Abstract
Rationale
Quitting smoking is often very challenging, leading to frequent relapse. Exposure to acute and chronic stress during abstinence increases the likelihood of relapse to smoking. In rodents, stress acutely reinstates nicotine seeking after extinction of nicotine self-administration (SA). However, whether reacquisition of nicotine taking is amplified by chronic stress during abstinence from nicotine SA has not been determined in animals.
Objectives
We sought to determine effects of repeated restraint stress during abstinence on reacquisition of nicotine SA.
Methods
Rats acquired nicotine SA (23h/day) under a fixed ratio (FR) 5 schedule of reinforcement, which was followed by an abstinence phase. Restraint (0, 2, and 4 times) was administered during abstinence. Animals reacquired nicotine SA, first under a progressive ratio (PR) schedule, beginning immediately after the final stress, followed by an FR5 schedule. In another experiment, reacquisition (FR5) began 24 h after the final stress. No PR testing was conducted.
Results
Four restraint stress exposures during abstinence, but not only 2, enhanced reacquisition of nicotine SA by increasing nicotine injections under a PR schedule beginning immediately after the final stress (p < 0.05) followed by increasing nicotine intake under an FR5 schedule (p < 0.05). This was observed even when the final stress and reacquisition trial were separated by 24 h. Moreover, repeated stress induced nicotine taking during the behaviorally inactive phase (i.e., lights on) of the 24 h diurnal cycle.
Conclusions
Chronic (i.e. repeated) stress during abstinence promotes reacquisition of nicotine SA and affects diurnal pattern of nicotine intake.
Keywords: abstinence, nicotine, reacquisition, self-administration, stress
Introduction
Cigarette smoking is a prevalent substance of abuse throughout the world. Smoking remains the leading cause of preventable morbidity and mortality in the USA, resulting in one hundred billion dollars of lost productivity annually (Centers for Disease Control and Prevention 2008). Smoking cessation can significantly reduce the risk of smoking-associated diseases, benefiting health and longevity at all ages (Samet 1990; Taylor et al. 2002). More than 50 percent of all smokers in the United States tried to quit smoking from 2001 to 2010 (Centers for Disease Control and Prevention 2011). However, only 3–5% of those who attempt to quit are successful for 6 to 12 months, while most attempts to quit fail within the first 8 days (Hughes et al. 2004). So smoking cessation is usually very challenging, most frequently ending in relapse. Improved treatment strategies that reduce the probability of relapse require a more thorough understanding of relapse behavior and the factors that impact it.
Many human studies have shown that exposure to acute and chronic stress during abstinence increases the likelihood of relapse to smoking (Cohen and Lichtenstein 1990; Falba et al. 2005; Matheny and Weatherman 1998; Perkins and Grobe 1992; Siahpush and Carlin 2006). Nicotine, the principal psychoactive agent in tobacco, is a major cause of tobacco dependence (D'Souza and Markou 2011). In both rats and mice, a relatively brief episode of acute intermittent footshock stress has been shown to reinstate nicotine seeking after extinction of cue-associated nicotine self-administration (SA). Yet animal studies that model the effects of chronic stress on relapse to smoking have not been reported.
Although the procedure for reinstatement of drug-seeking that is used in laboratory animals has similarities to relapse in humans, there are also important differences (Shaham et al. 2003). Animals undergo extinction learning (non-reinforced responding on the drug-associated lever). Whereas humans rarely undergo extinction while quitting smoking or other drugs. Instead, humans always undergo abstinence. Furthermore, during reinstatement trials, lever presses are not reinforced by drug, whereas, relapse is usually intimately associated with drug taking in humans. Although animal models of reacquisition of drug taking (e.g. cocaine, alcohol) after abstinence from drug SA have been developed (Reichel and Bevins 2009), very few animal studies have modeled reacquisition of nicotine SA after abstinence (George et al. 2007; Manhaes et al. 2008; O'Dell and Koob 2007). Furthermore, the effects of chronic stress during abstinence on reacquisition of nicotine SA have not been investigated. Therefore, we determined the effects of repeated restraint stress during abstinence on reacquisition of nicotine SA in rats with extended access (23h/day) to nicotine (Valentine et al. 1997).
Materials and Methods
Animals
Adult male Sprague-Dawley rats (300–350 g, Harlan, Madison, WI) were used for this study. The animals were housed in a reversed 12:12 h light/dark cycle (lights off at 10:00 AM). Standard rat chow and water were provided ad libitum throughout all experiments. All procedures conformed to NIH guidelines concerning the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee at The University of Tennessee Health Science Center.
