Abstract
The mechanisms of mechanical energy recovery during gait have been thoroughly investigated in healthy subjects, but never described in patients with Parkinson disease (PD). The aim of this study was to investigate whether such mechanisms are preserved in PD patients despite an altered pattern of locomotion. We consecutively enrolled 23 PD patients (mean age 64±9 years) with bilateral symptoms (H&Y ≥II) if able to walk unassisted in medication-off condition (overnight suspension of all dopaminergic drugs). Ten healthy subjects (mean age 62±3 years) walked both at their ‘preferred’ and ‘slow’ speeds, to match the whole range of PD velocities. Kinematic data were recorded by means of an optoelectronic motion analyzer. For each stride we computed spatio-temporal parameters, time-course and range of motion (ROM) of hip, knee and ankle joint angles. We also measured kinetic (Wk), potential (Wp), total (WtotCM) energy variations and the energy recovery index (ER). Along with PD progression, we found a significant correlation of WtotCM and Wp with knee ROM and in particular with knee extension in terminal stance phase. Wk and ER were instead mainly related to gait velocity. In PD subjects, the reduction of knee ROM significantly diminished both Wp and WtotCM. Rehabilitation treatments should possibly integrate passive and active mobilization of knee to prevent a reduction of gait-related energetic components.
Introduction
Gait disturbance is a relevant component of motor disability in subjects with Parkinson disease (PD) and a large amount of experimental work has been dedicated to investigate biomechanical abnormalities in these patients. While PD patients at an early disease stage can show exclusively a reduction of gait velocity and stride length [1–3], along with disease progression they usually exhibit shortened stride length, prolonged stance and double support phases [4,5] and reduced velocity [3–6]. Gait cadence might not be altered [7,8] or, in some cases, it appears to be increased as a possible adaptation to stride length reduction [6,8–10]. The range-of-motion (ROM) at lower limb joints is also usually reduced [8,11–15]. Very few studies, with unclear results, investigated energetic expenditure in PD patients. In particular, patients were investigated in unspecified meds-on state [16], or while walking on a treadmill [17], a condition which has been shown to alter the gait pattern with respect to over-ground walking [18,19]. In addition, PD patients were never compared to healthy subjects walking at similar velocities. Last but not least, the role of mechanical energy recovery was never taken into account in the analysis of energy expenditure along a stride cycle of PD patients.
In normal walking, the gravitational potential energy (Ep) of the center of mass (CM) is at maximum level during mid stance, when the kinetic energy (Ek) of the CM is minimum. From mid stance, the CM descends, and Ep is partially converted into Ek; forward acceleration occurs and the body lands on the contralateral limb. After this foot-ground contact, the CM again moves upward (as long as the limb remains relatively straight extended) and decreases its forward velocity. As a consequence, Ep increases again and Ek decreases [19–21]. Energy variation corresponds to mechanical work, so that ΔEk = Wk and ΔEp = Wp. In an ideal energy recovery mechanism, the work associated to changes of potential energy is exactly the same as the work associated to kinetic energy changes, but with different sign: Wp = -Wk. That means that work produced to increase the potential energy can be obtained by reducing the kinetic energy, and can again be returned to increase the kinetic energy at the next step-to-step transition. Actually, the conversion between Ep and Ek does not occur completely, but it is about 70% during normal walking at preferred speed [21].
Several studies have separately indicated that the metabolic cost of walking is primarily allocated towards raising the CM throughout the gait cycle [22–24]. Therefore, the mechanism of exchanging Ek and Ep aims to reduce the metabolic cost of locomotion by lowering the muscular effort required to accelerate and decelerate the CM [25].
Aim of this study was to investigate changes in the mechanical energy recovery, and its correlations with spatio-temporal gait parameters, in a carefully selected cohort of PD patients at different disease stages.
