6 (3.8) | 17.0 (4.5) |
Figure 4.
Example of UES movement drawn on pressure topography, derived from combined topography and videofluoroscopy data. The four white circles indicate the location of the midpoint of UES at key moments in the swallow: baseline, UES opening, maximum laryngeal elevation, and UES closure.
Large vs Small Diameter Catheter
Results of theMANOVAshowed statistically significant greater values of UES-IRP, PD-UESCI, UES-Max, and PCI in studies using a 4.2mm diameter catheter compared to the current study, using a 2.75mm diameter catheter. Effect sizes for these differences ranged from low to high (UES-IRP: d=0.5, PD-UESCI: d=0.9 UES-Max; d=0.6, and PCI: d=0.3)
Discussion
This study aimed to develop novel pressure topography metrics for clinical high-resolution pharyngeal-esophageal manofluorography. To our knowledge, this is the first collection of normative data from different age groups using high-resolution manometry concurrently with videofluorography.
Similar to previous research studies conducted in healthy adults, we observed occasional penetration of thin liquids into the airway and two instances of trace aspiration (28–31). Non-obstructing cervical osteophytes and cricopharyngeal (CP) bars were noted in three subjects each. Earlier research studies have reported that a CP bar is a radiographic finding that can be observed in some asymptomatic, non-dysphagic individuals (32–34).
Pressure topographic regions of interest pertinent to the oropharyngeal swallow were based on meticulous correspondence of pressure data with structures and swallow events visualized on videofluoroscopy. The devised pharyngeal metrics provide comprehensive measures quantifying the contractile vigor and stability of different components of the pharyngeal response modeled after analyses of esophageal pressure topography now codified in the Chicago Classification (18). Although we report on normative values for 9 metrics, one of these, the PhCI, would likely suffice as a summary measure of pharyngeal contractility should it be in the normal range with the more discrete measures (Ph-Max, VPCI, BTI, HPCI) adding resolution in situations of impairment. Identifying impairment in specific pharyngeal components (e.g. reduced tongue base retraction versus inadequate velopharyngeal closure) can guide the selection of rehabilitation strategies in therapy.
In this study, significantly greater values for indices of pharyngeal contractility were observed for the older group in all conditions, apart from the effortful swallow. The elimination of this difference in the effortful swallows suggests that with the natural (non-effortful) swallow older individuals utilize more pressure than do the younger adults, albeit within a similar range of capacity. This increase in pharyngeal contractile vigor may be a compensatory response for other age-related changes in the pharyngeal swallow previously observed (23,35,36). The greater transsphincteric intrabolus UES pressures observed in the elderly likely reflects decreased compliance of the cricopharyngeus muscle with age. Previous studies using conventional manometry systems have reported similar patterns of change in pharyngeal contractile pressures (15,17),, while others have reported different outcomes (23,37). These differences are likely related to methodological differences. Findings of this study provide further support for previous studies reporting increases in UES pressure measurements with age, when examined with conventional manometry systems (13,14,16,18,38). Age-related differences found in this study should be considered when applying these data to clinical populations. Furthermore, it will be important to collect normative HRPEPT from adults above the age of 80, since greater changes in swallow function may occur in later life.
With respect to UES function, we developed the 0.25 s UES-IRP, an adaptation of the esophagogastric junction IRP developed for esophageal HRM studies (18). The UES-IRP is reflective of deglutitive intra-sphincteric intrabolus pressure, indicative of both the completeness of UES relaxation and sphincter compliance. The 0.25 s period was chosen to be well within the normal interval of deglutitive UES relaxation so as not to be confounded by pre or post-swallow UES contraction. This current data compliment data reported by Weijenbrg et al. (39) on a 0.2s UES IRP measured from a group of young healthy adults in supine position. Additionally, this study filled a gap in the literature by devising and extracting measures of UES post-deglutitive response and proximal esophageal contraction. The role of the UES post-deglutitive swallow contraction in insuring safe passageway of the bolus from pharynx to the esophagus was highlighted by Pouderoux et al (40). Their study demonstrated the mechanism (termed the grabbing effect) by which the post-deglutive UES contraction traps swallowed content, pulling it down with laryngeal descent in order to prevent regurgitation and potential aspiration. Despite its contribution to the safety of the swallow, this factor isn’t often considered in dysphagia evaluation. Likewise, HRM data on the proximal esophageal contraction was also lacking. Although the clinical importance of PCI is incompletely defined, abnormalities of the proximal esophageal contraction have been noted to occur with specific pathologies (41–43). Future comparison of the normative PCI values reported in this study with those of patient populations may add clarity.
