SUMMARY
Cytokeratin-18 (CK-18) is a major intermediate filament protein in liver cells. The M30 fragment of CK-18 has been identified as a useful marker of apoptosis associated with fibrosis and steatosis in nonalcoholic steatohepatitis (NASH). We sought to assess the relationship of this marker and steatosis in a cohort of adult patients with chronic hepatitis C. The study cohort included sera from 267 treatment-naïve chronic hepatitis C (CHC) patients and 100 healthy controls with normal alanine aminotransferase (ALT). Biopsies from CHC patients were assessed for METAVIR fibrosis stage, Histology Activity Index (HAI) inflammation score and steatosis grade by expert histopathologists. The M30 fragment of CK-18 was quantified by ELISA. Wilcoxon Rank Sum, Spearman Correlation and Linear Regression tests were performed for statistical analysis. Median CK-18 levels were higher in CHC patients compared to controls (411 vs 196 U/L, P < 0.0001). Fibrosis stage was associated with increasing serum CK-18 levels (P = 0.015) and CK-18 levels were higher for F2–F4 vs F0–F1 (500 vs 344 U/L; P = 0.001). There was no association between CK-18 and increasing steatosis grade 1, 2 or 3 (460.7 vs 416.8 vs 508.3 U/L; P = 0.35) and presence or absence of steatosis (445.3 vs 365.8 U/L; P = 0.075). Fibrosis stage was independently associated with serum M30 in a multivariable linear regression model (P = 0.03). CK-18 levels were higher in CHC compared to healthy controls and associated with hepatic fibrosis. There was no difference in CK-18 M30 levels between CHC patients with and without steatosis. Although apoptosis may still contribute to hepatitis C virus (HCV)-mediated steatosis, our results suggest that serum CK-18 will not be a clinically useful test for identifying significant steatosis in CHC.
Keywords: apoptosis, chronic hepatitis C, cytokeratin 18, fibrosis, steatosis
INTRODUCTION
Chronic hepatitis C (CHC) is a global health problem infecting an estimated 170 million people [1]. Most patients develop chronic infection and are at risk for developing cirrhosis and complications of end-stage liver disease including hepatocellular carcinoma [2–4]. Steatosis is a common histologic finding in patients with chronic hepatitis C, seen in up to 70% of patients depending on viral genotype [5–7]. Steatosis has been associated with both fibrosis progression and decreased virologic response to treatment in patients with CHC [8–12]. Steatosis is a histological diagnosis, and although noninvasive methods for determination of steatosis are emerging, currently, the only reliable way to assess for steatosis is with a liver biopsy [13,14]. The identification of serologic markers specific for steatosis could provide additional prognostic information for patients with CHC without the risks associated with liver biopsy.
The M30 fragment of CK-18 is a serologic marker of apoptosis that has been associated with increased severity of liver disease in patients with CHC and nonalcoholic fatty liver disease (NAFLD) [15,16]. In contrast to studies in NAFLD that demonstrate minimal correlation between CK-18 and simple steatosis [17], a recent small study in 23 paediatric CHC patients indicated a significant association for CK-18 fragment M30 and increasing steatosis grade [18].
The aim of this study was to determine whether levels of M30 in the serum of adult patients with CHC were associated with steatosis independently of inflammation and fibrosis.
MATERIALS AND METHODS
Patient population
Treatment-naïve patients with CHC and matched serum and liver biopsy samples were selected from the Duke Clinical Research Institute liver disease biorepository. Serum from 100 age-matched healthy individuals with normal alanine aminotransferase (ALT) was obtained from a commercial repository and were included as controls, with assumption of minimal fibrosis and absence of steatosis (<3% fat) (Bioreclamation, Hicksville, NY, USA).
Chronic hepatitis C was defined as the presence of detectable hepatitis C virus (HCV) RNA in the serum. Liver biopsies from CHC patients were scored by expert histopathologists using the METAVIR fibrosis stage (F0–F4) [19], HAI inflammation score [20], (mild/0 = 0–5, moderate/1 = 6–10, severe/2 = 11–16) and steatosis grade according to the percentage of hepatocytes containing fat droplets (0 ≤ 3%, 1 = 3–30%, 2 = 31–59% and 3 ≥ 59%). Serum was analysed for the M30 fragment of CK-18 by ELISA (Peviva AB, Bromma, Sweden) according to the manufacturer’s recommendations.
