Abstract
Platelet adhesion, activation, and aggregation are central to the propagation of coronary thrombosis following rupture, fissure, or erosion of an atherosclerotic plaque. This chain of deleterious events underlies the pathophysiological process leading to an acute coronary syndrome. Therefore, oral antiplatelet therapy has become the cornerstone of therapy for the management of acute coronary syndrome and the prevention of ischemic complications associated with percutaneous coronary intervention. Landmark trials have established aspirin, and the addition of clopidogrel to aspirin, as key therapeutic agents in the context of acute coronary syndrome and percutaneous coronary intervention. Dual antiplatelet therapy has been the guideline-mandated standard of care in acute coronary syndrome and percutaneous coronary intervention. Despite the proven efficacy of dual antiplatelet therapy, adverse ischemic events continue to occur and this has stimulated the development of novel, more potent antiplatelet agents. We focus this state-of-the-art review on the most recent advances in oral antiplatelet therapy, treading the tightrope of potency versus bleeding risk, the quest to determine the optimal duration of dual antiplatelet therapy and future of personalized antiplatelet therapy.
Keywords: Antiplatelet, aspirin, clopidogrel, ticagrelor, prasugrel, acute coronary syndrome, percutaneous coronary intervention
Introduction
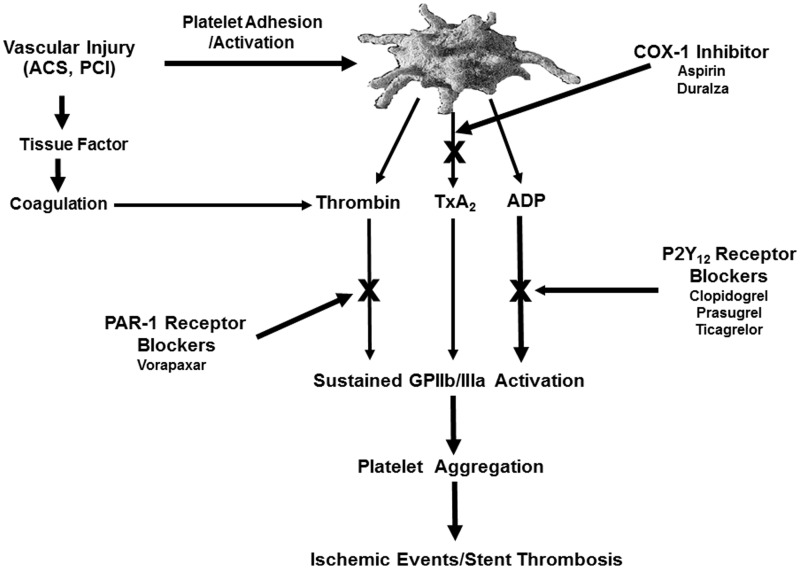
The pivotal roles played by platelets in thrombosis at sites of vascular injury provide a strong rationale for blocking their function in the setting of coronary artery disease (CAD). Following adhesion at the site of arterial vascular injury in the presence of shear force, platelets undergo activation and release adenosine diphosphate (ADP) from dense granules and generate arachidonic acid from membrane phospholipids via the cyclooxygenase-1 (COX-1)/thromboxane synthase pathway and thrombin through the coagulation pathway on the surface of activated platelets. ADP, thromboxane A2, and thrombin act on three important G-protein-coupled receptors: P2Y12, TP, and protease-activated receptor (PAR)-1, respectively, and a cascade of intracellular signaling events culminate in the activation of the glycoprotein (GP) IIb/IIIa receptor that then binds to the dimeric fibrinogen molecule to mediate platelet aggregation (Figure 1). The relative contributions of these upstream receptors to in vivo thrombosis remain incompletely defined. P2Y12, TP, and PAR-1 are associated with redundancy in their responses (signaling pathways). Therefore, targeting more than one of these receptor pathways by oral agents is an attractive antithrombotic strategy for acute as well as long-term prevention of recurrent cardiovascular (CV) events in patients with CAD and has been extensively explored in clinical trials.1
Figure 1.
Targets of oral antiplatelet agents.
Aspirin
Aspirin remains the bedrock of antiplatelet therapy for acute and long-term treatment of patients with coronary and cerebrovascular diseases. After absorption in the upper gastrointestinal (GI) tract, it rapidly and irreversibly acetylates platelet COX-1 serine residue 529 in the prehepatic circulation. Acetylation prevents arachidonic acid from accessing the active site of the enzyme, thereby preventing subsequent generation of TxA2 from thromboxane synthase and TxA2-induced platelet aggregation. Aspirin is highly effective at blocking COX-1. In addition, non-COX-1-mediated effects of aspirin in platelets and other pleiotropic effects may also contribute antithrombotic properties.2,3 Aspirin monotherapy has been recommended for primary prevention in patients at high CV risk, defined as ≥2 major CV events (death, myocardial infarction, or stroke) projected per 100 person-years, who are not at increased risk of bleeding.4 In most large-scale trials, novel antiplatelet agents have been administered as an adjunct to aspirin therapy. The net clinical benefit of aspirin for the secondary prevention of CV events is well demonstrated in multiple clinical trials, systematic reviews, and meta-analyses.
