Abstract
Background
Rigorous processes ensure quality of research and clinical care at NCI-designated Comprehensive Cancer Centers (NCICCC). Un-measurable elements of structure and process of cancer care delivery warrant evaluation. Impact of NCICCC care on survival and access to NCICCCs for vulnerable subpopulations remains unstudied.
Methods
Our population-based cohort of 69,579 patients had newly-diagnosed adult-onset (22–65 years) cancers reported to the Los Angeles County (LAC) cancer registry between 1998 and 2008. Geographic Information Systems was used for geospatial analysis.
Results
Overall Survival
Across multiple diagnoses, patients not receiving their first planned treatment at NCICCCs experienced poorer outcome compared to those treated at NCICCCs; differences persisted in multivariable analyses adjusting for clinical and sociodemographic factors (hepatobiliary [HR=1.5, 95%CI, 1.4–1.7, p<0.001]; lung [HR=1.4, 95%CI, 1.3–1.6, p<0.001]; pancreatic [HR=1.5, 95%CI, 1.3–1.7, p<0.001]; gastric [HR=1.3, 95%CI, 1.1–1.7, p=0.01]; oral [HR=1.2, 95%CI, 1.0–1.5, p=0.09]; breast [HR=1.3, 95%CI, 1.1–1.5, p<0.001]; and colorectal [HR=1.2, 95%CI, 1.0–1.4, p=0.05).
Barriers to care
Multivariable analyses revealed that a lower likelihood of treatment at NCICCCs was associated with: race/ethnicity (African-American: OR range across diagnoses: 0.4–0.7, p≤0.03; Hispanic: OR, 0.5–0.7, p≤0.04); lack of private insurance (public: OR, 0.6–0.8, p≤0.004; uninsured: OR, 0.1–0.5, p≤0.04); less than high SES (high-mid: OR, 0.4–0.7, p≤0.02; mid: OR, 0.3–0.5, p≤0.001; low: OR, 0.2–0.6, p≤0.01) and residing >9 miles from nearest NCICCC (OR 0.5–0.7, p≤0.02).
Conclusions
Among 22 to 65 year-olds with newly-diagnosed adult-onset cancer, LAC patients treated at NCICCCs experienced superior survival compared with those at non-NCICCC facilities. Barriers to care at NCICCCs included race/ethnicity, insurance, SES and distance.
Keywords: Cancer, Outcomes, NCI, Cancer Centers
INTRODUCTION
Despite therapeutic and supportive care advances, prognosis for many cancers remains poor. Outcome is assessed along the lines of disease biology, therapy and patient-specific sociodemographic factors (race/ethnicity1,2); nevertheless, sociodemographic factors necessitate consideration within the construct of the health care delivery system. Donabedian deconstructed health care delivery into structure, process and outcome,3 with structure connoting the scaffolding on which the health care delivery system is built – from physical and information systems, to payor structure; process encompassed the provision of the care – from decision making to implementation. Elements of structure have been evaluated including insurance and socioeconomic status (SES).4,5 Facility and the volume relationship with surgical diseases has been evaluated,6 as have surgical outcomes and facility safety net status,7 size, technology and academic status.8 Elements of process evaluated include guideline compliance,9,10 enrollment on clinical trials,11 and organizational affiliation12 in isolated malignancies. Many elements have been measured in the surgical setting with numerous unmeasured elements especially in the medical facets of oncology, including supportive care, multidisciplinary decision-making, and mechanism of therapy delivery. Without validated, systematic and widely-available measures at the granular level of the structure and process of cancer care delivery, we conceptually dichotomized care into that which is delivered at centers with National Cancer Institute (NCI) designation of comprehensiveness, and those who do not. “To facilitate discovery and its translation into direct benefit to patients and the general public, the NCI awards [the designation of comprehensive cancer center] to institutions that critically mass excellent cancer-relevant research; [NCICCC] focus on research derives from the belief that a culture of discovery, scientific excellence, transdisciplinary research and collaboration yields tangible benefits extending far beyond the generation of new knowledge.”13 To our knowledge, the impact of care at NCICCCs on survival remains unstudied outside of pediatric/adolescent cancer,14 post-operative mortality15,16 and older patients.17 Within this framework, access to NCICCCs for vulnerable populations (underrepresented minorities, low SES, public or no insurance, and distance to care) warrants comprehensive evaluation despite examination in isolated malignancies18, as sociodemographic factors are intrinsically linked to both health care delivery19 and quality,20 and impact where a patient receives general21 or cancer healthcare22.
Pending widespread development and implementation of detailed measures in response to the Institute of Medicine’s (IOM) call to action to measure quality23,24 a surrogate measure was employed to evaluate the outcome for patients diagnosed between the ages of 22–65 years with adult-onset cancers in Los Angeles County (LAC) by assessing care at NCICCCs as compared to non-NCICCC facilities. We further aimed to explore access to NCICCCs for patients from racial/ethnic minority groups, without private insurance, from low SES or facing potential geographic barriers to care.
METHODS
Patients
We assembled a population-based cohort of 75,987 patients newly diagnosed between 1998–2008 at 22–65 years of age with adult-onset cancers (breast, cervical, colorectal, gastric, hepatobiliary, lung, oral, and pancreatic) using the LAC cancer registry (Cancer Surveillance Project [CSP]). [Detail in supplementary materials]. Eligible patients resided and received care at facilities within LAC. This project was approved by the State of California’s and City of Hope’s institutional review boards.