Materials and Methods
Subjects
We consecutively enrolled 23 PD patients with bilateral symptoms (Hoehn and Yahr, HY stage ≥II) if able to walk unassisted in medication-off condition (overnight suspension of all dopaminergic drugs). All patients had stable dopaminergic treatment for at least six months and no levodopa-related motor fluctuations (e.g., dyskinesia). Ten age-matched healthy subjects (HC, mean age 62±3 years) also took part in the study. The diagnosis of PD was made according to the UK Brain Bank criteria and patients were evaluated with the Unified Parkinson Disease Rating Scale motor part (UPDRS-III). All PD patients improved (>20% at UPDRS-III score) after intake of 150–200 mg of L-Dopa (acute challenge test), thus further supporting the clinical diagnosis of idiopathic PD. Patients were not suffering from freezing of gait and did not show any freezing episodes during the acquisitions. No patients showed any atypical features of parkinsonism. Patients with cognitive decline (Mini-Mental State Examination <27) or any other signs of neurological or psychiatric disease other than PD were excluded. All patients did not suffer from any other disease than PD nor underwent any major surgery (e.g. orthopedic surgery). Patients were divided into two groups according to the HY stage: mild group (PDM: HY stage II), and severely affected group (PDS: HY stage III or IV). The local institutional review board (Section of Human Physiology, Department of Pathophysiology and Transplantation, University of Milan) approved the study and the consent procedure. All participants signed a written informed consent. All efforts were made to protect patient privacy and anonymity.
Experimental Setup and Protocol
Kinematic data were recorded using an optoelectronic system (SMART-E, BTS Bioengineering, Italy), consisting of six video cameras (sampling rate: 60 Hz; calibrated volume 4x2x1.5m). The position of the subjects’ main body segments was determined by means of 29 retro-reflective markers (diameter: 15 mm) according to a published protocol [1]. During the static calibration trial, eight additional “technical” markers were attached on the following bony landmarks, on both sides of the body: greater trochanter, medial femoral condyle, medial malleolus, and first metatarsal head. The position of these points, not visible to the cameras during gait, was computed offline by means of technical reference systems, assuming their relative position in relation to local reference frames was fixed. Anthropometric parameters of each subject were computed from the markers’ positions recorded during the calibration trial, and used for the estimation of internal joint centers, thus enabling calculation of lower limb kinematics. Subjects were asked to walk barefoot along a straight trajectory about 11.5 m long. All subjects started to walk from at least two strides behind the calibrated volume, without any starting command. Trials were repeated three to five times, according to patients' capabilities. All PD patients were evaluated after overnight suspension of all dopaminergic drugs (meds-off).
HC did two sets of eight walking trials at ‘preferred’ (HCN) and ‘slow’ (HCS) speeds, in random order, following verbal instructions in the absence of external feedback. The consistency of the two datasets was verified on the basis of the actual measured speeds.
Data Analysis and energy calculation
We used ad-hoc algorithms to compute the CM trajectory all along the gait cycle and to measure spatio-temporal gait parameters (i.e. walking speed, stride length and period, stance phase and double support phase duration), time courses of hip, knee and ankle joints angles during the stride cycle and their ROM.
For each subject, spatial parameters were normalized as a percentage of the body height (BH). Temporal parameters and all curves representing the time-course of kinematic variables were time normalized as a percentage of the stride duration (defined from heel contact of one foot to next heel contact of the same foot).
Subsequently, the mean values and the standard deviation (SD) for corresponding normalized time intervals were calculated for each variable of each subject. ROM values were computed as the difference between the maximum and the minimum values reached by each joint angle, within the stride.
The whole body center of mass (CM) was computed by estimating the displacement of the center of mass of each body segment (CMj), and then implementing the general formula:
| (1) |
where, YCM is the generic coordinate of CM; yj is the generic coordinate of center of mass of each anatomical segment (j), mj is the mass of each body segment (j) and M is the mass of the whole body. The position of CMj within each anatomical segment, as well as the mass of each body segment were obtained from the anthropometric tables and regression equations provided by Zatsiorsky and Seluyanov [26].
The kinetic energy associated to CM displacements was computed as follows:
| (2) |
where M is the whole body mass, vx, vy, vz are the three components of the velocity of CM.
