Abstract
Background
Oysters have important ecological functions in their natural environment, acting as global carbon sinks and improving water quality by removing excess nutrients from the water column. During their life-time oysters are exposed to a variety of pathogens that can cause severe mortality in a range of oyster species. Environmental stressors encountered in their habitat can increase the susceptibility of oysters to these pathogens and in general have been shown to impact on oyster immunity, making immune parameters expressed in these marine animals an important research topic.
Results
Paired-end Illumina high throughput sequencing of six S. glomerata tissues exposed to different environmental stressors resulted in a total of 484,121,702 paired-end reads. When reads and assembled transcripts were compared to the C. gigas genome, an overall low level of similarity at the nucleotide level, but a relatively high similarity at the protein level was observed. Examination of the tissue expression pattern showed that some transcripts coding for cathepsins, heat shock proteins and antioxidant proteins were exclusively expressed in the haemolymph of S. glomerata, suggesting a role in innate immunity. Furthermore, analysis of the S. glomerata ORFs showed a wide range of genes potentially involved in innate immunity, from pattern recognition receptors, components of the Toll-like signalling and apoptosis pathways to a complex antioxidant defence mechanism.
Conclusions
This is the first large scale RNA-Seq study carried out in S. glomerata, showing the complex network of innate immune components that exist in this species. The results confirmed that many of the innate immune system components observed in mammals are also conserved in oysters; however, some, such as the TLR adaptors MAL, TRIF and TRAM are either missing or have been modified significantly. The components identified in this study could help explain the oysters’ natural resilience against pathogenic microorganisms encountered in their natural environment.
Background
Oysters are an important ecological invertebrate species, carrying out a wide range of environmental functions. For instance, oysters filter nutrients, suspended solids and phytoplankton from the water column which lowers turbidity and increases water quality. Furthermore, they act as global carbon sinks, sequestering carbon from the ocean and depositing it in their shell matrix as calcium carbonate. Oysters and oyster reefs also protect shorelines and salt marshes from wave erosion, influence sediment distribution and can provide a habitat for other benthic and epibenthic species [1–5]. In addition to their ecological value, oysters such as the iconic Sydney rock oyster (Saccostrea glomerata) also have a substantial economic value, having contributed about AU$30 million to the New South Wales (Australia) economy in 2012/2013 [6]. In their natural habitat, oysters are exposed to a range of pathogens that can cause mass mortalities. Some of these pathogens are Haplosporidium nelsoni and Perkinsus marinus that cause MSX and Dermo, respectively in Crassostrea virginica Gmelin, with mortality rates of infected oysters between 50–90% [7]. Mortalities in bivalve hatcheries have been attributed to bacteria of the genus Aeromonas, Pseudomonas, Vibrio and Nocardia [8], and Ostreid herpesvirus 1 (OsHV-1) is the cause for repeated mass mortalities in Crassostrea gigas, with a variant of the virus associated with mortalities in Ruditapes phillipinarum, C. gigas and Pecten maximus [9]. The paramyxean protozoan of the genus Marteilia has also been shown to cause mass mortalities in several oyster species. For example, Marteilia refringens (Aber disease) was implicated in mass mortalities in Ostrea edulis and Marteilioides chungmuensis appears to be a pathogen of C. gigas [10]. Marteilia sydneyi is known to cause Queensland unknown (QX) disease in Sydney rock oysters, with mortality rates of up to 98% during an outbreak [11, 12]. While breeding of QX survivors has shown improvement in their ability to withstand QX disease, mortality in this breeding line was observed to increase during second season exposure to QX [13]. Previous studies have shown that environmental stressors (e.g. reduced salinity, pollution) to which oysters are exposed to in their natural habitat, can have detrimental effects on their immune functions. These in turn can increase their susceptibility to diseases such as QX or Dermo [14–17]. For instance, Cherkasov et al. [18] observed a significant increase in circulating haemocyte mortality in C. virginica exposed to elevated temperature and Kuchel et al. [19] reported a significant decrease in phagocytosis in response to air exposure, low salinity and mechanical stress. Considering the ecological and economical importance of oysters and their exposure to environmental stress and pathogens in their natural habitat, a strong innate immunity is essential for oyster health and survival. Furthermore, a better understanding of the molecular basics of the immune system in oysters and other molluscs is needed to develop strategies that increase oyster resilience to environmental stressors and diseases.
Next generation sequencing (NGS) provides large sequencing datasets at a fast sequencing speed and more lately at an affordable price [20]. This technology has already been used to produce two oyster genomes, the genome of C. gigas [21] and the draft genome of Pinctada fucata [22]. Furthermore, NGS provided insights into a range of biological functions in molluscs such as biomineralisation and immunity in Pinctada martensii [23], biomineralisation in Pinctada margaritifera [24], immunity in C. virginica and Mytilus edulis [25, 26] and sex differentiation in P. margaritifera [27]. While immunity has been assessed in some molluscs, it has not yet been assessed in the iconic Sydney rock oysters for which only limited sequencing information is publically available [28]. Therefore, in this study, we exposed S. glomerata to a range of environmental stressors and sequenced six tissues of stressed and non-stressed adult oysters (samples pooled per tissue type) to obtain a broad spectrum of genes expressed in this species. Resulting S. glomerata raw Illumina sequencing reads were cleaned, assembled and open reading frames (ORFs) analysed for potential immune and immune related genes. Furthermore, transcript expression patterns across the different tissues were examined. The results of the analysis are presented in this study.
Results and Discussion
S. glomerata transcriptome sequencing and assembly
In order to capture a broad spectrum of genes actively expressed in S. glomerata in response to stress, adult oysters were exposed to different potential stressors (CO2, salinity, temperature, copper and polycyclic aromatic hydrocarbons) and tissue samples (haemolymph, gill, mantle, adductor muscle, gonad and digestive) extracted at multiple sampling time-points (treatment details are presented in S1 File). Normalised strand-specific libraries, as well as non-normalised and non-strand specific libraries were prepared from each of the tissues and sequenced using the Illumina technology, resulting in a total of 484,121,702 paired-end reads with a GC content of 44–46%. Similar GC contents have been found in other molluscs, for example in the snail Bythinia siamensis goniomphalos (44.4%) and the oyster Pinctada maxima (43.2%) [29, 30]. Of the raw reads, 99.7% were retained past quality control and pre-processing and then assembled into contigs with Trinity RNASeq [31]. Two reference transcriptomes were produced: one derived only from the strand-specific data (strand-specific transcriptome) and one derived from all the data (combined transcriptome). Assembly statistics before and after redundancy removal are summarised in Table 1. As expected due to increased coverage, the combined transcriptome assembly had 20.7% more ORF predictions, a higher N50 value and longer transcripts. However, the increased coverage may have caused issues due to the high degree of polymorphism in this species. When assessing completeness using the CEGMA approach [32], more core eukaryotic genes were found to be present in the strand-specific than the combined transcriptome (Table 1). While the CEGMA software was originally developed to assess genomic assemblies for completeness, it has also been used to assess transcriptomes [33, 34]. In addition to the CEGMA analysis, the N50 values obtained for the S. glomerata transcriptomes were similar to the N50 values of other molluscan transcriptomes [25, 35, 36]. Based on these results, both assemblies were considered to be of a suitable quality for further analysis and therefore used in this study.
Table 1. Summary of S. glomerata assembly, ORF prediction and CEGMA analysis.
| Strand-specific transcriptome | Combined transcriptome | |
|---|---|---|
| Total transcripts (#) | 519,639 | 502,645 |
| N50 length (bp) | 949 | 1,238 |
| Mean transcript length (bp) | 633 | 701 |
| Min transcript length (bp) | 201 | 201 |
| Max transcript length (bp) | 27,590 | 30,775 |
| Non-redundant transcripts (#) | 414,578 | 453,096 |
| N50 length (bp) | 768 | 937 |
| Mean transcript length (bp) | 564 | 613 |
| Min transcript length (bp) | 201 | 201 |
| Max transcript length (bp) | 27,590 | 30,775 |
| n transcripts < 500 bp | 292,626 | 318,450 |
| n transcripts 500–1000 bp | 72,056 | 73,425 |
| n transcripts > 1000 bp | 49,896 | 61,221 |
| ORF predictions (#) | 85,786 | 108,130 |
| Min length (bp) | 300 | 300 |
| Max length (bp) | 25,917 | 29,883 |
| CEGMA | ||
| Complete proteins (%) | 95.56 | 91.94 |
| Partial proteins (%) | 99.19 | 95.56 |
C. gigas comparison and transcript annotation
Reads and reference transcriptomes from S. glomerata were aligned to the C. gigas genome to examine the level of similarity of the two oyster species. Strand-specific and normalised reads, as well as the strand-specific reference transcriptome showed higher mapping rates to the C. gigas genome than the non-normalised and non-strand specific reads and combined transcriptome (21.4% and 3.4% versus 15.6% and 2.1%, respectively). The observed low transcript alignment percentages (3.4% and 2.1%) indicate that the majority of S. glomerata transcripts overall have less than 60% similarity to the C. gigas genome at the nucleotide level. However, at the deduced protein level C. gigas was the closest match, with 81.2% and 75.7% of the best blast matches for the S. glomerata strand-specific and combined ORF’s, respectively being to protein sequences of C. gigas. Other molluscan best-hit matches were with Lottia gigantea, Aplysia californica, Ostrea edulis, Mytilus galloprovincialis and Crassostrea ariakensis (S1a and S1b Fig). Similar to our study, Zhang et al. [25] mapped C. virginica Illumina paired-end reads to the C. gigas genome, showing that only 3.43% of raw reads aligned to the genome, while over 99% of C. virginica contigs could be annotated by the C. gigas protein set, thus also showing a much higher similarity at the protein rather than nucleotide level.
Functional annotation was carried out on the 85,786 strand-specific and 108,130 combined S. glomerata ORFs, using Blast2GO. A total of 83.5% and 81.4% of the strand-specific and combined ORFs, respectively were annotated with NCBI’s non-redundant database, using an e-value cut-off of 1e-5. When all ORFs were searched against the InterProScan database, 80.1% and 79.6% of strand-specific and combined ORFs, respectively could be annotated. Of the functionally annotated ORFs for both transcriptomes, GO-terms associated with metabolic and cellular processes, binding and catalytic activity, and cells and membranes contained the most ORFs (S2 and S3 Figs). Similar GO-terms have been found in other molluscs such as C. virginica and the clam Meretrix meretrix [25, 37]. In addition, both S. glomerata transcriptomes had a range of GO-terms associated with responses to different stimuli/stress (e.g. response to chemical stimulus, detection of stimulus) and to immunity (e.g. immune response, death) (S2a and S3a Figs).
Tissue distribution of transcripts
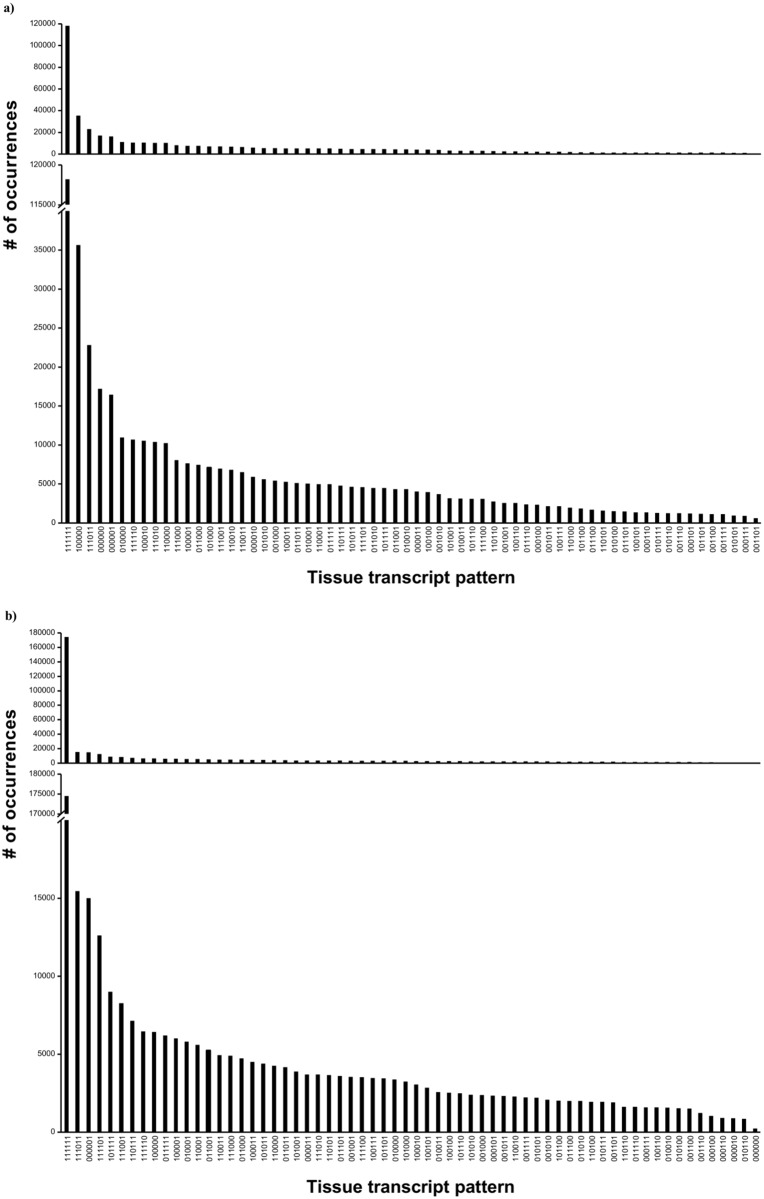
S. glomerata reads (S1 Table) were mapped to their respective reference transcriptome and the transcript expression pattern across the six tissues examined. The largest number of S. glomerata transcripts (42.1% and 26.1% for strand-specific and combined, respectively) were found to be expressed in all tissues (Fig 1a and 1b). As most eukaryotic cells express the same set of cell homeostasis related genes [38], it was expected to see a high number of transcripts expressed in all S. glomerata tissues tested, with a smaller amount of transcripts specifically expressed in certain cells and tissues depending on the function of these cells and tissues. This has been observed in S. glomerata, where transcripts expressed in all tissues but the adductor muscle, transcripts only expressed in the digestive system and transcripts only expressed in the haemolymph (Fig 1a and 1b) were among the ten highest tissue patterns found in the S. glomerata transcriptomes. Some of the transcripts found to be expressed only in the haemolymph coded for cathepsins (e.g. specific transcripts coding for cathepsin B and L), heat shock proteins (e.g. specific transcripts coding for heat shock protein 70 and 90), peroxiredoxin (e.g. specific transcripts coding for peroxiredoxin 6) and superoxide dismutase (e.g. some transcripts with mitochondrial manganese-superoxide dismutase domains). Of these transcripts, peroxiredoxin and superoxide dismutase have been shown to act as antioxidants [39, 40], while cathepsins are lysosomal proteolytic enzymes involved in phagocytosis [41]. Heat shock proteins, on the other hand, are involved in the response to stress and have also been shown to act as endogenous ligands for Toll-like receptors (TLRs) in mammals, which activate the TLR signalling pathway, resulting in inflammatory responses [42, 43].
Fig 1. Transcript patterns across six different S. glomerata tissues.