Nicotine self-administration
Rats were given extended access to nicotine SA (23 h/day, 7 days/week) without food deprivation and prior training. The nicotine SA procedure was conducted as previously described (Valentine et al. 1997). Briefly, rats were implanted with jugular catheter, and were placed into the operant conditioning chambers after 3 days of post-surgery recovery. The operant conditioning chamber contained two horizontal levers, and a green cue light above each lever illuminated when nicotine was available during SA sessions. Lever presses were recorded and syringe pumps were controlled by computers and interfaces, using L2T2 software (Coulbourn Instruments, Allentown, PA). Nicotine SA started immediately following the initiation of the dark cycle. Pressing the active lever, randomly designated, elicited an injection of nicotine solution (0.03 mg/kg , per 50 μl/0.81 s, free base, pH 7.2–7.4) through the jugular vein. To avoid over-dosing from nicotine, each injection was followed by a 7s timeout period during which the green cue light above the lever was extinguished and nicotine was unavailable. Pressing the alternate (inactive) lever had no programmed consequence. The final hour of the 12 h lights-on cycle (i.e., 9:00–10:00 AM) was reserved for animal husbandry, nicotine solution refreshing, and data recording. The patency of jugular catheters was checked periodically and assessed by methohexital sodium injection (0.2 ml; JHP pharmaceuticals, Rochester, MI). Rats with occluded catheters were excluded from the experiment.
Experimental design
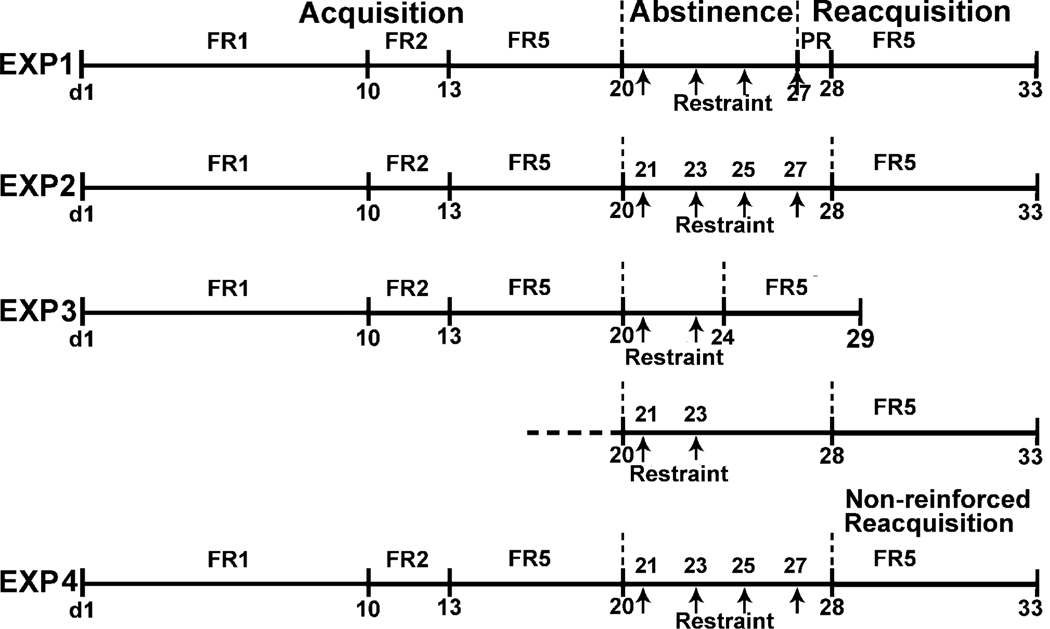
A schematic illustrating the overall design of experiments in this study is shown in Fig. 1.
Fig. 1.
Schematic illustrating time course of experiments in this study. Rats acquired nicotine SA (0.03 mg/kg, 23h/day) under an escalating fixed-ratio (FR) schedule of reinforcement from FR1 to FR5 for at least 20 days. Then, rats abstained from nicotine for 7 days during which, on alternating days, the restraint (30 min) was applied to rats in the stress group, while not in the non-stress group. Thereafter, in experiment 1 (EXP 1), reacquisition of nicotine SA was first elevated under a progressive ratio (PR) schedule during a 23h session that began immediately post-final stress (i.e. 4th session); this was followed by an FR5 schedule for 5 days. In EXP 2, the reacquisition sessions (FR5) began 24 h after the final stress (i.e. 4th session). No PR session was conducted. In EXP 3, only 2 sessions of restraint were administered during abstinence. Reacquisition was assessed beginning 24 h or 5 days after the second stress. In EXP 4, the time course was as same as in experiment 2, but nicotine reinforcement was not available during reacquisition.