The potential energy associated to CM was calculated as:
| (3) |
where g is the gravitational acceleration (m/s2) and h is the vertical distance of CM from the ground.
The total energy associated to CM was computed as function of time (t) as:
| (4) |
Over the stride period, the positive variations (difference between maximum and minimum) of respectively Ep,CM, Ek,CM, and Etot,CM were identified and calculated. They were named respectively Wp, Wk, and WtotCM. Then the energy recovery index (ER) was computed according to Cavagna et al. [21]:
| (5) |
All energetic parameters were computed for each stride collected from our subjects and averaged over the strides (left and right pooled together) for each gait velocity command and for each group of subjects. Energetic parameters were divided by the mass (M) of the subject.
Statistics
Statistical analysis was performed using JMP statistical package (version 12.0, SAS Institute, Inc., Cary, NC, USA). ROMs of left and right hemibodies were compared by means of matched pairs analysis. Differences between PD and HC groups were analyzed by means of Kruskal-Wallis and Steel-Dwass tests. To look for measurements that were predictive of energetic parameters, we used the Spearman correlation coefficient. Variables were then included in stepwise multiple linear regression. Strength of the correlations was defined according to the absolute value of ρ as “very weak” (.00-.19), “weak” (.20-.39), “moderate” (.40-.59), “strong” (.60-.79) and “very strong” (.80–1.0). A p<0.05 was considered to be statistically significant.
Results
Demographic and clinical data are listed in Table 1. As expected, PDS showed higher scores at UPDRS-III in meds-off and higher L-Dopa Equivalent Daily Dose (LEDD) [27] compared to PDM (p<0.05, in both cases). No significant differences were found for age and body mass index among PD sub-groups and HC. No difference was also found when comparing ROMs of the right and left hemibodies, both for HC and PD, and data were then pooled together.
Table 1. Demographic and clinical data.
| PDM | PDS | HC | |
|---|---|---|---|
| N. (male/female) | 10 (8/2) | 13 (7/6) | 10 (8/2) |
| Age (years) | 62 ± 9 | 65 ± 8 | 62 ± 3 |
| Weight (kg) | 80.2 ± 14.6 | 65.0 ± 13.7 | 80.8 ± 9.5 |
| Height (m) | 1.7 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.1 |
| BMI | 27.7 ± 5.2 | 24.5 ± 4.7 | 26.9 ± 3.1 |
| Disease duration (years) | 5 ± 2 | 12 ± 3 | |
| UPDRS-III | 20 ± 9 | 28 ± 9 | |
| L-Dopa daily dose | 325.0 ± 143.6 | 557.1 ± 225.0 | |
| LEDD | 443.3 ± 142.4 | 690.4 ± 205.6 |
LEDD = L-Dopa Equivalent Daily Dose; UPDRS-III = Unified Parkinson’s Disease Rating Scale motor part (III) in meds-off state; BMI = Body mass index. Disease duration was from motor symptoms onset. Values are means and standard deviation.
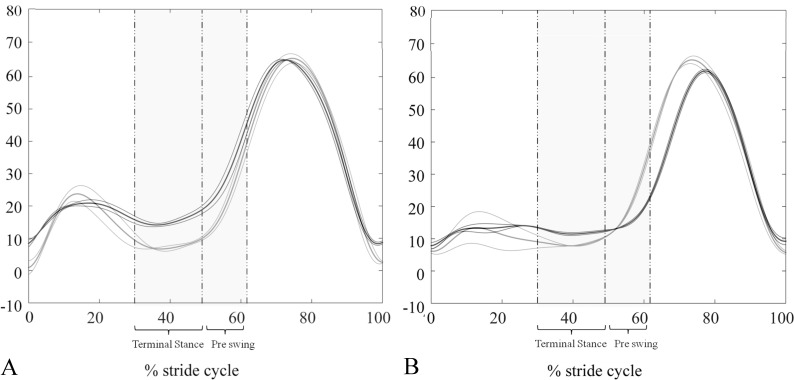
Spatio-temporal, kinematic and energetic parameters are listed in Table 2. Average gait velocity of HCN matched the homologous data of PDM, and the same hold true concerning HCS and PDS. Of relevance, PDS showed a significant reduction of stride length and knee ROM when compared to HCS, which was due to a more flexed knee in the stance phase (Fig 1). The average knee joint angles measured during terminal stance were: 12.74±5.32° and 6.22±4.62° for PDM and HCN (p<0.05), and 13.52±8.24° and 5.19±4.42° for PDS and HCS (p<0.05). Lastly, in PDS hip and ankle ROMs were reduced in comparison to PDM and both negatively correlated with UPDRS-III scores (hip ROM: ρ = -0.56, p<0.05 and ankle ROM: ρ = -0.54, p<0.05).