The antithrombotic trialists collaboration meta-analysis of 16 secondary prevention trials (N = 17,000 individuals with above-average risk) demonstrated that aspirin versus control therapy was associated with significant reduction in annual rates of serious vascular events (6.7% vs. 8.2%; P < 0.0001), total stroke (2.1% vs. 2.5%; P = 0.002), and major coronary events (4.3% vs. 5.3%; P < 0.0001). There was a non-significant increase in hemorrhagic stroke (risk ratio (RR), 1.67 (95% CI, 0.81–3.44)). However, in an aggregate of studies that included major bleeding as an endpoint, there was a significantly higher incidence of major bleeding in patients treated with aspirin versus controls (RR: 2.69 (95% CI, 1.25–5.76); P = 0.01).5 The net clinical benefit favored aspirin therapy in the secondary prevention of serious vascular events.
Many controversies exist regarding aspirin therapy. An optimal aspirin dose for secondary prevention has not truly been established. In the CURRENT OASIS-7 trial, in patients with acute coronary syndrome (ACS) and intended early percutaneous coronary intervention (PCI), there was no significant difference between low-dose aspirin (75–100 mg/d) and high-dose aspirin (300–325 mg/d) on 30-day MI, stroke, or CV mortality (4.1% vs. 4.2%; adjusted hazard ratio (HR), 0.98 (95% CI, 0.84–1.13); P = 0.8) or major bleeding (1.5% vs. 1.3%; HR 1.18 (95% CI, 0.92–1.53); P = 0.2). However, there was a trend toward higher rates of GI bleeding in the high- versus low-dose aspirin group (0.38% vs. 0.24%; P = 0.05). These findings suggested that the low-dose aspirin regimens were as efficacious as high-dose aspirin regimens for secondary prevention of cardiovascular disease, but exhibited a more favorable GI tolerability profile.6 The anti-ischemic benefit of long-term aspirin therapy has been shown to be similar for doses ≥ 75 mg/day in high-risk patients; however, increased bleeding events, particularly GI-related bleeding associated with ≥325 mg/day dose.7,8
Current guidelines for secondary prevention widely recommend indefinite 75–325 mg daily aspirin for all patients, and this has been generally implemented into current clinical practice. Whenever rapid and complete inhibition of TxA2-induced platelet aggregation is desired, a 150 - to 325-mg aspirin loading dose is favored.2 The new aspirin dosing: a patient-centric trial assessing benefits and long-term effectiveness (http://theaspirinstudy.org) study has been planned to determine the optimal aspirin dose that is associated with maximum anti-ischemic benefit and minimal bleeding risk. In this study, 20,000 patients with CV disease will be randomly treated with 81 versus 325 mg/d of aspirin for 30 months.
Table 1.
Comparison of platelet inhibitors.
| Aspirin | Clopidogrel | Prasugrel | Ticagrelor | Vorapaxar | |
|---|---|---|---|---|---|
| Target | COX-1 enzyme | P2Y12 receptor | P2Y12 receptor | P2Y12 receptor | PAR-1 receptor |
| Class | Acetyl salicylic acid | Thienopyridine | Thienopyridine | CTPT | Himbacine analogue |
| Metabolism | Direct drug | Prodrug | Prodrug | Direct drug | Direct drug |
| Administration | Oral | Oral | Oral | Oral | Oral |
| Metabolic pathway | Hepatic (salicylic acid) | Hepatic CYP P450 (1A2, 2C19, 3A4/5, 2B6, 2C9) | Intestine/hepatic CYP P450 (2C19, 3A4/5, 2B6, 2C9) | Hepatic CYP34/5 | CYP P450 (3A4, 2J2) |
| Conversion to active metabolite | ∼100% | 15% | 85% | 90–100% | ∼ 20% to M20 |
| Binding property | Irreversible Ser529 of COX-1 | Irreversible Free thiol of Cys97 | Irreversible Free thiol of Cys97 | Reversible At site distinct from ADP-binding site | Reversible |
| Half-life | Salicylate excretion dependent on urine pH | ∼ 20 min post-75 mg (active metabolite) | ∼ 30 min post- 10 mg ∼ 7.4 h post-60 mg (active metabolite) | ∼7 h | 3–5 d |
| Time to steady state inhibition | 30 min post-100 mg bolus | 8 h post- 600 mg | 1–2 h post-60 mg | 2 h post-180 mg | 7 d post-2.08 mg |
| Pharmacodynamic Off set | 7–10 d | 5–7 d | up to 9 d | 5–7 d | >4 wk |
| Level of inhibition at steady state | >95% inhibition of TxA2 | ∼40–50% wide response variability | ∼65–80% | ∼65–80% | ≥80% |
| Off target effects | Multiple | None significant | None significant | Inhibition of adenosine reuptake | None significant |
The stability of the antiplatelet effect over 24 h with widely used immediate-release aspirin in high-risk populations has been under scrutiny in recent years. Although, twice-daily dosing may provide improved antiplatelet activity coverage compared with once-daily dosing, the clinical significance and safety profile of twice-daily dosing have not been studied in a large-scale trial to date. To address the unmet medical need for 24-h antiplatelet coverage in high-risk populations, an extended-release aspirin (Durlaza®, New Haven Pharmaceuticals, Inc., North Haven, CT) was developed and approved in the United States in 2015.9 In addition, ongoing studies are now investigating the utility of deleting aspirin therapy in the setting of potent P2Y12 receptor blockade with ticagrelor or replacing it with a new oral anticoagulant.
