Abstract
Hepatitis C (HCV) coinfection is the leading cause of liver-related morbidity and is a leading cause of mortality in human immunodeficiency virus (HIV)-infected individuals in the antiretroviral therapy era. Direct-acting antiviral (DAA) therapies are transforming how HCV is treated with significant improvements in efficacy and tolerability. In this article, DAA agents expected to be available in 2014 are reviewed, including telaprevir, boceprevir, sofosbuvir, simeprevir, faldaprevir, and daclatasvir. Available data regarding clinical efficacy, adverse effects, and drug interactions in HIV-HCV coinfection are discussed. The management of adverse effects of HCV therapy and treatment considerations in patients with cirrhosis are also reviewed.
Keywords: Hepatitis C, HIV, HIV-HCV coinfection, Telaprevir, Boceprevir, Sofosbuvir, Simeprevir, Faldaprevir, Daclatasvir
Introduction
With an estimated 185 million people seropositive for hepatitis C virus (HCV) worldwide, HCV and its associated liver disease (cirrhosis, end stage liver disease, hepatocellular carcinoma) are a leading cause of death globally [1, 2]. In the United States, mortality related to HCV-associated liver disease recently eclipsed that related to human immunodeficiency virus (HIV), and modeling predictions suggest this number will only increase over the next two decades [3–5]. In fact, HCV-associated liver disease is a leading cause of death among HIV-infected persons [6–8].
The management of the HIV-HCV coinfected patient can be complicated by comorbidities, including substance abuse and psychiatric disease, which may limit or exclude HCV treatment [9, 10]. Often referred to as “difficult to treat,” this patient population has now become one in desperate need of new HCV therapies. As we enter the dawn of a new age in HCV therapeutics, it is of critical importance that HIV providers engage these patients in HCV specific care.
We will briefly review the natural history of chronic HCV infection in the setting of HIV and will discuss treatment candidacy considerations that will be common over the next year. We will then discuss current therapeutic targets with a focus on the compounds that will be clinically available for genotype 1 infection in 2014. Finally, we will briefly discuss the management of adverse events and specific challenges for patients with cirrhosis.
HCV-Associated Liver Disease in the HIV-Infected Person
HIV-HCV coinfection results in increased liver-related morbidity and mortality, non-hepatic organ dysfunction, and overall mortality. Even in the highly active antiretroviral era, HIV coinfection remains independently associated with advanced liver fibrosis and cirrhosis in patients with chronic HCV infection [11–13]. In the D:A:D study, liver-related death was the most common cause of non-AIDS death; this was primarily driven by HCV coinfection [8, 14]. Standardized and excess mortality rates in patients with HIV-HCV coinfection are higher than those with HIV-monoinfection and HCV-monoinfection [15]. Perhaps most importantly, overall and cause-specific mortality in HIV-infected patients that are HCV seropositive have not improved since the years of early combined antiretroviral therapy [16].
Treatment of HCV that results in a sustained virologic response (SVR, defined as undetectable HCV RNA 24 weeks after end of treatment) has a significant impact on the health of patients with HCV infection. Achievement of SVR has been associated with decreases in liver-related outcomes in patients with varied levels of fibrosis at time of cure [17, 18]. HIV-HCV coinfected patients cured with pegylated interferon-alfa and ribavirin (P/R) have lower rates of hepatic decompensation, hepatocellular carcinoma, and mortality [19, 20•, 21•].
Despite the substantial benefits of HCV treatment for HIV-HCV coinfected patients, there remain distinct and considerable challenges to effective HCV therapy. The previous standard of care, P/R, had poor efficacy in this population. The PARADIGM trial, a double-blinded international trial of HIV-HCV genotype 1 coinfection treated with P/R, reported SVR rates of only 19–22 % [22]. In addition, there has been limited uptake of therapy for HCV due to patient comorbidities, patient and provider perception, and the adverse events related to interferon (IFN)-based therapy [23, 24].
Determining Treatment Candidates
With the advent of potent direct acting antivirals (DAAs), HIV-HCV coinfected patients have a lot to gain. While comorbidities and potential drug reactions with antiretrovirals remain challenges, the risk-benefit ratio is shifting to favor treatment rather than deferral. As DAA regimens become more potent, less complex, and better tolerated, the balance will clearly favor treatment; thus, the primary decision for many providers will not be who to treat, but when to treat.
There are multiple variables to consider when assessing a patient for HCV treatment candidacy. Previous issues, such as comorbid psychiatric disease and poor CD4 reconstitution (or CD4 percentage/total discordance), will have less significance when regimens are free of IFN. Several key attributes that are likely to drive these decisions include: the patient’s willingness to wait for IFN-free regimens; the severity and stage of liver disease; and access to newly approved agents. Patients with severe fibrosis or cirrhosis should be prioritized for therapy and should undergo additional evaluation for complications of liver disease including hepatocellular carcinoma and esophageal varices screening, preventative vaccination, and monitoring for portal hypertension prior to treatment initiation.
Direct Acting Antivirals: The “How”
HCV Life Cycle and Therapeutic Targets
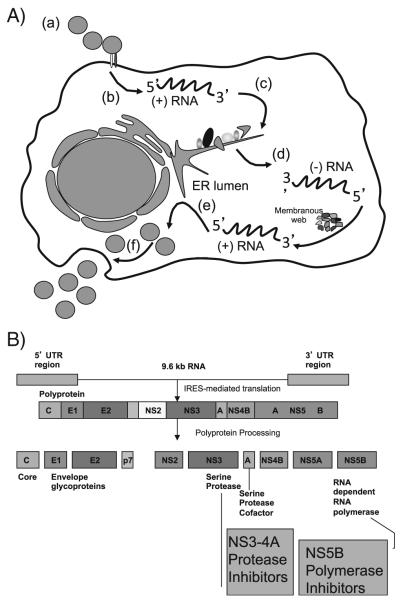
HCV is a flavivirus with significant genetic diversity, including seven genotypes and several subtypes that vary geographically; genotype 1 represents approximately 70 % of HCV infections in the United States [25, 26]. HCV contains a positive-sense RNA genome of 9.6 kb; translation of this genome produces a single polyprotein. Cleavage and processing of this polyprotein results in both structural proteins (core, E1, and E2) and non-structural proteins (p7, NS2, NS3, NS4A, NS4B, NS5A and NS5B) (Fig. 1). The primary targets of DAAs include the serine protease NS3 and its cofactor NS4, the viral RNA-dependent RNA polymerase NS5B, and the nonstructural protein NS5A [27–29].
Fig. 1.
a The life cycle of HCV: (a) virus– receptor binding and endocytosis; (b) cytoplasmic release and uncoating; (c) translation and polyprotein processing; (d) RNA replication; (e) virion assembly; and (f) virion release. b The HCV polyprotein. IRES, internal ribosome entry site. (Naggie S, Patel K, McHutchison J. Hepatitis C virus directly acting antivirals: current developments with NS3/4A HCV serine protease inhibitors. J Antimicrob Chemother. 2010;65(10):2063-9. Reproduced with permission of Oxford University Press.)
Treatment of HCV in 2014
HCV therapy has advanced since the use of type I IFN in 1986 for non-A, non-B hepatitis. Ribavirin (RBV) was added in 1998, and pegylated IFN-alfa was introduced in 2001 [29]. After a series of trials in 2004, pegylated IFN-alfa in combination with RBV (P/R) became the standard of care for HCV therapy in HIV-HCV coinfected patients [30–33]. Despite these advances, SVR rates remained very low in this population [22]. After the introduction of telaprevir and boceprevir in 2011, an HCV protease inhibitor in combination with P/R (an off-label triple therapy regimen) became the new standard of care for the treatment of HIV-HCV coinfected patients. With the anticipated approval of the first NS5B nucleotide polymerase inhibitor, sofosbuvir, and a second wave NS3/4A protease inhibitor, simeprevir, in December 2013, the standard of care will again change, demonstrating the rapid pace of drug discovery in HCV therapeutics today.
