Abstract
Many cellular stresses cause damages of intracellular proteins, which are eventually degraded by the ubiquitin and proteasome system. The proteasome is a multicatalytic protease complex composed of 20S core particle and the proteasome activators that regulate the proteasome activity. Extracellular mutants 29 (Ecm29) is a 200 kDa protein encoded by KIAA0368 gene, associates with the proteasome, but its role is largely unknown. Here, we generated KIAA0368-deficient mice and investigated the function of Ecm29 in stress response. KIAA0368-deficient mice showed normal peptidase activity and proteasome formation at normal condition. Under stressed condition, 26S proteasome dissociates in wild-type cells, but not in KIAA0368−/− cells. This response was correlated with efficient degradation of damaged proteins and resistance to oxidative stress of KIAA0368−/− cells. Thus, Ecm29 is involved in the dissociation process of 26S proteasome, providing clue to analyse the mechanism of proteasomal degradation under various stress condition.
Keywords: oxidative stress, proteasome, proteasome activators, protein degradation, ubiquitin
Various intracellular and extracellular stresses lead to modification on proteins, lipid and nucleic acids (1–3). Oxidative stress causes carbonylation of protein, and the abnormal accumulation of such modified proteins is related to Alzheimer’s disease, inflammatory bowel disease and age-related diseases such as proteopathy (4–8).
The proteasome is a multicatalytic protease complex that functions in eukaryotic intracellular protein degradation (9). The proteasome is composed of over 60 subunits, and the subunits can be divided into two particles, one is catalytic core particle (CP) and the other is proteasome activator. The CP is also called 20S proteasome. CP is a barrel shaped complex composed of four stacked rings. The rings are composed of two outer α rings and two inner β rings. Both of these two rings are composed of structurally resembled seven subunits named α1–7 and β1–7 subunits (10). Protein degradations are carried out by β1, β2 and β5 subunits in the CP. Each of these subunits is responsible for different peptidase activity, β1 for caspase-like activity, β2 for trypsin-like activity and β5 for chymotrypsin-like activity (11). Unless proteasome activators do not bind to the CP, α rings are generally closed and substrates are unable to enter to the inner core of the CP where the substrates are degraded (12–14).
The proteasome activators bind to both ends of CP and regulate the proteasome activity. Several kinds of proteasome activators were identified up to date, such as PA700 (also called 19S regulatory particle), PA28αβ hetero-heptamer, PA28γ homo-heptamer (also called 11S regulator or REG) and PA200 (15). 19S regulatory particle (RP) dedicated proteasome is called 26S proteasome and it fulfils degradation of ubiquitylated proteins. In ubiquitin-proteasome system, polyubiquitin chains are formed on the substrates and become a degradation signal for 26S proteasome (16). 19S RP has a role in the ubiquitin chain recognition, removal of ubiquitin chain from substrate and unfolding of the substrate (17–19). Binding of 19S RP to the CP requires ATP, but binding of other proteasome activators such as PA28 and PA200, does not. PA28αβ heterocomplex facilitates the antigen processing in MHC class Ι antigen presentation (20, 21). PA28γ complex plays important roles in growth and proliferation according to the knockout mice phenotypes (22). The function of PA200 is linked to spermatogenesis and DNA repair, because PA200-deficient mice show abnormalities in male reproduction and damaged DNA repair (23).
Extracellular mutants 29 (Ecm29), encoded by KIAA0368 gene, was identified as a 26S proteasome-associated protein. Ecm29 attaches on both the CP and the 19S RP (24). CP–RP interaction was destabilized in the ATP deprived condition in Ecm29 deletion mutant (25), therefore Ecm29 is thought to strengthen the tethering between 20S CP and 19S RP. On the other hand, other experiments suggested that Ecm29 has inhibitory effects against proteasome activity by inducing dissociation of CP–RP (26), or suppressing peptidase activities of aberrant proteasomes (27, 28). It is also proposed that Ecm29 plays a role in a checkpoint during CP maturation (29). In mammals, Ecm29 is located on the cytosolic surface of secretory components such as endoplasmic reticulum and golgi body and plays a role in the regulation of proteasome localization and signalling pathway (30–32).
Although Ecm29 in yeast has been reported to regulate the proteasomal CP–RP interactions, its physiological roles in mammals has not been analysed. Therefore, we generated KIAA0368-deficient mice and investigated its phenotypes and effects on the activities of mammalian proteasome.
Materials and Methods
Generation of KIAA0368-deficient mice
Mouse KIAA0368 gene was obtained from C57BL/6J mouse genomic DNA library. The targeting vector was constructed by cloning the 13.5 kb HindIII–HindIII fragment, and the insertion of 1.2 kb neomycin resistant gene cassette. The diphtheria toxin A gene (DTA) was used for negative selection. TT2 ES cells were transfected with the targeting vector and selected by G418 (SIGMA). ES cells heterozygous for KIAA0368 gene were microinjected to eight-cell stage ICR mice embryos to generate chimeric mice. Germline transmission of the mutant allele was identified by Southern blot analyses. Heterozygous mice were backcrossed for more than 10 generations on the C57BL/6 J background. KIAA0368 homozygous mice and their wild-type control littermates were obtained by heterozygous intercrossing. All animal care and experimental treatments were in accordance with the University of Tsukuba guidelines for animal care and use.
Preparation of mouse embryonic fibroblasts and cell culture
Mouse embryonic fibroblast (MEF) cells were isolated from 13.5 days post coitus embryos and cultured in Dulbecco’s modified Eagle’s medium (D-MEM, high glucose) (Wako) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Invitrogen) in 37°C, 5% CO2 incubator. MEF cells were immortalized by transfecting SV40 large T antigen as described previously (33).
Oxidative stress response
MEF cells were cultured in D-MEM containing various concentrations of H2O2 or H2O as control for indicated periods in the presence or absence of 20 μM MG132. Cells were subsequently subjected to cell viability assay and immunoblot analyses.
Cell viability assay
3 × 103 cells were stimulated with various concentrations of H2O2 for 2 h. After stimulation, medium was replaced with fresh complete medium and incubated for 16 h. Viable cells were measured by Cell Counting Kit-8 (Dojindo). The absorbance was measured at 450 nm. For trypan-blue exclusion assay, 1 × 105 cells were incubated in various concentrations of H2O2 for 2 h. After incubation, cells were trypsinized and mixed with equal volumes of 0.4% trypan-blue solution (SIGMA). Cells were counted on burkerturk hemocytometer, and the ratio of the blue-stained dead cells per total cells were calculated.