P2Y12 receptor blockers
Given the synergistic importance of the ADP-P2Y12 and TxA2-TP pathways in amplifying platelet activation, dual antiplatelet therapy with a P2Y12 inhibitor on top of aspirin is the most widely used strategy in high-risk patients. The currently available P2Y12 receptor blockers are the thienopyridines (ticlopidine, clopidogrel, and prasugrel) and ticagrelor.
Thienopyridines
Thienopyridines are orally administered prodrugs that require metabolic activation by the cytochrome P450 pathway. The active thiolactone metabolite of thienopyridines irreversibly prevents the ADP-induced receptor-mediated signaling and platelet aggregation for the life of platelets by binding covalently to the free thiol of Cys97 on P2Y12.1
Clopidogrel
The first generation thienopyridine, ticlopidine, was associated with hematologic and other side effects and has largely been superceded by the second generation agent, clopidogrel. The clinical benefit of clopidogrel added to aspirin versus aspirin therapy has been unequivocally demonstrated in patients with high risk CAD. Pharmacodynamic studies have conclusively demonstrated response variability and non-responsiveness to clopidogrel, the most widely prescribed P2Y12 inhibitor. Suboptimal active metabolite generation and pharmacodynamic effects have been linked to single nucleotide polymorphisms in genes encoding specific CYP P450 cytochromes associated with loss-of-function, drug–drug interactions between thienopyridines and other co-administered drugs that compete with or inhibit specific CYP P450 cytochromes, and specific demographic variables. Currently available evidence supports the concept of a threshold for on-treatment platelet reactivity to ADP in patients treated with dual antiplatelet therapy that may be used to stratify patient risk for ischemic/thrombotic events after PCI, including stent thrombosis.
In the gauging responsiveness with a VerifyNow assay-impact on thrombosis and safety (GRAVITAS) trial, 2214 patients shown to have high on-treatment platelet reactivity, using the VerifyNow point-of-care assay, 12 to 24 h post-PCI were randomized to a high-dose (600 mg loading followed by 150 mg daily) versus standard-dose (no additional loading dose, continue with 75 mg daily) clopidogrel strategy for six months. The primary endpoint was a composite of death from CV causes, non-fatal myocardial infarction, or stent thrombosis at six months. There was no significant difference in the primary endpoint between the two groups at six months. Neither was there a significant difference in severe or moderate bleeding based on the GUSTO definition.10
In the the assessment by a double randomization of a conventional antiplatelet strategy versus a monitoring-guided strategy for drug-eluting stent implantation and of treatment interruption versus continuation one year after stenting (ARCTIC) trial, 2440 patients scheduled for PCI were randomized to a conventional (without monitoring or drug adjustment) versus platelet-function monitoring (with subsequent drug adjustment for those with a poor response to antiplatelet therapy) strategy. Those in the monitoring group who demonstrated high platelet reactivity (HPR) despite clopidogrel administration (34.5% of patients) were given an additional bolus of clopidogrel or prasugrel along with GP IIb/IIIa inhibitors during the procedure. Overall there was no significant difference in the composite primary endpoint of death, myocardial infarction, stent thrombosis, stroke, or urgent revascularization one year after stent implantation (P = 0.10) between the two strategies. The rate of major bleeding did not differ significantly either confirming yet further the results of GRAVITAS.11
Platelet function testing may be considered in determining an antiplatelet strategy in patients with a history of stent thrombosis and in patients prior to undergoing high-risk PCI. However, tailored antiplatelet therapy according to platelet reactivity measured using point-of-care assays, cannot be recommended on the current evidence base. A diminished pharmacodynamic effect of clopidogrel has also been demonstrated in patients treated with proton pump inhibitors, particularly omeprazole. However, the clinical importance of this pharmacodynamic interaction remains highly controversial.9 These investigations failed to demonstrate the utility of platelet function testing in reducing the post-PCI ischemic risk. Major criticism for the latter neutral observation are: all these investigations used the VerifyNow P2Y12 assay to assess platelet reactivity to ADP and to identify HPR, these investigations included mostly low-risk patients undergoing PCI and had resultant low event rates irrespective of platelet reactivity. Although a major risk factor for post-PCI thrombotic event occurrence, HPR is not the sole factor responsible for these events. In contrast, the absence of HPR is the best reassurance thus far for a low likelihood of future ischemic events. Other factors, including demographic, clinical, and angiographic factors, must be taken into consideration to optimally identify the patients at greatest risk.12
In the CURRENT-OASIS 7 trial, a strategy of double-dose clopidogrel (600 mg on day 1, 150 mg on days 2–7, and then 75 mg daily) was compared with standard dose clopidogrel (300 mg on day 1 and then 75 mg daily) in patients with ACS. The 30-day primary outcome of CV death, MI, or stroke did not differ between the two clopidogrel groups; however, bleeding was greater in the double-dose group. In an analysis of 78% of patients who underwent PCI, double-dose clopidogrel therapy was associated with a 14% reduction in the rate of the primary outcome of CV death, MI or stroke in 30days (3.9% vs. 4.5%; adjusted HR 0·86, P = 0·039) and a 46% reduction in the secondary outcome of definite stent thrombosis (0.7% vs. 1.3%; adjusted HR 0·54, P = 0·0001). Double-dose clopidogrel therapy was associated with more major bleeding than standard-dose clopidogrel in the overall group and in the PCI cohort (1.6 vs. 1.1%; HR, 1.41; 95% CI, 1.09–1.83; P = 0.009). Interestingly, the maximum benefit in the PCI cohort was observed in patients who were treated with double-dose clopidogrel and high-dose aspirin (3.5 vs. 4.2%; HR, 0.81; 95% CI, 0.65–1.01; P = 0.058).6 Currently, a 600 mg loading dose during the acute setting of ACS and PCI followed by 75 mg per day for long-term therapy is widely implemented in clinical practice.
