Abstract
Caldicellulosiruptor bescii encodes at least six unique multimodular glycoside hydrolases crucial for plant cell wall polysaccharides degradation, with each having two catalytic domains separated by two to three carbohydrate binding modules. Among the six enzymes, three have one N- or C-terminal GH5 domain with identical amino acid sequences. Despite a few reports on some of these multimodular enzymes, little is known about how the conserved GH5 domains behave, which are believed to be important due to the gene duplication. We thus cloned a representative GH5 domain from the C-terminus of a multimodular protein, i.e. the bifunctional cellulase/mannanase CbCel9B/Man5A which has been reported, and expressed it in Escherichia coli. Without any appending CBMs, the recombinant CbMan5A was still able to hydrolyze a variety of mannan substrates with different backbone linkages or side-chain decorations. While CbMan5A displayed the same pH optimum as CbCel9B/Man5A, it had an increased optimal temperature (90°C) and moreover, was activated by heating at 70°C and 80°C, a property not ever reported for the full-length protein. The turnover numbers of CbMan5A on mannan substrates were, however, lower than those of CbCel9B/Man5A. These data suggested that evolution of CbMan5A and the other domains into a single polypeptide is not a simple assembly; rather, the behavior of one module may be affected by the other ones in the full-length enzyme. The differential scanning calorimetry analysis further indicated that heating CbMan5A was not a simple transition state process. To the best knowledge of the authors, CbMan5A is the first glycoside hydrolase with thermal activation property identified from a multimodular bifunctional enzyme.
Introduction
The thermophilic bacterium Caldicellulosiruptor bescii (previously classified as Anaerocellum thermophilum) optimally growing at 75°C is distinguished by its excellent capacity to degrade crystalline cellulose [1]. It is also able to acquire energy from hemicellulose including xylan and mannan and even untreated switchgrass and poplar [1]. These indicate that the microbe has an array of robust, thermophilic glycoside hydrolases that can efficiently deconstruct plant cell wall polysaccharides (PCWP) into simple sugars. Many endeavors have been carried out in the characterization of the cellulase and xylan-degrading enzymes of C. bescii [2,3,4,5].
One striking finding for the PCWP-degrading glycoside hydrolases of C. bescii is the existence of a large number of multimodular enzymes. The genome of C. bescii encodes at least six unique multimodular glycoside hydrolases bearing two catalytic domains separated by two to three carbohydrate binding modules (CBMs) [2]. Notably, these multimodular enzymes are encoded by genes within a single but crucial PCWP-utilization gene cluster [6,7]. Such a special domain organization in a single large polypeptide appears to represent a novel naturally occurring paradigm of glycoside hydrolases for hydrolysis of PCWP. The high efficiency of CelA (or CbCel9A/Cel48A [8]), a cellulase with one N-terminal GH9 endoglucanase and a C-terminal GH48 exoglucanase, has been ascribed to this special domain arrangement [4]. Interestingly, among the six multimodular glycoside hydrolases, three have one N- or C-terminal GH5 domain (Fig 1A) with the same amino acid sequence (S1 Fig). Despite a few reports on some of these multimodular enzymes, little is known about the biochemical behavior of the conserved GH5 domains, which are believed to be important due to the gene duplication.
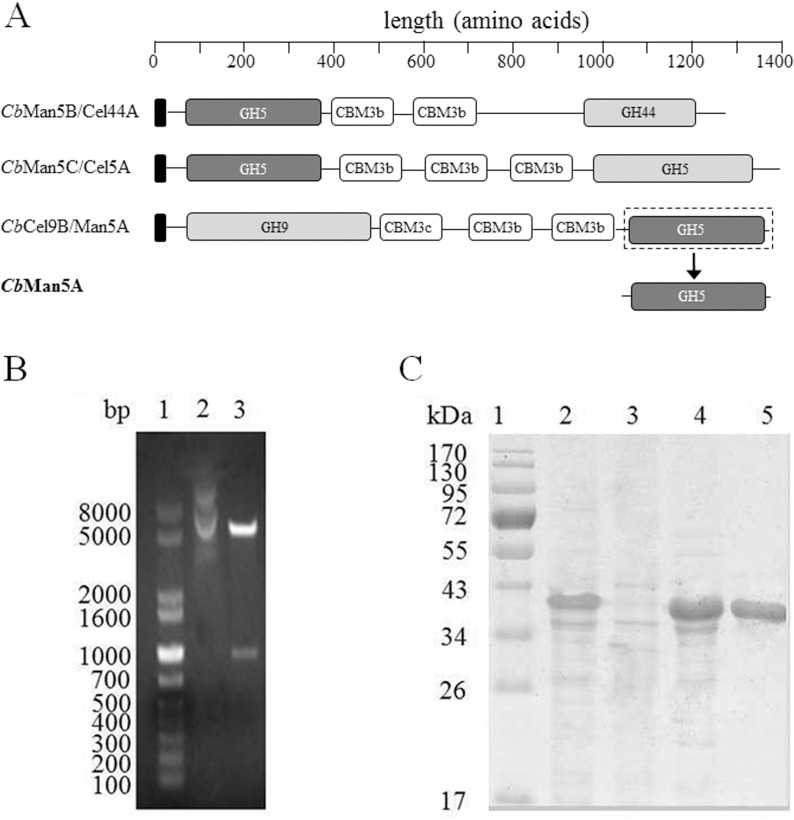
Fig 1. Cloning, expression, and purification of CbMan5A.