Immunoblot analyses
Organs were homogenized using Potter–Elvehjem homogenizer in five times volume per organ weight of homogenization buffer (25 mM Tris–HCl [pH 7.5], 250 mM Sucrose, 1 mM dithiothreitol [DTT] and 1 mM phenylmethylsulfonyl fluoride) and centrifuged at 20,000 × g for 30 min. Cells were lysed in ice-cold lysis buffer (50 mM Tris–HCl [pH 7.5], 5 mM MgCl2, 2 mM ATP, 0.5% NP-40 and 1 mM DTT) and centrifuged at 15,000 × g for 30 min. The supernatants were combined with sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer and boiled at 100°C for 5 min. Samples were subjected to SDS–PAGE and blotted onto PVDF membranes (PALL). Membranes were incubated in 5% non-fat dry milk in TBS-T (50 mM Tris–HCl [pH 7.5], 150 mM NaCl and 0.05% Tween 20) for blocking and treated with primary and secondary antibodies. Bands were visualized by Western Lightning Plus-ECL reagent (PerkinElmer Life Sciences).
Antibodies
Anti-PA28γ monoclonal (BD Transduction Laboratories; 611180), anti-α-tubulin monoclonal (SIGMA; T9026), anti-β5 rabbit polyclonal (Abcam; ab3330), anti-dinitrophenol (DNP) goat polyclonal (Bethyl Laboratories; A150-117 A) and anti-Multi Ubiquitin monoclonal (MBL, D058-3) antibodies were purchased. Anti-Ecm29, anti-β7 and anti-PA200 antibodies were raised by immunizing His-tagged recombinant Ecm29 (N-terminal 100 amino acids), β7 and PA200 (N-terminal 100 amino acids) proteins in rabbit, respectively. The quality of the antibodies were checked by enzyme-linked immunosorbent assay and immunoblot. Anti-PA28α and anti-Rpt1 antibodies are described previously (34, 35). Peroxidase-conjugated anti-rabbit and anti-mouse IgG antibodies (Jackson ImmunoResearch) were used as secondary antibodies for immunoblot analyses. Anti-ubiquitin rabbit polyclonal (Dako; Z0458) and Alexa Fluor 488-conjugated anti-rabbit IgG (Invitrogen) antibodies were used for immunohistological analyses.
Sedimentation velocity centrifugation
One milligram of tissue lysates or 500 μg of cell lysates were separated by glycerol density gradient ultracentrifugation (25 mM Tris–HCl [pH 7.5], 10–40% Glycerol, 5 mM MgCl2, 2 mM ATP and 1 mM DTT) at 25,000 rpm with SW41Ti rotor for 22 h. The gradients were fractionated using liquid layer injector fractionator (ADVANTEC) equipped with fraction collector (ATTO) into 23 or 32 fractions.
Measurement of proteasome peptidase activity
Ten microlitres of samples were incubated with 0.5 μM fluorogenic substrates in a final 100 μl of reaction mixture (50 mM Tris–HCl [pH 7.5], 25 mM KCl, 5 mM MgCl2, 1 mM ATP with or without 0.02% SDS) at 37°C. Succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarine (Suc-LLVY-AMC) (Peptide institute) was used as fluorogenic substrates. The fluorescence was measured using Multi label counter (PerkinElmer Life Sciences) at 355 nm excitation and 460 nm emission wavelengths. The results show fluorescence per protein amounts and reaction time.
Histology
Wild-type and KIAA0368-deficient mice of 8 weeks old were fixed in 4% paraformaldehyde in phosphate buffered saline by perfusion fixation. Fixed organs were paraffin embedded and sectioned at 2 µm. Sections were stained with haematoxylin and eosin (H&E) or subjected to immunohistochemical analyses.
Immunohistochemistry
Deparaffinized specimens were immersed in a citrate buffer (5 µM C6H8O7 and 5 µM Na3(C6H5O7) [pH 6.0]) and boiled for 3 min to retrieve antigens. The activated tissues were blocked in 2% goat serum and incubated with anti-ubiquitin rabbit polyclonal antibody followed by Alexa Fluor 488-conjugated anti-rabbit antibody. The slides were mounted with VECTASHIELD containing Hoechst 33342. The slides were observed using fluorescence microscope (Biorevo 9500).
DNA ladder assay
1 × 106 cells were incubated with various concentrations of H2O2 for 24 h. Cells were harvested and lysed in DNA extraction buffer (50 mM Tris–HCl [pH 8.0], 100 mM NaCl, 20 mM ethylenediaminetetraacetic acid (EDTA), 1% SDS, 2 mg/ml proteinase E and 0.3 mg/ml proteinase K) at 55°C for overnight. DNA were purified by phenol/chloroform extraction followed by ethanol precipitation and resuspended in a buffer (10 mM Tris–HCl [pH 8.0], 1 mM EDTA and 0.2 mg/ml RNase A). Approximately 0.5 μg of DNA were electrophoresed on 1% agarose gel at constant voltage of 200 V for 10 min. Gel was stained with ethidium bromide and visualized under UV-transilluminator.
Measurement of carbonylated protein
Carbonyl groups were detected as a marker of damaged proteins (36). Cells were lysed in RIPA buffer (50 mM Tris–HCl [pH 7.5], 150 mM NaCl, 1% NP-40, 1% Sodium deoxycholate, 0.1% SDS and 2 mM DTT). Five microlitres of lysates were denatured by adding 5 μl of 12% SDS and incubated for 5 min at room temperature. Samples were incubated with 5 μl of 2.5 mM 2,4-dinitrophenylhydrazine dissolved in 2.5% trifluoroacetic acid for 15 min for derivatization, and the reaction was stopped by adding 7.5 μl of 500 μM Tris and 10% glycerol. Approximately 7.5 μg of samples were subjected to immunoblot analyses using anti-DNP antibody.