Clinical Prognostic Variables
Cases were selected using ICDO-3-based histology codes with appropriate site codes; in-situ disease was excluded. Clinical variables included primary diagnosis, age at diagnosis, gender and stage. CSP summary staging was used, which is based on the Collaborative Staging system integrating TNM categories, stage groupings and SEER Extent of Disease coding. A histology variable accounted for differences between poor prognosis histology breast cancer (inflammatory, sarcoma) and others.
Sociodemographic Predictors
A combined race/ethnicity variable yielded the categories: Non-Hispanic white (NHW), Hispanic, African-American (AA), and Asian/Pacific-Islander (API). Due to small numbers (n=334, 0.4%), Alaskan Native/Other patients and those with unknown/missing ethnicity were excluded. We collapsed insurance into three categories: public, private, and no insurance; patients were excluded (n=2,551, 3.4%) if payor was missing/unknown. The SES variable was collapsed into four levels, combining the lowest SES levels.
Treatment Site
Systematic definition of care identified the facility associated with each episode of care. We prioritized the facility where the patient had all or part of the first course of treatment (detailed in supplemental materials). Patients were considered treated at NCICCCs if they were cared for at one of the three NCICCCs in LAC (UCLA/Jonsson, USC/Norris and City of Hope). All other patients were considered to have received care at non-NCICCC sites.
Geography
CSP provided patient address at diagnosis. Geographic Information Systems ([GIS]; ArcMap 10.1, esri, Redlands, CA) was used to geocode hospital address, and measure straight-line distance between patient residence and the nearest NCICCC. Euclidean distance is highly correlated with drive time 25 and has been used in distance to cancer care investigations in California.18
Statistical Analysis
Overall survival (OS) was calculated using Kaplan-Meier survival analysis, (log-rank tests detected differences between groups). Cox regression techniques determined hazard ratios of mortality with associated 95% confidence intervals (CI). Logistic regression analysis was determined odds ratios (OR) with associated 95%CI for multivariable modeling of the likelihood of receiving care at an NCICCC. Unless otherwise noted, multivariable models of survival adjusted for age at diagnosis, gender, race/ethnicity, stage, SES and payor while multivariable models for likelihood of receiving care at NCICCCs included the above variables and distance to nearest NCICCC. Two-sided tests with p<0.05 were considered statistically significant. SAS 9.3 (SAS Institute, Cary, NC) was used for all analysis. Patients with missing/unknown sociodemographic data comprised a small proportion of the cohort. Parallel univariable and multivariable analyses were performed in a cohort; including these patients had no impact on the hazard ratio nor significance (detail in supplemental material), thus they were excluded in the final analysis.
RESULTS
Patients
Characteristics of the cohort (n=69,579) are detailed in Table 1, overall and by treatment site. Racial and ethnic minorities, low SES and publicly insured and uninsured patients were well-represented.
Table 1.
Patient Characteristics Overall and by Treatment Site
| Total (n=69,579) | NCICCC (n=4,428) | Non-NCICCC (n=65,151) | p-value | |
|---|---|---|---|---|
| Age | ||||
| 22–39y | 5,873 (8.4%) | 429 (9.7%) | 5,444 (8.4%) | 0.002 |
| 40–65y | 63,706 (91.6%) | 3,999 (90.3%) | 59,707 (91.6%) | |
| Gender | ||||
| Female | 50,005 (71.9%) | 3,141 (70.9%) | 46,864 (71.9%) | 0.15 |
| Male | 19,574 (28.1%) | 1,287 (29.1%) | 18,287 (28.1%) | |
| Race/Ethnicity | ||||
| NHW | 32,040 (46.1%) | 2,601 (58.7%) | 29,439 (45.2%) | <0.001 |
| Black | 9,532 (13.7%) | 314 (7.1%) | 9,218 (14.1%) | |
| Hispanic | 17,412 (25.0%) | 702 (15.9%) | 16,710 (25.7%) | |
| API | 10,595 (15.2%) | 811 (18.3%) | 9,784 (15.0%) | |
| Payor | ||||