Experiment 1: Effects of repeated stress during abstinence on reacquisition of nicotine SA begun immediately after the final stress
(1) Acquisition
During the initial 10 days, rats acquired nicotine SA under an FR1 schedule of reinforcement (1 active lever press yields 1 nicotine injection); then rats acquired nicotine SA under an FR2 schedule for 3 days followed by an FR5 schedule for 7 days, until the following was achieved: during the last 3 days, average injections/23 h > 20 and a ratio of active/inactive lever presses >2. Rats that did not meet these criteria were excluded from the data analysis.
(2) Abstinence
After acquisition of nicotine SA, animals were withdrawn from nicotine in their home cages (not operant conditioning chambers) for 7 days (without nicotine, levers, and cue-lights). This interval was selected since the majority of attempts to quit smoking fail within the first 8 days (Hughes et al. 2004) and most withdrawal symptoms, in humans and rats, peak and subside within the first week (Hughes 2007; Malin et al. 1992).
(3) Restraint stress procedure
Rats were randomly assigned to treatment groups (i.e., stress and non-stress). The restraint was imposed in a 6.5 cm diameter plastic cylinder for 30 min between 9:00–10:00 AM on day 1, 3, 5, and 7 after initiation of abstinence. Rats in the non-stress group, were not restrained, but were otherwise handled comparably to the stress group.
(4) Reacquisition
Immediately after the final stress or non-stress, animals were returned to operant conditioning chambers and tested for reacquisition of nicotine SA. n = 5 for the non-stress and n = 7 for the stress group; a total of 6 rats in both groups were excluded because jugular catheters occluded during acquisition or abstinence. Firstly, rats reacquired nicotine SA under a PR schedule to measure the effort exerted to obtain nicotine during a single 23 h session. The lever press requirements for successive injections were 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145 etc., as derived from the exponential formula [5 exp (injection number × 0.2) – 5] (Arnold and Roberts 1997; Richardson and Roberts 1996). Breakpoints were defined as the final ratio completed during a 23 h session (Doyle et al. 2012). After PR testing, nicotine SA continued under an FR5 schedule for a total of 5 days, each consisting of 23 h sessions.
Experiment 2: Effects of repeated stress during abstinence on reacquisition of nicotine SA begun 24 h after the final stress
In a separate experiment, the procedures for the acquisition of nicotine SA and repeated stress during abstinence were the same as described above. However, reacquisition of nicotine SA was evaluated under an FR5 schedule for 5 consecutive days, beginning 24 h after the final stress (i.e., 4th session) or non-stress session. No PR testing was conducted. n = 5 per group (stress vs. non-stress); 5 rats in both groups were excluded from this experiment due to inoperable catheters.
Experiment 3: Effects of limited exposures to stress (i.e. only 2 sessions) during abstinence on reacquisition of nicotine SA
This experiment was conducted to determine whether 4 restraint stress exposures or 2 restraint stress exposures are necessary for enhanced reacquisition behavior. Therefore, 2 sessions of restraint were administered during abstinence (i.e., on day 1 and 3). In separate groups, reacquisition (FR5) was evaluated either 24 h or 5 days after the second restraint stress. n = 5–6 per group; total 9 rats were excluded from this experiment.
Experiment 4: Effects of repeated stress during abstinence on non-reinforced reacquisition
A separate group of animals (n = 5) receiving repeated stress during abstinence were returned to their operant conditioning chambers 24 h after the final stress (i.e. 4th session). Reacquisition behavior was tested under an FR5 schedule identical to that described above (e.g. the light cue is on and extinguished on a 7 s timeout period during SA session), except that active lever presses were not reinforced by nicotine.
Data analysis and statistics
Both active lever presses and nicotine injections, during the final 3 days of acquisition of nicotine SA and during the 5 days of reacquisition of nicotine SA under an FR5 schedule, were expressed as a percentage of the baseline, defined as the average number of presses or injections during the final 3 days of acquisition. The behavior data of reacquisition were analyzed by Repeated measures ANOVA, using SPSS 10.0 software (Chicago, IL), with the day of reacquisition designated as a within-subjects factor and the stress exposure designated as a between-subjects factor. Student t-test was used for comparisons between different time intervals within the same treatment group, and between stress and non-stress groups at the same time interval. Data were expressed as mean ± SEM. Statistical significance was assigned at p < 0.05.