Results
KIAA0368-deficient mice were viable and grew normally
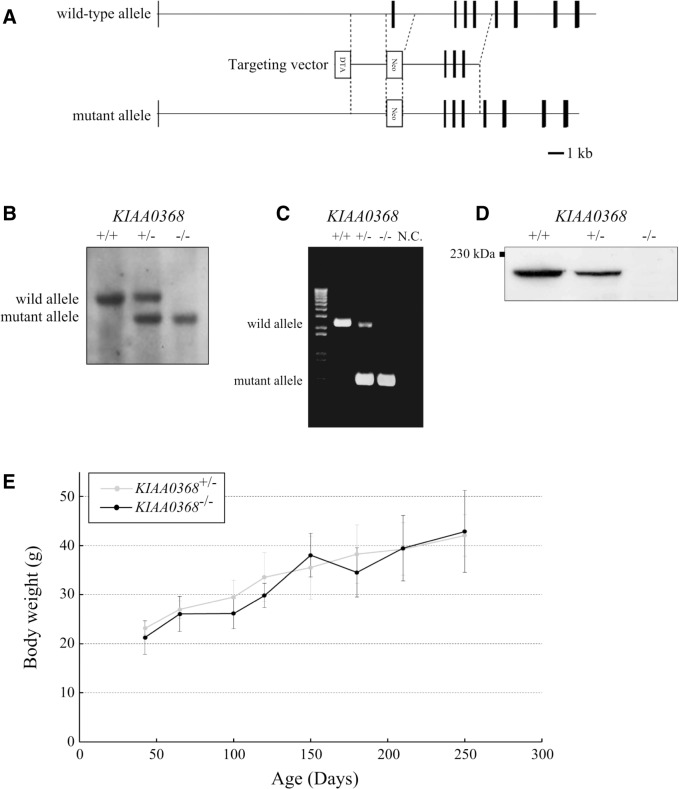
To investigate the physiological roles of Ecm29, we generated mice lacking KIAA0368 gene. The targeting vector was designed to replace exon 2 of the KIAA0368 gene with neomycin resistant gene cassette (Fig. 1A). The disruption of KIAA0368 gene was confirmed by Southern blot (Fig. 1B), and polymerase chain reaction (PCR) of genomic DNA (Fig. 1C). Heterozygous intercrossing yielded homozygous mice in predicted Mendelian ratio (Table I). Tissue blot of Ecm29 revealed its protein expression in various organs and the homozygous mice lacked the expression of Ecm29 suggesting that the mutation is a null mutation (Fig. 2D). The expression level of Ecm29 protein decreased dependent on the mutant gene dosage (Fig. 1D). The KIAA0368 homozygous mice grew normally (Fig. 1E), were fertile both male and female. Tissue morphologies on H&E staining were apparently normal at all major organs analysed, such as cerebrum, heart, kidney, testis, liver, pancreas and spleen (Supplementary Fig. S1).
Fig. 1.
Generation of KIAA0368-deficient mice. (A) Schematic structure of KIAA0368 wild-type allele, the targeting vector and the resulting mutant allele. The exons are illustrated by black boxes. Neo; neomycin-resistant gene cassette, DTA; diphtheria toxin A. Bar indicates 1 kb. (B) Southern blot analysis of genomic DNA. Tail genomic DNA were digested with HindIII. Wild-type allele was detected at 13 kb and the mutant allele at 10 kb. (C) Detection of wild-type and mutant allele by genomic PCR. Tail genomic DNA were subjected to PCR. Wild-type allele was detected at 2.2 kb and the mutant allele at 0.6 kb. NC; negative control. (D) Detection of Ecm29 protein by immunoblotting. Fifty micrograms of brain lysates of indicated genotypes were subjected to immunoblotting using antibody against Ecm29. (E) Body weights of mice with indicated genotypes. Both line charts represent the mean ± SD from five independent male mice.
Table I.
Number of offspring yielded from heterozygous intercrossing
| Sex | Genotype |
Total | ||
|---|---|---|---|---|
| +/+ | +/− | −/− | ||
| Male | ||||
| Numbers | 77 | 116 | 74 | 267 |
| Ratio (%) | 28.8 | 43.4 | 27.7 | 50.7 |
| Female | ||||
| Numbers | 67 | 133 | 60 | 260 |
| Ratio (%) | 25.8 | 51.2 | 23.1 | 49.3 |
| Total | ||||
| Numbers | 144 | 249 | 134 | 527 |
| Ratio (%) | 27.3 | 47.2 | 25.4 | 100.0 |
The numbers and ratio of offspring corresponding to their sex and genotype produced from heterozygous intercrossing. Data were acquired from 63 mating pairs.
Fig. 2.
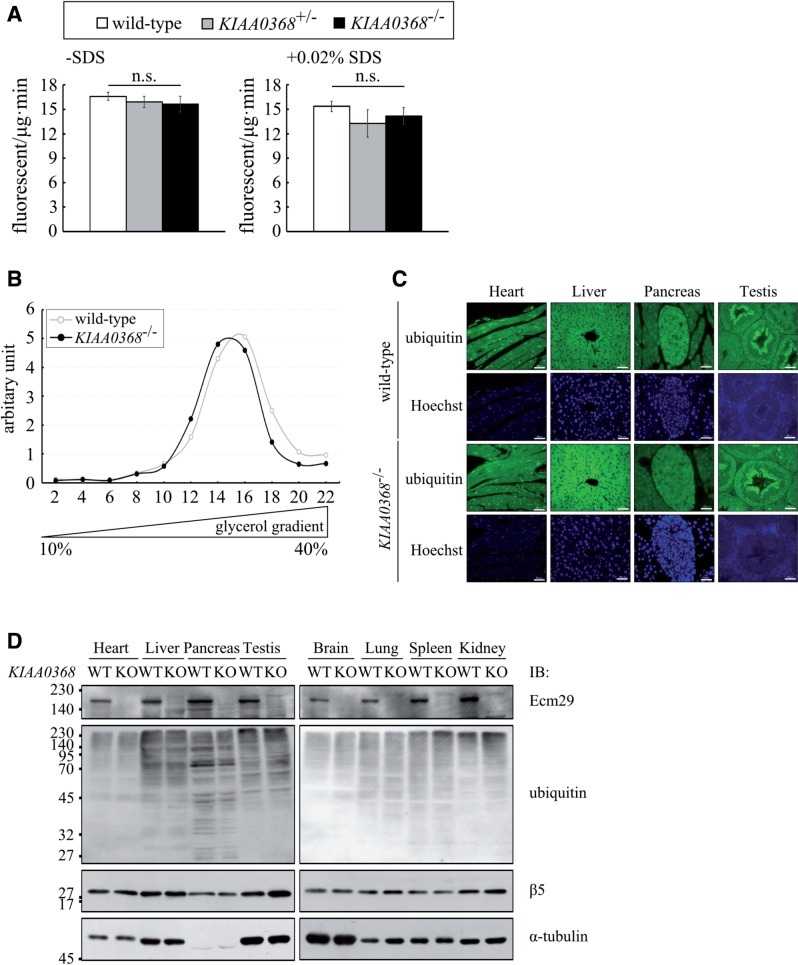
Proteasomal peptidase activities in KIAA0368-deficient mice. (A) Peptidase activities of testes lysates. Hundred micrograms of testes lysates from wild-type, heterozygous or homozygous mice for KIAA0368, were incubated with fluorescent substrates in the presence or absence of 0.02% SDS. The bar charts represent the mean ± SD of fluorescence/μg/min. LLVY; chymotrypsin-like activity. White bars; wild-type, grey bars; KIAA0368+/-, black bars; KIAA0368−/−. n.s.; not significant by student’s t-test. (B) Peptide hydrolysis activities of sedimentation velocity fractions from wild-type or KIAA0368-deficient testes lysates. 1 mg of testis lysates were fractionated by glycerol density gradient centrifugation (10–40% glycerol from 1 to 22 fractions). Suc-LLVY-AMC was used to measure the peptidase activities. The relative peptidase activities (fluorescence/min) normalized to the first fraction are shown. (C) Tissue sections from wild-type or KIAA0368-deficient mice were immunostained with ubiquitin antibody and counterstained with Hoechst 33342. Scale bars indicate 50 µm. (D) Tissue blots of wild-type (WT) and KIAA0368-deficient mice (KO). 50 μg of heart, liver, pancreas, testis, brain, lung, spleen and kidney lysates were subjected to immunoblot analyses using antibodies against Ecm29, Multi-ubiquitin, β5 and α-tubulin.