A: Domain structure showing the three unique multimodular proteins with a conserved GH5 domain. The conserved GH5 domains are filled with dark grey, the signal peptides are filled with black, and the other domains are filled with white (CBMs) or light grey (catalytic domains). CbMan5B/Cel44A, CbMan5C/Cel5A, and CbCel9B/Man5A are also referred as Athe_1859, Athe_1866, and Athe_1865, respectively. B: Restriction digestion of pET-cbMan5A. Lane 1: DNA molecular mass marker; 2: undigested pET-cbMan5A; 3: pET-cbMan5A digested with EcoRI and NotI. C: SDS-PAGE analysis of purified CbMan5A. Lane 1: protein molecular mass marker; 2: whole cell lysate of pET-cbMan5A/BL21(DE3); 3: cell debris; 4: supernatant; 5: purified CbMan5A.
We thus cloned a representative GH5 domain from the C-terminus of a multimodular protein, i.e. the bifunctional cellulase/mannanase CbCel9B/Man5A which has been reported [2], and expressed it in Escherichia coli. Biochemical analyses were carried out on the purified protein.
Results
Gene Cloning, Expression, and Purification of CbMan5A
Three multimodular enzymes were identified to bear one identical GH5 domain existing at either the N- (CbMan5B/Cel44A, or Athe_1859, GenBank accession number: ACM60947; CbMan5C/Cel5A, or Athe_1866, ACM60954) or C-terminus (CbCel9B/Man5A, or Athe_1865, ACM60953) (Fig 1A). The gene coding for the C-terminal GH5 domain of CbCel9B/Man5A was ligated into pET-28a(+) to obtain pET-CbMan5A. The insertion of cbMan5A was verified by restriction digestion of the recombinant plasmid using EcoRI and NotI (Fig 1B) and DNA sequencing (data not shown). The recombinant CbMan5A expressed in E. coli was purified by immobilized metal affinity chromatography (IMAC) as analyzed by SDS-PAGE (Fig 1C).
Substrate Specificity of CbMan5A
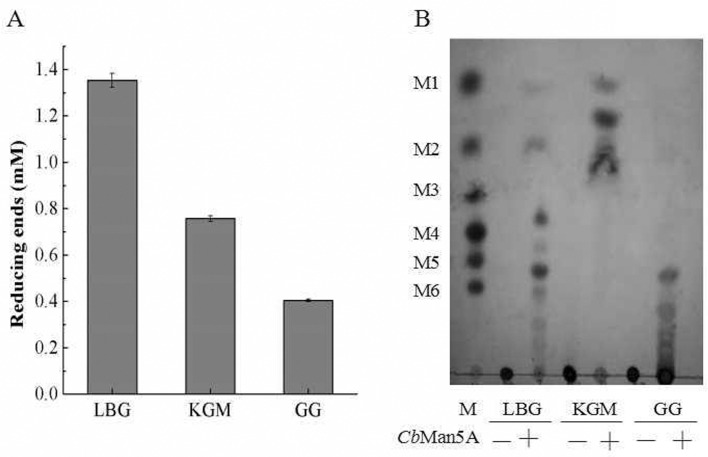
CbMan5A was most active in degrading the galactomannan locust bean gum (LBG), followed by konjac glucomannan (KGM, a polysaccharide with a mixed glucose-mannose linkage) and another galactomannan guar gum (GG) (Fig 2A), demonstrating that it could hydrolyze a variety of mannan substrates. However, no reducing sugars were released from cellulose and xylan in the short time (15 min) assay (Fig 2A) unless the incubation was prolonged to 12 h (data not shown). From LBG, CbMan5A released mannose, mannobiose, and mannooligosaccharides with higher degrees of polymerization or side chain modifications as end products (Fig 2B). For GG, the major products were a mannooligosaccharide appearing on the TLC plate between mannopentaose and mannohexaose and those with higher degrees of polymerization (Fig 2B). In contrast, CbMan5A released mannose and oligosaccharides with low degrees of polymerization from KGM (Fig 2B).
Fig 2. Hydrolysis of mannan substrates.
A: Substrate specificity of CbMan5A on mannans. Although mannan, cellulose, and xylan substrates were included in the screening, only the mannan substrates with significant signal of reducing sugars in the 15-min assay were shown here. B: Hydrolysis products of CbMan5A on various mannans as analyzed by thin-layer chromatography. M1: mannose; M2-M6: mannobiose to mannohexaose. LBG: locust bean gum; KGM: konjac glucomannan; GG: guar gum.