Telaprevir
Telaprevir is a first generation, first wave NS3/4A protease inhibitor. It received Food and Drug Administration (FDA) approved in 2011 for treatment of HCV monoinfection. The ADVANCE, ILLUMINATE, and REALIZE studies demonstrated that telaprevir in combination with P/R significantly increased SVR rates as compared to P/R alone [34–36]. However, these studies actively excluded HIV coinfection, and, as such, any current treatment of HIV-HCV coinfected patients with telaprevir is considered off-label.
Study VX08-950-110, also known as Study 110, was a phase IIa study of telaprevir use in HIV patients with treatment naïve, HCV genotype 1 coinfection [37]. Patients received telaprevir or placebo for 12 weeks in combination with P/R for 48 weeks. SVR occurred in 74 % (28/38) of patients on telaprevir versus 45 % (10/22) of patients treated with P/R alone. Pruritus, rash, anemia, and anorectal discomfort were more common in patients treated with telaprevir.
As a substrate and strong inhibitor of CYP3A4, telaprevir has exhibited significant drug interactions with some antiretrovirals (Table 1). In Study 110, atazanavir/ritonavir and efavirenz based therapy (with telaprevir dose adjustment) effectively controlled HIV during HCV therapy with no viral breakthrough [37•]. Darunavir, fosamprenavir, lopinavir, and rilpivirine are not currently recommended for co-administration with telaprevir, while etravirine and dolutegravir may be options on a case-by-case basis [38–42].
Table 1.
Direct Acting Antivirals in 2014: Approved and Investigational
| DAA | Mechanism of action | Application status | Genotype activity | Barrier to resistance |
Dosing | Length of therapy (DAA/PR) |
Antiretroviral considerations |
|---|---|---|---|---|---|---|---|
| Telaprevir | NS3/4A Protease Inhibitor (first generation, first wave) |
FDA approved | GT 1 only | Low | BID or TID | 12/48* weeks | Contraindicated with protease inhibitors and NNRTIs (except atazanavir and efavirenz). Requires increased dosage when coadministered with efavirenz. |
| Boceprevir | NS3/4A Protease Inhibitor (first generation, first wave) |
FDA approved | GT 1 only | Low | TID | 24-44/48* weeks | Contraindicated with protease inhibitors and efavirenz. |
| Sofosbuvir | NS5B RNA Polymerase Inhibitor |
FDA New Drug Application pending approval |
GT 1-6 | High | Daily | 12 weeks | No clinically significant interactions with antiretroviral therapy known. |
| Simeprevir | NS3/4A Protease Inhibitor (first generation, second wave) |
FDA New Drug Application pending approval |
GT 1, 2, 4, 5, and 6 | Low | Daily | 12/24-48 weeks | Concentrations significantly decreased in presence of efavirenz and significantly increased in presence of darunavir/ritonavir. No clinically significant interactions with raltegravir, rilpivirine, and tenofovir. |
| Faldaprevir | NS3/4A Protease Inhibitor (first generation, second wave) |
Awaiting FDA New Drug Application |
GT 1, 2, 4, 5, and 6 | Low | Daily | 12-24/24-48 weeks | Concentrations reduced by efavirenz and increased by boosted protease inhibitors. No clinically significant interactions with raltegravir and maraviroc. |
| Daclatasvir | NS5A Inhibitor | Awaiting FDA New Drug Application |
GT 1-6 | Low | Daily | 12-24 weeks | Concentrations reduced by efavirenz and increased by boosted protease inhibitors. No clinically significant interactions with tenofovir. |
There are no published data that support response-guided therapy in HIV-HCV coinfection, although this is an area of current investigation.
DAA Direct acting antiviral, FDA Food and drug administration, GT Genotype, NS Non-structural, NNRTIs Non-nucleoside reverse transcriptase inhibitors
Boceprevir
Boceprevir is a first generation, first wave NS3/4A protease inhibitor. It was also FDA approved in 2011 for treatment of HCV monoinfection. As with telaprevir, boceprevir therapy demonstrated higher SVR rates when combined with P/R in multiple registration trials including SPRINT-2, RESPOND-2, and PROVIDE [43–45]. Again, these studies excluded patients with HIV coinfection.
Study MK-3034 protocol 05411 was a phase II study of boceprevir in HIV patients with treatment naïve, HCV genotype 1 coinfection [46•]. All patients were treated with a “lead-in” phase with P/R for 4 weeks followed by randomization to either boceprevir or placebo in addition to P/R for 44 weeks. Forty (63 %) of the 64 patients in the boceprevir group achieved SVR versus ten (29 %) of the 34 patients in the control arm. Anemia, pyrexia, decreased appetite, dysgeusia, vomiting, neutropenia, and thrombocytopenia were more common in the boceprevir group [46•].
Boceprevir is a significant inhibitor of CYP3A4. Boceprevir is primarily metabolized by aldoketoreductases 1C2 and 1C3, although it is also partially metabolized by CYP3A4 [38]. Drug interaction studies have demonstrated that efavirenz reduces boceprevir concentrations; bi-directional pharmacokinetic interactions occur with concomitant administration of HIV protease inhibitors and boceprevir [47, 48]. The ANRS HC27 BocepreVIH Study Group showed reduced exposures of atazanavir (51 %) and boceprevir (34 %) during co-administration [49]. Raltegravir has variable but clinically insignificant changes in exposure, while boceprevir pharmacokinetic parameters were not significantly affected [49, 50]. Etravirine, maraviroc, and dolutegravir have not shown significant interactions with boceprevir in healthy volunteers [42, 51, 52]. While awaiting the results of phase III pharmacokinetic evaluations, we recommend using a raltegravir-based antiretroviral regimen if treating a coinfected patient with this HCV regimen [53, 54].
First generation, First wave Protease Inhibitors in 2014
There are increasing reports of real world use of telaprevir and boceprevir in both HCV monoinfected and HIV-HCV coinfected clinical cohorts. The experience across centers is similarly intense, requiring frequent clinic visits and laboratory assessments. The regimens are complex and have significant and frequent adverse events that often require additional interventions, including erythropoietin growth factors, blood transfusions, and hospitalizations, especially in patients with cirrhosis [55, 56]. With the approval of two new DAAs pending, the use of the first wave HCV protease inhibitors has already significantly declined. Presuming access to newer agents is not delayed, telaprevir and boceprevir are unlikely to play a large role in the treatment of HIV-HCV coinfected patients in 2014.
Sofosbuvir
Sofosbuvir (GS-7977) is a nucleotide analogue inhibitor of the NS5B polymerase. This agent provides pangenotypic activity, once daily dosing and an exceptional barrier to resistance [57]. Sofosbuvir has shown promise as part of both IFN-sparing and IFN-free regimens.
The NEUTRINO trial was a phase III trial that evaluated sofosbuvir plus P/R for 12 weeks in 327 treatment naïve patients infected with HCV genotypes 1, 4, 5, and 6 [58••]. Eighty-nine percent of genotype 1 patients achieved SVR [58••]. SVR rates were high across more difficult to treat subpopulations, including African Americans (87 %), cirrhotics (80 %), and unfavorable IL28B genotypes (87 %). Thus far, sofosbuvir has been well tolerated and does not increase the adverse event profile over that expected for P/R inclusive regimens [59]. This regimen performed similarly in a pilot study in HIV-HCV coinfected subjects, with 19 genotype 1 patients achieving a SVR12 of 89 % [60•]. The patients were not cirrhotic and had well controlled HIV on efavirenz (30 %), atazanavir/ritonavir (22 %), darunavir/ritonavir (17 %), raltegravir (26 %), or rilpivirine based (4 %) antiretroviral regimens. This appears to be an excellent option for coinfected, treatment naïve, genotype 1 patients.