A) non-normalised and non-strand specific tissue reads were mapped to the combined reference transcriptome and b) strand-specific and normalised tissue reads were mapped to the strand-specific reference transcriptome, using CLC Workbench version 7.5. Graph shows the number of transcripts that are common across individual tissue patterns, with 1 standing for “transcript present in tissue” and 0 for “transcript not present in tissue”. The order in which the tissues are listed is as follows: haemolymph (closest to the x-axis label), gill, mantle, muscle, gonad and digestive. The respective top graphs show the full-scale graph, with the respective lower graphs emphasising the fine scale features of the same graph by adding a graph break.
Immune and immune-related genes
Innate immunity is the first and only line of defence against invading pathogens in invertebrates such as oysters. Considering that aquatic habitats harbour a range of bacteria and viruses (approximately 1029 and 1010 cells/L of prokaryotes and viruses, respectively), aquatic animals such as oysters that inhabit this environment strongly rely on the protective functions of the innate immune system [44]. The innate immune response contains cellular (e.g. phagocytes) and humoral (e.g. enzymes) components that protect the bivalve host from harmful microorganisms that it encounters in its natural environment [45, 46]. In general terms, immune cells (e.g. haemocytes) are activated by the stimulation of receptors by pathogen-associated molecular patterns (PAMPs). This immune response involves signalling cascades and the release/production of various molecules such as hydrolytic enzymes and antimicrobial peptides that are part of the immune arsenal against pathogens [47]. In the following sections we describe the different components identified in this transcriptome study that are potentially involved in innate immunity in the Sydney rock oyster.
Pattern recognition receptors (PRRs)
Through interactions with their environment, oysters are continuously exposed to a broad range of microorganisms. Different to vertebrates, oysters lack an adaptive immune system and thus have to rely heavily on the innate immune system as protection from invading microorganisms [48]. PRRs are an important part of the innate immune defence as they are able to recognise PAMPs (e.g. lipopolysaccharide [LPS], bacterial DNA and viral RNA) and endogenous ligands (e.g. heat shock proteins), also termed damage-associated molecular patterns (DAMPs), that are generally released in response to tissue injury or cell stress [42, 44, 49, 50]. Stimulation of PRRs induces intracellular signalling pathways, leading to inflammatory immune responses. In addition, PRRs are also linked to phagocytosis and the complement pathway [42, 49]. Similar to vertebrates, several PRRs have been discovered in invertebrates such as crustaceans and molluscs. These include, for instance, Toll-like receptors (TLRs) and Down syndrome cell adhesion molecules (DSCAM) [43, 51]. Akin to the PRRs found in other invertebrates, a range of PRRs has also been detected in the S. glomerata transcriptomes of this study. Multiple transcripts of TLRs, peptidoglycan recognition proteins (PGRPs), gram-negative bacteria binding proteins (GNBPs), c-type lectins, collectins, ficolins, macrophage mannose receptors, galectins, RIG-I-like receptors (RLRs), thioester-containing proteins (TEPs), fibrinogen-related proteins (FREPs), scavenger receptors (SRs), as well as DSCAM were found to be expressed in the S. glomerata transcriptomes (Table 2). TLRs, which are a key component of the immune system, have been studied over many years and appear to be conserved across vertebrates and invertebrates alike and are expressed in mammals, flies, crustaceans and molluscs [43, 51, 52]. Along with the TLRs found in our study, TLR sequences have also been observed in other molluscs such as the mussels Bathymodiolus azoricus and M. edulis, the scallop Pecten maximus and the oyster C. virginica [25, 26, 53, 54]. Furthermore, most of the other PRRs detected in our study have also been reported in C. virginica, M. edulis and P. maximus, from SRs, PGRPs, GNBPs, c-type lectin and galectin to collectins and TEPs [25, 26, 53]. Contradictory to the observation of Zhang et al. [25], ficolins, which have been linked to the complement system [55], have already been detected in molluscs, with transcripts found in the scallop P. maximus [53] and the C. gigas genome [21], as well as in our S. glomerata transcriptomes. Xiang et al. [55] showed an increased expression of a ficolin-like gene in Crassostrea hongkongensis after the oyster was challenged with microbes, with the recombinant protein being able to agglutinate Escherichia coli K-12 in the presence of Ca2+ and raising the phagocytic activity of C. hongkongensis haemocytes. It is conceivable that the S. glomerata ficolins found in our study could have a similar functionality as the ficolin-like in C. hongkongensis, which would allow S. glomerata to not only recognise invading pathogens but to also act as opsonins, increasing the likelihood that the pathogens will be cleared from the host by phagocytosis.
Table 2. Potential S. glomerata immune and immune related ORFs determined by GO and/or InterProScan annotation.
| # of ORFs | Details | |
|---|---|---|
| Pattern recognition receptors (PRRs) | ||
| peptidoglycan recognition proteins (PGRPs) | 23 | |
| gram-negative bacteria binding proteins (GNBPs) | 3 | |
| Collectins | 53 | |
| Ficolins | 30 | |
| c-type lectins | 110 | |
| macrophage mannose receptors | 237 | |
| scavenger receptors (SRs) | 26 | based on domain information in [56] |
| thioester-containing proteins (TEPs) | 31 | |
| fibrinogen-related proteins (FREPs) | 40 | |
| down syndrome cell adhesion molecule (DSCAM) | 11 | |
| Galectins | 20 | |
| Toll-like receptors (TLRs) | 220 | |
| interferon-induced helicase c domain-containing protein 1 (MDA5)/(also RIG-I called) DDX58 | 22 | |
| stabilin-2 | 3 | |
| Immune signaling pathways | ||
| myeloid differentiation primary response protein 88 (MyD88) | 14 | |
| sterile alpha and TIR domain containing protein (SARM1) | 3 | |
| IL-1R-associated kinase (IRAK) | 1 | |
| tumor necrosis factor [TNF] receptor-associated factor (TRAF) | 31 | TRAF7 (8), TRAF6 (3), TRAF2 (3), TRAF4 (3), TRAF3 (1), TRAF6-like isoform 1 (1) |
| inhibitor of nuclear factor kappa-b kinase (IKK) | 6 | |
| transforming-growth-factor [TGF]-β-activated kinase 1 (TAK1, also called MEKK7) | 6 | |
| TAK1-binding protein (TAB1) | 3 | |
| TAK1-binding protein 2 (TAB2) | 2 | |
| nuclear factor [NF]-κB essential modulator (NEMO) | 2 | |
| TRAF-family-member-associated NF-κB activator-binding kinase 1 (TBK1) | 13 | |
| inhibitor of nuclear factor-κB (IκB) | 4 | |
| nuclear factor of kappa light polypeptide gene enhancer in b-cells 2, p49/p100 (NFκB2) | 2 | |
| activator protein 1 (AP-1) | 1 | |
| interferon regulatory factor (IRF) | 7 | |
| janus activated kinase (JAK) | 2 | |
| signal transducer and activator of transcription (STAT) | 4 | |
| mitogen-activated protein kinases (MAPK) | 13 | MAPK3 (1), MAPK14 (4), MAPK15 (2), MAPK6 (3) and MAPK7 (1) |
| mitogen-activated protein kinase kinase (MEK also called MKK) | 11 | |
| mitogen-activated protein kinase kinase kinase (MAP3K or MEKK) | 34 | MEKK1 (2), MEKK19 (8), MEKK2 (1), MEKK10 (1), MEKK15 (1), MEKK9 (1), MEKK13 (2) |
| mitogen-activated protein kinase kinase kinase MLT-like isoform 1 (also called ZAK isoform 1) | 1 | |
| mitogen-activated protein kinase kinase kinase kinase (MAP4K) | 10 | MAP4K3 (6) and MAP4K5 (4) |
| receptor-interacting protein 1 (RIP1) | 3 | |
| Pellino | 2 | |
| toll-interacting protein (TOLLIP) | 1 | |
| suppressor of cytokine signaling (SOCS) | 5 | |
| NF-kappa-B inhibitor-like protein 1 (NFKBIL1) | 1 | |
| interleukin-1 receptor-associated kinase 1-binding protein 1 (IRAK1BP1) | 1 | |
| mitochondrial antiviral-signaling protein (MAVS) | 1 | |
| nuclear factor of activated T-cells (NFAT) | 1 | |
| c-jun-amino terminal kinase-interacting protein 4 (JIP4) | 12 | |
| c-jun-amino terminal kinase-interacting protein 1 (JIP1) | 1 | |
| endogenous ligands | ||
| fibrinogen (α/β/γ-chain) | 8 | |
| heat shock protein [Hsp]20 family | 13 | |
| Hsp70 family | 40 | |
| Hsp90 family | 25 | |
| Hsp60 | 2 | |
| Hsp10 | 1 | |
| stress-induced-phosphoprotein 1 (STIP1) | 2 | |
| intraflagellar transport protein 25 homolog (Hspb11) | 2 | |
| Hypoxia-inducible factors (HIFs) | ||
| HIF-1α / HIF-2α | 6 | |
| HIF-1β | 2 | |
| hypoxia-inducible factor 1-α inhibitor (HIF1AN) | 1 | |
| prolyl hydorxylases (PHD) | 2 | |
| p300 / CREB-binding protein (CBP) | 13 | |
| Immune effectors | ||
| Antioxidant defence system/phagocytosis | ||
| superoxide dismutase (SOD) | 25 | with mitochondrial manganese-SOD domains (4) and copper/zinc-SOD domains (21) |
| catalase (CAT) | 7 | |
| glutathione peroxidase (GPX) | 7 | |
| nitric oxide synthase (NOS) | 2 | |
| Thioredoxin | 6 | |
| thioredoxin reductase | 4 | |
| Peroxiredoxin | 11 | |
| Glutaredoxin | 6 | |
| glutathione S-transferase (GST) | 53 | |
| glutathione synthase (GS) | 2 | |
| glutamate—cysteine ligase regulatory subunit | 1 | |
| glutamate—cysteine ligase catalytic subunit | 3 | |
| methionine sulfoxide reductase A | 3 | |
| methionine-R-sulfoxide reductase B3 | 4 | |
| glutathione reductase (mitochondrial) | 1 | |
| NADPH oxidase (NOX) | 4 | |
| p67phox | 1 | |
| cytochrome b-245 heavy chain-like (gp91phox) | 1 | |
| cytochrome b-245 light chain-like (p22phox) | 1 | |
| dual oxidase (DUOX) | 7 | |
| small GTPase Rac | 2 | |
| evolutionarily conserved signaling intermediate in toll pathways, mitochondrial (ECSIT) | 1 | |
| Cathepsin | 59 | cathepsin B (9), F (3), L (17), C (1), O (2), Z (1), D (3) |
| Lysozymes | 16 | |
| Antimicrobial peptides | ||
| big defensins | 5 | |
| Hydramacin | 1 | |
| bactericidal permeability increasing protein (BPI) | 27 | |
| cytokines and cytokine receptors | ||
| macrophage migration inhibitory factor (MIF) | 3 | |
| interleukin [IL]-17 | 3 | |
| interleukin-17 receptor d | 5 | |
| interleukin-6 receptor | 12 | |
| Others | ||
| Septin | 7 | |
| c4b-binding protein alpha/beta chain | 8 | |
| Apoptosis | ||
| FAS-associated death domain protein (FADD) | 3 | |
| TNF receptor superfamily members | 9 | |
| TNF superfamily members | 8 | |
| apoptosis-inducing factor 1, mitochondrial (AIF) | 1 | |
| Bax | 2 | |
| Caspases | 114 | |
| baculoviral IAP repeat-containing proteins (IAPs) | 75 | |
| direct IAP-binding protein of low isoelectric point (DIABLO) | 4 | |
| interferon alpha-inducible protein 27 (IFI27) | 25 |
RLRs (MDA5 and RIG-I), another important PRR group aside from TLRs were first observed in M. edulis [26]. RLRs are important sensors for viruses in the cytoplasm, with downstream immune signalling initiated after virus recognition [26, 49]. These PRRs have also been detected in the S. glomerata transcriptomes of our study (Table 2), suggesting that the immune system of S. glomerata might be able to defend the oyster from intracellular viruses. One other family of PRR found in mammals aside from TLRs are NOD-like receptors (NLRs) that are able to recognise and elicit an immune response to bacterial motifs in the cytoplasm [49]. While sequence homology searches revealed matches to NLRs in our S. glomerata transcriptomes, these matches could not be confirmed with InterProScan and comparison to curated NLR sequences on uniprot. Similar to our study, Philipp et al. [26] also did not find any NLRs in their M. edulis transcriptome, indicating that in contrast to TLRs and RLRs, NLRs might not be conserved in bivalves.
Some of the PRRs found in invertebrates have also been examined for their involvement in innate immunity. For instance, SR expression in the scallop Chlamys farreri was induced in response to PAMPs (LPS, PGN and β-glucan), with the SR recombinant protein able to bind LPS, PGN and the fungal particles mannan and zymosan in the presence of Ca2+ [56]. Other PRRs examined were TEP, FREPs and DSCAM, which were observed to respond to bacteria in invertebrates, with DSCAM also associated with phagocytosis [43, 51]. These results indicate that PRRs in invertebrates also function in PAMPs detection, as their mammalian counterparts do. Of the PRRs found in invertebrates and the S. glomerata transcriptome of this study, members of the c-type lectin superfamily have also been linked to the complement system in vertebrates [57–59]. This family consists of c-type lectins, collectins and macrophage mannose receptor, with the latter associated with the cell membrane and linked to phagocytosis [57]. Ficolins on the other hand are serum proteins (except M-ficolin) that have been shown to activate the complement pathway [58, 59]. Having a range of PRRs that act in different compartments (e.g. haemolymph), respond to a wide range of pathogens and activate different pathways (e.g. TLR signalling pathway) might give S. glomerata an edge against invading pathogens, especially as the innate immune system is the only defence system oysters possess, making it essential to have a broad range of PAMPs recognition molecules to activate downstream defence mechanisms (Fig 2).
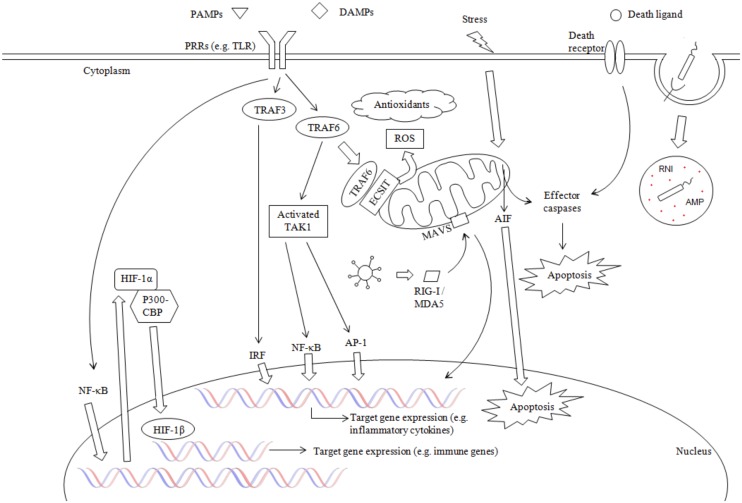
Fig 2. Schematic of the predicted innate immune processes of S. glomerata.