Effects of pH and Temperature on the Activity and Stability of CbMan5A
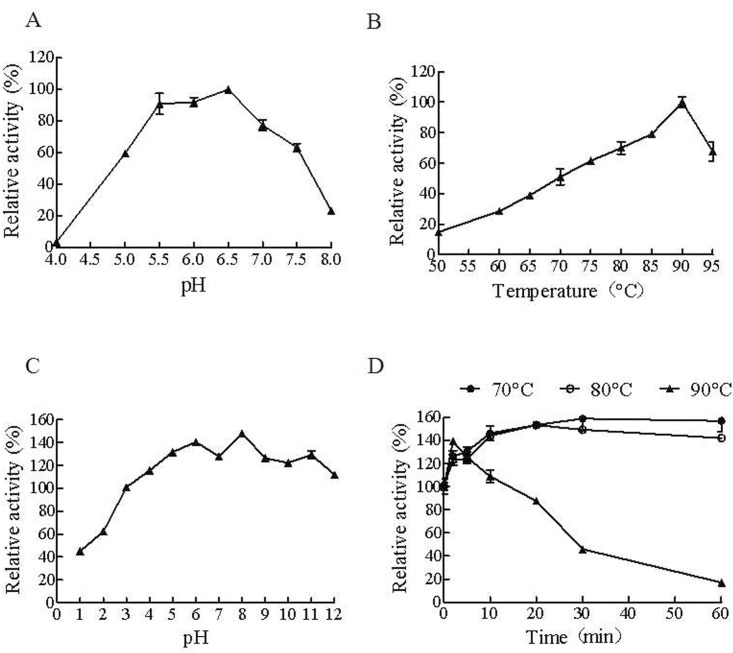
CbMan5A had an optimal pH of 6.5 (Fig 3A) identical to that of CbCel9B/Man5A. The optimal temperature of CbMan5A was 90°C, higher than that of CbCel9B/Man5A (85°C). At the temperatures from 75–95°C, the enzyme had relative activities of above 60% (Fig 3B). While pretreatment at pH1.0 and 2.0 decreased the activity of CbMan5A substantially, incubation at pH3.0 for 1 h appeared not to affect its activity, and pH4.0 to pH12.0 tended to increase the activity significantly (Fig 3C). At 90°C, CbMan5A quickly lost its activity to 17% after 60 min (Fig 3D). However, no loss of activity was observed for incubation at 70°C and 80°C, indicating that CbMan5A has a sound thermostability. Interestingly, significant increases of residual activity (up to 159% and 153%) were observed for CbMan5A pretreated at both 70°C and 80°C after 1 h (Fig 3D).
Fig 3. Effects of pH and temperature on the activity and stability of CbMan5A.
A: pH optimum of CbMan5A. B: Temperature optimum of CbMan5A. C: Stability of CbMan5A at various pHs. The enzyme was pretreated at a given pH for 1 h before activity measurement. D: Thermotolerance of CbMan5A. The activities of the CbMan5A under different conditions or treatments were represented as relative activity (in percentage) by dividing the activities against the reference activity, which was the maximal activity under a certain situation (for A, pH6.5: 1,713 U/mg; for B, 90°C: 1,566 U/mg) or the activity before treatment (for C, 1,482 U/mg; for D, 976 U/mg, 70°C; 1,082 U/mg, 80°C; 1,005 U/mg, 90°C).
Kinetic Analysis of CbMan5A
For LBG, KGM, and GG, the specific activities of CbMan5A were 1,691, 690, and 505 U/mg, respectively, while those for Avicel, filter paper, CMC-Na, and beechwod xylan were much lower (Table 1). CbMan5A had a kcat of 1,043 and 514 s-1, Km of 1.7 and 0.9 mg ml-1, and catalytic efficiency of 602.9 and 571.1 s-1 ml mg-1 on LBG and KGM, respectively. The kinetic parameters of CbMan5A on GG were not obtained since the reaction could not be saturated even at very high concentration of the substrate. Compared with other mannnases, CbMan5A is a robust mannanase degrading galactomannan (Table 1).
Table 1. Biochemical properties of CbMan5A in comparison with other mannanases.