Statistical analysis
Descriptive results were reported using medians and interquartile ranges. Wilcoxon Rank Sum and Kruskal–Wallis nonparametric tests were used to compare values among two or more groups. Spearman’s correlation was used to assess relationships between two continuous variables. Linear regression was performed for multivariable analysis. Continuous variables were log transformed when appropriate to approximate normality. Statistical significance was assessed at P = 0.05 and data were analysed using the SAS™ 9.1 software (Cary, NC, USA).
RESULTS
Patient demographics
The study cohort included 267 CHC patients and 100 healthy controls. Baseline characteristics of the patients and controls are reported in Table 1.
Table 1.
Patient characteristics
| Characteristic | CHC | Normal controls |
|---|---|---|
| Sex | ||
| Male | 188/267 (70%) | 56/100 (56%) |
| Female | 79/267 (30%) | 44/100 (44%) |
| Ethnicity | ||
| Caucasian | 213/267 (80%) | 65 (65%) |
| Black | 6/267 (2%) | 32 (32%) |
| Latino | 32/267 (12%) | |
| Other or unknown | 16/267 (6%) | 3 (3%) |
| Age (median, IQR) | 44 (40–48) | 41.5 (31–48) |
| BMI (median, IQR) | 26.8 (25–30) | 27 (24–30) |
| ALT IU/L (median, IQR) | 87.0 (50–130) | 9 (7–13) |