Results
Experiment 1: Effects of repeated stress during abstinence on reacquisition of nicotine SA begun immediately after the final stress
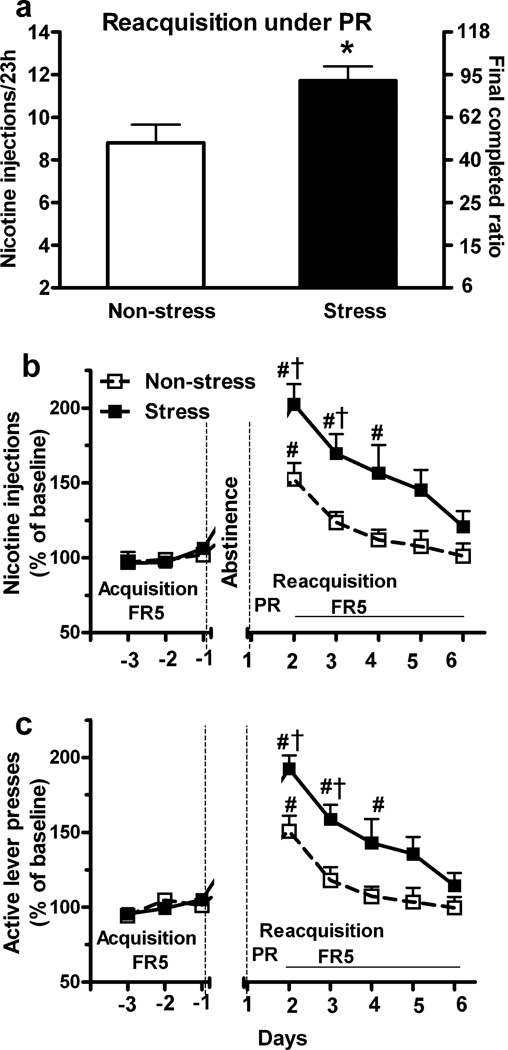
In this experiment, during the final 3 days of nicotine SA acquisition under FR5 schedule, the mean number of active lever presses/23 h elicited by rats randomly assigned to the non-stress and stress groups was similar (187 ± 21.6 vs. 207 ± 16.8; p > 0.05; t-test), as were inactive lever presses (34.4 ± 6.7 vs. 31.1 ± 2.1, p > 0.05) and nicotine injections (28 ± 2.6 vs. 31.5 ± 2.3; p > 0.05). Reacquisition of nicotine SA under a PR schedule began immediately after the final stress or non-stress. Compared to the non-stress group, repeated restraint stress significantly increased the number of nicotine injections (Fig. 2a; 12.0 ± 0.7 vs. 8.8 ± 0.8, p < 0.05; t-test) and the final completed ratio (Fig. 2a; 77.3 ± 12.8 vs. 41.2 ± 9.3, p < 0.05) during a 23 h session. In contrast, there was no difference in the time required to complete the final ratio by the non-stress and stress groups (8.4 ± 0.3 h vs. 9.6 ± 1.1 h from the beginning of the SA session, p > 0.05; t-test).
Fig. 2.
Repeated stress during abstinence increased reacquisition of nicotine SA under a PR schedule begun immediately after the final stress and followed by an FR5 schedule. (a) The number of nicotine injections and the final ratio completed under a PR schedule during a 23 h session of reacquisition were significantly increased by repeated restraint stress compared to the non-stress group. (b, c) After the PR session, both nicotine injections and active lever presses during 5 days of reacquisition under an FR5 schedule were also significantly increased by stress (Repeated measure ANOVA: p < 0.05). *p < 0.05 vs. non-stress groups (t-test). # p < 0.05 vs. baseline during the final 3 days of acquisition within group; † p < 0.05 stress vs. non-stress group at same time interval (t-test). n = 5 and 7 for non-stress and stress groups, respectively.
Following the PR schedule, rats reacquired nicotine SA under an FR5 schedule for 5 consecutive days (Fig. 2b and c). Compared to the non-stress group, repeated stress increased nicotine injections (Fig. 2b; Repeated measure ANOVA: effect of stress exposure, F1, 10 = 8.4, p < 0.05; interaction of stress × day, F4, 40 = 2.05, p > 0.05) and active lever presses (Fig. 2c; effect of stress exposure, F 1, 10 = 10.4, p < 0.05; interaction of stress × day, F4, 40 = 1.61, p > 0.05) during reacquisition. On the first 2 days of reacquisition (FR5), active lever presses and nicotine injections were significantly greater in the stress group (p < 0.05; t-test). In both stress and non-stress groups, active lever presses and nicotine injections declined from peak levels on the first day to levels approximating those recorded during last 3 days of acquisition. In the stress group, the increase in nicotine injections and active lever presses persisted throughout the first 3 days of reacquisition, in comparison to last 3 days of acquisition (p < 0.05), whereas an increase was only detected on the first day in the non-stress group (p < 0.05). Inactive lever presses were unaffected by stress (p > 0.05; data not shown).