Proteasomal activities were not impaired in KIAA0368-deficient mice
We then measured proteasomal peptidase activities in tissue lysates of wild-type, heterozygous and homozygous mice for KIAA0368 (Fig. 2A). Their peptidase activities were not significantly altered in KIAA0368 homozygous mice compared to wild-type and heterozygous counterparts. To further confirm this, we separated the 26S proteasome by glycerol density gradient centrifugation. The peptidase activities of 26S proteasome in both wild-type and mutant mice were observed at fractions 14–16, and their peptidase activities were comparable (Fig. 2B). These results suggest that loss of KIAA0368 does not affect peptidase activities of 26S proteasome.
Peptidase activities of proteasomes were not affected in lysates from KIAA0368-deficient mice, however, it may affect degradation of ubiquitylated proteins. Therefore, we immunostained the tissue sections with ubiquitin antibody to check whether ubiquitin level is changed or ubiquitin-positive aggregates could be observed in KIAA0368-deficient mice. The staining pattern of ubiquitin was normal in KIAA0368-deficient mice as compared with wild-type mice (Fig. 2C). The tissue blots of these organs also did not show marked differences in the amount of ubiquitylated protein (Fig. 2D). These results suggest that proteasomal protein degradation is not impaired in KIAA0368-deficient tissues, although the possibility of some defects in proteasome function(s) cannot be ruled out.
Other proteasome activators were not altered in KIAA0368-deficient mice
Although the differences in peptidase activities and protein degradation were not observed between wild-type and KIAA0368-deficient mice, the expression of proteasome activators and their interaction with CP might be affected by KIAA0368 deletion. To this end, we analysed the expression of proteasome activators in tissue lysates and samples fractionated by glycerol density gradient. The expression levels of PA28α, PA28γ and PA200 in tissue lysates were not altered in KIAA0368-deficient mice (Fig. 3A). Next, those expressions were analysed on fractionated samples (Fig. 3B). In wild-type lysate, subunit of CP (β7 subunit) was sedimented in fraction 8–12. The peak of Ecm29 (fractions 10–14) was observed at overlapping but slightly higher sedimentation fractions than those of β7 subunit. Subunit of 19S RP (Rpt1) was also distributed in higher sedimentation fractions (fractions 10–14). PA28αβ or γ complex were sedimented in the lower fractions (fractions 2–6), suggesting it was not associated to CP or dissociated during the sample preparation. PA200 was observed in fractions 8–12, similar to those of β7. In KIAA0368-deficient lysates, the subunit distributions were similar to those of wild-type counterpart, and any alteration was observed. These results demonstrate that 26S proteasome and the hybrid proteasome with PA200 is not impaired or affected by KIAA0368-deficiency.
Fig. 3.
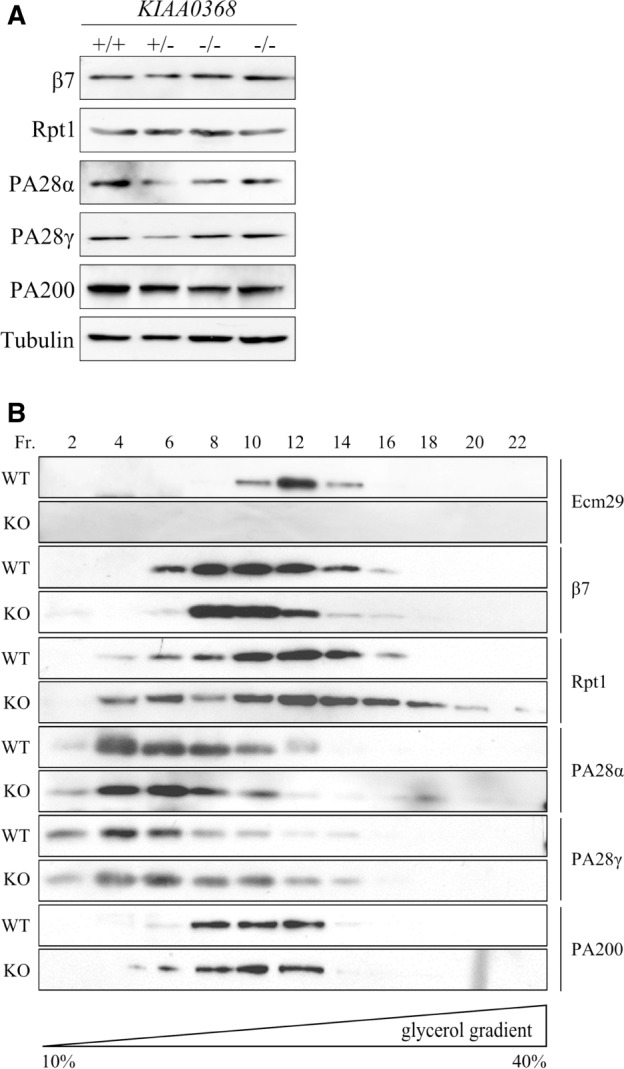
The expression of proteasome components in KIAA0368-deficient mice. (A) Expressions of CP subunit (β7) and proteasome activators (Rpt1, PA28α, PA28γ and PA200) in each KIAA0368 wild-type (+/+), heterozygous (+/−) and homozygous (−/−) littermates. Twenty micrograms of testis lysates of each genotype were subjected to immunoblot analyses. (B) Distribution of Ecm29, CP subunit (β7) and proteasome activators (Rpt1, PA28α, PA28γ and PA200) on sedimentation velocity centrifugation. One milligram of testis lysates were fractionated by glycerol density gradient centrifugation (10–40% glycerol from 1 to 22 fractions) and every two fractions were combined as one lane for immunoblot analyses. Antibodies used are indicated. WT; wild-type, KO; KIAA0368-deficient mice.