| Private | 46,843 (67.3%) | 3,468 (78.3%) | 43,376 (66.6%) | <0.001 |
| Public | 17,585 (25.3%) | 852 (19.3%) | 16,736 (25.7%) | |
| Uninsured | 5,151 (7.4%) | 108 (2.4%) | 5,043 (7.7%) | |
| SES | ||||
| High | 16,058 (23.1%) | 1,810 (40.9%) | 14,248 (21.9%) | <0.001 |
| High-Middle | 15,294 (22.0%) | 1,086 (24.5%) | 1,086 (24.5%) | |
| Middle | 14,357 (20.6%) | 723 (16.3%) | 13,634 (20.9%) | |
| Low | 23,870 (34.3%) | 809 (18.3%) | 23,061 (35.4%) | |
| Distance to Nearest NCICCC in miles | ||||
| Median (IQR) | 9.1 (5.8–13.9) | 7.5 (5.0–12.7) | 9.2 (5.9–14.0) | <0.001 |
| Mean (SD) | 10.8 (7.3) | 9.8 (7.4) | 10.9 (7.2) | |
| Primary Diagnosis | ||||
| Breast (n=31,762) | ||||
| Localized/Regional Extension | 18,583 | 1,199 (57.9%) | 17,384 (58.6%) | 0.58 |
| Regional Nodes +/− Extension | 11,738 | 770 (37.2%) | 10,968 (36.9%) | |
| Remote | 1,441 | 103 (4.9%) | 1,338 (4.5%) | |
| Colorectal (n=12,298) | ||||
| Localized/Regional Extension | 6,082 | 216 (43.2%) | 5,866 (49.7%) | 0.02 |
| Regional Nodes +/− Extension | 3,242 | 144 (28.8%) | 3,098 (26.3%) | |
| Remote | 2,974 | 140 (28.0%) | 2,834 (24.0%) | |
| Lung (n=10,844) | ||||
| Localized/Regional Extension | 1,957 | 101 (21.7%) | 1,856 (17.9%) | 0.05 |
| Regional Nodes +/− Extension | 1,663 | 78 (16.7%) | 1,585 (15.3%) | |
| Remote | 7,224 | 287 (61.6%) | 6,937 (66.8%) | |
| Hepatobiliary (n=4,181) | ||||
| Localized/Regional Extension | 2,619 | 488 (78.3%) | 2,131 (60.0%) | <0.001 |
| Regional Nodes +/− Extension | 239 | 27 (4.3%) | 212 (6.0%) | |
| Remote | 1,323 | 108 (17.4%) | 1,215 (34.2%) | |
| Cervix (n=3,691) | ||||
| Localized/Regional Extension | 2,876 | 108 (75.5%) | 2,768 (78.0%) | 0.78 |
| Regional Nodes +/− Extension | 421 | 18 (12.6%) | 403 (11.4%) | |
| Remote | 394 | 17 (11.9%) | 377 (10.6%) | |
| Gastric (n=2,664) | ||||
| Localized/Regional Extension | 550 | 29 (23.6%) | 521 (20.5%) | 0.65 |
| Regional Nodes +/− Extension | 810 | 38 (30.9%) | 772 (30.4%) | |
| Remote | 1,304 | 56 (45.5%) | 1,248 (49.1%) | |
| Pancreas (n=2,317) | ||||
| Localized/Regional Extension | 504 | 84 (33.6%) | 420 (20.3%) | <0.001 |
| Regional Nodes +/− Extension | 358 | 51 (20.4%) | 307 (14.9%) | |
| Remote | 1,455 | 115 (46.0%) | 1,340 (64.8%) | |
| Oral Cancer (n=1,822) | ||||
| Localized/Regional Extension | 978 | 132 (52.6%) | 846 (53.8%) | 0.93 |
| Regional Nodes +/− Extension | 628 | 88 (35.1%) | 540 (34.4%) | |
| Remote | 216 | 31 (12.3%) | 185 (11.8%) | |
Treatment Site
Clinical and sociodemographic details by treatment site are presented in Table 1. A majority of the cohort received their first planned treatment at a non-NCICCC facility. There was no difference in the proportion of newly-diagnosed patients treated at non-NCICCC vs. NCICCC facilities by clinical stage for breast, cervical, gastric and oral cancers. In colorectal cancer, there was a higher representation of lower stage cancers at the non-NCICCC facilities; in hepatobiliary, lung and pancreatic disease there was a higher representation of lower stage cancers at the NCICCCs.
Survival by site of care
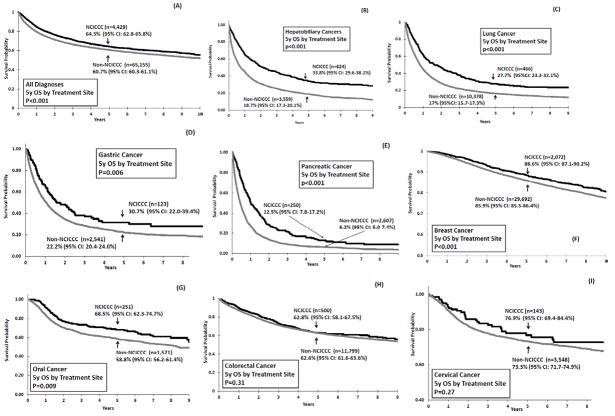
Patients treated at non-NCICCC facilities had poorer outcome as compared to NCICCCs in the following cancers (Figure 1; Table 2): hepatobiliary, lung, pancreatic, gastric, oral, and breast. After adjusting for clinical and sociodemographic factors, patients treated at non-NCICCC facilities continued to experience a higher likelihood of mortality in all but oral cancer. A group of breast cancer patients had hormone receptor (estrogen, progesterone) and HER2 status available (n=15,545, 48.9%); a combined hormone receptor (HR) variable [HR+/HER2−, HR+/HER2+, HR−/HER2−, HR−/HER2+, borderline/unknown] informed a subset analysis in these patients with identical results (Supplemental Table 1). Stratified by histology (non-small-cell, small-cell), lung cancer patients had identical findings.
Figure 1. Five-Year Overall Survival by Treatment Site in patients with adult-onset cancers diagnosed and treated in Los Angeles County.