Experiment 2: Effects of repeated stress during abstinence on reacquisition of nicotine SA begun 24 h after the final stress
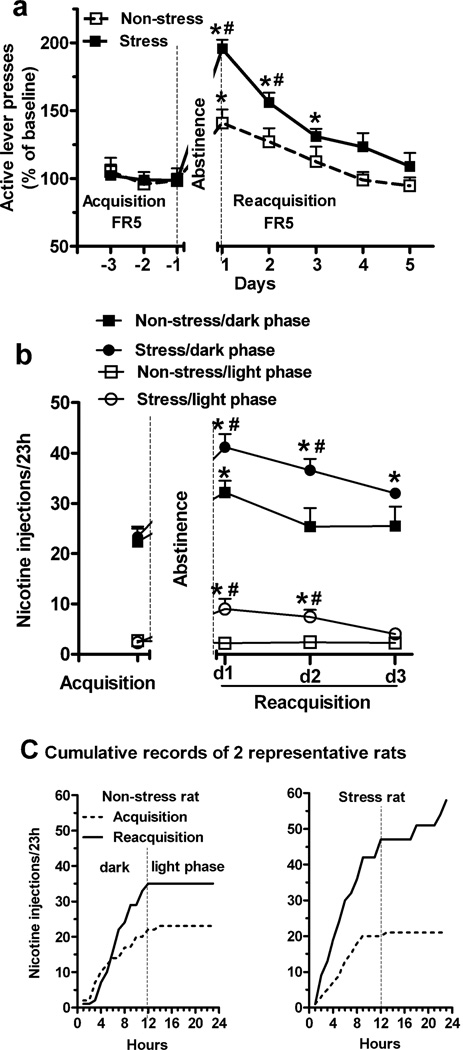
We determined whether repeated stress during abstinence affects reacquisition of nicotine SA when the final stress and reacquisition trial are separated by 24 h without an intervening PR test. Similar to experiment 1, the mean number of active lever presses (154.1 ± 21.4 vs. 165 ± 13.9) and nicotine injections (25.3 ± 3.2 vs. 25.8 ± 1.8) during final 3 days of acquisition for non-stress and stress groups were similar (p > 0.05, t-test). Repeated restraint stress increased active lever presses during 5 days of reacquisition compared to non-stress group (Fig. 3a; Repeated measure ANOVA: effect of stress exposure, F 1, 8 = 7.6, p < 0.05; interaction of stress × day, F4, 32 = 1.6, p > 0.05). On both day 1 and 2, active lever presses were significantly greater in the stress group (p < 0.05; t-test). Furthermore, compared to the basal levels during last 3 days of acquisition within group, stress elevated active lever presses during the first 3 days of reacquisition (p < 0.05), whereas abstinence per se increased active presses only on the first day of reacquisition (p < 0.05). Inactive lever presses were unaffected by stress (p > 0.05; data not shown).
Fig. 3.
Repeated stress during abstinence increased reacquisition of nicotine SA begun 24 h after the final stress and induced nicotine taking during the inactive phase of the light cycle. (a) Active lever presses during 5 days of reacquisition under an FR5 schedule were increased by repeated restraint stress compared to non-stress group (Repeated measure ANOVA: p < 0.05). (b) During first 3 days of reacquisition, stress significantly increased nicotine injections both in the dark and light phases (Repeated measure ANOVA: p < 0.05). (c) Cumulative (hourly) nicotine injections in 2 representative rats from non-stress and stress groups on day 1 of reacquisition. Nicotine taking during “lights on” (i.e., inactive phase) was only seen in stressed rats. * p < 0.05 vs. acquisition within group; # p < 0.05 stress vs. non-stress group at same time interval (t-test). n = 5 per group.
The nicotine injections obtained throughout the 24 h light/dark cycle on the last day of acquisition and day 1 to 3 of reacquisition are shown in Fig. 3b. During the first 3 days of reacquisition, stress significantly increased nicotine injections, both in the dark (Repeated measure ANOVA, effect of stress exposure, F 1, 8 = 5.7, p < 0.05) and light phase (effect of stress exposure, F 1, 8 = 6.4, p < 0.05). During acquisition, most nicotine injections were obtained during the dark phase in both stress (90.4 ± 3.8% of daily total) and non-stress (89.3 ± 2.9%) groups. In the non-stress group, the number of nicotine injections increased only in the dark phase of reacquisition day 1 (Fig. 3b; p < 0.05; t-test), and the percentage of total daily nicotine intake during the dark phase was not affected (89.3 ± 2.9% vs. 92.3 ± 2.9%; p > 0.05). In contrast, in both light and dark phases on day 1 and 2 of reacquisition, repeated stress increased the number of nicotine injections compared to the non-stress group (Fig. 3b; p < 0.05; t-test), but this was not observed on day 3. Moreover, stress shifted the diurnal pattern of nicotine intake by reducing the percentage of total daily nicotine intake during the dark phase (stress: 82.1 ± 1% vs. non-stress: 93.5 ± 2.4% in day 1; p < 0.01). Cumulative records of nicotine injections in 2 representative rats from the non-stress and stress groups on the last day of acquisition and day 1 of reacquisition are shown in Fig. 3c. Nicotine taking during “lights on” (i.e., inactive phase) was only seen in stressed rats.