| Enzyme | Organism | Optimal pH | Optimal temperature(°C) | Specific activity (unit, substrate) | kcat (s-1) | Km (mg ml-1) | Reference |
|---|---|---|---|---|---|---|---|
| CbMan5A | C. bescii | 6.5 | 90 | 1,691 (U/mg, LBG) | 1043 | 1.7 | This.work |
| 690 (U/mg, KGM) | 514 | 0.9 | This.work | ||||
| 505 (U/mg, GG) | – | – | This.work | ||||
| 1.4 (mU/mg, Avicel) | – | – | This.work | ||||
| 0.5 (mU/mg, Filter paper) | – | – | This.work | ||||
| 2.2 (mU/mg, CMC-Na) | – | – | This.work | ||||
| 5.9 (mU/mg, Beechwood xylan) | – | – | This.work | ||||
| Man5A1 | Talaromyces leycettanus | 4.5 | 90 | 2,160 (U/mg, LBG) | – | 1.9 | [19] |
| Man5A2 | Talaromyces leycettanus | 4.0 | 85–90 | 1,800 (U/mg, LBG) | – | 2.2 | [19] |
| Man5XZ3 | Aspergillus nidulans | 5.0 | 80 | 186 (U/mg, LBG) | – | 0.9 | [20] |
| ManBK | Aspergillus niger | 4.5 | 80 | – | 292 | 2.2 | [21] |
| MAN-P | Aspergillus niger | 4.5 | 80 | 3,049 (U/ml, LBG) | – | – | [22] |
| rMan5P1 | Neosartorya fischeri | 4.0 | 80 | 1,703 (U/mg, LBG) | – | 0.8 | [23] |
| Man5XZ7 | Thielavia arenaria | 5.0 | 75 | – | – | 5.3 | [24] |
| Man5A | Humicola insolens | 5.5 | 70 | 1,122 (U/mg, LBG) | – | 1.5 | [25] |
| AaManA | Alicyclobacillus acidocaldarius | 5.5 | 65 | – | 340 | 2.4 | [26] |
| ManA | Bacillus subtilis | 7.0 | 60 | 415.2 (U/ml, LBG) | – | – | [27] |
| MANN | Aspergillus sulphureus | 2.4 | 50 | 366 (U/mg, LBG) | – | 0.9 | [28] |
| ManA | Xanthomonas campestris | 7.0 | 37 | 282.55 (U/mg, LBG) | – | 0.88 | [29] |
Effect of Chemicals and Metal Ions on the Activity of CbMan5A
The presence of Co2+, K+, Na+, Ni2+, Mn2+, and Zn2+, and surprisingly β-mercaptoethanol, enhanced its activity by over 10% (S1 Table). Only Ag+ could strongly inhibit the enzyme by repressing the activity of CbMan5A to 9.0%.
Thermal Unfolding of CbMan5A Fitted Well to a Non-2 State Model
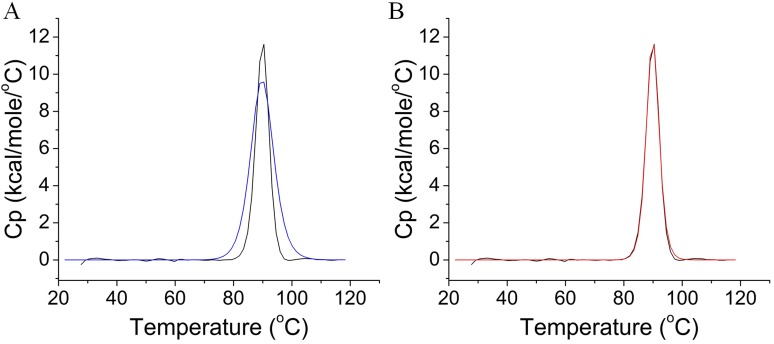
Fitting of the raw DSC data according to a simple 2-state model generated poor overlay of the two curves, as illustrated in Fig 4A. However, the DSC data fit much better to a non-2 state model with one transition stage (Fig 4B). The Tm (transition midpoint) was 89.9°C, with a ΔH of 6.903×104 cal/mol and ΔHv of 1.812×105 cal/mol, respectively.
Fig 4. Heating of CbMan5A as analyzed by DSC.
A and B: Fitting of the DSC data to either a simple 2-state (A) or a non-2-state model (B). The black solid lines in A and B are the raw DSC data, while the blue and red solid lines in A and B are the fitted curves with the 2-state or non-2-state model, respectively.
Discussion
The multiple GH5 endo-mannanase orthologs are thought to cooperate with a GH2 β-mannosidase in degrading mannans into mannose for metabolism by C. bescii [9]. With the optimal temperature of 90°C, CbMan5A is one of the most thermophilic mannanases that have ever been characterized (Table 1). CbMan5A is a robust endo-mannanase hydrolyzing a variety of mannan substrates and resistant to many metals and chemicals, indicating that it may serve as a good candidate enzyme with application potential in industry. Noticeably, heating CbMan5A at 70°C and 80°C for 1 h improved, rather than diminished, its enzymatic activity. In line with this, the DSC analysis indicated that heating CbMan5A is not a simple unfolding process with only two states. Thermal activation of enzymes is only discovered for a few enzymes such as an apple polyphenol oxidase [10], a glutamate dehydrogenase from Pyrococcus furiosus [11], and an alkaline lipase from Pseudomonas sp. [12]. The rare examples also include glycoside hydrolases such as an endoglucanase from Thermomonospora curvata [13] and a β-galactosidase from Thermotoga naphthophila [14]. To the best knowledge of the authors, CbMan5A is the first glycoside hydrolase with thermal activation property derived from a multimodular bifunctional enzyme.