KIAA0368-deficient cells were resistant to oxidative stress
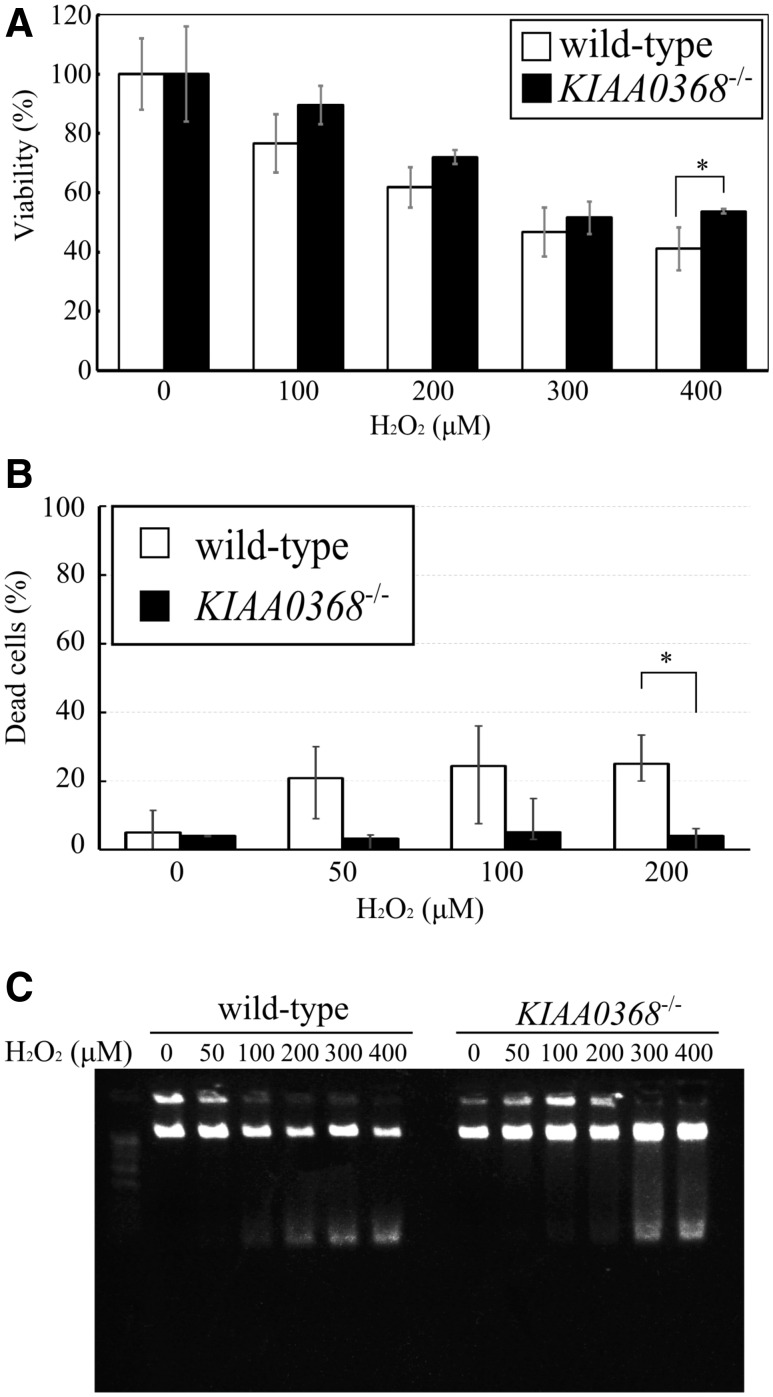
Prior investigation in yeast reported that yeast lacking ecm29 is sensitive to oxidative stress (26). Therefore, we analysed the cell viability and apoptosis using MEF cells derived from KIAA0368-deficient mice and wild-type littermate control, following H2O2 treatment. At all points of H2O2 concentrations, KIAA0368-deficient cells were more resistant to H2O2 than wild-type counterparts (Fig. 4A). Furthermore the H2O2-induced cell death was analysed by trypan blue exclusion assay (Fig. 4B). About 20% of cells were dead by 50 μM H2O2 treatment in wild-type cells. However, only 4% of cells were dead even by 200 μM H2O2 treatment in KIAA0368-deficient cells. These results indicate KIAA0368-deficient cells are more resistant to H2O2 than wild-type counterparts. When the DNA fragmentation was also analysed, apoptosis-induced DNA fragmentation was detected at 200 μM H2O2 on wild-type cells, but only at higher concentration in KIAA0368-deficient cells (Fig. 4C). Taken together, KIAA0368-deficient cells were more tolerant to oxidative stress-induced cell death.
Fig. 4.
Cell viability under oxidative stress. (A) Cell viability assay under oxidative stress. 3 × 103 cells were cultured in H2O2 of indicated concentrations and their viabilities were measured by cell viability assay. The bar charts represent the mean ± SD to untreated cells. White bars; wild-type, black bars; KIAA0368−/−. *P < 0.05 by student’s t-test. (B) Trypan blue exclusion assay on oxidative stress. Wild-type and KIAA0368-deficient MEF cells were treated with indicated concentrations of H2O2 for 2 h. Dead cells were counted and the ratio of dead cells per total cells were calculated. The bar charts represent the mean ± SD White bars; wild-type, black bars; KIAA0368−/−. *P < 0.05 by student’s t-test. (C) DNA ladder assay under oxidative stress. Wild-type and KIAA0368-deficient MEF cells were treated with indicated concentrations of H2O2 for 2 h. Genomic DNA were extracted and 0.5 μg of genomic DNA were electrophoresed on 1% agarose gel and visualized under UV illuminator.