A few additional findings stemming from this study may warrant consideration when utilizing HRPEPT measures. Studies using the larger catheter diameter resulted in significantly greater values of UES and proximal esophagus measures, though the magnitude of this difference was low-moderate in UES-IRP and PCI. This finding should be confirmed in the future by a within-subject comparison study. Greater proximal esophageal contraction was noted for males compared to females. The measures of UES-IRP, UES-PDCI and PCI showed an effect of viscosity, yet no consistent effect of liquid volume was seen for any of the measures. The effects of the effortful swallows were evident on all integral measures of the pharynx and appeared to extend, to some degree, to the proximal esophagus. This finding suggests that the effortful swallow could be clinically useful in volitionally influencing proximal esophageal contraction. In the older group, the effortful swallow resulted in a significant rise in UES intrabolus pressure, an effect that was not observed in the younger group. This observation raises the question of whether the clinical use of the effortful swallow in elders should be modified, since increased resistance to flow at the UES may mitigate the added benefit of an augmented pharyngeal contraction to drive the bolus.
In conclusion, the clinical utility of manofluorography has been demonstrated with both conventional and high-resolution pharyngeal manometry and has been used for investigating outcomes of medical treatment, assessing aspiration risk and categorizing impairments in order to guide treatment (8,12,44–50). It is hoped that HRPEPT will detect incremental changes in UES compliance and pharyngeal contractility at early stages of disorders, before these changes are evident on videofluorography. In light of the potential diagnostic advantage of HRPEPT, the data presented in this study provide an essential step in advancing its clinical application. The novel pressure topography metrics used in this study and the corresponding age-specific normative values should provide a useful foundation for future HRPEPT studies of patients with oropharyngeal dysphagia.
Supplementary Material
Figure 1.
Pharyngeal Contractile Integral (PhCI), Velopharyngeal Closure Integral (VPCI), Tongue base/pharyngeal wall Integral (TBI), Hypopharyngeal Contractile Integral (HPCI), Upper esophageal sphincter integrated relaxation pressure (UES-IRP) and post-deglutitive UES contractile integral (PD-UESCI) diagrammed on pressure topography in Manoview™ software.
Key Messages.
This study aimed to define normative values for novel pressure topography metrics of high-resolution pharyngeal-esophageal manometry with videofluoroscopy and examined the effects of age, gender and bolus properties on these measures.
Older individuals exhibited more vigorous contractility in the pharynx than did younger subjects in all but the effortful swallows, perhaps reflecting a compensatory response to other age-related physiological changes
Upper esophageal sphincter (UES) intrabolus pressure during sphincter opening was also greater in the older subjects, possibly indicating a decrease in compliance of the cricopharyngeal muscle with age.
Effects of gender, bolus consistency and catheter diameter were observed on some of the measures
Pressure topography metrics and the corresponding age-specific normative values should provide a useful foundation for clinical evaluation of patients with oropharyngeal dysphagia
Acknowledgments
The authors thank Kristin Larsen, Sharon Veis, Cory Atkinson, and Megan Schliep for assistance in conducting videofluoroscopic swallow study studies; Boris Lubomyr, Meghan Thompson and Chen-Yuan Lin for assistance with manometry study procedures; Muveddet Harris for technical support.