The following curves compare five-year overall survival between patients 22 to 65 years of age cared for at National Cancer Institute-designated Comprehensive Cancer Centers (NCICCC) versus patients cared for in the community (Non-NCICCC): (A) represents the overall cohort; (B) through (I) represent survival by disease entity.
Table 2.
Survival: NCICCCs vs. Non-NCICCC Facilities
| Primary Diagnosis | 5-year Overall Survival (OS)a | Likelihood of Mortalitya | ||
|---|---|---|---|---|
| OS (95% CI) | p-value | HR (95% CI) | p-value | |
| Full Cohort | ||||
| NCICCC | 64.3% (62.8–65.8%) | <0.001 | 1.0 | <0.001 |
| Non-NCICCC | 60.7% (60.3–61.1%) | 1.3 (1.2–1.3) | ||
| Hepatobiliary | ||||
| NCICCC | 33.8% (29.6–38.1%) | <0.001 | 1.0 | <0.001 |
| Non-NCICCC | 18.7% (17.3–20.1%) | 1.5 (1.4–1.7) | ||
| Lung | ||||
| NCICCC | 27.7% (23.3–32.1%) | <0.001 | 1.0 | <0.001 |
| Non-NCICCC | 17% (15.7–17.3%) | 1.4 (1.3–1.6) | ||
| Pancreas | ||||
| NCICCC | 12.5% (7.8–17.2%) | <0.001 | 1.0 | <0.001 |
| Non-NCICCC | 6.2% (5.0–7.4%) | 1.5 (1.3–1.7) | ||
| Gastric | ||||
| NCICCC | 30.7% (22.0–39.4%) | 0.007 | 1.0 | 0.01 |
| Non-NCICCC | 22.2% (20.4–24.0%) | 1.3 (1.1–1.7) | ||
| Breast b,c | ||||
| NCICCC | 88.6% (87.1–90.2%) | <0.001 | 1.0 | <0.001 |
| Non-NCICCC | 85.9% (85.5–86.4%) | 1.3 (1.1–1.5) | ||
| Cervix c | ||||
| NCICCC | 76.9% (69.4–84.4%) | 0.27 | 1.0 | 0.07 |
| Non-NCICCC | 73.3% (71.7–74.9% | 1.3 (0.9–1.9) | ||
| Oral | ||||
| NCICCC | 68.5% (62.3–74.7%) | 0.009 | 1.0 | 0.09 |
| Community | 58.8% (56.2–61.4%) | 1.2 (1.0–1.5) | ||
| Colorectal | ||||
| NCICCC | 62.8% (58.1–67.5%) | 0.31 | 1.0 | 0.05 |
| Non-NCICCC | 62.6% (61.6–63.6%) | 1.2 (1.0–1.4) | ||
Multivariable Cox regression, adjusted for age, gender, stage, race/ethnicity, SES, payor
Adjusted for histology.
Females.
In colorectal disease, patients treated at non-NCICCC and NCICCC facilities had similar outcomes in univariable analysis; however, after adjusting for sociodemographic and clinical characteristics, colorectal cancer patients treated at non-NCICCC facilities experienced a higher likelihood of mortality (Table 2). These findings persisted in parallel analyses stratified by SES. (Supplemental Table 2).
In cervical cancer, patients had similar outcomes at both facilities; adjustment for sociodemographic and clinical factors revealed a trend towards an increased risk of mortality in patients treated at non-NCICCC facilities (Table 2).
Likelihood of care at NCICCC
Across most diagnoses, patients were less likely to be treated at NCICCCs (Table 3) if they were from an underrepresented minority group [AA or Hispanic]. There was a ‘dose effect’ in SES in which the lowest SES patients had the lowest likelihood of being treated at NCICCCs and the highest SES had the highest likelihood; this was the case in all cancers except for oral and cervical. Uninsured patients were less likely to receive care at NCICCCs in all cancers (trend in breast); publicly insured patients were less likely to receive NCICCC care in the full cohort along with hepatobiliary, gastric and oral cancer. Patients living more than 9 miles from the nearest NCICCC were less likely to be treated at an NCICCC in the majority of cancers; distance did not impact the likelihood of treatment at NCICCCs in hepatobiliary or oral cancers. Older patients (40–65 years) were less likely to be treated at an NCICCC than younger patients (22–39 years) in oral and breast cancers, while age did not have a similar impact other cancers.
Table 3.