Disassembly of proteasomes under oxidative stress was impaired in KIAA0368-deficient cells
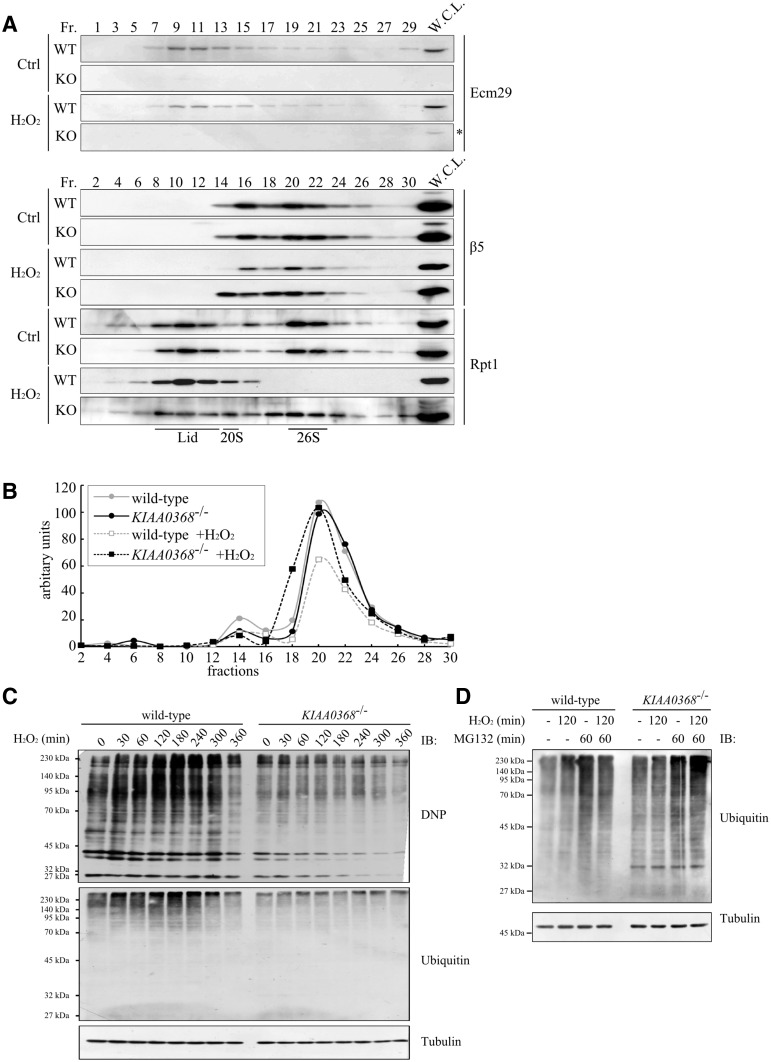
To test whether the oxidative stress tolerance is associated with any change in the proteasome composition, the sedimentation of CP and 19S RP under oxidative stress were analysed. Wild-type and KIAA0368-deficient MEF cells treated with H2O2 were subjected to glycerol density gradient fractionation followed by immunoblot analyses (Fig. 5A). At untreated normal condition, subunit of CP (β5) was sedimented around 16–22 fractions, and subunit of 19S RP (Rpt1) appeared at lower fraction and higher fractions (fractions 8–12 and 20–22) in both wild-type and KIAA0368-deficient cells. Rpt1 in fractions 8–12 appears to be 19S RP complex, and fractions 20–22 to be 26S proteasome. Note that the 19S RP complex in lighter fractions was not detected in tissue lysate (Figs 3B and 5A). It is known that tissues contain more 26S proteasome than proliferating cells, and the appearance of free lid complex may be associated with the cell proliferation (37). Ecm29 was detected at wider range of fractions (fractions 7–29). After exposure to H2O2, the distribution of Ecm29 and β5 was not significantly changed in both wild-type and mutant cells. However, Rpt1 was almost completely lost from the 26S fractions (fractions 20–22) on wild-type cells. These results suggest the possibility that 26S proteasome dissociates into 19S RP complex and CP in wild-type cells. On the other hand, Rpt1 still located at 26S fractions in KIAA0368-deficient cells after treatment of oxidants (fractions 20–22, Fig. 5A bottom 2 panels). These results suggest that the disassembly of 26S proteasome by the exposure of H2O2 is affected by the absence of Ecm29. The decrease in proteasomal peptidase activity on 26S fractions was also observed in wild-type, but not mutant MEF cells (Fig. 5B, fraction 20), supporting the notion that 26S proteasome is dissociated and inactivated in wild-type cells but not KIAA0368-deficient cells.
Fig. 5.
Analyses of proteasome complex upon oxidative stress. (A) Analysis of proteasome complexes under oxidative stress. Wild-type (WT) or KIAA0368-deficient (KO) MEF cells were treated with 100 μM H2O2 or H2O for 20 min. Five hundred micrograms of whole cell lysates were fractionated by glycerol density gradient (10–40% glycerol from 1 to 32 fractions) and immunoblotted with indicated antibodies. Asterisk indicates non-specific bands. W.C.L.; whole cell lysate. (B) Peptidase activities under oxidative stress. Odd fractions prepared in (A) were measured using Suc-LLVY-AMC. The vertical axis represent relative fluorescence normalized to peptidase activity of the first fraction. (C) Detection of oxidatively damaged proteins. Wild type and KIAA0368-deficient MEF cells were treated with 200 μM H2O2 for indicated times, then cell lysates were immunoblotted with indicated antibodies. (D) Accumulation of ubiquitylated proteins. Wild-type and KIAA0368-deficient MEF cells were treated with 200 μM H2O2 for 120 min in the presence or absence of 20 μM MG132 for 60 min, then the cell lysates were immunoblotted with indicated antibodies.
Oxidative stress is apprehended as to induce the formation of toxic misfolded proteins (38). To assess the effect of proteasome dissociation on degradation of damaged protein, we analysed the amount of carbonylated proteins (36). After exposure to H2O2, carbonylated proteins were immediately accumulated on wild-type cells, and subsequently decreased by 360 min post stimulation (Fig. 5C, upper panel). The ubiquitin blots also show the accumulation of ubiquitylated proteins and its subsequent degradation in wild-type cells. On the other hand, the accumulation of carbonylated proteins nor ubiquitylated proteins were not observed in KIAA0368-deficient cells (Fig. 5C, middle panel). These results suggest that carbonylated proteins are efficiently degraded by 26S proteasome in KIAA0368−/− cells. To confirm that this efficient degradation is indeed dependent on proteasome, we cultured cells with proteasome inhibitor MG132. In the presence of MG132, H2O2-induced accumulation of ubiquitylated proteins were observed even in KIAA0368−/− cells (Fig. 5D). Therefore, the proteasome has significant contribution to efficiently degrade damaged proteins. Taken together, KIAA0368-deficiency leads to efficient degradation of damaged and ubiquitylated proteins due to stabilized 26S proteasome under oxidative stress.
Discussion
In this study, we reported the phenotypes of KIAA0368-deficient mice. KIAA0368-deficient mice were viable, fertile, and did not show any obvious histological abnormalities. Therefore, Ecm29 is not essential for development and viability of mice.
Prior investigation reported that Ecm29 tethers both CP and 19S RP to stabilize the holoenzyme independent of ATP energy (24). We did not observe the instability of CP–RP in KIAA0368-deficient mice (Fig. 3B). The degradation of abnormal and ubiquitylated proteins was also not observed in KIAA0368-deficient mice (Fig. 2C). On the contrary, we unexpectedly observed the stabilization of 26S proteasome under oxidative stress condition. In yeast, Ecm29 recognizes immature proteasome species and functions as a scaffold for CP maturation (29). According to the model that Ecm29 is involved in the proteasome maturation checkpoint, it is possible that other protein(s) associated with the 26S proteasome confer the stability of the 26S proteasome in the absence of Ecm29.
Previous investigation demonstrated that yeast lacking ecm29 was more sensitive to oxidative stress (26). On the contrary, our experiment demonstrated that KIAA0368-deficient cells were more resistant to oxidative stress (Fig. 4A and B). In yeast, Ecm29 was reported to function in de novo synthesis of CP (29), while we could not observe defects in proteasome assembly and maturation (Fig. 2B). Therefore the difference in cell viability may be associated to the different requirement of Ecm29 on de novo synthesis of CP, which is responsible for efficient degradation of damaged proteins.