| Genotype | ||
| 1 | 156/267 (58%) | na |
| 2 | 40/267 (15%) | |
| 3 | 53 (20%) | |
| 4 | 3 (1%) | |
| 6 | 2 (<1%) | |
| Unknown | 13/267 (5%) | |
| Fat grade | ||
| 0 | 130 (49%) | na |
| 1 | 92 (35%) | |
| 2 | 31 (12%) | |
| 3 | 14 (5%) | |
| Fibrosis stage | ||
| 0 | 35 (13%) | na |
| 1 | 125 (47%) | |
| 2 | 42 (16%) | |
| 3 | 35 (13%) | |
| 4 | 30 (11%) | |
| Inflammation | ||
| 0–5 | 79 (31%) | na |
| 6–10 | 110 (44%) | |
| 11–16 | 62 (25%) |
CHC, chronic hepatitis C, BMI, body mass index, ALT, alanine aminotransferase; na, not applicable.
CK-18 [M30] levels and steatosis
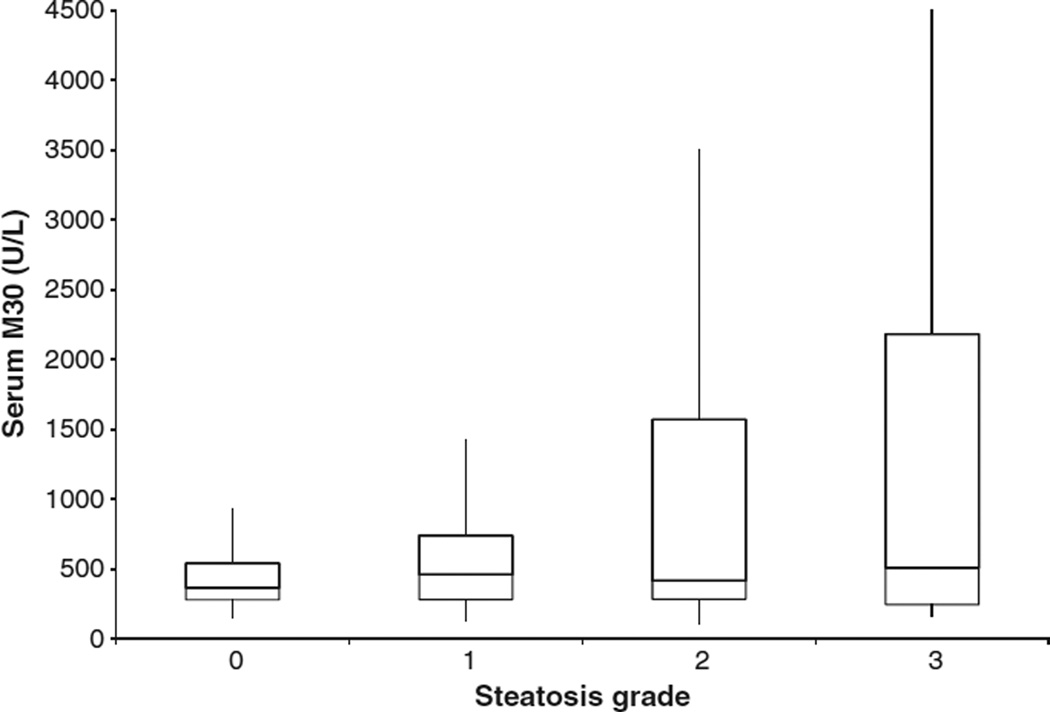
Compared to controls, patients with CHC had higher median serum M30 levels (196 vs 411 U/L; P < 0.0001). Median serum M30 levels were not different between the respective steatosis grades 0, 1, 2 or 3 (365.8 vs 460.7 U/L vs 416.8 U/L vs 508.3 U/L; P = 0.35) (Fig. 1), or between median serum M30 levels in patients with and without steatosis (445.3vs 365.8 U/L, respectively) (P = 0.075). There was no relationship between serum M30 levels and body mass index (BMI) (spearman correlation coefficient 0.11; P = 0.08).
Fig. 1.
Serum M30 levels and steatosis.