Likelihood of Receiving Care at NCICCC vs. Non-NCICCC Facilitya
| Primary Diagnosis | Race/Ethnicity (ref: NHW) | Payor (ref: private) | SES (ref: high) | Age (ref:22–39y) | Distance (ref <9 miles) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Black | Hispanic | API | Public | Uninsured | High-Mid | Mid | Low | 40–65y | >9 miles | |
| Full Cohort | ||||||||||
| OR (95% CI) | 0.6 (0.5–0.7) | 0.7 (0.6–0.8) | 1.0 (0.9–1.1) | 0.8 (0.8–0.9) | 0.3 (0.3–0.4) | 0.6 (0.6–0.7) | 0.5 (0.4–0.5) | 0.3 (0.3–0.4) | 0.7 (0.6–0.8) | 0.6 (0.55–0.63) |
| p-value | <0.001 | <0.001 | 0.48 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Hepatobiliary | ||||||||||
| OR (95% CI) | 0.7 (0.5–0.9) | 0.8 (0.6–1.0) | 0.9 (0.7–1.1) | 0.7 (0.6–0.9) | 0.2 (0.1–0.3) | 0.7 (0.5–0.8) | 0.5 (0.4–0.6) | 0.4 (0.3–0.5) | 0.9 (0.6–1.4) | 0.9 (0.7–1.1) |
| p-value | 0.02 | 0.1 | 0.20 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | 0.71 | 0.17 |
| Lung | ||||||||||
| OR (95% CI) | 0.5 (0.3–0.7) | 0.6 (0.4–0.9) | 0.3 (1.0–1.7) | 0.8 (0.7–1.0) | 0.2 (0.1–0.4) | 0.6 (0.5–0.8) | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) | 0.6 (0.4–1.1) | 0.6 (0.5–0.7) |
| p-value | <0.001 | 0.01 | 0.03 | 0.11 | <0.001 | <0.001 | <0.001 | <0.001 | 0.11 | <0.001 |
| Pancreatic | ||||||||||
| OR (95% CI) | 0.4 (0.2–0.7) | 0.5 (0.3–0.7) | 1.1 (0.7–1.6) | 0.8 (0.6–1.2) | 0.5 (0.2–1.0) | 0.4 (0.3–0.6) | 0.4 (0.2–0.5) | 0.3 (0.2–0.4) | 0.9 (0.4–1.7) | 0.7 (0.6–1.0) |
| p-value | 0.001 | 0.001 | 0.83 | 0.23 | 0.04 | <0.001 | <0.001 | <0.001 | 0.55 | 0.02 |
| Gastric | ||||||||||
| OR (95% CI) | 0.4 (0.2–1.0) | 0.6 (0.3–1.0) | 1.2 (0.8–1.9) | 0.5 (0.3–0.8) | 0.1 (0.03–0.5) | 0.5 (0.3–0.9) | 0.6 (0.4–1.0) | 0.3 (0.1–0.4) | 0.9 (0.5–1.6) | 0.6 (0.4–0.8) |
| p-value | 0.06 | 0.06 | 0.44 | 0.004 | 0.002 | 0.02 | 0.07 | <0.001 | 0.63 | 0.003 |
| Oral | ||||||||||
| OR (95% CI) | 0.6 (0.3–1.0) | 0.6 (0.4–1.0) | 1.0 (0.6–1.6) | 0.6 (0.4–0.9) | 0.2 (0.1–0.4) | 0.7 (0.5–1.0) | 0.5 (0.3–0.8) | 0.6 (0.4–0.9) | 0.4 (0.3–0.7) | 1.0 (0.8–1.4) |
| p-value | 0.06 | 0.04 | 0.93 | 0.005 | <0.001 | 0.08 | 0.001 | 0.01 | <0.001 | 0.83 |
| Breastb,c | ||||||||||
| OR (95% CI) | 0.6 (0.5–0.8) | 0.7 (0.6–0.8) | 1.1 (1.0–1.2) | 0.9 (0.8–1.1) | 0.8 (0.6–1.1) | 0.7 (0.6–0.8) | 0.5 (0.4–0.6) | 0.3 (0.3–0.4) | 0.7 (0.6–0.8) | 0.5 (0.4–0.5) |
| p-value | <0.001 | <0.001 | 0.24 | 0.27 | 0.055 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Colorectal | ||||||||||
| OR (95% CI) | 0.6 (0.4–0.8) | 0.7 (0.5–0.9) | 0.9 (0.7–1.2) | 0.9 (0.7–1.2) | 0.5 (0.3–0.8) | 0.5 (0.4–0.6) | 0.3 (0.3–0.4) | 0.2 (0.2–0.3) | 0.7 (0.5–1.0) | 0.7 (0.6–0.8) |
| p-value | <0.001 | <0.001 | 0.58 | 0.61 | 0.002 | <0.001 | <0.001 | <0.001 | 0.06 | <0.001 |
| Cervicalc | ||||||||||
| OR (95% CI) | 0.9 (0.4–1.6) | 0.5 (0.3–0.8) | 0.6 (0.5–1.0) | 0.7 (0.5–1.1) | 0.1 (0.04–0.4) | 0.7 (0.4–1.1) | 0.7 (0.4–1.2) | 0.5 (0.3–0.8) | 0.8 (0.6–1.1) | 0.6 (0.4–0.8) |
| p-value | 0.61 | 0.003 | 0.055 | 0.13 | <0.001 | 0.10 | 0.19 | 0.004 | 0.17 | 0.002 |
Logistic Regression, adjusted for all variables in addition to gender and stage.
Adjusted for histology.
Females.
Interactions were examined between distance and the following: SES, race/ethnicity and stage. The only significant interaction was between distance and SES in oral cancer, in which patients living closer to the NCICCCs were less likely to receive treatment at NCICCCs if they were in the low SES rather than the high SES group.
DISCUSSION
Our population-level findings demonstrate that patients newly diagnosed between 22–65 years of age in LAC with specific adult-onset cancers have superior survival when receiving initial therapy at NCICCCs rather than at non-NCICCC facilities. We identify race/ethnicity, lack of private insurance, low SES and distance from the nearest NCICCC as barriers to receiving treatment at NCICCCs.