Prasugrel
Prasugrel, a third generation thienopyridine, is more efficiently metabolized, potent and rapid acting than clopidogrel. Prasugrel is associated with markedly less response variability and non-responsiveness than clopidogrel.13 Prasugrel therapy is recommended in ACS patients managed by PCI only, based on the results of the TRITON TIMI 38 study where clopidogrel was the comparator. In this trial, prasugrel (60 mg load/10 mg daily maintenance) plus aspirin treatment (75–162 mg/d) was associated with a 19% reduction (9.9 vs. 12.1%; HR, 0.81; P = 0.0004) in the primary composite endpoint of CV death, non-fatal MI, and non-fatal stroke at a median 14.5-month follow-up compared with clopidogrel (300 mg load/75 mg daily maintenance) plus aspirin treatment in patients with NSTE ACS and STEMI undergoing planned PCI. However, these benefits were associated with significantly increased key safety endpoints of thrombolysis in myocardial infarction (TIMI) major bleeding, including life-threatening and fatal bleeding in patients treated with prasugrel (2.4 vs. 1.8%; P < 0.03).14
A maintenance dose of 5 mg has been approved by the Food and Drug Administration (FDA) in patients < 60 kg because of a potential increased risk of bleeding; however, the effectiveness and safety of the 5 mg dose have not been studied prospectively. Prasugrel is not recommended in patients with active pathologic bleeding or a history of transient ischemic attack (TIA) or stroke. In patients ≥ 75 years of age, prasugrel is generally not recommended because of the increased risk of fatal and intracranial bleeding. It is recommended not to start prasugrel therapy in patients likely to undergo urgent CABG. When possible, prasugrel should be discontinued at least seven days before any surgery.14
In the targeted platelet inhibition to clarify the optimal strategy to medically manage acute coronary syndromes (TRILOGY ACS) study, patients with NSTE ACS receiving aspirin and managed without revascularization were randomized to therapy with prasugrel (10 mg daily in patients < 75 years old and 5 mg daily in patients ≥ 75 years of age) versus 75 mg daily clopidogrel. At a median follow-up of 17 months, there was no significant difference in the primary endpoint of CV death, MI, or stroke among patients < 75 years (13.9 vs. 16.0%; HR, 0.91; 95% CI, 0.79–1.05; P = 0.21). Similar results were observed in the overall population. Severe and intracranial bleeding rates were similar in the two groups in all age groups. This trial failed to demonstrate the superiority of prasugrel over clopidogrel in medically managed patients; therefore, prasugrel is not recommended for the treatment of ACS patients managed without revascularization.15
In the comparison of prasugrel at the time of PCI or as pretreatment at the time of diagnosis in patients with non-ST elevation myocardial infarction (ACCOAST) trial, patients admitted with NSTE ACS who were scheduled to undergo coronary angiography within 2–48 h after randomization were treated with prasugrel (30 mg loading dose) before angiography (pretreatment group) or placebo (control group), and an additional 30 mg of prasugrel was given in the pretreatment group at the time of PCI, and 60 mg of prasugrel was given in the control group at the time of PCI. The median duration of pretreatment prior to PCI was approximately 4 h, a factor that may limit the detectability of pretreatment benefit. The rate of the primary composite efficacy endpoint of CV death, MI, stroke, urgent revascularization, or GPIIb/IIIa bailout through day seven did not differ significantly between the two treatment groups (HR = 1.02; P = 0.81). The key safety endpoint of all TIMI major bleeding (CABG or non-CABG) was increased in patients pretreated with prasugrel (HR, 1.90; P = 0.006). These results suggest that pretreatment with prasugrel in NSTE ACS patients undergoing PCI is not beneficial in reducing ischemic risk but is associated with elevated bleeding risk.16
Ticagrelor
Ticagrelor, a cyclopentyltriazolopyrimidine, is reversibly binding, selective, direct acting, and orally administered. Ticagrelor does not prevent ADP binding to P2Y12, but instead reversibly inhibits the ADP-induced receptor conformational change and G-protein activation by binding to a site distinct from the ADP-binding site. These characteristics keep the receptor in an inactive state and after ticagrelor unbinding, the receptor can be reactivated by ADP. Ticagrelor is metabolized rapidly by hepatic CYP3A4/5 to produce AR-C124910XX, the main metabolite of ticagrelor that is equipotent in inhibiting the P2Y12 receptor. Ticagrelor is associated with a rapid onset of action, a greater level of inhibition and a more rapid offset of pharmacodynamic action compared with clopidogrel.1