Several investigations reported that, 20S CP (39), immunoproteasome (40), 26S proteasome (41), PA28- and PA200-dedicated proteasomes (42) are responsible for efficient recognition and degradation of oxidatively damaged proteins. In support of the importance of proteasomes other 26S proteasome, our data shows the dissociation of 26S proteasome under oxidative stress (Fig. 5A). KIAA0368-deficient cells did not show the dissociation of 26S proteasome and demonstrated efficient degradation of damaged proteins (Fig. 5C). These results suggest that, even though 20S CP can degrade oxidatively damaged proteins, and the contribution of other PA28-, and PA200-conjugated proteasomes cannot be ruled out, 26S proteasome can efficiently eliminate damaged proteins.
A large part of Ecm29 protein is composed of HEAT-like repeat motifs. Like Ecm29, proteasome activator PA200 is also composed of HEAT-like motifs (43). It was previously reported that both Ecm29 and Blm10 (yeast homologue of PA200) function in CP assembly pathways (29, 44) and that deletion of both ecm29 and blm10 shows synthetic sensitivities to high temperature and canavanine-induced proteotoxicity in yeast (45). In this regard, although we did not observe the up-regulation of PA200 in the mutant mice, KIAA0368-deficiency might be compensated in part by PA200. The analysis of double knockout mice lacking both KIAA0368 and Psme4 (which encodes PA200) would be important, which is under investigation.
Supplementary Data
Supplementary Data are available at JB Online.
Acknowledgements
We thank to T. Naganuma (University of Tsukuba) for critical reading of the manuscripts and members of the Chiba laboratory for helpful discussions and technical supports.
Funding
This work was supported in part by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) in Japan (23570221 to T.C.).
Conflict of Interest
None declared.
Glossary
Abbreviations
- CP
core particle
- Ecm29
Extracellular mutants
- RP
regulatory particle
References
- 1.Sedelnikova O.A., Redon C.E., Dickey J.S., Nakamura A.J., Georgakilas A.G., Bonner W.M. (2010) Role of oxidatively induced DNA lesions in human pathogenesis. Mutant Res. 704, 152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adibhatla R.M., Hatcher J.F. (2010) Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox. Signal. 12, 125–169 [DOI] [PubMed] [Google Scholar]
- 3.Bochkov V.N., Oskolkova O.V., Birukov K.G., Levonen A.L., Binder C.J., Stöckl J. (2010) Generation and biological activities of oxidized phospholipids. Antioxid. Redox. Signal. 12, 1009–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reznick A.Z., Packer L. (1998) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 233, 357–363 [DOI] [PubMed] [Google Scholar]
- 5.Smith C.D., Carney J.M., Starke-Reed P.E., Oliver C.N., Stadtman E.R., Floyd R.A., Markesbery W.R. (1991) Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc. Natl. Acad. Sci. USA 88, 10540–10543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lih-Brody L., Powell S.R., Collier K.P., Reddy G.M., Cerchia R., Kahn E., Weissman G.S., Katz S., Floyd R.A., McKinley M.J., Fisher S.E., Mullin G.E. (1996) Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig. Dis. Sci. 41, 2078–2086 [DOI] [PubMed] [Google Scholar]
- 7.Aiken C.T., Kaake R.M., Wang X., Huang L. (2011) Oxidative stress-mediated regulation of proteasome complexes. Mol. Cell. Proteomics 10, R110.006924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page M.M., Robb E.L., Salway K.D., Stuart J.A. (2010) Mitochondrial redox metabolism: aging, longevity and dietary effects. Mech. Ageing Dev. 131, 242–252 [DOI] [PubMed] [Google Scholar]
- 9.Goldberg A.L. (1990) ATP-dependent proteases in prokaryotic and eukaryotic cells. Semin. Cell Biol. 1, 423–432 [PubMed] [Google Scholar]
- 10.Groll M., Ditzel L., Löwe J., Stock D., Bochtler M., Bartunik H.D., Huber R. (1997) Structure of 20S proteasome from yeast at 2.4 Å resolution. Nature 386, 463–471 [DOI] [PubMed] [Google Scholar]
- 11.Heinemeyer W., Ramos P.C., Dohmen R.J. (2004) The ultimate nanoscale mincer: assembly, structure and active sites of the 20S proteasome core. Cell. Mol. Life Sci. 61, 1562–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitby F.G., Masters E.I., Kramer L., Knowlton J.R., Yao Y., Wang C.C., Hill C.P. (2000) Structural basis for the activation of 20S proteasomes by 11S regulators. Nature 408, 115–120 [DOI] [PubMed] [Google Scholar]
- 13.Groll M., Bajorek M., Köhler A., Moroder L., Rubin D.M., Huber R., Glickman M.H., Finley D. (2000) A gated channel into the proteasome core particle. Nat. Struct. Biol. 7, 1062–1067 [DOI] [PubMed] [Google Scholar]
- 14.Köhler A., Cascio P., Leggett D.S., Woo K.M., Goldberg A.L., Finley D. (2001) The axial channel of the proteasome core particle is gated by the Rpt2 ATPase and controls both substrate entry and product release. Mol. Cell 7, 1143–1152 [DOI] [PubMed] [Google Scholar]
- 15.Stadtmueller B.M., Hill C.P. (2011) Proteasome activators. Mol. Cell 41, 8–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hershko A., Ciechanover A. (1998) The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 [DOI] [PubMed] [Google Scholar]
- 17.Deveraux Q., Ustrell V., Pickart C., Rechsteiner M. (1994) A 26 S protease subunit that binds ubiquitin conjugates. J. Biol. Chem. 269, 7059–7061 [PubMed] [Google Scholar]
- 18.Lam Y.A., Lawson T.G., Velayutham M., Zweler J.L., Pickart C.M. (2002) A proteasomal ATPase subunit recognizes the polyubiquitin degradation signal. Nature 416, 763–767 [DOI] [PubMed] [Google Scholar]
- 19.Finley D. (2002) Ubiquitin chained and crosslinked. Nat. Cell. Biol. 4, E121–E123 [DOI] [PubMed] [Google Scholar]
- 20.Groettrup M., Soza A., Eggers M., Kuehn L., Dick T.P., Schild H., Rammensee H.G., Koszinowski U.H., Kloetzel P.M. (1996) A role for the proteasome regulator PA28alpha in antigen presentation. Nature 381, 166–168 [DOI] [PubMed] [Google Scholar]