The efficacy of this triple regimen in treatment experienced, genotype 1 patients is unknown and is not being evaluated in a registration trial. However, the FDA advisory committee document suggests that consideration will be given to the use of this triple regimen in patients who have a failed a prior P/R regimen due in part to the improved efficacy of this regimen in other difficult to treat subpopulations, including patients with cirrhosis and unfavorable IL28B genotype [73]. To date, P/R null responders and treatment experienced patients with cirrhosis have received 48 weeks of P/R in combination with variable lengths of a DAA (i.e., telaprevir=12 weeks; boceprevir=44 weeks; simeprevir=12 weeks). The lack of viral breakthrough on any sofosbuvir containing regimen and sofosbuvir’s exceptional barrier to resistance means that patients who fail treatment could be retreated with other future DAA regimens, even those including sofosbuvir or another nucleotide analogue [88].
Although the pending approval of sofosbuvir for HCV genotype 1 infection will include P/R, there are phase II studies in HCV monoinfected patients that support the use of sofosbuvir with RBV or in combination with other DAAs with or without RBV in select genotype 1 patients. The COSMOS study combined sofosbuvir with simeprevir with or without RBV for 12 or 24 weeks in non-cirrhotic P/R null responders (cohort 1) and treatment naïve and P/R null responders with F3/4 severe fibrosis (cohort 2) [61]. In cohort 1, >90 % of patients in three of four treatment arms achieved SVR12. In cohort 2, 96 % patients (RBV inclusive) and 100 % patients (RBV-free) in the 12 week arm have achieved SVR4 [61]. In trial AI-444040, 41 non-cirrhotic patients with HCV genotype 1 chronic infection who experienced virologic failure after telaprevir- or boceprevir-based therapy were treated with sofosbuvir and daclatasvir (a NS5A inhibitor) with or without RBV for 24 weeks. All 41 patients achieved SVR4, and 40 of 41 patients achieved SVR12 [62]. This same regimen was also studied in genotype 1, 2, and 3 treatment naïve patients for 24 weeks with near perfect SVR rates [63]. Lastly, the SPARE trial investigated sofosbuvir plus RBV for 24 weeks in genotype 1, treatment naïve patients. This study included a more difficult to treat clinical trial population with 71 % African American, 84 % unfavorable IL28B genotype, and 24 % cirrhosis. Sixty-eight percent of patients (N=25) achieved SVR24 in those receiving weight-based RBV [64].
These, albeit small, studies offer evidence for IFN-free DAA combination therapies that will otherwise not be available for HCV mono-infected patients via the FDA approval pathway until 2015.
For HIV-HCV coinfected patients, there is additional evidence to support the use of IFN-free therapy. Interim data for the first IFN-free regimen (sofosbuvir in combination with RBV) studied in HIV-HCV coinfection (PHOTON-1) recently reported excellent response rates for treatment naïve genotype 1, 2, and 3 patients [65•]. Treatment naïve genotype 2 (N=26) and 3 (N=42) patients received 12 weeks of the all-oral therapy while genotype 1 (N=114) patients received 24 weeks of therapy. SVR12 rates were 88 %, 67 %, and 76 %, respectively, and no HCV viral breakthroughs occurred. Ninety percent of genotype 1 patients completed therapy and only three patients discontinued the regimen due to adverse effects. Healthy volunteer drug interaction studies of sofosbuvir with efavirenz, rilpivirine, boosted darunavir, raltegravir, tenofovir, and emtricitabine have not identified clinically significant interactions [66] (Table 1). A majority of patients were on antiretrovirals including efavirenz (34 %), atazanavir/ritonavir (17 %), darunavir/ritonavir (32 %), and raltegravir (16 %). Two patients experienced HIV breakthrough due to poor adherence to antiretrovirals. Grade 3/4 adverse events were uncommon (9 %) as was discontinuation of therapy (3 %). These very promising data suggest that HIV-HCV coinfected patients can achieve high SVR rates with IFN-free therapies.
Simeprevir
Simeprevir (TMC435) is a first generation, second wave NS3/4A protease inhibitor. While its pharmacologic activity is similar to telaprevir and boceprevir, simeprevir is dosed once daily and has an improved safety profile. It has demonstrated antiviral activity against HCV genotype 1, 2, 4, 5, and 6 [67, 68]. The QUEST studies (QUEST-1 and QUEST-2) were phase III trials comparing 12 weeks of simeprevir plus 24 or 48 weeks of P/R versus a placebo/P/R arm [69, 70]. Response guided therapy (RGT) criteria were used to shorten therapy to 24 weeks in approximately 90 % of patients; of these, 81–86 % achieved SVR12. In contrast, only 32 % of those who did not meet RGT criteria achieved SVR12. There were differences in SVR across subgroups including: high viral load; severe fibrosis and cirrhosis; African American race; presence of the unfavorable IL28B genotypes; and presence of the baseline Q80K mutation. In subjects with a baseline Q80K mutation there was no benefit with simeprevir over placebo (58 % vs 55 %, respectively). The Q80K is a common polymorphism found in the NS3 gene of genotype 1a viruses in the US (48 %), thus baseline genotype testing will be required in this subgroup prior to treatment [73]. Adverse effects were similar to other trials including P/R; however, rash, photosensitivity, and hyperbilirubinemia (attributed to the drug’s effect on hepatic transport of bilirubin) did occur more often with simeprevir than placebo [69, 70].
The ASPIRE (phase IIb) and PROMISE (phase III) trials investigated the efficacy of simeprevir with P/R in treatment experienced patients. In the ASPIRE trial (12 weeks of simeprevir/P/R followed by 36 weeks of P/R), relapse, partial response, and null response patients achieved 85 %, 75 %, and 51 % SVR24 in the simeprevir arm, as compared to 19 %, 9 %, and 37 % in the placebo arm, respectively [71]. The PROMISE study investigated simeprevir combined with P/R in HCV genotype 1 P/R relapse patients. Ninety-three percent of patients met RGT criteria and were treated for a total of 24 weeks; 83 % achieved SVR12. Only 32 % of those who did not meet RGT criteria achieved SVR12 [72]. Again, the baseline Q80K mutation predicted a lower SVR12 of 47 % as compared to genotype 1b (86 %) or genotype 1a without the Q80K (78 %) [73].
The C212 study is an ongoing phase III trial of simeprevir in combination with P/R in HIV-HCV coinfected patients. This study is investigating the efficacy of RGT in both HCV treatment naïve and P/R relapse patients. Partial response, null response, and cirrhotic patients receive simeprevir for 12 weeks in combination with P/R for 48 weeks. The primary analysis reported an overall SVR12 of 74 % (treatment naïve 79 %; prior relapser 87 %; prior partial responder 70 %; prior null responder 57 %). Eighty-nine percent of patients eligible for RGT were able to shorten therapy to 24 weeks; of those treated with RGT, 78 % achieved SVR12. Some subgroups had poorer response including: genotype 1a (71 % vs 89 % in genotype 1b); genotype 1a with the Q80K mutation at baseline (67 %); advanced fibrosis or cirrhosis (64 %); and subjects not on ART (62 % vs 75 % of subjects on ART) [74•]. Healthy volunteer studies suggest simeprevir has no significant drug interactions with tenofovir, rilpivirine, and raltegravir. Simeprevir concentrations were significantly decreased when dosed with efavirenz and significantly increased when dosed with darunavir/ritonavir, resulting in the exclusion of efavirenz and all HIV protease inhibitors from the trial [75] (Table 1).
Based on the available data, simeprevir may be an alternative to sofosbuvir in HIV-HCV genotype 1 coinfected patients on compatible ARV regimens, especially if cost plays a role in access to treatment. The once daily dosing and improved adverse event profile will make it a favored choice over telaprevir or boceprevir. Furthermore, the ability to shorten therapy in a majority of treatment naïve or P/R relapse patients will improve acceptance of this regimen. The increased failure in genotype 1a patients with the baseline Q80K mutation (and thus the need for baseline resistance testing) and the longer course of P/R required will limit this therapy as compared to the sofosbuvir-containing regimens. In particular for HIV-HCV coinfected patients, the drug interaction profile will also limit this regimen to those who can maintain HIV viral suppression on a compatible ARV regimen.