In 2013, the IOM deemed the US cancer care system in crisis with evolving disparities.24,26 Recommendations thus included (1) national quality measurement and (2) reduction of disparities in access for vulnerable and underserved populations. Quality measurement is integral to achieving these objectives,24 but current measurement systems fall short in breadth and specificity.26 We utilized NCICCC designation as an externally validated, peer-reviewed and re-reviewed population-level surrogate measure to encompass unmeasurable facets of health care delivery’s structure and process. This designation is based in breadth of research capabilities in which clinical, laboratory and population cancer research are integrated into a transdisciplinary approach, and serves as an externally validated, rigorous designation. Key elements also include state-of-the-art facilities, translation of findings from bench to bedside and then curbside, and outreach to underserved populations.13 This investigation represents a hypothesis-generating, preliminary assessment of quality of care.
After adjusting for key sociodemographic and clinical variables, there was a 20–50% increased risk of mortality associated with treatment at non-NCICCC facilities rather than NCICCCs in newly-diagnosed hepatobiliary, lung, pancreatic, gastric, breast and colorectal cancers. The trend toward superior survival in NCICCC patients with cervical cancer did not achieve statistical significance, primarily due to a small number of these patients seeking initial treatment at NCICCCs. The NCICCC impact on outcome is likely multifactorial; while some of these have been measured (surgical outcome15, organizational affiliation12, guideline compliance10) there are many aspects which have not been measured and include aspects of comprehensiveness of care, therapy, clinical trial availability, supportive care and other elements of the designation which contribute to the structure and process of health care delivery. Disease entities in which care models have shown disparities in outcome between centers of excellence and community care include genetic diseases (cystic fibrosis27 and hemophilia28), where outcomes likely reflect provider/staff expertise, comprehensiveness of care and institution of quality metrics; surgical volume stands on solid evidence.6,29 The examined diseases are not purely surgical nor guideline-dependent, thus current data support the hypothesis that currently unmeasurable differences contribute to outcome disparities between facilities operating on a model of NCICCC designation and those who are not.
With NCICCC care significantly predicting outcome, access to care warranted exploration. Compared to NHW patients, Hispanic and AA patients were less likely to receive treatment at NCICCCs across cancers. This contradicts the attention that NCICCCs direct towards underserved populations13 and previous investigations in older patients,18 underlining the IOM recommendation to ameliorate disparities in access for vulnerable and underserved populations. With evidence that patients from underrepresented minorities and other underserved populations lack equal access to centers providing comparable outcomes, evaluation of cancer care provision within the NCICCC system and the greater cancer community is crucial.
Lack of private insurance was associated with a lower likelihood of NCICCC treatment; uninsured status was significant across cancers and public insurance in many. Insurance influences location of health care delivery in different ways; current data were from an era of evolving health care and insurance frameworks. Payor/organizational contracts establish initial contact points within the system, but numerous factors drive referrals from that point, insurance being only one. To this end, the ‘dose effect’ of SES in which the lowest SES was associated with the lowest likelihood of treatment at NCICCCs and the highest SES with the highest likelihood points to the importance of the multiple facets of this variable. With insurance emerging as a predictor independent of SES, the significance of SES and its ‘dose effect’ represents income and educational status; education likely impacts health literacy including the ability to self-refer and/or discuss options with one’s initial point of contact within the system.
Finally, distance from an NCICCC was associated with a lower likelihood of utilizing an NCICCC in all but hepatobiliary and oral cancers. Likely this represents familiarity in treating the examined cancers without needing to refer to NCICCCs; hepatobiliary and oral cancers represent either a therapeutic or surgical niche requiring a perceived expertise available at NCICCCs. How to apply the statistically significant, yet modest difference in absolute distance between groups to other counties will vary; LAC has more NCICCCs than most states, and is physically challenging to navigate with 9 miles representing a transportation challenge and time commitment distinct from other regions, as presented in other distance evaluations.18,30 Studying LAC alone provides a geographic and sociodemographic landscape with a robust multi-ethnic population, 3 NCICCCs within 4,752 square miles spanning rural and urban areas, and a population that would rank as the eighth most populous state.
Clinical characteristics were comparable between NCICCC and non-NCICCC patients. The anecdotal belief that referral bias leads towards a dominance of high-grade cancers at the NCICCCs was not unilaterally confirmed in data regarding newly diagnosed patients. We saw a higher representation of lower stage colorectal cancers in non-NCICCCs, with higher representation of lower stage hepatobiliary, lung and pancreatic disease at NCICCCs, and no differences in the remaining diseases. Colorectal cancer staging may be influenced by community-based screening strategies and referrals. It is plausible that surgical expertise in less disseminated stages of hepatobiliary, pancreatic and lung cancers require specialized surgical services, and that nonspecific symptoms in these may be associated with earlier evaluations in patients more often at NCICCCs (private insurance, high SES), thus presenting in lower stages.