Without any CBM3 modules, CbMan5A is still able to degrade a variety of mannans as CbCel9B/Man5A does, indicating that the CBM3s are not the primary determinant of the substrate specificity of CbMan5A for these model substrates. However, the biochemical properties of CbMan5A apparently differed from those of the full-length enzyme CbCel9B/Man5A. The Km of CbMan5A on LBG (1.7 mg/ml) was higher, while that on KGM (0.9 mg/ml) was lower, than those of CbCel9B/Man5A (0.62 and 1.84 mg/ml, respectively). The kcats of CbMan5A on LBG and KGM were 1,043 and 514 s-1, respectively, both lower than those of CbCel9B/Man5A (1,420 s-1 and 1,068 s-1, respectively). Notably, the thermal activation property is not observed for the full-length enzyme CbCel9B/Man5A (S2 Fig and [2]). Therefore, the data suggested that evolution of CbMan5A and the other domains (one GH9 and three CBM3s) into a single polypeptide is not a simple assembly since the missing of the N-terminal CBMs and GH9 catalytic module has complex effects on the enzyme characters of CbMan5A. Our finding further supports the hypothesis that the catalytic and accessory modules in the multi-domain glycoside hydrolases may not be simply tandem-linked but rather functionally coupled [2,15].
The recurring of GH5 mannanases is not the only case of gene duplication in multimodular glycoside hydrolases in C. bescii. The rest three among the six multimodular enzymes (CelA, GenBank accession number: ACM60955; CbXyn10C/Cel48B, ACM60945; and Athe_1860, ACM60948) all have an identical C-terminal GH48 cellobiohydrolase [16]. The recruiting of CbMan5A into the multimodular CbCel9B/Man5A apparently enhanced its activity in terms of the turnover numbers (kcat); however, the thermal activation character, a property that would be more favorable for adaptation to high temperature environment, is not retained. Since there is no evidence of significant cleavage of CbMan5A from the full-length enzyme CbCel9B/Man5A [4], the heat activation property of CbMan5A is an interesting phenomenon but may be non-biologically relevant. Note, in contrast, there is significant cleavage of GH48 modules from the multimodular cellulases [4]. Therefore, it may be interesting to explore how differently the GH48 module would behave either as a single domain protein or in the context of a multimodular protein. It may also be inferred that, the thermodynamics effect in addition to the enzymology of associated domains has to be considered when one designs artificial multimodular bifuntional glycoside hydrolases.
In conclusion, we identified the C-terminal GH5 mannanase of CbCel9B/Man5A as the first glycoside hydrolase with heat activation property from a multimodular bifunctional enzyme. CbMan5A is a highly thermophilic, robust endo-mannanase hydrolyzing a variety of mannans, potentiating it as a candidate for industry application. CbMan5A displays similar but still differing characteristics to those of the full-length CbCel9B/Man5A, suggesting that the multimodular and bifunctional enzymes are more than a simple assembly of several independent domains; rather, the behavior of one module may be affected by the other ones in the full-length enzyme.
Materials and Methods
Plasmid Construction
The gene encoding the C-terminal GH5 (aa 1048 to 1360, designated CbMan5A) of CbCel9B/Man5A was amplified from the genomic DNA of C. bescii DSM 6725 (DSMZ, Braunschweig, Germany) with the primer pairs of Pman5A_F (5’-GGCGAATTCCAGGAGCCGAGTGGAGCGACACCAAC-3’, underlined for EcoRI) and Pman5A_R (5’-ATGGGCGGCCGCTTATTCAGCACcaatcgcattag-3’, underlined for NotI) using the PrimeSTAR HS DNA Polymerase (TaKaRa, Dalian, China). The PCR products were digested with EcoRI and NotI, and then ligated into the pET-28a(+) plasmid (Merck, Darmstadt, Germany) to obtain pET-cbMan5A.
Gene Expression and Protein Purification
The pET-cbMan5A plasmid was transformed into BL21(DE3) (Transgen, Beijing, China) chemically competent cells. Five to six colonies were inoculated into 200 ml of LB medium and the culture was shaken at 37°C. When the OD600 of the culture reached 0.6, IPTG was added to the culture at a final concentration of 0.2 mM, and the culture was continued for 16 h. The E. coli cells were harvested by centrifugation and re-suspended in a binding buffer containing 20 mM Tris-HCl, 500 mM NaCl, pH7.6. The cytosol containing the recombinant CbMan5A was released by sonication. CbMan5A was purified by using immobilized-metal affinity chromatography (IMAC) with an column-equilibration buffer of NTA0 containing 50 mM Tris-HCl, 500 mM NaCl, pH7.5 and elution buffers with the NTA0 containing a series of step gradient concentrations of imidazole (40 to 400 mM).
Determination of the Substrate Specificity of CbMan5A
All polysaccharides, unless otherwise mentioned, were purchased from Sigma-Aldrich (St. Louis, MO). Ten μM of CbMan5A was incubated in a McIlvaine buffer containing 200 mM sodium phosphate, 100 mM sodium citrate, pH 6.0 with a series of plant cell wall polysaccharides including 5 mg/ml of locust bean gum (LBG), guar gum (GG), and konjac glucomannan (KGM, from Megazyme, Wicklow, Ireland), and 10 mg/ml of beechwood xylan, sodium carboxymethyl cellulose (CMC-Na), Avicel, and filter paper. The reaction was carried out at 70°C for 15 min. For the last four polysaccharides, a prolonged reaction was also carried out at 70°C for 12 h. The released reducing sugars were determined using the 3,5-dinitrosalicylic acid (DNS)method [17].