Faldaprevir
Faldaprevir is a first generation, second wave NS3/4A protease inhibitor. Like simeprevir, faldaprevir is a once-daily medication that has demonstrated activity against HCV genotypes 1, 2, 4, 5, and 6 [76]. The phase III studies, STARTVerso™ 1 and 2 (treatment naïve) and STARTVerso™ 3 (treatment experienced), reported SVR12 rates of 72–73 %, 70 %, 48–57 %, and 33 % when combined with P/R in treatment naïve, P/R relapse, P/R partial response, and P/R null response patients, respectively [77, 78].
The STARTVerso™ 4 study is a phase III trial of faldaprevir plus P/R in HIV-HCV coinfected patients with genotype 1 [79•]. This open-label, sponsor-blinded study is in the process of evaluating two doses and lengths of treatment of faldaprevir in combination with P/R in HCV treatment naïve and P/R relapse patients using a RGT design. An interim analysis has shown an overall SVR4 of 74 % (genotype 1a 74 %; unfavorable IL28B genotype 67 %; cirrhosis 76 %; relapse 87 %). For those patients eligible for RGT, 80 % were able to receive a shortened course of therapy and 91-93 % achieved SVR4. Hemoglobin ≤8.5 g/dL occurred in only 6 % of patients, a rate less than that observed with telaprevir and boceprevir [79•].
Faldaprevir is a mild inhibitor of CYP2CP and an inhibitor of UGT1A1 [80]. STARTVerso™ 4 has allowed raltegravir, maraviroc, efavirenz, darunavir/ritonavir, and atazanavir/ritonavir in addition to common nucleoside analogues. Faldaprevir doses must be increased when given with efavirenz and decreased when given with boosted protease inhibitors [79•] (Table 1). Faldaprevir may be an alternative option for HIV-HCV coinfected patients based on its efficacy, side effect profile, and compatibility with multiple antiretroviral regimens; faldaprevir should be submitted for FDA approval in 2014.
Daclatasvir
Daclatasvir is a NS5A inhibitor with pangenotypic activity and is likely to play a primary role as a component of IFN-free regimens. Daclatasvir has been studied in combination with asunaprevir (NS3/4A inhibitor) as dual (RBV-free) therapy and as quad (in combination with P/R) therapy in P/R null response patients with excellent SVR12 rates in genotype 1b (78 %) and genotype 1a and 1b (95 %) infections, respectively [81]. Daclatasvir has also been studied in combination with sofosbuvir, with or without RBV, achieving SVR12 in >90 % of treatment naïve, genotype 1 patients with 12 weeks of treatment and in >90 % of triple therapy protease inhibitor failures with 24 weeks of treatment [82]. This DAA is in phase II study with simeprevir and is being studied in an IFN-free, RBV-free regimen with asunaprevir and BMS-791325. Daclatasvir may be co-administered with tenofovir, efavirenz, nevirapine, rilpivirine, abacavir, lamivudine, emtricitabine, raltegravir, maraviroc and boosted atazanavir; however, the dose may need to be increased when taken with efavirenz and decreased when taken with boosted protease inhibitors [83] (Table 1). This agent is currently in Phase III study in HIV-HCV coinfected patients and should be submitted for FDA approval in 2014.
HCV Therapy Beyond 2014
While approved DAA regimens for genotype 1 HCV infection will likely include P/R in 2014, there are several exciting DAA regimens in development that may be submitted and possibly approved by the FDA in late 2014 or beyond. A combination of ABT-450 (a NS3/4A protease inhibitor) boosted with ritonavir, ABT-267 (a NS5A inhibitor), ABT-333 (a non-nucleoside NS5B polymerase inhibitor) ± RBV has demonstrated SVR24 in >90 % of treatment naïve and experienced patients with HCV genotype 1 chronic infection; a phase III study in HIV-HCV coinfection is currently enrolling (NCT01939197) [84]. Ledipasvir is a NS5A inhibitor with evidence of increased SVR rates in combination with P/R in both treatment naïve and treatment experienced patients with HCV genotype 1 [85, 86]. Ledipasvir is now in phase III study in combination with sofosbuvir ± RBV as part of the ION studies which will include coinfected patients; this regimen is expected to be the first fixed dose DAA combination therapy for HCV genotype 1 [87, 88]. Daclatasvir in combination with asunaprevir and BMS-791325 (non-nucleoside NS5B polymerase inhibitor) achieved SVR in 94 % of treatment naïve, HCV genotype 1 patients and looks to be a very promising all-oral regimen [89]. Faldaprevir and deleobuvir ± PPI-668 (a pangenotypic NS5A inhibitor) ± RBV is another oral combination currently in phase II study [90, 91]. The promise of all-oral, highly effective and well-tolerated regimens is a primary confounder in current decisions of when to treat HCV infection.
Managing On-Treatment Adverse Events and Side Effects
Despite the increased efficacy of first wave DAA therapies, current HCV treatments are limited by significant adverse effects. Although future DAA therapies may offer IFN-free options, DAA therapy in combination with P/R will likely be the standard of care for HIV-HCV genotype 1 coinfected patients in 2014. To date, anemia is one of the most common adverse effects related to IFN, RBV, and DAA therapy. Real world cohorts receiving current triple therapies have reported rates of anemia greater than those reported in trials of non-cirrhotic patients [55]. RBV dose reduction is the primary management strategy; blood transfusion and erythropoietin administration should be reserved for refractory cases [92–94] (Table 2). Neutropenia occurs with IFN therapy; however, neutropenia has not been identified as a risk for infection while on treatment [95, 96]. Management includes IFN dose reduction and filgrastim when necessary [92]. Rash, depression, and flu-like symptoms are other common adverse effects attributed to P/R that providers will continue to manage in 2014 [97] (Table 2).
Table 2.
Adverse Effects of HCV Therapy in the DAA Era
| Adverse effect | Related medications | Treatment and management | Comments |
|---|---|---|---|
| Flu-like symptoms | Interferon Ribavirin |
Regular daily schedule Adequate sleep Hydration Exercise Acetaminophen |
May include fatigue, myalgias, weakness, fever, and insomnia. |
| Anemia | Interferon Ribavirin Telaprevir Boceprevir Faldaprevir |
Ribavirin dose reduction Erythropoietin or other erythrocyte-stimulating agents Blood transfusions |
DAA association with anemia is greatest for boceprevir, telaprevir, and faldaprevir. |
| Neutropenia | Interferon Ribavirin |
Interferon dose reduction Filgrastim |
Unclear clinical significance of neutropenia due to lack of association with infection. |
| Rash | Telaprevir Boceprevir Simeprevir Faldaprevir |
Preventative skin care and hydration Topical corticosteroids Topical anti-histamine Oral anti-histamines DAA withdrawal |
Severe rash (>50 % of body surface area or systemic symptoms) requires DAA withdrawal and progression warrants discontinuation of all medications. |
| Anorectal discomfort | Telaprevir | Diet modification (increased fat intake) Barrier creams Sitz baths Topical corticosteroids Topical lidocaine |
Diarrhea may require additional workup and evaluation. |
| Dysgeusia | Boceprevir | Hydration Candies or lozenges Using plastic utensils |
|
| Depression | Interferon | Patient selection Support groups and services Anti-depressants |
Monitoring for suicidality is essential while on interferon based therapies for at-risk patients. |
DAA Direct acting antiviral
Challenges in Patients with Cirrhosis
Patients with cirrhosis exhibit less tolerance and have poorer response to HCV therapies. Due to the risk of hepatic decompensation, co-management with hepatology is recommended. Several real world cohorts have reported results of currently available triple therapies with a similar message: cirrhotic patients have more serious adverse events, higher discontinuation rates, and can suffer severe outcomes including hepatic decompensation and death [55, 56]. While newer DAA therapies may be safer and have fewer side effects, patients with cirrhosis will remain higher risk and should be monitored vigilantly. Studies in the hepatic decompensation and liver transplant populations are ongoing and will help guide future management in these special populations.