Experiment 3: Effects of limited exposure to restraint stress (i.e. only 2 sessions) during abstinence on reacquisition of nicotine SA
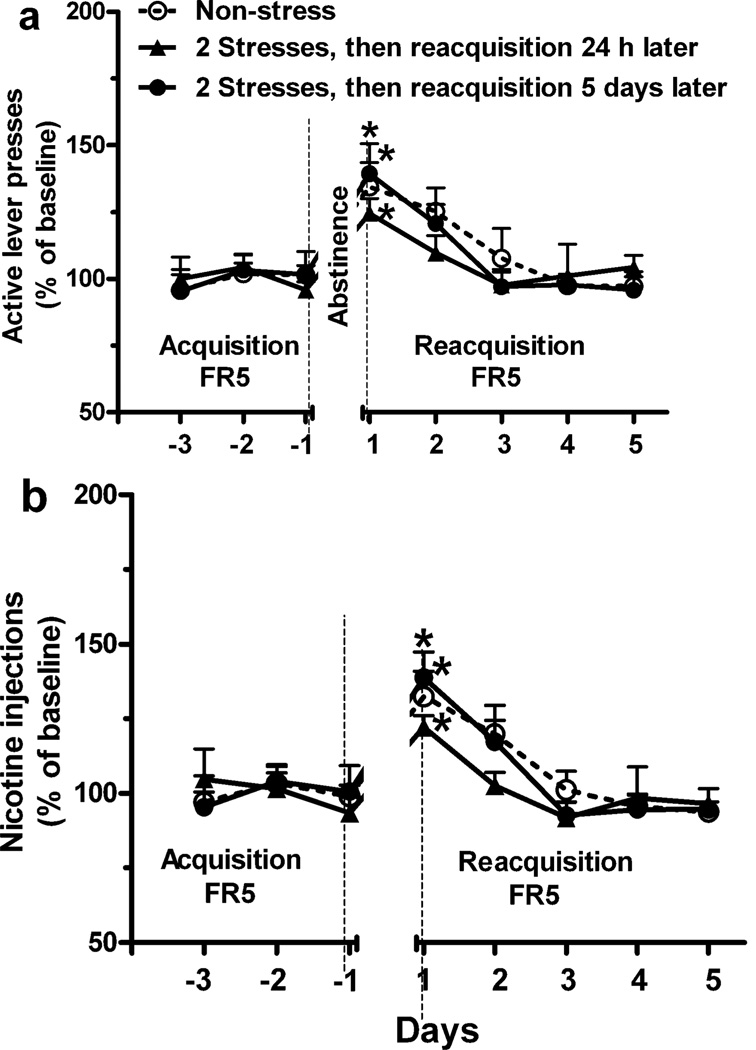
We also determined the effects of only 2 sessions of restraint stress on reacquisition of nicotine SA. After the second stress, reacquisition was evaluated either 24 h or 5 days later. Two sessions of restraint stress were not sufficient to increase active lever presses (Fig 4a; Repeated measure ANOVA: effect of stress exposure, F 1, 9 = 0.5 for 24 h interval between the final stress and reacquisition, p > 0.05; F 1, 9 = 0.01 for 5 days interval, p > 0.05) or nicotine injections (Fig. 4b; effect of stress exposure, F 1, 9 = 1.2 for 24 h interval p > 0.05; F 1, 9 = 0.02 for 5 days interval, p > 0.05) compared to the non-stress group (i.e., reacquisition determined on the same day corresponding to 5 days post stress) during reacquisition. The mean number of active lever presses (172.9 ± 18.3 vs. 187.8 ± 16.8 and 154.8 ± 12.2) and nicotine injections (29.2 ± 2.4 vs. 31.9 ± 3.0 and 28.8 ± 1.4) during final 3 days of acquisition for non-stress and two stress groups in this experiment were similar (p > 0.05, t-test).
Fig. 4.