S. glomerata innate immune transcripts observed in this study are predicted to be involved in a range of different innate immune processes, (components regulating the different pathways are not visualised in this figure). Abbreviations are as follows: PPRs (pattern recognition receptors), TLR (Toll-like receptor), PAMPs (pathogen-associated molecular patterns), DAMPs (damage-associated molecular patterns), TRAF (tumor necrosis factor receptor-associated factor), TAK1 (transforming-growth-factor-β-activated kinase 1), ROS (reactive oxygen species), ECSIT (evolutionary conserved signalling intermediate in toll pathways), RIG-1/MDA5 (interferon-induced helicase c domain-containing protein 1), MAVS (mitochondrial antiviral-signalling protein), AIF (apoptosis-inducing factor 1), AP-1 (activator protein 1), NF-κB (nuclear factor kappa B), IRF (interferon regulatory factor), HIF (hypoxia-inducible factor), CBP (CREB-binding protein), RNI (reactive nitrogen intermediates), AMP (antimicrobial peptide). ↑ arrows used in the figure (excluding the ones used in the nucleus) depict signalling pathways.
TLR signalling pathway
TLRs are well characterised and have been found in both vertebrates and invertebrates. They have a key role in the recognition of microbes and their stimulation by a range of exogenous and endogenous ligands (e.g. double-stranded viral RNA, LPS, HSP60 and HSP70) leads to the activation of a highly conserved signalling pathway [26, 42, 44, 51, 60]. Once the ligand interacts with its respective TLR, the receptor is activated, after which the TLR signalling domain dimerises and recruits adaptors [42, 50]. Five adaptor proteins have been found so far, myeloid differentiation primary-response protein 88 (MyD88), MyD88-adaptor-like protein (MAL, also called TIRAP), toll/interleukin-1 receptor (TIR) domain-containing adaptor protein inducing interferon [IFN]-β (TRIF or TICAM1), TRIF-related adaptor molecule (TRAM, also termed TIRP) and sterile-α-and armadillo-motif-containing protein 1 (SARM1) [42, 49, 50]. While the first four adaptor proteins lead to downstream signalling, SARM1 has been found to inhibit TRIF [52]. Of the five adaptor proteins, only MyD88 and SARM1 were found in the S. glomerata transcriptome along with multiple ORFs coding for TLRs (Table 2). Even though MAL appears to be important in mammalian TLR signalling, this adaptor, as well as TRIF and TRAM have not been found in S. glomerata, which overall appears to be consistent with results in M. edulis and Strongylocentrotus purpuratus where distinct orthologues of TRAM and TRIF could not be found [26, 61]. Furthermore, as MAL, TRIF and TRAM have only been detected in chordates so far [62], it seems that neither of these adaptors might be used in molluscs. Although SARM1 acts as an inhibitor in the mammalian TLR signalling pathway, contradictory functions of SARM1 have been observed in horseshoe crabs and Caenorhabditis elegans [63]. In the crustacean, SARM1 functioned as inhibitor, whereas in C. elegans its function resulted in the production of antimicrobial peptides [63], suggesting that SARM1 could potentially act as a second adaptor next to MyD88 in S. glomerata, leading to downstream signalling and the induction of the innate immune system. However, further studies are needed to clarify the exact function(s) of SARM1 in invertebrates and especially in molluscs. Research into the expression levels of nine TLRs and three MyD88s in M. edulis challenged with the bacteria Vibrio splendidus, V. anguillarum and Micrococcus luteus and the fungus Fusarium oxysporum, showed that one TLR responded to the bacteria and fungus with an increase in gene expression [64]. In addition, all three MyD88 had increased levels of expression after challenge with bacteria, with only one of the MyD88 also stimulated by the fungus [64]. A similar pattern was shown in M. galloprovincialis, where bacterial challenge resulted in an increase in expression levels in one out of five TLRs and all three tested MyD88s [65]. These results indicate that both TLRs and the adaptor protein MyD88 respond to a range of pathogens and play an important role in the innate immunity of bivalves such as S. glomerata.
In mammals, the specific adaptor(s) recruited to the individual TLR determines the downstream signalling pathway, activating either the MyD88-dependent or the TRIF-dependent pathway. All TLRs but TLR3 have been shown to utilise the MyD88-dependent pathway that eventually results in the production of inflammatory cytokines. TLR3 on the other hand uses the TRIF-dependent pathway that leads to the production of inflammatory cytokines as well as the expression of type I IFNs [49, 50]. Different to most TLRs, TLR4 has been shown to be able to initiate both, the MyD88-dependent and the TRIF-dependent pathway [49, 50]. TLR4 is also different in that it needs the adaptor protein TRAM to link with TRIF, whereas TLR3 only interacts with TRIF to activate the TRIF-dependent signalling pathway [49, 52]. Previous studies in bivalves [64, 65] have shown that both, TLRs and MyD88, are induced by a variety of pathogens. More importantly, in vitro studies using TIR-domains of Toll receptor and MyD88 genes from the mussel Hyriopsis cumingii showed that the domains were able to induce the expression of antimicrobial peptides, suggesting that a MyD88-dependent signalling pathway is present in molluscs [66, 67]. Considering these results and that our S. glomerata transcriptomes contain multiple TLR and MyD88 transcripts, it is likely that a MyD88-dependent TLR signalling pathway also exists in S. glomerata.
MyD88-dependent pathway
Once MyD88 has associated with the receptor, IL-1R-associated kinase 4 (IRAK4) is recruited to the receptor complex, with the interaction between IRAK4 and MyD88 leading to the phosphorylation and subsequent activation of IRAK 1 and/or IRAK 2[50, 52]. Phosphorylation of IRAK 1 allows TRAF 6 (tumor necrosis factor [TNF] receptor-associated factor 6) to bind to the complex, after which IRAK 1 –TRAF 6 dissociates from the receptor complex [50, 52, 60]. Under certain circumstances (signalling through TLR1, TLR2 or TLR4), TRAF 6 can move to the mitochondria where it interacts with ECSIT (evolutionarily conserved signalling intermediate in Toll pathways), resulting in the production of mitochondrial reactive oxygen species (ROS) [68]. ORFs coding for ECSIT, TRAF and IRAK were observed in our S. glomerata transcriptomes (Table 2), indicating that both TLR signalling pathways (MyD88-dependent and signalling through ECSIT) appear to exist in S. glomerata (Fig 2). This would enable S. glomerata to trigger the production and release of the important innate immune components ROS and cytokines in response to pathogens. While cytokine release can lead to the activation of other immune cells, ROS can kill pathogens as well as act as a secondary messenger in cellular signalling pathways, such as the MyD88-dependent pathway (involvement in the activation of transcription factors activator protein 1 and nuclear factor-κB) and the RLR signalling pathway [39, 69, 70]. Simulation of both pathways would lead to a strong, protective immune response in S. glomerata.
In the general signalling pathway, IRAK 1 –TRAF 6 interact with the TAK1 (transforming-growth-factor-β-activated kinase 1)–TAB1 (TAK1-binding protein)–TAB2/TAB3 protein complex, leading to the phosphorylation of TAK1 and TAB2/TAB3. The complex then moves into the cytoplasm of the cell where TAK1 is activated [50, 60, 71]. It has been shown that TAK1 is able to phosphorylate IKKβ (an inhibitor of nuclear factor-κB [IκB]-kinase) and that both, TAB2 and TAB3, appear to be involved in the downstream NF-κB (nuclear factor-κB) activation [50, 71]. Furthermore, it is known that in order to activate NF-κB, IKKα and IKKβ need to be activated by phosphorylation of two serine residues. In addition, along with the catalytically active IKKα and IKKβ, the regulatory subunit NEMO (NF-κB essential modulator) has been shown to be important for NF-κB activation [71]. The active IKK complex (IKKα, IKKβ and NEMO) then phosphorylates IκBα, after which IκBα will eventually be degraded by the proteasome [50, 71, 72]. As IκBα is bound to the inactive NF-κB in the cytosol, phosphorylation of IκBα leads to the release of NF-κB, which then translocates to the nucleus where it actives the transcription of inflammatory cytokine genes [68, 71, 72]. AP-1 (activator protein 1), another transcription factor, can also be activated through the action of TAK1 [49, 52, 68]. AP-1 activation appears to involve a phosphorylation cascade that moves from mitogen-activated protein kinase kinase kinase (MEKK) to MEK to the MAPKs JNK (c-JUN N-terminal kinase) and p38, ultimately leading to the activation of the transcription factor [68]. All the components involved in the MyD88-dependent pathway have been found in our S. glomerata transcriptomes. Aside from the previously described TLRs, MyD88, IRAK and TRAF transcripts, the S. glomerata transcriptomes contained ORFs coding for TAK1, TAB1, TAB2, IKK, NF-κB2, NEMO, IκB, AP-1 and a range of MAPKs, MEK, MAP4K and MEKKs (Table 2). This suggests that the MyD88-dependent TLR signalling pathway is conserved in S. glomerata (Fig 2). Similar results have been seen in high throughput sequencing datasets of mussel (M. edulis, Bathymodiolus azoricus) and oyster (C. virginica) tissues [25, 26, 54]. Aside from TLRs, MyD88, IκB, TRAF, IRAK and AP-1 were observed in all three studies [25, 26, 54]. In addition, IKK, NF-κB, TAK1 and SARM were found in C. virginica and M. edulis, ECSIT in M. edulis and various mitogen-activated kinases across all three studies [25, 26, 54]. Many of the MyD88-dependent pathway components found in our S. glomerata study, were also examined in M. galloprovincialis after a bacterial challenge [65]. The authors analysed the expression levels of IRAK-a and -b, ECSIT, TRAF3, TRAF6, TAK1, IKK-1 and -2, NEMO, IκB-1 and -2, Rel and NF-κB and found no response to bacteria challenge for TAK1. ECSIT and TRAF3 expression was shown to be decreased after Gram-negative challenge, while the rest was found to be up-regulated in response to bacteria [65]. The results of these studies suggest that the TLR signalling pathway in molluscs also functions in innate immunity in response to invading pathogens, using similar pathways to those described in mammals.
TRIF-dependent pathway
After interaction of TRIF with its receptor, TRIF associates with TRAF 3 that links TRIF with the IKK-related protein complex IKKε –TBK1 (TRAF-family-member-associated NF-κB activator [TANK]-binding kinase 1). The complex then phosphorylates IRF3 (IFN-regulatory factor 3), which leads to IRF3 dimer formation and its localisation into the nucleus. There, IRF3 triggers the expression of type I IFN genes with the aid of p300 and CBP (cAMP-responsive-element-binding protein [CREB]-binding protein) [50, 60]. Type I IFN in turn appears to be able to increase IFN production through the activation of the JAK-STAT (Janus activated kinase–signal transducer and activator of transcription) signalling pathway and subsequent induction of IRF7 [60].
TRIF has also been shown to activate NF-κB through two pathways, involving TRIF’s N- and C-terminal region. In one pathway, TRAF 6 is recruited to TRIF, which eventually leads to the activation of NF-κB. In the second pathway, NF-κB is activated after TRIF associates with RIP1 (receptor-interacting protein 1) through its C-terminal region [50, 60]. Although TRIF has not been found in our study, it is interesting to note that components of the TRIF-dependent pathways were detected in the S. glomerata transcriptomes. These components were TBK1, TRAF, IRF, p300/CBP, RIP1, JAK and STAT (Table 2). TBK was also found in C. virginica and M. edulis, STAT in M. edulis and B. azoricus, and IRF and JAK2 in M. edulis [25, 26, 54], with RIP-like not induced by bacteria, while the expression level of TRAF3 decreased after Gram-negative challenge in the mussel M. galloprovincialis [65]. Furthermore, similar to findings in M. edulis [26], no transcript for interferon was found in our S. glomerata transcriptomes. However, based on GO annotation results, Bettencourt et al. [54] detected interferon in their study on B. azoricus, indicating that interferon might be expressed in molluscs, even though it was not found in S. glomerata or M. edulis. Considering that most components of the TRIF-dependent TLR signalling pathway (excluding TRIF and TRAM) have been found in our study, as well as in other molluscs, it is possible that a TRIF-related pathway exists in molluscs that can be triggered either independently of the adaptor protein TRIF or through the actions of an as yet unknown adaptor protein (Fig 2).
Other molecules involved in TLR signalling pathways
While inflammatory responses induced by TLR signalling are an important protective mechanism, an excessive response can be detrimental to the health of the host and therefore needs to be tightly regulated [73, 74]. Aside from cytokines and cytokine receptors, components such as pellino, Toll-interacting protein (TOLLIP), interleukin-1 receptor-associated kinase 1-binding protein 1 (IRAK1BP1), suppressor of cytokine signalling (SOCS), NF-κB inhibitor-like protein 1 (NFKBIL1) and heat shock proteins, all thought to be involved in the regulation of TLR signalling pathways and inflammatory responses, were found in our S. glomerata transcriptome (Table 2). In mammalian TLR signalling pathways, pellino is thought to act as a type of scaffolding protein by forming a complex with IRAK 1, once the TLRs have been activated through interaction with their ligand. Similar to pellino, TOLLIP appears to carry out its function by interacting with IRAK 1. In non-stimulated cells, TOLLIP inhibits the phosphorylation of IRAK 1, effectively stopping the activation of NF-κB [60]. Different to pellino and TOLLIP, IRAK1BP1 appears to act on NF-κB, resulting in the down-regulation of proinflammatory cytokine transcription [75]. A study in the abalone Haliotis diversicolor showed that the strongest expression of IRAK1BP1 was in the haemocytes of this mollusc, with the expression levels of IRAK1BP1 increased after H. diversicolor was challenged with the bacterium Vibrio parahaemolyticus [74]. These results suggest that IRAK1BP1 is involved in the immune response of molluscs. NFKBIL1, another regulatory component is believed to be a member of the IκB family with an NF-κB inhibitory function [76, 77]. SOCS proteins can be induced through the stimulation of TLRs and are able to modulate the sensitivity of immune cells such as macrophages to cytokines and therefore mediate inflammatory responses [73]. Similar to pellino and TOLLIP, heat shock proteins, which can stimulate TLRs by acting as endogenous ligands [42], appear to also be linked to the IKK complex of the TLR signalling pathway. While Hsp 90 seems to have a stabilising function on the kinases, interaction of Hsp 70 with NEMO negatively affects NF-κB signalling [71]. All these components have been found in our S. glomerata study (Table 2), as well as in the C. gigas genome (SOCS, NFKBIL1, heat shock proteins) [21], in C. virginica (pellino) [25], M. edulis (TOLLIP, SOCS) [26] and H. diversicolor (IRAK1BP1) [74], indicating that these proteins are also important in molluscs, with a potential function in immunity. Further studies are needed to determine their exact function in the innate immunity of S. glomerata and other molluscs.