Table 2. Energetic, spatio-temporal and kinematic parameters.
| Parameters | HCN | HCS | PDM | PDS |
|---|---|---|---|---|
| Stride Velocity (%BH/s) | 67.4±6.0 1* | 43.3±8.1 1 | 64.2±5.6 2* | 41.1±8.7 2 |
| Stride Period (s) | 1.1±0.1 1* | 1.4±0.2 1 | 1.1±0.1 2 | 1.2±0.1 2 |
| Stride Length (%BH) | 71.6±5.2 1 | 59.3±5.9 1, 3 | 68.8±5.8 2* | 49.0±6.6 2, 3 |
| % Stance Phase | 61.8±2.1 1* | 66.5±2.8 1 | 61.1±1.8 2 | 64.7±3.4 2 |
| % Double Support Phase | 12.1±2.2 1 | 16.4±3.2 1 | 11.4±1.9 2* | 16.0±2.5 2 |
| Hip ROM (°) | 40.9±2.9 1* | 35.8±2.1 1 | 38.0±6.6 2* | 31.0±8.2 2 |
| Knee ROM (°) | 56.0±4.7 | 53.8±3.8 3 | 49.6±8.3 | 42.0±9.3 3 |
| Ankle ROM (°) | 24.5±4.3 | 22.1±5.1 | 26.8±4.7 2* | 20.4±6.2 2 |
| ER index (%) | 65.4±5.7 1 | 49.1±10.2 1 | 68.2±4.3 2 | 52.5±12.13 2 |
| WtotCM (J/kg) | 0.36±0.08 | 0.37±0.05 3* | 0.34±0.05 2* | 0.24±0.04 2, 3 |
| Wp (J/kg) | 0.57±0.14 | 0.47±0.09 3 | 0.57±0.06 2* | 0.33±0.09 2, 3 |
| Wk (J/kg) | 0.51±0.08 1* | 0.32±0.07 1, 3 | 0.5±0.09 2* | 0.21±0.06 2, 3 |
Superscript numbers indicate statistically significant differences (p<0.05 or p<0.01 when * is present) between HCN and HCS (1), PDS and PDM (2), HCS and PDS (3). We did not find any statistical difference between HCN and PDM. Values are means and standard deviation. See text for statistical analysis.
Fig 1. Time courses of knee flexion/extension angles.
(A) Comparison between one representative PDM (black lines) and one HCN (grey lines). (B) Comparison between one representative PDS (black lines) and one HCS (grey lines). Thick and thin lines refer to the average time courses ±SD of different trials, respectively. The intervals of maximum knee extension, reached during the stance phase, are highlighted in grey.