Thin-Layer Chromatorgraphy (TLC)
The TLC method described by Moon et al. [18] was employed to analyze the hydrolysis products of CbMan5A on different polysaccharides. Two nM of CbMan5A were incubated with 2.5 mg/ml of LBG, KGM, and GG in 5 mM McIlvaine buffer (pH 6.5) in a total volume of 500 μl at 80°C for 12 h. Appropriate amounts of the hydrolysates were applied onto a silica gel (Silica gel 60 F254, Merck, Darmstadt, Germany) and air dried. The plate was developed using a solution containing n-butanol-acetic acid-H2O with a volumetric ratio of 10:5:1. The plate was air dried, sprayed with a solution containing H2SO4-ethanol with a volumetric ration of 1:19, and heated at 110°C for 10 min until the products could be clearly visualized.
Effect of pH and Temperature on the Activity and Stability of CbMan5A
For optimal pH determination, 0.2 μM CbMan5A was incubated with 5 mg/ml of LBG in the McIlvaine buffers with different pHs (pH4.0–8.0) at 70°C for 10 min. For optimal temperature, CbMan5A was incubated with 5 mg/ml of LBG in the McIlvaine buffers at pH6.5 at temperatures ranging from 50°C to 90°C with a gradient of 5°C. The released reducing sugars were determined using the DNS method. To determine the pH-stability, 0.2 μM CbMan5A was first pretreated in the buffers with different pHs (pH1.0–2.0: 100 mM glycine-HCl; pH 3.0–8.0: 100mM McIlvaine buffer; pH 9.0–12.0: 100 mM glycine-NaOH) at 37°C for 1 h. To determine the thermostability, CbMan5A was pre-incubated at 70°C, 80°C, and 90°C for different time periods (2 min, 5 min, 10 min, 20 min, 30 min, and 1 h). Thereafter, for both pH-stability and thermostability experiments, the residual activity was determined at its optimal reaction conditions (pH 6.5 and 90°C). The released reducing sugars were measured using the DNS method. The experiments were repeated for three times.
Determination of the Enzyme Kinetics
Appropriate amounts of CbMan5A were incubated in the McIlvaine buffer with 0.5 to 5 mg/ml of LBG or KGM at its optimal pH and temperature for 5 min when the released reducing sugars versus time were linear. The Km and Vmax were calculated according to the Michaelis-Menten equation using the software GraphPad Prism 5.01 (La Jolla, CA). The specific activity was determined by incubating 2 nM of CbMan5A with 5 mg/ml of LBG, GG or KGM at its optimal pH and temperature for 10 min. One unit (U) of specific activity was defined as the amount of enzyme that released one μmol of reducing sugars per minute.
Effect of Metal Ions and Chemicals on the Enzyme Activity
The salts including NaCl, KCl, CaCl2, CoCl2, CrCl3, NiCl2, CuCl2, MgCl2, FeCl3, MnCl2, ZnCl2, Pb(CH3COO)2, and AgNO3 containing different metal ions, and three chemicals including sodium dodecyl sulfate (SDS), ethylene diamine tetraacetic acid (EDTA), and β-mercaptoethanol, were added with a final concentration of 5 mM into the reaction mixture. The mannanase activity of CbMan5A was measured using 5 mg/ml of LBG under its optimal conditions.
Differential Scanning Calorimetry (DSC) Analysis
DSC was performed on a Nano-DSC (TA Instruments, New Castle, DE) at a heating rate of 1°C/min and a scanning rate of 1°C/min. The sample (0.2 mg/ml of CbMan5A) were dissolved in 100 mM McIlvaine buffers (pH 7.5).The samples were degassed for 7 min before measurements in a dewar vessel. Then 400 μl of the sample with a 400 μl reference were loaded into the calorimeter cells. As the baseline, the same amount of buffer was measured and then subtracted from measured scan. During all DSC experiments, a constant pressure of 3 atm was maintained to prevent possible degassing of the solution on heating. The test was repeated twice at temperatures between 20°C and 120°C.
Supporting Information
Athe_1866 and Athe_1859 represent CbMan5C/Cel5A and CbMan5B/Cel44A, respectively.
(DOCX)
CbCel9B/Man5A was incubated at 70°C, 80°C, and 90°C for 1 h. At different time, samples were taken out and measured for the residual mannanase activity. Locust bean gum was used as the substrate. The activities of CbCel9B/Man5A were represented as relative activity (in percentage) by dividing the activities against the reference activity, which was the activity before treatment.