The platelet inhibition and patient outcomes (PLATO) trial was a phase III, randomized, multicenter, double-blind study designed to evaluate the efficacy of ticagrelor (180 mg loading dose/90 mg bid) compared with clopidogrel (300–600 mg loading dose/75 mg qd) for the prevention of vascular events and death in patients with ACS. Ticagrelor therapy was associated with a significant reduction in the primary efficacy endpoint compared with clopidogrel at 30 days (4.8 vs. 5.4%; P = 0.045), and the superiority of ticagrelor was maintained throughout 12 months, with a 16% relative risk reduction (9.8 vs. 11.7%, respectively; P < 0.001). CV death (5.1% clopidogrel vs. 4.0% ticagrelor; P = 0.001), and MI (6.9% clopidogrel vs. 5.8% ticagrelor; P = 0.005) but not stroke (1.5% vs. 1.3%, P = 0.22) were significantly reduced by ticagrelor treatment.17
There were no differences in the primary safety endpoint of major bleeding as defined by both the study protocol (ticagrelor 11.6% vs. clopidogrel 11.2%; P = 0.43) and TIMI criteria (7.9 vs. 7.7%; P = 0.57). Despite the fact that patients in the ticagrelor treatment group were allowed to undergo CABG within 24–72 h following discontinuation of study medication (compared with five days in the clopidogrel group), CABG-related bleeding event rates were similar between the two groups. Non-CABG-related major bleeding event rates were higher following ticagrelor treatment (4.5 vs. 3.8%; P = 0.026 and 2.8 vs. 2.2%; P = 0.025 for protocol- and TIMI study group-defined bleeding events, respectively).15
One of the most remarkable observations of the PLATO trial was a significant reduction in mortality associated with ticagrelor therapy. Anti-ischaemic benefits associated with ticagrelor versus clopidogrel were consistent regardless of whether the management strategy selected upfront was invasive or conservative, age, risk factors, body weight, prior medical history (including TIA or stroke), type of ACS, or genotype.17 However, clinical benefit associated with ticagrelor treatment was absent in the North American patient population enrolled in the PLATO trial. The latter has been attributed to the concomitant use of high-dose aspirin (aspirin > 100 mg/d). Ticagrelor therapy is associated with side effects, including dyspnea (which occurs in up to 15% of patients within the first week of treatment but is rarely severe enough to cause discontinuation of treatment) and bradycardia. When possible, ticagrelor should be discontinued at least five days before surgery.
In the prevention of CV events in patients with prior heart attack using ticagrelor compared to placebo on a background of aspirin–thrombolysis in myocardial infarction 54 (PEGASUS-TIMI 54) trial, long-term therapy with ticagrelor added to low-dose aspirin to reduce the risk of major adverse cardiac events (MACE) among stable patients with a history of MI was studied. Patients who had had an MI 1–3 years earlier were randomized to therapy with 90 mg twice-daily ticagrelor, 60 mg twice-daily ticagrelor, or placebo in addition to low-dose aspirin for a median of 33 months. Overall, ticagrelor was associated with a significantly reduced risk of the primary composite endpoint of CV death, MI, or stroke; Kaplan–Meier rates at three years were 7.85% in the 90 mg of ticagrelor twice-daily group, 7.77% in the 60 mg of ticagrelor twice-daily group, and 9.04% in the placebo group (90 mg of ticagrelor vs. placebo: HR, 0.85; 95% CI, 0.75–0.96; P = 0.008; 60 mg of ticagrelor vs. placebo: HR, 0.84; 95% CI, 0.74–0.95; P = 0.004). TIMI major bleeding was higher with ticagrelor (2.6% with 90 mg, and 2.30% with 60 mg) than with placebo (1.06%) (P < 0.001 for each dose vs. placebo), whereas intracranial hemorrhage or fatal bleeding was similar (0.63, 0.71, and 0.60%, respectively) between groups.18 Whether the results of this trial will lead to prolonged use of ticagrelor to prevent further MACE in a high-risk population is yet to materialize.
Duration of dual antiplatelet therapy
The optimal duration of dual antiplatelet therapy is not well established. Current guideline recommendations are based primarily on the results of large-scale clinical trials and observational studies and expert consensus. Recently, randomized trials in patients treated with new generation coronary artery stents and meta-analyses have suggested that shorter duration dual antiplatelet therapy is associated with similar clinical efficacy compared with one-year duration. However, these trials were underpowered.