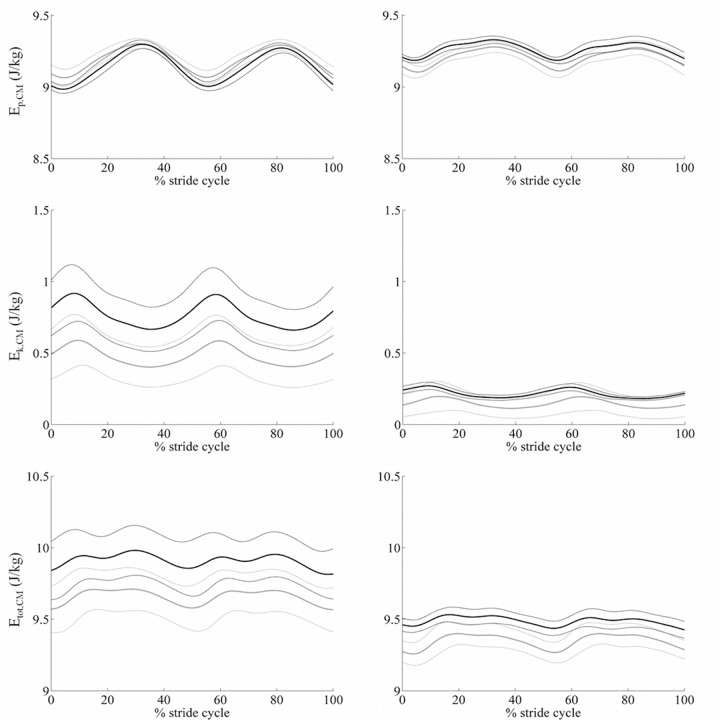
In Table 2 we listed all energetic measurements. Fig 2 shows time courses of kinetic, potential and total energy associated to CM during the stride cycle. As expected, we found low values of ER and Wk in slow walking subjects (i.e. PDS and HCS) being both measurements strictly related to gait velocity. Indeed, ER and Wk correlated with stride velocity in HC (ρ = 0.82, p<0.0001 and ρ = 0.88, p<0.001, respectively) and in PD patients (ρ = 0.69, p<0.001 and ρ = 0.91, p<0.0001, respectively).
Fig 2. Energy components.
Left column: PDM and HCN. Right column: PDS and HCS. Black lines refer to one representative PD and grey lines to one HC. Thick and thin lines refer to the average time courses ±SD of different trials, respectively.
PDS showed lower Wp values in comparison with PDM and, more interestingly, with HCS, although walking at comparable velocities. Wp positively correlated with hip ROM in HC (ρ = 0.73, p<0.01) while with knee ROM in PD (ρ = 0.68, p<0.01). Wk values were lower in PDS than HCS. Besides the aforementioned correlation with stride velocity, in PD patients Wk also correlated with hip ROM (ρ = 0.79, p<0.001). WtotCM matched closely with Wp findings. All mean data per subject were listed in S1 Table.
Discussion
The main finding of our study was a reduction of WtotCM and Wp along with PD progression. These changes were greatly dependent on knee ROM reduction and in particular on knee extension in the terminal stance phase of the stride. Wk was also reduced in advanced PD patients primarily due to low gait velocities, but also due to a reduction of hip ROM, possibly reflecting a greater rigidity and stopped posture in more advanced stages of the disease (i.e. PDS).
The direct correlation of all energetic parameters with gait velocity [28,29], as seen also when comparing cohorts matched for gait velocity (Table 2), is reasonable if we consider that Wk is the variation of kinetic energy along the stride, and thus it depends on the square of velocity, and Wp is the variation of potential energy, which relies upon the vertical excursion of CM. Of note, the minimum height of CM excursion is reached during the double support phase, and depends on step length, which in turn is related to hip joint excursion and on gait velocity. The maximum height of CM excursion depends instead on how much the knee is extended in the mid stance phase.
In PDS, we found two main conditions to justify a reduction of all energetic components. In particular, (i) a reduced gait velocity, mainly as a result of short stride length (stride time was even shorter than in HCS) and (ii) a reduced hip and knee ROM, the latter resulting mainly from a lack of full extension in the terminal stance. Of note, in normal subjects the rate of knee extension in terminal stance should be approximately half that of flexion during limb loading [28]. In PDS, a deeply flexed knee during stance resulted in a reduced rising and a more flat path of the CM [25] which in turns reduced the amount of stored gravitational Ep [25,29,30]. We speculate that such an increased knee flexion could be related to an altered activity of plantar flexors muscles [9], which normally play a role to accelerate the knee into extension [12]. Indeed, in terminal stance the triceps surae muscle increases its activity and contracts vigorously as an ankle stabilizer [28]. The lack of EMG recording prevents us from confirming this hypothesis, but a reduction in amplitude of gastrocnemius activity was previously described in PD patients [12,13].