Conclusion
HCV remains a critically important cause of morbidity and mortality in HIV-HCV coinfected patients. Therapy for HCV genotype 1 in HIV-HCV coinfected patients is in the midst of a transformation with two new DAA therapies pending FDA approval before 2014. While IFN and RBV remain part of HCV genotype 1 therapies for now, new regimens will provide improved efficacy, reduced complexity, better tolerance, and shorter courses of treatment. Sofosbuvir, given its excellent pharmacologic profile, will be an ideal DAA for HIV-HCV coinfected patients on antiretrovirals, while simeprevir will serve as an excellent alternative therapy for patients on compatible antiretroviral regimens. Faldaprevir and daclatasvir may provide additional therapeutic options later in 2014. With multiple phase III studies in HIV-HCV coinfection underway, we look forward to 2014 and the advances it will bring.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest Cody A. Chastain declares no conflict of interest.
Susanna Naggie declares roles as a Scientific Advisory Board/Consultant for Gilead, Vertex, Boehringer Ingleheim, Abbott, Janssen, and Achillion. Susanna Naggie also declares involvement with research related activities for Gilead, Vertex, AbbVie, BMS, Achillion, and Scynexis.
Human and Animal Rights and Informed Consent Susanna Naggie is an investigator at the Duke Clinical Research Institute and has participated in studies included in this manuscript.
Contributor Information
Cody A. Chastain, Email: cody.a.chastain@vanderbilt.edu, Division of Infectious Diseases, Vanderbilt University Medical Center, A-2200 MCN, 1161 21st Avenue, Nashville, TN 37232-2582, USA
Susanna Naggie, Division of Infectious Diseases, Duke Clinical Research Institute, DUMC 102359, Durham, NC 27710, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–42. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ly KN, Xing JJ, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271–8. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 4.Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43(1):66–72. doi: 10.1016/j.dld.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Zalesak M, Francis K, Gedeon A, Gillis J, Hvidsten K, Kidder P, et al. Current and future disease progression of the chronic HCV Population in the United States. PLoS ONE. 2013;8(5):e63959. doi: 10.1371/journal.pone.0063959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34(6):831–7. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 7.Sulkowski MS, Mast EE, Seeff LB, Thomas DL. Hepatitis C virus infection as an opportunistic disease in persons infected with human immunodeficiency virus. Clin Infect Dis. 2000;30(Suppl 1):S77–84. doi: 10.1086/313842. [DOI] [PubMed] [Google Scholar]
- 8.Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166(15):1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 9.Butt AA, Khan UA, Shaikh OS, McMahon D, Dorey-Stein Z, Tsevat J, et al. Rates of HCV treatment eligibility among HCV-monoinfected and HCV/HIV-coinfected patients in tertiary care referral centers. HIV Clin Trials. 2009;10(1):25–32. doi: 10.1310/hct1001-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butt AA, McGinnis K, Skanderson M, Justice AC. A comparison of treatment eligibility for hepatitis C virus in HCV-monoinfected versus HCV/HIV-coinfected persons in electronically retrieved cohort of HCV-infected veterans. AIDS Research and Human Retroviruses. 2011;27(9):973–9. doi: 10.1089/aid.2011.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thein H-H, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22(15):1979–91. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- 12.De Ledinghen V, Barreiro P, Foucher J, Labarga P, Castera L, Vispo ME, et al. Liver fibrosis on account of chronic hepatitis C is more severe in HIV-positive than HIV-negative patients despite antiretroviral therapy. J Viral Hepatitis. 2008;15(6):427–33. doi: 10.1111/j.1365-2893.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- 13.Fierer DS, Dieterich DT, Fiel MI, Branch AD, Marks KM, Fusco DN, et al. Rapid progression to decompensated cirrhosis, liver transplant, and death in HIV-infected men after primary hepatitis C virus infection. Clin Infect Dis. 2013;56(7):1038–43. doi: 10.1093/cid/cis1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study Group Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS. 2010;24(10):1537–48. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 15.Hernando V, Perez-Cachafeiro S, Lewden C, Gonzalez J, Segura F, Oteo JA, et al. All-cause and liver-related mortality in HIV positive subjects compared to the general population: differences by HCV coinfection. J Hepatol. 2012;57(4):743–51. doi: 10.1016/j.jhep.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Berenguer J, Alejos B, Hernando V, Viciana P, Salavert M, Santos I, et al. Trends in mortality according to hepatitis C virus serostatus in the era of combination antiretroviral therapy. AIDS. 2012;26(17):2241–6. doi: 10.1097/QAD.0b013e3283574e94. [DOI] [PubMed] [Google Scholar]
- 17.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. YJCGH. 2011;9(6):509–16. doi: 10.1016/j.cgh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour J-F, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–93. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 19.Berenguer J, Álvarez-Pellicer J, Martín PM, López-Aldeguer J, Von-Wichmann MA, Quereda C, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50(2):407–13. doi: 10.1002/hep.23020. [DOI] [PubMed] [Google Scholar]
- 20 •.Limketkai BN, Mehta SH, Sutcliffe CG, Higgins YM, Torbenson MS, Brinkley SC, et al. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA. 2012;308(4):370–8. doi: 10.1001/jama.2012.7844. This prospective cohort noted fewer liver-related clinical events in HIV-HCV coinfected patients on antiretroviral therapy. No events were detected in a subset of 51 patients treated with HCV therapy who achieved SVR.
- 21 •.Mira JA, Rivero-Juarez A, Lopez-Cortes LF, et al. Benefits from sustained virologic response to pegylated interferon plus ribavirin in HIV/hepatitis C virus-coinfected patients with compensated cirrhosis. Clin Infect Dis. 2013;56(11):1646–53. doi: 10.1093/cid/cit103. In this prospective cohort of HIV-HCV coinfected patients with cirrhosis, 43 of 166 patients achieved SVR after treatment with pegylated interferon and ribavirin. Patients who achieved an SVR had a significantly lower rate of liver decompensation and overall mortality.
- 22.Rodriguez-Torres M, Slim J, Bhatti L, Sterling R, Sulkowski M, Hassanein T, et al. Peginterferon alfa-2a plus ribavirin for HIV-HCV genotype 1 coinfected patients: a randomized international trial. HIV Clin Trials. 2012;13(3):142–52. doi: 10.1310/hct1303-142. [DOI] [PubMed] [Google Scholar]
- 23.Mehta SH, Lucas GM, Mirel LB, Torbenson M, Higgins Y, Moore RD, et al. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS. 2006;20(18):2361–9. doi: 10.1097/QAD.0b013e32801086da. [DOI] [PubMed] [Google Scholar]
- 24.Thomas DL. The challenge of hepatitis C in the HIV-infected person. Annual Rev Med. 2008;59:473–85. doi: 10.1146/annurev.med.59.081906.081110. [DOI] [PubMed] [Google Scholar]
- 25.Zein NN, Rakela J, Krawitt EL, Reddy KR, Tominaga T, Persing DH. Hepatitis C virus genotypes in the United States: epidemiology, pathogenicity, and response to interferon therapy. Ann Intern Med. 1996;125(8):634–9. doi: 10.7326/0003-4819-125-8-199610150-00002. [DOI] [PubMed] [Google Scholar]
- 26.Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84(11):1744–50. doi: 10.1002/jmv.23399. [DOI] [PubMed] [Google Scholar]
- 27.Pawlotsky JM, Chevaliez S, McHutchison JG. The hepatitis C virus life cycle as a target for new antiviral therapies. Gastroenterology. 2007;132(5):1979–98. doi: 10.1053/j.gastro.2007.03.116. [DOI] [PubMed] [Google Scholar]
- 28.Naggie S, Patel K, McHutchison J. Hepatitis C virus directly acting antivirals: current developments with NS3/4A HCV serine protease inhibitors. J Antimicrob Chemother. 2010;65(10):2063–9. doi: 10.1093/jac/dkq284. [DOI] [PubMed] [Google Scholar]
- 29.Scheel TK, Rice CM. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nature Med. 2013;19(7):837–49. doi: 10.1038/nm.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351(5):451–9. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-García J, Lazzarin A, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351(5):438–50. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 32.Laguno M, Murillas J, Blanco JL, Martínez E, Miquel R, Sánchez-Tapias JM, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for treatment of HIV/HCV coinfected patients. AIDS. 2004;18(13):F27–36. doi: 10.1097/00002030-200409030-00003. [DOI] [PubMed] [Google Scholar]
- 33.Carrat F, Bani-Sadr F, Pol S, Rosenthal E, Lunel-Fabiani F, Benzekri A, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA. 2004;292(23):2839–48. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 34.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–16. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 35.Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365(11):1014–24. doi: 10.1056/NEJMoa1014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364(25):2417–28. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 37 •.Sulkowski MS, Sherman KE, Dieterich DT, Bsharat M, Mahnke L, Rockstroh JK, et al. Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann Intern Med. 2013;159(2):86–96. doi: 10.7326/0003-4819-159-2-201307160-00654. This phase 2 trial demonstrated the clinical efficacy of telaprevir in HIV-HCV coinfected patients. HIV coinfected, HCV treatment naïve patients treated with telaprevir plus P/R achieved an SVR of 74% while patients treated with placebo plus P/R achieved an SVR of 45%.