(DOCX)
(DOCX)
Acknowledgments
This research was supported by the Elite Youth Program of Chinese Academy of Agricultural Sciences, the National Natural Science Foundation of China (31400067), and the Fundamental Research Funds for Central Public Interest Institutes of the Chinese Academy of Agricultural Sciences (2015ZL045), the National High Technology Research, and Development Program of China 863 program (no. 2012AA022208), and the China Modern Agriculture Research System (CARS-42).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
1.Elite Youth Program of Chinese Academy of Agricultural Sciences. Grant number:n/a. http://www.caas.net.cn/kjcxgczl/zytz/237653.shtml 2. National Natural Science Foundation of China (31400067). http://www.nsfc.gov.cn/ 3. Fundamental Research Funds for Central Public Interest Institutes of the Chinese Academy of Agricultural Sciences (2015ZL045). http://www.caas.cn 4. National High Technology Research, and Development Program of China 863 program (no. 2012AA022208), http://www.863.gov.cn/ 5. China Modern Agriculture Research System (CARS-42), http://www.moa.gov.cn/
References
- 1. Yang SJ, Kataeva I, Hamilton-Brehm SD, Engle NL, Tschaplinski TJ, Doeppke C, et al. (2009) Efficient degradation of lignocellulosic plant biomass, without pretreatment, by the thermophilic anaerobe "Anaerocellum thermophilum" DSM 6725. Appl Environ Microbiol 75: 4762–4769. 10.1128/AEM.00236-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su X, Mackie RI, Cann IK (2012) Biochemical and mutational analyses of a multidomain cellulase/mannanase from Caldicellulosiruptor bescii. Appl Environ Microbiol 78: 2230–2240. 10.1128/AEM.06814-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su X, Han Y, Dodd D, Moon YH, Yoshida S, Mackie RI, et al. (2013) Reconstitution of a thermostable xylan-degrading enzyme mixture from the bacterium Caldicellulosiruptor bescii. Appl Environ Microbiol 79: 1481–1490. 10.1128/AEM.03265-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunecky R, Alahuhta M, Xu Q, Donohoe BS, Crowley MF, Kataeva IA et al. (2013) Revealing nature's cellulase diversity: the digestion mechanism of Caldicellulosiruptor bescii CelA. Science 342: 1513–1516. 10.1126/science.1244273 [DOI] [PubMed] [Google Scholar]
- 5.Velikodvorskaia GA, Chekanovskaia LA, Lunina NA, Sergienko OV, Lunin VG, Dvortsov IA, et al. (2013) The family 28 carbohydrate-binding module of the thermostable endo-1,4-β-glucanase CelD Caldicellulosiruptor bescii maximizes the enzyme's activity and binds irreversibly to amorphous cellulose. Mol Biol (Mosk) 47: 667–673. [DOI] [PubMed] [Google Scholar]
- 6.Young J, Chung D, Bomble YJ, Himmel ME, Westpheling J (2014) Deletion of Caldicellulosiruptor bescii CelA reveals its crucial role in the deconstruction of lignocellulosic biomass. Biotechnol Biofuels 7: 142 10.1186/s13068-014-0142-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung D, Pattathil S, Biswal AK, Hahn MG, Mohnen D, Westpheling J. (2014) Deletion of a gene cluster encoding pectin degrading enzymes in Caldicellulosiruptor bescii reveals an important role for pectin in plant biomass recalcitrance. Biotechnology for biofuels 7: 147 10.1186/s13068-014-0147-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi Z, Su X, Revindran V, Mackie RI, Cann I (2013) Molecular and biochemical analyses of CbCel9A/Cel48A, a highly secreted multi-modular cellulase by Caldicellulosiruptor bescii during growth on crystalline cellulose. PloS One 8: e84172 10.1371/journal.pone.0084172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang D, Gong L, Yao B, Xue X, Qin X, Ma R, et al. (2015) Implication of a galactomannan-binding GH2 β-mannosidase in mannan utilization by Caldicellulosiruptor bescii. Biochem Biophys Res Commun 467: 334–340. 10.1016/j.bbrc.2015.09.156 [DOI] [PubMed] [Google Scholar]
- 10.Soysal C (2008) Kinetics and thermal activation/inactivation of starking apple polyphenol oxidase. J Food Process Pres 32: 1034–1046. [Google Scholar]
- 11.Klump H, Di Ruggiero J, Kessel M, Park JB, Adams MW, Robb FT. (1992) Glutamate dehydrogenase from the hyperthermophile Pyrococcus furiosus. Thermal denaturation and activation. J Biol Chem 267: 22681–22685. [PubMed] [Google Scholar]
- 12.Rathi P, Bradoo S, Saxena RK, Gupta R (2000) A hyper-thermostable, alkaline lipase from Pseudomonas sp. with the property of thermal activation. Biotechnol Lett 22: 495–498. [Google Scholar]
- 13.Stutzenberger F, Lupo D (1986) pH-dependent thermal activation of endo-1,4-β-glucanase in Thermomonospora curvata. Enzyme Microb Technol 8: 205–208. [Google Scholar]
- 14.Kong F, Wang Y, Cao S, Gao R, Xie G (2014) Cloning, purification and characterization of a thermostable β-galactosidase from Thermotoga naphthophila RUK-10. Process Biochem 49: 775–782. [Google Scholar]