Funding
Peter J Kahrilas was supported by public health service, grant # R01 DK56033 (PJK).
Nogah Nativ-Zeltzer was supported by the Northwestern University Graduate Research Grant and the Wendy R. Schall Research Fund Grant.
Footnotes
Conflict of Interest
No competing interests declared
Author Contribution
NNZ was involved in concept development, design, data collection, data analysis and writing of the manuscript. JAL was involved in concept development, design, and supervision of data collection. SGZ was involved in statistical analysis and writing of the manuscript. PJK was involved in concept development, design, supervision of data collection and writing of the manuscript.
References
- 1.Kahrilas PJ. Esophageal motor disorders in terms of high-resolution esophageal pressure topography: what has changed? Am J Gastroenterol. 2010 May;105(5):981–987. doi: 10.1038/ajg.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJPM, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2015 Feb;27(2):160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandolfino JE, Fox MR, Bredenoord AJ, Kahrilas PJ. High-resolution manometry in clinical practice: utilizing pressure topography to classify oesophageal motility abnormalities. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2009 Aug;21(8):796–806. doi: 10.1111/j.1365-2982.2009.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandolfino JE, Kahrilas PJ. New technologies in the gastrointestinal clinic and research: impedance and high-resolution manometry. World J Gastroenterol WJG. 2009 Jan 14;15(2):131–138. doi: 10.3748/wjg.15.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Kahrilas PJ. Deglutitive upper esophageal sphincter relaxation: a study of 75 volunteer subjects using solid-state high-resolution manometry. Am J Physiol Gastrointest Liver Physiol. 2006 Sep;291(3):G525–G531. doi: 10.1152/ajpgi.00081.2006. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman MR, Ciucci MR, Mielens JD, Jiang JJ, McCulloch TM. Pharyngeal swallow adaptations to bolus volume measured with high-resolution manometry. The Laryngoscope. 2010 Dec;120(12):2367–2373. doi: 10.1002/lary.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mielens JD, Hoffman MR, Ciucci MR, Jiang JJ, McCulloch TM. Automated analysis of pharyngeal pressure data obtained with high-resolution manometry. Dysphagia. 2011 Mar;26(1):3–12. doi: 10.1007/s00455-010-9320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omari TI, Dejaeger E, Van Beckevoort D, Goeleven A, De Cock P, Hoffman I, et al. A Novel Method for the Nonradiological Assessment of Ineffective Swallowing. [cited 2011 Jul 10];Am J Gastroenterol [Internet] 2011 May 10; doi: 10.1038/ajg.2011.143. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21556039. [DOI] [PubMed]
- 9.Pal A, Williams RB, Cook IJ, Brasseur JG. Intrabolus pressure gradient identifies pathological constriction in the upper esophageal sphincter during flow. Am J Physiol Gastrointest Liver Physiol. 2003 Nov;285(5):G1037–G1048. doi: 10.1152/ajpgi.00030.2003. [DOI] [PubMed] [Google Scholar]
- 10.Rommel N, Omari T. Abnormal pharyngoesophageal function in infants and young children: diagnosis with high-resolution manometry. J Pediatr Gastroenterol Nutr. 2011 May;52(Suppl 1):S29–S30. doi: 10.1097/MPG.0b013e318213a4b8. [DOI] [PubMed] [Google Scholar]