Limited exposure to stress (i.e., only 2 sessions) during abstinence was not sufficient to increase reacquisition of nicotine SA. Two sessions of restraint were administered on day 1 and 3 of abstinence. Reacquisition (FR5), determined either 24 h or 5 days after the second stress, was not increased by 2 sessions of stress exposure compared to the non-stress group (i.e., reacquisition determined on the same day corresponding to 5 days post stress; Repeated measure ANOVA: p > 0.05). * p < 0.05 vs. final 3 days of acquisition within group. n = 5 or 6 per group.
Experiment 4: Effects of repeated stress on non-reinforced reacquisition behavior
In a separate group of animals receiving repeated stress during abstinence, reacquisition was elevated under an FR5 schedule begun 24 h after the final stress, but active lever presses were not reinforced by nicotine. Compared to the number of active lever presses elicited on the final day of acquiring nicotine SA under FR5, in the absence of nicotine reinforcement, active presses on day 1 of reacquisition were markedly reduced (168.6 ± 16.3 vs. 40.4 ± 3.3; p < 0.01) and were similar to inactive presses. Inactive lever presses were unaffected (52.4 ± 11.7 vs. 42.8 ± 7.3; p > 0.05).
Discussion
Stress is known to promote relapse to cigarette smoking (Falba et al. 2005; Perkins and Grobe 1992; Siahpush and Carlin 2006), and to reinstate nicotine seeking after extinction of cue-associated nicotine SA in rodents (Bruijnzeel et al. 2009; Plaza-Zabala et al. 2010). Herein, we have demonstrated that 4 restraint stress exposures during abstinence, but not only 2 sessions, enhances reacquisition of nicotine SA evaluated immediately or following an interval of 24 h after the final stress, by heightening the operant conditioning responses under both PR and FR5 schedules. Moreover, daily nicotine intake was increased and nicotine-taking behavior was induced in the inactive phase (i.e., light phase) of the diurnal light cycle.
Repeated stress during abstinence amplified several parameters of reacquisition. The breakpoint (i.e. number of nicotine injections) under a PR schedule was significantly increased during a 23 h session begun immediately after the final stress. This indicates that repeated stress during abstinence enhanced the motivation to nicotine (Arnold and Roberts 1997; Doyle et al. 2012). On an FR5 schedule that followed the PR session, stress-enhanced responses were emitted for 3 days. This was observed even when reacquisition sessions began 24 h after the final stress. In contrast, abstinence per se (non-stress) only increased nicotine SA on the first day of requisition (FR5 schedule). These observations indicate that repeated stress has a prolonged effect on nicotine taking during reacquisition. Analysis of the effects of stress vs. non-stress (i.e. abstinence per se) offers insight into the stress-enhanced reacquisition of nicotine SA. No significant interaction between stress exposure and the day of reacquisition was observed. Hence, repeated restraint stress most likely exerts an additive and qualitatively similar effect on responding to that induced by abstinence per se.
Consistent with previous reports (O'Dell et al. 2007; Valentine et al. 1997), in this study, most nicotine injections were obtained during the dark phase of the light cycle when rats are most active. However, with very prolonged access to nicotine SA (e.g., 40 days), nicotine intake was reported to be less circadian, and the difference in nicotine intake between dark and light phases diminished (O'Dell et al. 2007). In the current study, abstinence per se increased nicotine intake during reacquisition, but only in the dark (active) phase of the circadian cycle. Whereas repeated stress during abstinence further increased nicotine intake during reacquisition not only in the dark phase, but also in the light (inactive) phase. This induction of nicotine-taking behavior during the inactive phase of the circadian cycle by stress most likely reflects greater motivation to obtain nicotine (Hoffman and Simmons 2011).
In the present study, abstinence per se enhanced active lever presses and nicotine intake only on the first day of reacquisition under FR5 schedule, and rapidly reverted to baseline levels after day 1 of reacquisition. These escalation and reversion accords with previous studies of withdrawal from multiple intermittent 4 days cycles of prolonged nicotine SA, each separated by 3 days of abstinence (George et al. 2007; O'Dell and Koob 2007). Moreover, in the current studies, the return of active lever presses and nicotine injections during reacquisition to acquisition levels was also observed in rats that were stressed, but after a significantly longer interval (3 days) compared to abstinence per se. At present, the neuronal mechanisms governing this escalation and its reversion are not clearly defined.
It is known that acute stress (i.e. foot shock stress) reliably induces the reinstatement of nicotine seeking after extinction of nicotine SA (Bruijnzeel et al. 2009; Plaza-Zabala et al. 2010; Shaham et al. 1997; Zislis et al. 2007). In contrast, the current study utilized forced abstinence without extinguishing, and effect of chronic stress (i.e. repeated) during abstinence on reacquisition of nicotine SA was detected. Four stress exposures were required to enhance reacquisition of nicotine SA. Moreover, stress-enhanced reacquisition behavior was not observed if nicotine was not available for reinforcement. Therefore, the abstinence model of stress-enhanced reacquisition of nicotine SA in present study is different from previous extinction model of stress-induced reinstatement of nicotine seeking.