Two recent large-scale randomized trials, the PEGASUS-TIMI 54 trial and dual antiplatelet therapy (DAPT) study, addressed the efficacy of P2Y12 inhibitor therapy beyond 12 months.18,19 In the PEGASUS-TIMI 54 trial, the Kaplan–Meier primary endpoint curves diverged over three years, indicating continuous accrual of efficacy from ticagrelor and low-dose aspirin over aspirin monotherapy. However, the antithrombotic benefit of dual antiplatelet therapy in the PEGASUS-TIMI 54 study was offset by significantly greater bleeding, such that the net clinical benefit (MACE reduction – TIMI major bleeding increase) was essentially neutral.18
In the DAPT study, 12 months after treatment with standard thienopyridine therapy (clopidogrel or prasugrel) and aspirin, patients who had undergone angioplasty with a drug-eluting stent were randomly assigned to continue receiving thienopyridine treatment or to receive placebo for another 18 months in addition to aspirin. The continued treatment with thienopyridine was associated with significantly lower rates of stent thrombosis (0.4 vs. 1.4%; HR, 0.29; P < 0.001) and major adverse CV and cerebrovascular events (a composite of death, MI, or stroke) (4.3 vs. 5.9%; HR, 0.71; P < 0.001), but increased rate of moderate or severe bleeding according to the global utilization of streptokinase and tissue plasminogen activator for occluded coronary arteries (GUSTO) trial criteria (2.5 vs. 1.6%, P = 0.001). In this trial, 3206 patients had NSTE ACS. There was no apparent heterogeneity for prior MI history. All-cause death was greater in the DAPT study and was attributed to more non-CV death. The underlying reason(s) for this observation remains unclear.19
Thus, the results of DAPT and PEGASUS suggest prolonged therapy with more potent P2Y12 inhibitors without interruption in selected patients with high risk for ischemic events and lower risk for bleeding may be an option. Although this strategy may appear attractive on the surface the “selection” of potential high-risk patients may not be so easy. A subanalysis of the PEGASUS trial demonstrated that thrombotic risk is greater in patients who recently discontinued P2Y12 therapy compared to those who discontinued therapy over 30 days previously and had been event free.20 In general, although patients who recently discontinued antiplatelet therapy appear to be at “high risk”, the prevalence of risk factors such as hypertension, hyperlipidemia, and PCI for index MI was equally high in all studied groups (>70%). Only multivessel disease was higher (68% vs. ∼60%) in the patients who discontinued a P2Y12 inhibitor ≤ 30 days, therefore making it difficult to characterize which factors push patients towards the very limit of the “high-risk” spectrum.20
A planned extension of the ARCTIC trial – ARCTIC-interruption – further randomized patients enrolled into the original trial with no contraindications to interruption of DAPT after a year of follow up. As such, 1259 eligible patients were randomized in a 1:1 fashion to an interruption group in which the thienopyridine was stopped, and aspirin monotherapy was continued indefinitely versus a continuation group in which DAPT was continued for a further 6–18 months. There was no significant difference in the primary composite endpoint of death, MI, stent thrombosis, stroke, or urgent target revascularization between the two groups (HR 1.17 (95% CI 0.68–2.03); P = 0.58). Both major and minor bleeding events occurred more frequently in the continuation group, although the difference did not reach significance. The investigators concluded that there was no apparent benefit in prolonging the duration of DAPT beyond one year after coronary stenting with drug-eluting stents, and indeed there was a trend towards harm with such a strategy.21
It is important to note that atherothrombosis is a dynamic process and the providing regional observations to study predictors of events in the coronary tree (PROSPECT) study demonstrated that long-term post-PCI thrombotic events can occur equally in the culprit and non-culprit vessels.22 Moreover, the stability of the platelet reactivity phenotype over time, that is pivotal for the thrombotic event occurrence, remains unclear. The underlying cause of “rebound” ischemic events also remains unclear. In this line, it has been hypothesized that the perfect storm scenario including demographic and clinical variables, systemic factors and most importantly heightened platelet reactivity is required for an acute coronary event to occur.23 Furthermore, unlike the strong association between platelet reactivity and coronary ischemic events, the underlying mechanisms of bleeding are more complex and heterogeneous in origin. In addition, most of the risk factors associated with heightened ischemic risk are also associated with bleeding. Thus, although the PEGASUS study provided some important insights into the durability of long-term DAPT, the selection of patients with high risk for ischemic events and lower risk for bleeding still remains an enigma and must be further explored in future studies with more emphasis on biomarkers including platelet reactivity phenotype in addition to other variables.
Vorapaxar
Despite superior antiplatelet effects compared to clopidogrel, therapy with prasugrel or ticagrelor has been associated with a ∼10% treatment failure rate and greater bleeding. The latter observations suggest a ceiling of net clinical benefit associated with a strategy of COX-1 and potent P2Y12 inhibition. Vorapaxar, a synthetic analogue of himbacine, is a first in class, selective, reversibly binding, orally administered, high-affinity PAR-1 inhibitor that selectively inhibits thrombin-induced platelet aggregation. Its antiplatelet effect is virtually irreversible due to a very slow dissociation constant.24