- 11.Williams RBH, Wallace KL, Ali GN, Cook IJ. Biomechanics of failed deglutitive upper esophageal sphincter relaxation in neurogenic dysphagia. Am J Physiol Gastrointest Liver Physiol. 2002 Jul;283(1):G16–G26. doi: 10.1152/ajpgi.00189.2001. [DOI] [PubMed] [Google Scholar]
- 12.Lan Y, Xu G, Dou Z, Wan G, Yu F, Lin T. Biomechanical changes in the pharynx and upper esophageal sphincter after modified balloon dilatation in brainstem stroke patients with dysphagia. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2013 Dec;25(12):e821–e829. doi: 10.1111/nmo.12209. [DOI] [PubMed] [Google Scholar]
- 13.Van Herwaarden MA, Katz PO, Gideon RM, Barrett J, Castell JA, Achem S, et al. Are manometric parameters of the upper esophageal sphincter and pharynx affected by age and gender? Dysphagia. 2003;18(3):211–217. doi: 10.1007/s00455-002-0099-7. [DOI] [PubMed] [Google Scholar]
- 14.Shaw DW, Cook IJ, Gabb M, Holloway RH, Simula ME, Panagopoulos V, et al. Influence of normal aging on oral-pharyngeal and upper esophageal sphincter function during swallowing. Am J Physiol. 1995 Mar;268(3 Pt 1):G389–G396. doi: 10.1152/ajpgi.1995.268.3.G389. [DOI] [PubMed] [Google Scholar]
- 15.Wilson JA, Pryde A, Macintyre CC, Maran AG, Heading RC. The effects of age, sex, and smoking on normal pharyngoesophageal motility. Am J Gastroenterol. 1990 Jun;85(6):686–691. [PubMed] [Google Scholar]
- 16.Butler SG, Stuart A, Russell GB, Koch K, Kemp S. Effects of age, gender, bolus condition, viscosity, and volume on pharyngeal and upper esophageal sphincter pressure and temporal measurements during swallowing. J Speech Lang Hear Res JSLHR. 2009 Feb;52(1):240–253. doi: 10.1044/1092-4388(2008/07-0092). [DOI] [PubMed] [Google Scholar]
- 17.Perlman AL, Schultz JG, VanDaele DJ. Effects of age, gender, bolus volume, and bolus viscosity on oropharyngeal pressure during swallowing. J Appl Physiol Bethesda Md 1985. 1993 Jul;75(1):33–37. doi: 10.1152/jappl.1993.75.1.33. [DOI] [PubMed] [Google Scholar]
- 18.Shaker R, Ren J, Podvrsan B, Dodds WJ, Hogan WJ, Kern M, et al. Effect of aging and bolus variables on pharyngeal and upper esophageal sphincter motor function. Am J Physiol. 1993 Mar;264(3 Pt 1):G427–G432. doi: 10.1152/ajpgi.1993.264.3.G427. [DOI] [PubMed] [Google Scholar]
- 19.Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol A Biol Sci Med Sci. 1995 Sep;50(5):M257–M262. doi: 10.1093/gerona/50a.5.m257. [DOI] [PubMed] [Google Scholar]
- 20.Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J Speech Lang Hear Res JSLHR. 2000 Oct;43(5):1264–1274. doi: 10.1044/jslhr.4305.1264. [DOI] [PubMed] [Google Scholar]
- 21.Cook IJ, Weltman MD, Wallace K, Shaw DW, McKay E, Smart RC, et al. Influence of aging on oral-pharyngeal bolus transit and clearance during swallowing: scintigraphic study. Am J Physiol - Gastrointest Liver Physiol. 1994 Jun 1;266(6):G972–G977. doi: 10.1152/ajpgi.1994.266.6.G972. [DOI] [PubMed] [Google Scholar]
- 22.Logemann JA, Pauloski BR, Rademaker AW, Kahrilas PJ. Oropharyngeal swallow in younger and older women: videofluoroscopic analysis. J Speech Lang Hear Res JSLHR. 2002 Jun;45(3):434–445. doi: 10.1044/1092-4388(2002/034). [DOI] [PubMed] [Google Scholar]
- 23.Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992 Sep;103(3):823–829. doi: 10.1016/0016-5085(92)90013-o. [DOI] [PubMed] [Google Scholar]
- 24.Sonies BC, Parent LJ, Morrish K, Baum BJ. Durational aspects of the oral-pharyngeal phase of swallow in normal adults. Dysphagia. 1988;3(1):1–10. doi: 10.1007/BF02406274. [DOI] [PubMed] [Google Scholar]