The conceptual model driving this investigation is that NCICCC treatment yields superior survival not otherwise explained by disease severity, structure or process variables; a number of unmeasurable variables would contribute to a full case mix control model if available including comorbidity, lifestyle, environment and nutrition in terms of outcome and lifestyle, deprivation, English proficiency and physician referral patterns in terms of access. Population-level cancer registry data enabled us to study a large sample size across institution types and cancer diagnoses, without the bias of data collection occurring only at research institutions; nevertheless, registry data lack granularity. Minimal detailed treatment data precludes examination of therapeutic differences. Without comorbidity data, limiting our study to adults <65 years aimed to limit comorbidities. The findings are generalizable to newly-diagnosed patients.
The question emerges, how do NCICCCs deliver superior outcomes? We hypothesize this encompasses multiple aspects of comprehensiveness, as the findings span cancer stages (localized to remote), and diagnoses (with/without a role for surgery and with/without clear guidelines). To this end, guideline compliance,9,10 enrollment on clinical trials,11 organizational affiliation12 and surgical expertise6 likely contribute in select malignancies along with supportive care, multidisciplinary decision-making, mechanism of therapy delivery and availability of investigator-initiated clinical trials allowing the direct benefit of cutting-edge research. We posit that requirements for high-quality research13 are associated with delivery of high-quality clinical care with clinicians and administrators serving either as investigators or alongside investigators; these sites are mandated to lead, clinical trials, exchange ideas, disseminate findings, and maintain facility requirements. The NCI operates on the belief that a culture of discovery, scientific excellence, transdisciplinary research and collaboration yields tangible benefits extending far beyond the generation of new knowledge.13
These population-level findings indicate the presence of significant differences in outcome according to care at NCICCCs in several newly-diagnosed cancers and highlight the need for ongoing investigations into the structure and process of cancer care delivery. Prior studies addressing the payor, socioeconomic or surgical volume elements have been integral to piecing together the model, but drawing broad conclusions from one facet of the model ignores the interconnectedness that Donabedian31 laid out for health care delivery. Each element contributes to overall outcome, and is not mutually exclusive. The identification of barriers to receiving treatment at specialized cancer centers underscores a crucial gap in provision of cancer care for vulnerable populations. The evolving health care delivery system has been separately focused on providing access and improving quality; these findings suggest that the IOM recommendations regarding development and implementation of robust cancer-focused quality measures are crucial, and that such measurement should delve into granular measures as it continues to assess whether all patients have access to cancer care that promises comparable outcomes.
Supplementary Material
Acknowledgments
Funding: NIH (K12CA001727; P30CA33572 [COH Survey Research Core]); St. Baldrick’s Foundation.
Footnotes
No conflicts of interest [JAW, CLS, LW and SB]. AH consults [GTX; Seattle Genetics] and leads investigator-initiated research [GSK; Celgene].
References
- 1.Shavers VL, Brown ML. Racial and Ethnic Disparities in the Receipt of Cancer Treatment. Journal of the National Cancer Institute. 2002 Mar 6;94(5):334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 2.Kadan-Lottick NS, Ness KK, Bhatia S, Gurney JG. Survival Variability by Race and Ethnicity in Childhood Acute Lymphoblastic Leukemia. JAMA. 2003 Oct 15;290(15):2008–2014. doi: 10.1001/jama.290.15.2008. [DOI] [PubMed] [Google Scholar]
- 3.Donabedian A. The quality of care: How can it be assessed? JAMA. 1988;260(12):1743–1748. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
- 4.Kent E, Sender L, Largent J, Anton-Culver H. Leukemia survival in children, adolescents, and young adults: influence of socioeconomic status and other demographic factors. Cancer Causes and Control. 2009;20(8):1409–1420. doi: 10.1007/s10552-009-9367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez CP, Baz R, Jawde RA, et al. Impact of socioeconomic status and distance from treatment center on survival in patients receiving remission induction therapy for newly diagnosed acute myeloid leukemia. Leukemia Research. 2008;32(3):413–420. doi: 10.1016/j.leukres.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Archives of surgery (Chicago, Ill: 1960) 2003 Jul;138(7):721–725. doi: 10.1001/archsurg.138.7.721. discussion 726. [DOI] [PubMed] [Google Scholar]
- 7.Sabik LM, Bradley CJ. Differences in Mortality for Surgical Cancer Patients by Insurance and Hospital Safety Net Status. Medical care research and review : MCRR. 2013;70(1):84–97. doi: 10.1177/1077558712458158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghaferi AA, Osborne NH, Birkmeyer JD, Dimick JB. Hospital Characteristics Associated with Failure to Rescue from Complications after Pancreatectomy. Journal of the American College of Surgeons. 2010;211(3):325–330. doi: 10.1016/j.jamcollsurg.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 9.Buchholz TA, Theriault RL, Niland JC, et al. The Use of Radiation As a Component of Breast Conservation Therapy in National Comprehensive Cancer Network Centers. Journal of Clinical Oncology. 2006 Jan 20;24(3):361–369. doi: 10.1200/JCO.2005.02.3127. [DOI] [PubMed] [Google Scholar]
- 10.Bilimoria KY, Balch CM, Wayne JD, et al. Health Care System and Socioeconomic Factors Associated With Variance in Use of Sentinel Lymph Node Biopsy for Melanoma in the United States. Journal of Clinical Oncology. 2009 Apr 10;27(11):1857–1863. doi: 10.1200/JCO.2008.18.7567. [DOI] [PubMed] [Google Scholar]
- 11.Bleyer WA. Cancer in older adolescents and young adults: Epidemiology, diagnosis, treatment, survival, and importance of clinical trials. Medical and Pediatric Oncology. 2002;38(1):1–10. doi: 10.1002/mpo.1257. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter WR, Reeder-Hayes K, Bainbridge J, et al. The Role of Organizational Affiliations and Research Networks in the Diffusion of Breast Cancer Treatment Innovation. Medical Care. 2011;49(2):172–179. doi: 10.1097/MLR.0b013e3182028ff2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NCI. [Accessed July 6, 2014];NCI-Designated Cancer Centers. 2012 http://www.cancer.gov/researchandfunding/extramural/cancercenters/about.