Another molecule of interest that has been connected to several components of the TLR signalling pathway is the adaptor molecule MAVS (mitochondrial antiviral signalling protein), which has also been detected in our S. glomerata transcriptomes (Table 2). MAVS has been found to be linked to the outer mitochondrial membrane where it functions in antiviral signalling by activating NK-κB, IRF3 and IRF7 in response to viruses. However, the exact mechanism of this activation has not yet been fully elucidated. Current knowledge is that MAVS can interact with cytosolic RIG-I (retinoic acid-inducible gene I, or DDX58) and MDA5 (melanoma differentiation-associated gene 5), which are RIG-I-like receptors that can recognize viruses. MAVS has also been shown to bind TRAFs (e.g. TRAF 6, TRAF 3), as well as interact with TRADD (TNFR1-associated death domain protein) to activate IRF3 and IRF7 through a signalling pathway that involves TRAF3, TANK, IKKε and/or TBK1. NF-κB activation is believed to involve TRADD, FADD (FAS-associated death domain protein) and RIP1, with the kinases IKKα and IKKβ also indicated in MAVS based signalling [78]. While the exact pathway involved in MAVS signalling has not been fully elucidated in mammals, the main components so far associated with MAVS have also been found in our S. glomerata study. Along with MAVS, ORFs coding for MDA5/DDX58, RIP1 and FADD were detected in our S. glomerata study (Table 2), suggesting that S. glomerata could potentially recognise and respond to viruses (Fig 2). While Philipp et al. [26] only observed MDA5 and DDX58 but not MAVS in M. edulis, MAVS was also found in C. gigas, where its inhibition led to a decrease in TRAF3 expression [79]. The results of this recent study not only showed that MAVS exists in other oyster species aside from S. glomerata, but also that MAVS expression seems to be linked to some degree with the expression of TRAF3 as has been observed in mammals. With mass mortalities of oysters caused by viral diseases such as Ostreid herpesvirus-1 (OsHV-1) [80], an effective innate immune response against viruses would be essential for the health and survival of oysters such as S. glomerata. Considering that mammalian MAVS and RIG-I-like receptors can recognise and respond to viruses, eventually leading to an innate immune response against these pathogens, MAVS might be a promising target for further research in oysters.
Hypoxia-inducible factors (HIFs)
Oxygen availability is important for vertebrate and invertebrate alike and has roles in many biological functions (e.g. energy production). Oysters such as S. glomerata are often exposed to hypoxia in their natural habitat (e.g. during low tide or high nutrient load in the water) and need mechanisms that allow it to adapt to a low oxygen environment [81, 82]. HIFs are genes that have been shown to play a role in adaptation to hypoxia in animals [81], with HIF-1α/HIF-2α as well as HIF-1β observed in our S. glomerata transcriptome (Table 2). While the observation of HIF genes in S. glomerata would be expected, HIF have recently been linked to the innate immune response in mammals and could potentially have a similar secondary role in S. glomerata next to their protective function against hypoxia. The heterodimeric HIF functions as a transcription factor, and through the genes whose expression is regulated by HIF, has a role in various cellular pathways such as metabolism, cell differentiation and apoptosis [83]. HIF is comprised of two subunits, HIF-α and HIF-1β, with HIF-α encompassing the following 3 genes: HIF-1α, HIF-2α and HIF-3α [84]. In a normoxic environment and without an activating stimulus (e.g. insulin-like growth factor, interleukin-1β or LPS), HIF-α is quickly degraded by prolyl hydroxylases (PHDs) or otherwise regulated by hypoxia-inducible factor 1-alpha inhibitor (HIF1AN) [83, 85]. However, bacterial LPS for instance, has been shown to be able to increase HIF-1α mRNA expression not only in hypoxic but also in normoxic conditions [86, 87]. This LPS induction of HIF-1α expression appears to involve TLR4 and p44/42 MAPK and NF-κB signalling pathways [83, 86, 87]. Under hypoxic conditions, for example during inflammation/infection where the metabolism of pathogens and host inflammatory cells and decreased perfusion creates a localised low oxygen environment, PHDs and HIF1AN are inhibited and HIF-α associates with p300-CBP (CREB-binding protein). HIF-α–p300-CBP then moves to the nucleus where the HIF heterodimer forms and the HIF complex binds to the hypoxic-response elements (HREs), resulting in the regulation of target gene expression. In addition, HIF is thought to boost neutrophil and macrophage migration to areas of infection, PAMPs detection, phagocytosis, bactericidal activity (e.g. antimicrobial peptides, tumour necrosis factor and nitric oxide) and the survival of neutrophils, macrophages and monocytes [83–85]. HIF-1 has also been indicated in the transcriptional regulation of TLR4 expression in macrophages exposed to hypoxia and in the direct regulation of TLR2 and TLR6 expression [88, 89]. The S. glomerata reference transcriptomes of this study contain ORFs for all components of the HIF pathway, from HIF-1α/ HIF-2α, HIF-1β, HIF1AN to PHD and p300/CBP (Table 2) based on sequence homology as well as InterProScan domain and family matches. Some of these genes have also been found in other molluscs, such as HIF-α and PHD that have been cloned from C. virginica and C. gigas [81, 82] and HIF1AN that was found in the C. gigas genome [21]. However, to the best of our knowledge, HIF-1β has not been previously found in oysters and is therefore reported here for the first time in the oyster S. glomerata. While most research into HIF’s roles in immunity appears to have been carried out in vertebrates, one study in C. gigas has observed a link between HIF-α expression and respiratory burst activity in haemocytes, indicating that HIF-α has a role in reactive oxygen species (ROS) production in this oyster species [90]. Although HIF’s main role might be in allowing S. glomerata to adapt to short-term hypoxia, the study in C. gigas suggests that HIF could also have a role in immunity, similar to mammalian HIF (Fig 2). Furthermore, with ORFs for all components of the HIF pathway found in the S. glomerata transcriptomes, as well as ORFs for the TLR signalling pathway, a dual role of HIF in S. glomerata could be possible (Fig 2). Further research is necessary to determine HIF’s exact role(s) in S. glomerata and other oyster species. Sequence information gained in our transcriptome study could be beneficial for such future research endeavours.
Phagocytosis and antioxidant defence
Phagocytes, such as macrophages are an important part of the innate immune defence in response to microorganisms and phagocytic activity of immune cells has been shown not only in vertebrates, but also in invertebrates such as M. galloprovincialis and S. glomerata [11, 91, 92]. In these and other bivalves, phagocytosis is the most important cellular defence mechanism, with a large number of immune cells (2–4 x 106 cells/ml) contained within the haemolymph [46, 92]. The basic principle behind phagocytosis is the recognition of microorganisms or cells, their internalisation and destruction [91]. Recognition of particles occurs through a variety of receptors, for example, TLRs and other PRRs (e.g. mannose receptor), opsonic receptors (e.g. complement receptors) or apoptotic corpse receptors (e.g. stabilin-2) [91, 93]. Some of these receptors, such as TLRs, macrophage mannose receptors, SRs and the apoptotic corpse receptor stabilin-2 have also been found in our S. glomerata transcriptomes (Table 2). Receptors that bind to immunoglobulin G (IgG) (e.g. FcγRI) or IgAs (FcαRI), however, have not been found in S. glomerata and also do not appear to have been observed in the C. gigas genome [21]. As oysters do not possess adaptive immunity, it is likely that these receptors are not used in oysters. The receptors found in S. glomerata (e.g. TLR, SRs) could potentially fulfil the important innate immune function of foreign particle recognition for phagocytic clearance, therefore ensuring that the host is protected from potentially harmful microorganisms or accumulating apoptotic bodies (Fig 2). Furthermore, as described previously, a ficolin-like recombinant protein from C. hongkongensis was shown to increase the phagocytic activity of C. hongkongensis haemocytes by acting as an opsonin [55]. Having these multiple receptors that directly or indirectly (e.g. through opsonins) result in the successful phagocytic clearance of cells or invading microbes would be beneficial for S. glomerata as these oysters are continuously exposed to bacteria in their natural environment and would therefore need a strong protective immune mechanism such as phagocytosis to maintain their health.
Depending on the receptor(s) that recognise the particles, specific signalling pathways are triggered that eventually lead to the engulfment of the particles and their internalisation into the phagosome [93]. The phagosome then undergoes maturation in which it becomes increasingly more acidic before it fuses with lysosomes to form a phagolysosome [93]. Along with the acidic (pH 4.5–5.0) environment, phagolysosomes contain active cathepsins, ROS (e.g. superoxide anions, hydrogen peroxide and hydroxyl radicals), reactive nitrogen intermediates (RNI) and antimicrobial proteins and peptides (e.g. lysozyme, defensins) that allow phagocytes to destroy the engulfed targets [69, 93]. Enzymes that are involved in the production of ROS and RNI as well as ORFs coding for cathepsins, antimicrobial proteins and peptides have been found in the S. glomerata transcriptome of this study (Table 2) and are discussed in detail in the following sections.
ROS and RNI
In response to proinflammatory cytokines or PAMPs, phagocytes express nitric oxide synthase (NOS) which catalyses the reaction that produces nitrous oxide. Nitrous oxide then reacts with ROS to create a range of potent RNI (e.g. peroxynitrite) that are effective against lipids, proteins and nucleic acids [70, 93]. NOS activity has been detected in a variety of molluscs, for example, in the haemocytes of the freshwater snail Viviparus ater and the mussel M. galloprovincialis and NOS sequences have been found not only in our study (Table 2) but also in A. californica and other marine invertebrates [94–96]. With NOS activity observed in other molluscs, it is likely that the NOS found in this S. glomerata study also functions in the production of nitrous oxide.
ROS, which can interact with nitrous oxide to form toxic RNI, are produced as a by-product of biological reactions as well as specifically formed by phagocyte NADPH oxidase (NOX). To date, seven NOX isoforms have been discovered, NOX 1–5, dual oxidase 1 (DUOX 1) and DUOX 2, with all members of the family functioning as electron transporter, reducing oxygen to superoxide in the process [97]. Similar to mammalian studies, multiple transcripts coding for NOX and DUOX have been found in the S. glomerata of our study (Table 2). In addition, DUOX has been found in the C. gigas genome [21] and also in the scallop Mizuhopecten yessoensis, where DUOX expression was found to be induced in the gill after exposure to copper [98]. While NOX 2 has also been shown to be expressed in non-phagocytic cells, its function appears to be the production of ROS in phagocytes [70, 93, 97]. Proinflammatory stimuli trigger the formation of the NOX 2 (also known as gp91phox) complex in phagocytes that forms between NOX 2 and p22phox, a transmembrane protein. Once the two proteins have associated with each other to form flavocytochrome b558 the complex is activated by cytosolic components (p47phox, p67phox and p40phox) that translocate once p47phox has been phosphorylated, allowing p47phox to interact with p22phox [93, 97]. Another important molecule NOX 2 interacts with during complex assembly and activation is the small GTPase Rac [93, 97], which has also been detected in the transcriptomes of this study (Table 2). As one transcript coding for gp91phox (NOX 2) has also been found in our study (Table 2), it is possible, that NOX 2 and Rac could interact in a similar way with each other in S. glomerata as it does in mammals, potentially resulting in the production of ROS.
Aside from NOX 2, mitochondria can also produce ROS that aid macrophages in their bactericidal activity. Mitochondrial ROS is generated through TLR signalling (TLR1, TLR2 and TLR4) during which TRAF6 translocates to the mitochondria where it interacts with ECSIT (evolutionarily conserved signalling intermediate in Toll pathways), leading to a rise in mitochondrial ROS production [78]. One ORF coding for a 451 amino acid long ECSIT has been detected in this study (Table 2), as well as in C. gigas. In their study, Zhang et al. [99] showed ECSIT expression levels were significantly up-regulated in the haemolymph of C. gigas challenged with the bacterium V. anguillarum when compared to control oysters, indicating that ECSIT plays a role in innate immune defence of oysters. Additionally, the authors observed ECSIT gene expression in the haemolymph, gill, digestive gland, muscle, mantle and gonad of C. gigas [99]. This is in accord with our data, where the ECSIT transcript was detected in the haemolymph, gill, mantle, adductor muscle, gonad and digestive system of S. glomerata. Furthermore, comparison of the C. gigas ECSIT with the S. glomerata ECSIT, using Clustal Omega showed that the 452 amino acid long S. glomerata ECSIT shared a 72.3% sequence identity with the C. gigas ECSIT (S4 Fig) and a 38.2% sequence identity with a M. galloprovincialis ECSIT (AHI17287). Based on the results of the study by Zhang [99], as well as the sequence identity of the C. gigas and S. glomerata ECSIT, it is likely that S. glomerata ECSIT also functions in innate immune defence (Fig 2).
In addition to Rac, ECSIT, NOX, DUOX and gp91phox, p22phox and p67phox have also been found in our study (Table 2). Interestingly, based on amino acid (aa) length, S. glomerata NOX and NOX-related genes appear to be very similar to the mammalian orthologous genes. For instance, mammalian NOX are 564–747 aa long [97] and S. glomerata NOX between 568 and 851 aa. Similarly, S. glomerata DUOX (396 to 1,627 aa, with the majority between 1,392–1,627 aa), gp91phox (568 aa), p22phox (172 aa) and p67phox (185 aa—partial sequence) transcripts are comparable to DUOX (1,548–1,551 aa), gp91phox (570 aa), p22phox (195 aa) and p67phox (526 aa) gene length in mammals [97]. Research into non-mammalian species indicates that ROS production by NADPH oxidase might not be limited to mammals. For instance, Adema et al. [100] used haemocytes of the pond snail Lymnaea stagnalis to test inhibitors of NADPH-oxidase and compounds that inhibit NOX complex assembly in mammalian cells and showed that phagocytosis was inhibited by these compounds in the pond snail. Another study assessed the effect of V. splendidus challenge of C. gigas haemocytes on the expression of genes involved in phagocytosis and observed a significant increase of NADPH oxidase after a secondary challenge with the bacterium [101]. While these studies suggest that NADPH oxidase also produces ROS in non-mammalian cells, we only found an ORF for one of the three cytosolic components (p67phox) of the NOX2-complex in our transcriptome, but no ORFs for p47phox or p40phox. In addition, no apparent hits for p40phox (alternative name: SH3 and PX domain-containing protein 4) or p47phox (alternative name: SH3 and PX domain-containing protein 1A) could be found in the C. gigas genome [21]. This suggests that these two cytosolic components might not be needed for NOX2-complex formation in oysters. Alternatively, other molecules not yet found might carry out the roles of p40phox and p47phox in oysters, or the sequences have diverged in molluscs and therefore could not be detected in our S. glomerata study. Further research is needed to elucidate the exact process by which the NOX2-complex forms and functions in oysters. In addition, while one p22phox was observed in the C. gigas genome [21], p67phox and gp91phox do not appear to have been found in any other oyster yet and have been reported for the first time in our S. glomerata study.
Considering the environment S. glomerata inhabit, immune components with strong bactericidal activity (e.g. ROS) are essential to the health and survival of these bivalves. In this study we have found genes necessary to produce both nitrous oxide and ROS. Furthermore, fundamental components of two ROS production pathways were found in S. glomerata: NOX 2 and ECSIT. Possessing two pathways that potentially lead to the synthesis of ROS could allow S. glomerata oysters to produce sufficient amounts of ROS, nitrous oxide and RNIs to successfully protect themselves from pathogens (Fig 2). Furthermore, ROS production by the mitochondria through TLR signalling, whose individual components have also been found in this study, would allow S. glomerata to directly respond to endogenous (stress related) or exogenous (bacterial challenge) ligands (Fig 2).