- 25.Logemann JA, Pauloski BR, Rademaker AW, Lazarus CL, Mittal B, Gaziano J, et al. Xerostomia: 12-month changes in saliva production and its relationship to perception and performance of swallow function, oral intake, and diet after chemoradiation. Head Neck. 2003 Jun;25(6):432–437. doi: 10.1002/hed.10255. [DOI] [PubMed] [Google Scholar]
- 26.Rogus-Pulia NM, Pierce MC, Mittal BB, Zecker SG, Logemann JA. Changes in swallowing physiology and patient perception of swallowing function following chemoradiation for head and neck cancer. Dysphagia. 2014 Apr;29(2):223–233. doi: 10.1007/s00455-013-9500-y. [DOI] [PubMed] [Google Scholar]
- 27.Rogus-Pulia NM, Pierce M, Mittal BB, Zecker SG, Logemann J. Bolus effects on patient awareness of swallowing difficulty and swallow physiology following chemoradiation for head and neck cancer. Head Neck. 2014 May 19; doi: 10.1002/hed.23720. [DOI] [PubMed] [Google Scholar]
- 28.Allen JE, White CJ, Leonard RJ, Belafsky PC. Prevalence of penetration and aspiration on videofluoroscopy in normal individuals without dysphagia. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2010 Feb;142(2):208–213. doi: 10.1016/j.otohns.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Daggett A, Logemann J, Rademaker A, Pauloski B. Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia. 2006 Oct;21(4):270–274. doi: 10.1007/s00455-006-9051-6. [DOI] [PubMed] [Google Scholar]
- 30.Butler SG, Stuart A, Kemp S. Flexible endoscopic evaluation of swallowing in healthy young and older adults. Ann Otol Rhinol Laryngol. 2009 Feb;118(2):99–106. doi: 10.1177/000348940911800204. [DOI] [PubMed] [Google Scholar]
- 31.Butler SG, Stuart A, Markley L, Rees C. Penetration and aspiration in healthy older adults as assessed during endoscopic evaluation of swallowing. Ann Otol Rhinol Laryngol. 2009 Mar;118(3):190–198. doi: 10.1177/000348940911800306. [DOI] [PubMed] [Google Scholar]
- 32.Ekberg O, Nylander G. Cineradiography of the pharyngeal stage of deglutition in 150 individuals without dysphagia. Br J Radiol. 1982 Apr;55(652):253–257. doi: 10.1259/0007-1285-55-652-253. [DOI] [PubMed] [Google Scholar]
- 33.Leaper M, Zhang M, Dawes PJD. An anatomical protrusion exists on the posterior hypopharyngeal wall in some elderly cadavers. Dysphagia. 2005;20(1):8–14. doi: 10.1007/s00455-004-0018-1. [DOI] [PubMed] [Google Scholar]
- 34.Leonard R, Kendall K, McKenzie S. UES opening and cricopharyngeal bar in nondysphagic elderly and nonelderly adults. Dysphagia. 2004;19(3):182–191. doi: 10.1007/s00455-004-0005-6. [DOI] [PubMed] [Google Scholar]
- 35.Humbert IA, Robbins J. Dysphagia in the Elderly. Phys Med Rehabil Clin N Am. 2008 Nov;19(4):853 – x. doi: 10.1016/j.pmr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sura L, Madhavan A, Carnaby G, Crary MA. Dysphagia in the elderly: management and nutritional considerations. Clin Interv Aging. 2012;7:287–298. doi: 10.2147/CIA.S23404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tracy JF, Logemann JA, Kahrilas PJ, Jacob P, Kobara M, Krugler C. Preliminary observations on the effects of age on oropharyngeal deglutition. Dysphagia. 1989;4(2):90–94. doi: 10.1007/BF02407151. [DOI] [PubMed] [Google Scholar]
- 38.Yokoyama M, Mitomi N, Tetsuka K, Tayama N, Niimi S. Role of laryngeal movement and effect of aging on swallowing pressure in the pharynx and upper esophageal sphincter. The Laryngoscope. 2000 Mar;110(3 Pt 1):434–439. doi: 10.1097/00005537-200003000-00021. [DOI] [PubMed] [Google Scholar]