CK-18 [M30] levels with inflammation and fibrosis
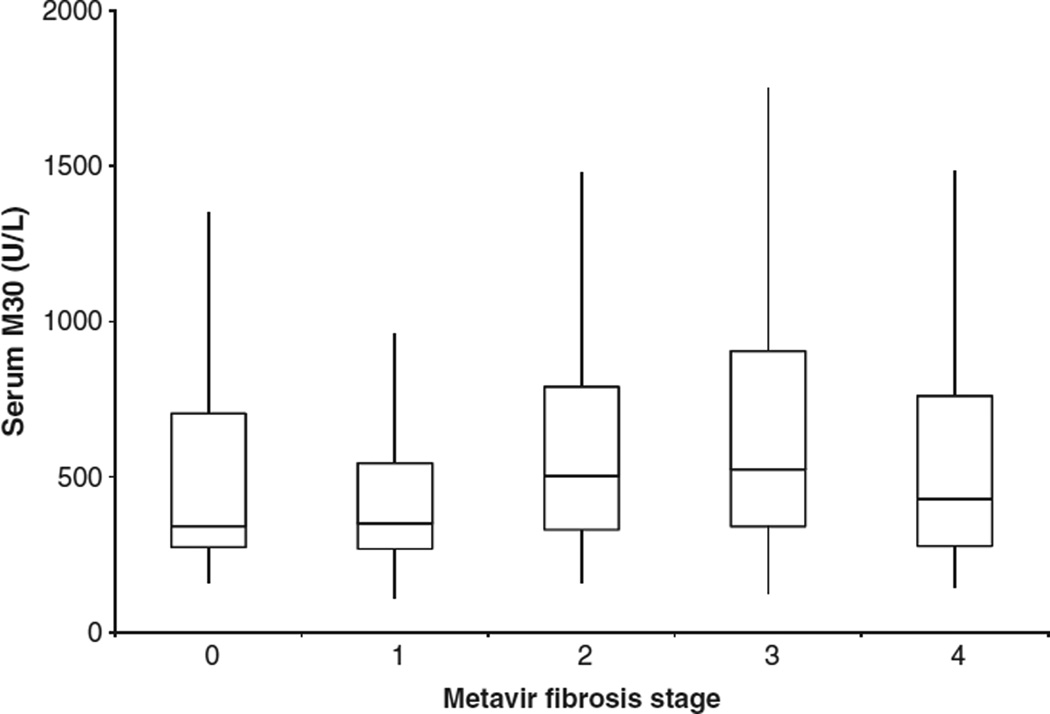
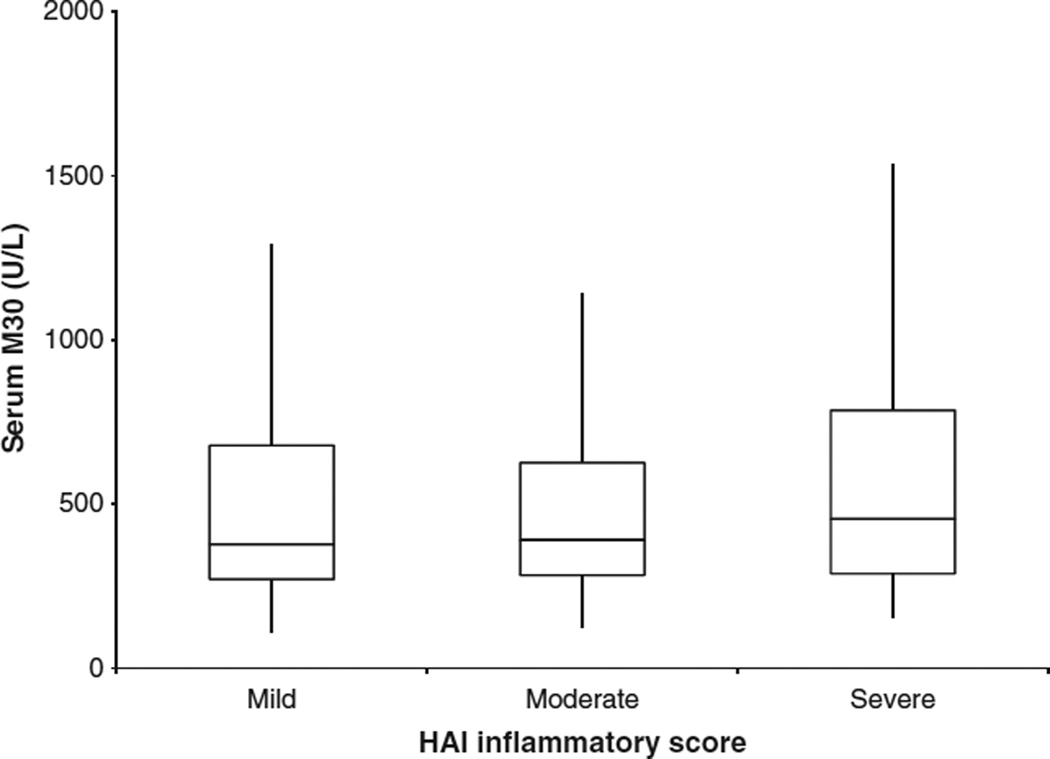
Median M30 levels differed by fibrosis stage; stage F0 341.5 U/L, F1 350 U/L, F2 502.4 U/L, F3 524 U/L and F4 427.9 U/L (P = 0.015) (Fig. 2). Patients with fibrosis stage F0–1 had lower M30 levels compared to significant fibrosis stage F2–4 (343.7 vs 500.1 U/L; P = 0.0014). Additionally, there was a relationship between ALT values and serum M30 (spearman coefficient 0.17; P = 0.007). There was no relationship between overall HAI inflammation and serum M30 (P = 0.44) (Fig. 3). In a linear regression model with serum M30 log transformed as the dependent variable and BMI, fat grade, fibrosis score, HAI score and serum ALT log transformed, fibrosis was the only variable independently associated with serum M30 (P = 0.03) (Table 2).
Fig. 2.
Serum M30 levels and fibrosis.
Fig. 3.
Serum M30 and inflammation.
Table 2.
Multivariable linear regression model of serum M30
| Variable | P value |
|---|---|
| BMI | 0.97 |
| Steatosis grade | 0.09 |
| METAVIR fibrosis stage | 0.03 |
| HAI inflammation score | 0.86 |
| Log ALT | 0.09 |
ALT, alanine aminotransferase; BMI, body mass index.