- 21.Dick T.P., Ruppert T., Groettrup M., Kloetzel P.M., Kuehn L., Koszinowski U.H., Stevanović S., Schild H., Rammensee H.G. (1996) Coordinated dual cleavages induced by the proteasome regulator PA28 lead to dominant MHC ligands. Cell 86, 253–262 [DOI] [PubMed] [Google Scholar]
- 22.Murata S., Kawahara H., Tohma S., Yamamoto K., Kasahara M., Nabeshima Y., Tanaka K., Chiba T. (1999) Growth retardation in mice lacking the proteasome activator PA28gamma. J. Biol. Chem. 274, 38211–38215 [DOI] [PubMed] [Google Scholar]
- 23.Qian M.X., Pang Y., Liu C.H., Haratake K., Du B.Y., Ji D.Y., Wang G.F., Zhu Q.Q., Song W., Yu Y., Zhang X.X., Huang H.T., Miao S., Chen L.B., Zhang Z.H., Liang Y.N., Liu S., Cha H., Yang D., Zhai Y., Komatsu T., Tsuruta F., Li H., Cao C., Li W., Li G.H., Cheng Y., Chiba T., Wang L., Goldberg A.L., Shen Y., Qiu X.B. (2013) Acetylation-mediated proteasomal degradation of core histones during DNA repair and spermatogenesis. Cell 153, 1012–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leggett D.S., Hanna J., Borodovsky A., Crosas B., Schmidt M., Baker R.T., Walz T., Ploegh H., Finley D. (2002) Multiple associated proteins regulate proteasome structure and function. Mol. Cell 10, 495–507 [DOI] [PubMed] [Google Scholar]
- 25.Kleijnen M.F., Roelofs J., Park S., Hathaway N.A., Glickman M., King R.W., Finley D. (2007) Stability of the proteasome can be regulated allosterically through engagement of its proteolytic active sites. Nat. Struct. Mol. Biol. 14, 1180–1188 [DOI] [PubMed] [Google Scholar]
- 26.Wang X., Yen J., Kaiser P., Huang L. (2010) Regulation of the 26S proteasome complex during oxidative stress. Sci. Signal. 3, ra88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park S., Kim W., Tian G., Gygi S.P., Finley D. (2011) Structural defects in the regulatory particle-core particle interface of the proteasome induce a novel proteasome stress response. J. Biol. Chem. 286, 36652–36666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De La Mota-Peynado A., Lee S.Y., Pierce B.M., Wani P., Singh C.R., Roelofs J. (2013) The proteasome-associated protein Ecm29 inhibits proteasomal ATPase activity and in vivo protein degradation by the proteasome. J. Biol. Chem. 288, 29467–29481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehmann A., Niewienda A., Jechow K., Janek K., Enenkel C. (2010) Ecm29 fulfils quality control functions in proteasome assembly. Mol. Cell 38, 879–888 [DOI] [PubMed] [Google Scholar]
- 30.Gorbea C., Goellner G.M., Teter K., Holmes R.K., Rechsteiner M. (2004) Characterization of mammalian Ecm29, a 26 S proteasome-associated protein that localizes to the nucleus and membrane vesicles. J. Biol. Chem. 279, 54849–54861 [DOI] [PubMed] [Google Scholar]
- 31.Gorbea C., Pratt G., Ustrell V., Bell R., Sahasrabudhe S., Hughes R.E., Rechsteiner M. (2010) A protein interaction network for Ecm29 links the 26 S proteasome to molecular motors and endosomal components. J. Biol. Chem. 285, 31616–31633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorbea C., Rechsteiner M., Vallejo J.G., Bowles N.E. (2013) Depletion of the 26S proteasome adaptor Ecm29 increases Toll-like receptor 3 signaling. Sci. Signal. 6, ra86. [DOI] [PubMed] [Google Scholar]
- 33.Kigoshi Y., Tsuruta F., Chiba T. (2011) Ubiquitin ligase activity of Cul3-KLHL7 protein is attenuated by autosomal dominant retinitis pigmentosa causative mutation. J. Biol. Chem. 286, 33613–33621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanahashi N., Yokota K., Ahn J.Y., Chung C.H., Fujiwara T., Takahashi E., DeMartino G.N., Slaughter C.A., Toyonaga T., Yamamura K., Shimbara N., Tanaka K. (1997) Molecular properties of the proteasome activator PA28 family proteins and gamma-interferon regulation. Genes Cells 2, 195–211 [DOI] [PubMed] [Google Scholar]
- 35.Tanahashi N., Suzuki M., Fujiwara T., Takahashi E., Shimbara N., Chung C.H., Tanaka K. (1998) Chromosomal localization and immunological analysis of a family of human 26S proteasomal ATPases. Biochem. Biophys. Res. Commun. 243, 229–232 [DOI] [PubMed] [Google Scholar]
- 36.Dalle-Donne I., Rossi R., Giustarini D., Milzani A., Colombo R. (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta. 40, 23–38 [DOI] [PubMed] [Google Scholar]
- 37.Tai H.C., Besche H., Goldberg A.L., Schuman E.M. (2010) Characterization of the brain 26S proteasome and its interacting proteins. Front. Mol. Neurosci. 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stadtman E.R. (2006) Protein oxidation and aging. Free Radic. Res. 40, 1250–1258 [DOI] [PubMed] [Google Scholar]
- 39.Davies K.J. (2001) Degradation of oxidized proteins by the 20S proteasome. Biochimie 83, 301–310 [DOI] [PubMed] [Google Scholar]
- 40.Seifert U., Bialy L.P., Ebstein F., Bech-Otschir D., Voigt A., Schröter F., Prozorovski T., Lange N., Steffen J., Rieger M., Kuckelkorn U., Aktas O., Kloetzel P.M., Krüger E. (2010) Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell 142, 613–624 [DOI] [PubMed] [Google Scholar]
- 41.Shang F., Gong X., Taylor A. (1997) Activity of ubiquitin-dependent pathway in response to oxidative stress: ubiquitin-activating enzyme is transiently up-regulated. J. Biol. Chem. 272, 23086–23093 [DOI] [PubMed] [Google Scholar]
- 42.Pickering A.M., Davies K.J. (2012) Differential roles of proteasome and immunoproteasome regulators Pa28αβ, Pa28γ and Pa200 in the degradation of oxidized proteins. Arch. Biochem. Biophys. 523, 181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kajava A.V., Gorbea C., Ortega J., Rechsteiner M., Steven A.C. (2004) New HEAT-like repeat motifs in proteins regulating proteasome structure and function. J. Struct. Biol. 146, 425–430 [DOI] [PubMed] [Google Scholar]
- 44.Fehlker M., Wendler P., Lehmann A., Enenkel C. (2003) Blm3 is part of nascent proteasomes and is involved in a late stage of nuclear proteasome assembly. EMBO Rep. 4, 959–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt M., Haas W., Crosas B., Santamaria P.G., Gygi S.P., Walz T., Finley D. (2005) The HEAT repeat protein Blm10 regulates the yeast proteasome by capping the core particle. Nat. Struct. Mol. Biol. 12, 294–303 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.