- 38.Rodriguez-Torres M. Focus on drug interactions: the challenge of treating hepatitis C virus infection with direct-acting antiviral drugs in the HIV-positive patient. Current Opin Inf Dis. 2013;26(1):50–7. doi: 10.1097/QCO.0b013e32835c2027. [DOI] [PubMed] [Google Scholar]
- 39.Van Heeswijk R, Garg V, Boogaerts G, Vandebosch A, Luo D, Witek J, et al. The pharmacokinetic interaction between telaprevir and raltegravir in healthy volunteers. 51st Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); Chicago, IL. September 17-20, 2011; [Abstract A1-1738a] [Google Scholar]
- 40.Van Heeswijk R, Vandevoorde A, Boogaerts G, et al. Pharmacokinetic interactions between ARV agents and the investigational HCV protease inhibitor TVR in healthy volunteers. 18th Conference on Retroviruses and Opportunistic Infections (CROI 2011); Boston, MA. February 27 – March 2, 2011; [Abstract 119] [Google Scholar]
- 41.Kakuda T, Leopold L, Nijs S, et al. Pharmacokinetic interaction between etravirine or rilpivirine and telaprevir in healthy volunteers: a randomized, two-way crossover trial. 13th International Workshop on Clinical Pharmacology of HIV Therapy; Barcelona, Spain. April 16-18, 2012; [Abstract O18] [DOI] [PubMed] [Google Scholar]
- 42.Johnson M, Borland J, Chen S-J, Savina P, Wynne B, Piscatelli S. Dolutegravir, boceprevir, telaprevir PK: the effect of boceprevir and telaprevir on dolutegravir pharmacokinetics, in healthy adult subjects. 14th International Workshop on Clinical Phamacology of HIV Therapy; Amsterdam, the Netherlands. April 22-24, 2013; [Abstract O16] [Google Scholar]
- 43.Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195–206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1207–17. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flamm SL, Lawitz E, Jacobson I, Bourlière M, Hezode C, Vierling JM, et al. Boceprevir with peginterferon alfa-2a–ribavirin is effective for previously treated chronic hepatitis C genotype 1 infection. YJCGH. 2013;11(1):81–7. doi: 10.1016/j.cgh.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 46 •.Sulkowski M, Pol S, Mallolas J, Fainboim H, Cooper C, Slim J, et al. Boceprevir versus placebo with pegylated interferon alfa-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double-blind, controlled phase 2 trial. Lancet Inf Dis. 2013;13(7):597–605. doi: 10.1016/S1473-3099(13)70149-X. This phase 2 trial demonstrated the clinical efficacy of boceprevir in HIV-HCV coinfected patients. HIV coinfected, HCV treatment naïve patients treated with boceprevir plus P/R achieved an SVR of 63% while patients treated with placebo plus P/R achieved an SVR of 29%.
- 47.Kassera C, Hughes E, Treitel M, et al. Clinical pharmacology of BOC: metabolism, excretion, and drug-drug interactions. 18th Conference on Retroviruses and Opportunistic Infections (CROI 2011); Boston, MA. February 27 – March 2, 2011; [Abstract 118] [Google Scholar]
- 48.Hulskotte E, Feng H, Xuan F, et al. Pharmacokinetic interaction between the HCV protease inhibitor boceprevir and ritonavir-boosted HIV-1 protease inhibitors atazanavir, lopinavir, and darunavir. 19th Conference on Retroviruses and Opportunistic Infections (CROI 2012); Seattle, WA. March 5-8, 2012; [Abstract 771LB] [Google Scholar]
- 49.Garraffo R, Poizot-Martin I, Piroth L, Pol TLS, Bellissant E, Teicher E, et al. Pharmacokinetic (PK) interactions between boceprevir (BOC) and atazanavir/r (ATV/r) or raltegravir (RAL) in HIV/HCV coinfected patients (pts). 14th International Workshop on Clinical Pharmacology on HIV Therapy; Amsterdam, the Netherlands. April 22-24, 2013; [Abstract O-15] [Google Scholar]
- 50.De Kanter C, Blonk M, Colbers A, et al. Influence of the HCV protease inhibitor boceprevir on the pharmacokinetics of the HIV integrase inhibitor raltegravir. 19th Conference on Retroviruses and Opportunistic Infections (CROI 2012); Seattle, WA. March 5-8, 2012; [Abstract 772LB] [Google Scholar]
- 51.Hammond KP, Wolfe P, Burton JR, Jr, Predhomme JA, Ellis CM, Ray ML, et al. Pharmacokinetic interaction between boceprevir and etravirine in HIV/HCV seronegative volunteers. J Acquir Immune Defic Syndr. 2013;62(1):67–73. doi: 10.1097/QAI.0b013e318275da93. [DOI] [PubMed] [Google Scholar]
- 52.Vourvahis M, Plotka A, Kantaridis C, Fang A, Heera J. Maraviroc, boceprevir, telaprevir PK in healthy volunteers: the effect of boceprevir and telaprevir on the pharmacokinetics of maraviroc: an open-label, fixed-sequence study in healthy volunteers. 14th International Workshop on Clinical Pharmacology of HIV Therapy; Amsterdam, the Netherlands. April 22-24, 2013; [Abstract O17] [Google Scholar]
- 53.Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Last accessed: September 19, 2013]. Accessed at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. [Google Scholar]
- 54.European Medicines Agency [Last accessed: September 19, 2013];Victrelis: EPAR - Product Information. Annex I: Summary of Product Characteristics. Accessed at: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002332/WC500109786.pdf.
- 55.Hezode C, Dorival C, Zoulim F, et al. Safety of telaprevir or boceprevir in combination with peginterferon alfa/ribavirin, in cirrhotic non responders: first results of the French early access program (ANRS CO20-CUPIC) J Hepatol. 2012;56:S4. [Google Scholar]
- 56.Fried M, Reddy K, Di Bisceglie A, Jensen D, Jacobson I, Sulkowski MS, et al. HCV-TARGET: a longitudinal, observational study of North American patients with chronic hepatitis C treated with boceprevir or telaprevir. 48th Annual Meeting of the European Association for the Study of the Liver (EASL); Amsterdam, the Netherlands. April 24-28, 2013; [Abstract 818] [Google Scholar]
- 57.Lawitz E, Lalezari JP, Hassanein T, Kowdley KV, Poordad FF, Sheikh AM, et al. Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naïve patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Inf Dis. 2013;13(5):401–8. doi: 10.1016/S1473-3099(13)70033-1. [DOI] [PubMed] [Google Scholar]
- 58 ••.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–87. doi: 10.1056/NEJMoa1214853. The NEUTRINO trial, reported in concert with the FISSION trial in this article, demonstrated the efficacy of sofosbuvir in combination with P/R. This regimen may become part of the new standard of care for HCV genotype 1 infection.