Of relevance, the finding that Wk, Wp, and WtotCM were reduced in PDS in comparison to both patients walking faster (i.e. PDM) and control subjects walking at a similar velocity (i.e. HCS) suggests that such a reduction is mainly related to different kinematic patterns (such as altered ROMs) rather than to gait velocity per se.
At this point, it was quite unexpected that, despite a considerable reduction of all energetic parameters, the ER index itself was not reduced in PDS when compared to HCS subjects. This may suggest that the basic energy recovery mechanism, as adopted by normal subjects, which is an efficient way to reduce the energetic cost of walking, is still exploited in PD patients, also at advanced disease stages. Still, in PDS the ER was relatively low when compared to subjects walking faster (both PDM and HCN). Therefore, it can be argued that any intervention aimed at increasing gait velocity would be beneficial from the energetic point of view. However, it must be considered that lower limb can only roughly be approximated to an inverted pendulum for which perfect out-of-phase kinetic and potential energy variations occur. Actually, among the many factors that can reduce energy cost during walking, the knee flexion-extension at load acceptance, the ankle plantarflexion-dorsiflexion at early stance phase and pelvis tilt in the frontal plane in mid stance are the most important [31,32]. They all must be regarded with great attention if walking efficiency, as manifested by the ER index, is to be preserved.
A limitation of our study is the relative low number of patients recruited. This relied mainly upon our inclusion criteria. In particular, only few PD patients at HY stage III or IV were willing and able to suspend overnight all dopaminergic medications and even fewer were able to complete unassisted all walking trials in meds-off state. In this study, we recruited solely subjects with PD at HY stage II or higher as patients with mild motor symptoms can show normal ROM of hip, knee and ankle joints during linear walking at preferred speed ([1] and Table 2).
At last, our findings could help developing a tailored rehabilitation treatment of gait in PD subjects. Indeed, PD patients could benefit from passive and active mobilization of the knee to possibly normalize knee extension and consequently improve WtotCM and Wp. Knee extension should be also monitored and possibly reinforced during treadmill training, which has been proved useful in the rehabilitation of gait disorders in PD, in particular at an early stage of the disease [33–35].
Supporting Information
(PDF)
Acknowledgments
The authors would like to thank Drs. Margherita Canesi and Roberto Cilia for patient referral. We wish to thank also Eng. Alberto Marzegan for his help with algorithms writing.
The study was funded in part by the Grigioni Foundation for Parkinson’s disease, the Fondazione Europea Ricerca Biomedica FERB ONLUS and the Interdisziplinäres Zentrum für Klinische Forschung (IZKF) of the University Hospital Würzburg.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
The study was funded in part by the Fondazione Grigioni per la Malattia di Parkinson (IUI), Fondazione Europea Ricerca Biomedica FERB – ONLUS (IUI, GF), and the Interdisziplinäres Zentrum für Klinische Forschung (IZKF F-255) of the University Hospital Würzburg (MD, EEP, AC, IUI). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Carpinella I, Crenna P, Calabrese E, Rabbuffetti M, Mazzoleni P, Nemni R, et al. Locomotor function in the early stage of Parkinson’s disease. IEEE Trans Neural Syst Rehabil Eng. 2007;15(4):543–551. 10.1109/TNSRE.2007.908933 [DOI] [PubMed] [Google Scholar]