- 14.Howell DL, Ward KC, Austin HD, Young JL, Woods WG. Access to Pediatric Cancer Care by Age, Race, and Diagnosis, and Outcomes of Cancer Treatment in Pediatric and Adolescent Patients in the State of Georgia. J Clin Oncol. 2007 Oct 10;25(29):4610–4615. doi: 10.1200/JCO.2006.07.6992. [DOI] [PubMed] [Google Scholar]
- 15.Birkmeyer NJ, Goodney PP, Stukel TA, Hillner BE, Birkmeyer JD. Do cancer centers designated by the National Cancer Institute have better surgical outcomes? Cancer. 2005 Feb 1;103(3):435–441. doi: 10.1002/cncr.20785. [DOI] [PubMed] [Google Scholar]
- 16.Paulson EC, Mitra N, Sonnad S, et al. National Cancer Institute designation predicts improved outcomes in colorectal cancer surgery. Annals of surgery. 2008 Oct;248(4):675–686. doi: 10.1097/SLA.0b013e318187a757. [DOI] [PubMed] [Google Scholar]
- 17.Onega T, Duell EJ, Shi X, Demidenko E, Gottlieb D, Goodman DC. Influence of NCI cancer center attendance on mortality in lung, breast, colorectal, and prostate cancer patients. Med Care Res Rev. 2009 Oct;66(5):542–560. doi: 10.1177/1077558709335536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang LC, Ma Y, Ngo JV, Rhoads KF. What factors influence minority use of National Cancer Institute-designated cancer centers? Cancer. 2014 Feb 1;120(3):399–407. doi: 10.1002/cncr.28413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen RM, Davidson PL. Improving Access to Care in America: Individual and Contextual Indicators. In: Andersen RM, Rice TH, Kominski GF, editors. Changing the US Health Care System. 3. San Francisco: John Wiley & Sons, Inc; 2007. pp. 3–31. [Google Scholar]
- 20.Ayanian JZ, Weissman JS, Chasan-Taber S, Epstein AM. Quality of care by race and gender for congestive heart failure and pneumonia. Med Care. 1999 Dec;37(12):1260–1269. doi: 10.1097/00005650-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Mayberry RM, Mili F, Ofili E. Racial and ethnic differences in access to medical care. Med Care Res Rev. 2000;57(Suppl 1):108–145. doi: 10.1177/1077558700057001S06. [DOI] [PubMed] [Google Scholar]
- 22.Onega T, Duell EJ, Shi X, Demidenko E, Goodman DC. Race versus place of service in mortality among Medicare beneficiaries with cancer. Cancer. 2010;116(11):2698–2706. doi: 10.1002/cncr.25097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ensuring Quality Cancer Care. The National Academies Press; 1999. [PubMed] [Google Scholar]
- 24.Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. The National Academies Press; 2013. [PubMed] [Google Scholar]
- 25.Bliss RL, Katz JN, Wright EA, Losina E. Estimating proximity to care: are straight line and zipcode centroid distances acceptable proxy measures? Med Care. 2012 Jan;50(1):99–106. doi: 10.1097/MLR.0b013e31822944d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spinks TE, Ganz PA, Sledge GW, Jr, et al. Delivering high-quality cancer care: The critical role of quality measurement. Healthcare. 2014;2(1):53–62. doi: 10.1016/j.hjdsi.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahadeva R, ADJ, Kevin W, et al. Clinical outcome in relation to care in centres specialising in cystic fibrosis: cross sectional studyCommentary: Management in paediatric and adult cystic fibrosis centres improves clinical outcome. BMJ. 1998;316(7147):1771–1775. doi: 10.1136/bmj.316.7147.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker JR, Crudder SO, Riske B, Bias V, Forsberg A. A Model for a Regional System of Care to Promote the Health and Well-Being of People with Rare Chronic Genetic Disorders. American Journal of Public Health 2005. 2005 Nov 01;95(11):1910–1916. doi: 10.2105/AJPH.2004.051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002 Apr 11;346(15):1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 30.Wolfson J, Sun C-L, Kang T, Wyatt L, D’Appuzzo M, Bhatia S. Impact of Treatment Site in Adolescents and Young Adults With Central Nervous System Tumors. Journal of the National Cancer Institute. 2014 Aug 1;106(8) doi: 10.1093/jnci/dju166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donabedian A, Wheeler JR, Wyszewianski L. Quality, cost, and health: an integrative model. Med Care. 1982 Oct;20(10):975–992. doi: 10.1097/00005650-198210000-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.