- 15.Dodd D, Kocherginskaya SA, Spies MA, Beery KE, Abbas CA, Mackie RI, et al. (2009) Biochemical analysis of a β-D-xylosidase and a bifunctional xylanase-ferulic acid esterase from a xylanolytic gene cluster in Prevotella ruminicola 23. J Bacteriol 191: 3328–3338. 10.1128/JB.01628-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dam P, Kataeva I, Yang SJ, Zhou F, Yin Y, Chou W, et al. (2011) Insights into plant biomass conversion from the genome of the anaerobic thermophilic bacterium Caldicellulosiruptor bescii DSM 6725. Nucleic Acids Res 39: 3240–3254. 10.1093/nar/gkq1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31: 426–428. [Google Scholar]
- 18.Moon YH, Iakiviak M, Bauer S, Mackie RI, Cann IK (2011) Biochemical analyses of multiple endoxylanases from the rumen bacterium Ruminococcus albus 8 and their synergistic activities with accessory hemicellulose-degrading enzymes. Appl Environ Microbiol 77: 5157–5169. 10.1128/AEM.00353-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, Luo H, Niu C, Shi P, Huang H, Meng K, et al. (2015) Biochemical characterization of a thermophilic β-mannanase from Talaromyces leycettanus JCM12802 with high specific activity. Appl Microbiol Biotechnol 99: 1217–1228. 10.1007/s00253-014-5979-x [DOI] [PubMed] [Google Scholar]
- 20.Lu H, Luo H, Shi P, Huang H, Meng K, Yang P, et al. (2014) A novel thermophilic endo-β-1,4-mannanase from Aspergillus nidulans XZ3: functional roles of carbohydrate-binding module and Thr/Ser-rich linker region. Appl Microbiol Biotechnol 98: 2155–2163. 10.1007/s00253-013-5112-6 [DOI] [PubMed] [Google Scholar]
- 21.Do BC, Dang TT, Berrin JG, Haltrich D, To KA, Sigoillot J, et al. (2009) Cloning, expression in Pichia pastoris, and characterization of a thermostable GH5 mannan endo-1,4-β-mannosidase from Aspergillus niger BK01. Microb Cell Fact 8: 59 10.1186/1475-2859-8-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu S, Li Z, Wang Y, Chen W, Fu L, Tang W, et al. (2015) High-level expression and characterization of a thermophilic β-mannanase from Aspergillus niger in Pichia pastoris. Biotechnol Lett. [DOI] [PubMed] [Google Scholar]
- 23.Yang H, Shi P, Lu H, Wang H, Luo H, Huang H, et al. (2015) A thermophilic β-mannanase from Neosartorya fischeri P1 with broad pH stability and significant hydrolysis ability of various mannan polymers. Food Chem 173: 283–289. 10.1016/j.foodchem.2014.10.022 [DOI] [PubMed] [Google Scholar]
- 24.Lu H, Zhang H, Shi P, Luo H, Wang Y, Yang P, et al. (2013) A family 5 β-mannanase from the thermophilic fungus Thielavia arenaria XZ7 with typical thermophilic enzyme features. Appl Microbiol Biotechnol 97: 8121–8128. 10.1007/s00253-012-4656-1 [DOI] [PubMed] [Google Scholar]
- 25.Luo H, Wang K, Huang H, Shi P, Yang P, Yao B. (2012) Gene cloning, expression, and biochemical characterization of an alkali-tolerant β-mannanase from Humicola insolens Y1. J Ind Microbiol Biotechnol 39: 547–555. 10.1007/s10295-011-1067-8 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Gao F, Xue Y, Zeng Y, Peng H, Qi J, et al. (2008) Crystallization and preliminary X-ray study of native and selenomethionyl beta-1,4-mannanase AaManA from Alicyclobacillus acidocaldarius Tc-12-31. Acta Crystallogr Sect F Struct Biol Cryst Commun 64: 209–212. 10.1107/S1744309108002832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Summpunn P, Chaijan S, Isarangkul D, Wiyakrutta S, Meevootisom V (2011) Characterization, gene cloning, and heterologous expression of β-mannanase from a thermophilic Bacillus subtilis. J Microbiol 49: 86–93. 10.1007/s12275-011-0357-1 [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Cao Y, Ding Y, Lu W, Li D (2007) Cloning, functional expression and characterization of Aspergillus sulphureus β-mannanase in Pichia pastoris. J Biotechnol 128: 452–461. [DOI] [PubMed] [Google Scholar]
- 29.Hsiao YM, Liu YF, Fang MC, Tseng YH (2010) Transcriptional regulation and molecular characterization of the manA gene encoding the biofilm dispersing enzyme mannan endo-1,4-β-mannosidase in Xanthomonas campestris. J Agric Food Chem 58: 1653–1663. 10.1021/jf903637s [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Athe_1866 and Athe_1859 represent CbMan5C/Cel5A and CbMan5B/Cel44A, respectively.
(DOCX)
CbCel9B/Man5A was incubated at 70°C, 80°C, and 90°C for 1 h. At different time, samples were taken out and measured for the residual mannanase activity. Locust bean gum was used as the substrate. The activities of CbCel9B/Man5A were represented as relative activity (in percentage) by dividing the activities against the reference activity, which was the activity before treatment.
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.