- 2.Shulman LM, Gruber-Baldini AL, Anderson KE, Vaughan CG, Reich SG, Fishman PS, et al. The evolution of disability in Parkinson disease. Mov Disord. 2008;23(6):790–796. 10.1002/mds.21879 [DOI] [PubMed] [Google Scholar]
- 3.Morris ME, Iansek R, Matyas TA, Summers JJ. The pathogenesis of gait hypokinesia in Parkinson’s disease. Brain. 1994;117(5):1169–1181. [DOI] [PubMed] [Google Scholar]
- 4.Ferrarin M, Lopiano L, Rizzone M, Lanotte M, Bergamasco B, Recalcati M, et al. Quantitative analysis of gait in Parkinson’s disease: a pilot study on the effects of bilateral sub-thalamic stimulation. Gait Posture. 2002;16(2):135–148. [DOI] [PubMed] [Google Scholar]
- 5.Vieregge P, Stolze H, Klein C, Heberlein I. Gait quantitation in Parkinson’s disease—locomotor disability and correlation to clinical rating scales. J Neural Transm. 1997;104(2–3):237–248. [DOI] [PubMed] [Google Scholar]
- 6.Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain. 1996;119(2):551–568. [DOI] [PubMed] [Google Scholar]
- 7.Morris ME, McGinley J, Huxham F, Collier J, Iansek R. Constraints on the kinetic, kinematic and spatiotemporal parameters of gait in Parkinson’s disease. Hum Mov Sci. 1999;18(2–3):461–483. [Google Scholar]
- 8.Morris M, Iansek R, McGinley J, Matyas T, Huxham F. Three-dimensional gait biomechanics in Parkinson’s disease: Evidence for a centrally mediated amplitude regulation disorder. Mov Disord. 2005;20(1):40–50. [DOI] [PubMed] [Google Scholar]
- 9.Kimmeskamp S, Hennig EM. Heel to toe motion characteristics in Parkinson patients during free walking. Clin Biomech. 2001;16(9):806–812. [DOI] [PubMed] [Google Scholar]
- 10.Morris ME, Iansek R, Matyas TA, Summers JJ. Ability to modulate walking cadence remains intact in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1994;57(12):1532–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrarin M, Rizzone M, Bergamasco B, Lanotte M, Recalcati M, Pedotti A, et al. Effects of bilateral subthalamic stimulation on gait kinematics and kinetics in Parkinson’s disease. Exp Brain Res. 2005;160(4):517–527. [DOI] [PubMed] [Google Scholar]
- 12.Mitoma H, Hayashi R, Yanagisawa N, Tsukagoshi H. Characteristics of parkinsonian and ataxic gaits: A study using surface electromyograms, angular displacements and floor reaction forces. J Neurol Sci. 2000;174(1):22–39. [DOI] [PubMed] [Google Scholar]
- 13.Dietz V, Zijlstra W, Prokop T, Berger W. Leg muscle activation during gait in Parkinson’s disease: adaptation and interlimb coordination. Electroencephalogr Clin Neurophysiol. 1995;97(6):408–415. [DOI] [PubMed] [Google Scholar]
- 14.Morris ME, Huxham F, McGinley J, Dodd K, Iansek R. The biomechanics and motor control of gait in Parkinson disease. Clin Biomech. 2001;16(6):459–470. [DOI] [PubMed] [Google Scholar]
- 15.Delval A, Salleron J, Bourriez JL, Bleuse S, Moreau C, Krystkowiak P, et al. Kinematic angular parameters in PD: Reliability of joint angle curves and comparison with healthy subjects. Gait Posture. 2008;28(3):495–501. 10.1016/j.gaitpost.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 16.Maggioni MA, Veicsteinas A, Rampichini S, Cè E, Nemni R, Riboldazzi G, et al. Energy cost of spontaneous walking in Parkinson’s disease patients. Neurol Sci. 2012;33(4):779–784. 10.1007/s10072-011-0827-6 [DOI] [PubMed] [Google Scholar]