In conclusion, these experiments demonstrate that chronic (i.e. repeated) stress during abstinence amplified reacquisition of nicotine SA. Although stress-induced reinstatement of nicotine seeking after extinction is well established, this study provides the first experimental model demonstrating the amplification of reacquisition of nicotine taking by chronic stress during abstinence in laboratory rats. Further studies of this model may elucidate the underlying neurobiological mechanism(s), dissect susceptibility factors and identify preventive treatment to ameliorate the effects of chronic stress on relapse to smoking.
Acknowledgements
This research was supported by DA-03977 (B.M.S.) from NIDA. The authors thank Zaifang Huang for her excellent technical assistance.
Footnotes
Conflict of interest: None
References
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Prado M, Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol Psychiatry. 2009;66:110–117. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses--United States: 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;57:1226–1228. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Quitting smoking among adults--United States: 2001–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1513–1519. [PubMed] [Google Scholar]
- Cohen S, Lichtenstein E. Perceived stress, quitting smoking, and smoking relapse. Health Psychol. 1990;9:466–478. doi: 10.1037//0278-6133.9.4.466. [DOI] [PubMed] [Google Scholar]
- D'Souza MS, Markou A. Neuronal mechanisms underlying development of nicotine dependence: implications for novel smoking-cessation treatments. Addict Sci Clin Pract. 2011;6:4–16. [PMC free article] [PubMed] [Google Scholar]
- Doyle SE, Breslin FJ, Rieger JM, Beauglehole A, Lynch WJ. Time and sex-dependent effects of an adenosine A2A/A1 receptor antagonist on motivation to self-administer cocaine in rats. Pharmacol Biochem Behav. 2012;102:257–263. doi: 10.1016/j.pbb.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falba T, Teng HM, Sindelar JL, Gallo WT. The effect of involuntary job loss on smoking intensity and relapse. Addiction. 2005;100:1330–1339. doi: 10.1111/j.1360-0443.2005.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O'Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AC, Simmons D. Menthol cigarette smoking and nicotine dependence. Tob Induc Dis. 2011;9(Suppl 1):S5. doi: 10.1186/1617-9625-9-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS, Wilson OB. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43:779–784. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- Manhaes AC, Guthierrez MC, Filgueiras CC, Abreu-Villaca Y. Anxiety-like behavior during nicotine withdrawal predict subsequent nicotine consumption in adolescent C57BL/6 mice. Behav Brain Res. 2008;193:216–224. doi: 10.1016/j.bbr.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Matheny KB, Weatherman KE. Predictors of smoking cessation and maintenance. J Clin Psychol. 1998;54:223–235. doi: 10.1002/(sici)1097-4679(199802)54:2<223::aid-jclp12>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Markou A, Zorrilla EP, Koob GF. Extended access to nicotine self-administration leads to dependence: Circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007;320:180–193. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Koob GF. 'Nicotine deprivation effect' in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol Biochem Behav. 2007;86:346–353. doi: 10.1016/j.pbb.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE. Increased desire to smoke during acute stress. Br J Addict. 1992;87:1037–1040. doi: 10.1111/j.1360-0443.1992.tb03121.x. [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A, Martin-Garcia E, de Lecea L, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J Neurosci. 2010;30:2300–2310. doi: 10.1523/JNEUROSCI.5724-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Bevins RA. Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Curr Drug Abuse Rev. 2009;2:184–194. doi: 10.2174/1874473710902020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self- administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Samet JM. The 1990 Report of the Surgeon General: The Health Benefits of Smoking Cessation. Am Rev Respir Dis. 1990;142:993–994. doi: 10.1164/ajrccm/142.5.993. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Siahpush M, Carlin JB. Financial stress, smoking cessation and relapse: results from a prospective study of an Australian national sample. Addiction. 2006;101:121–127. doi: 10.1111/j.1360-0443.2005.01292.x. [DOI] [PubMed] [Google Scholar]
- Taylor DH, Jr, Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of smoking cessation for longevity. Am J Public Health. 2002;92:990–996. doi: 10.2105/ajph.92.6.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine JD, Hokanson JS, Matta SG, Sharp BM. Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology (Berl) 1997;133:300–304. doi: 10.1007/s002130050405. [DOI] [PubMed] [Google Scholar]
- Zislis G, Desai TV, Prado M, Shah HP, Bruijnzeel AW. Effects of the CRF receptor antagonist D-Phe CRF(12-41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007;53:958–966. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]