- 59.Kowdley K, Hassanein T, Gane E, Hyland R, Ma J, Symonds W, et al. Sofosbuvir safety and tolerability in 741 patients treated for up to 24 weeks. 48th Annual Meeting of the European Association for the Study of the Liver (EASL); Amsterdam, the Netherlands. April 24-28, 2013; [Abstract 842] [Google Scholar]
- 60 •.Rodriguez-Torres M, Rodriguez-Orengo J, Gaggar A, Shen G, Symonds B, McHutchison J, et al. Sofosbuvir and peginterferon alfa-2a/ribavirin for treatment-naïve genotype 1-4 HCV-infected patients who are coinfected with HIV. IDWeek 2013 (Infectious Diseases Society of America); San Francisco, CA. October 2-6, 2013; [Abstract 714] This recent report highlights the effectiveness of sofosbuvir in combination with P/R in HIV-HCV coinfected patients. This regimen may become part of the new standard of care for HIV-HCV coinfected patients.
- 61.Jacobson I, Ghalib R, Rodriguez-Torres M, Younossi Z, Corregidor A, Sulkowski M, et al. SVR results of a once-daily regimen of simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in cirrhotic and non-cirrhotic HCV genotype 1 treatment-naïve and prior null responder patients: the COSMOS study. 64th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); Washington, DC. November 1-5, 2013; [Abstract LB-3] [Google Scholar]
- 62.Sulkowski M, Gardiner DF, Rodriguez-Torres M, et al. Sustained virologic response with daclatasvir plus sofosbuvir±ribavirin (RBV) in chonic HCV genotype (GT) 1-infected patients who previously failed telaprevir (TVR) or boceprevir (BOC). 48th Annual Meeting of the European Association for the Study of the Liver (EASL); Amsterdam, the Netherlands. April 24-28, 2013; [Abstract 1417] [Google Scholar]
- 63.Sulkowski M, Gardiner D, Rodriguez-Torres M, Reddy K, Hassanein T, Jacobson I, et al. High rate of sustained virologic response with the all-oral combination of daclatasvir (NS5A inhibitor) plus sofosbuvir (nucelotide NS5B inhibitor), with or without ribavirin, in treatment-naïve patients chronically infected with HCV GT 1, 2, or 3. 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); Boston, MA. November 9-12, 2012; [Abstract LB-2] [Google Scholar]
- 64.Osinusi A, Meissner EG, Lee Y-J, et al. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA. 2013;310(8):804. doi: 10.1001/jama.2013.109309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65 •.Sulkowski M, Rodriguez-Torres M, Lalezari J, Fessel W, Mounzer K, Shuhart M, et al. All-oral therapy with sofosbuvir plus ribavirin for the treatment of HCV genotype 1, 2, and 3 infection in patients coinfected with HIV (PHOTON-1). 64th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); Washington, DC. November 1-5, 2013; [Abstract 66] This study is one of the first to evaluate an all-oral DAA regimen in HIV-HCV coinfected patients. This regimen may become part of the new standard of care for HIV-HCV coinfected patients.
- 66.Kirby B, Mathias A, Rossi S, et al. No clinically significant pharmacokinetic drug interactions between sofosbuvir (GS-7977) and HIV antiretrovirals Atripla, rilpivirine, darunavir/ritonavir, or raltegravir in healthy volunteers. 63rd Annual Meeting of the American Association of the Study of Liver Diseases (AASLD); Boston, MA. November 9-13, 2012; [Abstract 1877] [Google Scholar]
- 67.Reesink HW, Fanning GC, Farha KA, et al. Rapid HCV-RNA decline with once daily TMC435: a phase I study in healthy volunteers and hepatitis C patients. YGAST. 2010;138(3):913–21. doi: 10.1053/j.gastro.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 68.Moreno C, Berg T, Tanwandee T, Thongsawat S, Van Vlierberghe H, Zeuzem S, et al. Antiviral activity of TMC435 monotherapy in patients infected with HCV genotypes 2-6: TMC435-C202, a phase IIa, open-label study. J Hepatol. 2012;56(6):1247–53. doi: 10.1016/j.jhep.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 69.Jacobson I, Dore G, Foster GR, et al. Simeprevir (TMC435) with peginterferon/ribavirin for chronic HCV genotype 1 infection in treatment-naïve patients: results from QUEST-1, a phase III trial. 48th Annual Meeting of the European Association for the Study of the Liver (EASL); Amsterdam, the Netherlands. April 24-28, 2013; [Abstract 1525] [Google Scholar]
- 70.Manns M, Marcellin P, Poordad F, et al. Simeprevir (TMC435) with peginterferon/ribavirin for treatment of chronic HCV genotype-1 infection in treatment-naïve patients: results from QUEST-2, a phase III trial. 48th Annual Meeting of the European Association for the Study of the Liver (EASL); Amsterdam, the Netherlands. April 24-28, 2013; [Abstract 1413] [Google Scholar]
- 71.Zeuzem S, Berg T, Gane E, et al. TMC435 in HCV genotype 1 patients who have failed previous pegylated interferon/ribavirin treatment: final SVR24 results of the ASPIRE trial. 47th Annual Meeting of the European Association for the Study of the Liver (EASL); Barcelona, Spain. April 18-22, 2012; [Abstract 2] [Google Scholar]
- 72.Lawitz E, Forns X, Zeuzem S, Gane E, Bronowicki J-P, Andreone P, et al. Simeprevir (TMC435) with peginterferon/ribavirin for treatment of chronic HCV genotype 1 infection in patients who relapsed after previous interferon-based therapy: results from PROMISE, a phase III trial. Digestive Disease Week 2013; Orlando, FL. May 18-21, 2013; [Abstract 869b] [Google Scholar]
- 73. [Last accessed 8 Nov 2013];FDA Antiviral Drugs Advisory Committee Meeting Briefing Information. http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/ucm371875.htm.
- 74 •.Dieterich D, Rockstroh J, Orkin C, et al. Simeprevir (TMC435) plus pegylated interferon/ribavirin in patients co-infected with HCV genotype-1 and HIV-1: primary analysis of the TMC435-C212 study. 14th European AIDS Conference (EACS 2013); Brussels, Belgium. October 16-19, 2013; [Abstract PS9/5] These results highlight the use of simeprevir in HIV-HCV coinfected patients. This study supports the use of simeprevir in HIV-HCV coinfected patients.
- 75.Ouwerkerk-Mahadevan S, Sekar V, Peeters M, Beumont-Mauviel M. The pharmacokintic interactions of HCV protease inhibitor TMC435 with rilpivirine, tenofovir, efavirenz or raltegravir in healthy volunteers. 19th Conference on Retroviruses and Opportunistic Infections (CROI 2012); Seattle, WA. March 5-8, 2012; [Abstract 49] [Google Scholar]
- 76.White P, Llinas-Brunet M, Amad M, et al. Preclinical characterization of non covalent HCV NS3/4A protease inhibitor BI 201335. J Hepatol. 2010;52(Suppl 1):S302. [Google Scholar]
- 77.Jensen D, Asselah T, Dieterich D, Foster G, Sulkowski M, Zeuzem S, et al. A pooled analysis of two randomized, double-blind placebo-controlled phase III trials (STARTVerso1&2) of faldaprevir plus pegylated interferon alfa-2a and ribavirin in treatment-naïve patients with chronic hepatitis C genotype-1 infection. 64th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); Washington, DC. November 1-5, 2013; [Abstract 1088] [Google Scholar]
- 78.Jacobson I, Asselah T, Ferenci P, Foster G, Jensen D, Negro F, et al. STARTVerso3: A randomized, double-blind, placebo-controlled phase III trial of faldaprevir in combination with pegylated interferon alfa-2a and ribavirin in treatment-experienced patients with chronic hepatitis C genotype-1 infection. 64th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); Washington, DC. November 1-5, 2013; [Abstract 1100] [Google Scholar]
- 79 •.Rockstroh J, Nelson M, Soriano V, Arasteh K, Guardiola J, Bhagani S, et al. STARTVerso 4 phase III trial of faldaprevir plus peg interferon alfa-2a and ribavirin (PR) in patients with HIV and HCV genotype 1 coinfection: end of treatment response. 64th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); Washington, DC. November 1-5, 2013; [Abstract 1099] These interim results from this phase 2 trial demonstrate another DAA regimen in HIV-HCV coinfected patients. Early results reveal high end of treatment responses that vary regarding to genotype and prior treatment experience.