Antioxidants
While RNI and ROS are an important part of the innate immune defence mechanism, ROS have also been shown to play roles in, for instance, cellular signalling, apoptosis, cell growth and differentiation. However, while ROS can be beneficial, a fine balance has to be maintained of the level of ROS produced and removed, as any disruption in the balance can lead to oxidative stress which may result in damage to a variety of macromolecules like DNA, RNA and proteins [39, 40, 69]. This balance is maintained by non-enzymatic (e.g. minerals and carotenoids) and enzymatic antioxidants such as superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT), as well as redox proteins like thioredoxin, glutaredoxin and peroxiredoxin, which allow the host organism to protect itself from the harmful effects of ROS [39, 40, 102]. In this S. glomerata transcriptome study, ORFs encoding the proteins SOD, GPX and CAT were found (Table 2). These three enzymatic antioxidants protect organisms from oxidative stress, caused by ROS such as superoxide anion and hydrogen peroxide (Fig 2). SOD, which exists in three forms in humans (cytosolic copper/zinc-SOD, mitochondrial manganese-SOD and extracellular SOD), reacts with superoxide anions, generating hydrogen peroxide in the process. Hydrogen peroxide in turn is catalysed by CAT and GPX, resulting in the formation of water and molecular oxygen. Whereas both CAT and GPX can remove hydrogen peroxide, only GPX needs glutathione (GSH) to catalyse the reaction [39, 40]. GSH is synthesised in the cytosol from glutamate, cysteine and glycine through the sequential catalytic action of the two enzymes γ-glutamylcysteine synthetase (GCS) that consists of a catalytic (also called glutamate—cysteine ligase catalytic subunit) and a regulatory polypeptide (also called glutamate—cysteine ligase regulatory subunit) and glutathione synthetase (GS) [103, 104]. Once GSH has been oxidised to glutathione disulfide (GSSG) during the reduction of hydrogen peroxide, it can be reduced back to GSH by the action of glutathione reductase [39]. These enzymes (GS, both GCS subunits and glutathione reductase) involved in the synthesis and reduction of GSH have also been found in the transcriptomes of this study (Table 2), indicating that GSH synthesis and GSSG reduction are conserved and also provide the necessary GSH for the removal of hydrogen peroxide in S. glomerata. In addition, we have also found thioredoxin, thioredoxin reductase, peroxiredoxin, glutaredoxin, glutathione S-transferase (GST) and two methionine sulfoxide reductases (MSRs) in the S. glomerata transcriptomes (Table 2). The thioredoxin system is another major antioxidant system that functions, among others, in oxidative stress defence by transferring electrons to, for instance peroxiredoxin, that is able to react with and remove H2O2, ROOH and ONOO- [39, 105]. Reaction efficiency of peroxiredoxin in terms of H2O2 removal appears to be on par with GPX and CAT. Once peroxiredoxin has disposed of H2O2, it is reduced to its active state by the thioredoxin antioxidant system. Similar to peroxiredoxin, MSRs also receive electrons from the thioredoxin system; however, MSRs function only indirectly as ROS scavengers. Oxidative stress can oxidise methionine to methionine sulfoxide, impacting on the protein’s function. MSRs are able to transform methionine sulfoxide back into methionine, restoring protein function [105]. More importantly, one MSR was found to be up-regulated in the clam Ruditapes decussates in response to the parasite Perkinsus oleni, indicating that MSR is involved in the immune response of this mollusc [106]. Oxidised thioredoxin itself can be reduced by thioredoxin reductase, as well as by GSH and glutaredoxin. In turn, the thioredoxin system appears to be able to reduce oxidised GSH, interlinking the GSH and thioredoxin antioxidant systems [105]. Another more indirectly acting antioxidant family are GSTs that protect against molecules, such as epoxides and hydroperoxides produced by oxidative stress [103].
Of the antioxidants and antioxidant related genes found in the S. glomerata transcriptomes of this study, peroxiredoxin, GPX and GST have also been detected in Akoya pearl oysters (Pinctada fucata), when the oysters were exposed to stressors such as mechanical agitation and air [107]. Green et al. [108] also found peroxiredoxin 6 and SOD differentially expressed in S. glomerata selected for disease resistance when compared to wild oysters. In addition, CAT and GPX have been cloned in C. gigas and their expression and activity along with SOD measured in C. gigas, Saccostrea cucullata, and Bathymodiolus azoricus [109–111]. Furthermore, GSH levels have been measured in S. glomerata and C. gigas [112, 113]. Taken together, these results suggest that the main antioxidants CAT, SOD and GPX might function similarly in vertebrates and invertebrates such as oysters. In addition to the main antioxidants, the activity of glutathione reductase, thioredoxin reductase and GST was measured in the gills of C. gigas [113] and an EST for thioredoxin found in C. virginica [114]. Expressed sequence tags were found for GST, thioredoxin, thioredoxin reductase, GPX and SOD in M. galloprovincialis exposed to stressors such as pollutants, bacteria and temperature [115]. Furthermore, sequences for thioredoxin reductase, glutaredoxin, GS, both glutamate—cysteine ligase subunits, glutathione reductase and both MSRs were also found in the C. gigas genome [21]. These and our S. glomerata results show that antioxidants and antioxidant related genes are conserved across a wide range of molluscs, indicating that antioxidant defence is an important mechanism for S. glomerata and other molluscs. Moreover, taking into account the complex set of antioxidant and antioxidant related genes found in our S. glomerata transcriptomes, antioxidant defence appears to be well developed in this oyster species. This will allow S. glomerata oysters to guard themselves against the detrimental effects of ROS and RNIs, while benefiting from their protective functions. Additionally, as S. glomerata appear to not only have the GSH but also the thioredoxin system and GSTs to defend themselves from oxidative stress, they might still be sufficiently protected should a specific pathway or component be inhibited (Fig 2).
Proteases and antimicrobial proteins and peptides
Among the defensive arsenal of host cells are cysteine (cathepsins B, C, F, H, K, L, O, S, V, X and W), aspartic (cathepsins D and E) and serine proteases (cathepsins A and G), whose gene expression is controlled by a range of inflammatory stimuli [41, 116, 117]. The main function of cathepsins in the lysosomal system is the degradation of proteins; however, their proteolytic activity depends on the pH of their environment [116, 117]. While most studies into cathepsins have been carried out in mammals, 59 transcripts coding for cathepsins have also been found in this S. glomerata transcriptome study (Table 2), as well as in the haemocytes of C. virginica and the mussel Cristaria plicata [114, 118]. Furthermore, cathepsin B and C expression was shown to be induced in the clam Sinonovacula constricta by the bacterium V. anguillarum [119, 120]. These results suggest that cathepsins could also play an important role in the innate immune defence of oysters and other molluscs. Moreover, the range of cathepsins found in our S. glomerata transcriptomes indicates that these proteases are an important component of the S. glomerata innate immunity (Fig 2).
In addition to cathepsins, the S. glomerata transcriptomes contained transcripts coding for lysozyme, big defensin, hydramacin and bactericidal permeability increasing protein (BPI) (Table 2). Lysozymes are hydrolytic enzymes that act on bacterial peptidoglycan to destroy bacterial pathogens, with cDNA sequences for this enzyme also isolated from a variety of other molluscs, such as Bathymodiolus thermophilus, Calyptogena sp. 1, M. galloprovincialis, Mactra veneriformis and others [121, 122]. Gene expression levels of an i-type lysozyme increased in the mussel C. plicata after exposure with Aeromonas hydrophila, and the recombinant lysozyme produced in the same study demonstrated bacteriolytic activity against a range of bacteria (e.g. A. hydrophilia, Bacillus subtilis, Staphylococcus aureus and Escherichia coli) [123]. Moreover, Zhao et al. [124] cloned a lysozyme from the scallop Chlamys farreri with sequence similarity to g-type lysozymes. The authors also assessed the lytic activity of the recombinant lysozyme protein, showing that the protein was bactericidal against the Gram-positive bacteria Micrococcus lysodikicus and Micrococcus luteus, with only weak lytic activity against Gram-negative bacteria (e.g. V. parahaemolyticus and V. anguillarum) and no activity against E. coli and S. aureus [124]. These studies showed that not only vertebrate, but also invertebrate lysozymes have a bactericidal ability against a wide range of bacterial targets. Considering that invertebrates do not have adaptive immunity, expressing lysozymes with lytic activity towards a variety of bacterial pathogens could allow S. glomerata oysters to protect themselves from the bacteria they encounter in their natural environment.
Aside from lysozymes, the invertebrate innate immune defence system also depends on antimicrobial peptides (AMPs) like defensins. These cationic antimicrobial peptides carry out their defence function by forming pores in the bacterial membrane, which ultimately leads to an osmotic imbalance in the bacterial cell [93, 125]. Research into big defensins in the haemolymph of horseshoe crabs indicated that this invertebrate AMP is active against fungi as well as Gram-positive and Gram-negative bacteria [126]. A similar pattern was observed in the scallop Argopecten irradians, where big defensin was shown to be active against Gram-positive bacteria, weakly against Gram-negative bacteria (no effect on E. coli), and a potential fungicidal activity against the yeast Pichia pastoris [127]. Furthermore, big defensin expression levels increased in the haemolymph of the clam Venerupis philippinarum after V. anguillarum challenge. In addition, big defensin also showed an inhibitory activity against other Gram-negative and Gram-positive bacteria [128]. Interestingly, one study assessed the expression levels of three big defensins in the haemolymph of C. gigas and found two of them up-regulated in response to bacterial challenge. It was hypothesised by the authors that the third big defensin might be constitutively expressed in the haemocytes of this oyster [126]. Together these results suggest that big defensins in invertebrates are active against a wide range of targets and that not all big defensins might be regulated in the same manner. When the S. glomerata big defensins of this transcriptome study were compared with each other, two of the five big defensin ORFs (m.22175 and m.22176) found in this transcriptome showed 87% sequence identity with each other, but only between 33 to 48% with the other three ORFs (m.6735, m.28084 and m.24530) (S5 Fig). These results show that S. glomerata big defensin protein sequences display a fairly high variability. Interestingly, the five S. glomerata big defensins also appeared to be expressed in different tissues. While transcripts of three of the big defensins (m.22175, m.22176 and m.24530) were expressed in all six tissues, m.6735 was only expressed in the digestive tissue and transcripts of m.28084 were detected in all tissues but the gill and adductor muscle. It is unclear why some S. glomerata big defensins appear to be only expressed in some but not all of the tested tissues. Nevertheless, it was interesting to note that only the S. glomerata digestive tissue had all five big defensins expressed. Considering that the particles that are filtered from the water column by S. glomerata also contain bacteria, it might be beneficial for this oyster to have an as broad as possible range of big defensins expressed in the digestive system to protect itself from ingested bacteria. Alignment of the five S. glomerata big defensins to the putative protein sequences of the big defensins of the scallop A. irradians, clam V. philippinarum and C. gigas studies above showed that S. glomerata big defensins mapped closer to the big defensins of C. gigas (sequence identities of 27–30% with m.6735, 46–54% with the other four) than to the big defensins of V. philippinarum and A. irradians (sequence identities between 22% (m.6735) and 18–35%) (S5 Fig). This was expected to be seen as S. glomerata are more closely related to C. gigas then to either clam or scallop.
Bactericidal permeability increasing protein (BPI), another AMP found in our study (Table 2), has been shown to counteract the effects of LPS (e.g. TNF-α production), has anti-inflammatory properties and a role in opsonisation that leads to an increase in phagocytosis. In addition, BPI expression can be triggered by LPS (TLR—MyD88-independent pathway) and carries out its bactericidal activity by causing damage to the bacterial membrane [129, 130]. This antimicrobial peptide has been identified in C. gigas haemocytes, where the recombinant protein was able to bind LPS, damage bacterial membranes and had antibacterial activity against the strain E. coli SBS363. BPI expression levels were also observed to increase in response to bacterial challenge [130], indicating that C. gigas BPI had similar functionality to vertebrate BPI. While the majority (18) of the S. glomerata BPIs appear to be expressed in all six tissues studied, six of the BPIs were expressed in all tissues but the adductor muscle, and one each were expressed in a) haemolymph, gill and mantle, b) haemolymph and mantle and c) all but adductor muscle and digestive tissue. Hydramacin, an AMP detected for the first time in the clam V. philippinarum [131] was also expressed in all S. glomerata tissues of this study. This coincides with the tissue expression pattern of hydramacin in V. philippinarum, where it was found in the adductor muscle, mantle, gill, haemocytes, shiphon and foot of the clam [131]. Similar to other AMPs, hydramacin transcript expression was shown to increase after clams were challenged with the bacterium Vibrio tapetis [131]. Alignment of the S. glomerata and V. philippinarum hydramacin showed a sequence identity of 37% (S6 Fig) indicating that the two putative protein sequences are not closely related.
Considering that S. glomerata oysters are directly exposed to a variety of microorganisms in their natural environment, phagocytosis with its various protective components (e.g. AMPs, hydrolytic enzymes) is an important innate immune defence mechanism that allows the animals to combat the harmful effects of invading pathogens (Fig 2). Furthermore, expressing more than one type of AMP in different tissues, as has been seen in this study, would allow S. glomerata oysters to protect themselves from a diversity of bacterial pathogens. Future research into expression levels of different AMPs and different types of the same AMP in S. glomerata in response to stress or bacterial challenge would give us a more detailed view on the protective function of AMPs in this animal, with potential implications for other molluscs.
Apoptosis
Apoptosis is an evolutionary conserved mechanism with essential functions in homeostasis and innate immunity that removes damaged cells without triggering inflammation [132, 133]. This was shown in a study in C. virginica, where a basic level of apoptosis of up to 25% was observed in circulating haemocytes. Furthermore, infection of the oyster with the parasite Perkinsus marinus resulted in increased haemocyte apoptosis levels, indicating that apoptosis is an important immune response in oysters such as C. virginica that allows the immune system to remove infected haemocytes [134]. Current knowledge suggests that vertebrate and invertebrate apoptosis share many components of apoptotic cascades, with detailed descriptions of the apoptotic cascades in vertebrates and to some degree in molluscs available [132, 135, 136]. In short, the apoptotic signalling cascades are generally split into two interconnected pathways, an intrinsic and an extrinsic cell death pathway [135, 136], with environmental stressors (e.g. pollution, salinity fluctuations) believed to stimulate the intrinsic pathway in molluscs [132]. Induction of the intrinsic pathway involves pro-apoptotic proteins (e.g. Bax and interferon alpha-inducible protein 27 [IFI27]) and results in the release of proteins (cytochrome c, Smac/DIABLO [second mitochondrial activator of caspases/direct IAP-binding protein of low isoelectric point] and AIF [apoptosis-inducing factor]) from the mitochondria into the cytosol [135, 137]. These mitochondrial factors interact with other components of the signalling cascade (e.g. apoptotic protease-activating factor-1 [Apaf-1], caspases) to promote apoptosis. Baculoviral IAP repeat-containing proteins (IAPs) inhibit caspase activity, with the inhibitory action of IAPs removed through binding with Smac/DIABLO. AIF in comparison causes apoptosis in a caspase-independent manner [135]. Our S. glomerata transcriptomes contained multiple transcripts of caspases, IAPs, DIABLO and one transcript for AIF, along with transcripts for the two pro-apoptotic proteins, Bax and IFI27 (Table 2). Similar to our study, caspases and AIFs were found in P. maximus and M. edulis [26, 53], caspases, IAPs and Bax in C. virginica [25], IFI27 in C. gigas [21] and DIABLO in A. californica [GenBank:XP_005110230] (http://www.ncbi.nlm.nih.gov). While these results show that many of the components of the intrinsic apoptosis pathway are also found in molluscs, we did not find any transcripts for Apaf-1 in S. glomerata. Moreover, to the best of our knowledge, no Apaf-1 transcripts have been observed yet in any other mollusc. This suggests that while the majority of the intrinsic pathway appears to be conserved in S. glomerata, this pathway either acts independent of the action of Apaf-1 or contains an as of yet unknown component with similar functions to Apaf-1 in mammals.