- 39.Weijenborg PW, Kessing BF, Smout AJPM, Bredenoord AJ. Normal values for solid-state esophageal high-resolution manometry in a European population; an overview of all current metrics. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2014 May;26(5):654–659. doi: 10.1111/nmo.12314. [DOI] [PubMed] [Google Scholar]
- 40.Pouderoux P, Kahrilas PJ. Function of upper esophageal sphincter during swallowing: the grabbing effect. Am J Physiol. 1997 May;272(5 Pt 1):G1057–G1063. doi: 10.1152/ajpgi.1997.272.5.G1057. [DOI] [PubMed] [Google Scholar]
- 41.Dantas RO, Alves LMT, Nascimento WV. Effect of bolus volume on proximal esophageal contractions of patients with Chagas’ disease and patients with idiopathic achalasia. Dis Esophagus Off J Int Soc Dis Esophagus ISDE. 2010 Nov;23(8):670–674. doi: 10.1111/j.1442-2050.2010.01066.x. [DOI] [PubMed] [Google Scholar]
- 42.Huang MH, King KL, Chien KY. Esophageal manometric studies in patients with myasthenia gravis. J Thorac Cardiovasc Surg. 1988 Feb;95(2):281–285. [PubMed] [Google Scholar]
- 43.Paterson WG, Goyal RK, Habib FI. Esophageal motility disorders. [cited 2015 Mar 21];GI Motil Online [Internet] 2006 Available from: http://www.nature.com/gimo/contents/pt1/full/gimo20.html. [Google Scholar]
- 44.Bammer T, Salassa JR, Klingler PJ. Comparison of methods for determining cricopharyngeal intrabolus pressure in normal patients as possible indicator for cricopharyngeal myotomy. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2002 Oct;127(4):299–308. doi: 10.1067/mhn.2002.128554. [DOI] [PubMed] [Google Scholar]
- 45.Hammer MJ, Jones CA, Jones CA, Mielens JD, Kim CH, McCulloch TM. Evaluating the tongue-hold maneuver using high-resolution manometry and electromyography. Dysphagia. 2014 Oct;29(5):564–570. doi: 10.1007/s00455-014-9545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higo R, Nakahira M, Sugasawa M, Nakatsuka T. Manometric assessment of pharyngeal swallowing pressure after mandibular reconstruction. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2011 Jun;268(6):941–944. doi: 10.1007/s00405-011-1559-1. [DOI] [PubMed] [Google Scholar]
- 47.Hoffman MR, Jones CA, Geng Z, Abelhalim SM, Walczak CC, Mitchell AR, et al. Classification of high-resolution manometry data according to videofluoroscopic parameters using pattern recognition. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2013 Jul;149(1):126–133. doi: 10.1177/0194599813489506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knigge MA, Thibeault S, McCulloch TM. Implementation of High-resolution Manometry in the Clinical Practice of Speech Language Pathology. Dysphagia. 2014 Feb 1;29(1):2–16. doi: 10.1007/s00455-013-9494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Omari TI, Dejaeger E, Tack J, Van Beckevoort D, Rommel N. Effect of bolus volume and viscosity on pharyngeal automated impedance manometry variables derived for broad Dysphagia patients. Dysphagia. 2013 Jun;28(2):146–152. doi: 10.1007/s00455-012-9423-z. [DOI] [PubMed] [Google Scholar]
- 50.O’Rourke A, Morgan LB, Coss-Adame E, Morrison M, Weinberger P, Postma G. The effect of voluntary pharyngeal swallowing maneuvers on esophageal swallowing physiology. Dysphagia. 2014 Apr;29(2):262–268. doi: 10.1007/s00455-013-9505-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.