- 80.Sabo J, Kort J, Haschke M, et al. Pharmacokinetic interactions of darunavir/ritonavir, efavirenz, and tenofovir with the hepaitis C virus protease inhibitor faldaprevir in healthy volunteers. 20th Conference on Retroviruses and Opportunistic Infections (CROI 2013); Atlanta, GA. March 3-6, 2013; [Abstract 35] [Google Scholar]
- 81.Lok A, Gardiner D, Hezode C, et al. Sustained virologic response in chronic HCV genotype (GT) 1-infected null responders with combination of daclatasvir (DCV; NS5A inhibitor) and asunaprevir (ASV; NS3 inhibitor) with or without peginterferon alfa-2a/ribavirin (PEG/RBV). 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); Boston, MA. November 9-13, 2012; [Abstract 79] [Google Scholar]
- 82.Sulkowski M, Gardiner D, Rodriguez-Torres M, Reddy K, Hassanein T, Jacobson I, et al. High rate of sustained virologic response with the all-oral combination of daclatasvir (NS5A inhibitor) plus sofosbuvir (nucleotide NS5B inhibitor), with or without ribavirin, in treatment-naïve patients chronically infected with HCV GT 1, 2, or 3. 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); Boston, MA. November 9-12, 2012; [Abstract LB-2] [Google Scholar]
- 83.Bifano M, Hwang C, Oosterhuis B, Hartstra J, Grasela D, Tiessen R, et al. [Last accessed: October 7, 2013];Assessment of pharmacokinetic interactions of the HCV NS5A replication complex inhibitor daclatasvir with antiretroviral agents: ritonavir-boosted atazanavir, efavirenz and tenofovir. Antiviral Ther. doi: 10.3851/IMP2674. Epub August 20, 2013. [DOI] [PubMed] [Google Scholar]
- 84.Kowdley K, Lawitz E, Poordad F, et al. Safety and efficacy of interferon-free regimens of ABT-450/R, ABT-267, ABT-333±ribavirin in patients with chronic HCV GT1 infection: results from the AVIATOR study. 48th Annual Meeting of the European Association for the Study of the Liver (EASL); Amsterdam, the Netherlands. April 24-28, 2013; [Abstract 3] [Google Scholar]
- 85.Thompson A, Hans S, Shiffman M, et al. GS-5885 (ledipasvir)+GS-9451+peginterferon and ribavirin (PR) for six or twelve weeks achieves high SVR12 rates in treatment-naïve genotype 1 IL28B CC patients. 48th Annual Meeting of the European Association for the Study of the Liver (EASL); Amsterdam, the Netherlands. April 24-28, 2013; [Abstract 64] [Google Scholar]
- 86.Everson G, Di Bisceglie A, Vierling J, et al. Combination of the NS5A inhibitor, GS5885, the NS3 protease inhibitor, GS-9451, and pegylated interferon plus ribavirin in treatment experienced patients with genotype 1 hepatitis C infection. 48th Annual Meeting of the European Association for the Study of the Liver (EASL); Amsterdam, the Netherlands. April 24-28, 2013; [Abstract 13] [Google Scholar]
- 87.Gane E, Stedman CA, Hyland RH, et al. Once daily sofosbuvir/ledipasvir fixed dose combination with or without ribavirin: the ELECTRON trial. 64th Annual Meeting of the American Society for the Study of Liver Diseases (AASLD); Washington, DC. Nov 1-5, 2013; [Abstract 73] [Google Scholar]
- 88.Lawitz E, Poordad F, Hyland RH, et al. Once daily sofosbuvir/ledipasvir fixed dose combination with or without ribavirin resulted in >95 % sustained virologic response in patients with HCV genotype 1, including patients with cirrhosis: the LONESTAR trial. 64th Annual Meeting of the American Association for the Study of Liver Diseases; Washington, DC. Nov 1-5, 2013; [Abstract 215] [Google Scholar]
- 89.Everson G, Sims K, Thuluvath PJ, et al. Phase 2b study of the interferon-free and ribavirin-free combination of daclatasvir, asunaprevir, and BMS-791325 for 12 weeks in treatment-naive patients with chronic HCV genotype 1 infection. 64th Annual Meeting of the American Society for the Study of Liver Diseases (AASLD); Washington, DC. November 1-5, 2013; [Abstract LB-1] [Google Scholar]
- 90.Zeuzem S, Soriano V, Asselah T, Bronowicki J-P, Lohse AW, Müllhaupt B, et al. Faldaprevir and deleobuvir for HCV genotype 1 infection. N Engl J Med. 2013;369(7):630–9. doi: 10.1056/NEJMoa1213557. [DOI] [PubMed] [Google Scholar]
- 91.Boehringer Ingelheim Pharmaceuticals, Inc. [Last accessed: September 19, 2013];Boehringer Ingelheim expands investigation of interferon-free hepatitis C treatment regimens to reach more patient types through Presidio Pharmaceuticals clinical collaboration. Accessed at: us.boehringer-ingelheim.com/news_events/press_release_archive/2013/09-10-13-boehringeriongelheim-expands-investigation-interferon-free-hepatitis-c-treatment-regimens-reach-more-patient-types-presidio-pharmaceuticals-clinical-collaboration.html.
- 92.Sherman KE. Managing adverse effects and complications in completing treatment for hepatitis C virus infection. Top Antivir Med. 2012;20(4):125–8. [PMC free article] [PubMed] [Google Scholar]
- 93.Jacobson IM, Kowdley KV, Kwo PY. Anemia management in the era of triple combination therapy for chronic HCV. Gastroenterol Hepatol. 2012;8(9; Suppl 6):1–16. [PMC free article] [PubMed] [Google Scholar]
- 94.Sulkowski M, Roberts S, Afdhal N, et al. Ribavirin dose modification in treatment-naïve and previously treated patients who received telaprevir combination treatment: no impact on sustained virologic response in phase 3 studies. J Hepatol. 2011;54(Suppl 1):S195. [Google Scholar]
- 95.Yu JW, Sun LJ, Zhao YH, Kang P, Yan BZ. The study of relationship between neutropenia and infection during treatment with peginterferon α and ribavirin for chronic hepatitis C. Eur J Gastroenterol Hepatol. 2011;23(12):1192–9. doi: 10.1097/MEG.0b013e32834c5b32. [DOI] [PubMed] [Google Scholar]
- 96.Payer BA, Reiberger T, Breitenecker F, Aichelburg MC, Schuster C, Heil PM, et al. The risk of infections in HIV-HCV coinfected patients during antiviral therapy with pegIFN+RBV. J Infection. 2012;65(2):142–9. doi: 10.1016/j.jinf.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 97.Chopra A, Klein PL, Drinnan T, Lee SS. How to optimize HCV therapy in genotype 1 patients: management of side-effects. Liver Int. 2013;33:30–4. doi: 10.1111/liv.12080. [DOI] [PubMed] [Google Scholar]