In the TRA 2°P-TIMI 50 trial, patients who had a history of MI, ischemic stroke, or peripheral arterial disease (PAD) received vorapaxar or placebo and were followed for a median of 30 months. Overall, >80% of patients were on aspirin therapy and, in the MI cohort, nearly 80% were on a thienopyridine with the vast majority of these patients being treated with clopidogrel. After two years, treatment in patients with a history of stroke was stopped due to increased intracranial hemorrhage. In patients with prior MI or PAD without a previous stroke or TIA, vorapaxar added to standard therapy was effective for long-term secondary prevention of thrombotic CV events, while also increasing moderate or severe bleeding.25 In a subgroup analysis of the proposed label population, defined as the patients in the post-MI group with no history of stroke or TIA (n = 17,769; 67% of the overall population), vorapaxar therapy was associated with a significant reduction in the primary composite endpoint of CV death, MI, or stroke (8.1 vs. 9.7%; HR, 0.80; P < 0.0001) at three years, that was mainly attributed to a significant reduction in MI (5.7 vs. 7.0%; HR, 0.79; P = 0.0003). There was a significant reduction in the secondary endpoint of CV death, MI, stroke, or urgent coronary revascularization (10.5 vs. 12.1%; HR, 0.83; P = 0.0001), but no significant difference in CV death between the vorapaxar group and placebo.26
There was an increase in the primary safety endpoint occurrence of GUSTO moderate or severe bleeding (3.4 vs. 2.1%; HR, 1.61; P < 0.0001), TIMI non-CABG major or minor bleeding (3.6 vs. 2.2%; HR, 1.60; P < 0.0001), and non-significantly increased intracranial hemorrhage (0.6 vs. 0.4%; HR, 1.54; P = 0.076). Finally, the net clinical outcome favored vorapaxar therapy in the proposed population with five fewer fatal events and 45 fewer non-fatal serious events, but 33 additional GUSTO moderate bleeding events.26 In the proposed label population, 7375 patients had NSTEMI, and vorapaxar therapy in these patients was associated with a reduction in the primary efficacy endpoint (10.4 vs. 11.2%; HR, 0.88; 95% CI, 0.76–1.02). Based on these favorable results, the FDA approved the use of vorapaxar to reduce the risk of MI, stroke, CV death, and revascularization in patients with a previous MI. In addition, vorapaxar was approved for patients with PAD. It is recommended that vorapaxar be used with aspirin and/or clopidogrel according to their indications. There is limited clinical experience with other antiplatelet agents and no experience with vorapaxar as monotherapy. In Europe, vorapaxar is approved to be co-administered with aspirin and, where appropriate, clopidogrel, in patients with a history of MI.
Conclusions
Since thrombotic complications of CAD are platelet-centric, oral antiplatelet therapy with aspirin and a P2Y12 inhibitor constitute the predominant strategy to prevent recurrent ischemic event in the acute and long-term treatment of atherothrombotic disease. Current US and European guidelines advocate the use of dual antiplatelet therapy for 12 months (unless there are contraindications such as excessive bleeding risk) in the context of both the invasive and/or conservative management of unstable angina/NSTE ACS, STEMI, and elective coronary revascularization with drug-eluting coronary stents.27–29 Aspirin remains the cornerstone of oral antiplatelet therapy and has been assigned a Class I, level of evidence (LoE) A recommendation by these guidelines. However, recent studies are focused on deleting aspirin, particularly in the presence of more potent P2Y12 receptor blocker or new oral anticoagulant. Clopidogrel, ticagrelor, or prasugrel complete the dual antiplatelet combination and have all been assigned a Class I, LoE B recommendation. The recent guidelines prefer ticagrelor or prasugrel over clopidogrel. Despite its comparatively weaker antiplatetlet potency, slower onset of action, and propensity towards response variability, clopidogrel continues to maintain its relevance in the routine practice. This is especially the case in those patients receiving conservative ACS management and in the secondary prevention of cerebrovascular disease and PAD. Furthermore, the availability of clopidogrel in its generic form has slightly tipped the cost-benefit balance in its favor with respect to ticagrelor and prasugrel.
Much of the focus in recent times has turned to the potential benefit of tailoring antiplatelet therapy to the individual, particularly in those with high on-treatment platelet reactivity. Although intuitive, a strategy of increasing the clopidogrel maintenance dose or changing to a more potent thienopyridine in patients with HPR during clopidogrel therapy has not resulted in the efficacy benefit.
Another area of increasing interest has been the duration of dual antiplatelet therapy to provide net clinical benefit in those at high CV risk. Again the data have been equivocal and indeed have suggested a tendency toward causing harm in terms of increased bleeding events in those assigned to a strategy of dual antiplatelet therapy continued beyond one year. As with any form of antithrombotic therapy, bleeding is the Achilles heel of oral antiplatelet drugs. Bleeding risk must always be borne in mind when initiating DAPT, especially in those with low body weight, renal dysfunction, age ≥ 75 years, and a previous history of bleeding.
Despite their undoubted efficacy, patients continue to suffer recurrent atherothrombotic events on dual antiplatelet therapy. More potent antiplatelet therapy has reduced the incidence of ischemic events at the expense of higher bleeding rates. It is this double-edged sword of risk versus benefit that continues to stimulate the need for further clinical trials investigating novel antiplatelet drugs and strategies to establish the best therapeutic regimen for those patients presenting with ACS and undergoing coronary revascularization.
Declaration of conflicting interests
Gurbel reports personal fees from AstraZeneca, Merck, New Haven Pharmaceuticals, Bayer, and Haemonetics; grants from Haemonetics, Merck, Duke Clinical Research Institute, Harvard Clinical Research Institute, National Institutes of Health, New Haven Pharmaceuticals, MedImmune and Coramed Technologies; a patent for platelet function testing; and stock options in Merck outside the submitted. Other authors report no conflict of interest work.
Funding
This study was supported by Inova Heart and Vascular Institute, 3300 Gallows road, Falls Church, VA 22042, USA.
Ethical approval
None.
Guarantor
Inova Heart and Vascular Institute, Falls Church, VA, USA.
Contributorship
Udaya S Tantry, Paul A Gurbel, Aung Myat – Original manuscript draft, critical revision. Jacek kubica – Critical revision.