Apart from the intrinsic apoptosis pathway, the extrinsic apoptosis pathway also seems to exist in molluscs [136]. Ligand binding (e.g. TNF) to a death receptor of the TNF receptor superfamily induces apoptosis through the extrinsic pathway that involves the recruitment of FAS-associated death domain protein (FADD) and the action of several caspases [135, 136]. These components of the extrinsic pathway have also been detected in our S. glomerata transcriptome, with ORFs potentially coding for FADD, TNF receptor superfamily members and TNF superfamily members (Table 2). FADD, TNF and TNF receptor superfamily members have also been found in C. virginica [25], indicating that these proteins are important in oysters. Overall, having found components of both, the intrinsic and extrinsic cell death signalling pathways, in our S. glomerata transcriptomes, suggests that apoptosis is an integral part of the S. glomerata immune response to maintain its health (Fig 2).
Conclusions
In this study, we have exposed the economically and ecologically important oyster species S. glomerata to a range of environmental stressors to obtain sequencing data for six different tissues (haemolymph, gill, mantle, adductor muscle, gonad and digestive). Many of the transcripts expressed in S. glomerata were found across all six tissues, with a smaller number of transcripts specifically expressed in the haemolymph of the oyster, suggesting a function in the innate immune system of S. glomerata. Some of the transcripts exclusive to the haemolymph were involved in phagocytosis (e.g. cathepsins), antioxidant defence (e.g. SOD, peroxiredoxin) and potentially TLR signalling (e.g. heat shock proteins). Closer examination of the S. glomerata transcriptomes revealed a wide range of transcripts potentially involved in the innate immune defence against invading pathogens. A schematic visualising the innate immune arsenal stimulated in S. glomerata in response to stress is shown in Fig 2. Pathways such as TLR signalling or apoptosis appear to be largely conserved in S. glomerata, indicating that these pathways are also important in invertebrates such as oysters. In addition, HIF genes, generally associated with hypoxia, but recently also linked to immunity in mammals, have been found in S. glomerata, with HIF-1β reported for the first time in oysters. Components of other equally important immune functions such as phagocytosis and antioxidant defence were also observed in S. glomerata, with to the best of our knowledge p67phox and gp91phox reported for the first time in oysters. Overall, this study examined for the first time the complex machinery of the S. glomerata innate immune system and will be a valuable source for further research into the innate immune system of S. glomerata and other oyster species.
Methods
Illumina sequencing
Wild, adult S. glomerata oysters were collected from Cromarty Bay (NSW, Australia) and exposed to a variety of environmental stressors in a controlled setting (S1 File). Different levels of a) CO2 and temperature (n = 90 oysters), b) salinity and temperature (n = 174 oysters), c) copper (n = 24 oysters) and d) pyrene and fluoranthene (n = 63 oysters) were chosen as experimental stressors. In addition, wild and selectively bred (for fast growth and disease resistance) adult oysters were exposed to different levels of CO2, spawned and their offspring also exposed to different levels of CO2 as larvae and adult. This was done for three generations of oysters. Six tissues (haemolymph, gill, mantle, adductor muscle, digestive system and gonad) were extracted from each of the third generation adult offspring (n = 108 oysters) from this experiment, from the wild oysters of the above four experiments, and from non-stressed control oysters at multiple time-points throughout the experiments. Small amounts of tissue were pooled per tissue type from all collected samples (n = 459 oysters total) and total RNA extracted, using TRIsure™ (Bioline, Australia) according to the manufacturer’s protocol. Quantity and quality of the total RNA was tested with the NanoDrop2000 spectrophotometer (Thermo Fisher Scientific, USA) and gel electrophoresis before samples were freeze dried and sent to BGI (Hong Kong) for sequencing. On arrival, BGI checked the quality of the total RNA samples with an Agilent Bioanalyzer®, carried out a DNase I digestion for each of the samples, then prepared strand-specific and normalised, as well as non-normalised and non-strand specific libraries for paired-end 101 bp sequencing for each of the six pooled tissue samples, using Illumina HiSeq-2000. Normalisation of the strand-specific libraries was chosen to increase the possibility of detecting low expressed genes. This, in addition to sequencing non-normalised and non-strand specific libraries, coupled with using tissue from non-stressed and stressed oysters, should result in a most comprehensive transcriptome. Raw sequencing reads were deposited in the NCBI sequence read archive under the BioProject number PRJNA272756.
Quality control and de novo assembly
Quality of the sequencing data of the six tissues was assessed with FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) pre- and post-trimming, after which adapter sequences were removed and the sequences trimmed with Trimmomatic [138], using the following parameters: TRAILING:3, MINLEN:36 and HEADCROP:1. Furthermore, the sequences were filtered for artefacts such as E. coli, plasmid or human sequences, as well as ribosomal sequences. Post-processed reads of the six tissues were combined into two reference data sets and both sets assembled with Trinity [31]. The two data sets were comprised of: a) strand-specific and normalised reads only and b) non-normalised and non-strand specific reads and reverse complement of strand-specific and normalised reads, with the resulting two reference assemblies considered as strand-specific transcriptome and combined transcriptome, respectively throughout the rest of the text. Redundancy was removed from both data sets with cd-hit-est [139], using a 99% cut-off, and potential coding regions determined with TransDecoder (http://transdecoder.sourceforge.net/) and used for further analysis. Completeness of both non-redundant assemblies was assessed with CEGMA (Core Eukaryotic Genes Mapping Approach) [32].
Functional annotation and tissue distribution
Blastp similarity searches were carried out on the open reading frames (ORFs) of both transcriptomes against the NCBI non-redundant (nr) database (downloaded 01 October 2013) with an e-value cut-off of 1e-5 and a hit number threshold of 25. Blast2GO [140] with default parameters (hit adjusted to 25) was used for mapping and annotation of the ORFs, with InterProScan results merged with the already existing annotations. Furthermore, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotations were also obtained via Blast2GO. To obtain information on the transcript expression pattern across different tissues, reads of the six individual tissues were mapped back to the non-redundant strand-specific and combined reference transcriptomes, using the CLC Genomics Workbench version 7.5 (CLC Bio, USA) with default parameters except for length and similarity fraction that were set to 1.0 and 0.9, respectively. General transcript patterns across the six tissues were determined, with the tissue distribution of specific transcripts determined based on the CLC mapping information and the results presented using Microsoft Excel graphs.
Sequence comparison to Crassostrea gigas
Non-normalised and non-strand specific and normalised and strand-specific reads were mapped to the C. gigas genome [21] with Bowtie 2 [141]. Furthermore, both non-redundant reference transcriptomes were mapped to the C. gigas genome with CLC Genomics Workbench version 7.5 to determine similarity at the nucleotide level. S. glomerata transcripts had to match over their entire length with a similarity of at least 60% to the C. gigas genome to be considered a valid alignment.
Immune and immune-related genes
In the context of this study, only S. glomerata ORFs with the following characteristics were considered potential immune genes: 1) sequence homology to known immune and immune-related genes, and 2) annotated with an immune or immune-related GO term (e.g. GO:0006955, GO:0016265 and GO:0006950) and/or containing typical domain organisations as determined with InterProScan. Typical domain organisations for each potential immune and immune-related gene were determined based on curated (reviewed) entries in uniprot (https://www.uniprot.org). Redundancy in the ORFs of interest, caused by using ORFs from both reference transcriptomes, was removed with cd-hit [139], using a sequence identity threshold of 1.0 at the protein level. In addition, where sequence alignments are shown or % sequence identities given, they have been carried out with Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/).
Supporting Information
Left graphs for both, a) and b) show the respective full-scale graphs, with the respective right graphs emphasising the fine scale features of the same graphs by adding a graph break.
(DOCX)
GO-terms were determined with Blast2GO, using default parameters except for seq filter that was set to 50. Terms listed are based on a) biological process (Level 3), b) molecular function (Level 3) and c) cellular component (Level 4).
(DOCX)
GO-terms were determined with Blast2GO, using default parameters except for seq filter that was set to 50. Terms listed are based on a) biological process (Level 3), b) molecular function (Level 3) and c) cellular component (Level 4).
(DOCX)
Alignment of S. glomerata ECSIT transcript (c383034.graph_c0_seq1|m.66044) with ECSIT from C. gigas [GenBank:HQ225834] and M. galloprovincialis [GenBank:AHI17287].
(DOCX)
Alignment of S. glomerata big defensin transcripts (c211420.graph_c0_seq1|m.6735, c349903.graph_c0_seq1|m.22175, c349903.graph_c0_seq2|m.22176, c359623.graph_c0_seq5|m.28084 and c354390.graph_c0_seq1|m.24530) with big defensins from C. gigas [GenBank:AEE92778, GenBank:AEE92768 and GenBank:AEE92775], A. irradians [GenBank:DQ334340] and V. philippinarum [GenBank:HM562672].
(DOCX)
Alignment of S. glomerata hydramacin transcript (c328639.graph_c0_seq1|m.16116) with V. philippinarum hydramacin [GenBank:AGM14601].
(DOCX)
(DOCX)
CLC Genomics Workbench (version 7.5) mapping statistics of individual tissue reads for determination of transcript distribution across the six tissues.
(DOCX)
Acknowledgments
We are grateful to Mr John Wright, Ms Grace Bourke and Dr Laura Parker for giving us access to their CO2 and temperature, copper, and CO2 generational exposure experiments, respectively. We also would like to thank Mr Stephan O’Connor, Mr Kyle Johnston and the rest of the DPI hatchery team for their help and advice with oyster husbandry, Dr Anna Kuballa and Dr Peter Brooks for experimental advice and Mr Daniel Powell for expert laboratory management and computational support. We also acknowledge CSIRO for a postgraduate studentship for NGE.
Data Availability
Raw sequencing reads were deposited in the NCBI sequence read archive under the BioProject number PRJNA272756.
Funding Statement
This project was funded by the Fisheries Research & Development Corporation (FRDC – http://frdc.com.au/), the Australian Seafood Cooperative Research Centre (CRC – http://www.seafoodcrc.com/) (2011/718 to NGE, AE and WAO), and the University of the Sunshine Coast, Australia. NGE was supported by an Australian Seafood CRC and University of the Sunshine Coast scholarship, as well as a scholarship from Queensland Education and Training International (QETI). The funding parties had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Beck MW, Brumbaugh RD, Airoldi L, Carranza A, Coen LD, Crawford C, et al. Oyster reefs at risk and recommendations for conservation, restoration, and management. Bioscience. 2011;61(2):107–16. [Google Scholar]
- 2.Coen LD, Brumbaugh RD, Bushek D, Grizzle R, Luckenbach MW, Posey MH, et al. Ecosystem services related to oyster restoration. Mar Ecol Prog Ser. 2007;341:303–7. [Google Scholar]
- 3.Higgins CB, Stephenson K, Brown BL. Nutrient bioassimilation capacity of aquacultured oysters: quantification of an ecosystem service. J Environ Qual. 2011;40(1):271–7. [DOI] [PubMed] [Google Scholar]
- 4.Peterson CH, Grabowski JH, Powers SP. Estimated enhancement of fish production resulting from restoring oyster reef habitat: quantitative valuation. Mar Ecol Prog Ser. 2003;264:249–64. [Google Scholar]
- 5.Ruesink JL, Lenihan HS, Trimble AC, Heiman KW, Micheli F, Byers JE, et al. Introduction of non-native oysters: ecosystem effects and restoration implications. Annu Rev Ecol Evol Syst. 2005;36:643–89. [Google Scholar]
- 6.Creese A, Trenaman R. Aquaculture production report 2012–2013. New South Wales, Australia: NSW Department of Primary Industries, Industries NDoP; 2014. [Google Scholar]
- 7.Yu Z, Guo X. Identification and mapping of disease-resistance QTLs in the eastern oyster, Crassostrea virginica Gmelin. Aquaculture. 2006;254(1–4):160–70. [Google Scholar]
- 8.Bachère E, Mialhe E, Noёl D, Boulo V, Morvan A, Rodriguez J. Knowledge and research prospects in marine mollusc and crustacean immunology. Aquaculture. 1995;132:17–32. [Google Scholar]
- 9.Segarra A, Pépin JF, Arzul I, Morga B, Faury N, Renault T. Detection and description of a particular Ostreid herpesvirus 1 genotype associated with massive mortality outbreaks of Pacific oysters, Crassostrea gigas, in France in 2008. Virus Res. 2010;153(1):92–9. 10.1016/j.virusres.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 10.Berthe FCJ, Roux FL, Adlard RD, Figueras A. Marteiliosis in molluscs: a review. Aquat Living Resour. 2004;17:433–48. [Google Scholar]
- 11.Dang C, Lambert C, Soudant P, Delamare-Deboutteville J, Zhang MM, Chan J, et al. Immune parameters of QX-resistant and wild caught Saccostrea glomerata hemocytes in relation to Marteilia sydneyi infection. Fish Shellfish Immunol. 2011;31(6):1034–40. 10.1016/j.fsi.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 12.Butt D, Raftos D. Immunosuppression in Sydney rock oysters (Saccostrea glomerata) and QX disease in the Hawkesbury River, Sydney. Mar Freshw Res. 2007;58(2):213–21. [Google Scholar]
- 13.Nell JA, Perkins B. Evaluation of the progeny of third-generation Sydney rock oyster Saccostrea glomerata (Gould, 1850) breeding lines for resistance to QX disease Marteilia sydneyi and winter mortality Bonamia roughleyi. Aquac Res. 2006;37(7):693–700. [Google Scholar]
- 14.Peters R, Raftos DA. The role of phenoloxidase suppression in QX disease outbreaks among Sydney rock oysters (Saccostrea glomerata). Aquaculture. 2003;223:29–39. [Google Scholar]