- 17.Christiansen CL, Schenkman ML, McFann K, Wolfe P, Kohrt WM. Walking economy in people with Parkinson’s disease. Mov Disord. 2009;24(10):1481–1487. 10.1002/mds.22621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carpinella I, Crenna P, Rabuffetti M, Ferrarin M. Coordination between upper- and lower-limb movements is different during overground and treadmill walking. Eur J Appl Physiol. 2010;108(1):71–82. 10.1007/s00421-009-1168-5 [DOI] [PubMed] [Google Scholar]
- 19.Cavagna GA, Heglund NC, Taylor CR. Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. Am J Physiol—Regul Integr Comp Physiol. 1977;233:R243–R261. [DOI] [PubMed] [Google Scholar]
- 20.Margaria R. Biomechanics and Energetics of Muscular Exercise (Clarendon Press, Oxford: ).; 1976. [Google Scholar]
- 21.Cavagna GA, Thys H, Zamboni A. The sources of external work in level walking and running. J Physiol. 1976;262(3):639–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grabowski A, Farley CT, Kram R. Independent metabolic costs of supporting body weight and accelerating body mass during walking. J Appl Physiol. 2005;98(2):579–583. [DOI] [PubMed] [Google Scholar]
- 23.Duff-Raffaele M, Kerrigan DC, Corcoran PJ, Saini M. The proportional work of lifting the center of mass during walking. Am J Phys Med Rehabil. 1996;75(5):375–379. [DOI] [PubMed] [Google Scholar]
- 24.Neptune RR, Zajac FE, Kautz SA. Muscle mechanical work requirements during normal walking: The energetic cost of raising the body’s center-of-mass is significant. J Biomech. 2004;37(6):817–825. [DOI] [PubMed] [Google Scholar]
- 25.Sparling TL, Schmitt D, Miller CE, Guilak F, Somers TJ, Keefe FJ, et al. Energy recovery in individuals with knee osteoarthritis. Osteoarthr Cartil. 2014;22(6):747–755. 10.1016/j.joca.2014.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zatsiorsky V, Seluyanov V. The mass and inertia characteristics of the main segments of the human body. Biomech VIII-B Proc Eight Int Congr Biomech. 1983:1152–1159. [Google Scholar]
- 27.Isaias IU, Marotta G, Hirano S, Canesi M, Benti R, Righini A, et al. Imaging essential tremor. Mov Disord. 2010;25(6):679–686. 10.1002/mds.22870 [DOI] [PubMed] [Google Scholar]
- 28.Perry J. Gait Analysis: Normal and Pathological Function. J Pediatr Orthop. 1992;12:815. [Google Scholar]
- 29.Fisher NM, White SC, Yack HJ, Smolinski RJ, Pendergast DR. Muscle function and gait in patients with knee osteoarthritis before and after muscle rehabilitation. Disabil Rehabil. 1997;19(2):47–55. [DOI] [PubMed] [Google Scholar]
- 30.Stauffer RN, Chao EY, Györy a N. Biomechanical gait analysis of the diseased knee joint. Clin Orthop Relat Res. 1977;(126):246–255. [PubMed] [Google Scholar]
- 31.Inman VT, Ralson HJ, Todd F. Human Walking Baltimore (MD): Williams & Wilkins; 1981. [Google Scholar]
- 32.INMAN VT. Human locomotion. Can Med Assoc J. 1966;94(20):1047–1054. [PMC free article] [PubMed] [Google Scholar]
- 33.Mehrholz J, Friis R, Kugler J, Twork S, Storch A, Pohl M. Treadmill training for patients with Parkinson’s disease. Cochrane Database Syst Rev. 2010;(1). [DOI] [PubMed] [Google Scholar]
- 34.Frazzitta G, Maestri R, Uccellini D, Bertotti G, Abelli P. Rehabilitation treatment of gait in patients with Parkinson’s disease with freezing: A comparison between two physical therapy protocols using visual and auditory cues with or without treadmill training. Mov Disord. 2009;24(8):1139–1143. 10.1002/mds.22491 [DOI] [PubMed] [Google Scholar]
- 35.Frazzitta G, Maestri R, Bertotti G, Riboldazzi G, Boveri N, Perini M, et al. Intensive Rehabilitation Treatment in Early Parkinson’s Disease: A Randomized Pilot Study With a 2-Year Follow-up. Neurorehabil Neural Repair. 2015;29(2):123–131. 10.1177/1545968314542981 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.