References
- 1.Gurbel PA, Kuliopulos A, Tantry US. G-protein-coupled receptors signaling pathways in new antiplatelet drug development. Arterioscler Thromb Vasc Biol 2015; 35: 500–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eikelboom JW, Hirsh J, Spencer FA, et al. Antiplatelet drugs: antithrombotic therapy and prevention of thrombosis. 9th ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141: e89S–e119S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tantry US, Mahla E, Gurbel PA. Aspirin resistance. Prog Cardiovasc Dis 2009; 52: 141–152. [DOI] [PubMed] [Google Scholar]
- 4.Halvorsen S, Andreotti F, ten Berg JM, et al. Aspirin therapy in primary cardiovascular disease prevention: a position paper of the European Society of Cardiology working group on thrombosis. J Am Coll Cardiol 2014; 64: 319–327. [DOI] [PubMed] [Google Scholar]
- 5.Baigent C, Blackwell L, Collins R, et al. Antithrombotic Trialists' (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373: 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta SR, Tanguay JF, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet 2010; 376: 1233–1243. [DOI] [PubMed] [Google Scholar]
- 7.Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324: 71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger JS, Sallum RH, Katona B, et al. Is there an association between aspirin dosing and cardiac and bleeding events after treatment of acute coronary syndrome? A systematic review of the literature. Am Heart J 2012; 164: 153–162. [DOI] [PubMed] [Google Scholar]
- 9.Bliden KP, Patrick J, Pennell AT, et al. Drug delivery and therapeutic impact of extended-release acetylsalicylic acid. Future Cardiol 2016; 12: 45–58. [DOI] [PubMed] [Google Scholar]
- 10.Price MJ, Berger PB, Teirstein PS, et al. GRAVITAS Investigators. Standard- vs. high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 201; 305: 1097–105. [DOI] [PubMed]
- 11.Collet JP, Cuisset T, Rangé G, et al. ARCTIC Investigators. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med 2012; 367: 2100–2109. [DOI] [PubMed] [Google Scholar]
- 12.Tantry US, Bonello L, Aradi D, et al. Working group on on-treatment platelet reactivity. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol 2013; 62: 2261–2273. [DOI] [PubMed] [Google Scholar]
- 13.Jeong YH, Tantry US, Gurbel PA. Importance of potent P2Y(12) receptor blockade in acute myocardial infarction: focus on prasugrel. Expert Opin Pharmacother 2012; 13: 1771–1796. [DOI] [PubMed] [Google Scholar]
- 14.Wiviott SD, Braunwald E, McCabe CH, et al. TRITON-TIMI 38 investigators. prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357: 2001–2015. [DOI] [PubMed] [Google Scholar]
- 15.Roe MT, Armstrong PW, Fox KA, et al. TRILOGY ACS investigators. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med 2012; 367: 1297–1309. [DOI] [PubMed] [Google Scholar]
- 16.Montalescot G, Bolognese L, Dudek D, et al. ACCOAST Investigators. Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N Engl J Med 2013; 369: 999–1010. [DOI] [PubMed] [Google Scholar]
- 17.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361: 1045–1057. [DOI] [PubMed] [Google Scholar]
- 18.Bonaca MP, Bhatt DL, Cohen M, et al. PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372: 1791–1800. [DOI] [PubMed] [Google Scholar]
- 19.Mauri L, Kereiakes DJ, Yeh RW, et al. DAPT Study Investigators. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014; 371: 2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magnani G, Storey RF, Steg G, et al. Efficacy and safety of ticagrelor for long-term secondary prevention of atherothrombotic events in relation to renal function: insights from the PEGASUS-TIMI 54 trial. Eur Heart J 2016; 37: 400–408. [DOI] [PubMed] [Google Scholar]
- 21.Collet JP, Silvain J, Barthélémy O, et al. ARCTIC investigators. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet 2014; 384: 1577–1585. [DOI] [PubMed] [Google Scholar]
- 22.Stone GW, Maehara A, Lansky AJ, et al. PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011; 364: 226–235. [DOI] [PubMed] [Google Scholar]
- 23.Arbab-Zadeh A, Nakano M, Virmani R, et al. Acute coronary events. Circulation 2012; 125: 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tantry US, Liu F, Chen G, et al. Vorapaxar in the secondary prevention of atherothrombosis. Expert Rev Cardiovasc Ther 2015; 13: 1293–1305. [DOI] [PubMed] [Google Scholar]
- 25.Morrow DA, Braunwald E, Bonaca MP, et al. TRA 2P–TIMI 50 Steering Committee and Investigators. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med 2012; 366: 1404–1413. [DOI] [PubMed] [Google Scholar]
- 26.Scirica BM, Bonaca MP, Braunwald E, et al. TRA 2°P-TIMI 50 Steering Committee Investigators. Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA 2°P-TIMI 50 trial. Lancet 2012; 380: 1317–1324. [DOI] [PubMed] [Google Scholar]
- 27.Amsterdam EA, Wenger NK, Brindiset RG, et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64: e139–e228. [DOI] [PubMed] [Google Scholar]
- 28.Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2014; 35: 2541–2619.25173339 [Google Scholar]
- 29.Levine GN, O'Gara PT, Bates ER, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2016; 67: 1235–1250. [DOI] [PubMed]