- 15.Green TJ, Barnes AC. Reduced salinity, but not estuarine acidification, is a cause of immune-suppression in the Sydney rock oyster Saccostrea glomerata. Mar Ecol Prog Ser. 2010;402:161–70. [Google Scholar]
- 16.Chu FLE, Hale RC. Relationship between pollution and susceptibility to infectious disease in the Eastern oyster, Crassostrea virginica. Mar Environ Res. 1994;38:243–56. [Google Scholar]
- 17.Butt D, Shaddick K, Raftos D. The effect of low salinity on phenoloxidase activity in the Sydney rock oyster, Saccostrea glomerata. Aquaculture. 2006;251(2–4):159–66. [Google Scholar]
- 18.Cherkasov AS, Grewal S, Sokolova IM. Combined effects of temperature and cadmium exposure on haemocyte apoptosis and cadmium accumulation in the eastern oyster Crassostrea virginica (Gmelin). J Therm Biol. 2007;32(3):162–70. [Google Scholar]
- 19.Kuchel RP, Raftos DA, Nair S. Immunosuppressive effects of environmental stressors on immunological function in Pinctada imbricata. Fish Shellfish Immunol. 2010;29(6):930–6. 10.1016/j.fsi.2010.07.033 [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Chiodini R, Badr A, Zhang G. The impact of next-generation sequencing on genomics. J Genet Genomics. 2011;38(3):95–109. 10.1016/j.jgg.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang G, Fang X, Guo X, Li L, Luo R, Xu F, et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature. 2012;490:49–54. 10.1038/nature11413 [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi T, Kawashima T, Koyanagi R, Gyoja F, Tanaka M, Ikuta T, et al. Draft genome of the pearl oyster Pinctada fucata: a platform for understanding bivalve biology. DNA Res. 2012;19:117–30. 10.1093/dnares/dss005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao X, Wang Q, Jiao Y, Huang R, Deng Y, Wang H, et al. Identification of genes potentially related to biomineralization and immunity by transcriptome analysis of pearl sac in pearl oyster Pinctada martensii. Mar Biotechnol. 2012;14(6):730–9. 10.1007/s10126-012-9438-3 [DOI] [PubMed] [Google Scholar]
- 24.Joubert C, Piquemal D, Marie B, Manchon L, Pierrat F, Zanella-Cléon I, et al. Transcriptome and proteome analysis of Pinctada margaritifera calcifying mantle and shell: focus on biomineralization. BMC Genomics. 2010;11:613 10.1186/1471-2164-11-613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Li L, Zhu Y, Zhang G, Guo X. Transcriptome analysis reveals a rich gene set related to innate immunity in the Eastern oyster (Crassostrea virginica). Mar Biotechnol. 2014;16(1):17–33. 10.1007/s10126-013-9526-z [DOI] [PubMed] [Google Scholar]
- 26.Philipp EER, Kraemer L, Melzner F, Poustka AJ, Thieme S, Findeisen U, et al. Massively parallel RNA sequencing identifies a complex immune gene repertoire in the lophotrochozoan Mytilus edulis. PLoS ONE. 2012;7(3):e33091 10.1371/journal.pone.0033091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teaniniuraitemoana V, Huvet A, Levy P, Klopp C, Lhuillier E, Gaertner-Mazouni N, et al. Gonad transcriptome analysis of pearl oyster Pinctada margaritifera: identification of potential sex differentiation and sex determining genes. BMC Genomics. 2014;15:491 10.1186/1471-2164-15-491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hook SE, Johnston EL, Nair S, Roach AC, Moncuquet P, Twine NA, et al. Next generation sequence analysis of the transcriptome of Sydney rock oysters (Saccostrea glomerata) exposed to a range of environmental stressors. Mar Genomics. 2014;18:109–11. 10.1016/j.margen.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 29.Cantacessi C, Prasopdee S, Sotillo J, Mulvenna J, Tesana S, Loukas A. Coming out of the shell: building the molecular infrastructure for research on parasite-harbouring snails. PLoS Negl Trop Dis. 2013;7(9):e2284 10.1371/journal.pntd.0002284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng Y, Lei Q, Tian Q, Xie S, Du X, Li J, et al. De novo assembly, gene annotation, and simple sequence repeat marker development using Illumina paired-end transcriptome sequences in the pearl oyster Pinctada maxima. Biosci Biotechnol Biochem. 2014:1–8. Epub Jul 22. [DOI] [PubMed] [Google Scholar]
- 31.Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc. 2013;8(8):1494–512. 10.1038/nprot.2013.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parra G, Bradnam K, Korf I. CEGMA: a pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics. 2007;23(9):1061–7. [DOI] [PubMed] [Google Scholar]
- 33.Nakasugi K, Crowhurst RN, Bally J, Wood CC, Hellens RP, Waterhouse PM. De novo transcriptome sequence assembly and analysis of RNA silencing genes of Nicotiana benthamiana. PLoS ONE. 2013;8(3):e59534 10.1371/journal.pone.0059534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu H, Bandyopadhyay PK, Olivera Bm, Yandell M. Characterization of the Conus bullatus genome and its venom-duct transcriptome. BMC Genomics. 2011;12:60 10.1186/1471-2164-12-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho KKY, Leung PTY, Ip JCH, Qiu JW, Leung KMY. De novo transcriptomic profile in the gonadal tissues of the intertidal whelk Reishia clavigera. Mar Pollut Bull. 2014;85(2):499–504. 10.1016/j.marpolbul.2014.02.023 [DOI] [PubMed] [Google Scholar]
- 36.Prentis PJ, Pavasovic A. The Anadara trapezia transcriptome: A resource for molluscan physiological genomics. Mar Genomics. 2014;18:113–5. 10.1016/j.margen.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 37.Huan P, Wang H, Liu B. Transcriptomic analysis of the clam Meretrix meretrix on different larval stages. Mar Biotechnol. 2012;14(1):69–78. 10.1007/s10126-011-9389-0 [DOI] [PubMed] [Google Scholar]
- 38.Glick BR, Pasternak JJ. DNA, RNA, and protein synthesis Molecular biotechnology: principles and applications of recombinant DNA. 3rd ed Washington DC, USA: ASM Press; 2003. p. 23–46. [Google Scholar]
- 39.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. 10.1097/WOX.0b013e3182439613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matés JM, Pérez-Gómez C, Castro INd. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32(8):595–603. [DOI] [PubMed] [Google Scholar]
- 41.Conus S, Simon H-U. Cathepsins: key modulators of cell death and inflammatory responses. Biochem Pharmacol. 2008;76:1374–82. 10.1016/j.bcp.2008.07.041 [DOI] [PubMed] [Google Scholar]
- 42.Kutikhin AG, Yuzhalin AE. The biology of toll-like receptors and NOD-like receptors: the toggles of inflammation Genomics of pattern recognition receptors: applications in oncology and cardiovascular diseases. Basel: Springer; 2013. p. 1–26. [Google Scholar]
- 43.Wang L, Qiu L, Zhou Z, Song L. Research progress on the mollusc immunity in China. Dev Comp Immunol. 2013;39:2–10. 10.1016/j.dci.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 44.Rauta PR, Samanta M, Dash HR, Nayak B, Das S. Toll-like receptors (TLRs) in aquatic animals: signaling pathways, expressions and immune responses. Immunol Lett. 2014;158:14–24. [DOI] [PubMed] [Google Scholar]
- 45.Gosling E. Bivalve molluscs: biology, ecology and culture. Oxford: Fishing News Books; 2003. [Google Scholar]
- 46.Ellis RP, Parry H, Spicer JI, Hutchinson TH, Pipe RK, Widdicombe S. Immunological function in marine invertebrates: responses to environmental perturbation. Fish Shellfish Immunol. 2011;30:1209–22. 10.1016/j.fsi.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 47.Venier P, Varotto L, Rosani U, Millino C, Celegato B, Bernante F, et al. Insights into the innate immunity of the mediterranean mussel Mytilus galloprovincialis. BMC Genomics. 2001;12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hancock REW, Brown KL, Mookherjee N. Host defence peptides from invertebrates—emerging antimicrobial strategies. Immunobiology. 2006;211:315–22. [DOI] [PubMed] [Google Scholar]
- 49.Lee MS, Kim Y-J. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–80. [DOI] [PubMed] [Google Scholar]
- 50.Feng Y, Chao W. Toll-like receptors and myocardial inflammation. Int J Inflam. 2011;2011:170352 10.4061/2011/170352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X-W, Wang J-X. Pattern recognition receptors acting in innate immune system of shrimp against pathogen infections. Fish Shellfish Immunol. 2013;34:981–9. 10.1016/j.fsi.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 52.O'Neill LAJ, Golenbock D, Bowie AG. The history of toll-like receptors—redefining innate immunity. Nat Rev Immunol. 2013;13:453–60. 10.1038/nri3446 [DOI] [PubMed] [Google Scholar]
- 53.Pauletto M, Milan M, Moreira R, Novoa B, Figueras A, Babbucci M, et al. Deep transcriptome sequencing of Pecten maximus hemocytes: a genomic resource for bivalve immunology. Fish Shellfish Immunol. 2014;37:154–65. 10.1016/j.fsi.2014.01.017 [DOI] [PubMed] [Google Scholar]
- 54.Bettencourt R, Pinheiro M, Egas C, Gomes P, Afonso M, Shank T, et al. High-throughput sequencing and analysis of the gill tissue transcriptome from the deep-sea hydrothermal vent mussel Bathymodiolus azoricus. BMC Genomics. 2010;11:559 10.1186/1471-2164-11-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiang Z, Qu F, Wang F, Li J, Zhang Y, Yu Z. Characteristic and functional analysis of a ficolin-like protein from the oyster Crassostrea hongkongensis. Fish Shellfish Immunol. 2014;40:514–23. 10.1016/j.fsi.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 56.Liu L, Yang J, Qiu L, Wang L, Zhang H, Wang M, et al. A novel scavenger receptor-cysteine-rich (SRCR) domain containing scavenger receptor identified from mollusk mediated PAMP recognition and binding. Development & Comparative Immunology. 2011;35(2):227–39. [DOI] [PubMed] [Google Scholar]
- 57.Weis WI, Taylor ME, Drickamer K. The c-type lectin superfamily in the immune system. Immunol Rev. 1998;163:19–34. [DOI] [PubMed] [Google Scholar]
- 58.Fujita T, Matsushita M, Endo Y. The lectin-complement pathway—its role in innate immunity and evolution. Immunol Rev. 2004;198:185–202. [DOI] [PubMed] [Google Scholar]
- 59.Holmskov U, Thiel S, Jensenius JC. Collectins and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–78. [DOI] [PubMed] [Google Scholar]
- 60.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. [DOI] [PubMed] [Google Scholar]
- 61.Hibino T, Loza-Coll M, Messier C, Majeske AJ, Cohen AH, Terwilliger DP, et al. The immune gene repertoire encoded in the purple sea urchin genome. Dev Biol. 2006;300(1):349–65. [DOI] [PubMed] [Google Scholar]
- 62.Wu B, Xin B, Jin M, Wei T, Bai Z. Comparative and phylogenetic analyses of three TIR domain-containing adaptors in metazoans: implications for evolution of TLR signaling pathways. Dev Comp Immunol. 2011;35(7):764–73. 10.1016/j.dci.2011.02.009 [DOI] [PubMed] [Google Scholar]
- 63.Poole AZ, Weis VM. TIR-domain-containing protein repertoire of nine anthozoan species reveals coral-specific expansions and uncharacterized proteins. Dev Comp Immunol. 2014;46:480–8. 10.1016/j.dci.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 64.Toubiana M, Gerdol M, Rosani U, Pallavicini A, Venier P, Roch P. Toll-like receptors and MyD88 adaptors in Mytilus: complete cds and gene expression levels. Dev Comp Immunol. 2013;40:158–66. 10.1016/j.dci.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 65.Toubiana M, Rosani U, Giambelluca S, Cammarata M, Gerdol M, Pallavicini A, et al. Toll signal transduction pathway in bivalves: complete cds of intermediate elements and related gene transcription levels in hemocytes of immune stimulated Mytilus galloprovincialis. Dev Comp Immunol. 2014;45(2):300–12. 10.1016/j.dci.2014.03.021 [DOI] [PubMed] [Google Scholar]
- 66.Ren Q, Zhong X, Yin S-W, Hao F-Y, Hui K-M, Zhang Z, et al. The first Toll receptor from the triangle-shell pearl mussel Hyriopsis cumingii. Fish Shellfish Immunol. 2013;34:1287–93. 10.1016/j.fsi.2013.02.014 [DOI] [PubMed] [Google Scholar]
- 67.Ren Q, Chen Y-H, Ding Z-F, Huang Y, Shi Y-R. Identification and function of two myeloid differentiation factor 88 variants in triangle-shell pearl mussel (Hyriopsis cumingii). Dev Comp Immunol. 2014;42:286–93. 10.1016/j.dci.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 68.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12(8):695–708. 10.1038/ni.2065 [DOI] [PubMed] [Google Scholar]
- 69.Yang Y, Bazhin AV, Werner J, Karakhanova S. Reactive oxygen species in the immune system. Int Rev Immunol. 2013;32:249–70. 10.3109/08830185.2012.755176 [DOI] [PubMed] [Google Scholar]
- 70.Kohchi C, Inagawa H, Nishizawa T, Soma G-I. ROS and innate immunity. Anticancer Res. 2009;29:817–22. [PubMed] [Google Scholar]
- 71.Israёl A. The IKK complex, a central regulator of NF-κB activation. Cold Spring Harb Perspect Biol. 2010;2(3):a000158 10.1101/cshperspect.a000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86–100. 10.1186/1476-4598-12-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dalpke A, Heeg K, Bartz H, Baetz A. Regulation of innate immunity by suppressor of cytokine signaling (SOCS) proteins. Immunobiology. 2008;213:225–35. [DOI] [PubMed] [Google Scholar]
- 74.Ge H, Wang G, Zhang L, Wang S, Zou Z, Yan S, et al. Characterization of interleukin-1 receptor-associated kinase 1 binding protein 1 gene in small abalone Haliotis diversicolor. Gene. 2012;506:417–22. 10.1016/j.gene.2012.06.038 [DOI] [PubMed] [Google Scholar]
- 75.Conner JR, Smirnova II, Moseman AP, Poltorak A. IRAK1BP1 inhibits inflammation by promoting nuclear translocation of NF-κB p50. Proc Natl Acad Sci USA. 2010;107(25):11477–82. 10.1073/pnas.1006894107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cordeiro Q, Cappi C, Sampaio AS, Palácios SA, Pereira CAdB, Shavitt RG, et al. Association study between the -62A/T NFKBIL1 polymorphism and obsessive-compulsive disorder. Rev Bras Psiquiatr. 2009;31(2):131–5. [DOI] [PubMed] [Google Scholar]
- 77.Taylor JM, Wicks K, Vandiedonck C, Knight JC. Chromatin profiling across the human tumour necrosis factor gene locus reveals a complex, cell type-specific landscape with novel regulatory elements. Nucleic Acids Res. 2008;36(15):4845–62. 10.1093/nar/gkn444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11(6):389–402. 10.1038/nri2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang B, Zhang L, Du Y, Li L, Qu T, Meng J, et al. Alternative splicing and immune response of Crassostrea gigas tumor necrosis factor receptor-associated factor 3. Mol Biol Rep. 2014;41:6481–91. 10.1007/s11033-014-3531-9 [DOI] [PubMed] [Google Scholar]
- 80.Paul-Pont I, Evans O, Dhand NK, Rubio A, Coad P, Wittington RJ. Descriptive epidemiology of mass mortality due to Ostreid herpesvirus-1 (OsHV-1) in commercially farmed Pacific oysters (Crassostrea gigas) in the Hawkesbury River estuary, Australia. Aquaculture. 2014;422–423:146–59. [Google Scholar]