DISCUSSION
This retrospective single-centre cohort study indicated that CK-18 M30 levels were higher in CHC patients compared to healthy controls and were associated with significant hepatic fibrosis. However, there was no difference in CK-18 M30 levels between CHC patients with and without steatosis. Although apoptosis may still be associated with HCV-mediated steatosis, our results suggest that measuring serum CK-18 M30 alone will not be a clinically useful noninvasive tool for identifying significant steatosis in CHC patients. Additionally, although we found a statistically significant difference in serum M30 levels by degree of fibrosis, there is considerable overlap in the distribution of serum M30 levels. This suggests that the positive and negative predictive value for any given value of serum M30 will be of limited usefulness to the practicing clinician for assessing steatosis or fibrosis.
In patients with NAFLD, hepatocyte apoptosis plays a role in steatohepatitis progression and hepatic damage [21,22]. CK-18 M30 levels have been reported to differentiate patients with simple steatosis and steatohepatitis. Additionally, there has been a correlation described between elevated M30 levels and degree of liver fibrosis in patients with nonalcoholic steatohepatitis (NASH) and CHC [15,23]. Caspase activation may occur early in CHC and correlates with inflammatory liver injury [24]. We did not detect any significant association between CK-18 M30 and histologic inflammatory activity. The mechanisms leading to caspase activation and CK 18 M30 release into blood are not well understood and are dependent on relative degrees of hepatocyte apoptosis and necroinflammation.
Our study did not find a relationship between steatosis and serum CK18 M30 levels, although there were several limitations to our study cohort. The majority of patients in our cohort had low-level grades of steatosis and high levels of inflammation and fibrosis. Other studies that have selectively included cohorts with lesser degrees of inflammation and fibrosis have found a relationship between histologic measures of apoptosis and severe steatosis and stellate cell activation in CHC [25,26]. Perhaps more severe grades of steatosis may be associated with pro-apoptotic lipid peroxidation that leads to caspase activation independently of CHC-mediated inflammatory injury. Another limitation of our study is that we did not assess for histologic measures of apoptosis to further delineate these differences in relation to steatosis and necroinflammatory or fibrotic injury.
In summary, our study supports the finding that CK-18 M30 levels are higher in patients with more advanced fibrosis in CHC infection. However, CK-18 M30 levels were not significantly related to the degree of steatosis. This is consistent with the findings of differential CK-18 elevations in NAFLD patients with simple steatosis or steatohepatitis. Further studies will be required to determine the diagnostic and prognostic utility of apoptosis markers in addition to available serum and imaging methods for detection of significant steatosis and fibrosis in CHC infection.
Acknowledgments
None of the authors have served as a speaker, consultant or advisory board member for an organization related to the content of this manuscript. None of the authors are employed by or own stocks or shares in an organization related to the content of this manuscript. No authors have a patent related to the content of this manuscript.
Abbreviations
- ALT
alanine aminotransferase
- BMI
body mass index
- CHC
chronic hepatitis C
- HCV
hepatitis C virus
- NASH
nonalcoholic steatohepatitis.
Footnotes
DISCLOSURES
There are no relevant funding interests or grants related to this manuscript.
REFERENCES
- 1.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5(9):558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.Strader DB, Seeff LB. The natural history of chronic hepatitis C infection. Eur J Gastroenterol Hepatol. 1996;8(4):324–328. doi: 10.1097/00042737-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(5 Suppl 1):S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 4.Levine RA, Sanderson SO, Ploutz-Snyder R, et al. Assessment of fibrosis progression in untreated Irish women with chronic hepatitis C contracted from immunoglobulin anti-D. Clin Gastroenterol Hepatol. 2006;4(10):1271–1277. doi: 10.1016/j.cgh.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 5.Bach N, Thung SN, Schaffner F. The histological features of chronic hepatitis C and autoimmune chronic hepatitis: a comparative analysis. Hepatology. 1992;15(4):572–577. doi: 10.1002/hep.1840150403. [DOI] [PubMed] [Google Scholar]