- 81.Piontkivska H, Chung JS, Ivanina AV, Sokolov EP, Techa S, Sokolova IM. Molecular characterization and mRNA expression of two key enzymes of hypoxia-sensing pathways in eastern oysters Crassostrea virginica (Gmelin): Hypoxia-inducible factor α (HIF-α) and HIF-prolyl hydroxylase (PHD). Comp Biochem Physiol Part D Genomics Proteomics. 2011;6(2):103–14. 10.1016/j.cbd.2010.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kawabe S, Yokoyama Y. Role of hypoxia-inducible factor α in response to hypoxia and heat shock in the Pacific oyster Crassostrea gigas. Mar Biotechnol. 2012;14:106–19. 10.1007/s10126-011-9394-3 [DOI] [PubMed] [Google Scholar]
- 83.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–17. 10.1038/nri2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhandari T, Nizet V. Hypoxia-inducible factor (HIF) as a pharmacological target for prevention and treatment of infectious diseases. Infect Dis Ther. 2014. Epub 24 June 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harris AJ, Thompson AR, Whyte MK, Walmsley SR. HIF-mediated innate immune responses: cell signaling and therapeutic implications. Hypoxia (Auckl). 2014;14(2):47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frede S, Stockmann C, Freitag P, Fandrey J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-κB. Biochem J. 2006;396:517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jantsch J, Wiese M, Schödel J, Castiglione K, Gläsner J, Kolbe S, et al. Toll-like receptor activation and hypoxia use distinct signaling pathways to stabilize hypoxia-inducible factor 1α (HIF1A) and result in differential HIF1A-dependent gene expression. J Leukoc Biol. 2011;90:551–62. 10.1189/jlb.1210683 [DOI] [PubMed] [Google Scholar]
- 88.Kim SY, Choi YJ, Joung SM, Lee BH, Jung Y-S, Lee JY. Hypoxic stress up-regulates the expression of Toll-like receptor 4 in macrophages via hypoxia-inducible factor. Immunology. 2009;129(4):516–24. 10.1111/j.1365-2567.2009.03203.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuhlicke J, Frick JS, Morote-Garcia JC, Rosenberger P, Eltzschig HK. Hypoxia inducible factor (HIF)-1 coordinates induction of toll-like receptors TLR2 and TLR6 during hypoxia. PLoS ONE. 2007;2(12):e1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi SH, Lee BY, Lee SJ, Cho MY, Lee SJ, Kim JW, et al. Effects of RNA interference-mediated knock-down of hypoxia-inducible factor-α on respiratory burst activity of the Pacific oyster Crassostrea gigas hemocytes. Fish Shellfish Immunol. 2013;35:476–9. 10.1016/j.fsi.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 91.Underhill DM, Goodridge HS. Information processing during phagocytosis. Nat Rev Immunol. 2012;12(7):492–502. 10.1038/nri3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Malagoli D, Casarini L, Sacchi S, Ottaviani E. Stress and immune response in the mussel Mytilus galloprovincialis. Fish Shellfish Immunol. 2007;23(1):171–7. [DOI] [PubMed] [Google Scholar]
- 93.Flannagan RS, Jaumouillé V, Grinstein S. The cell biology of phagocytosis. Annu Rev Pathol. 2012;7:61–98. 10.1146/annurev-pathol-011811-132445 [DOI] [PubMed] [Google Scholar]
- 94.Conte A, Ottaviani E. Nitric oxide synthase activity in molluscan hemocytes. FEBS Lett. 1995;365(2–3):120–4. [DOI] [PubMed] [Google Scholar]
- 95.Gourdon I, Guérin M-C, Torreilles J, Roch P. Nitric oxide generation by hemocytes of the mussel Mytilus galloprovincialis. Nitric Oxide. 2001;5(1):1–6. [DOI] [PubMed] [Google Scholar]
- 96.Palumbo A. Nitric oxide in marine invertebrates: a comparative perspective. Comp Biochem Physiol, Part A Mol Integr Physiol. 2005;142(2):241–8. [DOI] [PubMed] [Google Scholar]
- 97.Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. [DOI] [PubMed] [Google Scholar]
- 98.Meng X, Tian X, Liu M, Nie G, Jiang K, Wang B. The transcriptomic response to copper exposure by the gill tissue of Japanese scallops (Mizuhopecten yessoensis) using deep-sequencing technology. Fish Shellfish Immunol. 2014;38:287–93. 10.1016/j.fsi.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 99.Zhang L, Li L, Zhang G. The first identification of molluscan ECSIT in the Pacific oyster, Crassostrea gigas, and its expression against bacterial challenge. Aquac Res. 2012;43:1071–80. [Google Scholar]
- 100.Adema CM, Deutekom-Mulder ECv, Knaap WPWvd, Sminia T. NADPH-oxidase activity: the probable source of reactive oxygen intermediate generation in hemocytes of the gastropod Lymnaea stagnalis. J Leukoc Biol. 1993;54(379–383). [DOI] [PubMed] [Google Scholar]
- 101.Zhang T, Qiu L, Sun Z, Wang L, Zhou Z, Liu R, et al. The specifically enhanced cellular immune responses in Pacific oyster (Crassostrea gigas) against secondary challenge with Vibrio splendidus. Dev Comp Immunol. 2014;45(1):141–50. 10.1016/j.dci.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 102.Carocho M, Ferreira ICFR. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. 2013;51:15–25. 10.1016/j.fct.2012.09.021 [DOI] [PubMed] [Google Scholar]
- 103.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinated regulated defence against oxidative stress. Free Radic Res. 1999;31(4):273–300. [DOI] [PubMed] [Google Scholar]
- 104.Wu G, Fang Y-Z, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134(3):489–92. [DOI] [PubMed] [Google Scholar]
- 105.Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med. 2014;66:75–87. 10.1016/j.freeradbiomed.2013.07.036 [DOI] [PubMed] [Google Scholar]
- 106.Leite RB, Milan M, Coppe A, Bortoluzzi S, Anjos Ad, Reinhardt R, et al. mRNA-Seq and microarray development for the Grooved carpet shell clam, Ruditapes decussatus: a functional approach to unravel host -parasite interactions. BMC Genomics. 2013;14:741 10.1186/1471-2164-14-741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kuchel RP, Nair S, Raftos DA. Changes in the transcriptional expression of oxidative stress response genes in Akoya pearl oysters (Pinctada fucata) exposed to air and mechanical agitation. Aquaculture. 2012;362–363:33–8. [Google Scholar]
- 108.Green TJ, Dixon TJ, Devic E, Adlard RD, Barnes AC. Differential expression of genes encoding anti-oxidant enzymes in Sydney rock oysters, Saccostrea glomerata (Gould) selected for disease resistance. Fish Shellfish Immunol. 2009;26:799–810. 10.1016/j.fsi.2009.03.003 [DOI] [PubMed] [Google Scholar]
- 109.Niyogi S, Biswas S, Sarker S, Datta AG. Antioxidant enzymes in brackishwater oyster, Saccostrea cucullata as potential biomarkers of polyaromatic hydrocarbon pollution in Hooghly Estuary (India): seasonality and its consequences. Sci Total Environ. 2001;281:237–46. [DOI] [PubMed] [Google Scholar]
- 110.Jo PG, Choi YK, Choi CY. Cloning and mRNA expression of antioxidant enzymes in the Pacific oyster, Crassostrea gigas in response to cadmium exposure. Comp Biochem Physiol C, Comp Pharmacol Toxicol. 2008;147(4):460–9. [DOI] [PubMed] [Google Scholar]
- 111.Bebianno MJ, Company R, Serafim A, Camus L, Cosson RP, Fiala-Médoni A. Antioxidant systems and lipid peroxidation in Bathymodiolus azoricus from mid-atlantic ridge hydrothermal vent fields. Aquat Toxicol. 2005;75:354–73. [DOI] [PubMed] [Google Scholar]
- 112.Edge KJ, Johnston EL, Roach AC, Ringwood AH. Indicators of environmental stress: cellular biomarkers and reproductive responses in the Sydney rock oyster (Saccostrea glomerata). Ecotoxicology. 2012;21(5):1415–25. 10.1007/s10646-012-0895-2 [DOI] [PubMed] [Google Scholar]
- 113.Trevisan R, Arl M, Sacchet CL, Engel CS, Danielli NM, Mello DF, et al. Antioxidant deficit in gills of Pacific oyster (Crassostrea gigas) exposed to chlorodinitrobenzene increases menadione toxicity. Aquat Toxicol. 2012;108:85–93. 10.1016/j.aquatox.2011.09.023 [DOI] [PubMed] [Google Scholar]
- 114.Jenny MJ, Ringwood AH, Lacy ER, Lewitus AJ, Kempton JW, Gross PS, et al. Potential indicators of stress response identified by expressed sequence tag analysis of hemocytes and embryos from the American oyster, Crassostrea virginica. Mar Biotechnol. 2002;4:81–93. [DOI] [PubMed] [Google Scholar]
- 115.Venier P, Pittà CD, Bernante F, Varotto L, Nardi BD, Bovo G, et al. MytiBase: a knowledgebase of mussel (M. galloprovincialis) transcribed sequences. BMC Genomics. 2009;10:72 10.1186/1471-2164-10-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, et al. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta. 2012;1824(1):68–88. 10.1016/j.bbapap.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guha S, Padh H. Cathepsins: fundamental effectors of endolysosomal proteolysis. Indian J Biochem Biophys. 2008;45:75–90. [PubMed] [Google Scholar]
- 118.Hu X, Hu X, Hu B, Wen C, Xie Y, Wu D, et al. Molecular cloning and characterization of cathepsin L from freshwater mussel, Cristaria plicata. Fish Shellfish Immunol. 2014;40(2):446–54. 10.1016/j.fsi.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 119.Niu D, Jin K, Wang L, Sun F, Li J. Identification of cathepsin B in the razor clam Sinonovacula constricta and its role in innate immune responses. Dev Comp Immunol. 2013;41(1):94–9. 10.1016/j.dci.2013.04.014 [DOI] [PubMed] [Google Scholar]
- 120.Niu D, Xie S, Bai Z, Wang L, Jin K, Li J. Identification, expression, and responses to bacterial challenge of the cathepsin C gene from the razor clam Sinonovacula constricta. Development and Comparative Immunology. 2014;46(2):241–5. [DOI] [PubMed] [Google Scholar]
- 121.Bachali S, Jager M, Hassanin A, Schoentgen F, Jollès P, Fiala-Medioni A, et al. Phylogenetic analysis of invertebrate lysozymes and the evolution of lysozyme function. J Mol Evol. 2002;54:652–64. [DOI] [PubMed] [Google Scholar]
- 122.Fang Y, Yang H, Liu B, Zhang L. Transcriptional response of lysozyme, metallothionein, and superoxide dismutase to combined exposure to heavy metals and bacteria in Mactra veneriformis. Comp Biochem Physiol C, Comp Pharmacol Toxicol. 2013;157(1):54–62. [DOI] [PubMed] [Google Scholar]
- 123.Wu D, Hu B, Wen C, Lin G, Tao Z, Hu X, et al. Gene identification and recombinant protein of a lysozyme from freshwater mussel Cristaria plicata. Fish Shellfish Immunol. 2013;34(5):1033–41. 10.1016/j.fsi.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 124.Zhao J, Song L, Li C, Zou H, Ni D, Wang W, et al. Molecular cloning of an invertebrate goose-type lysozyme gene from Chlamys farreri, and lytic activity of the recombinant protein. Mol Immunol. 2007;44:1198–208. [DOI] [PubMed] [Google Scholar]
- 125.Dimarcq J-L, Bulet P, Hetru C, Hoffmann J. Cysteine-rich antimicrobial peptides in invertebrates. Biopolymers. 1998;47(6):465–77. [DOI] [PubMed] [Google Scholar]
- 126.Rosa RD, Santini A, Fievet J, Bulet P, Destoumieux-Garzón D, Bachère E. Big defensins, a diverse family of antimicrobial peptides that follows different patterns of expression in hemocytes of the oyster Crassostrea gigas. PLoS ONE. 2011;6(9):e25594 10.1371/journal.pone.0025594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao J, Song L, Li C, Ni D, Wu L, Zhu L, et al. Molecular cloning, expression of a big defensin gene from bay scallop Argopecten irradians and the antimicrobial activity of its recombinant protein. Mol Immunol. 2007;44:360–8. [DOI] [PubMed] [Google Scholar]
- 128.Zhao J, Li C, Chen A, Li L, Su X, Li T. Molecular characterization of a novel big defensin from clam Venerupis philippinarum. PLoS ONE. 2010;5(10):e13480 10.1371/journal.pone.0013480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Balakrishnan A, Marathe SA, Joglekar M, Chakravortty D. Bactericidal/permeability increasing protein: a multifaceted protein with functions beyond LPS neutralization. Innate Immun. 2012;19(4):339–47. 10.1177/1753425912465098 [DOI] [PubMed] [Google Scholar]
- 130.Gonzalez M, Gueguen Y, Destoumieux-Garzón D, Romestand B, Fievet J, Pugnière M, et al. Evidence of a bactericidal permeability increasing protein in an invertebrate, the Crassostrea gias Cg-BPI. Proc Natl Acad Sci USA. 2007;104(45):17759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee Y, Revathy SK, Lee S, Whang I, Oh C, Kang D-H, et al. First molluscan antimicrobial peptide hydramacin in Manila clam: molecular characterization and expression analysis. Journal of Coastal Life Medicine. 2014;2(6):447–52. [Google Scholar]
- 132.Sokolova IM. Apoptosis in molluscan immune defense. Invertebrate Surviv J. 2009;6:49–58. [Google Scholar]
- 133.Cho S-G, Choi E-J. Apoptotic signaling pathways: caspases and stress-activated protein kinases. J Biochem Mol Biol. 2002;35(1):24–7. [DOI] [PubMed] [Google Scholar]
- 134.Hughes FM, Foster B, Grewal S, Sokolova IM. Apoptosis as a host defense mechansims in Crassostrea virginica and its modulation by Perkinsus marinus. Fish Shellfish Immunol. 2010;29:247–57. 10.1016/j.fsi.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 135.Ashe PC, Berry MD. Apoptotic signaling cascades. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:199–214. [DOI] [PubMed] [Google Scholar]
- 136.Kiss T. Apoptosis and its functional significance in molluscs. Apoptosis. 2010;15:313–21. 10.1007/s10495-009-0446-3 [DOI] [PubMed] [Google Scholar]
- 137.Cheriyath V, Leaman DW, Borden EC. Emerging roles of FAM14 family members (G1P3/ISG 6–16 and ISG12/IFI27) in innate immunity and cancer. J Interferon Cytokine Res. 2011;31(1):173–81. 10.1089/jir.2010.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22(13):1658–9. [DOI] [PubMed] [Google Scholar]
- 140.Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–6. [DOI] [PubMed] [Google Scholar]
- 141.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Left graphs for both, a) and b) show the respective full-scale graphs, with the respective right graphs emphasising the fine scale features of the same graphs by adding a graph break.
(DOCX)
GO-terms were determined with Blast2GO, using default parameters except for seq filter that was set to 50. Terms listed are based on a) biological process (Level 3), b) molecular function (Level 3) and c) cellular component (Level 4).
(DOCX)
GO-terms were determined with Blast2GO, using default parameters except for seq filter that was set to 50. Terms listed are based on a) biological process (Level 3), b) molecular function (Level 3) and c) cellular component (Level 4).
(DOCX)
Alignment of S. glomerata ECSIT transcript (c383034.graph_c0_seq1|m.66044) with ECSIT from C. gigas [GenBank:HQ225834] and M. galloprovincialis [GenBank:AHI17287].
(DOCX)
Alignment of S. glomerata big defensin transcripts (c211420.graph_c0_seq1|m.6735, c349903.graph_c0_seq1|m.22175, c349903.graph_c0_seq2|m.22176, c359623.graph_c0_seq5|m.28084 and c354390.graph_c0_seq1|m.24530) with big defensins from C. gigas [GenBank:AEE92778, GenBank:AEE92768 and GenBank:AEE92775], A. irradians [GenBank:DQ334340] and V. philippinarum [GenBank:HM562672].
(DOCX)
Alignment of S. glomerata hydramacin transcript (c328639.graph_c0_seq1|m.16116) with V. philippinarum hydramacin [GenBank:AGM14601].
(DOCX)
(DOCX)
CLC Genomics Workbench (version 7.5) mapping statistics of individual tissue reads for determination of transcript distribution across the six tissues.
(DOCX)
Data Availability Statement
Raw sequencing reads were deposited in the NCBI sequence read archive under the BioProject number PRJNA272756.