- 6.Scheuer PJ, Ashrafzadeh P, Sherlock S, Brown D, Dusheiko GM. The pathology of hepatitis C. Hepatology. 1992;15(4):567–571. doi: 10.1002/hep.1840150402. [DOI] [PubMed] [Google Scholar]
- 7.Lefkowitch JH, Schiff ER, Davis GL, et al. Pathological diagnosis of chronic hepatitis C: a multicenter comparative study with chronic hepatitis B. The Hepatitis Interventional Therapy Group. Gastroenterology. 1993;104(2):595–603. doi: 10.1016/0016-5085(93)90432-c. [DOI] [PubMed] [Google Scholar]
- 8.Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33(6):1358–1364. doi: 10.1053/jhep.2001.24432. [DOI] [PubMed] [Google Scholar]
- 9.Poynard T, Ratziu V, McHutchison J, et al. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38(1):75–85. doi: 10.1053/jhep.2003.50267. [DOI] [PubMed] [Google Scholar]
- 10.Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126(2):586–597. doi: 10.1053/j.gastro.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Patton HM, Patel K, Behling C, et al. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol. 2004;40(3):484–490. doi: 10.1016/j.jhep.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Rubbia-Brandt L, Fabris P, Paganin S, et al. Steatosis affects chronic hepatitis C progression in a genotype specific way. Gut. 2004;53(3):406–412. doi: 10.1136/gut.2003.018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams LA, Feldstein AE. Non-invasive diagnosis of nonalcoholic fatty liver and nonalcoholic steatohepatitis. J Dig Dis. 2011;12(1):10–16. doi: 10.1111/j.1751-2980.2010.00471.x. [DOI] [PubMed] [Google Scholar]
- 14.Poynard T, Ratziu V, Charlotte F, et al. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:34. doi: 10.1186/1471-230X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papatheodoridis GV, Hadziyannis E, Tsochatzis E, et al. Serum apoptotic caspase activity in chronic hepatitis C and nonalcoholic Fatty liver disease. J Clin Gastroenterol. 2010;44(4):e87–e95. doi: 10.1097/MCG.0b013e3181c0945a. [DOI] [PubMed] [Google Scholar]
- 16.Bantel H, Lugering A, Heidemann J, et al. Detection of apoptotic caspase activation in sera from patients with chronic HCV infection is associated with fibrotic liver injury. Hepatology. 2004;40(5):1078–1087. doi: 10.1002/hep.20411. [DOI] [PubMed] [Google Scholar]
- 17.Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for non-alcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50(4):1072–1078. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valva P, De Matteo E, Galoppo MC, Gismondi MI, Preciado MV. Apoptosis markers related to pathogenesis of pediatric chronic hepatitis C virus infection: M30 mirrors the severity of steatosis. J Med Virol. 2010;82(6):949–957. doi: 10.1002/jmv.21699. [DOI] [PubMed] [Google Scholar]
- 19.Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20(1 pt 1):15–20. [PubMed] [Google Scholar]
- 20.Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1(5):431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 21.Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125(2):437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 22.Bantel H, Ruck P, Gregor M, Schulze-Osthoff K. Detection of elevated caspase activation and early apoptosis in liver diseases. Eur J Cell Biol. 2001;80(3):230–239. doi: 10.1078/0171-9335-00154. [DOI] [PubMed] [Google Scholar]
- 23.Younossi ZM, Jarrar M, Nugent C, et al. A novel diagnostic biomarker panel for obesity-related nonalcoholic steatohepatitis (NASH) Obes Surg. 2008;18(11):1430–1437. doi: 10.1007/s11695-008-9506-y. [DOI] [PubMed] [Google Scholar]
- 24.Bantel H, Lugering A, Poremba C, et al. Caspase activation correlates with the degree of inflammatory liver injury in chronic hepatitis C virus infection. Hepatology. 2001;34(4 pt 1):758–767. doi: 10.1053/jhep.2001.28229. [DOI] [PubMed] [Google Scholar]
- 25.Walsh MJ, Vanags DM, Clouston AD, et al. Steatosis and liver cell apoptosis in chronic hepatitis C: a mechanism for increased liver injury. Hepatology. 2004;39(5):1230–1238. doi: 10.1002/hep.20179. [DOI] [PubMed] [Google Scholar]
- 26.Seidel N, Volkmann X, Langer F, et al. The extent of liver steatosis in chronic hepatitis C virus infection is mirrored by caspase activity in serum. Hepatology. 2005;42(1):113–120. doi: 10.1002/hep.20747. [DOI] [PubMed] [Google Scholar]